Introduction
Ovarian cancer represents the leading cause of
deaths by women's malignancy in Western countries, without specific
symptoms and reliable diagnosis in its early stage, ovarian cancer
mortality reaches a high level of 70% in 5 years of diagnosis
(1). The lack of effective
treatment for recurrent cases contributes to the problem. One big
challenge is the development of resistance in as many as 60%
patients treated with conventional chemotherapy drugs. In recent
years, the molecular targeting therapy applying gefitinib and
bevacizumab has shown potential, although the heterogeneous
response by different patients continues to be a major obstacle for
its clinical application (2). The
development of new targeting modalities is essential for the
improvement on the management of recurrent/drug-resistant ovarian
cancer patients.
Since 2001, novel nanomaterials have attracted
attention of biomedical investigators. The coupling of chemotherapy
drugs with nanocarriers opened a new avenue in cancer therapy
(3). Based on the physiological
characteristics of tumor tissue, nanocarriers can be designed to
reduce the toxicity and drug resistance (4). The ideal nanocarriers can protect the
activities of drugs, prolong the plasma half-life, and selective
releasing of drug in the tumor tissue. The suitable particle size
capable of achieving the enhanced permeability and retention (EPR)
is the foundation of the targeting therapy by nanocarriers
(5). Drug release is controlled by
a switch, which is usually an environment condition such as
temperature, pH and magnetic (6).
Specific chemical groups, such as folate receptor, monoclonal
antibodies, nucleic acid and polypeptide, was used as targeting
ligands to modify the nanocarriers to promote their cellular
uptake. The high abundance of receptors on cancer cell membrane may
specifically enhance the accumulation of the drug in cancer
cells.
In this study the hollow mesoporous silica
nanoparticles (HMSN) were chosen as the nanocarriers due to their
larger aperture, good histocompatibility, stable chemical property
and suitable diameter (50 nm) (4).
pH value was chosen as the switch. The pH of the blood circulation
system is between 7.35 and 7.45, whereas the pH will decrease to
6.0–6.5 in the tumor tissues and further reduce to 4.0–6.0 in tumor
cells (7). This can change the
interaction between HMSN and drugs from electrostatic attraction to
electrostatic repulsion. NVP-AEW541 (NVP) is a small molecule
inhibitor of insulin-like growth factor receptor (IGF-1R). NVP was
the targeting ligands that are modified on HMSN. Insulin-like
growth factor (IGF) pathway is involved in the regulation of cancer
stem cells and associated with the progression of ovarian cancer
(8–10). Thus, HMSN modified with NVP tend to
internalized into cancer cells via the specific recognition.
Doxorubicin (DOX) is one of the most widely used anticancer drugs
for various treatment of tumor. Due to the red fluorescence of
emission DOX, the localization of nanocarriers can be clearly
observed in tumor cells.
In this study, the enhancement on the uptake of
HMSN-COOH@DOX fluorescence NVP particle by ovarian cancer stem
cells was determined. Moreover, we examined whether the co-delivery
of NVP and DOX could achieve a synergism in the killing of cancer
cells. These studies on the new targeting methods have a clear
basic science and clinical significance.
Materials and methods
CD117+CD44+A2780
cell culture
The CD117+CD44+A2780 cell
line, were all obtained from KeyGen Co., Ltd. (Nanjing, Jiangsu,
China). This cell line displayed the characters of cancer stem
cells (11,12). Cells were cultured in serum-free
medium at 37°C in a 5% CO2 atmosphere.
Main reagents and drugs
Insulin like growth factor I (IGF-1), mouse
monoclonal antibody against IGF-1, IGF-2 and IGF-1R, and
fluorescent NVP, DOX were obtained from KeyGen Co., Ltd. The HMSN
and N-[(3-trimethoxysily)prop]ethylenediamine triacetic acid
trisodium salt were kindly donated by the Department of Clinical
Laboratory, School of Medicine, Southeast University, Nanjing.
Immunohistochemical analysis
The CD117+CD44+A2780 cells
were cultured in complete medium overnight. The medium was replaced
with serum-free medium and culture continued for 48 h.
Immunohistochemistry was performed as previously published
(13,14). Briefly, cells were fixed with
methanol and cultured with primary antibody (diluted at 1:200)
overnight at 4°C and followed by secondary antibody for 2 h at room
temperature. Color development was carried out with
diaminobenzidine and cells were counterstained with hematoxylin.
The expression of IGF-1, IGF-2 and IGF-1R in cells was examined
under an optical microscope.
Determination of the optimal
concentration of IGF-1
Cells were cultured in complete medium overnight,
the medium was replaced by serum-free medium. After 12 h, 200 µl of
IGF-1 solutions with different concentrations (10, 25, 40 and 55
ng/ml) was added, respectively. The number of cells was counted
after 24 h, and the optimal IGF-1 concentration inducing the
strongest proliferation was used for subsequent experiments.
Determining the role of IGF-1R and NVP
in cell cycle regulation and apoptosis
The experiments were performed with three groups,
the IGF-1 stimulation group, the NVP (10 µM)-inhibition group and
control group. Cells were seeded in 6-well plates
(5×105), serum-starved for 24 h, and exposed to IGF-1 or
NVP for 24 h. Following treatment, cells were stained with 50 µg/ml
propidium iodide and 30 µg/ml RNase A in 1X PBS. The percentage of
cells in specific cell cycle phases was determined with a flow
cytometer equipped with a 488-nm argon laser (BD Biosciences, San
Jose, CA, USA). Annexin V-FITC apoptosis detection kit (BD
Biosciences) was used to detect cell apoptosis according to the
manufacturer's instructions. To detect the expression of cyclin B1,
the rabbit polyclonal antibodies raised against cyclin B1 (Boster,
Wuhan, Hubei, China) was applied for 12 h. Following extensive
washing, samples were incubated with HRP labeled goat anti-rabbit
IgG for 1 h, before color development with chemiluminescence kit
(Boster).
Synthesis of HMSN-COOH@DOX
fluorescence NVP co-delivery system
N-[(3-trimethoxysily)propy]ethylenediamine triacetic
acid trisodium (100 µl), which was chosen as the donator of
carboxyl, was dissolved in 10 ml anhydrous ethanol, and HMSN (10
mg) were added and the mixture was incubated at 80°C for 24 h.
Finally, the modified HMSN (HMSN-COOH) were collected by
centrifugation and rinsed with ethanol and deionized water. DOX (2
mg) was dissolved in 10 ml PBS (pH 7.4), and 10 mg HMSN-COOH was
added and the mixture was stirred for 24 h at room temperature.
HMSN-COOH@DOX and supernatant were, respectively collected by
centrifugation. Fluorescence NVP (0.5 mg) was suspended in 10 ml
PBS (pH 8.0) and subsequently mixed with 10 mg of HMSN-COOH@DOX.
Following continued stirring for 24 h, the HMSN-COOH@DOX
fluorescence NVP was collected by centrifugation, and resuspended
in 10 ml PBS and stored in 4°C.
Determining the entrapment efficiency
and loading efficiency of DOX and fluorescence NVP.10 mg of DOX was
dissolved in 10 ml PBS (pH 7.4) to obtain the stock solution (1
mg/ml)
Serial dilution was performed with PBS to obtain
solutions with concentrations of 500, 400, 300, 200, 100, 50 and 25
µg/ml. Infrared absorption of DOX was measured by UV
spectrophotometer at 480 nm, and the calibration curve of
absorbance (Y) and concentration (X) was constructed by linear
regression. The following formula was used to calculate the
entrapment efficiency and loading efficiency: loading efficiency
(%) = final loaded DOX/total quality (drug and nanocarrier) × 100;
entrapment efficiency (%) = final loaded DOX/initial feed DOX ×
100.
Stock solution of fluorescence NVP
(0.5 mg/ml) was prepared and diluted to 100, 80, 60, 40, 20 and 10
µg/ml with DMSO
The absorbance of fluorescence NVP was measured with
UV spectrophotometer at 260 nm. The absorbance (Y) and the
fluorescence NVP (X) were used to obtain the calibration curve.
HMSN-COOH@DOX fluorescence NVP (1 mg) was dispersed in 5 ml DMSO.
Then the loading rate and encapsulation rate were calculated by the
following formula: loading efficiency (%) = final loaded
fluorescence NVP/total quality (drug and nanocarrier) × 100;
entrapment efficiency (%) = final loaded fluorescence NVP/initial
feed NVP × 100.
The release rate of DOX and
fluorescence NVP at different pH
HMSN-COOH@DOX fluorescence NVP (1 mg) was
resuspended in 10 ml PBS at pH 7.4, 6.5, 5.5 and 4.5 respectively.
The OD values of each group were measured every 20 h for 120 h. The
experiment was repeated three times to calculate the mean
DOX-release rate. HMSN-COOH@DOX fluorescence NVP (1 mg) was
resuspended in PBS at pH 7.4, 6.5 and 5.5, and OD values were
measured every 10 h for 80 h. The experiment was repeated three
times to calculate the mean fluorescence NVP-release rate.
Detection of the ζ-potential of
nanocarriers at different pH
The carboxylic group modified on the surface of HMSN
was detected by infrared spectrometer (Shimadzu, Japan). The form
and size of HMSN drug delivery system ware observed by transmission
electron microscopy (TEM) and scanning electron microscopy (SEM)
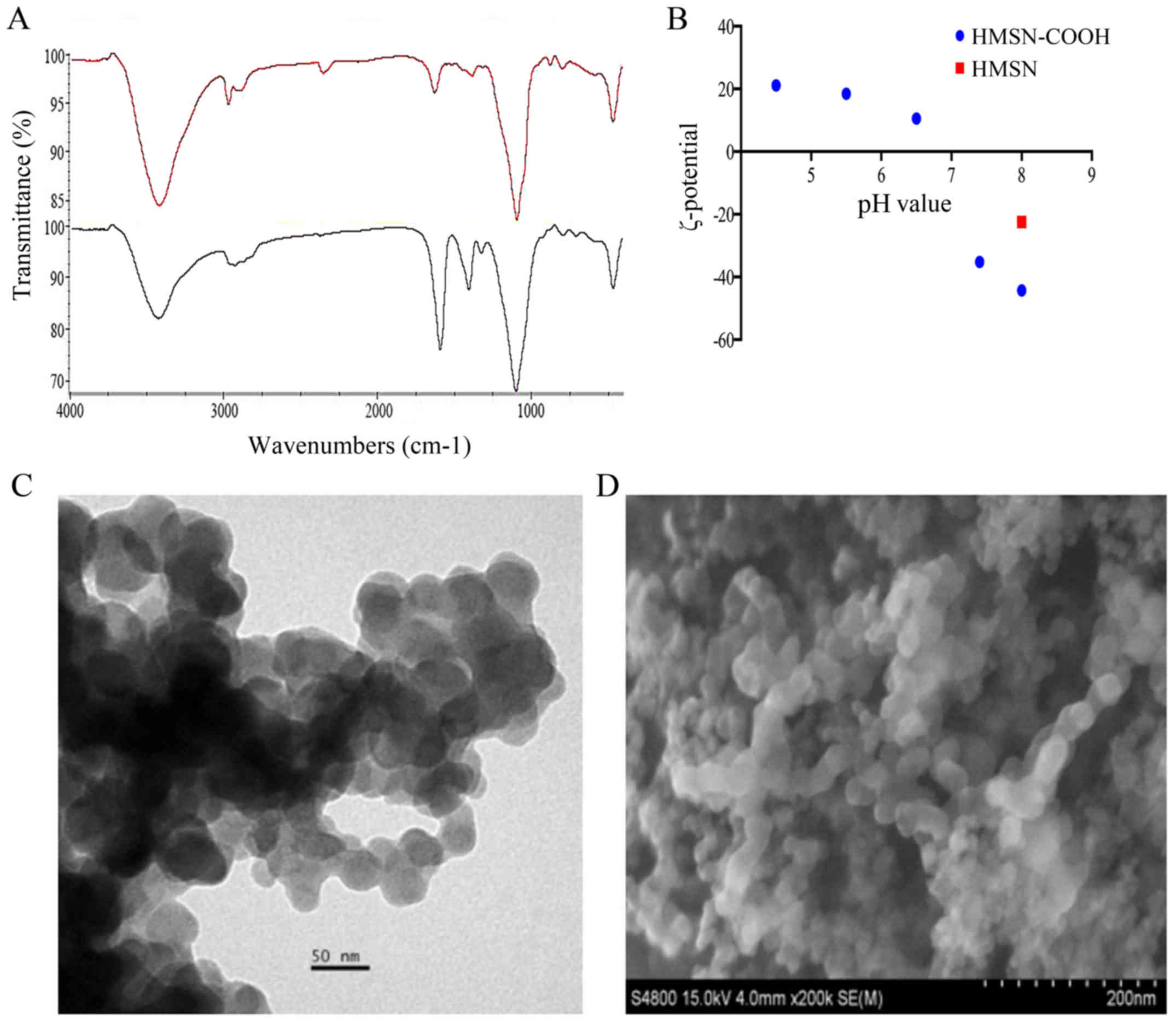
(Hitachi, Japan). The ζ-potential of HMSN and HMSN-COOH in
different pH environment was detected by dynamic light scattering
(MAL1043118, Malvern Instruments Ltd.).
In vitro effect of HMSN-COOH@DOX
fluorescence NVP co-delivery system
Intracellular accumulation, apoptosis ratio,
inhibition rate and protein level of Bax, Bcl-xl and p-Akt were
used as the indexes to determine the in vitro effects of
this system. Cells were plated in petri dish at a density of
5×105 per well. HMSN-COOH@DOX fluorescence NVP (1 mg) or
HMSN-COOH@DOX was resuspended in 1 ml PBS, and 200 µl was applied
to each well. Equivalent amount of free DOX and fluorescence NVP
were added to control groups. Medium was removed at one, two and
three hours, the intracellular accumulation of HMSN-COOH@DOX
fluorescence NVP was observed by using laser confocal microscopy.
Cells were collected to detect the apoptosis ratio with the methods
described above.
Three groups, HMSN-COOH@DOX fluorescence NVP,
HMSN-COOH@DOX and free DOX NVP group, were used to examine the
inhibition rate. Cells were inoculated to 96-well culture plates at
a density of 5,000 cells per well, cultured for 24 h with complete
medium, before drugs were added to each group as described above.
After 8 h, 10 µl of MTT (5 mg/ml) was added to each well and
incubation was continued for 4 h at 37°C. DMSO (100 µl) was added
to each well to dissolve the crystalline formazan after removing
MTT solution. The absorbance of each well was detected on
microplate reader (Enspire Instruments, Perkin-Elmer, USA) at a
wavelength of 490 nm.
The expression of Bax, Bcl-xl and p-Akt in
experimental and control groups were detected by western blotting.
Rabbit polyclonal antibodies raised against p-Akt, Bax and Bcl-xl
(Boster) were used as the primary antibodies cultured with cells
for 12 h. Following extensive washing, samples were incubated with
HRP labeled goat anti-rabbit IgG for 1 h, before color development
with chemiluminescence kit (Boster).
Results
Immunohistochemical analysis
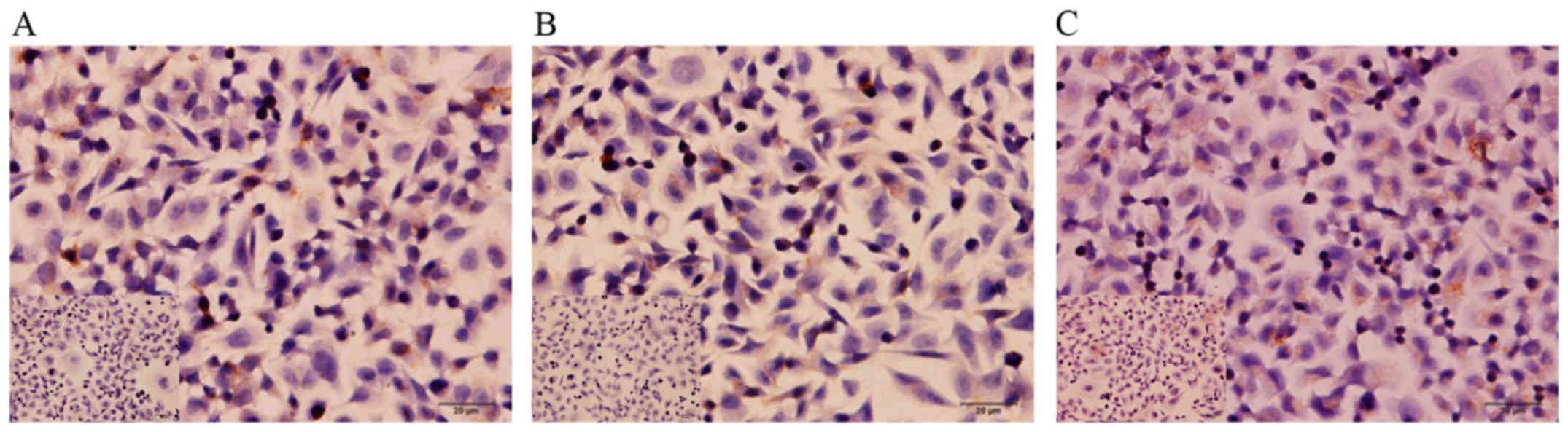
The expression of IGF-1, IGF-2 and IGF-1R in
CD117+CD44+A2780 cells was examined with the
use of immunohistochemistry. The expression of these proteins, as
indicated by the brown staining, was detected in cell membrane and
cytoplasm (Fig. 1). The result
demonstrated that this cell line expresses high levels of ligands
and receptors of the IGF pathway. Therefore, these cells can be
used in this experiment.
The optimal concentration of
IGF-1
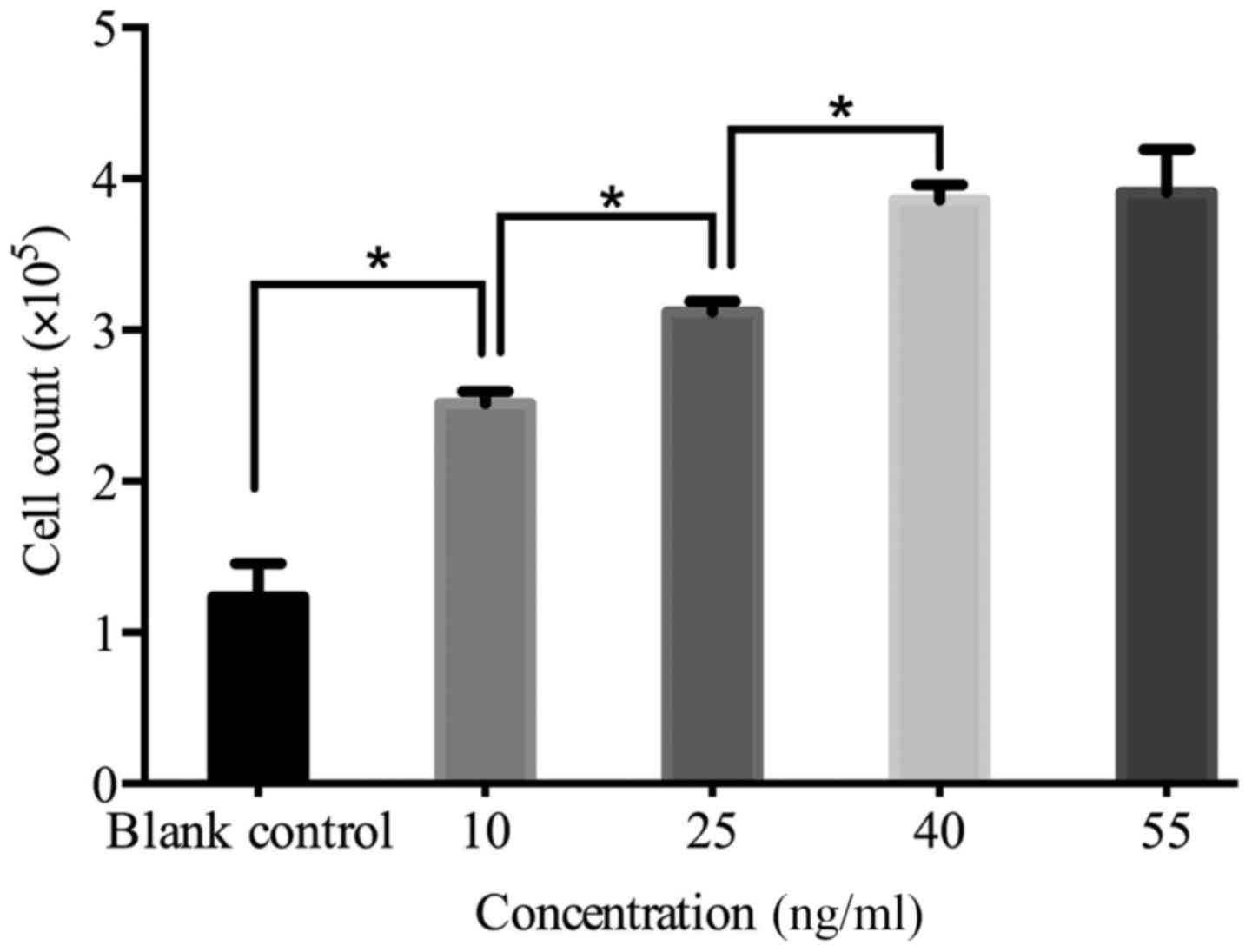
Our results showed that even at a relatively low
concentration IGF-1 can effectively stimulate cell proliferation,
and the effect is time- and concentration-dependent. As shown in
Fig. 2, the proliferation was
enhanced with the increasing concentration of IGF-1. At
concentrations >40 ng/ml no further increase in cell
proliferation was observed. The optimal concentration chosen and
applied in later experiments was 40 ng/ml.
IGF-1 and NVP affect cell cycle
regulation and apoptosis
To examine the effects of IGF-1 and IGF-1R inhibitor
on cell cycle and cell apoptosis, cancer cells were divided into
IGF-1 stimulating group, NVP inhibiting group and control group.
When compared with the control group, the cells in S phase were
significantly increased (P≤0.05), whereas relatively percentages of
G1 and G2 phases were significantly decreased in the IGF-1
stimulating group (P≤0.05). The NVP inhibiting group displayed an
accumulation of cells in G2 phase and reduction of percentages in
G1 phase and S phases (P≤0.05) (Table
I).
 | Table I.The cell cycle distribution of
CD117+CD44+A2780. |
Table I.
The cell cycle distribution of
CD117+CD44+A2780.
|
| Cell count (%) |
|---|
|
|
|
|---|
| Group | G1 phase | S phase | G2 phase |
|---|
| IGF-1 |
36.1±1.30a |
56.5±1.45a |
7.30±0.55a |
| NVP |
49.0±0.70a |
40.6±0.65a |
10.3±0.37a |
| Control | 52.4±0.37 | 37.8±0.56 | 9.67±0.63 |
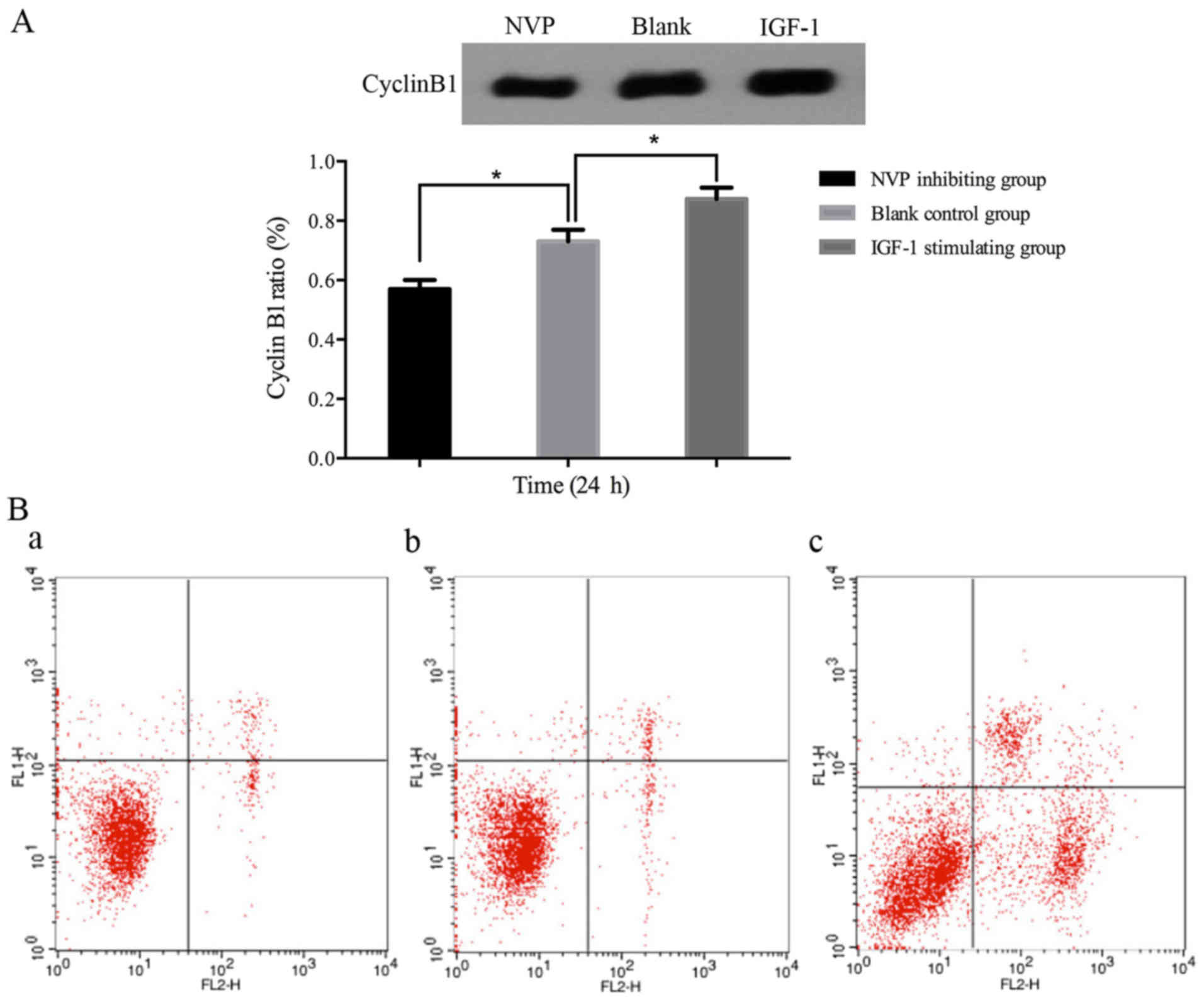
The expression of cyclin B1 was consistent with the
cell cycle distribution. Cyclin B1 was significantly higher in
IGF-1 stimulating group (P≤0.05), while in NVP inhibiting group it
was lower than that in other groups (P≤0.05) (Fig. 3). Therefore, IGF-1 can promote
ovarian cancer stem cell proliferation by activating IGF-1R, and
NVP can block this effect.
As expected, the apoptosis rate in the NVP
inhibiting group (32.79±0.34%) was significantly increased when
compared to the IGF-1 stimulating (6.53±0.36%) and control
(8.38±0.25%) groups (P≤0.05) (Fig.
3). Thus, IGF-1R is a potential target for cancer stem-like
cells, and inhibition of IGF-1R led to cell apoptosis.
Characterization of HMSN-COOH@DOX
fluorescence NVP co-delivery system
The HMSN and -COOH were characterized by IR: a
spectral peak at 400–1,400 cm−1 was mainly silicon
oxygen tetrahedron frame; the wide and strong absorption peak at
1,104 cm−1 was Si-O-Si asymmetric stretching vibration
peak; the amino vibration absorption peak was at 3,430
cm−1. Besides the characteristics of HMSN, the special
absorption peak of carboxylic can be clearly demonstrated in the
spectrum: the asymmetric and symmetric stretching vibration peak at
1,596 cm−1 and 1,412 cm−1 (15) confirm the carboxylic acid was
modified on the surface of HMSN (Fig.
4).
The result of SEM and TEM imaging showed that the
size and form of HMSN were not changed after drug loading. However,
the negative charge was increased after modification of carboxyl
group on the surface of HMSN, which could be beneficial to drug
loading by electrostatic attraction. The carboxyl group was
protonated and the electrostatic attraction of DOX was weakened
along with the gradually reduced pH. As a result, the release rate
of drugs was increased (Fig. 4).
Thus, the modification on the surface of HMSN provided a platform
for the control of drug loading and release through changes of the
electrostatic charge.
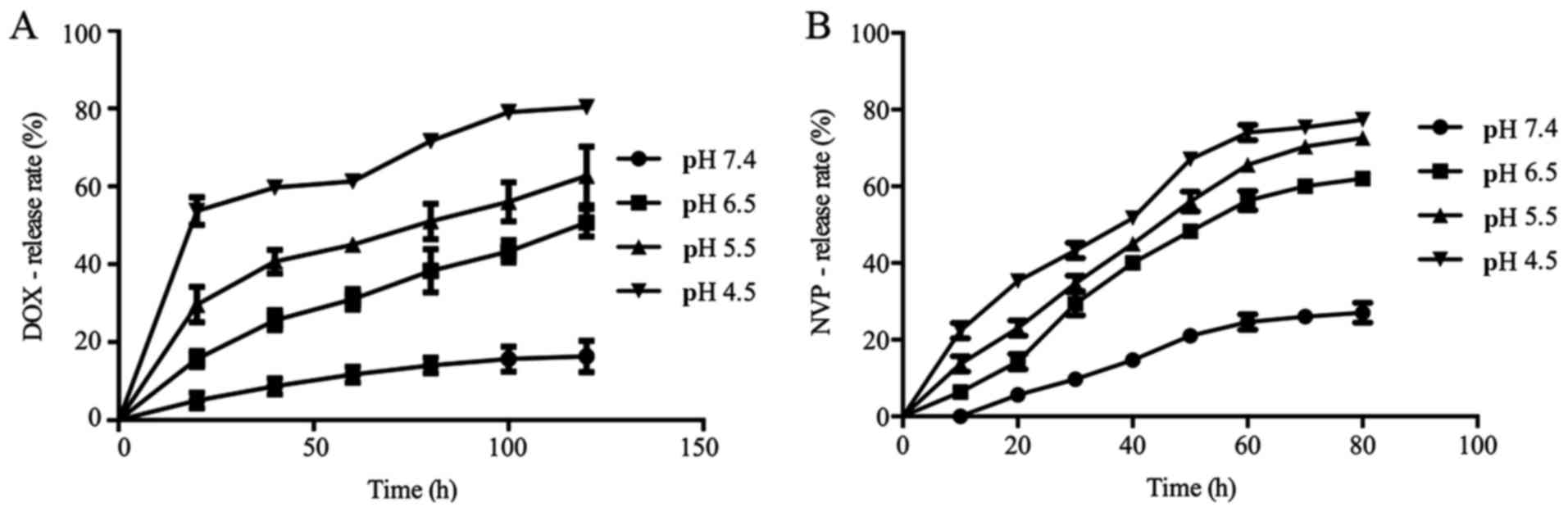
The loading quantity, encapsulation
efficiency and release rate of drugs
There is a linear relationship between OD value and
the concentration of DOX. The calibration curve is Y =0.032 × X +
0.071 (R2=0.998), where Y represents the OD value and X
represents concentration. According to the formula described above,
the DOX-encapsulation efficiency was 37%, and loading quantity was
6.17%. In alkaline environment DOX almost did not release, when the
pH decreases to <7.4, the release significantly accelerated
(P≤0.05). Similarly, the calibration curve of fluorescence NVP was
Y=0.0681 × X (R2=0.991), where Y represents the OD value
and X represents concentration. The encapsulation efficiency was
44%, and the loading quantity was 2.10%. When pH was at 6.5 or 5.5,
the release rates of fluorescence NVP were significantly higher
than that at pH 7.4 (P≤0.05) (Fig.
5). Therefore, we successfully synthesized a NVP-modified and
pH-sensitive co-delivery system.
Efficiency of the HMSN-COOH@DOX
fluorescence NVP co-delivery system
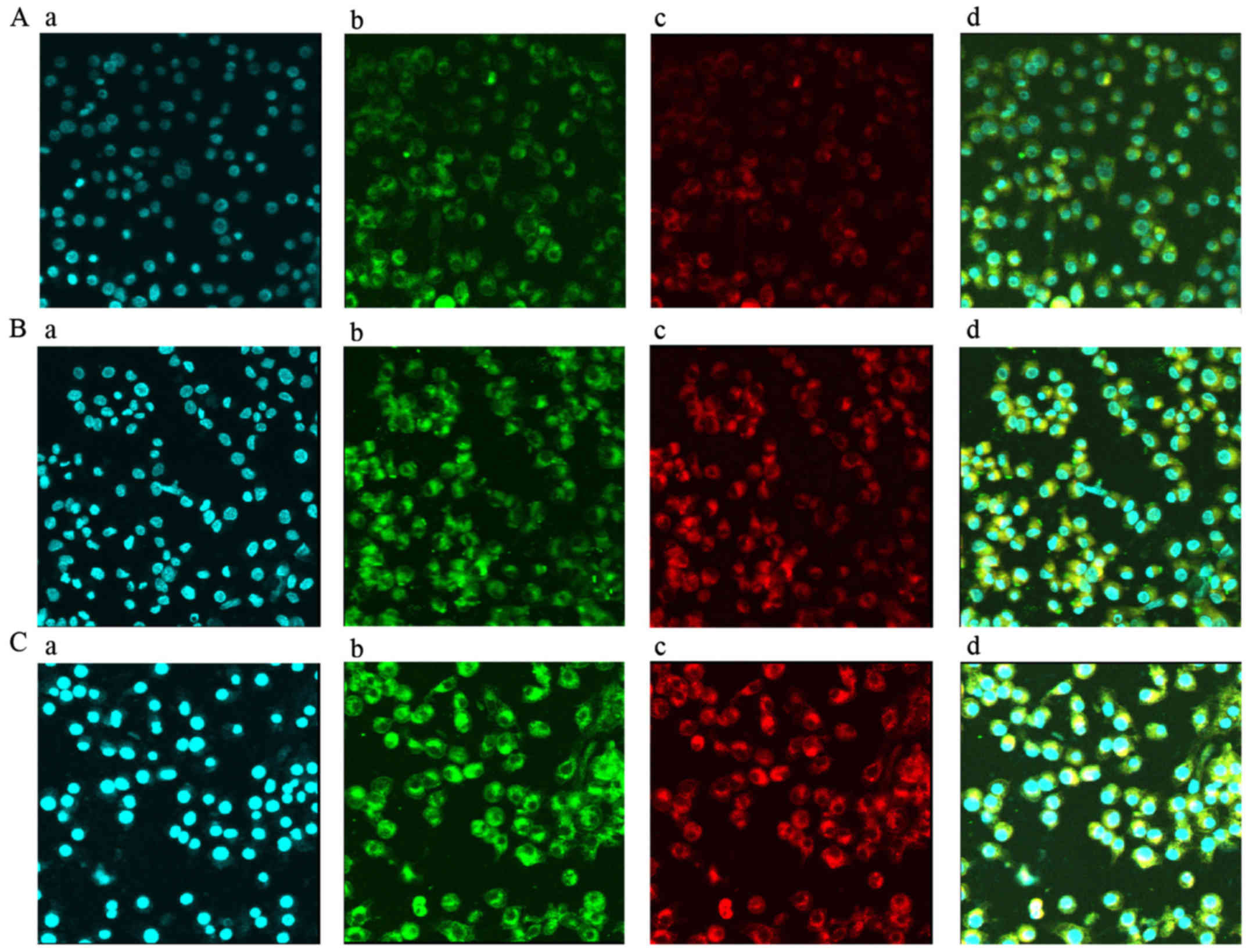
HMSN@DOX fluorescence NVP co-delivery system can be
gradually gathered in the intracellular, and penetrated into the
nucleus with extension of time (Fig.
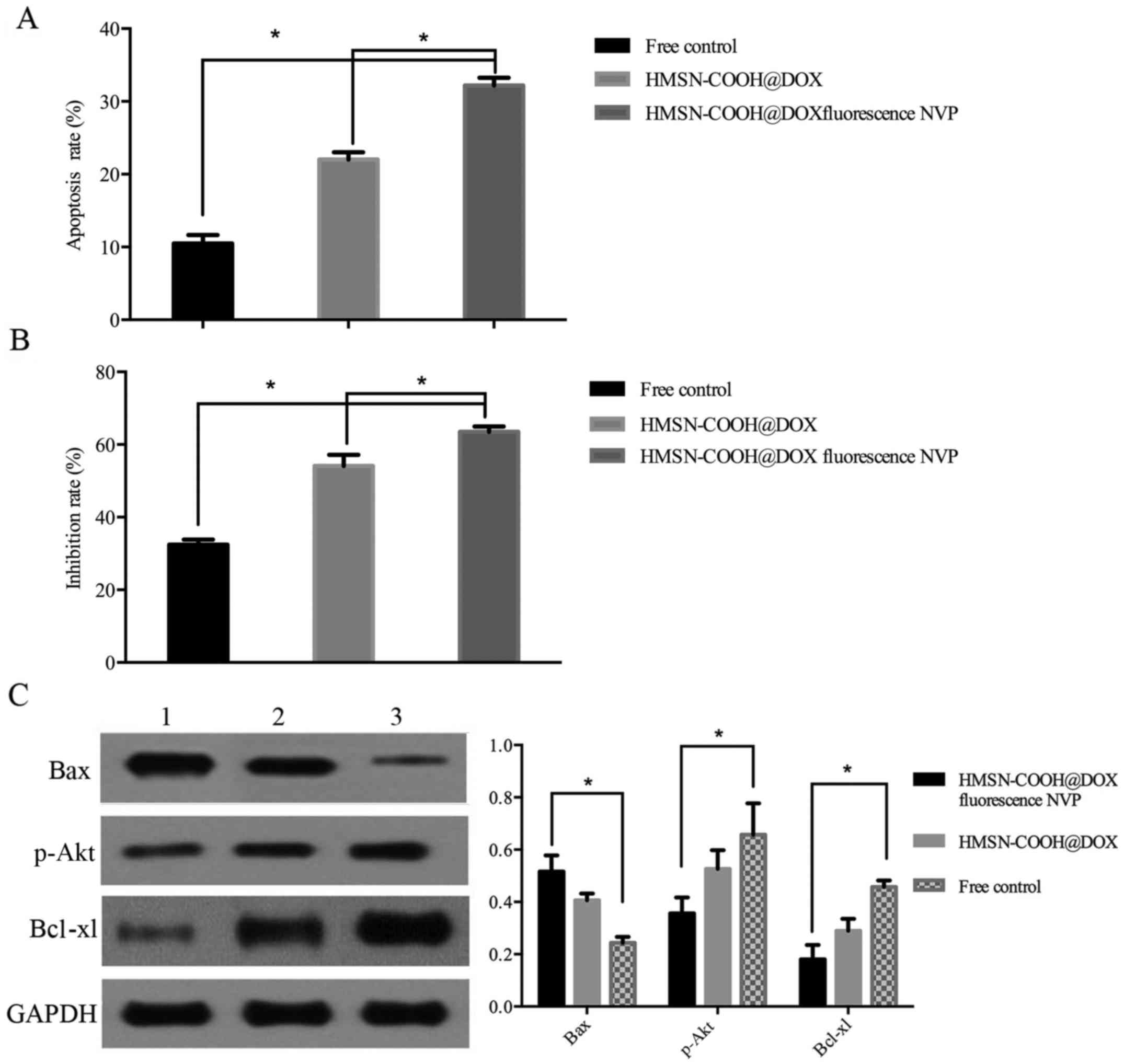
6). Cell apoptosis was significantly increased in the
HMSN-COOH@DOX fluorescence NVP group than that of HMSN-COOH@DOX
group (P≤0.05). NVP combined with DOX can achieve more efficient
cell killing. Compared with the control group, DOX and NVP loaded
in the HMSN had stronger apoptotic effect (P≤0.05) (Fig. 7). Similarly, the cell growth
inhibition rate of HMSN-COOH@DOX fluorescence NVP group was higher
than that of the other two groups (P≤0.05) (Fig. 7). These results showed that HMSN@DOX
fluorescence NVP co-delivery system could be potentially applied
for cancer therapy.
The expression of Bax was significantly increased in
HMSN-COOH@DOX fluorescence NVP group (P≤0.05), while the expression
of Bcl-xl and p-Akt were significantly decreased (P≤0.05) (Fig. 7). The results showed that the
proliferation and apoptosis of
CD117+CD44+A2780 cells were regulated by
PI3K/AKT pathway, which can be suppressed by NVP.
Discussion
Tumor recurrence represents a serious challenge for
cancer therapy. For primary ovarian cancer patients who have
received cytoreductive surgery and platinum-based combination
chemotherapy, a long period of complete remission is expected.
However, almost 70% of patients will experience recurrence
(1). According to previous studies,
the proliferation of cancer stem cells directly contribute to tumor
recurrence. IGF-1 is a multifunctional regulatory factor
extensively involved in various cell functions. Activation of IGF-1
pathway induces DNA synthesis and mitosis through promotion of G2/M
transition. Numerous studies showed that the IGF signal axis is
involved in malignant transformation and exerts an anti-apoptotic
effect (16). In this study, we
chose the IGF-1 as the therapy target of HMSN@DOX fluorescence NVP
co-delivery system to build and investigate the in vitro
effect of this system in a tumor stem-like cell line.
Targeting accumulation of nanocarriers is the basis
of the co-delivery system. With nanocarriers the size of ~100 nm is
easier to achieve EPR effect, and the smaller the nanocarriers are,
the less likely they are to be recognized and deprived by
macrophages, the longer their retention time in the bloodstream
(17–20). The HMSN synthesized in our
laboratory with a size of 50 nm can not only achieve EPR effect,
but also reduce the probability of phagocytosis and achieve the
enrichment in tumor tissue.
However, depending on the classification and
differentiation of tumor tissue, EPR effect may vary significantly
and actively targeting methods are still required. NVP was used as
the active targeting group because of the specific binding with
IGF-1R. In this system, the low pH value in tumor tissues caused by
anaerobic glycolysis was used as pH-sensitive switch to achieve the
targeted drug release (21). The
HMSN system enters into the tumor cells via a non-specific
endocytosis, then the carboxyl group on the surface will be
protonated, leading to a change from electrostatic attraction
between the loaded drugs and HMSN into a repulsion mode, which
results in drug release. According to this mechanism, drug loaded
in the HMSN is minimally released in the circulatory system, which
effectively reduces the systemic side effects of chemotherapy
drugs. In addition, in contrast to the conventional chemotherapy
regimens, HMSN drug delivery system can avoid the recognition of
ABC superfamily, which can pump out the free drug and lead to
multidrug resistance (22,23).
Tumor stem cells can exists in body for a long time
in the stationary state, which may be associated with immune escape
(11). The significantly higher
apoptosis rate in NVP inhibiting group showed that blockade of
IGF-1 can inhibit the proliferation and promote apoptosis of
ovarian cancer stem-like cells. From this point of view, IGF-1R can
be used as a target for the prevention of tumor recurrence. The
phosphorylation of IGF-1R activates multiple pathways including the
PI3K-AKT signaling cascade, which upregulates the expression of
cyclin B1 (24). Cyclin B1 promotes
the G2/M transition, and associated with tumor invasion and tumor
malignant degree (25,26).
The in vitro experiment was carried out to
compare the efficiency of the co-delivery system and free drugs.
Significantly higher cell apoptosis and more inhibition of cell
proliferation were observed in the co-delivery system. The level of
phosphorylated aspartic acid specificity cysteine protease AKT,
apoptosis-promoting protein Bax and apoptosis-inhibiting protein
Bcl-xl were mostly affected following cell treatment with the
co-delivery system. Thus, compared with HMSN-COOH@DOX, the enhanced
cell apoptosis by HMSN-COOH@DOX fluorescence NVP is associated with
the active targeting as well as blocking effect on IGF-1 by NVP.
These results indicated the HMSN-COOH@DOX fluorescence NVP
co-delivery system can achieve more potent antitumor effect on
ovarian cancer stem cells than conventional regimens (27,28).
In conlusion, we constructed a HMSN-COOH@DOX
fluorescence NVP co-delivery module and preliminarily verified the
potential efficacy of the system in a stem-like ovarian cancer cell
line. Through accumulation effect, intracellular release and
delayed efflux, stronger antitumor effect could be achieved by this
system. In addition, as doxorubicin mainly release inside tumor
tissue and reduces the drug amount in the circulation
theoretically, the effective dose of doxorubicin packaged in the
HMSN co-delivery system may be far below the current clinical
dosage, thus relieving the accumulated toxicity of doxorubicin.
However, the above needs to be verified in vivo experiments,
which are under investigation in our laboratory.
However, there are still some deficiencies. More
research should be carried out in the future to test and verify the
antitumor effect of the co-delivery system in other ovarian cancer
cell lines, such as SKOV-3 and HO8910, which also have highly
expressed IGF-1 and IGF-1R (29–31).
Adverse effects and safety concerns should also be addressed before
clinical application of the system.
References
|
1
|
Beauchamp MC, Yasmeen A, Knafo A and
Gotlieb WH: Targeting insulin and insulin-like growth factor
pathways in epithelial ovarian cancer. J Oncol. 2010:2570582010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mantia-Smaldone GM, Corr B and Chu CS:
Immunotherapy in ovarian cancer. Hum Vaccin Immunother.
8:1179–1191. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Boccardi E, Philippart A, Juhasz-Bortuzzo
JA, Beltrán AM, Novajra G, Vitale-Brovarone C, Spiecker E and
Boccaccini AR: Uniform surface modification of 3D bioglass
(®)-based scaffolds with mesoporous silica particles
(MCM-41) for enhancing drug delivery capability. Front Bioeng
Biotechnol. 3:1772015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu J, Luo Z, Zhang J, Luo T, Zhou J, Zhao
X and Cai K: Hollow mesoporous silica nanoparticles facilitated
drug delivery via cascade pH stimuli in tumor microenvironment for
tumor therapy. Biomaterials. 83:51–65. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li N, Huang C, Luan Y, Song A, Song Y and
Garg S: Active targeting co-delivery system based on pH-sensitive
methoxy-poly (ethylene glycol)2K-poly (ε-caprolactone)4K-poly
(glutamic acid)1K for enhanced cancer therapy. J Colloid Interface
Sci. 472:90–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang Y, Jiang Y, Wang H, Wang J, Shin MC,
Byun Y, He H, Liang Y and Yang VC: Curb challenges of the ‘Trojan
Horse’ approach: Smart strategies in achieving effective yet safe
cell-penetrating peptide-based drug delivery. Adv Drug Deliv Rev.
65:1299–1315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Han L, Tang C and Yin C: Dual-targeting
and pH/redox-responsive multi-layered nanocomplexes for smart
co-delivery of doxorubicin and siRNA. Biomaterials. 60:42–52. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bowker SL, Majumdar SR, Veugelers P and
Johnson JA: Increased cancer-related mortality for patients with
type 2 diabetes who use sulfonylureas or insulin: Response to
Farooki and Schneider. Diabetes Care. 29:1990–1991. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brokaw J, Katsaros D, Wiley A, Lu L, Su D,
Sochirca O, de la Longrais IA, Mayne S, Risch H and Yu H: IGF-I in
epithelial ovarian cancer and its role in disease progression.
Growth Factors. 25:346–354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Attias-Geva Z, Bentov I, Fishman A, Werner
H and Bruchim I: Insulin-like growth factor-I receptor inhibition
by specific tyrosine kinase inhibitor NVP-AEW541 in endometrioid
and serous papillary endometrial cancer cell lines. Gynecol Oncol.
121:383–389. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhan Q, Wang C and Ngai S: Ovarian cancer
stem cells: A new target for cancer therapy. BioMed Res Int.
2013:9168192013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Burgos-Ojeda D, Rueda BR and Buckanovich
RJ: Ovarian cancer stem cell markers: Prognostic and therapeutic
implications. Cancer Lett. 322:1–7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kryczek I, Zou L, Rodriguez P, Zhu G, Wei
S, Mottram P, Brumlik M, Cheng P, Curiel T, Myers L, et al: B7-H4
expression identifies a novel suppressive macrophage population in
human ovarian carcinoma. J Exp Med. 203:871–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Curiel TJ, Coukos G, Zou L, Alvarez X,
Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L,
Burow M, et al: Specific recruitment of regulatory T cells in
ovarian carcinoma fosters immune privilege and predicts reduced
survival. Nat Med. 10:942–949. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Trinchero A, Bonora S, Tinti A and Fini G:
Spectroscopic behavior of copper complexes of nonsteroidal
anti-inflammatory drugs. Biopolymers. 74:120–124. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gallagher EJ and LeRoith D: The
proliferating role of insulin and insulin-like growth factors in
cancer. Trends Endocrinol Metab. 21:610–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jun YW, Lee JH and Cheon J: Chemical
design of nanoparticle probes for high-performance magnetic
resonance imaging. Angew Chem Int Ed Engl. 47:5122–5135. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Peer D, Karp JM, Hong S, Farokhzad OC,
Margalit R and Langer R: Nanocarriers as an emerging platform for
cancer therapy. Nat Nanotechnol. 2:751–760. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gullotti E and Yeo Y: Extracellularly
activated nanocarriers: A new paradigm of tumor targeted drug
delivery. Mol Pharm. 6:1041–1051. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Meng H, Xue M, Xia T, Ji Z, Tarn DY, Zink
JI and Nel AE: Use of size and a copolymer design feature to
improve the biodistribution and the enhanced permeability and
retention effect of doxorubicin-loaded mesoporous silica
nanoparticles in a murine xenograft tumor model. ACS Nano.
5:4131–4144. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu N, Han J, Zhang X, Yang Y, Liu Y, Wang
Y and Wu G: pH-responsive zwitterionic polypeptide as a platform
for anti-tumor drug delivery. Colloids Surf B Biointerfaces.
145:401–409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Zhang H, Assaraf YG, Zhao K, Xu X,
Xie J, Yang DH and Chen ZS: Overcoming ABC transporter-mediated
multidrug resistance: Molecular mechanisms and novel therapeutic
drug strategies. Drug Resist Updat. 27:14–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multi-drug resistance in cancer chemotherapeutics: Mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Whitley BR, Beaulieu LM, Carter JC and
Church FC: Phosphatidylinositol 3-kinase/Akt regulates the balance
between plasminogen activator inhibitor-1 and urokinase to promote
migration of SKOV-3 ovarian cancer cells. Gynecol Oncol.
104:470–479. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang B, Zhao Y, Lou C and Zhao H:
Eupalinolide O, a novel sesquiterpene lactone from Eupatorium
lindleyanum DC., induces cell cycle arrest and apoptosis in
human MDA-MB-468 breast cancer cells. Oncol Rep. 36:2807–2813.
2016.PubMed/NCBI
|
|
26
|
Wang LH, Jiang XR, Chen GL, Guo W, Zhang
JY, Cui LJ, Li HH, Li M, Liu X, Yang JY, et al: Anti-tumor activity
of SL4 against breast cancer cells: Induction of G2/M arrest
through modulation of the MAPK-dependent p21 signaling pathway. Sci
Rep. 6:364862016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Subramaniam S and Unsicker K:
Extracellular signal-regulated kinase as an inducer of
non-apoptotic neuronal death. Neuroscience. 138:1055–1065. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Andersen JL and Kornbluth S: The tangled
circuitry of metabolism and apoptosis. Mol Cell. 49:399–410. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Singh RK, Gaikwad SM, Jinager A, Chaudhury
S, Maheshwari A and Ray P: IGF-1R inhibition potentiates cytotoxic
effects of chemotherapeutic agents in early stages of
chemoresistant ovarian cancer cells. Cancer Lett. 354:254–262.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jia J, Zhang Y, Cai J, Wang J, Ding H,
Zhou J, Fang F and Wang Z: A novel function of protein kinase B as
an inducer of the mismatch repair gene hPMS2 degradation. Cell
Signal. 25:1498–1504. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rao W, Li H, Song F, Zhang R, Yin Q, Wang
Y, Xi Y and Ge H: OVA66 increases cell growth, invasion and
survival via regulation of IGF-1R-MAPK signaling in human cancer
cells. Carcinogenesis. 35:1573–1581. 2014. View Article : Google Scholar : PubMed/NCBI
|