Introduction
Apolipoprotein C-I (apoC-I) is a small soluble
lipoprotein that can be found within HDL or VLDL (1). The gene encoding apoC-I is located in
chromosome 19 along with the genes for apoE, apoC-IV and apoC-II
(2). The apoC-I mRNA is translated
into an 83 amino acid protein, including 26 residues as an
N-terminal signal peptide (3) that
may be removed as the nascent polypeptide is translocated into the
endoplasmic reticulum (4) and 57
residues in the mature peptide (5,6) from
the C-terminus. A post-translationally modified form of the protein
characterized by the loss of a threonine and a proline from the
N-terminus of the mature peptide is known as truncated apoC-I
(7) and it is found in the
circulatory system. The appearance of the truncated isoform is most
likely due to a widely distributed post-proline cleaving enzyme
(8). This phenomenon is not limited
to humans (2).
ApoC-I can lead to atherosclerosis by reducing the
selective uptake of cholesteryl esters (9). In addition, apoC-I binds free fatty
acids and inhibits the activity of lipoprotein lipase, and
dysregulation of apoC-I is correlated with cutaneous abnormalities
or even obesity in humans (10). In
malignant diseases such as lung (11) and prostate (12) cancers, apoC-I could serve as a novel
diagnostic and prognostic biomarker. In pancreatic cancer (13), two isoforms of the mature and
truncated apoC-I peptide are overexpressed in the preoperative
serum and associated with poor prognosis, and play a key role in
maintaining cell survival. In a previous study (14), we showed that truncated apoC-I was
downregulated in the serum of patients with nephroblastoma in
correlation with tumor activity. Therefore, the truncated peptide
(TP) and the mature isoform may be involved in tumorigenesis.
Neuroblastoma (NB) is the most commonly abdominal
solid tumor in children. Distant metastasis is common in newly
diagnosed NB patients (15). Early
diagnosis and effective treatment strategies are urgently needed.
In addition to surgery, certain inhibitors (16,17)
play an important role in inducing apoptosis and suppressing
metastasis in patients with NB. However, there are currently no
effective treatments that can accurately target the tumor without
any side effects. apoC-I is secreted mainly from the liver and
widely distributed in the circulatory system. This kind of
endogenous peptide may show low toxicity to the human body and
apoC-I could be a promising therapeutic target in cancer. In the
present study, the TP (55 amino acids) was chemically synthesized
to examine its effect on NB and elucidate the underlying
mechanism.
Materials and methods
Cell line and cell culture
SH-SY5Y was obtained from the American Type Culture
Collection (ATCC, Rockville, MD, USA) and cultured in RPMI-1640
medium with 2 mM L-glutamine and 10% FBS. Cells were cultured at
37°C in a humidified incubator containing 5% CO2. The
medium, L-glutamine, and FBS were purchased from Sangong Biotech
(Shanghai, China).
Polypeptide by chemical synthesis
All the synthetic products were purchased from
LifeTein (Beijing, China). Solid phase peptide synthesis technology
was used from carboxy to amino terminus. The TP was synthesized
with 55 amino acids from the C-terminus of proapoC-I and the TP
labeled with FITC at the amino terminus. The signal peptide (SP)
contained 26 residues from the N-terminus of proapoC-I. Peptides
were isolated to a purity >95% and identified by HPLC, and
sequence information was confirmed by mass spectrometry.
Cell Counting Kit-8 (CCK-8) assay
The Cell Counting Kit-8 (CCK-8; Dojindo, Tokyo,
Japan) was used to study cell viability according to the
manufacturer's instructions. A cell suspension was inoculated into
a 96-well plate (3,000 cells/well), and incubated overnight. The
next day, the medium was replaced by new medium containing the
peptide at different concentrations (0, 0.1, 0.5, 1.0, 1.5 and 20
mg/ml) and incubated for 24, 48 and 72 h. The cells cultured with
TP and SP were in the same plate. At every time-point, 10 µl CCK-8
was added to each well and the plate was incubated for 3 h at 37°C
and 5% CO2. Absorbance was measured at 450 nm using a
microplate reader. The assay was performed with three replicates
for each group and repeated at least three times.
Wound healing assay
The wound assay was performed to determine the cell
migration capability. The cells were inoculated into 6-well plates
and incubated overnight. The next day, three parallel straight
lines were generated using a 200-µl pipette tip. After rinsing
three times with serum-free medium, the cells were incubated in
serum-free medium with various TP concentrations (0, 0.1 and 0.5
mg/ml) for 24 h. The wound healing area was photographed and
analyzed by IPP software. The relative migration was calculated as
a percentage of the area covered by the migrated cells compared to
the original wound area.
Cell migration and invasion assay
Migration and invasion assays were performed using
24-well Transwell cell culture chambers (Corning, NY, USA) or
Matrigel coated invasion chambers. The cells (2×104 for
migration and 4×104 for invasion) were seeded into the
upper chamber. The next day, plates were rinsed in serum-free
medium three times and the medium was replaced with fresh medium
with TP at various concentrations (0, 0.1 and 0.5 mg/ml). Medium
containing 10% FBS was placed in the lower chamber. After 24 h of
incubation, the cells in the upper layer of the membrane were
removed with a cotton-tipped swab and cells migrated to the lower
layer were fixed and stained with 0.1% crystal violet. Cells were
photographed and counted in six random microscopic fields.
Cell apoptosis and cell cycle
assay
Cell Apoptosis (Annexin V-FITC/PI) and Cell Cycle
(PI) Detection kits were purchased from KeyGen Biotech (Nanjing,
China). The cells were inoculated into 6-well plates and incubated
overnight. The medium was replaced by fresh medium with various TP
concentrations (0, 1.0 and 1.5 mg/ml) the next day and incubated
for 24 h. Cells were then harvested and analyzed using a
FACSCalibur cytometer (BD Biosciences, San Jose, CA, USA) according
to the manufacturer's instructions. CFlow and FlowJo software was
used for data analysis.
Mitochondrial membrane potential assay
by JC-1
A mitochondrial membrane potential assay kit was
purchased from Beyotime (Haimen, China). The cells were treated as
described for the cell apoptosis and cell cycle assays and then
collected and stained with JC-1 working solution at 37°C for 20 min
according to the manufacturer's instructions. FlowJo software was
used to analyze the results by flow cytometry.
Distribution of TP labeled with FITC
in the cell
The cells were incubated with TP (1.5 mg/ml) labeled
with FITC for 24 h, followed by incubation with β-tubulin rabbit
antibody at 4°C overnight and then donkey anti-rabbit IgG
conjugated with Cy3 at room temperature for 2 h (Sangong, Shanghai,
China). Finally, the cells were stained with DAPI working solution
at 37°C for 5 min, and the distribution of peptide in the cells was
observed under fluorescence microscope (Olympus, Tokyo, Japan).
Cell death detection by TUNEL
technology
A Cell Death Detection kit was purchased from Roche
(Mannheim, Germany). The cells were inoculated into 96-well plates
(2×103 cells/well) and incubated overnight. The medium
was replaced with fresh medium with various TP concentrations (0,
1.0, 1.5, and 2.0 mg/ml) the next day. After 24 h, the cells were
fixed and incubated with TUNEL working solution at 37°C for 60 min
followed by counterstaining with DAPI working solution at 37°C for
5 min. The results were observed under a fluorescence
microscope.
Western blotting
The cells were incubated with various TP
concentrations (0, 0.5, 1.0 and 1.5 mg/ml) for 24 h and collected
into centrifuge tubes. After washing the cells with cold PBS twice,
the proteins were extracted from the whole cell lysate by RIPA
buffer (50 mM Tris-HCl at pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% NP-40,
1% sodium deoxycholate, 1% Triton X-100, 10 mM NaF and 1 mM PMSF)
containing a protease inhibitor cocktail (Sangong). Proteins were
separated by SDS-PAGE and transferred onto nitrocellulose
membranes. After blocking, the membranes were incubated with
primary antibodies overnight at 4°C, including anti-Bcl-2,
anti-Bcl-xl, anti-Bax, anti-Bim, anti-tBid, anti-caspase-8,
anti-caspase-9, anti-caspase-3, anti-PARP, anti-cleaved-PARP, and
anti-β-actin, and then incubated with anti-mouse or rabbit IgG
conjugated with horseradish peroxidase at room temperature for 2 h.
All the antibodies were purchased from CST (Beverly, MA, USA). The
ECL western blot detection kit (Proteintech, Wuhan, China) was used
for chemiluminescent visualization. β-actin was used as a loading
control for whole cell extracts.
Xenograft tumor mouse model
The 4-week-old BALB/C nude mice were purchased from
Vital River (Beijing, China) and maintained at the SPF laboratory
animal room. A total of 5×106 SH-SY5Y cells in 0.1 ml of
PBS were injected subcutaneously into the left sides of the dorsal
area. After 2 weeks, mice bearing tumors of similar sizes were
randomly divided into two groups: a PBS control group and a
TP-treated group (100 mg/kg by intraperitoneal injection once daily
for 21 days). After the start of treatment, tumor volume was
measured and calculated every 7 days using the formula A ×
B2/2, where A is the longest diameter and B is the
shortest. At the end of the treatment, all mice were sacrificed.
Tumors were harvested, weighed and photographed. The present study
was approved by the Ethics Committee of Zhengzhou University.
Immunohistochemistry
Tumors were fixed and embedded with paraffin. The SP
immunohistochemistry method was used to assay protein expression.
The results were analyzed using IPP software. Primary anti-rabbit
antibodies (Bax, Bcl-2, Ki67 and PCNA) and secondary goat
anti-rabbit IgG conjugated with biotin and streptavidin conjugated
with HRP were purchased from Abcam (Cambridge, UK).
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software. The data were expressed as the mean ± SD. The Student's
t-test was used for comparisons between two groups of quantitative
data. The one-way ANOVA test was used for multigroup comparisons.
P<0.05 was considered statistically significant.
Results
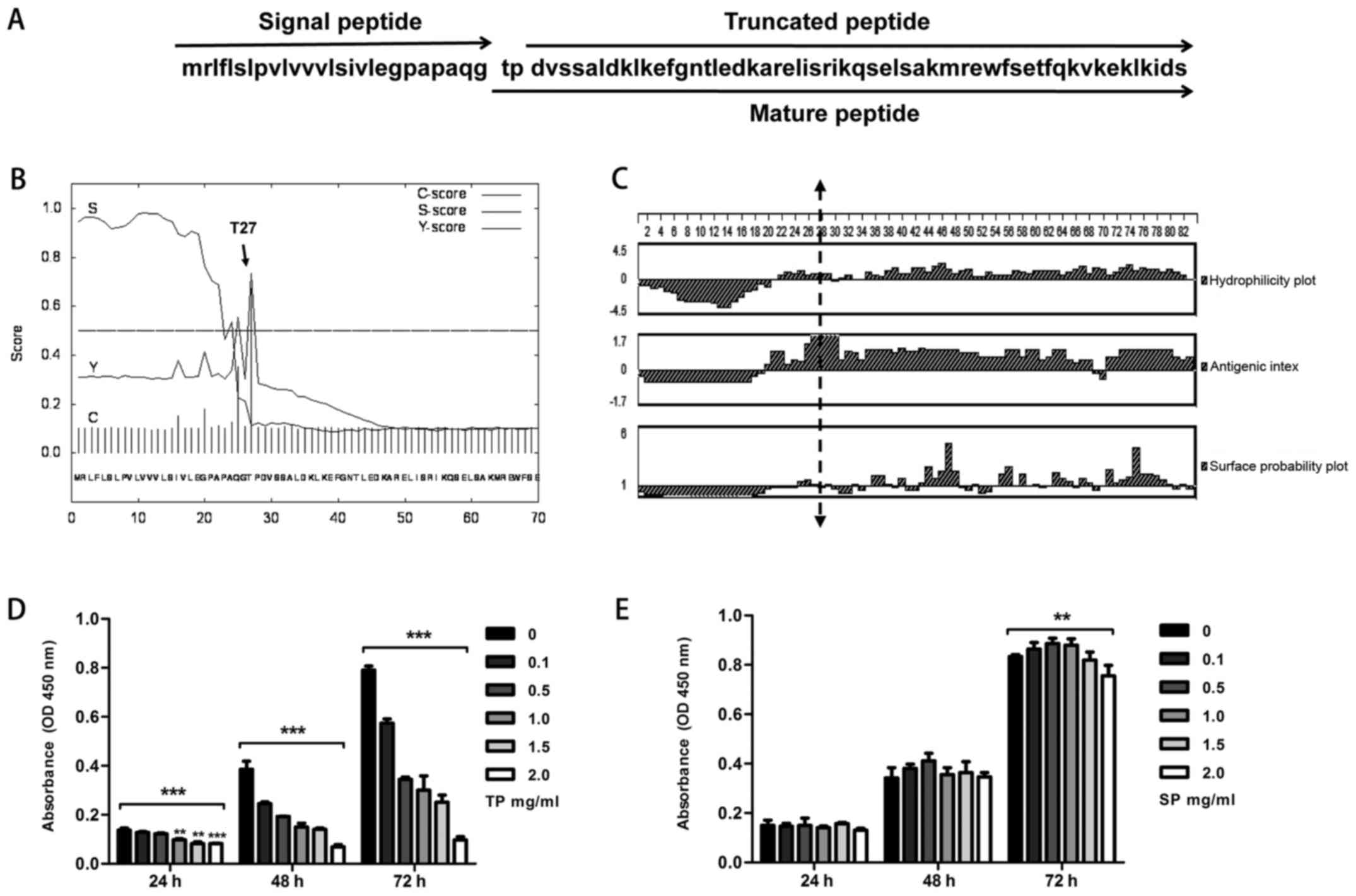
Peptide sequence analysis and
synthesis
The proapoC-I has 83 amino acids including the SP
(Met1-Gly26) and mature peptide (Thr27-Ser83). The loss of a
threonine and a proline from the N-terminus of the mature peptide
results in the TP (7). The sequence
information is shown in Fig. 1A. In
addition to what is reported in the literature, we found that the
site between G26 and T27 is the cleavage site from signal to mature
peptide using a signal peptide prediction website (http://www.cbs.dtu.dk/services/SignalP)
(Fig. 1B). DNAStar software was
used to further analyze sequence activity. Regions (above the
baseline) with high hydrophilicity, antigen index, and surface
probability were mainly found in the TP, suggesting that the TP is
a potentially active peptide with high hydrophilicity, antigenic
index, and surface probability compared with the SP (Fig. 1C). Therefore, we synthesized the TP
as experimental group and the SP as control group used for CCK-8
assay.
CCK-8 assay
For the TP group, increasing concentrations were
correlated with the inhibition of cell proliferation at 24, 48 and
72 h (all P-values <0.001). At 24 h, the groups treated with
concentrations of 0.1 and 0.5 mg/ml showed no statistically
significant differences from the control group (0 mg/ml) (Fig. 1D). In the SP group, cell
proliferation was not affected by different SP concentrations at 24
and 48 h (all P-values >0.05). However, at 72 h, various SP
concentrations inhibited cell proliferation to some degree
(P=0.001) (Fig. 1E).
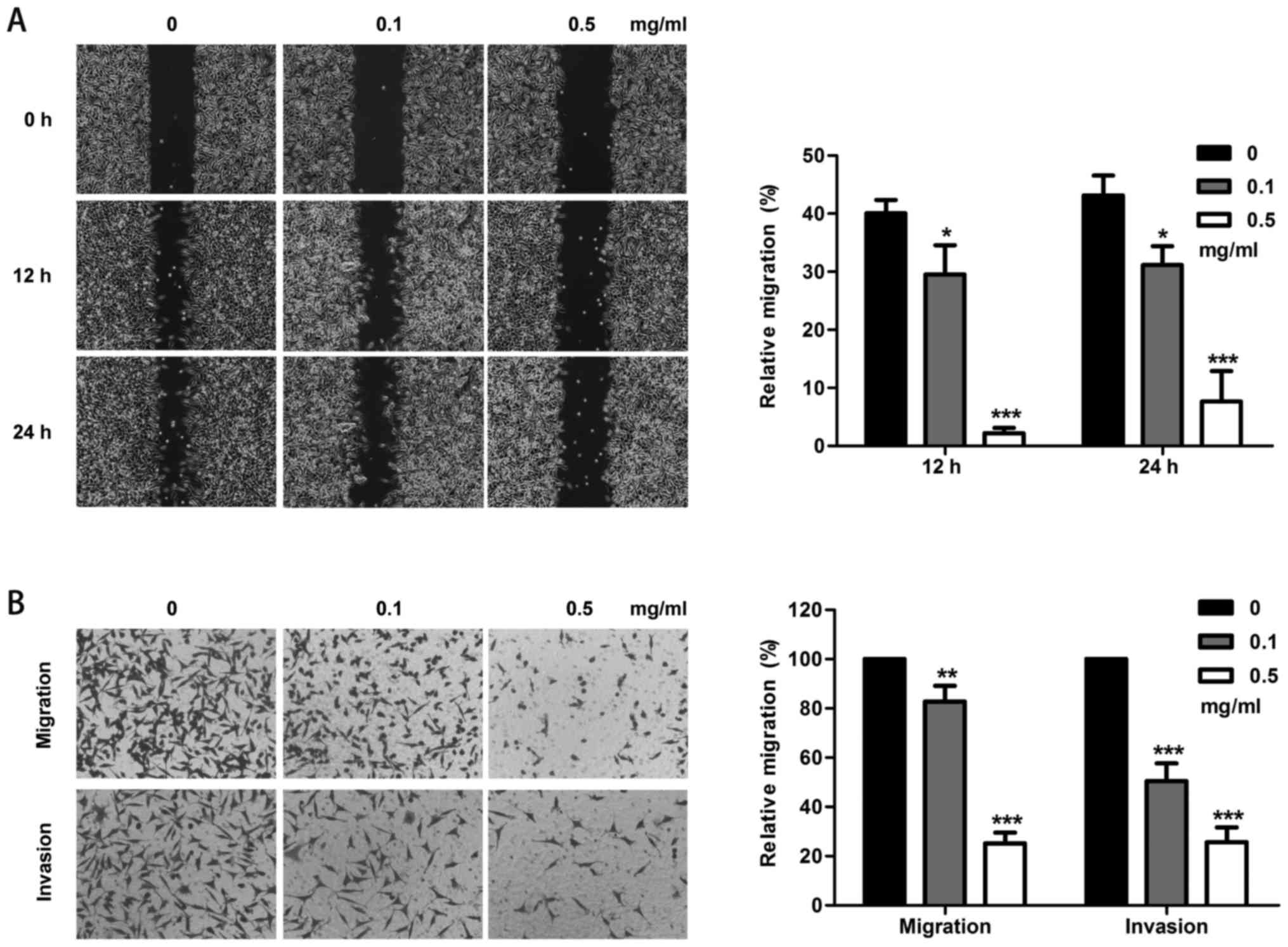
Cell migration and invasion assay
In the wound healing assay, TP at 0.1 and 0.5 mg/ml
inhibited the wound healing ability at 12 h compared with that in
the control group (P=0.03 and P<0.001). At 24 h, the wound
healing ability was also inhibited by 0.1 and 0.5 mg/ml TP (P=0.012
and P<0.001) (Fig. 2A). In the
Transwell migration assay, the migration ability was reduced to
82.7 and 25.1% in response to increasing TP concentrations compared
with the values in the control group (P=0.01 and P<0.001). In
the invasion assay, the invasion ability was reduced to 50.4 and
25.6% compared with that in the control group (all P-values
<0.001) (Fig. 2B).
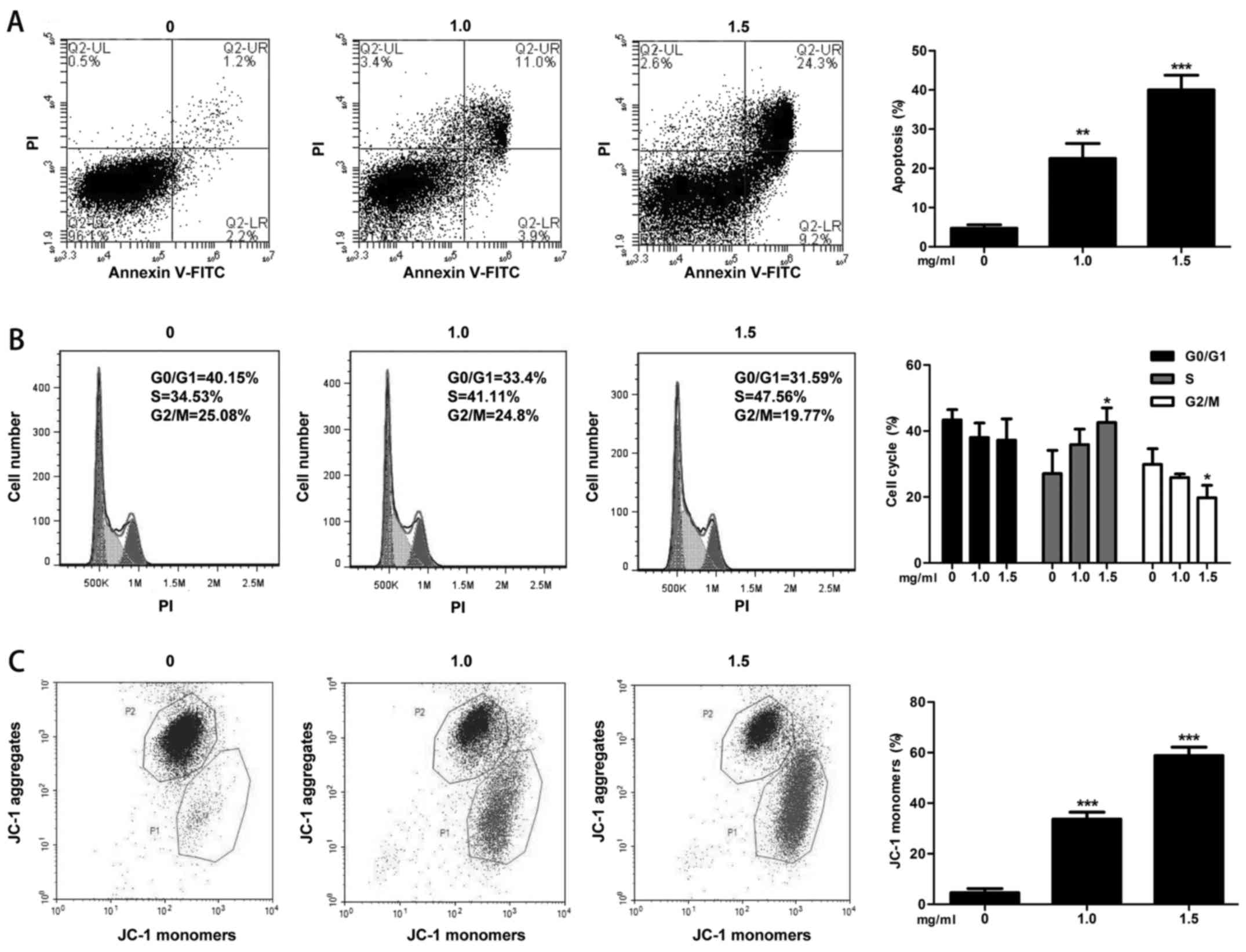
Flow cytometry
In the cell apoptosis assay, increasing TP
concentrations resulted in an increase in the rates of apoptosis by
22.5 and 40% (all P-values <0.01, Fig. 3A). The percentage of cells in S
phase increased to 35.9% (P>0.05) and 42.6% (P=0.03) compared
with that in the control group at 27.1%, and the percentage of
cells in G2/M decreased to 25.9% (P>0.05) and 19.8% (P=0.04)
compared with that in the control group at 29.9% (Fig. 3B). In the mitochondrial membrane
potential assay, increasing TP concentrations increased the
percentage of cells with JC-1 monomers to 33.7 and 58.8% (all
P-values <0.001, Fig. 3C).
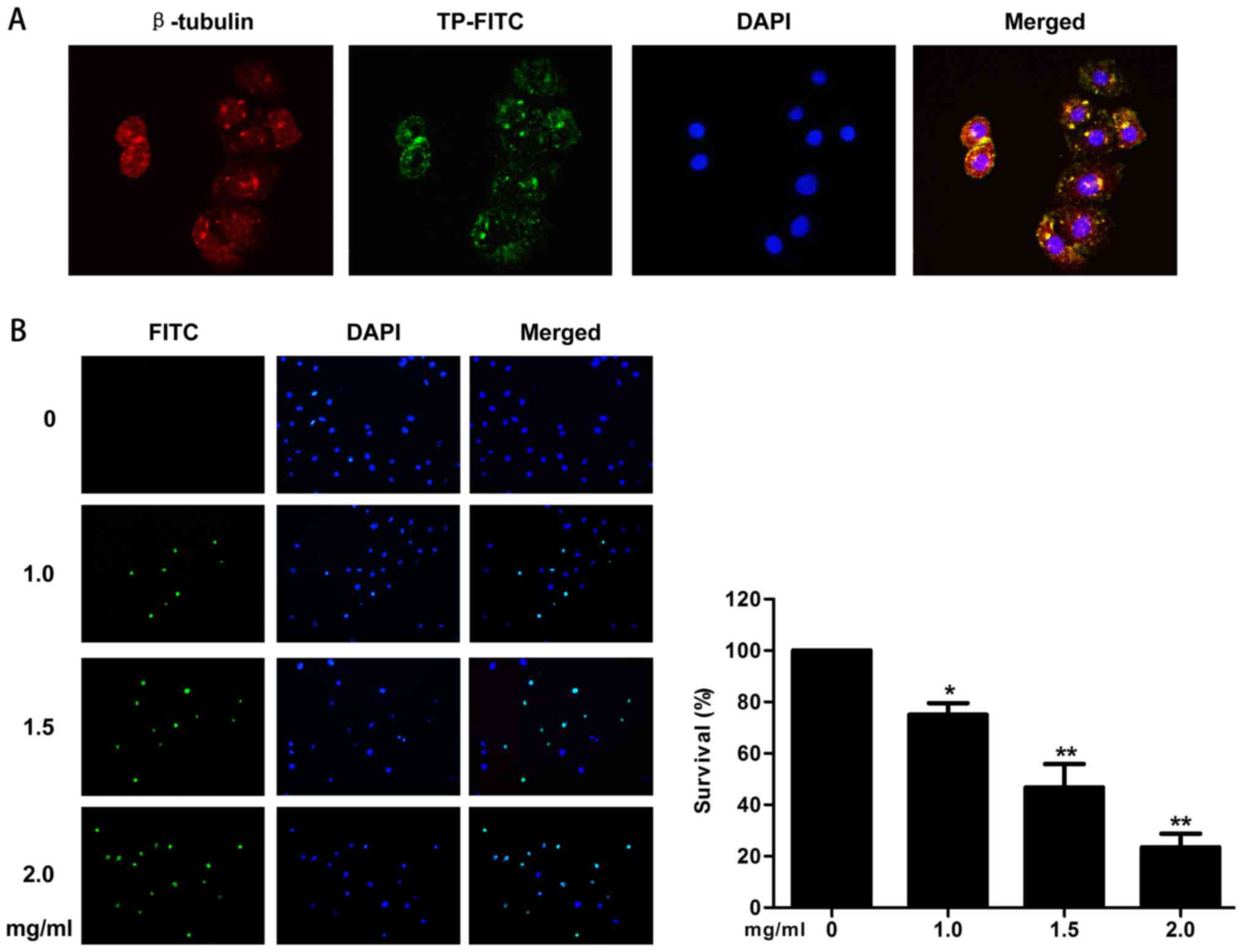
Distribution of FITC-labeled TP in the
cell
Incubation with FITC labeled TP at 1.5 mg/ml for 24
h resulted in the entry of the peptide into the cells. The result
showed that the TP was mainly concentrated in the cytoplasm and
cell membrane, with a fraction detected in the nucleus (Fig. 4A).
Cell death detection by TUNEL
technology
In the TUNEL assay, increasing TP concentrations
increased DNA cleavage during apoptosis. The results showed that
the survival rate of the cells was reduced to 75% (P=0.011), 46.7%
(P=0.009), and 23.5% (P=0.002) in response to increasing TP
concentrations (Fig. 4B). These
data provided direct evidence that the TP induced apoptosis.
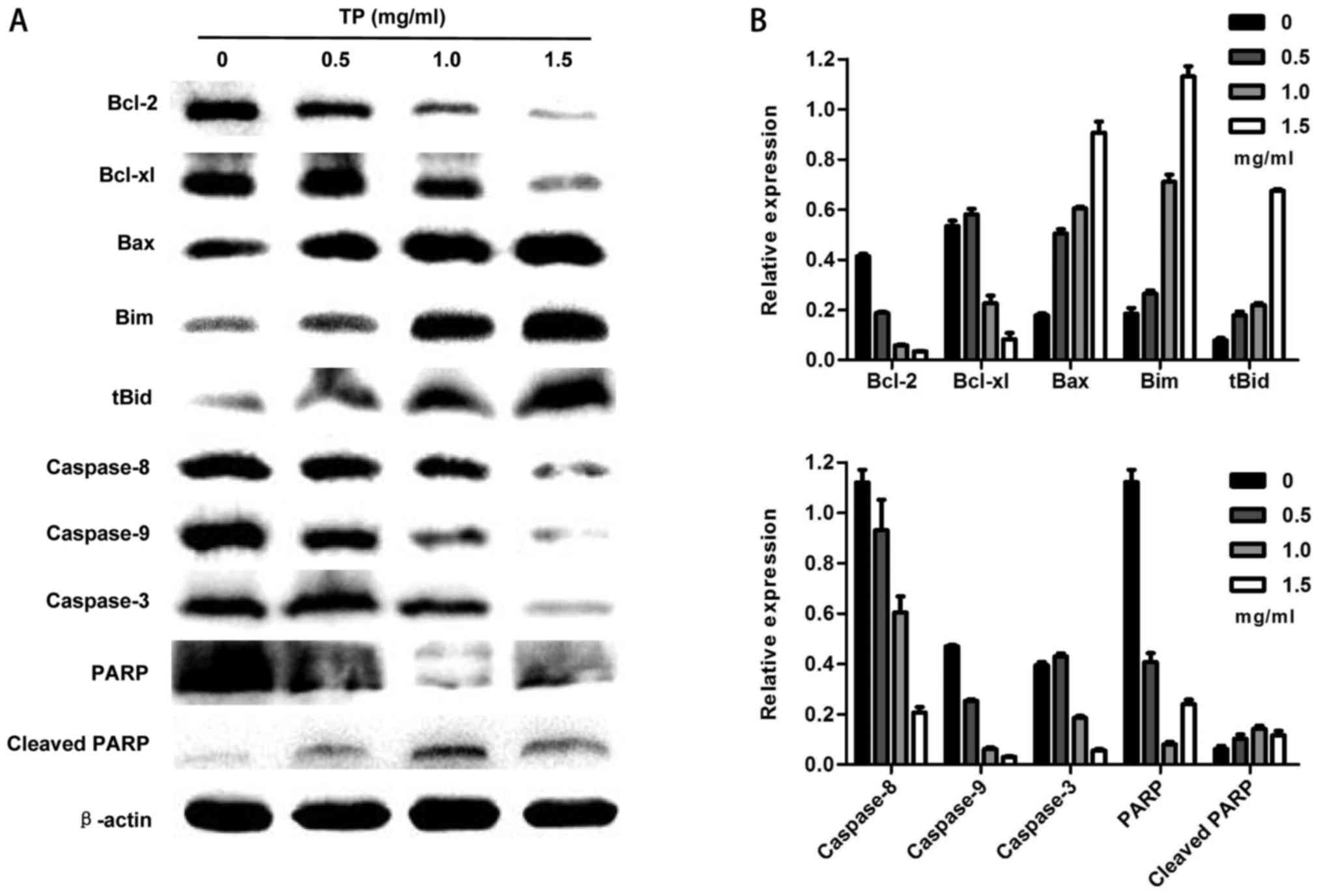
TP induces apoptosis by the intrinsic
and extrinsic pathways
Increasing TP concentrations resulted in the
downregulation of Bcl-2 and Bcl-xl which are anti-apoptotic
proteins of the Bcl-2 family. Downregulation of Bcl-2 or Bcl-xl
affects mitochondrial function. Upregulation of Bax causes the
release of cytochrome c through oligomerization from the
cytosol to the mitochondrial outer membrane. Bim accelerates this
process by inhibiting anti-apoptotic proteins such as Bcl-2 or
Bcl-xl. The release of cytochrome c activates caspase-9, and
cleaved caspase-9 further activates caspase-3. Finally PARP is
cleaved into its active form resulting in DNA damage. Disruption of
mitochondrial function by the intrinsic apoptosis pathway was
observed. On the other hand, activation of caspase-8 is most likely
associated with the stimulation of cell surface death receptors
such as tumor necrosis factor receptors (TNF-R). Active caspase-8
directly cleaves caspase-3 to induce apoptosis. Furthermore, Bid is
cleaved by active caspase-8 into tBid, which becomes an integral
membrane protein in mitochondria and recruits Bax from the cytosol
to the outer membrane, eventually causing the release of cytochrome
c. The results showed that the TP induced apoptosis through
the intrinsic and extrinsic pathways (Fig. 5).
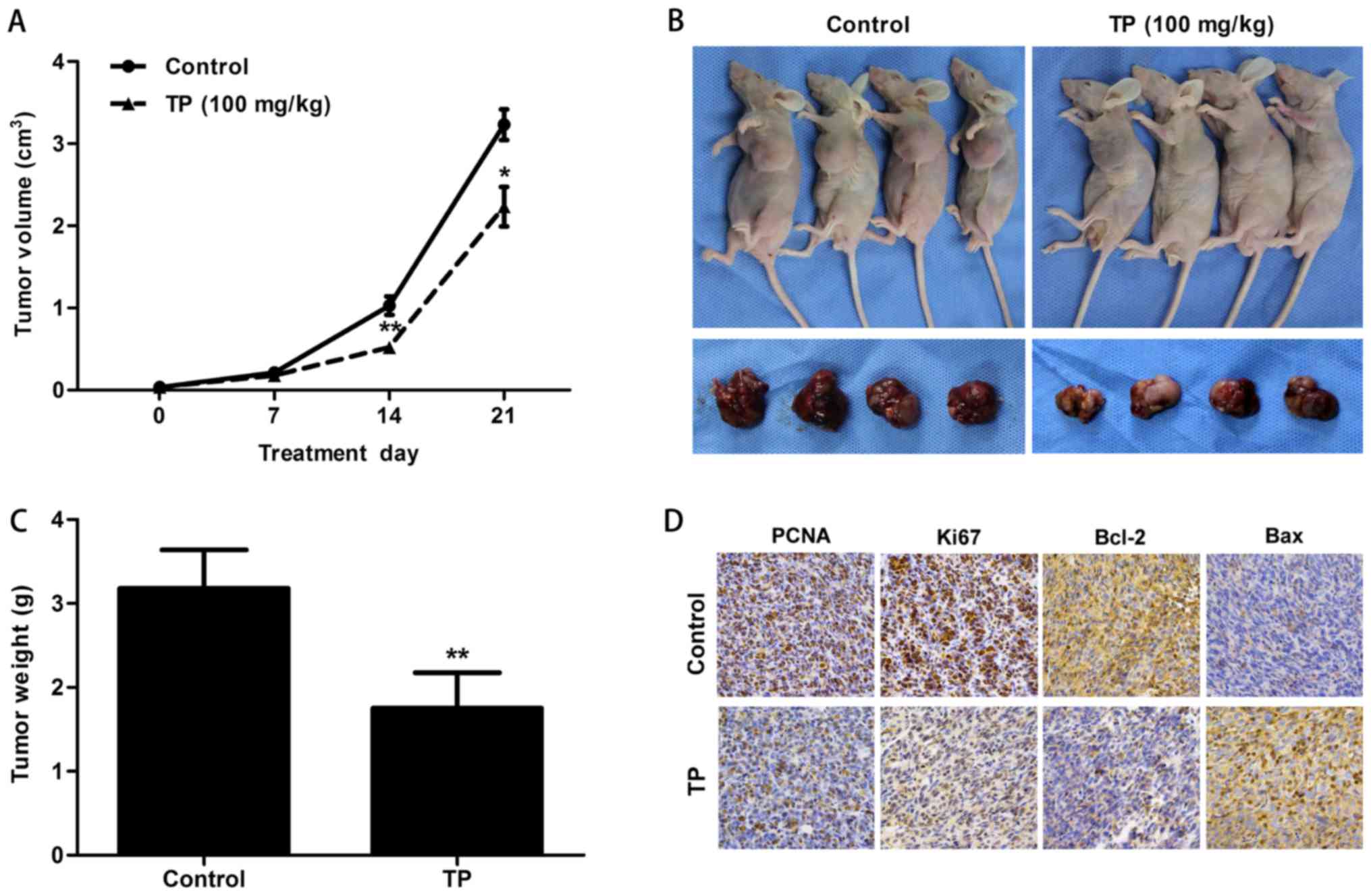
TP inhibited tumor proliferation and
induced apoptosis in a xenograft mouse model
Tumor volume was measured every 7 days in a PBS
control group and a TP-treated group (100 mg/kg by intraperitoneal
injection once daily for 21 days). After 7 days, the tumors in the
control group began to grow rapidly. Measurements at 14 days showed
a significantly greater tumor volume in the control group than in
the TP group (P=0.005) (Fig. 6A).
Mice were sacrificed at the end of treatment after 21 days. Tumors
were harvested, weighed and photographed. The results showed that
the tumor volume (P=0.017) and weight (P=0.004) were reduced in the
TP group (Fig. 6A-C). Tumors were
fixed and embedded with paraffin, and the SP immunohistochemistry
method was used to examine relative protein expression (Fig. 6D). Downregulation of PCNA and Ki67
in the TP group indicated the inhibition of tumor cell
proliferation. Upregulation of Bax and downregulation of Bcl-2
probably enhanced the permeabilization of the mitochondrial
membrane. The release of cytochrome c eventually induced
apoptosis in the tumor.
Discussion
The present study examined the role of apoC-I in the
tumorigenesis of NB. Although the TP is characterized by the loss
of two residues compared with the mature peptide, the two isoforms
have a similar sequence and probably play the same role in
vivo. The hydrophilicity, antigen index, and surface
probability predicted by DNAStar software indicated that the active
sites were mainly concentrated in the TP. The SP may be an inactive
form except for its function in guiding the nascent polypeptide
into the endoplasmic reticulum for further processing (3,4). Based
on these data, we synthesized the TP and used the SP as the
negative control. The results of the CCK-8 assay showed that the TP
selectively inhibited cell proliferation compared with the effect
of the SP.
Based on the results of the CCK-8 assay at 24 h, we
selected two TP concentrations (0.1 and 0.5 mg/ml) that did not
induce cell apoptosis to study the effect of the TP on cell
migration and invasion. The wound healing assay showed that the
wound healing ability was reduced by various TP concentrations. The
results of the Transwell assay showed that the migration ability
was reduced, respectively, to 82.7 and 25.1% and the invasion
ability was reduced to 50.4 and 25.6% compared with the control
group. Therefore, the TP not only inhibited cell proliferation but
also reduced the migration and invasion abilities. These results
suggested that the TP is a promising therapeutic agent against
metastasis in NB. However, the mechanism by which the TP reduced
the metastatic ability is not clear, and further studies are
necessary to clarify this.
The results of flow cytometry assays confirmed that
the TP inhibited cell proliferation and induced apoptosis in NB.
The Annexin V/PI assay showed that the apoptosis rates increased to
22.5 and 40%. The cell cycle assay demonstrated that cells were
arrested in the S phase in response to increasing TP
concentrations. JC-1 (18,19) is a sensitive method to detect the
mitochondrial membrane potential. When the potential is high, JC-1
is mainly concentrated in the matrix of the mitochondrion in the
form of aggregates. JC-1 monomers increase in response to
alterations in the mitochondrial membrane potential. Our results
showed that the TP induced apoptosis probably through the
mitochondrial pathway.
TUNEL technology is an effective way to detect DNA
cleavage during apoptosis (20,21).
Increasing TP concentrations led to an increase in the percentage
of cells with DNA breaks, indicating the induction of apoptosis. In
addition, we observed that the peptide was mainly distributed in
the membrane and cytoplasm, with a fraction detected in the
nucleus. This suggested that the TP may have directly stimulated
cell death receptors such as TNFRs (22) or entered into the cells to cause
changes in downstream signals, leading to the induction of
apoptosis through the extrinsic and intrinsic pathways.
Bcl-2 and Bcl-xl are important anti-apoptotic
members of the Bcl-2 family (23).
Downregulation of Bcl-2 and Bcl-xl by the TP would reduce the
stability of the mitochondrion. Meanwhile, upregulation of Bax
enhances the permeability of the mitochondrion through
oligomerization into the outer membrane from the cytosol (24). Overexpression of Bim promotes the
process by antagonizing anti-apoptotic members of the Bcl-2 family
(25–30). These factors together cause the
release of cytochrome c (31) and the subsequent activation of
procaspase-9 (32). Caspase-3 and
PARP are further activated, leading to apoptosis. On the other
hand, activation of caspase-8 (33)
was most likely due to the stimulation of cell death receptors such
as TNF-R by the TP. Active caspase-8 can directly cleave caspase-3
to induce apoptosis. Bid is also cleaved into the active form tBid
by caspase-8. tBid (34,35) becomes an integral membrane protein
in the mitochondrion and can strongly recruit Bax from the cytosol
to the outer membrane, eventually causing the release of cytochrome
c. The results showed that the TP induced apoptosis probably
through the intrinsic and extrinsic pathways. In addition, the
xenograft tumor mouse model confirmed that the TP inhibited tumor
proliferation and induced apoptosis in vivo.
The truncated apoC-I is a post-translationally
modified protein that mainly depends on the positively charged
lysine residues (10,36,37)
within the KVKEKLK motif to function. Whether the lysine residues
play a role in suppressing NB remains unknown, and further research
is necessary. Our results suggested that enrichment of the TP in
the tumor could induce apoptosis in NB. However, one report in
pancreatic cancer (13) indicated
that inhibition of apoC-I expression suppresses cell proliferation
and induces apoptosis. Therefore, dysfunction of apoC-I including
upregulation or downregulation may affect the growth of tumors.
Targeting apoC-I could therefore be a novel promising therapeutic
approach for the treatment of malignant tumors.
Glossary
Abbreviations
Abbreviations:
|
NB
|
neuroblastoma
|
|
apoC-I
|
apolipoprotein C-I
|
|
TP
|
truncated peptide
|
|
SP
|
signal peptide
|
|
TNFR
|
tumor necrosis factor receptor
|
References
|
1
|
Puppione DL: Higher primates, but not New
World monkeys, have a duplicate set of enhancers flanking their
ApoC-I genes. Comp Biochem Physiol Part D Genomics Proteomics.
11:45–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Puppione DL, Ryan CM, Bassilian S, Souda
P, Xiao X, Ryder OA and Whitelegge JP: Detection of two distinct
forms of ApoC-I in great apes. Comp Biochem Physiol Part D Genomics
Proteomics. 5:73–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Knott TJ, Robertson ME, Priestley LM,
Urdea M, Wallis S and Scott J: Characterisation of mRNAs encoding
the precursor for human apolipoprotein CI. Nucleic Acids Res.
12:3909–3915. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sabatini DD, Kreibich G, Morimoto T and
Adesnik M: Mechanisms for the incorporation of proteins in
membranes and organelles. J Cell Biol. 92:1–22. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jackson RL, Sparrow JT, Baker HN,
Morrisett JD, Taunton OD and Gotto AM Jr: The primary structure of
apolopoprotein-serine. J Biol Chem. 249:5308–5313. 1974.PubMed/NCBI
|
|
6
|
Shulman RS, Herbert PN, Wehrly K and
Fredrickson DS: Thf complete amino acid sequence of C-I
(apoLp-Ser), an apolipoprotein from human very low density
lipoproteins. J Biol Chem. 250:182–190. 1975.PubMed/NCBI
|
|
7
|
Bondarenko PV, Cockrill SL, Watkins LK,
Cruzado ID and Macfarlane RD: Mass spectral study of polymorphism
of the apolipoproteins of very low density lipoprotein. J Lipid
Res. 40:543–555. 1999.PubMed/NCBI
|
|
8
|
Gotoh H, Hagihara M, Nagatsu T, Iwata H
and Miura T: Activity of dipeptidyl peptidase IV and post-proline
cleaving enzyme in sera from osteoporotic patients. Clin Chem.
34:2499–2501. 1988.PubMed/NCBI
|
|
9
|
Krasteva V, Brodeur MR, Tremblay FL,
Falstrault L and Brissette L: Apolipoprotein C-I reduces
cholesteryl esters selective uptake from LDL and HDL by binding to
HepG2 cells and lipoproteins. Biochim Biophys Acta. 1801:42–48.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Westerterp M, Berbée JF, Delsing DJ, Jong
MC, Gijbels MJ, Dahlmans VE, Offerman EH, Romijn JA, Havekes LM and
Rensen PC: Apolipoprotein C-I binds free fatty acids and reduces
their intracellular esterification. J Lipid Res. 48:1353–1361.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ko HL, Wang YS, Fong WL, Chi MS, Chi KH
and Kao SJ: Apolipoprotein C1 (APOC1) as a novel diagnostic and
prognostic biomarker for lung cancer: A marker phase I trial.
Thorac Cancer. 5:500–508. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamamoto-Ishikawa K, Suzuki H, Nezu M,
Kamiya N, Imamoto T, Komiya A, Sogawa K, Tomonaga T, Nomura F and
Ichikawa T: The isolation and identification of apolipoprotein C-I
in hormone-refractory prostate cancer using surface-enhanced laser
desorption/ionization time-of-flight mass spectrometry. Asian J
Androl. 11:299–307. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takano S, Yoshitomi H, Togawa A, Sogawa K,
Shida T, Kimura F, Shimizu H, Tomonaga T, Nomura F and Miyazaki M:
Apolipoprotein C-1 maintains cell survival by preventing from
apoptosis in pancreatic cancer cells. Oncogene. 27:2810–2822. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang J, Guo F, Wang L, Zhao W, Zhang D,
Yang H, Yu J, Niu L, Yang F, Zheng S, et al: Identification of
apolipoprotein C-I as a potential Wilms' tumor marker after
excluding inflammatory factors. Int J Mol Sci. 15:16186–16195.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang HH, Liu YL, Lu MY, Jou ST, Yang YL,
Lin DT, Lin KH1, Tzen KY, Yen RF, Lu CC, et al: A multidisciplinary
team care approach improves outcomes in high-risk pediatric
neuroblastoma patients. Oncotarget. 17:4360–4372. 2017.
|
|
16
|
Mao X, Chen Z, Zhao Y, Yu Y, Guan S,
Woodfield SE, Vasudevan SA, Tao L, Pang JC, Lu J, et al: Novel
multi-targeted ErbB family inhibitor afatinib blocks EGF-induced
signaling and induces apoptosis in neuroblastoma. Oncotarget.
8:1555–1568. 2017.PubMed/NCBI
|
|
17
|
Chen Z, Wang L, Yao D, Yang T, Cao WM, Dou
J, Pang JC, Guan S, Zhang H, Yu Y, et al: Wip1 inhibitor GSK2830371
inhibits neuroblastoma growth by inducing Chk2/p53-mediated
apoptosis. Sci Rep. 6:380112016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang F, Kong DS, Zhang ZL, Lei N, Zhu XJ,
Zhang XP, Chen L, Lu Y and Zheng SZ: Tetramethylpyrazine induces
G0/G1 cell cycle arrest and stimulates mitochondrial-mediated and
caspase-dependent apoptosis through modulating ERK/p53 signaling in
hepatic stellate cells in vitro. Apoptosis. 18:135–149. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhao P, Han T, Guo JJ, Zhu SL, Wang J, Ao
F, Jing MZ, She YL, Wu ZH and Ye LB: HCV NS4B induces apoptosis
through the mitochondrial death pathway. Virus Res. 169:1–7. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nagamine A, Hasegawa H, Hashimoto N,
Yamada-Inagawa T, Hirose M, Kobara Y, Tadokoro H, Kobayashi Y and
Takano H: The effects of DPP-4 inhibitor on hypoxia-induced
apoptosis in human umbilical vein endothelial cells. J Pharmacol
Sci. 133:42–48. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu L, Deng Y, Feng L, Li D, Chen X, Ma C,
Liu X, Yin J, Yang M, Teng F, et al: Cardio-protection of
salvianolic acid B through inhibition of apoptosis network. PLoS
One. 6:e240362011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Derakhshan A, Chen Z and Van Waes C:
Therapeutic small molecules target inhibitor of apoptosis proteins
in cancers with deregulation of extrinsic and intrinsic cell death
pathways. Clin Cancer Res. 23:1379–1387. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cory S and Adams JM: The Bcl2 family:
Regulators of the cellular life-or-death switch. Nat Rev Cancer.
2:647–656. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murphy KM, Ranganathan V, Farnsworth ML,
Kavallaris M and Lock RB: Bcl-2 inhibits Bax translocation from
cytosol to mitochondria during drug-induced apoptosis of human
tumor cells. Cell Death Differ. 7:102–111. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bouillet P, Purton JF, Godfrey DI, Zhang
LC, Coultas L, Puthalakath H, Pellegrini M, Cory S, Adams JM and
Strasser A: BH3-only Bcl-2 family member Bim is required for
apoptosis of autoreactive thymocytes. Nature. 415:922–926. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Whitfield J, Neame SJ, Paquet L, Bernard O
and Ham J: Dominant-negative c-Jun promotes neuronal survival by
reducing BIM expression and inhibiting mitochondrial cytochrome
c release. Neuron. 29:629–643. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Connor L, Strasser A, O'Reilly LA,
Hausmann G, Adams JM, Cory S and Huang DC: Bim: A novel member of
the Bcl-2 family that promotes apoptosis. EMBO J. 17:384–395. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ley R, Balmanno K, Hadfield K, Weston C
and Cook SJ: Activation of the ERK1/2 signaling pathway promotes
phosphorylation and proteasome-dependent degradation of the
BH3-only protein, Bim. J Biol Chem. 278:18811–18816. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lei K and Davis RJ: JNK phosphorylation of
Bim-related members of the Bcl2 family induces Bax-dependent
apoptosis. Proc Natl Acad Sci USA. 100:2432–2437. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu JW, Chandra D, Tang SH, Chopra D and
Tang DG: Identification and characterization of Bimgamma, a novel
proapoptotic BH3-only splice variant of Bim. Cancer Res.
62:2976–2981. 2002.PubMed/NCBI
|
|
31
|
Wei H, Li Z, Hu S, Chen X and Cong X:
Apoptosis of mesenchymal stem cells induced by hydrogen peroxide
concerns both endoplasmic reticulum stress and mitochondrial death
pathway through regulation of caspases, p38 and JNK. J Cell
Biochem. 111:967–978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zou H, Li Y, Liu X and Wang X: An
APAF-1.cytochrome c multimeric complex is a functional
apoptosome that activates procaspase-9. J Biol Chem.
274:11549–11556. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Strasser A, Jost PJ and Nagata S: The many
roles of FAS receptor signaling in the immune system. Immunity.
30:180–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Korsmeyer SJ, Wei MC, Saito M, Weiler S,
Oh KJ and Schlesinger PH: Pro-apoptotic cascade activates BID,
which oligomerizes BAK or BAX into pores that result in the release
of cytochrome c. Cell Death Differ. 7:1166–1173. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bus P, Pierneef L, Bor R, Wolterbeek R,
van Es LA, Rensen PC, de Heer E, Havekes LM, Bruijn JA, Berbée JF,
et al: Apolipoprotein C-I plays a role in the pathogenesis of
glomerulosclerosis. J Pathol. 241:589–599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Berbée JF, van der Hoogt CC, Kleemann R,
Schippers EF, Kitchens RL, van Dissel JT, Bakker-Woudenberg IA,
Havekes LM and Rensen PC: Apolipoprotein CI stimulates the response
to lipopolysaccharide and reduces mortality in gram-negative
sepsis. FASEB J. 20:2162–2164. 2006. View Article : Google Scholar : PubMed/NCBI
|