Introduction
Cancer is an incurable disease, which not only
brings enormous burden to the countries, especially the developing
countries, but also affects the economic development to a certain
degree (1). As per the latest
researches, the morbidity and morality of cancer has been
increasing year after year, and gastric cancer is one of main
causes of death among all types of cancers, ranking the 3rd in
China (2). Gastric cancer in the
advanced stage tends to metastasize, and although the cancer is
treated by surgery, radiotherapy or chemotherapy or other means
(3), the results are unsatisfactory
as the survival time of the patients after operation is short and
relapse is common. There is increased research on the molecular
targeted treatment of gastric cancer, and it has become more
important to explore the molecular mechanism of the occurrence and
development of gastric cancer (4,5). Based
on the above, our research group, by referring to the literature,
discovered Kankl (6), as one
possible ideal and reliable molecular target for future molecular
targeted treatment, providing new significance to the clinical
understanding of the biological behavior of gastric cancer.
In 2002, the Japanese scholars cloned the candidate
tumor suppressor gene Kank1 (7).
Kankl plays the role of inhibiting the occurrence and development
of tumors to a certain extent (8).
The low expression of Kankl has been found in kidney (9), brain glioma (10), cervical (11), bladder (12), liver (13,14),
pancreatic cancer (15) and various
tumor tissues including lung cancer (16,17).
It mainly exists in the cytoplasm, adjusts the actin polymerization
in the cytoskeleton and participates in the cell motility (18). It also forms a compound with
β-catenin to shuttle among the nucleoplasm to regulate the
development of many malignant tumors. It has been found in some
research that downregulation of Kankl gene may participate in the
genesis and development of bone tumor subcellular distribution of
β-catenin, playing a key role in the genesis and via activating
JAK2-Stat (19) tumor-promoting
signaling pathway. The mechanism of the tumor genesis and
development is complicated and varied, while the missing or
mutation of cancer suppressor gene has significant impact on the
tumor genesis (20). We have found
low expression of Kankl in gastric cancer cells. It was found after
upregulating Kankl gene that the proliferation of tumor cells was
significantly inhibited, and with apoptosis. At the same time,
upregulation of Kankl gene can lead to the increase of Bax
expression and decrease of Bcl-2 expression, and the expression of
caspase family is changed, mainly with caspase-3 and caspase-9
activation. In addition, after the upregulation of Kankl gene, the
ability of tumor cell invasion and metastasis was reduced. In
vivo experiments showed upregulation of Kank1 may lead to lower
tumorigenicity rate and significant reduction in tumor size. In
summary, we conclude that the upregulation of Kankl gene can
inhibit gastric cancer development in vivo, and the
mechanism is closely related to apoptosis and tumor invasion and
metastasis.
Materials and methods
Cell strain
Human gastric cancer cell strain SGC-7901, MKN-49P
cells and normal human gastric epithelial cell line GES-1 and RGM-1
cells were cultured in the RPMI-1640 culture medium containing 10%
fetal bovine serum (FBS) in the incubator containing 5%
CO2 at 37°C of saturated humidity. Both the culture
medium RPMI-1640 and FBS were from Gibco (Carlsbad, CA, USA).
Antibodies and reagents
Kank1, Bax, Bcl-2 and MMP-7 antibodies were all from
Cell Signaling Technology Inc. (Danvers, MA, USA). siRNA and
plasmid were synthesized by Shanghai GenePharma, Co., Ltd.
(Shanghai, China). Caspase-3 Assay kit and caspase-9 Assay kit were
from (Abcam, Cambridge, UK). RT-PCR kit was obtained from (Takara
Bio, Shiga, Japan). PI was from Sigma-Aldrich (St. Louis, MO, USA)
and Annexin V-FITC apoptosis assays kit was from BD Biosciences
(San Jose, CA, USA).
Cell culture
Human gastric cancer cell strain SGC-7901 and
MKN-49P and normal gastric mucosa epithelial cell strains GES-1 and
RGM-1 were cultured in the RPMI-1640 culture medium containing 10%
fetal calf serum (FCS), 100 µg/ml penicillin and 100 µg/ml
streptomycin, and then were cultured in the incubator containing 5%
CO2 at 37°C.
Cell transfection
Human gastric cancer cells in logarithmic growth
phase (SGC-7901 and MKN-49P) were collected by trypsin, and
SGC-7901 and MKN-49P cells were plated at the density of
6×105/ml in 6-well plates for culture. Transfection was
carried out when the cells grew to >75% confluence. Negative
oligonucleotide was used as control. The culture medium was
replaced without serum and antibiotics, add respectively the mixed
solution of siRNA and plasmid with Lipofectamine 2000, adjusted to
a predetermined concentration by referring to the transfection
reagent instructions. We used an RT-PCR kit for PCR analysis.
RNA extraction and RT-PCR
analysis
RNA cells were collected and extracted for purity
and integrity analysis before the reverse transcription. After
calculating the concentration of RNA, we used the RT-PCR Kit
(Takara) for RT-PCR reaction with steps according to the
instructions. Kank1 and β-actin gene primers were designed and
synthesized by Invitrogen. The forward primer of Kank1 gene was
5′-CTTGACACAGTATTTTCACGCTTTTG-3 and the reverse primer,
5-AAGTAAATGTGACACGGTAAAAAGG-3; β-actin gene forward primer,
5-CTGGGACGACATGGAGAAAA-3 and reverse primer, 5-AAGGAAGGCTG
GAAGAGTGC-3. PCR reaction system 25 µl at the following reaction
conditions: 94°C for 2 min, 94°C degeneration for 30 sec, 60°C
annealing for 30 sec, 72°C extension for 30 sec, total 31 cycles.
The PCR product was electrophoresed with 1.5% agarose gel, then
scanned and analyzed with gel imaging system.
Cell viability
Cells were collected with trypsin and inoculated
into 96-well plates, with 5 double wells in each group. For the
transfection group, complete culture solution was used after 24 h
and 20 µl MTT solution was added 48 h later (0.5 mg/ml)/well. After
the cells were incubated for 4 h at 37°C, the supernatant was
removed and 150 µl of dimethyl sulfoxide (DMSO) was added to each
well for 10 min oscillation until the complete dissolution of the
purple crystal. The optical density value of the 490 nm wavelength
of each well was measured by the microplate reader.
Caspase viability
Human gastric cancer cells after transfection, human
gastric cancer cells without transfection and blank plasmid
transfected cells were collected to determine the activity of
caspase-3 and caspase-9 according to the caspase activity analysis
kit. The fluorescence spectrophotometer was used at an excitation
wavelength of 460 nm.
Analysis of apoptosis with flow
cytometry
We digested and collected the cells of all groups
for PBS cell suspension and pipetting preparation into single cell
suspension. According to Annexin V-FITC cell apoptosis analysis kit
steps, 10 µl of Annexin V and 5 µl of PI staining were added,
respectively for staining in the dark at room temperature for 15
min and flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA)
was used to detect cell apoptosis, and each experiment was repeated
3 times.
Cell invasion and migration ability
analysis
The cells of all groups were cultured in 6-well
Transwell culture plate of BD, the upper chamber of 8 µm membrane
was covered with Matrigel diluted at 1:6 and each culture well was
evenly laid with ~2×106 cells to culture for 36 h. The
cells that had gone through the membrane were fixed to the bottom
of the culture well with formalin and stain with Crystal violet.
The number of cells of 10 fields (×200) was counted for each
well.
Western blot assay
Human gastric cancer cells and their stably
transfected cell strain were collected, washed with PBS twice, and
1 ml protein lysate was added (Sigma-Aldrich) containing 10 µl
protease inhibitor for cell lysis. The concentration of protein was
measured by BSA method. Equal amount of protein was added to each
well for loading and separated with 10% SDS-PAGE gel, and then the
protein was transferred to PVDF membrane with semi-dry method and
5% skim milk powder was used for sealing at 4°C overnight. TBST
solution was diluted and the first antibody added at 37°C with the
first antibody dilution ratio (phosphorylated PI3K antibody 1:500,
phosphorylated-Akt antibody 1:1000, MMP-7 antibody 1:800). The
membrane was washed with TBST 3 times, second antibody incubation
at 37°C for 1 h, and TBST solution shaking and washing 4 times. ECL
luminescence reagent was added, with X exposure imaging, scanning
strip, and the gray analysis and β-actin were used as the reference
standard.
Inoculation in nude mice
The animal experiment program has been approved by
the Ethics Committee of Zhengzhou City People's Hospital. The nude
mice were purchased from the Animal Experimental Center of Henan
Medical University, and there were 20 male mice of 6–8 weeks,
weighing ~20 g. The mice were randomly divided into 2 groups with
10 mice in each group. The human gastric cancer cells were divided
into 2 groups: the constructed Kank1 gene stable expression of
MKN-49P cells were divided into a group, and non-transfection
group, and they were, respectively, planted in 2 groups of nude
mice via subcutaneous injection; the impact of Kank1 on the
transplanted tumor formation ability of nude mice was observed. The
effect of upregulating Kank1 gene on the growth of transplanted
tumor in nude mice was also observed and the tumor growth was
measured 21 and 28 days. The nude mice were sacrificed 4 weeks
later with cervical spine method, and the subcutaneous
transplantation tumor tissue under sterile condition was cut-off
for indicator analysis.
Statistical analysis
All the experimental data were analyzed with SPSS
18.0 statistical software. T-test and variance analysis were used.
P<0.05 was considered to indicate the significant statistical
difference.
Results
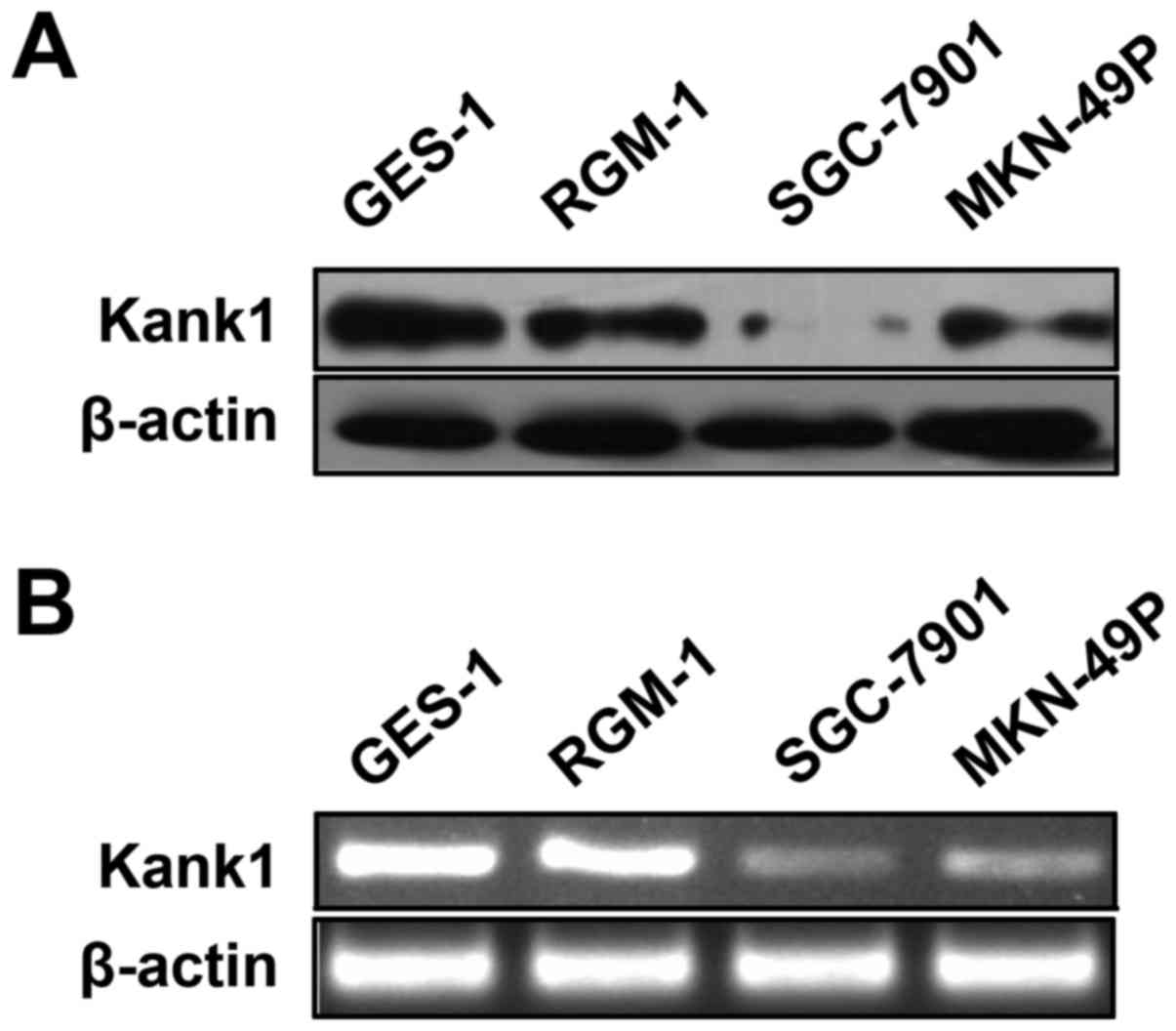
Kank1 gene expression is low in human
gastric cancer cells
We used RT-PCR and western blot assay to determine
the expression of Kank1 mRNA and protein in human gastric cancer
cells (SGC-7901 and MKN-49P cells) and normal human gastric
epithelial cell line GES-1 cells and RGM-1 cells. The results
showed that, as compared with normal human gastric mucosal
epithelial cell lines, the expression of Kank1 mRNA and protein in
SGC-7901 and MKN-49P cells also decreased. These results indicated
that both protein and gene of Kank1 were expressed in gastric
cancer at low levels (Fig. 1).
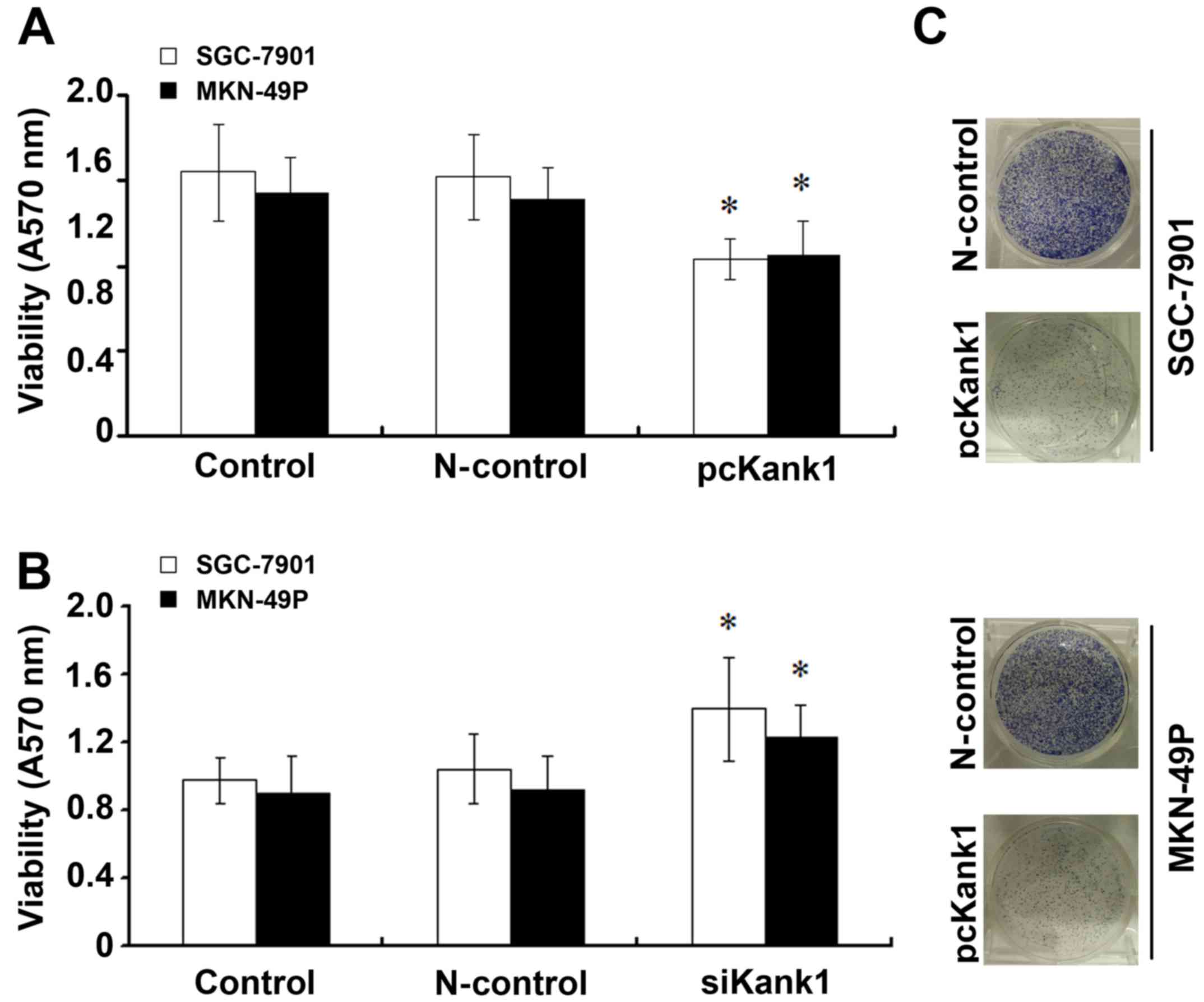
Kank1 gene inhibits growth of human
gastric cancer cells
In order to further confirm the effect of Kank1 gene
on the growth of human gastric cancer cells, we used transfection
experiments, respectively for the transfection of siKank1 and
successfully constructed and transfected Kank1 plasmid, upregulated
the expression level of Kank1 gene in human gastric cancer cells
and analyzed using the MTT method. We found that as compared with
the control group and transfection negative oligonucleotide control
group, the proliferation ability of SGC-7901 and MKN-49P cells
declined significantly after the upregulation of the expression of
Kank1 and when silencing Kank1, compared with the control group and
transfection negative oligonucleotide control group, cell
proliferation rate increased significantly which suggests that
Kank1 gene can inhibit the growth of human gastric cancer cells
(Fig. 2).
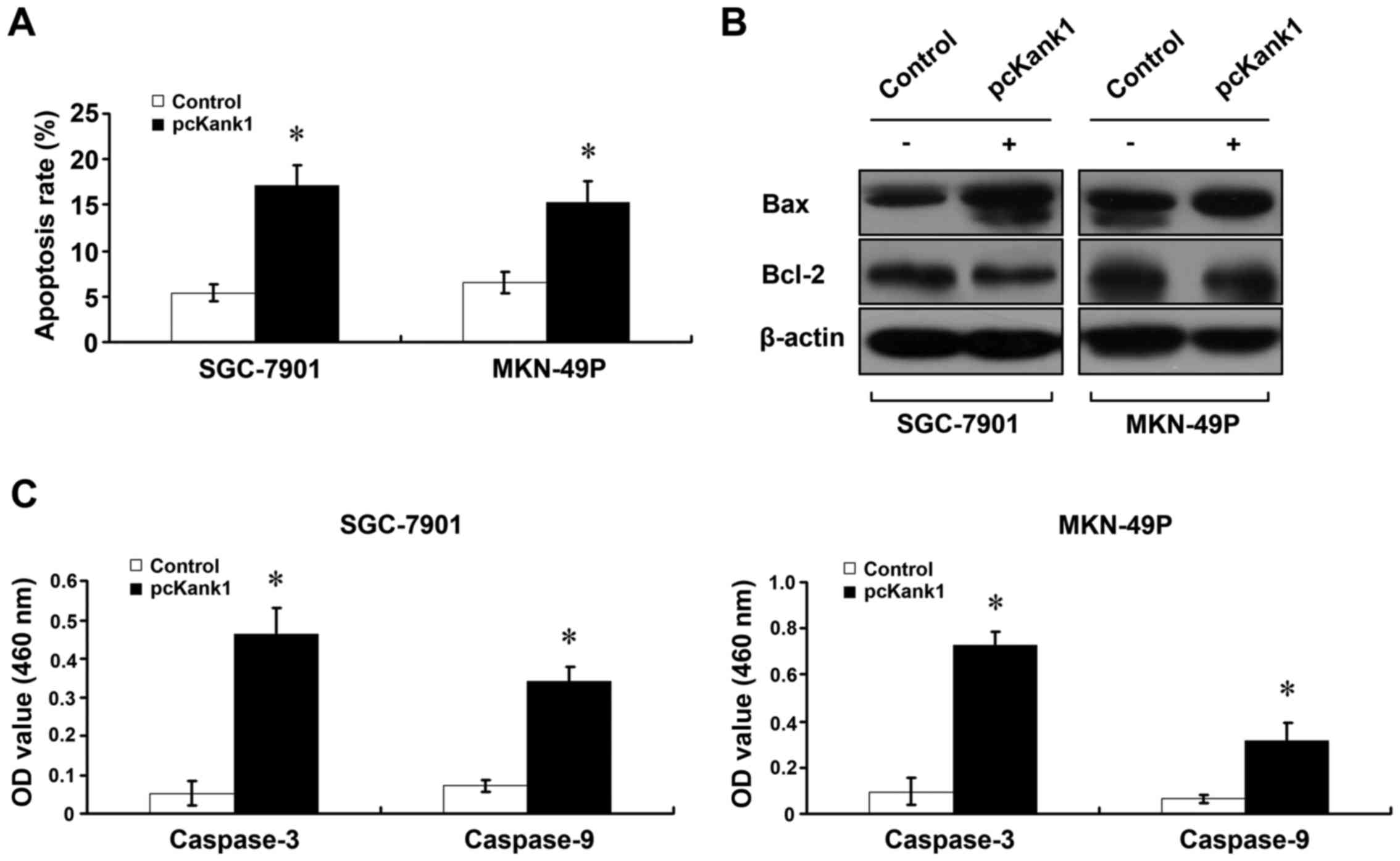
Upregulation of Kank1 gene induces
apoptosis of human gastric cancer cells
In order to investigate whether the Kank1 gene is
associated with apoptosis of human gastric cancer cells, we
increased the expression level of Kank1 gene, and used Annexin
V-FITC/PI double labeling method to determine the cell apoptosis
rate. We found that after the expression of Kank1 was upregulated,
the apoptosis rate of SGC-7901 and MKN-49P cells increased
significantly as compared with the control group (Fig. 3A). The changes of expression level
of Bel-2 and Bax proteins were determined with western blot assay
(Fig. 3B). In addition, we used
caspase activity assay kit to upregulate Kank1 gene expression
level and found viability enhancement of caspase-3 and caspase-9
(Fig. 3C). These results indicate
that upregulation of Kank1 gene can lead to apoptosis of human
gastric cancer cells.
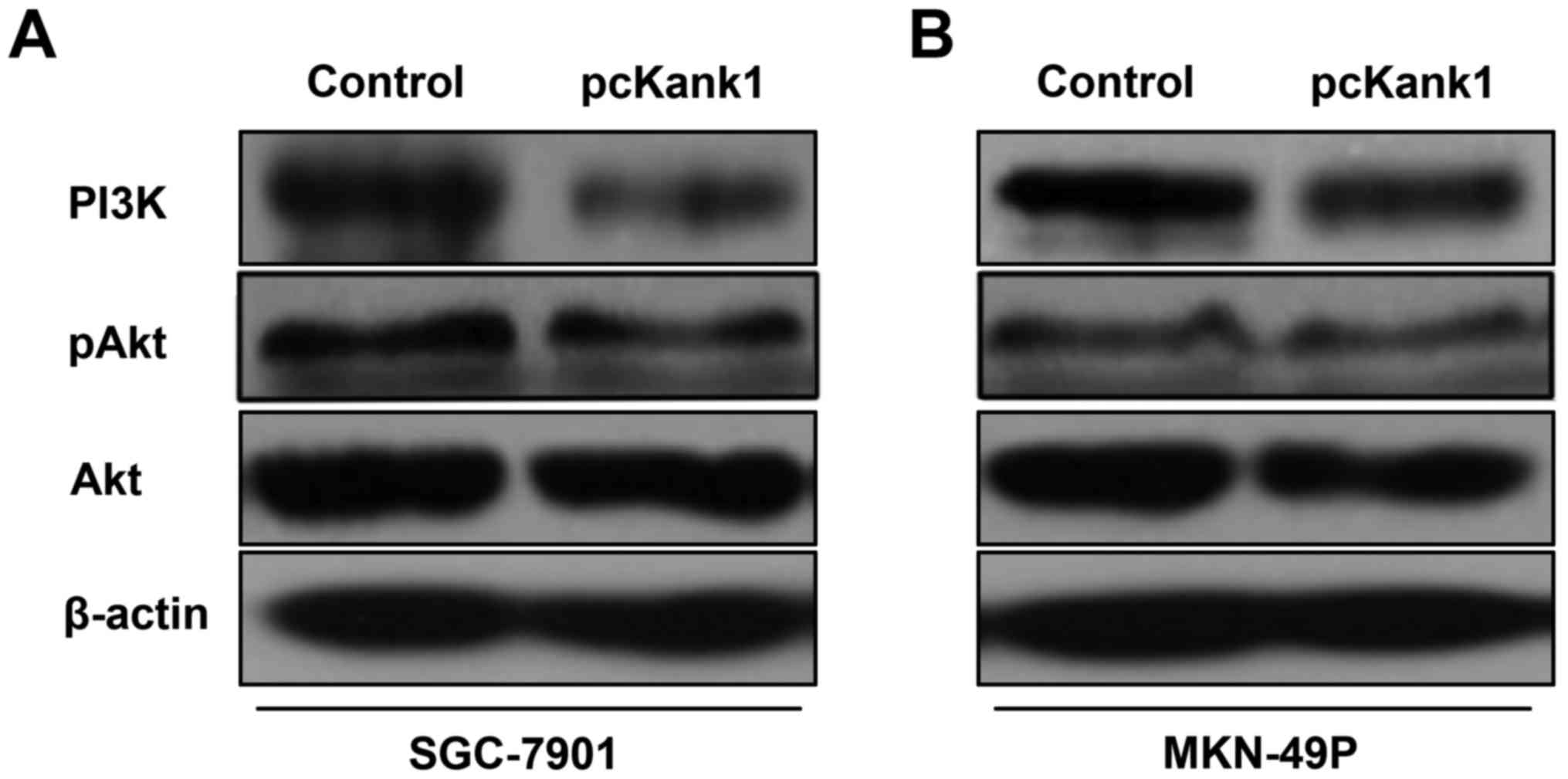
Upregulation of Kank1 gene induces
apoptosis of human gastric cancer cells by inhibiting PI3K/Akt
pathway
In order to investigate the molecular mechanism of
apoptosis of human gastric cancer cells induced by of upregulating
Kank1 gene, we referred to the relevant literature and found that
Kank1 gene may induce apoptosis of human gastric cancer cells by
inhibiting the PI3K/Akt pathway. We used western blot assay to
determine the changes in the expression level of PI3K/Akt pathway
proteins PI3K and Akt, and we tested the changes in their
phosphorylation level at the same time. We found reduction in the
expression level of PI3K protein after the upregulation of Kank1
gene. Moreover, phosphorylation of Akt protein also decreased after
the upregulation of the Kank1 gene (Fig. 4). Our results indicated that
upregulation of Kank1 gene can induce apoptosis of human gastric
cancer cells by inhibiting the PI3K/Akt signaling pathway.
Upregulation of Kank1 gene inhibits
invasion and metastasis of gastric cancer cells
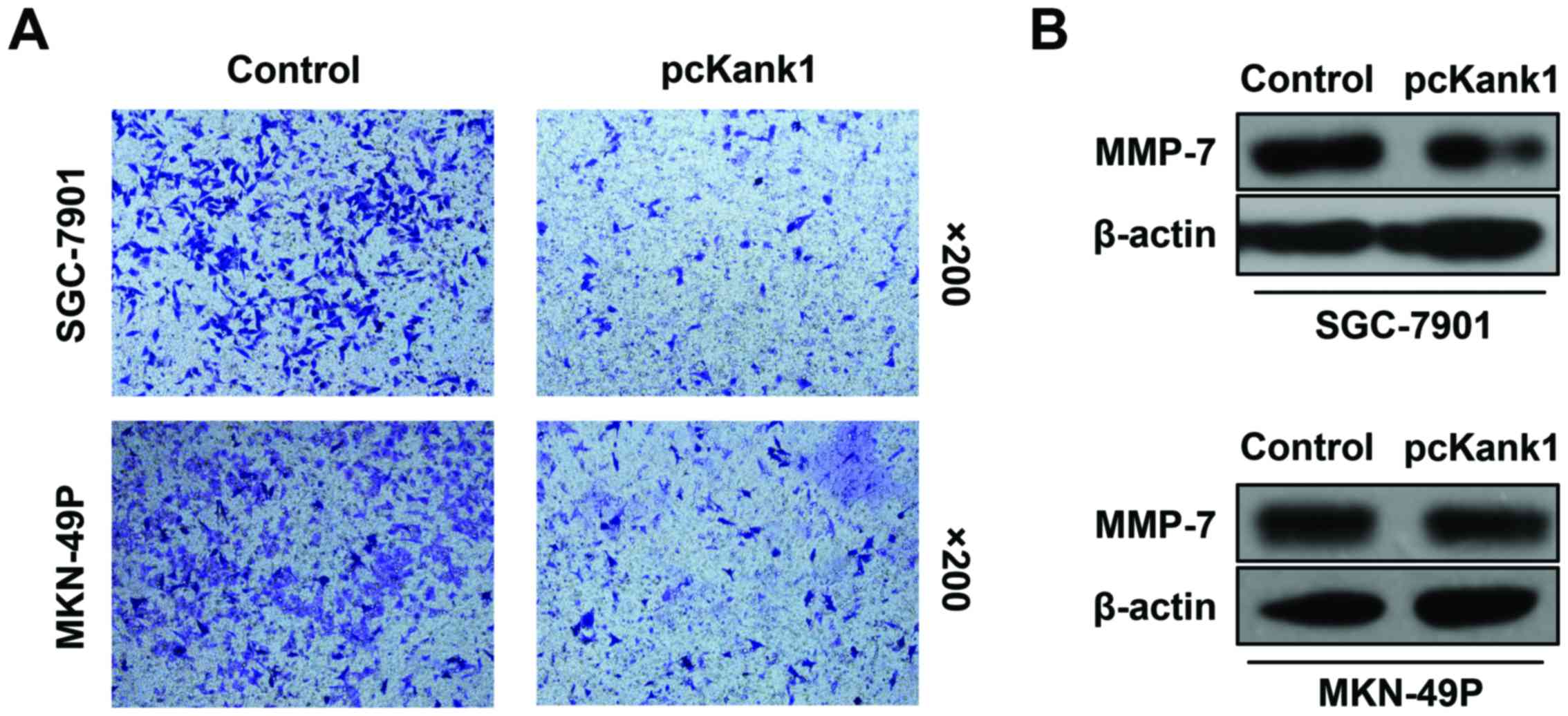
We have also found that upregulation of the Kank1
gene can inhibit human gastric cancer cell invasion and metastasis.
We analyzed the change in the invasion and migration ability of
gastric cancer cells via cell invasion and metastasis test. We have
found that invasion and metastasis ability of the human gastric
cancer cells (SGC-7901 and MKN-49P cells) with upregulated Kank1
gene, both declined significantly as compared with the control
group of oligonucleotides (Fig.
5A). We have found by western blot assay that the expression
level of MMP-7 protein in human gastric cancer cells declined by
upregulation of Kank1 gene (Fig.
5B). Our results indicated that upregulation of Kank1 gene can
inhibit the invasion and metastasis of human gastric cancer cells
and downregulate the expression of MMP-7 protein.
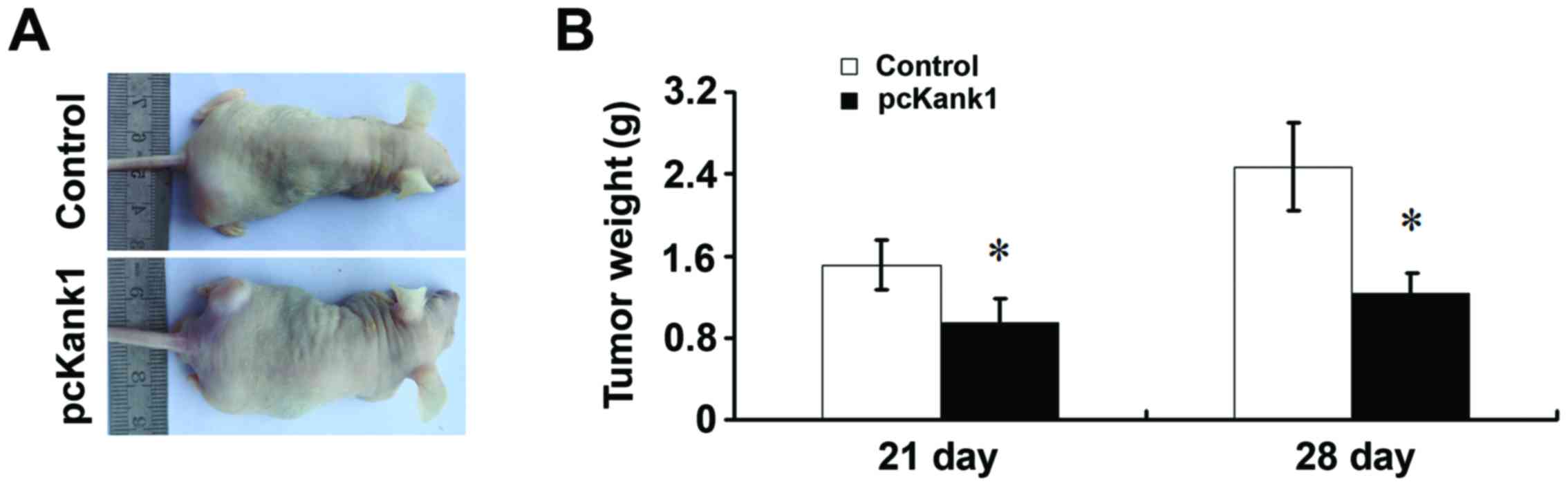
Effects of upregulation of Kank1 gene
on the growth of transplanted tumor in nude mice
We found that at any time, the tumor volume of the
group with upregulated Kank1 gene were significantly lower than
that of the control group (Fig.
6A). At the same time, the weight of tumor harvested at surgery
in the group of upregulated Kank1 gene was significantly lower than
the control group (Fig. 6B). These
results strongly suggest that upregulating Kank1 gene can
significantly inhibit the progress of gastric cancer in
vivo.
Discussion
At present, gastric cancer is a major cause of
cancer death in the world, although early detection and standard
treatment approaches have improved, gastric cancer mortality rates
have remained high (21). As is
known, the complicated pathogenesis of malignant tumor is closely
related to the missing or mutation of tumor suppressor genes or the
overexpression of cancer gene and some signaling pathways. The
research explores the occurrence and development of gastric cancer
gene from the perspective of tumor suppressor gene deletion or
mutation. Kankl is one of the key members of Kank family gene
(22). Kankl gene is kocated in
human chromosome 9p24.3 containing 12 exons with the total length
of ~27.7 kp (5). Kankl protein
consists of three parts, in which ankyrin repeat domain and coiled
coil domain are the functional domains where Kankl protein and
other proteins combine for their biological roles (6,10).
Kank1 is mainly distributed in the cytoplasm, and associated with
cell motility. In the development, invasion and metastasis of a
variety of diseases including malignant tumors, Kank1 often forms a
compound with β-catenin to shuttle in the nucleoplasm to adjust the
distribution of β-catenin in the nucleus and strengthen the
transcription of β-catenin to affect the occurrence and development
of tumor to a certain extent (23).
As a candidate tumor suppressor gene, Kank1 gene is
lowly expressed in a variety of malignant tumors, such as kidney
(24) and liver cancer (14). No report on the research of gastric
cancer in this aspect is available yet. The incidence and mortality
of gastric cancer is ranking top three in malignant tumors, but its
pathogenesis is still unclear. Therefore, it is imperative to
explore the effect of Kank1 gene on tumor development and
metastasis of gastric cancer. We found through research that the
Kank1 gene and protein expression in human gastric cancer cells are
low as compared with normal gastric cells. Thus, we can draw the
conclusion that there is a certain close relationship between Kank1
gene and gastric cancer development process, and the Kank1 gene may
well be a potential therapeutic target for gastric cancer. In order
to further elucidate the relationship between Kank1 gene and the
existence and development of gastric cancer as well as its detailed
mechanism of action, we used transfection and other methods to
upregulate Kank1 gene expression in human gastric cancer cells, and
observed the biological changes of human gastric cancer cell with
Kank1 gene overexpression through a series of experiments. We found
that upregulating the expression of Kank1 can inhibit the
proliferation of human gastric cancer cells and promote cell
apoptosis. Therefore, we believe that the low expression of Kank1
in gastric cancer promotes the proliferation of cancer cells to a
certain extent.
The mechanism of how Kank1 gene regulates the
process of gastric cancer cell proliferation is still unknown. In
order to clarify this key issue, we found that Kank1 gene and
PI3K/AKT signaling pathway are related (25). To a certain extent, PI3K/AKT
signaling pathway regulates the proliferation, differentiation,
migration and infiltration or other functions of gastric cancer
cells (26). When we upregulated
the expression level of Kank1 gene in human gastric cancer cells,
we found that the phosphorylation expression level of PI3K protein
and AKT protein was significantly inhibited. Therefore, from the
cell proliferation signaling pathway we found that Kank1 could
inhibit the proliferation of tumor cells and promote cell apoptosis
by inhibiting PI3K/AKT signaling pathway.
The mitochondrial pathway and death receptor pathway
of apoptosis is the classical pathway of apoptosis of various tumor
cells (27). Bcl-2 and Bax play a
key role in mitochondrial apoptotic pathway (28), the expression of Bcl-2 in a variety
of tumors, including gastric cancer, is high while Bax is the
opposite (29). The knowledge on
the mechanism of how Kank1 gene regulates apoptosis of human
gastric cancer is still very limited. Thus, after upregulating
Kank1 gene expression in human gastric cancer cells, we found via
western blot assay and RT-PCR, the shift of Bax and Bcl-2, the
decline in the expression level of Bax, and Bax translocation from
the cytosol to the mitochondrial membrane, which could change the
permeability of the mitochondrial membrane, promote the release of
cytochrome c from mitochondria into cytoplasm, then start
the apoptosis cascade, eventually leading to cell apoptosis. Thus,
we speculated that the Kank1 gene promoted apoptosis in gastric
cancer cells through regulating Bel-2 family of anti/pro-apoptotic
proteins (Bcl-2 and Bax), we also demonstrated that the phase of
Kank1 gene and mitochondrial pathway leading to apoptosis of tumor
cells. As known, caspase family activation and cascade
amplification is a necessary condition for cell apoptosis
regardless whether apoptosis is regulated externally or internally
(30). We upregulated Kank1 gene in
human gastric cancer cells and found that the expression level of
pro-caspase-9 and pro-caspase-3 both decreased significantly. In
conclusion, we found that the Kank1 gene can promote apoptosis of
human gastric cancer cells by regulating the change of
mitochondrial membrane potential, and activating mitochondria to
release apoptosis enzyme activation factor to activate
caspases.
Tumor invasion and metastasis is one of the
important causes of cancer death, as it is found that ~90% of the
tumor patients died of metastatic tumor (31). We found that overexpression of the
Kank1 gene can significantly inhibit the invasion and metastasis of
human gastric cancer cells. MMP-7, as an important member of the
MMP family, on the one hand, its physiological function can degrade
certain proteins in the extracellular matrix, such as elastic
protein and fibronectin (32), on
the other hand, it can activate protease activity, and promote the
release of the growth factor (33).
MMP-7 promotes the invasion and metastasis ability of various
tumors, and the latest research has found that MMP-7 is used as a
biomarker to evaluate the proliferation, differentiation and
metastasis of gastric cancer (34,35).
We found that the upregulation of Kank1 gene can reduce the
expression of MMP-7 in gastric cancer. These results indicated that
upregulation of Kank1 gene can inhibit the invasion and metastasis
of human gastric cancer cells through inhibition of MMP-7
expression in gastric cancer.
In conclusion, we found in the present study that
the reduction of Kank1 gene expression in gastric cancer, and the
upregulation of Kank1 gene can inhibit the PI3K/AKT signaling
pathway, and acts on the mitochondria pathway to regulate Bcl-2/Bax
to further induce the inhibition of the proliferation of human
gastric cancer cells leading to apoptosis. Kank1 gene in the future
may become a potential therapeutic target and provides theoretical
basis for clinical treatment of gastric cancer.
References
|
1
|
Jönsson B, Hofmarcher T, Lindgren P and
Wilking N: The cost and burden of cancer in the European Union
1995–2014. Eur J Cancer. 66:162–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zuo T, Zeng H, Zhang S
and He J: National cancer incidence and mortality in China, 2012.
Chin J Cancer Res. 28:1–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee JH, Lim JK, Kim MG and Kwon SJ: The
influence of post-operative surveillance on the prognosis after
curative surgery for gastric cancer. Hepatogastroenterology.
61:2123–2132. 2014.PubMed/NCBI
|
|
4
|
Yazici O, Sendur MA, Ozdemir N and Aksoy
S: Targeted therapies in gastric cancer and future perspectives.
World J Gastroenterol. 22:471–489. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee SY and Oh SC: Changing strategies for
target therapy in gastric cancer. World J Gastroenterol.
22:1179–1189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu Y, Kakinuma N, Wang Y and Kiyama R:
Kank proteins: A new family of ankyrin-repeat domain-containing
proteins. Biochim Biophys Acta. 1780:128–133. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sarkar S, Roy BC, Hatano N, Aoyagi T,
Gohji K and Kiyama R: A novel ankyrin repeat-containing gene (Kank)
located at 9p24 is a growth suppressor of renal cell carcinoma. J
Biol Chem. 277:36585–36591. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kakinuma N, Zhu Y, Wang Y, Roy BC and
Kiyama R: Kank proteins: Structure, functions and diseases. Cell
Mol Life Sci. 66:2651–2659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hatano N, Nishikawa NS, McElgunn C, Sarkar
S, Ozawa K, Shibanaka Y, Nakajima M, Gohiji K and Kiyama R: A
comprehensive analysis of loss of heterozygosity caused by
hemizygous deletions in renal cell carcinoma using a subtraction
library. Mol Carcinog. 31:161–170. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo X, Fan W, Bian X and Ma D:
Upregulation of the Kank1 gene-induced brain glioma apoptosis and
blockade of the cell cycle in G0/G1 phase. Int J Oncol. 44:797–804.
2014.PubMed/NCBI
|
|
11
|
Zimonjic DB, Simpson S, Popescu NC and
DiPaolo JA: Molecular cytogenetics of human papillomavirus-negative
cervical carcinoma cell lines. Cancer Genet Cytogenet. 82:1–8.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Simon R, Burger H, Semjonow A, Hertle L,
Terpe HJ and Bocker W: Patterns of chromosomal imbalances in muscle
invasive bladder cancer. Int J Oncol. 17:1025–1029. 2000.PubMed/NCBI
|
|
13
|
Huang SF, Hsu HC and Fletcher JA:
Investigation of chromosomal aberrations in hepatocellular
carcinoma by fluorescence in situ hybridization. Cancer Genet
Cytogenet. 111:21–27. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shao J, Li Y, Li H, Wu Q, Hou J and Liew
C: Deletion of chromosomes 9p and 17 associated with abnormal
expression of p53, p16/MTS1 and p15/MTS2 gene protein in
hepatocellular carcinomas. Chin Med J (Engl). 113:817–822.
2000.PubMed/NCBI
|
|
15
|
Heidenblad M, Schoenmakers EF, Jonson T,
Gorunova L, Veltman JA, van Kessel AG and Höglund M: Genome-wide
array-based comparative genomic hybridization reveals multiple
amplification targets and novel homozygous deletions in pancreatic
carcinoma cell lines. Cancer Res. 64:3052–3059. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato M, Takahashi K, Nagayama K, Arai Y,
Ito N, Okada M, Minna JD, Yokota J and Kohno T: Identification of
chromosome arm 9p as the most frequent target of homozygous
deletions in lung cancer. Genes Chromosomes Cancer. 44:405–414.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lo KC, Stein LC, Panzarella JA, Cowell JK
and Hawthorn L: Identification of genes involved in squamous cell
carcinoma of the lung using synchronized data from DNA copy number
and transcript expression profiling analysis. Lung Cancer.
59:315–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roy BC, Kakinuma N and Kiyama R: Kank
attenuates actin remodeling by preventing interaction between
IRSp53 and Rac1. J Cell Biol. 184:253–267. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kralovics R, Teo SS, Buser AS, Brutsche M,
Tiedt R, Tichelli A, Passamonti F, Pietra D, Cazzola M and Skoda
RC: Altered gene expression in myeloproliferative disorders
correlates with activation of signaling by the V617F mutation of
Jak2. Blood. 106:3374–3376. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boroughs LK and DeBerardinis RJ: Metabolic
pathways promoting cancer cell survival and growth. Nat Cell Biol.
17:351–359. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fitzmaurice C, Dicker D, Pain A, Hamavid
H, Moradi-Lakeh M, MacIntyre MF, Allen C, Hansen G, Woodbrook R,
Wolfe C, et al: Global Burden of Disease Cancer Collaboration: The
Global Burden of Cancer 2013. JAMA Oncol. 1:505–527. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clohisey SM, Dzhindzhev NS and Ohkura H:
Kank is an EB1 interacting protein that localises to muscle-tendon
attachment sites in Drosophila. PLoS One. 9:e1061122014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Kakinuma N, Zhu Y and Kiyama R:
Nucleo-cytoplasmic shuttling of human Kank protein accompanies
intracellular translocation of beta-catenin. J Cell Sci.
119:4002–4010. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Roy BC, Aoyagi T, Sarkar S, Nomura K,
Kanda H, Iwaya K, Tachibana M and Kiyama R: Pathological
characterization of Kank in renal cell carcinoma. Exp Mol Pathol.
78:41–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kakinuma N, Roy BC, Zhu Y, Wang Y and
Kiyama R: Kank regulates RhoA-dependent formation of actin stress
fibers and cell migration via 14-3-3 in PI3K-Akt signaling. J Cell
Biol. 181:537–549. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Chen S, Xue R, Zhao J and Di M:
Mefloquine effectively targets gastric cancer cells through
phosphatase-dependent inhibition of PI3K/Akt/mTOR signaling
pathway. Biochem Biophys Res Commun. 470:350–355. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ghavami S, Hashemi M, Ande SR, Yeganeh B,
Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, et
al: Apoptosis and cancer: Mutations within caspase genes. J Med
Genet. 46:497–510. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ghasemian M, Mahdavi M, Zare P and Ali
Hosseinpour Feizi M: Spiroquinazolinone-induced cytotoxicity and
apoptosis in K562 human leukemia cells: Alteration in expression
levels of Bcl-2 and Bax. J Toxicol Sci. 40:115–126. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sela B: Survivin: Anti-apoptosis protein
and a prognostic marker for tumor progression and recurrence.
Harefuah. 141:103–107, 123. 2002.(In Hebrew). PubMed/NCBI
|
|
30
|
Jia J, Furlan A, Gonzalez-Hilarion S,
Leroy C, Gruenert DC, Tulasne D and Lejeune F: Caspases shutdown
nonsense-mediated mRNA decay during apoptosis. Cell Death Differ.
22:1754–1763. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun Y, Ai X, Shen S and Lu S:
NF-κB-mediated miR-124 suppresses metastasis of non-small-cell lung
cancer by targeting MYO10. Oncotarget. 6:8244–8254. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang B, Gao J, Rao Z, Zhang B, Ouyang W
and Yang C: Antisense oligonucleotide targeting matrix
metalloproteinase-7 (MMP-7) changes the ultrastructure of human
A549 lung adenocarcinoma cells. Ultrastruct Pathol. 35:256–259.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu D, Nakano J, Ishikawa S, Yokomise H,
Ueno M, Kadota K, Urushihara M and Huang CL: Overexpression of
matrix metalloproteinase-7 (MMP-7) correlates with tumor
proliferation, and a poor prognosis in non-small cell lung cancer.
Lung Cancer. 58:384–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Soleyman-Jahi S, Nedjat S, Abdirad A,
Hoorshad N, Heidari R and Zendehdel K: Prognostic significance of
matrix metalloproteinase-7 in gastric cancer survival: A
meta-analysis. PLoS One. 10:e01223162015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ye Y, Zhou X, Li X, Tang Y, Sun Y and Fang
J: Inhibition of epidermal growth factor receptor signaling
prohibits metastasis of gastric cancer via downregulation of MMP7
and MMP13. Tumour Biol. 35:10891–10896. 2014. View Article : Google Scholar : PubMed/NCBI
|