Introduction
In recent years, research regarding the role of cell
apoptosis in cardiovascular diseases has advanced the understanding
of numerous cardiovascular diseases (1). It was previously believed that
myocardial ischemia, as well as ischemia/reperfusion (IR)
injury-induced myocardial death is a type of necrosis (2). It was demonstrated in recent research
that oxidative stress, overloading, ischemia, hypoxia, and
reperfusion injury will not only induce myocardial necrosis, but
also cause myocardial apoptosis (3). Myocardial ischemia,
ischemia/reperfusion, and heart failure are all related to
myocardial apoptosis (3). As cell
apoptosis is a pathological process controlled by a series of
programs and mediated by signaling pathways, blocking its signaling
pathways will contribute to blocking apoptosis, preventing a
reduction in the number of myocardial cells, and sustaining or
improving cardiac function (4).
Since the discovery that the caspase-3 protein,
which promotes cell apoptosis in mammals and nematodes, possesses
high homology in 1993, research has focused on the mechanism of
action of such proteases on cell apoptosis. The caspase family
plays a major role in apoptosis, among which, caspase-3 is the
final effector of the caspase cascade that is involved in
apoptosis, which plays a core role in the protease cascade cutting
process. Various proteases will cut the caspase-3 zymogen, which
activates caspase-3, and the activated caspase-3 will further cut
various substrates, resulting in final cell apoptosis (5). It is the marker as well as the
executor of cell apoptosis, the major mechanism of which is to
digest and destroy multiple intracellular protease complexes,
activate the intranuclear nuclease, lead to DNA pyrolysis, destroy
the calcium pump function of cells, resulting in intracellular
calcium overload (6).
p53 gene expression plays a leading role in the
myocardial apoptosis of IR. Myocardial apoptosis in hypoxia-induced
newborn rats is markedly increased, and expression of both p53
protein and mRNA is notably increased, as can be found in research
on cultured myocardial cells in newborn rats (7). Bcl-2 gene expression can block the
p53-induced cell apoptosis, which is independent of changes in p53
expression and its location in the nucleus; in addition, it can
inhibit the bax transcription of p53 (7,8). Bcl-2
is an important anti-apoptotic factor (9).
Iodine-131 is a type of radionuclide dominated by γ-
and β-decay, and is used to kill multi-layer tumor cells and cover
the leukemia proliferation (10).
During the process of applying iodine to treat differentiated
thyroid carcinoma, accompanying with cell morphological and
functional degeneration; besides, the expression of sodium-iodine
symporter and thyroid-stimulating hormone receptor gene is reduced,
with the decrease or even the loss of iodine uptake capacity
(11). In the present study, we
investigated the effects of iodine-131 on the induction of
apoptosis in human cardiac muscle cells and the underlying
molecular mechanisms.
Materials and methods
Cell culture and cell viability
assay
H9c2 cells were provided by Shanghai Cell Bank
(Shanghai, China) and maintained in complete Dulbecco's modified
Eagle's medium (DMEM) at 37°C in a humidified incubator containing
5% CO2. The cells were seeded in 96-well plates for 24 h
with culture medium per well containing 7.4, 14.8 and 29.6 MBq/ml
of iodine-131 for 24, 48 and 72 h. Following addition of 40 µl/well
MTT, the cells were incubated for 4 h and the medium was discarded.
DMSO (150 µl/well) was added and incubated for 20 min. The sample
absorbance was measured using a Multiskan plate reader (BioTek
Instruments, Inc., Winooski, VT, USA) at 570 nm.
Cell apoptosis assay
The cells were seeded in 96-well plates for 24 h
with culture medium per well containing 7.4, 14.8 and 29.6 MBq/ml
of iodine-131 for 48 h. Subsequently, the cells were washed with
phosphate-buffered saline (PBS) and centrifuged at 200 × g for 5
min. The cells were stained with 100 µl of Annexin V-PI labeling
solution (BD Biosciences, San Jose, CA, USA) for 15 min in
darkness. The apoptosis rate was analyzed by flow cytometry (BD
Biosciences).
Caspase-2/−3/−9 activity colorimetric
protease assay
Cells were harvested and lysed in RIPA lysis buffer,
and then protein content was extracted using the
radioimmunoprecipitation assay buffer (Wolsen, Xi'an, China). Equal
amount of protein was incubated with caspase-2/−3/−9 activity
colorimetric protease assay for 2 h at 37°C. The sample absorbance
was measured using a Multiskan plate reader (BioTek Instruments,
Inc.) at 405 nm.
Western blot analysis
Cells were harvested and lysed in RIPA lysis buffer,
and then the protein content was extracted using
radioimmunoprecipitation assay buffer (Wolsen). Equal amount of
protein was electrophoresed using 8–10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by
transfer of the proteins to a polyvinylidene difluoride membrane
(EMD Millipore, Billerica, MA, USA). The membranes were blocked
with 5% bovine serum albumin in TBAT (MP Biomedicals LLC, Santa
Ana, CA, USA) for 1 h at room temperature and incubated overnight
at 4°C with Bax, cytochrome c, PIDD, t-BID, cytochrome
c and GAPDH (Santa Cruz Biotechnology, Inc.). Subsequently,
the membranes were washed with TBAT and incubated with horseradish
peroxidase-labeled anti-rabbit or anti-mouse immunoglobulin (Ig)G
(Santa Cruz Biotechnology, Heidelberg, Germany) at 37°C for 1
h.
Statistical analysis
Data are presented as the mean ± standard deviation.
Experimental results were assessed using Chi-square test, t-test or
ANOVA as appropriate. Results were considered significant at
p≤0.05.
Results
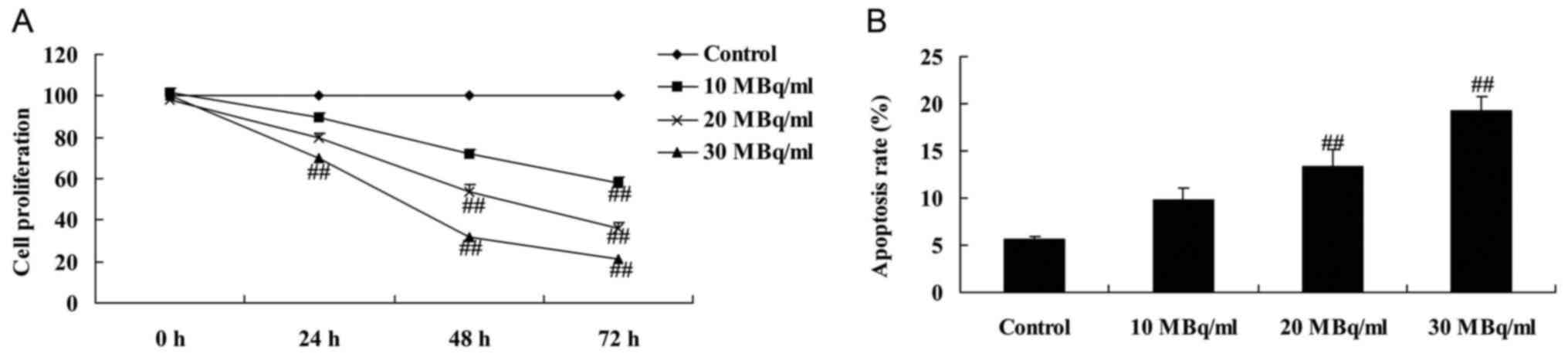
Iodine-131 reduces cell proliferation
and induces apoptosis in human cardiac muscle cells
To determine whether iodine-131 affects human
cardiac muscle cell growth, MTT assay and flow cytometry were used
to assess cell proliferation and apoptosis, respectively. As showed
in Fig. 1A, 30 MBq/ml of iodine-131
significantly reduced cell proliferation and induced apoptosis of
human cardiac muscle cells at 24, 48 and 72 h. Meanwhile, 20 MBq/ml
of iodine-131 significantly reduced cell proliferation and induced
apoptosis of human cardiac muscle cells at 48 and 72 h (Fig. 1A). Moreover, 10 MBq/ml of iodine-131
significantly reduced cell proliferation and induced apoptosis of
human cardiac muscle cells at 72 h (Fig. 1). Iodine-131 (20 and 30 MBq/ml)
significantly induced the apoptosis of human cardiac muscle cells
(Fig. 1B).
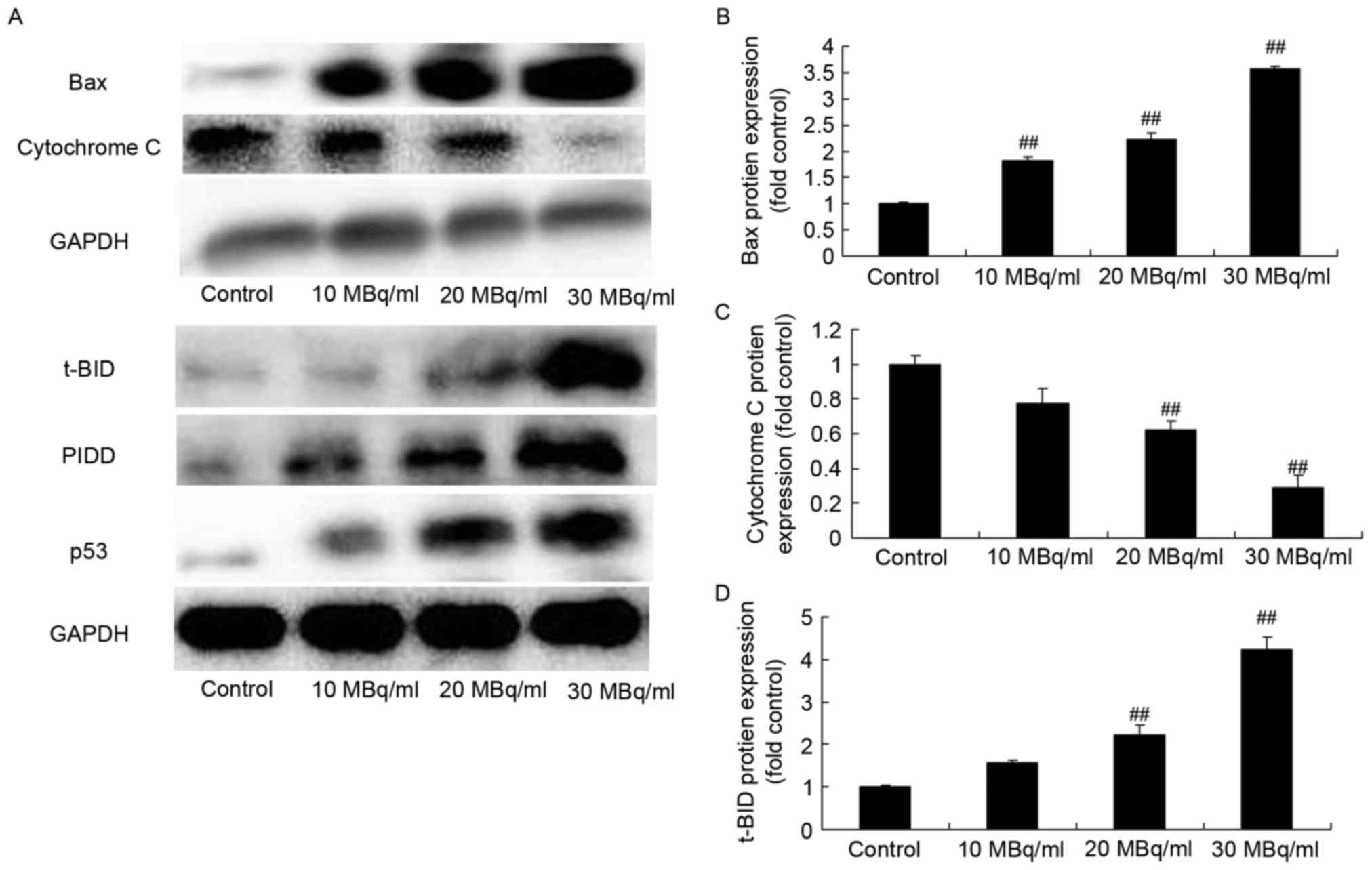
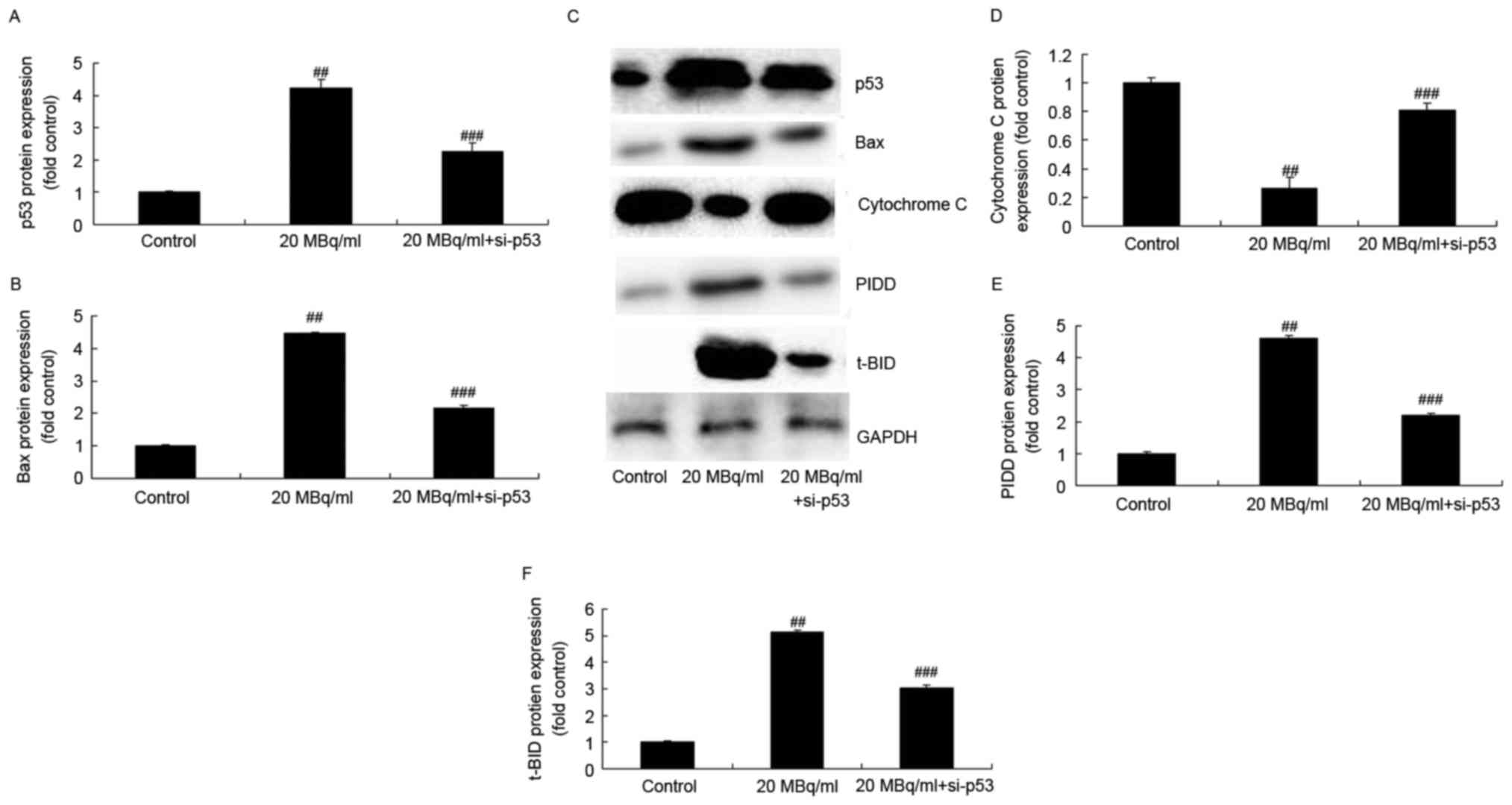
Iodine-131 induces p53, PIDD, t-BID
and Bax protein expression, and inhibits cytochrome c protein
expression in human cardiac muscle cells
In order to investigate the mechanism involved in
the human cardiac muscle cell apoptosis induced by iodine-131, we
used western blot analysis to analyze p53, PIDD, t-BID and Bax
protein expression, and cytochrome c protein expression in
human cardiac muscle cells. As showed in Fig. 2, iodine-131 significantly induced
p53, PIDD, t-BID and Bax protein expression, and decreased
cytochrome c protein expression in human cardiac muscle
cells.
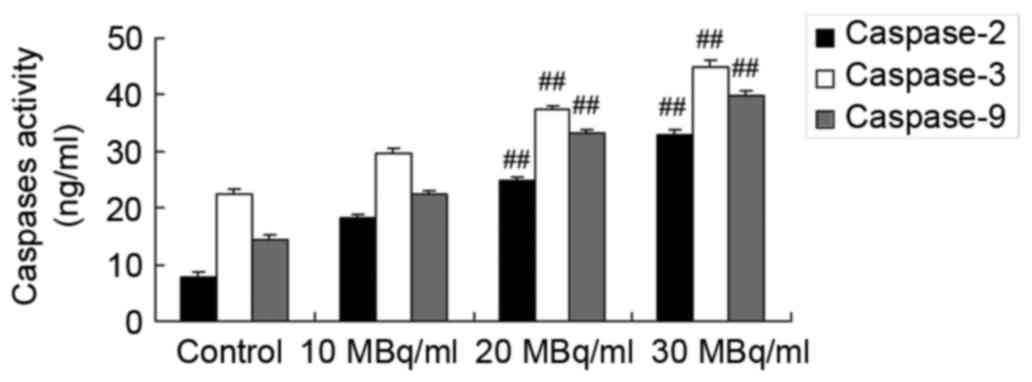
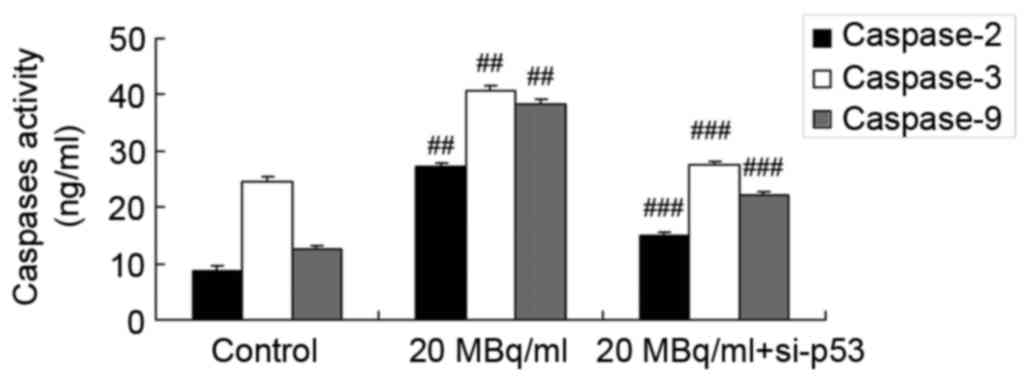
Iodine-131 promotes caspase-2, −3 and
−9 expression levels in human cardiac muscle cells
To determine the mechanism underlying the effects of
iodine-131 on caspase-2, −3 and −9 expression levels in human
cardiac muscle cells, commercial kits were used to analyze
caspase-2, −3 and −9 expression levels. As showed in Fig. 3, iodine-131 significantly promoted
caspase-2, −3 and −9 expression levels in human cardiac muscle
cells.
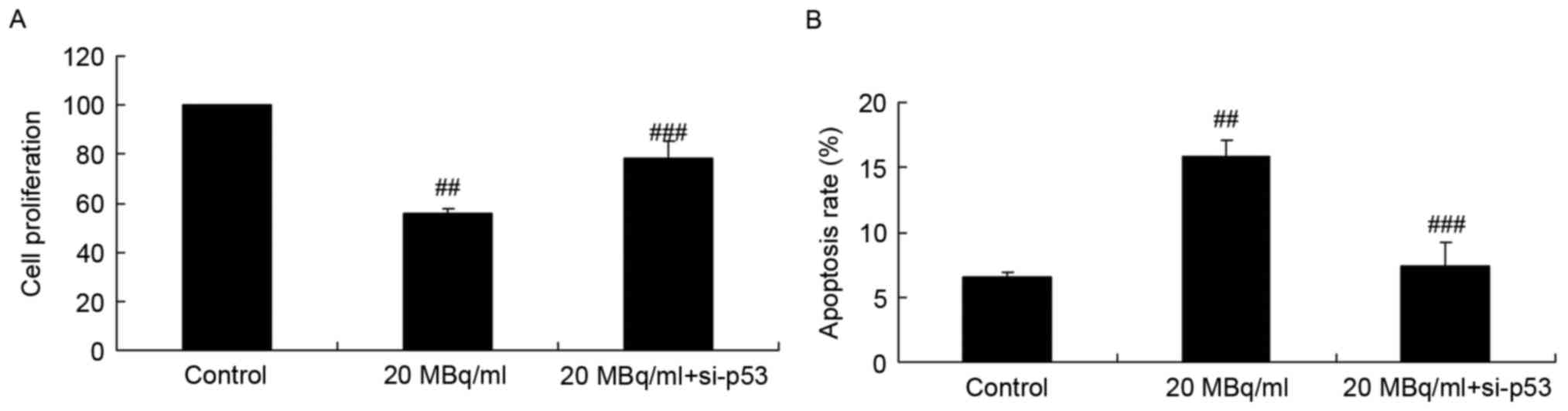
si-p53 inhibits the effects of
iodine-131 on cell proliferation and apoptosis in human cardiac
muscle cells
To assess the function of p53 on the effects of
iodine-131 on human cardiac muscle cell apoptosis, si-p53 was used
to inhibit p53 expression in human cardiac muscle cells. Fig. 4 indicates that si-p53 significantly
reversed the effects of iodine-131 and increased cell proliferation
and inhibited apoptosis of human cardiac muscle cells following
treatment with 20 MBq/ml of iodine-131.
si-p53 inhibits the effects of
iodine-131 on the regulation of the PIDD/caspase-2/t-BID/caspase-3
signaling pathway in human cardiac muscle cells
In addition, we investigated whether p53 is involved
in the effects of iodine-131 on apoptosis. Figs. 5A, C, E and F and 6 show that si-p53 significantly inhibited
p53, PIDD, t-BID protein expression and caspase-2 expression levels
in human cardiac muscle cells treated with 20 MBq/ml of
iodine-131.
si-p53 inhibits the effects of
iodine-131 on the regulation of Bax/cytochrome c/caspase-3
signaling pathway in human cardiac muscle cells
We investigated whether p53 is involved in the
effects of iodine-131 on apoptosis. Figs. 5B-D and 6 show that si-p53 significantly inhibited
Bax protein expression and caspase-3/−9 expression levels, and
induced cytochrome c protein expression in human cardiac
muscle cells treated with 20 MBq/ml of iodine-131.
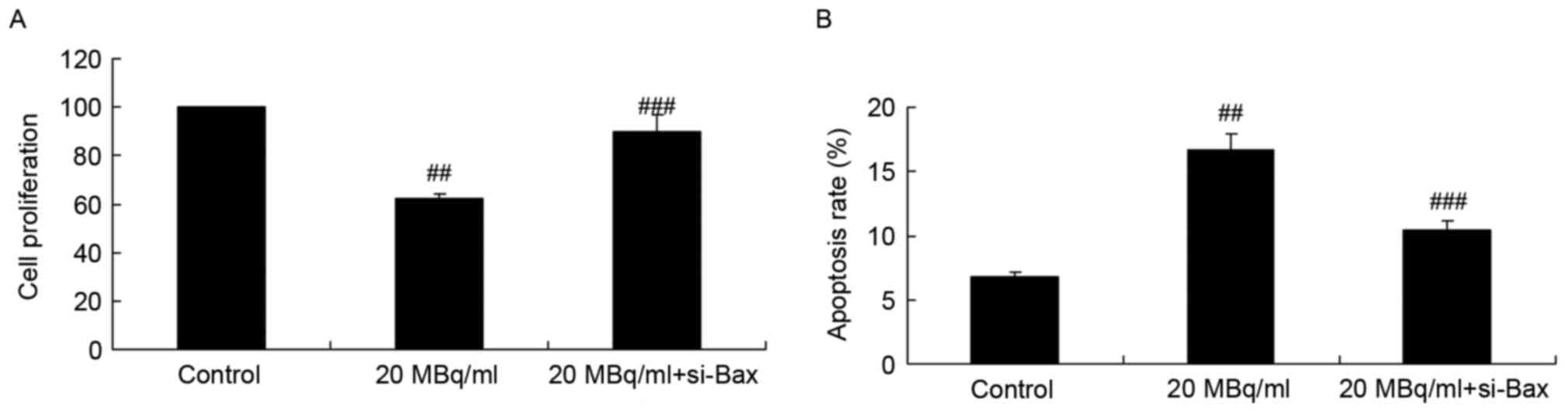
si-Bax reduces the effects of
iodine-131 on cell proliferation and apoptosis in human cardiac
muscle cells
Next, we investigated the function of si-Bax in the
effects of iodine-131 on apoptosis. si-Bax was used to inhibit Bax
expression in human cardiac muscle cells treated with iodine-131.
As shown in Fig. 7, si-Bax
significantly increased the cell proliferation and reduced the
apoptosis of human cardiac muscle cells treated with 20 MBq/ml of
iodine-131.
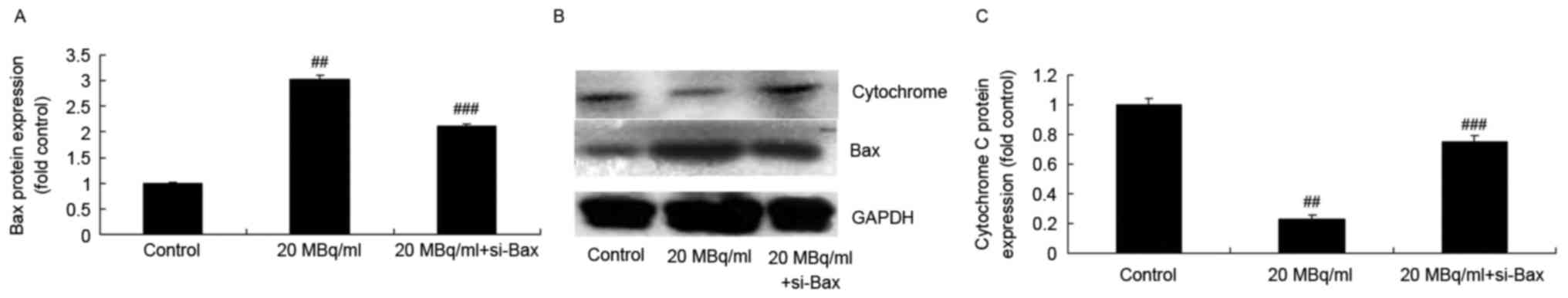
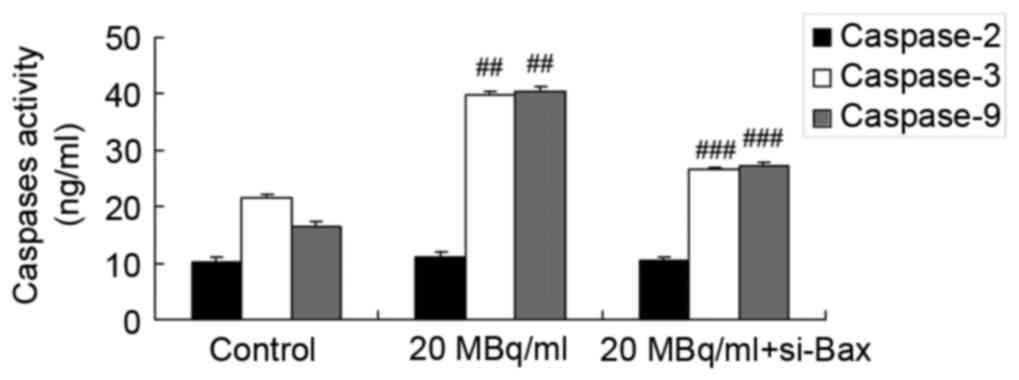
si-Bax reduces the effects of
iodine-131 on the cytochrome c/caspase-3 signaling pathway in human
cardiac muscle cell
To determine whether si-Bax has a function in the
effects of iodine-131 on apoptosis, the cytochrome
c/caspase-3 signaling pathway in human cardiac muscle cells
was analyzed. As shown in Figs. 8
and 9, si-Bax significantly
inhibited Bax protein expression and caspase-3/−9 activities, and
promoted cytochrome c protein expression in human cardiac
muscle cells treated with 20 MBq/ml of iodine-131. Yet, si-Bax did
not affect caspase-2 expression levels in human cardiac muscle
cells following treatment with 20 MBq/ml of iodine-131 (Fig. 9).
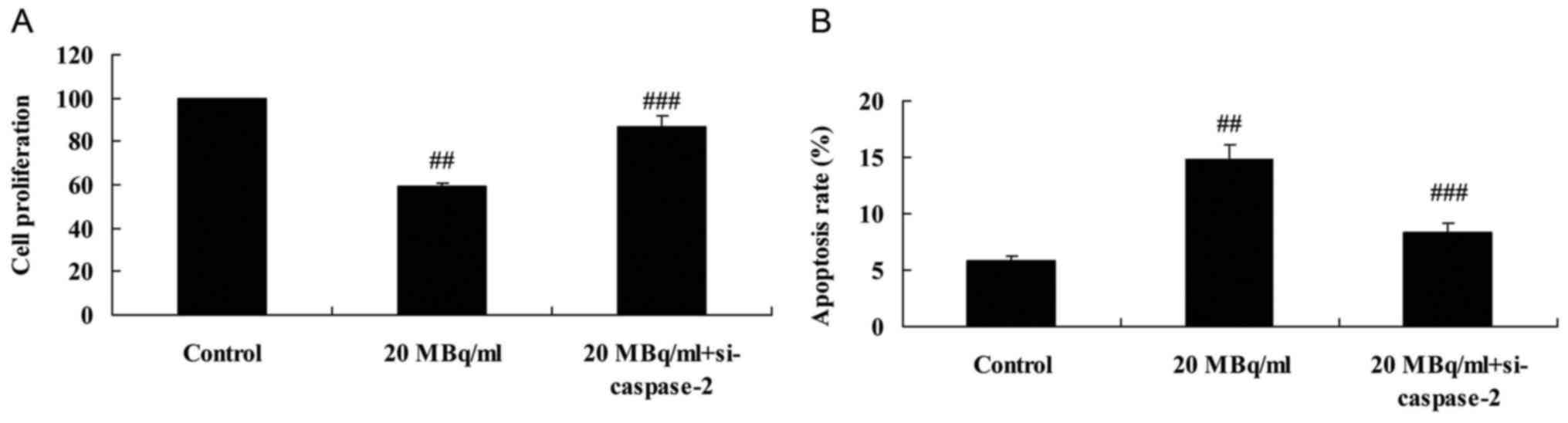
si-caspase-2 weakens the effects of
iodine-131 on cell proliferation and apoptosis in human cardiac
muscle cells
In order to investigate the function of caspase-2 in
the effects of iodine-131 on apoptosis and cell proliferation in
human cardiac muscle cells, expression of caspase-2 was assessed.
Fig. 10A and B indicates that
si-caspase-2 significantly increased cell proliferation and reduced
apoptosis of human cardiac muscle cells following treatment with 20
MBq/ml of iodine-131. Meanwhile, si-caspase-2 also significantly
suppressed caspase-2 protein expression of human cardiac muscle
cells following treatment with 20 MBq/ml of iodine-131 (Fig. 10C and D).
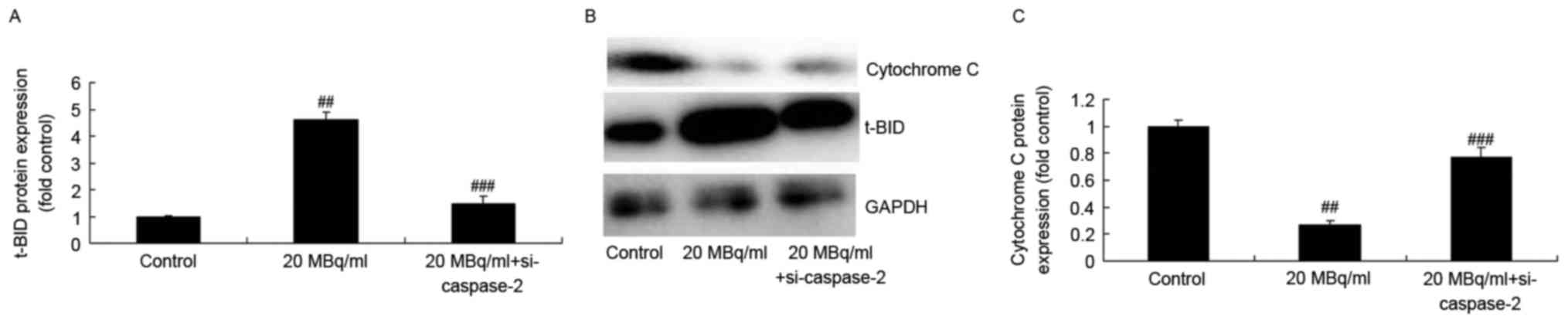
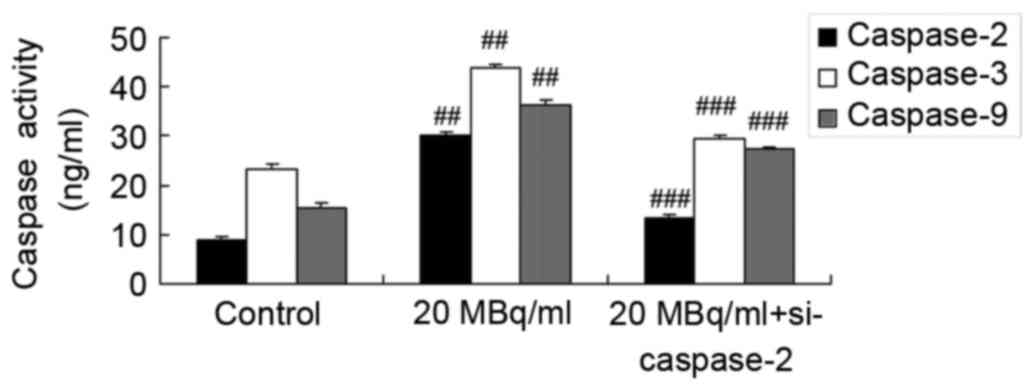
si-caspase-2 weakens the effects of
iodine-131 on the t-BID/cytochrome c/caspase-3 signaling pathway in
human cardiac muscle cells
We determined the function of si-caspase-2 in the
effects of iodine-131 on apoptosis. Figs. 11 and 12 indicate that si-caspase-2 inhibited
t-BID protein expression and caspase-2/−3/−9 expression levels and
promoted cytochrome c protein expression in human cardiac
muscle cells treated with 20 MBq/ml of iodine-131.
Discussion
Myocardial ischemia reperfusion injury is not only
the most common complication of ischemic cardiomyopathy, but also
an important reason responsible for insufficiency or failure of the
heart and other organs as a result of myocardial impairment after
severe trauma (12). As has been
reported in the literature, a large degree of myocardial apoptosis
is noted in the event of myocardial ischemia reperfusion (13). Therefore, in-depth investigation of
the myocardial apoptosis mechanism in myocardial ischemia
reperfusion injury is of certain theoretical and practical
significance to the prevention and treatment of ischemic
cardiomyopathy (13).
It has also been confirmed in human autopsy reports
after myocardial infarction that apoptosis does participate in the
pathophysiological process of infarction, and the apoptosis process
can be further promoted accompanied by the extension of myocardial
infarction duration, which thus injures myocardial cells and
affects myocardial function (13).
p53 is a type of cell cycle regulatory gene that promotes cell
apoptosis (14). Caspase-3 is the
final executor of cell apoptosis, p53 can directly act on the Bcl-2
regulatory domain, and the p53 protein interacts with the TATAAbos
binding protein (TBP), which results in inhibition of Bcl-2 gene
expression, causing myocardial cell injury (13,14).
The results of the present study identified that iodine-131 reduced
cell proliferation, induced apoptosis, induced p53, PIDD, t-BID
protein expression, suppressed cytochrome c protein
expression, and increased Bax protein expression, and promoted
caspase-2, −3 and −9 expression levels in human cardiac muscle
cells.
p53, an important tumor suppressor gene regulating
the cell cycle and apoptosis signaling pathway, is closely
associated with the occurrence of apoptosis. As an important
pro-apoptotic factor, p53 promotes cell apoptosis mainly through
two pathways, namely, the transcription-dependent and
transcription-independent pathways (15). Firstly, p53 can specifically induce
expression of cell apoptosis target genes, such as Noxa and Bax,
and participates in the endogenous and exogenous apoptosis pathways
through these proteins. Secondly, p53 protein allows for
transposition from the cytoplasm to mitochondria, and exerts a
pro-apoptotic effect by activating the mitochondrial pathway
(16). The results of the present
study revealed that si-p53 inhibited the effects of
iodine-131-reduced cell proliferation and induction of apoptosis in
human cardiac muscle cells through regulation of Bax/cytochrome
c/caspase-3 and PIDD/caspase-2/t-BID/cytochrome
c/caspase-3 signaling pathway.
The occurrence of cell apoptosis is regulated by
intracellular apoptosis-regulating proteins, which can be divided
into pro-apoptotic and anti-apoptotic proteins (17). The occurrence of cell apoptosis is
attributable to the loss of balance between these two types of
mutual antagonistic proteins. Among the established
apoptosis-regulating proteins at present, the Bcl-2 family (B-cell
leukemia/lymphoma 2-like proteins) plays a crucial role in
apoptosis induced by a variety of stimulating signals (17). It is indicated in research that the
Bcl-2 and Bax protein levels are directly related to apoptosis
regulation; increased Bax promotes cell apoptosis; while increased
Bcl-2 inhibits cell apoptosis (18). Consequently, it is proposed that
cell survival after stimulation by apoptosis is determined by the
Bcl-2/Bax ratio, which can be upregulated in the case of a high
Bcl-2 expression level, forms the Bcl-2/Bax heterodimer, inhibiting
cell apoptosis. However, the ratio is downregulated in the presence
of a high Bax expression level, which forms the Bax/Bax homodimer
leading to cell apoptosis (19). In
the present study, we found that si-Bax reduced the effects of
iodine-131 on cell proliferation and induced apoptosis in human
cardiac muscle cells through the cytochrome c/caspase-3
signaling pathway.
Bid is a pro-apoptotic protein in the Bcl-2 family
proteins, which exists in the cytoplasm in the form of inactive
p22Bid. Caspase-8 mediates the hydrolysis of inactive p22Bid in the
cytoplasm to produce a major functional fragment p15 (truncated
Bid, tBid), together with 2 smaller fragments, p13 and p11
(20). tBid activates the
mitochondria to release cytochrome c through transposition
to the mitochondrial membrane; the latter acts on effector
caspases, and finally mediates the apoptosis of multiple cells,
including lymphocytes and nerve cells (21). tBid can also be applied in gene
therapy, and induces apoptosis of tumor cells or viral-infected
cells (22).
Cytochrome c can activate the
Apaf-1-caspase-9 apoptosis system, and thus activates downstream
caspases, including caspase-3, caspase-2, caspase-6, caspase-8 and
caspase-10; among these, caspase-8 can cut Bid to form tBid and
activates tBid. tBid recruits Bax to embed into the mitochondria
after transposition to the mitochondrial membrane and forms the
transmembrane channel, which promotes cytochrome c to be
further released from the mitochondria, forming a positive feedback
pathway, and eventually inducing cell apoptosis (23,24).
As a downstream target gene of p53, PIDD is
essential in regulating cell apoptosis and stress repair (23). PIDD can bind with RIADD and
pro-caspase-2 to form a ternary complex, which gives rise to
caspase-2 activation, thus promoting cell apoptosis through the
mitochondrion-dependent pathway (25). The elevated PIDD expression may be
an important pathway for the apoptosis induced by the activated p53
pathway (23).
PIDD not only promotes cell apoptosis, but also
participates in cell repair under stress (26). Meanwhile, PIDD can directly activate
the NF-κΒ pathway that can promote DNA injury repair of cells, thus
protecting cells from apoptosis. From this point of view, PIDD has
certain protective effects on myocardial apoptosis (23). Therefore, PIDD is a gene that
possesses dual functions; it can promote cell apoptosis, and
prevent myocardial apoptosis by activating the protective signaling
pathway of cells.
The caspase family is the initiator and executor of
cell apoptosis in mammals, among which, caspase-3 is the most
crucial apoptotic protease in the downstream of the caspase cascade
reactions (24). Bcl-2 blocks the
activation of the upstream caspase protease by interfering with the
release of cytochrome c, thus inhibiting cell apoptosis
(24). As a composition of the ion
channel on the mitochondrial membrane, Bax protein allows
cytochrome c to pass through the mitochondrial membrane,
activating caspase-9, and further activating caspase-3, thus
resulting in cell apoptosis (9).
The results revealed that si-caspase-2 also reduced the effects of
iodine-131-reduced cell proliferation and induced apoptosis in
human cardiac muscle cells through the t-BID/cytochrome
c/caspase-3 signaling pathway.
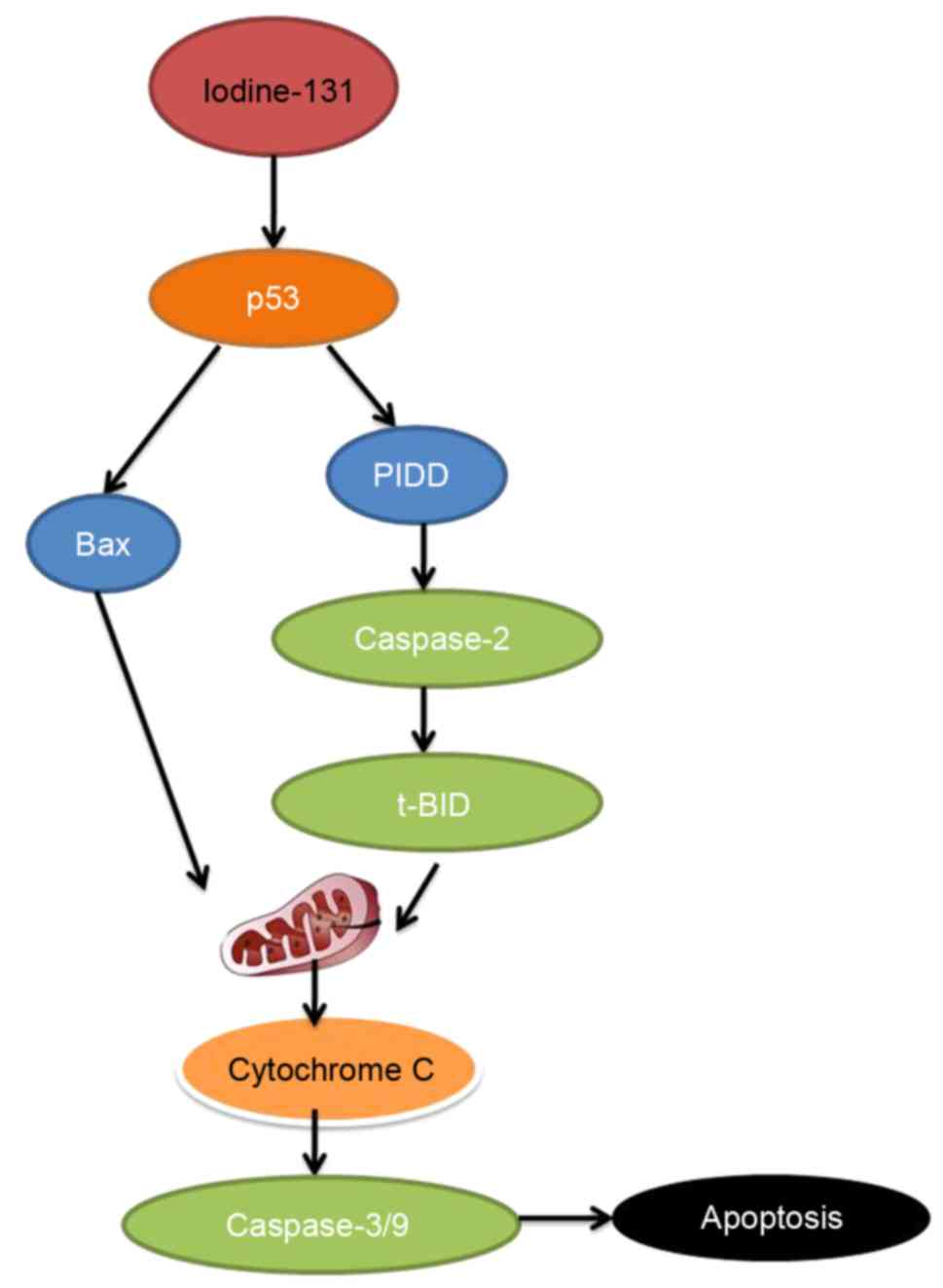
In conclusion, the present study demonstrated that
iodine-131 reduced cell proliferation and induced apoptosis of
human cardiac muscle cells through the p53/Bax/caspase-3 and
PIDD/caspase-2/t-BID/cytochrome c/caspase-3 signaling
pathway (Fig. 13). Iodine-131 with
its effects on the apoptosis of human cardiac muscle cells may
prove to be of potential therapeutic significance for designing
novel strategies to improve efficacy in myocardial disease.
References
|
1
|
Li P, Zhang L, Zhou C, Lin N and Liu A:
Sirt 1 activator inhibits the AGE-induced apoptosis and p53
acetylation in human vascular endothelial cells. J Toxicol Sci.
40:615–624. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tang JY, Jin P, He Q, Lu LH, Ma JP, Gao
WL, Bai HP and Yang J: Naringenin ameliorates
hypoxia/reoxygenation-induced endoplasmic reticulum stress-mediated
apoptosis in H9c2 myocardial cells: Involvement in ATF6, IRE1α and
PERK signaling activation. Mol Cell Biochem. 424:111–122. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piegari E, Russo R, Cappetta D, Esposito
G, Urbanek K, Dell'Aversana C, Altucci L, Berrino L, Rossi F and De
Angelis A: MicroRNA-34a regulates doxorubicin-induced
cardiotoxicity in rat. Oncotarget. 7:62312–62326. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang N, Ye F, Zhu W, Hu D, Xiao C, Nan J,
Su S, Wang Y, Liu M, Gao K, et al: Cardiac ankyrin repeat protein
attenuates cardiomyocyte apoptosis by upregulation of Bcl-2
expression. Biochim Biophys Acta. 1863:3040–3049. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao J, Zhao L, Wang Y, Teng Q, Liang L and
Zhang J: Effect of limb ischemic preconditioning on myocardial
apoptosis-related proteins in ischemia-reperfusion injury. Exp Ther
Med. 5:1305–1309. 2013.PubMed/NCBI
|
|
6
|
Song CL, Liu B, Diao HY, Shi YF, Zhang JC,
Li YX, Liu N, Yu YP, Wang G, Wang JP, et al: Down-regulation of
microRNA-320 suppresses cardiomyocyte apoptosis and protects
against myocardial ischemia and reperfusion injury by targeting
IGF-1. Oncotarget. 7:39740–39757. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Forini F, Kusmic C, Nicolini G, Mariani L,
Zucchi R, Matteucci M, Iervasi G and Pitto L: Triiodothyronine
prevents cardiac ischemia/reperfusion mitochondrial impairment and
cell loss by regulating miR30a/p53 axis. Endocrinology.
155:4581–4590. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin CL, Tseng HC, Chen RF, Chen WP, Su MJ,
Fang KM and Wu ML: Intracellular zinc release-activated
ERK-dependent GSK-3β-p53 and Noxa-Mcl-1 signaling are both involved
in cardiac ischemic-reperfusion injury. Cell Death Differ.
18:1651–1663. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang C, Shi J, Qian L, Zhang C, Wu K,
Yang C, Yan D, Wu X and Liu X: Nucleostemin exerts anti-apoptotic
function via p53 signaling pathway in cardiomyocytes. In Vitro Cell
Dev Biol Anim. 51:1064–1071. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu S, Feng F, Wang K, Men C, Lin C, Liu Q,
Yang D and Gao Z: The therapeutic efficacy of I131-PSCA-mAb in
orthotopic mouse models of prostate cancer. Eur J Med Res.
18:562013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Fang L, Zhang Q, Zheng Q, Tong J,
Fu X, Jiang X, Su C and Zheng J: An oncolytic adenovirus regulated
by a radiation-inducible promoter selectively mediates hSulf-1 gene
expression and mutually reinforces antitumor activity of
I131-metuximab in hepatocellular carcinoma. Mol Oncol. 7:346–358.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Alves MG, Machado NG, Sardão VA, Carvalho
RA and Oliveira PJ: Anti-apoptotic protection afforded by
cardioplegic celsior and histidine buffer solutions to hearts
subjected to ischemia and ischemia/reperfusion. J Cell Biochem.
112:3872–3881. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ran X, Diao JX, Sun XG, Wang M, An H,
Huang GQ, Zhao XS, Ma WX, Zhou FH, Yang YG, et al: Huangzhi oral
liquid prevents arrhythmias by upregulating caspase-3 and apoptosis
network proteins in myocardial ischemia-reperfusion injury in rats.
Evid Based Complement Alternat Med. 2015:5189262015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Formigli L, Ibba-Manneschi L, Perna AM,
Nediani C, Liguori P, Tani A and Zecchi-Orlandini S: Ischemia -
reperfusion-induced apoptosis and p53 expression in the course of
rat heterotopic heart transplantation. Microvasc Res. 56:277–281.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dai CQ, Luo TT, Luo SC, Wang JQ, Wang SM,
Bai YH, Yang YL and Wang YY: p53 and mitochondrial dysfunction:
Novel insight of neurodegenerative diseases. J Bioenerg Biomembr.
48:337–347. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang DG, Zheng JN and Pei DS:
P53/microRNA-34-induced metabolic regulation: New opportunities in
anticancer therapy. Mol Cancer. 13:1152014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Şentürk T, Çavun S, Avcı B, Yermezler A,
Serdar Z and Savcı V: Effective inhibition of cardiomyocyte
apoptosis through the combination of trimetazidine and
N-acetylcysteine in a rat model of myocardial ischemia and
reperfusion injury. Atherosclerosis. 237:760–766. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Gu H, Turrentine M and Wang M:
Estradiol treatment promotes cardiac stem cell (CSC)-derived growth
factors, thus improving CSC-mediated cardioprotection after acute
ischemia/reperfusion. Surgery. 156:243–252. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Hu Z, Sun B, Xu J, Jiang J and Luo
M: Ginsenoside Rg3 attenuates myocardial ischemia/reperfusion
injury via Akt/endothelial nitric oxide synthase signaling and the
B cell lymphoma/B cell lymphoma associated X protein pathway. Mol
Med Rep. 11:4518–4524. 2015.PubMed/NCBI
|
|
20
|
Ghate NB, Das A, Chaudhuri D, Panja S and
Mandal N: Sundew plant, a potential source of anti-inflammatory
agents, selectively induces G2/M arrest and apoptosis in MCF-7
cells through upregulation of p53 and Bax/Bcl-2 ratio. Cell Death
Dis. 2:150622016. View Article : Google Scholar
|
|
21
|
Singh N, Sarkar J, Sashidhara KV, Ali S
and Sinha S: Anti-tumour activity of a novel coumarin-chalcone
hybrid is mediated through intrinsic apoptotic pathway by inducing
PUMA and altering Bax/Bcl-2 ratio. Apoptosis. 19:1017–1028. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lo YL and Liu Y: Reversing multidrug
resistance in Caco-2 by silencing MDR1, MRP1, MRP2, and
BCL-2/BCL-xL using liposomal antisense oligonucleotides. PLoS One.
9:e901802014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ray P, Guha D, Chakraborty J, Banerjee S,
Adhikary A, Chakraborty S, Das T and Sa G: Crocetin exploits
p53-induced death domain (PIDD) and FAS-associated death domain
(FADD) proteins to induce apoptosis in colorectal cancer. Sci Rep.
6:329792016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li Y, Jia Z, Zhang L, Wang J and Yin G:
Caspase-2 and microRNA34a/c regulate lidocaine-induced dorsal root
ganglia apoptosis in vitro. Eur J Pharmacol. 767:61–66. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wan C, Jiang J, Mao H, Cao J, Wu X and Cui
G: Involvement of upregulated p53-induced death domain protein
(PIDD) in neuronal apoptosis after rat traumatic brain injury. J
Mol Neurosci. 51:695–702. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shi W, Huang W, Chen Y, Zhang S, Xu P, Gu
X, Fan H, Xu J, Chen Y, Ni R, et al: Low expression of PIDD is
associated with cell proliferation and apoptosis in hepatocellular
carcinoma. Tumour Biol. 37:10447–10457. 2016. View Article : Google Scholar : PubMed/NCBI
|