Introduction
Hepatocellular carcinoma (HCC) is the second leading
cause of cancer mortality worldwide. In 2012, there were
approximately 782,500 new cases and 745,500 deaths of HCC globally,
with more than half of both occurring in China (1). Although liver resection or
transplantation provides the best chances of long-term survival for
selected patients with early stage HCC, almost all HCC patients
diagnosed at advanced stage are always deprived of surgery,
resulting in even poorer prognosis with a 5-year survival rate of
5.1% (2,3). The reasons are ascribed to relapse and
metastasis, which remain hard to conquer (4). Complex signaling inside and outside
cancer cells orchestrate the considerably varied metastatic
potential (5), leading to invalid
staging systems for predicting prognosis of HCC (6). Currently only limited drugs have been
proved to have some effect on HCC patients, and even the best
characterized and unique FDA-approved, Sorafenib efficacy for
unresectable HCC, is often disappointing (7). Therefore, more useful tumor biomarkers
to identify HCC patients at high risk for poor survival and
subsequently to render precise adjuvant therapies need to be
established urgently.
Synaptophysin-like 1 (SYPL1), encoded by the SYPL1
gene, belongs to the SYP family proteins (8). Although originally reported as a
protein related to the neuroendocrine specific synaptophysin, SYPL1
has recently been identified in non-neuronal tissues, and its mRNA
was significantly abundant in adipose tissues and accordingly
increased during adipogenesis of 3T3-L1 cells (9). SYPL1 exerts transporter activity and
is a broadly distributed membrane component of small cytoplasmic
transport vesicles associated with GLUT4-containing vesicles in
adipocytes (10) and also
extracellular exosome (11).
Recently, SYPL1 was also reported as a probable regulator of NF-κB
signaling pathway, though its exact functional role remains unknown
(12). Although a previous study
demonstrated that SYPL1 staining was restricted to cells
surrounding sinusoids in liver, and undetectable in hepatocytes
(9), upregulation of SYPL1 has been
observed according to the data from high-resolution, array-based
comparative genomic hybridization and transcriptome analysis of HCC
samples (13). Collectively, the
expression pattern and known functional role of SYPL1 prompted us
to wonder whether and how SYPL1 contributed to HCC progress.
In the present study, quantitative real-time
polymerase chain reaction (qRT-PCR), western blot analysis (WB) and
immunohistochemical (IHC) staining were performed revealing that
SYPL1 was upregulated in HCC tissues when compared with the matched
adjacent non-tumor liver tissues (ANLTs) from the same patients. In
addition, the ectopic SYPL1 expression in HCC was further
demonstrated to be correlated with multiple malignant
clinicopathological features and was identified as an independent
prognostic factor for the OS and DFS of HCC patients.
Mechanistically, SYPL1 might be associated with
epithelial-mesenchymal transition (EMT) of HCC cells via
NF-κB/Snail signaling.
Materials and methods
Ethics
The present study conformed to the ethical
guidelines of the World Medical Association Declaration of
Helsinki-Ethical Principles for Medical Research Involving Human
Subjects and was approved by the Institutional Research Board at
the First Affiliated Hospital of Xiamen University, China. Written
informed consent was obtained from each patient. Institutional
Ethics Committee approval for this project was provided before the
commencement of the study. All specimens were handled according to
the ethical and legal standards.
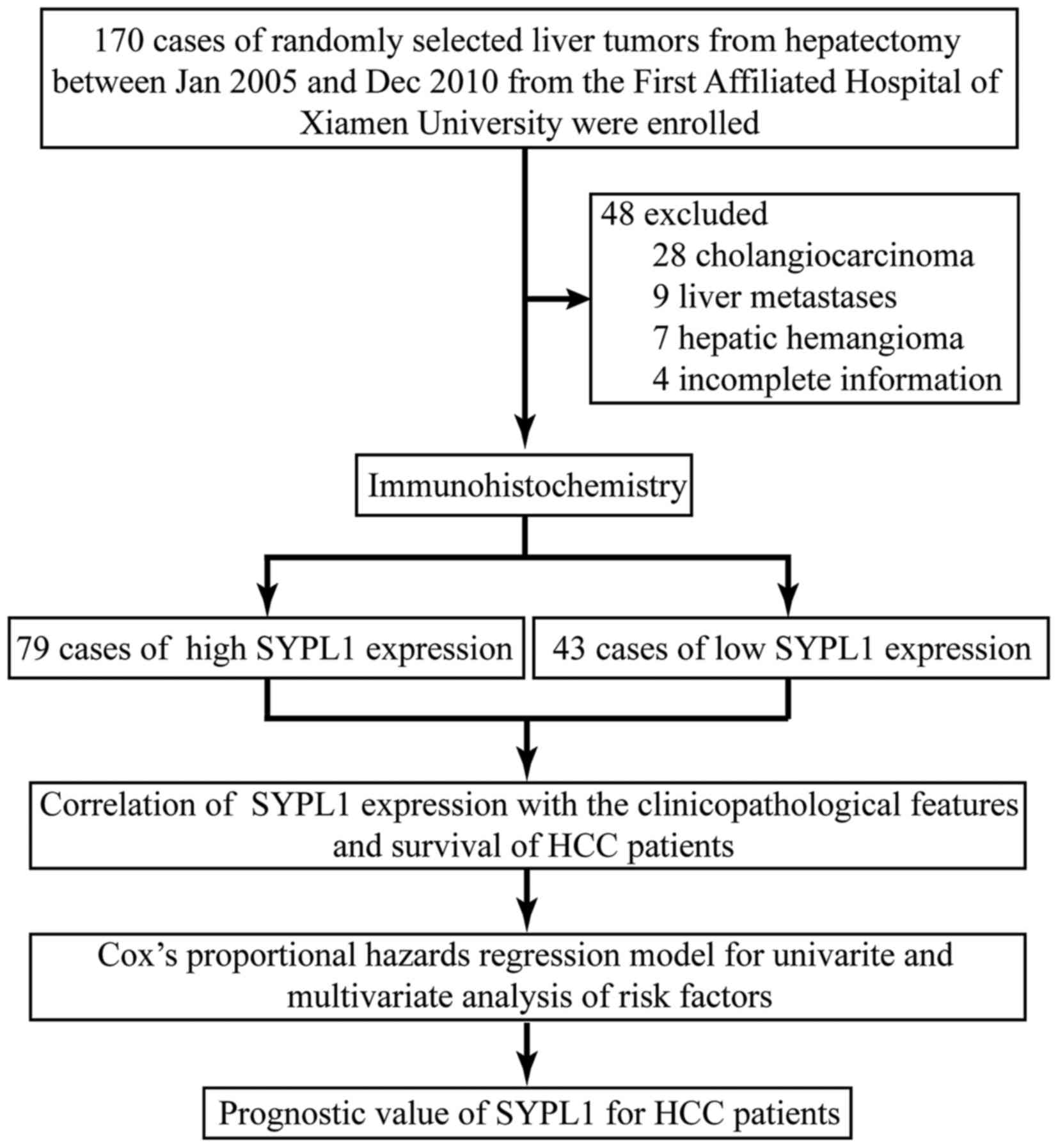
Patients and tissue specimens
Specimens, including HCC tissues and the matched
adjacent non-tumor liver tissues (ANLTs) which were collected from
areas >2 cm away from the tumor edge, were from 122 HCC patients
receiving curative hepatectomy at the Department of
Hepato-Biliary-Pancreatic and Vascular Surgery of the First
Affiliated Hospital of Xiamen University from January 2005 to
December 2010 (Fig. 1). None of the
patients had received preoperative chemotherapy or radiotherapy.
Each specimen was divided into two parts; one was snap-frozen in
liquid nitrogen and stored at −80°C for later RNA extraction, the
other was formalin-fixed and paraffin embedded for IHC. Among these
specimens, 30 and 16 of fresh HCC tissues and the matched ANLTs
were randomly selected for qRT-PCR and WB, respectively. The
clinicopathological data including age, sex, tumor size,
alpha-fetoprotein (AFP) level, nodal status, histological grade,
microvascular invasion (MVI) and TNM, were collected from patient
files. These patients included 101 (82.8%) males and 21 (17.2%)
females, with a median age of 43 years (range, 21–79 years). The
detailed clinical features of the 122 patients are presented in
Table I. Histopathology was
evaluated by two certified pathologists, and staged according to
the criteria of the seventh edition of the American Joint Committee
on Cancer/International Union against Cancer TNM classification
system.
 | Table I.The correlations of SYPL1 with
clinicopathological features of HCC. |
Table I.
The correlations of SYPL1 with
clinicopathological features of HCC.
|
|
| SYPL1 |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
variables | n | Low expression | High expression | P-value |
|---|
| Sex |
|
|
| 0.840 |
|
Female | 21 | 7 | 14 |
|
|
Male | 101 | 36 | 65 |
|
| Age (years) |
|
|
| 0.953 |
|
≤60 | 94 | 33 | 61 |
|
|
>60 | 28 | 10 | 18 |
|
| AFP (ng/ml) |
|
|
| 0.458 |
|
<20 | 32 | 13 | 19 |
|
|
≥20 | 90 | 30 | 60 |
|
| HBsAg |
|
|
| 0.119 |
|
Negative | 20 | 4 | 16 |
|
|
Positive | 102 | 39 | 63 |
|
| Liver
cirrhosis |
|
|
| 0. 109 |
|
Absence | 32 | 15 | 17 |
|
|
Presence | 90 | 28 | 62 |
|
| Tumor size
(cm) |
|
|
| 0.049 |
| ≤5 | 48 | 22 | 26 |
|
|
>5 | 74 | 21 | 53 |
|
| Tumor nodule
no. |
|
|
| 0.002 |
|
Solitary | 65 | 31 | 34 |
|
|
Multiple (≥2) | 57 | 12 | 45 |
|
| Capsular
formation |
|
|
| 0.029 |
|
Presence | 66 | 29 | 37 |
|
|
Absence | 56 | 14 | 42 |
|
| Edmondson-Steiner
grade |
|
|
| 0.048 |
| I +
II | 59 | 26 | 33 |
|
| III +
IV | 63 | 17 | 46 |
|
| Microvascular
invasion |
|
|
| 0.002 |
|
Absence | 62 | 30 | 32 |
|
|
Presence | 60 | 13 | 47 |
|
| TNM |
|
|
| 0.032 |
| Early
(I + II) | 91 | 37 | 54 |
|
| Late
(III + IV) | 31 | 6 | 25 |
|
| BCLC staging |
|
|
| 0.026 |
| 0 +
A | 41 | 20 | 21 |
|
| B +
C | 81 | 23 | 58 |
|
| Liver function |
|
|
| 0.778 |
|
Child-Pugh A | 73 | 25 | 48 |
|
|
Child-Pugh B | 49 | 18 | 31 |
|
Quantitative real-time
reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA of fresh specimens were extracted from
frozen materials by TRIzol reagent according to the manufacturers
protocol (Invitrogen, Carlsbad, CA, USA). Reverse transcription of
total RNA into complementary DNA was conducted using the Takara
reverse transcription reagents (Takara Bio, Inc., Shiga, Japan) at
37°C for 15 min followed by 85°C for 5 sec. Primers were designed
using Primer Premier 5.0 software (Premier Biosoft, Waterloo, ON,
Canada) and synthesized by Invitrogen. The primers of SYPL1 were as
follows: forward, 5-TATGTTGGCTACA CGAGTCTGT-3 and reverse,
5-ACAAGGCGGAAGTTCA TCAATAA-3; ACTB was used as a control with the
following primers: forward, 5-ACTCGTCATACTCCTGCT-3 and reverse,
5-GAAACTACCTTCAACTCC-3. The fold-change of SYPL1 mRNA expression in
HCC tissues compared to adjacent non-tumorous tissues was analyzed
using 2−ΔΔCt method (14).
Western blot analysis
Total protein of fresh HCC specimens were extracted
and quantified by BCA protein assay kit (Thermo Fisher Scientific,
Waltham, MA, USA) for protein concentrations. Then, proteins were
separated on 10% sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene
fluoride membranes (Millipore). After blocking with 5% skimmed milk
in Tris-buffered saline containing 0.05% Tween-20, the membranes
were incubated with a rabbit anti-SYPL1 monoclonal antibody
(ab184176, 1:1,000 dilution; Abcam, Cambridge, MA, USA) overnight,
followed by 20-min incubation with horseradish
peroxidase-conjugated secondary antibody. β-actin protein
determined by a mouse anti-β-actin monoclonal antibody (ab8226,
1:4,000 dilution; Abcam) was used as a loading control. The band
density was measured by ImageJ software, which was repeated three
times (15).
Immunohistochemical staining
IHC was performed to further determine the
expression pattern of SYPL1 in HCC tissues. The tissue specimens
were fixed in 10% formalin and then embedded in paraffin; 4 mm
sections were incised and placed on silane-coated slides. The
V-9000 Polymer Detection System, DAB and hematoxylin (Beijing
Zhongshan Golden Bridge Biotechnology, Co., Ltd., Beijing, China)
were used for IHC staining according to the manufacturers
recommendations. Some specimens were stained with H&E to
confirm the diagnosis. Other sections of representative blocks were
deparaffinized and dehydrated using gradient solvents. Following
antigen retrieval in 0.01 M sodium citrate buffer (pH 6.0) for 15
min at 95°C, endogenous peroxidase was blocked with 3%
H2O2. Ten percent of goat serum was used as
blocking liquid for 15 min. Thereafter, slides were incubated
overnight at 4°C respectively with a rabbit anti-SYPL1 monoclonal
antibody (ab184176, 1:250 dilution; Abcam), a mouse anti-vimentin
monoclonal antibody (ZM-0260, 1:200 dilution; Beijing Zhongshan
Golden Bridge Biotechnology) and a mouse anti-E-cadherin monoclonal
antibody (ZM-0092, 1:300 dilution; (Beijing Zhongshan Golden Bridge
Biotechnology), a rabbit anti-NF-κB p65 polyclonal antibody
(ab86299, 1:200 dilution; Abcam), a rabbit anti-Snail polyclonal
antibody (ab85936, 1:200 dilution; Abcam). Further incubation was
carried out with a secondary antibody followed by counterstaining
with DAB and hematoxylin, and then ethanol dehydration was
conducted by grade followed by addition of xylene to make the
sections transparent. Finally, mounting was performed with neutral
balsam. The staining intensity of proteins was evaluated on a
four-step scale (0, no staining; 1+, weak intensity; 2+, moderate
intensity; and 3+, strongest intensity). The staining fraction was
scored according to the following criteria: 0, no positive cancer
cells; 1, <25% positive cancer cells; 2, 26–50% positive cancer
cells; and 3, >50% positive cancer cells. Both staining
intensity and fraction determined the overall staining score
(16). Negative control slides were
treated with the same original but non-immunologic serum followed
by the secondary antibody under the same conditions. Two
pathologists simultaneously evaluated immunostaining on a multihead
microscope (Olympus BX43; Olympus, Tokyo, Japan).
Patient follow-up and prognostic
study
The follow-up period was defined as an interval
between the date of operation and the date patient died or the last
follow-up, from January 2005 to December 2015. The follow-up of all
surviving patients were performed periodically, including serum AFP
levels, liver computed tomography (CT), ultrasonography and chest
radiography every 1–2 months. For those highly suspected of
relapsing or metastatic patients, other available diagnostic
modalities such as hepatic angiography, magnetic resonance imaging
(MRI), high-resolution chest CT and positron emission tomography
(PET) were also comprehensively applied. We censored survival at 5
years after the initial resection surgery. Patients who died from
other causes were defined as censored cases. The OS was defined as
the interval between surgery and death regardless of etiology, and
the DFS was defined as the interval between surgery and tumor
relapse or metastasis.
Statistical analysis
All data were analyzed using the SPSS statistical
software, version 18.0, for Windows (SPSS, Inc., Chicago, IL, USA).
The correlation between SYPL1 expression and clinicopathological
parameters was evaluated using the Pearson χ2 test.
Survival curves were constructed by the Kaplan-Meier method and
evaluated by the log-rank test. The independent risk factors
associated with the overall and disease-free survival of HCC
patients were identified by establishing the Cox proportional
hazards regression model. A two-tailed P<0.05 was considered
statistically significant.
Results
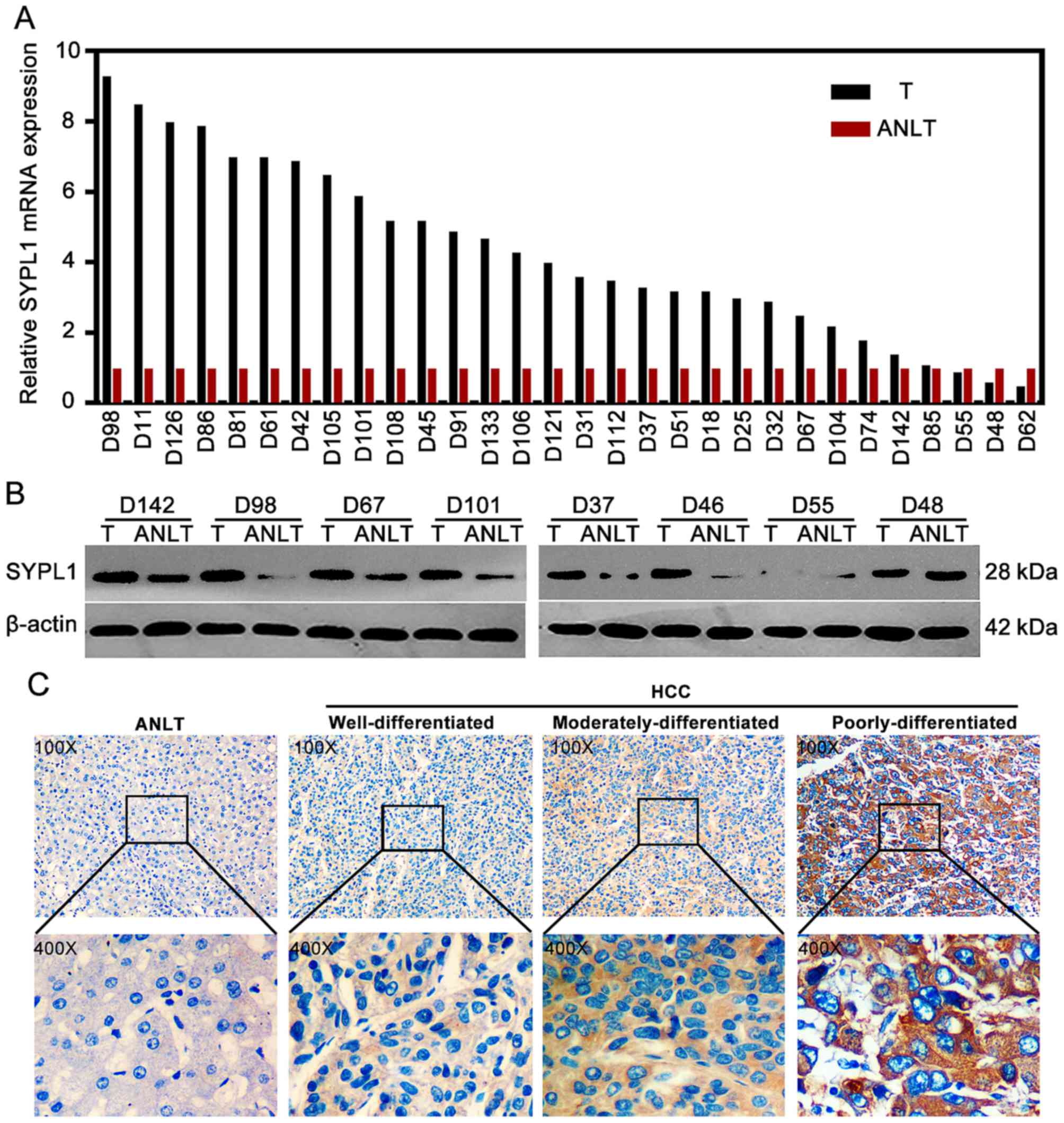
SYPL1 is significantly overexpressed
in HCC tissues
The results from qRT-PCR showed that the relative
SYPL1 mRNA expression level in HCC tissues was obviously higher
than that in the matched ANLT tissues (mean ± SD, 3.69±0.45 vs.
0.88±0.17; P<0.001) (Fig. 2A).
Consistently, the western blot results showed that the expression
of SYPL1 protein in HCC tissues was also significantly higher than
that in the matched ANLT tissues (P=0.026) (Fig. 2B). To further determine the
relationship between the expression pattern of SYPL1 and the
clinical significance in HCC patients, IHC staining was performed
and showed SYPL1 was broadly and significantly upregulated in HCC
tissues, compared with the matched ANLT tissues (P<0.001)
(Fig. 2C). The subcellular location
of SYPL1 was mainly in the cytoplasm, plasma membrane and
extracellularly located. Based on the SYPL1 staining in HCC
tissues, these patients were divided into two groups: low (IHC
level 0–1) vs. high (IHC level 2–3)-SYPL1 expression groups,
respectively. Of the 122 HCC specimens, 79 (64.8%) manifested
significantly high expression level of SYPL1 protein, while 43
(35.2%) expressed relatively low level of the protein. Moreover,
significantly higher expression level of SYPL1 was observed in the
poorly differentiated HCC tissues than that in well or moderately
differentiated HCC (P=0.048) (Fig.
2C).
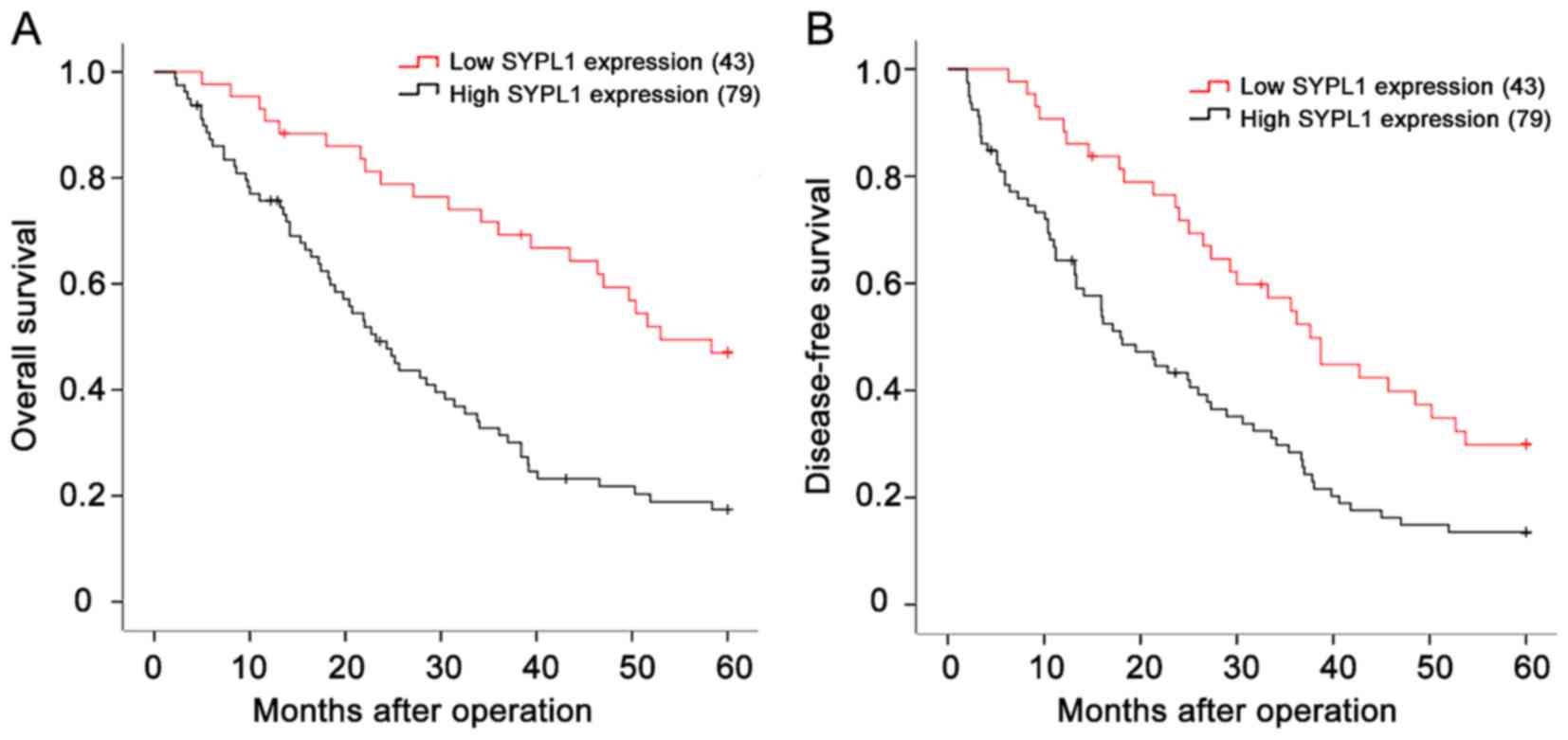
Elevated SYPL1 expression is
associated with malignant clinicopathological features and predicts
poor prognosis of HCC patients
Then we evaluated the relationship between SYPL1
expression and clinicopathological features of HCC patients. Of
note, the expression level of SYPL1 was significantly associated
with tumor size, tumor nodule number, capsular formation,
Edmondson-Steiner grade, MVI, Barcelona Clinic Liver Cancer (BCLC)
stage and tumor node metastasis (TNM) stage (all P<0.05;
Table I). HCC patients with high
SYPL1 expression in the tumor specimens had reduced OS (1-, 3- and
5-year OS: 74.7, 30.4 and 15.2 vs. 90.7, 67.4 and 44.2%;
P<0.001) and DFS (1-, 3- and 5-year DFS: 70.9, 26.6 and 12.7 vs.
88.4, 62.8 and 27.9%; P=0.002) than those with relatively low SYPL1
expression (Fig. 3A and B).
Furthermore, univariate and multivariate analysis indicated that
high SYPL1 expression was an independent risk factor for both OS
and DFS of HCC patients after liver resection (Tables II and III). These results adequately revealed
that SYPL1 was closely associated with malignant
clinicopathological features, and could serve as a novel
independent prognosis biomarker for HCC patients after hepatic
resection.
 | Table II.Univariate and multivariate analysis
of factors associated with overall survival. |
Table II.
Univariate and multivariate analysis
of factors associated with overall survival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | n | RR (95% CI) | P-value | RR (95% CI) | P-value |
|---|
| Sex |
|
Female | 21 | 1 |
|
|
|
|
Male | 101 | 1.122
(0.632–1.992) | 0.694 | n.a. | n.a. |
| Age (years) |
|
≤60 | 94 | 1 |
|
|
|
|
>60 | 28 | 1.455
(0.887–2.385) | 0.138 | n.a. | n.a. |
| AFP (ng/ml) |
|
<20 | 32 | 1 |
|
|
|
|
≥20 | 90 | 0.999
(0.618–1.614) | 0.997 | n.a. | n.a. |
| HBsAg |
|
Negative | 20 | 1 |
|
|
|
|
Positive | 102 | 0.957
(0.529–1.729) | 0.884 | n.a. | n.a. |
| Liver
cirrhosis |
|
Absence | 32 | 1 |
|
|
|
|
Presence | 90 | 1.920
(1.137–3.243) | 0.015 | n.a | n.a |
| Tumor size
(cm) |
| ≤5 | 48 | 1 |
|
|
|
|
>5 | 74 |
2.074(1.301–3.305) | 0.002 | n.a. | n.a. |
| Tumor nodule
number |
|
Solitary | 65 | 1 |
|
|
|
|
Multiple (≥2) | 57 |
1.645(1.065–2.542) | 0.025 | n.a | n.a |
| Capsular
formation |
|
Presence | 66 | 1 |
|
|
|
|
Absence | 56 | 1.666
(1.083–2.563) | 0.020 | n.a | n.a |
| Edmondson-Steiner
grade |
|
I-II | 59 | 1 |
|
|
|
|
III-IV | 63 | 1.568
(1.015–2.425) | 0.043 | n.a. | n.a. |
| Microvascular
invasion |
|
Absence | 62 | 1 |
| 1 |
|
|
Presence | 60 | 2.455
(1.573–3.831) | 0.002 | n.a | n.a |
| TNM |
| Early
(I-II) | 91 | 1 |
|
|
|
| Late
(III-IV) | 31 | 3.194
(2.011–5.072) |
<0.001 | 2.479
(1.480–4.151) | 0.001 |
| BCLC staging |
|
0-A | 41 | 1 |
| 1 |
|
| B +
C | 81 |
2.192(1.353–3.551) | 0.001 | 1.760
(1.013–3.059) | 0.045 |
| Liver function |
|
Child-Pugh A | 73 | 1 |
|
|
|
|
Child-Pugh B | 49 | 1.512
(0.980–2.332) | 0.062 | n.a. | n.a. |
| SYPL1
expression |
|
Low | 43 | 1 |
| 1 |
|
|
High | 79 | 2.637
(1.612–4.314) |
<0.001 | 2.443
(1.429–4.177) | 0.001 |
 | Table III.Univariate and multivariate analysis
of factors associated with disease-free furvival. |
Table III.
Univariate and multivariate analysis
of factors associated with disease-free furvival.
|
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|---|
| Variables | n | RR (95% CI) | P-value | RR (95% CI) | P-value |
|---|
| Sex |
|
Female | 21 | 1 |
|
|
|
|
Male | 101 | 1.200
(0.690–2.085) | 0.518 | n.a. | n.a. |
| Age (years) |
|
≤60 | 94 | 1 |
|
|
|
|
>60 | 28 | 1.398
(0.879–2.221) | 0.157 | n.a. | n.a. |
| AFP (ng/ml) |
|
<20 | 32 | 1 |
|
|
|
|
≥20 | 90 | 1.074
(0.680–1.697) | 0.758 | n.a. | n.a. |
| HBsAg |
|
Negative | 20 | 1 |
|
|
|
|
Positive | 102 | 0.978
(0.563–1.698) | 0.936 | n.a. | n.a. |
| Liver
cirrhosis |
|
Absence | 32 | 1 |
|
|
|
|
Presence | 90 | 1.944
(1.192–3.172) | 0.008 | n.a | n.a |
| Tumor size
(cm) |
| ≤5 | 48 | 1 |
| 1 |
|
|
>5 | 74 | 2.267
(1.458–3.523) |
<0.001 | 1.705
(1.019–2.853) | 0.042 |
| Tumor nodule
number |
|
Solitary | 65 | 1 |
|
|
|
|
Multiple (≥2) | 57 | 1.525
(1.014–2.293) | 0.043 | n.a | n.a |
| Capsular
formation |
|
Presence | 66 | 1 |
|
|
|
|
Absence | 56 | 1.859
(1.233–2.802) | 0.003 | n.a | n.a |
| Edmondson-Steiner
grade |
|
I-II | 59 | 1 |
|
|
|
|
III-IV | 63 |
1.560(1.035–2.350) | 0.034 | n.a. | n.a. |
| Microvascular
invasion |
|
Absence | 62 | 1 |
|
|
|
|
Presence | 60 | 2.454
(1.614–3.734) | 0.001 | n.a | n.a |
| TNM |
| Early
(I-II) | 91 | 1 |
| 1 |
|
| Late
(III-IV) | 31 | 2.998
(1.903–4.722) |
<0.001 | 2.306
(1.385–3.841) | 0.001 |
| BCLC staging |
|
0-A | 41 | 1 |
| 1 |
|
| B +
C | 81 | 2.495
(1.579–3.942) |
<0.001 | 1.962
(1.163–3.309) | 0.012 |
| Liver function |
|
Child-Pugh A | 73 | 1 |
|
|
|
|
Child-Pugh B | 49 | 1.739
(1.148–2.633) | 0.009 | n.a. | n.a. |
| SYPL1
expression |
|
Low | 43 | 1 |
| 1 |
|
|
High | 79 | 2.006
(1.292–3.113) | 0.002 | 1.680
(1.012–2.788) | 0.045 |
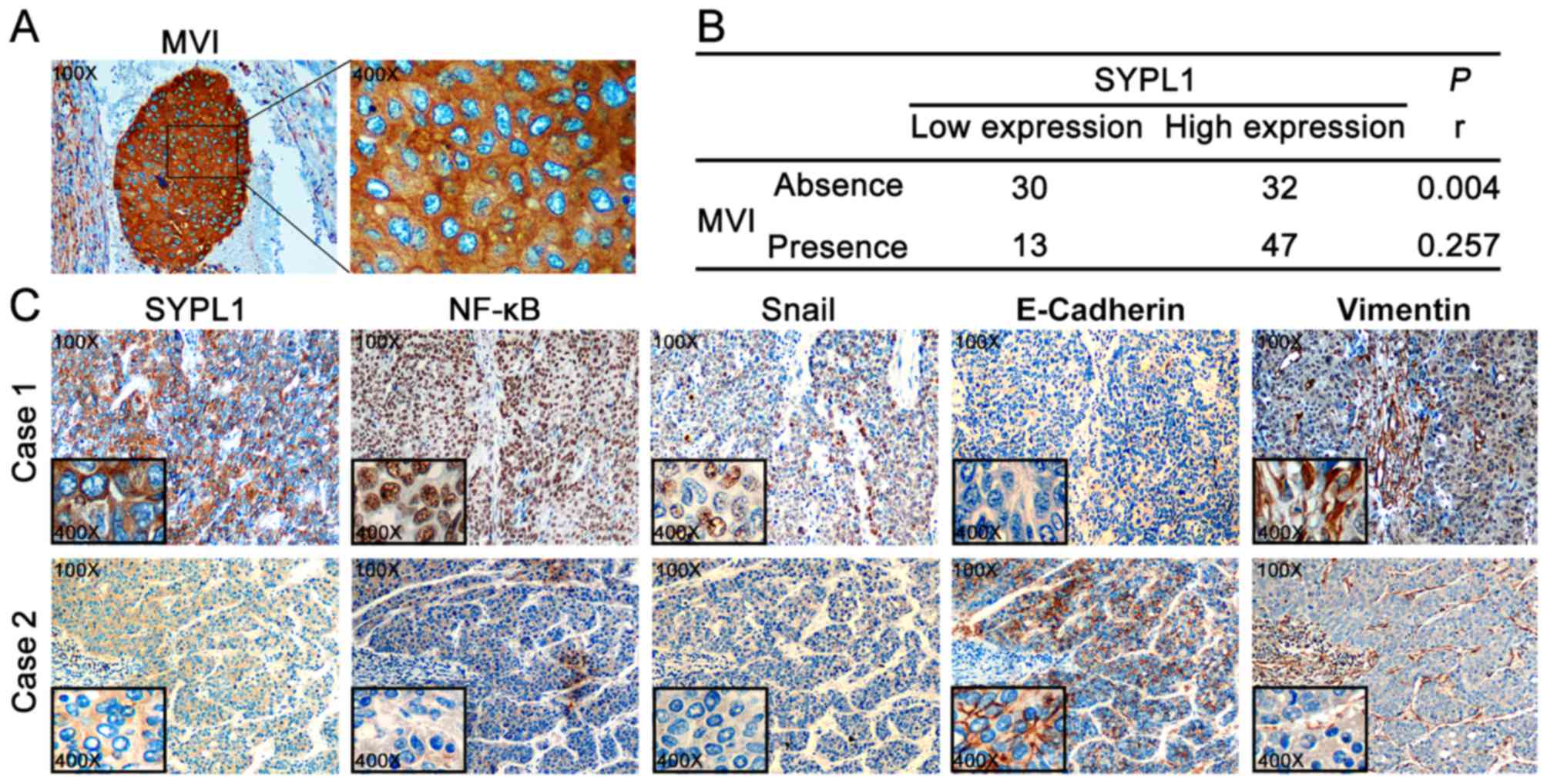
Elevated SYPL1 expression is
associated with the presence of MVI and EMT of HCC cells
Among the aforementioned malignant
clinicopathological features that closely associated with SYPL1
expression, the presence of MVI interested us greatly, because the
HCC cells in MVI displayed significant ectopic SYPL1 expression
(Fig. 4A). The significance of MVI
is drawing increasing attention, not only from clinicians but also
researchers, to predict the prognosis of HCC patients. Moreover,
MVI has been recognized as a better predictor of tumor recurrence
and OS rate following surgical resection compared to the Milan
Criteria. Therefore, we analyzed the MVI in specimens obtained from
HCC patients with liver resection, and the prevalence of MVI was
49.2% (60 in 122). Astonishingly, the presence of MVI was much more
frequently present in the high-SYPL1 expression group than in the
low-SYPL1 expression group (59.5 vs. 30.2%, P=0.002) (Table I). Furthermore, Spearman test was
performed and showed that ectopic SYPL1 was significantly
correlated with the presence of MVI (rho=0.257, P=0.004), (Fig. 4B). Therefore, these results
indicated that ectopic SYPL1 expression might be involved in HCC
cells invading to microvessels.
During the metastatic cascade, tumor cells acquire
enhanced ability to infiltrate into blood stream, and some settle
to proliferate and form MVI. EMT, a critical mechanism which
endowed motility to tumor cells, was associated with formation of
MVI (17). As mentioned above, we
had found ectopic SYPL1 was significantly correlated with the
presence of MVI, therefore, we tried to determine the correlation
between the expression pattern of SYPL1 and EMT markers, E-cadherin
and vimentin. Notably, HCC tissues which had elevated expression of
SYPL1 protein were also detected high level of vimentin,
accompanying with little or even no E-cadherin expression; and vice
versa (Fig. 4C). These results
indicated that ectopic SYPL1 expression was associated with EMT of
HCC cells, which might facilitate MVI formation, and the underlying
mechanism of which is still worthy of further interpretation.
Elevated SYPL1 expression is
associated with EMT of HCC cells, which might be mediated via
NF-κB/Snail signaling pathway
We then tried to gain insight into the mechanism,
which linked SYPL1 expression and the EMT of HCC cells. In a recent
study screening for regulators of the NF-κB signaling pathways via
performing a genome-wide siRNA, SYPL1 was identified as a potential
regulator of NF-κB (12). Since the
critical role of NF-κB/Snail signaling in HCC progress, especially
the significant role on EMT-inducing (18), we inquired into whether SYPL1
associated with NF-κB/Snail signaling and EMT of HCC cells. IHC
staining was performed to further determine the co-expression
patterns of SYPL1, NF-κB, Snail, E-cadherin and vimentin in HCC
tissues. These staining results from serial section showed an
obviously positive expression of SYPL1, NF-κB, Snail and vimentin,
whereas there was notably negative expression of E-cadherin
(Fig. 4C). These results suggest
that SYPL1 might be involved in NF-κB/Snail signaling pathway, via
which SYPL1 facilitated EMT of HCC cells and even MVI
formation.
Discussion
In the present study, we demonstrated that the
ectopic expression of SYPL1 in HCC tissues predicted poor prognosis
of HCC patients with reduced OS and DFS rates. Mechanistically,
SYPL1 might be involved in NF-κB/Snail signaling pathway and
associate with EMT of HCC cells, which may lead to an enhanced
mobility of HCC cells to invade into vascular system to form MVI,
resulting in relapse and metastasis (19). To the best of our knowledge, these
observations are the first evidence supporting the role of SYPL1 in
tumor progress. In previous studies, SYPL1 was reported to be
involved with cellular exosomes (11), which are considered as critical
factors of HCC progress (20).
Moreover, as a phosphoprotein component of adipocyte transport
vesicles, SYPL1 associated with GLUT4-containing vesicles (10), while GLUT-4 was associated with the
development of several diseases, including tumor progress and EMT
(21). In addition, SYPL1 was
suggested to be a potential regulator of NF-κB (12). NF-κB signaling is a well-known and
critical regulator contributing to tumorigenesis and progress. The
above indicated that SYPL1 might play a critical role in
cancer.
EMT, recognized as a central process in the complex
metastatic cascade of HCC, can dissociate collective HCC cells and
endow them more motile and invasive properties (22), facilitate their invading through the
basement membrane into the vascular system and then surviving or
proliferating, followed by extravasating out of the circulation
system at distant organ sites to settle and constitute
premetastatic niches, ultimately forming macroscopic metastases
(5,23). The hallmarks of EMT include
disruption of EMT-related transcript factors, such as Snail, TWIST
and ZEB; and loss of epithelial adherent junctions, concomitant
with a development of a migratory phenotype (24). These changes are characterized as
downregulation of epithelial markers of E-cadherin and upregulation
of mesenchymal markers, such as N-cadherin, vimentin and
fibronectin. In the present study, we found an obviously positive
correlation among SYPL1, NF-κB, Snail and vimentin expression,
whereas a negative correlation of E-cadherin expression in HCC
tissues with the four proteins mentioned above. These results
suggested that SYPL1 might associate with EMT of HCC cells via
NF-κB/Snail signaling pathway.
MVI, defined as a microscopic evidence of cancer
cell clusters existing in vessels of the tumor capsule and/or in
surrounding liver parenchyma (25),
has been included in the AJCC staging system (26). As a histopathologic feature, the
presence of MVI is a verified indicator for aggressive behavior of
HCC, and is directly related to early recurrence within 2 years
after curative liver resection or/and even orthotopic liver
transplantation (27,28). According to previous reports, the
prevalence of MVI in the specimens obtained from liver resection or
transplantation of HCC patients was between 15.0 and 57.1%
(29,30). However, in this study, the
prevalence of MVI in the specimens obtained from liver resection of
HCC patients was 49.2% generally, with a significantly higher
prevalence in the high-SYPL1 expression group than that in the
low-SYPL1 expression group (59.5 vs. 30.2%, P=0.002; Table I). There have been many efforts on
preoperative estimation of MVI over the past decade. The ‘typical
dynamical pattern’ (i.e., arterial enhancement and washout) on
contrast-enhanced magnetic resonance imaging (MRI) was reported to
be closely associated with MVI (31), even though other investigators
questioned the potential inter-observer variability before further
prospective validation for these results to avoid it (29). More recently, a preoperative
prediction model was developed, in which variables associated with
MVI in patients who underwent resection of HCC were used, with a
secondary aim to confirm the importance of MVI on long-term
outcomes (32). Moreover, a
research group suggested Nomogram for preoperative estimation of
MVI risk in Hepatitis B Virus-Related HCC, but they also recognized
that Nomogram estimation, to some extent, might lack reliability
and accuracy; therefore, they suggested that the combined usage of
specific markers to estimate MVI might further improve the accuracy
of Nomogram (19). Some experts
have also proposed the use of serum or other biomarkers to estimate
preoperative prediction of MVI risk for HCC patients (33). Disappointingly, these biomarkers
failed to identify HCC patients with MVI from the patients with
benign liver disease (34). In
spite of this, we demonstrated a close correlation between ectopic
SYPL1 expression and the presence of MVI in HCC patients with poor
prognosis, which suggest SYPL1 might be a good tumor biomarker for
preoperative MVI prediction to identify patients at high risk of
relapse and metastasis.
This study has some limitations. Firstly, it was
totally based on data from clinical tissues, without further
verified in the HCC cell lines. More studies are needed to
determine the function of SYPL1 by manipulating its expression
level in HCC cells. Secondly, activation or inactivation of NF-κB
as well as the translocation of NF-κB complexes, all of which would
obviously influence the NF-κB signaling pathway, subsequently
affect the expression level of downstream targeted genes through
regulating chromatin structure (35). The correlation between SYPL1 and
NF-κB/Snail signaling we found here may be the complement of NF-κB
signaling pathway, which require further functional tests to
explore the underlying mechanism.
In conclusion, we provided the first evidence that
SYPL1 overexpression is associated with the progress and the
clinicopathological features of HCC mentioned above, especially the
presence of MVI. Furthermore, mechanistically, ectopic SYPL1
expression might be associated with EMT of HCC cells via
NF-κB/Snail signaling pathway. Therefore, SYPL1 may be a valuable
biomarker for understanding metastatic mechanisms in individual
patient; and hence, to be a potential target for tailoring
treatment on an individual basis.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81672595 to
Z.-M.Z. and no. 81302287 to Q.-W.W.), the Youth Innovation Project
of Fujian Province Natural Science Foundation (no. 2014D011 to
S.-J.W.) and the Health Industry Joint Project of Fujian Provincial
Natural Science Foundation (no. 2015J01554 to S.-J.W.).
Glossary
Abbreviations
Abbreviations:
|
AFP
|
alpha-fetoprotein
|
|
HBsAg
|
hepatitis B surface antigen
|
|
TNM
|
tumor node metastasis
|
|
BCLC
|
Barcelona Clinic Liver Cancer
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM and Bruix J: Molecular targeted
therapies in hepatocellular carcinoma. Hepatology. 48:1312–1327.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Njei B, Rotman Y, Ditah I and Lim JK:
Emerging trends in hepatocellular carcinoma incidence and
mortality. Hepatology. 61:191–199. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrer-Fàbrega J, Forner A, Liccioni A,
Miquel R, Molina V, Navasa M, Fondevila C, García-Valdecasas JC,
Bruix J and Fuster J: Prospective validation of ab initio
liver transplantation in hepatocellular carcinoma upon detection of
risk factors for recurrence after resection. Hepatology.
63:839–849. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roayaie S, Blume IN, Thung SN, Guido M,
Fiel MI, Hiotis S, Labow DM, Llovet JM and Schwartz ME: A system of
classifying microvascular invasion to predict outcome after
resection in patients with hepatocellular carcinoma.
Gastroenterology. 137:850–855. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
NCCN Clinical Practice Guidelines in
Oncology Hepatobiliary Cancers. Version 2. 2015.http://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf
|
|
8
|
Andersen Ø, Johnsen H, De Rosa MC, Præbel
K, Stjelja S, Kirubakaran TG, Pirolli D, Jentoft S and Fevolden SE:
Evolutionary history and adaptive significance of the polymorphic
Pan I in migratory and stationary populations of Atlantic cod
(Gadus morhua). Mar Genomics. 22:45–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Windoffer R, Borchert-Stuhlträger M, Haass
NK, Thomas S, Hergt M, Bulitta CJ and Leube RE: Tissue expression
of the vesicle protein pantophysin. Cell Tissue Res. 296:499–510.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Brooks CC, Scherer PE, Cleveland K,
Whittemore JL, Lodish HF and Cheatham B: Pantophysin is a
phosphoprotein component of adipocyte transport vesicles and
associates with GLUT4-containing vesicles. J Biol Chem.
275:2029–2036. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prunotto M, Farina A, Lane L, Pernin A,
Schifferli J, Hochstrasser DF, Lescuyer P and Moll S: Proteomic
analysis of podocyte exosome-enriched fraction from normal human
urine. J Proteomics. 82:193–229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Warner N, Burberry A, Franchi L, Kim YG,
McDonald C, Sartor MA and Núñez G: A genome-wide siRNA screen
reveals positive and negative regulators of the NOD2 and NF-κB
signaling pathways. Sci Signal. 6:rs32013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Roessler S, Long EL, Budhu A, Chen Y, Zhao
X, Ji J, Walker R, Jia HL, Ye QH, Qin LX, et al: Integrative
genomic identification of genes on 8p associated with
hepatocellular carcinoma progression and patient survival.
Gastroenterology. 142:957–966.e12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Carvajal-Vergara X, Sevilla A, DSouza SL,
Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R,
et al: Patient-specific induced pluripotent stem-cell-derived
models of LEOPARD syndrome. Nature. 465:808–812. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Bu F, Royer C, Serres S, Larkin
JR, Soto MS, Sibson NR, Salter V, Fritzsche F, Turnquist C, et al:
ASPP2 controls epithelial plasticity and inhibits metastasis
through β-catenin-dependent regulation of ZEB1. Nat Cell Biol.
16:1092–1104. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang X, Ma C, Zong Z, Xiao Y, Li N, Guo C,
Zhang L and Shi Y: A20 inhibits the motility of HCC cells induced
by TNF-α. Oncotarget. 7:14742–14754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qin Y, Zhao D, Zhou HG, Wang XH, Zhong WL,
Chen S, Gu WG, Wang W, Zhang CH, Liu YR, et al: Apigenin inhibits
NF-κB and snail signaling, EMT and metastasis in human
hepatocellular carcinoma. Oncotarget. 7:41421–41431. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lei Z, Li J, Wu D, Xia Y, Wang Q, Si A,
Wang K, Wan X, Lau WY, Wu M, et al: Nomogram for preoperative
estimation of microvascular invasion risk in hepatitis B
virus-related hepatocellular carcinoma within the Milan criteria.
JAMA Surg. 151:356–363. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wei JX, Lv LH, Wan YL, Cao Y, Li GL, Lin
HM, Zhou R, Shang CZ, Cao J, He H, et al: Vps4A functions as a
tumor suppressor by regulating the secretion and uptake of exosomal
microRNAs in human hepatoma cells. Hepatology. 61:1284–1294. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Q, Qin Y, Wei P, Lian P, Li Y, Xu Y, Li
X, Li D and Cai S: Gas1 inhibits metastatic and metabolic
phenotypes in colorectal carcinoma. Mol Cancer Res. 14:830–840.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xiao S, Chang RM, Yang MY, Lei X, Liu X,
Gao WB, Xiao JL and Yang LY: Actin-like 6A predicts poor prognosis
of hepatocellular carcinoma and promotes metastasis and
epithelial-mesenchymal transition. Hepatology. 63:1256–1271. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nieto MA: Epithelial plasticity: A common
theme in embryonic and cancer cells. Science. 342:12348502013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shirabe K, Toshima T, Kimura K, Yamashita
Y, Ikeda T, Ikegami T, Yoshizumi T, Abe K, Aishima S and Maehara Y:
New scoring system for prediction of microvascular invasion in
patients with hepatocellular carcinoma. Liver Int. 34:937–941.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sobin LH and Compton CC: TNM seventh
edition: whats new, whats changed: communication from the
International Union Against Cancer and the American Joint Committee
on Cancer. Cancer. 116:5336–5339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Roayaie S, Frischer JS, Emre SH, Fishbein
TM, Sheiner PA, Sung M, Miller CM and Schwartz ME: Long-term
results with multimodal adjuvant therapy and liver transplantation
for the treatment of hepatocellular carcinomas larger than 5
centimeters. Ann Surg. 235:533–539. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yao FY, Ferrell L, Bass NM, Bacchetti P,
Ascher NL and Roberts JP: Liver transplantation for hepatocellular
carcinoma: Comparison of the proposed UCSF criteria with the Milan
criteria and the Pittsburgh modified TNM criteria. Liver Transpl.
8:765–774. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rodríguez-Perálvarez M, Luong TV, Andreana
L, Meyer T, Dhillon AP and Burroughs AK: A systematic review of
microvascular invasion in hepatocellular carcinoma: Diagnostic and
prognostic variability. Ann Surg Oncol. 20:325–339. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Portolani N, Coniglio A, Ghidoni S,
Giovanelli M, Benetti A, Tiberio GA and Giulini SM: Early and late
recurrence after liver resection for hepatocellular carcinoma:
Prognostic and therapeutic implications. Ann Surg. 243:229–235.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim MJ, Lee M, Choi JY and Park YN:
Imaging features of small hepatocellular carcinomas with
microvascular invasion on gadoxetic acid-enhanced MR imaging. Eur J
Radiol. 81:2507–2512. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schlichtemeier SM, Pang TC, Williams NE,
Gill AJ, Smith RC, Samra JS, Lam VW, Hollands M, Richardson AJ,
Pleass HC, et al: A pre-operative clinical model to predict
microvascular invasion and long-term outcome after resection of
hepatocellular cancer: The Australian experience. Eur J Surg Oncol.
42:1576–1583. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gouw AS, Balabaud C, Kusano H, Todo S,
Ichida T and Kojiro M: Markers for microvascular invasion in
hepatocellular carcinoma: Where do we stand? Liver Transpl.
17:(Suppl 2). S72–S80. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sterling RK, Wright EC, Morgan TR, Seeff
LB, Hoefs JC, Di Bisceglie AM, Dienstag JL and Lok AS: Frequency of
elevated hepatocellular carcinoma (HCC) biomarkers in patients with
advanced hepatitis C. Am J Gastroenterol. 107:64–74. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen LF and Greene WC: Shaping the nuclear
action of NF-kappaB. Nat Rev Mol Cell Biol. 5:392–401. 2004.
View Article : Google Scholar : PubMed/NCBI
|