Introduction
Acid ceramidase (ASAH1), a lysosomal cysteine
amidase, helps metabolize ceramides into sphingosine and free fatty
acids. Ceramides promote senescence and apoptosis, while
sphingososine-1-phospate (Sph-1P), the immediate metabolite of
sphingosine, promotes cell survival, proliferation, inflammation,
and angiogenesis (1). As such,
overexpression of ASAH1 confers resistance to apoptosis. Its levels
have been shown to be elevated in several cancers, including breast
(2), prostate (3,4), head
and neck (5), colon, and melanoma
(6). Moreover, downregulation or
inhibition of ASAH1 may improve anticancer treatments (5,7,8).
Glioblastoma multiforme (GBM) is the most common
primary, intracranial malignancy of the central nervous system. The
standard treatment protocol, which involves surgical resection,
concurrent radiation/temozolomide (TMZ), and adjuvant TMZ, still
imparts a grim prognosis, where median overall survival (OS) is
less than 15 months (9).
Ultimately, all GBM develop resistance to standard treatment with
recurrence or progression, where additional therapies yield a
median survival of ~30 weeks (10–12).
Though ASAH1 appears to be a promising therapeutic target in other
tumors, no studies have explored its role in recurrent GBM.
It has been postulated that GBM cancer stem cells
(CSCs) are the culprits that promote resistance to radiation, as
CD133-carrying glioma cells are increased in proportion following
ionizing radiation (13). Recent
studies with prostate cancer suggest that upregulation of ASAH1
confers resistance to radiation by altering the sphingolipid
metabolism pathway (14,15). This study examines whether ASAH1
plays a similar role in recurrent or irradiated GBM.
Materials and methods
Reagents and cells
Mouse antibody against ASAH1 (612302) was purchased
from BD Biosciences (San Jose, CA, USA). Anti-actin, carmofur,
temozolomide (TMZ), and N-oleoylethanolamine (OE),
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich (St. Louis, MO, USA).
HRP-conjugated goat anti-mouse IgG was supplied by R&D Systems,
Inc. (Minneapolis, MN, USA). SDS-PAGE and western blot materials
were obtained from Life Technologies, Inc. (Grand Island, NY, USA).
Murine anti-Sph-1P monoclonal antibody, (LT1002) and humanized
anti-Sph-1P monoclonal antibody (LT1009) were obtained from Lpath,
Inc. (16,17).
Cells
The pediatric glioblastoma cell line (SJGBM2) was
obtained from the Children's Oncology Group (COG) Cell Culture and
Xenograft Repository. These cells were grown in Iscove's modified
Dulbecco's medium supplemented with 20% fetal bovine serum, 4 mM
L-glutamine, and 1X ITS (5 µg/ml insulin, 5 µg/ml transferrin, 5
ng/ml selenous acid). The U87 glioblastoma cell line was cultured
in Eagle's minimum essential medium (MEM) containing 10% (v/v)
fetal bovine serum (FBS).
Radiation
Cells (U87 and SJGBM2) were grown to confluence, and
then radiated with a Pantak HF320 X-ray machine (Agfa NDT Ltd.,
Reading, UK) operating at 300 kV at a dosage of 2.09 Gy/min to a
total radiation dose of 10 Gy, to generate the U87-10gy and
SJGBM2-10gy cell lines. Following radiation, these irradiated cells
were allowed to grow to confluence over a period of ~1 month prior
to any experiments that were performed.
Tissue collection
All human brain samples were collected after
informed written consent was obtained from the GBM patients. The
research protocol was approved by the Institutional Review Board
(IRB) at the Medical College of Wisconsin (MCW), Milwaukee, WI,
USA. Briefly, glioblastoma tumor tissues from consented patients
were collected at the time of tissue resection and snap-frozen in
liquid nitrogen within 30 min of removal and stored at −80°C in the
Brain and Spinal Tissue Bank at MCW until use. All tissues were
evaluated by routine histologic, immunohistochemical, and
angiogenic measurements. Each tissue biopsy sample was fixed in 10%
buffered formalin, processed, embedded in paraffin, cut, stained
with hematoxylin and eosin and any other histochemical or
immunohistochemical stains needed to fully evaluate the tissue. The
diagnostic evaluation of each biopsy was performed in the
Department of Pathology at MCW. Diagnosis of glioblastoma was based
on morphologic features that are considered histological hallmarks
of glioblastoma, including high cellularity, nuclear
hyperchromatism and pleomorphism, abundant mitoses, endothelial
proliferation, and necrosis with or without pseudo-palisades per
the WHO classification.
Tissue homogenization
GBM primary tumor samples were homogenized and
powdered in liquid nitrogen using a mixer mill (Retsch Inc., Haan,
Germany). Samples were maintained at liquid N2 temperature
throughout the process. Homogenized and powdered tissue samples
were then re-suspended in 5X volume of the weight of the tissue
sample in a reducing buffer (125 mM Tris pH 6.8, 4% SDS (w/v), 10%
glycerol (v/v), 5% 2-mercaptoethanol (v/v), complete protease
inhibitor (Roche Diagnostics Corp., Indianapolis, IN, USA), HALT
phosphatase inhibitor (Thermo Fisher Scientific, Grand Island, NY,
USA). Samples were then heated to 70°C with mixing at 1,400 rpm for
10 min, sonicated with a tip sonicator for 30 sec at power level 4,
and then centrifuged at 16,000 × g for 10 min at room temperature.
The supernatant was then collected.
Western blot analysis and
quantification
Equal amounts (15 µg) of protein from each of the
tumor samples were loaded onto the 4–12% gel. SDS-PAGE and western
blots were performed using standard methods. Gels were blocked with
5% bovine serum albumin. A 1:500 dilution was used for primary
antibody and 1:10,000 for secondary antibody. ImageJ software was
used to quantify western blot images.
Acid ceramidase immunohistochemistry
(IHC) methodology
All immunohistochemical (IHC) staining was performed
on a Dako Autostainer Plus using the Dako Envision™ FLEX High pH
detection kit. Briefly, after deparaffinization and rehydration of
the tissue, antigen retrieval was performed with Tris/EDTA pH 9.
After blocking of non-target epitopes, anti-acid ceramidase primary
antibody (Santa Cruz Biotechnology Inc., Dallas, TX, USA) was
applied at a concentration of 1:100 for 30 min, secondary antibody
for 20 min, and DAB for 10 min. Hematoxylin was used as
counterstain.
Immunohistochemistry (IHC)
scoring
Photomicrographs of stained tissues were acquired
and graded blindly using the Allred scoring system as follows. For
each patient, we determined the proportion of positively stained
tumor cells (proportion score ‘PS’), as well as the staining
intensity (mean intensity score ‘IS’) (18). Both scores were added together to
obtain the final Allred score, which was then matched with the
individual WHO pathology diagnoses.
Sphingolipid quantification
Electrospray ionization tandem mass spectrometry
(ESI/MS/MS) analysis of endogenous (phyto)ceramide species were
performed on a Thermo Fisher Quantum triple quadrupole mass
spectrometer, operating in a multiple reaction monitoring (MRM)
positive ionization mode, using the modified version of published
methods (19). Briefly, biological
materials were fortified with the internal standards (ISs:
C17 base D-erythro-sphingosine (17CSph), C17
sphingosine-1-phosphate (17CSph-1P),
N-palmitoyl-D-erythro-C13 sphingosine (13C16-Cer) and
heptadecanoyl-D-erythro-sphingosine (C17-Cer) and
C6-Phyto-ceramide), then extracted with an ethyl
acetate/iso-propanol/water (60/30/10 %v/v) solvent system. After
evaporation and reconstitution in 150 µl of methanol, samples were
injected on the HP1100/TSQ Quantum LC/MS system and gradient eluted
from the BDS Hypersil C8, 150×3.2 mm, 3 µm particle size column,
with a 1.0 mM methanolic ammonium formate/2 mM aqueous ammonium
formate mobile phase system. Peaks corresponding to the target
analytes and internal standards were collected and processed using
the Xcalibur software system. Quantitative analysis was based on
the calibration curves generated by spiking an artificial matrix
with the known amounts of the target analyte synthetic standards
and an equal amount of the internal standards (ISs). The target
analyte/IS peak area ratios were plotted against analyte
concentration. The target analyte/IS peak area ratios from the
samples were similarly normalized to their respective ISs and
compared to the calibration curves, using a linear regression
model. Introduction of the internal standards to the samples prior
to extraction, yielded results already ‘recovery corrected’,
therefore, no further data manipulation was necessary.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assays
Cells were plated onto a 96-well plate at the
density of 1×105 cells/ml. Media was exchanged to
serum-free media following overnight incubation. Cells were treated
with various antibodies for 48 h. MTT reagents were added after 48
h of incubation, followed by acidic-isopropanol 4 h later to
dissolve formazan. The absorbance values were recorded at
wavelengths 570 and 630 nm. IC50 values were calculated
with the GraphPad Prism software.
Immunohistochemistry (IHC) of
Sph-1P
Cells were grown on Nunc Lab-Tek Chamber slides
overnight then fixed and stained according to the published
protocol, with the exception that the staining of U87 and U87-10gy
cells was performed with LT1002 at the concentration of 22 µg/ml
(16,20). Slides were imaged with a Nikon
Eclipse 80i microscope at the mentioned magnification level.
Results
U87-10gy and SJGBM2-10gy cells
overexpress ASAH1
A previous study demonstrated the upregulation of
ASAH1 in irradiated prostate cancer cells, suggesting a mechanism
of cancer cell resistance to radiation (14). To evaluate the role of ASAH1 in
promoting radioresistance, native U87 and SJGBM2 cells were
irradiated with 10 Gy of radiation to generate U87-10gy and
SJGBM2-10gy cell lines. Less than 1% of total cells survived
radiation. Irradiated cells were allowed to grow to confluence
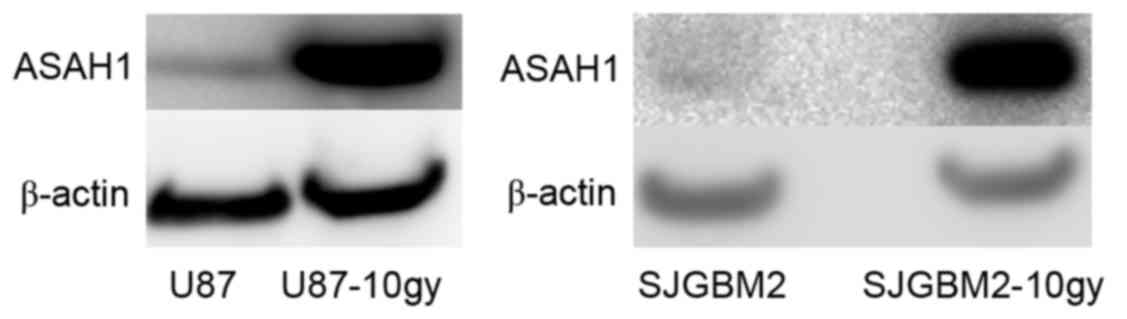
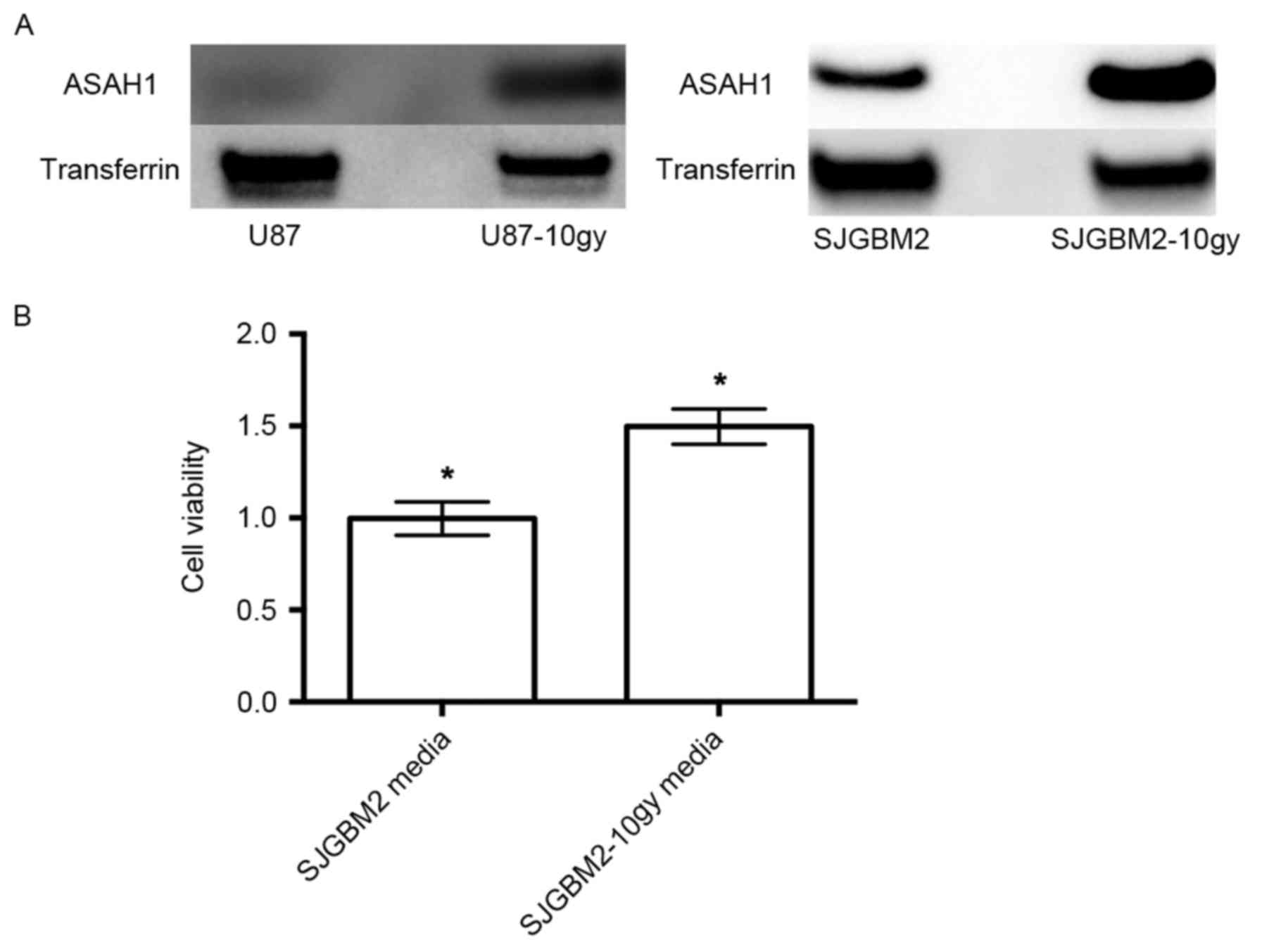
prior to being harvested for assays. Western blots of the cells
demonstrated U87-10gy and SJGM2-10gy cell lines expressed much
higher levels of ASAH1 compared to their native counterparts
(Fig. 1). These findings indicate
that these survived cells naturally overexpress ASAH1, which may be
an important mechanism for cell survival after radiation.
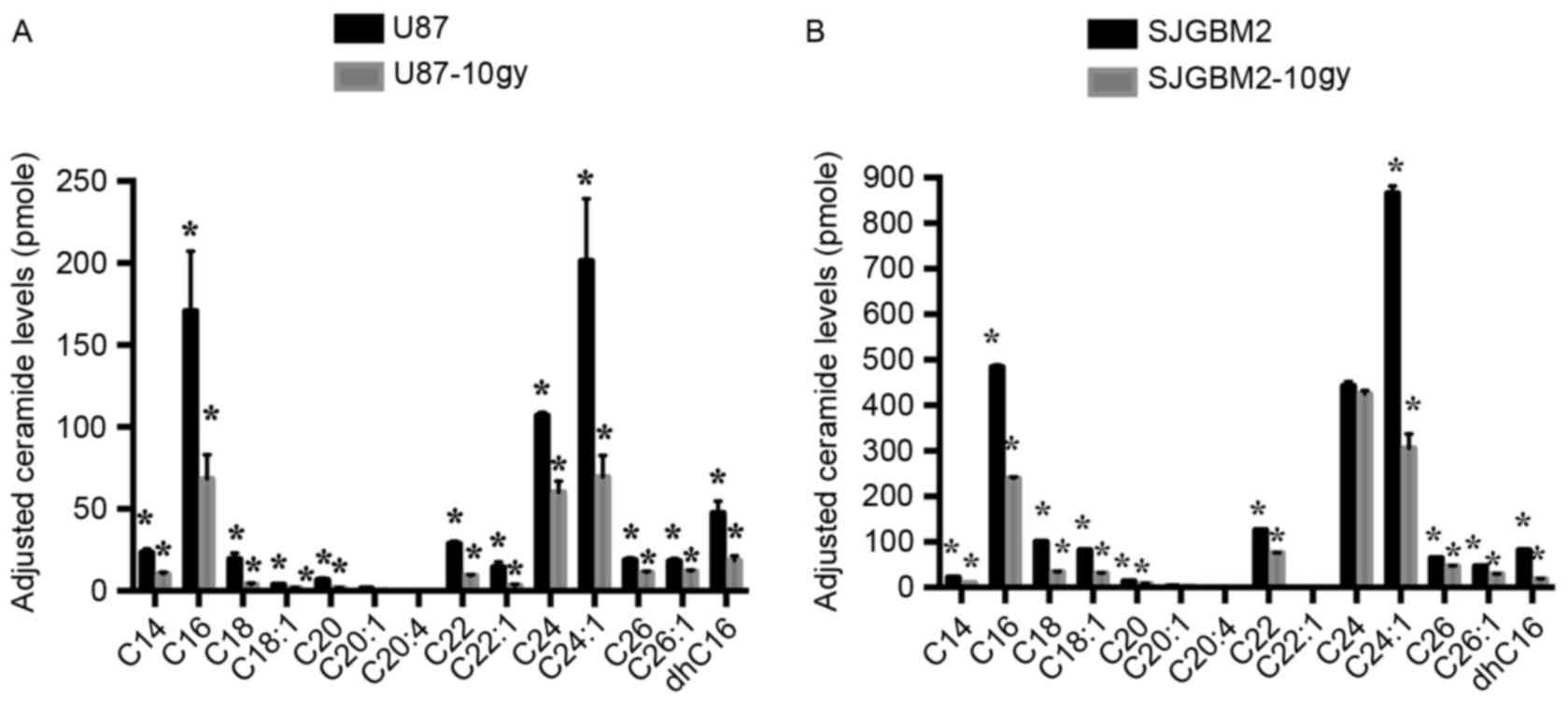
Cell radiation results in reduced
accumulation of ceramides in both U87-10gy and SJGBM2-10gy
To determine whether upregulation of ASAH1 modulates
sphingolipid metabolism, sphingolipid levels were determined in
native and irradiated GBM cells. In addition to the accumulation of
ASAH1, cells that survived radiation contained substantially
reduced levels of all ceramides measured compared to native cells,
potentially making them much less susceptible to cell death or
apoptosis induced by chemo- or radiotherapy (Fig. 2).
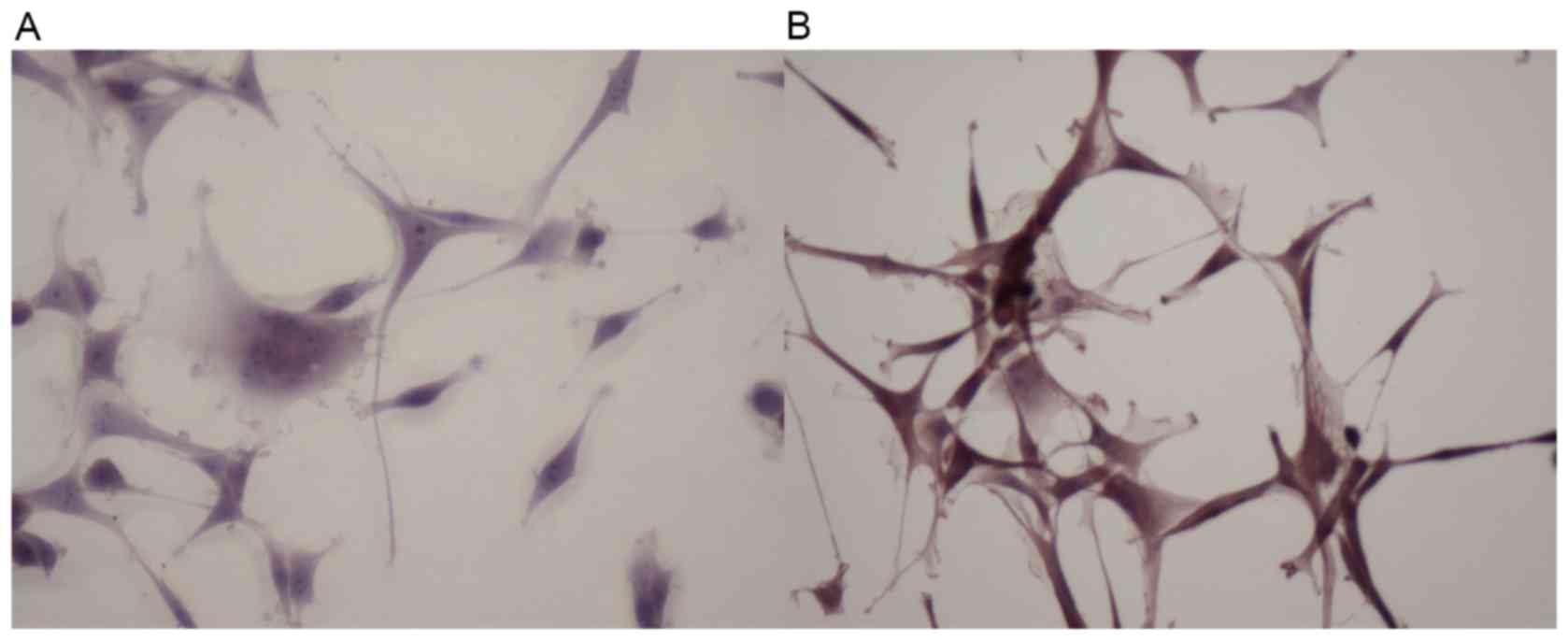
Sphingosine-1-phosphate is upregulated
following radiation as detected by IHC
To study the effect of radiation on the Sph-1P
level, we elected to perform IHC, using the anti-Sph-1P murine
monoclonal antibody to detect Sph-1P levels in U87 and U87-10gy
cells. As shown in Fig. 3, U87-10gy
cells exhibited a much greater staining intensity than U87 cells,
suggesting the presence of a higher amount of Sph-1P in U87-10gy
cells. A plausible interpretation is that radio-resistant cells
contain a substantially elevated level of ASAH1, whose enzyme
activity is known to be involved in the pathway leading to the
accumulation of Sph-1P (as seen in Fig.
3) (4).
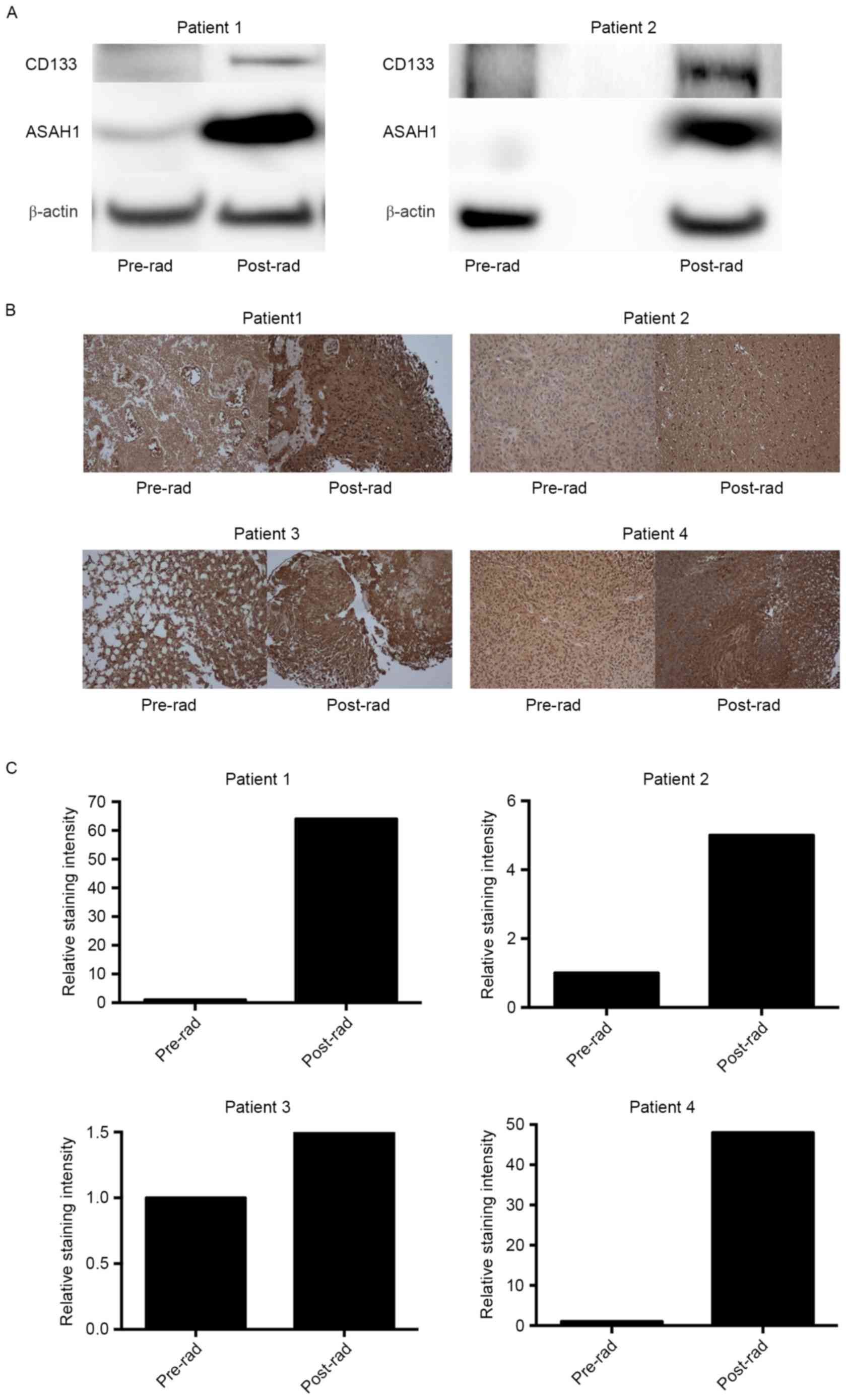
Irradiated or recurrent patient GBM
tissues exhibited upregulation of ASAH1 and CD133 based on western
blotting and IHC
We have now shown that irradiated cell culture
tissues (U87-10gy and SJGBM2-10gy) have higher expression levels of
ASAH1 than non-irradiated culture tissues. However, whether similar
findings can be seen in native human GBM samples that had undergone
prior radiation remains to be answered. To address this question,
we obtained pre- and post-radiation GBM samples from the same
patients who had undergone radiotherapies and eventually were
diagnosed with recurrent GBM. Western blots of these homogenized
patient tissues also demonstrated upregulation, to various degrees,
of ASAH1 in post-radiation tissues in comparison to pre-radiation
tissues, which paralleled the findings in tissue culture studies
(Figs. 1 and 4A). CD133, a glioma cancer stem cell
marker, was also found to be upregulated in post-radiation tissues
(Fig. 4A). Similarly, IHC of these
GBM samples revealed far greater ASAH1 staining in post-radiation
samples when compared to pre-radiation samples (Fig. 4B and C). Post-radiation GBMs also
demonstrated higher ASAH1 staining in the background or
extracellular space and this could be due to the secretion of ASAH1
into the extracellular space by irradiated GBMs, as had been shown
to be the case in irradiated U87 and SJGBM2 cells (Fig. 5).
Radiation induces over-secretion of
ASAH1 and SJGBM2-10gy media containing a high amount of secreted
ASAH1 stimulated 50% more cell growth than SJGBM2 media with a
lower amount of secreted ASAH1
Western blots of serum-free media previously used to
culture U87-10gy and SJGBM2-10gy demonstrated that U87-10gy and
SJGM2-10gy cell lines secreted much higher levels of ASAH1 compared
to their native counterparts (Fig.
5A). Far more ASAH1 was detected in the serum-free media of
SJGBM2-10gy cells than from SJGBM2. To test the effect of ASAH1 on
cell growth, serum-free media that were used to cultivate SJGBM2
and SJGBM2-10gy cells over a period of 48 h were collected.
Irradiated SJGBM2-10gy cells were allowed to grow to confluence
over a period of a week prior to being cultured for another 48 h in
serum-free media. U87 cells were then grown in these serum-free
media from irradiated SJGBM2-10gy cells for 48 h following by cell
growth analysis with MTT assays. Consistent with its function,
SJGBM2-10y media, which is rich in secreted ASAH1, promoted 50%
more cell growth than SJGBM2 media containing a lower level of
secreted ASAH1 (Fig. 5B).
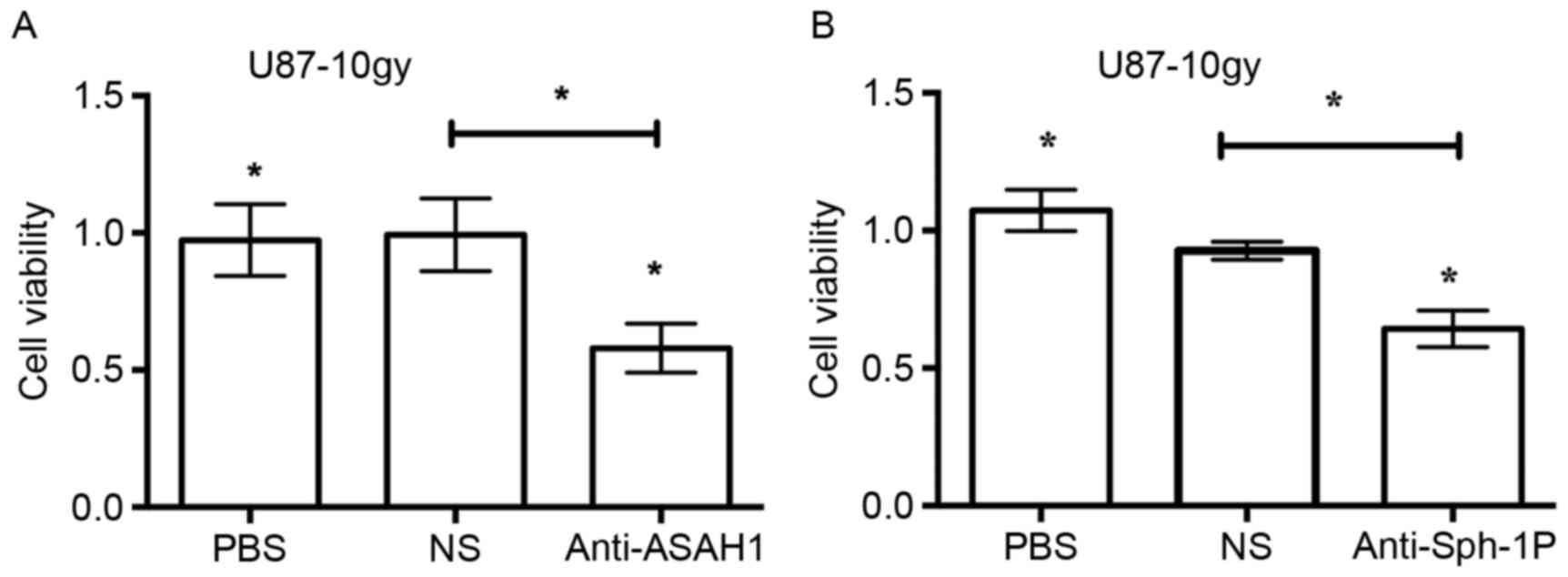
Neutralization of secreted ASAH1 and
Sph-1P with anti-ASAH1 and anti-Sph-1P antibodies, respectively,
resulted in reduced cell growth
ASAH1, as shown in this study, can be secreted into
the culture media. Similarly, Sph-1P can, as reported by others, be
secreted into the media as well (21). Given their known roles in the
promotion of cell growth (as shown in Fig. 5), we sought to determine whether
neutralization of ASAH1 and Sph-1P with antibodies would lead to
decreased cell growth. We treated U87-10gy with anti-ASAH1 and
anti-Sph-1P antibodies for 48 h and measured cell growth patterns
with MTT assays. Treatment of U87-10gy cells with 3 µg of either
anti-ASAH1 or anti-Sph-1P decreased cell growth by ~50% (Fig. 6). The data imply that the
neutralization of secreted ASAH1 or Sph-1P can prevent these
molecules from promoting cell growth and proliferation.
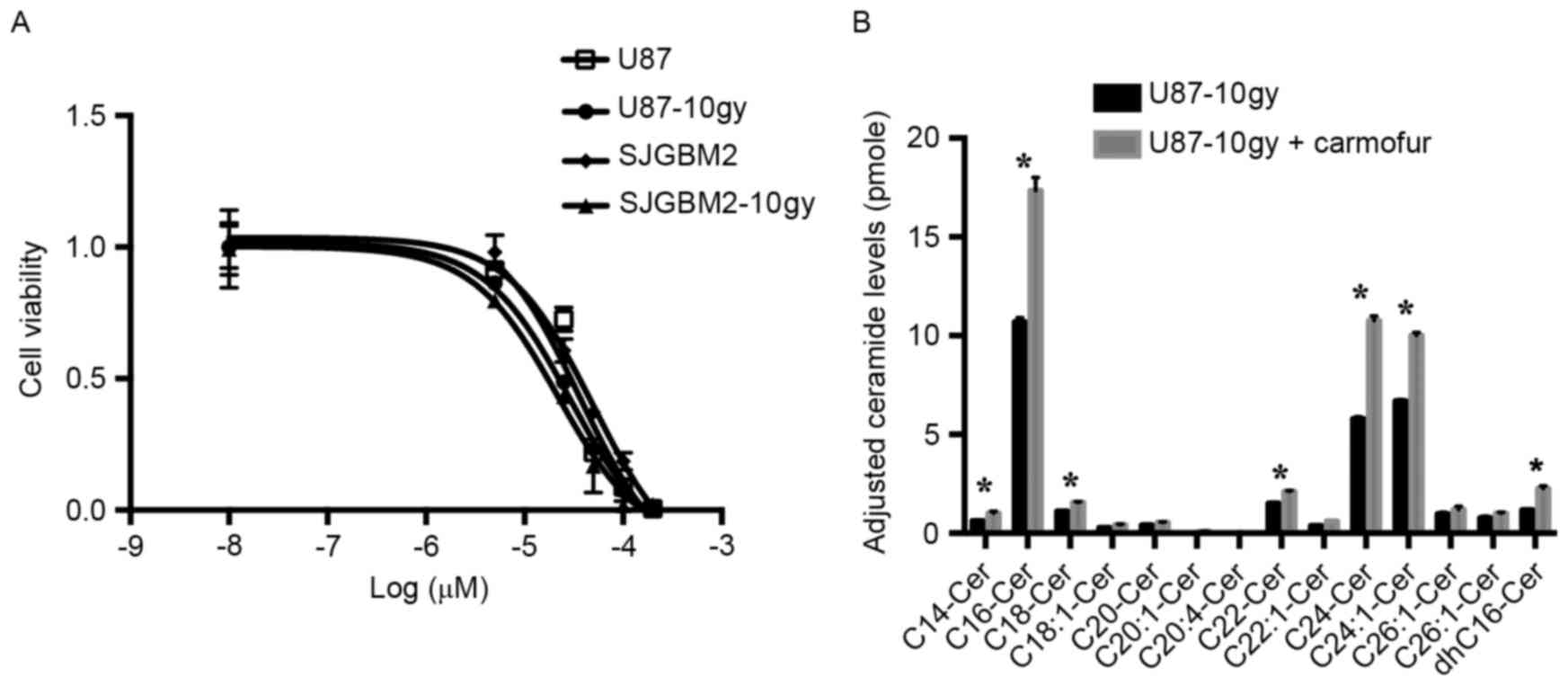
Treatment of U87-10gy and SJGBM2-10gy
cells with carmofur, an ASAH1 inhibitor, resulted in cell death and
elevated levels of ceramides
Other researchers have demonstrated that an ASAH1
inhibitor, such as carmofur, can effectively target cancers
(22). Previous data suggested that
carmofur inhibits ASAH1 activity and elevates tissue ceramide
levels, which in turn induces apoptosis (22). To test whether ASAH1 inhibition
contributes to cell death, we evaluated the effects of carmofur on
U87, SJGBM2, U87-10gy, and SJGBM-10gy cells. When exposed for 12 h
to carmofur, all cell lines showed markedly increased cell death
relative to control cells subjected to the same treatment. We
observed a median inhibitory concentration (IC50) of 37,
50, 28 and 21 µM for U87, SJGBM2, U87-10gy and SJGBM2-10gy,
respectively (Fig. 7). Importantly,
targeting U87-10gy cells with carmofur was accompanied by marked
intracellular accumulation of various ceramide species compared to
control cells (Fig. 7). This
suggested that ASAH1 inhibition by carmofur contributes to
cytotoxicity.
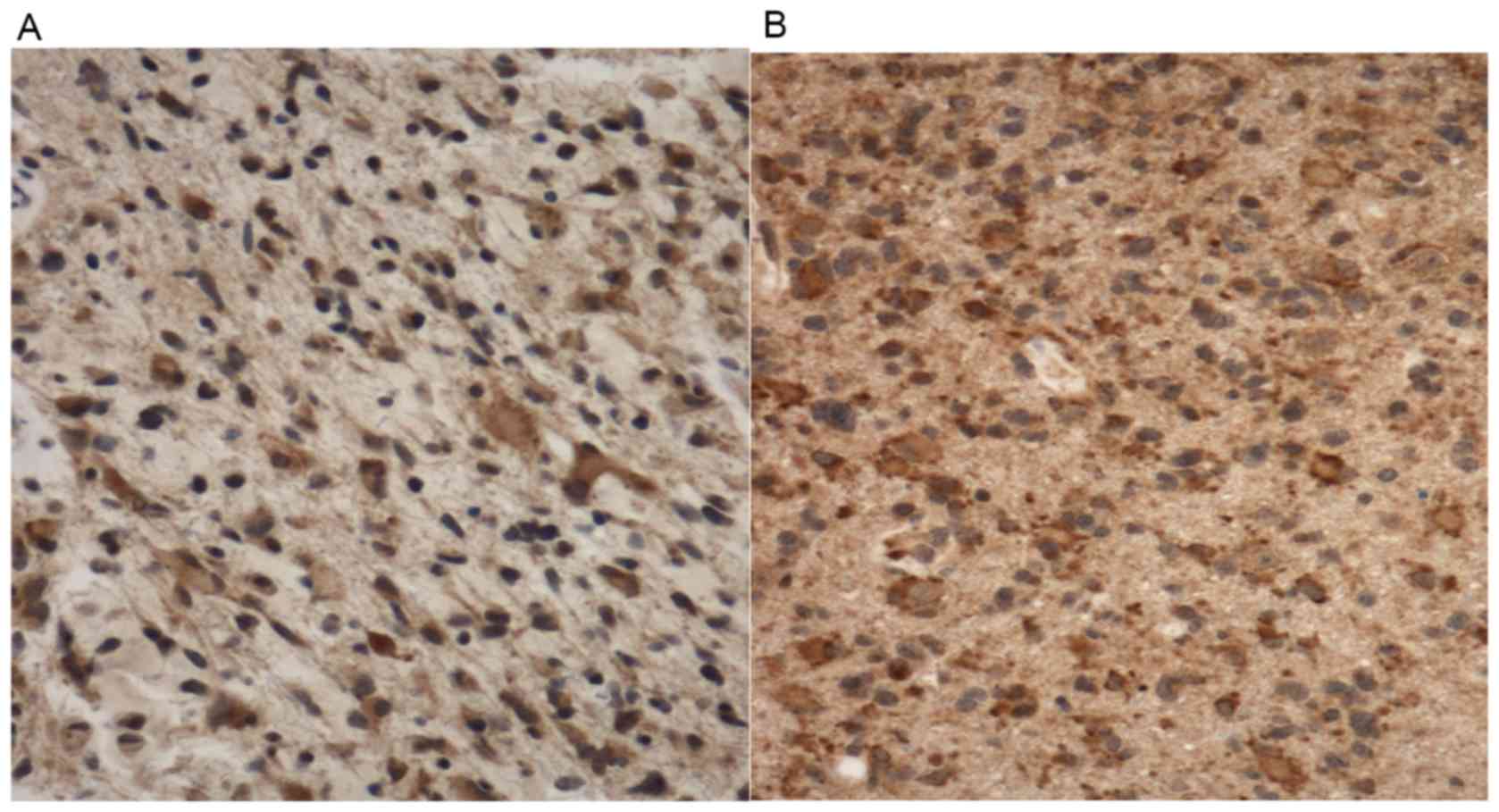
Irradiated GBMs exhibit significantly
greater ASAH1 staining than non-irradiated GBMs
To evaluate the difference in ASAH1 IHC staining
between irradiated and non-irradiated GBMs, we performed ASAH1 IHC
on 6 irradiated and 5 non-irradiated GBMs. We scored the level of
staining using the Allred scoring system, in which a higher score
suggests more staining (18).
Utilizing this system, we obtained the score of 5 for
non-irradiated vs. 7 for irradiated GBMs, with a statistically
significant p-value (Table I and
Fig. 8). Similar to Fig. 4, irradiated GBMs also exhibited
higher ASAH1 staining in the extracellular space, possibly due to
the secretion of ASAH1 into the extracellular space by irradiated
GBMs (Fig. 8).
 | Table I.Irradiated GBMs exhibit a
statistically significantly higher Alfred ASAH1 staining score than
non-irradiated GBMs. |
Table I.
Irradiated GBMs exhibit a
statistically significantly higher Alfred ASAH1 staining score than
non-irradiated GBMs.
|
Characteristics | Non-irradiated
GBM | Irradiated GBM | P-value |
|---|
| Male | 3 | 3 |
|
| Female | 2 | 3 |
|
| Mean age
(years) | 63±7 | 57±6 |
|
| Alfred median IHC
score | 5 | 7 | 0.036 |
Discussion
The current standard treatment protocol for GBM
consists of surgery followed by radiation and chemotherapy
(9,23). Despite treatment, all GBMs will
inevitably develop resistance and recur (12,24,25).
It was postulated that GBM cancer stem cells (CSCs) are the culprit
that promotes radioresistance, as evidenced by CD133-carrying
glioma cells that were increased in proportion following ionizing
radiation (13). A recent study in
prostate cancer suggested that upregulation of ASAH1 confers
resistance to radiation by altering the sphingolipid metabolism
pathway (14). Our work addresses
whether ASAH1 plays a similar role in GBM.
Western blot analysis of GBM cell cultures that
survived 10 Gy of radiation (U87-10gy and SJGBM-10gy) revealed that
protein expression of ASAH1 is significantly increased, while
ceramide levels correspondingly decreased, when compared to
non-irradiated GBM cells (Figs. 1
and 2). It has been shown in
prostate cancers that upregulation of ASAH1 (following radiation)
is mediated by radiation-induced c-Jun/AP-1, transcription factors
that have been implicated in the DNA-repair pathway (15). ASAH1 is the principal, rate-limiting
enzyme that metabolizes ceramides into sphingosine (3,26,27).
As expected from this mechanism, ceramide levels were decreased in
cells containing a high level of ASAH1, as shown in U87-10gy and
SJGBM2-10gy cells. However, Mahdy et al reported that
upregulation of ASAH1 in prostate cancer cells did not result in
lower ceramide levels (14). This
discrepancy can be due to the timing of the measurement following
radiation. In their study, the sphingolipid analysis was performed
within hours following radiation; on the other hand, in our study,
the analysis was performed once survived cells grew to confluence,
a process that took approximately one month. Following radiation,
most cells died within one week; <1% of cells survived ionizing
radiation and grew to confluence after a month of culture. The data
suggest that only cells that express a high level of ASAH1 could
survive radiation. Since Mahdy et al performed sphingolipid
analysis within hours following radiation, their results likely
included cells that would not survive radiation long-term (those
that contained lower levels of ASAH1 and higher levels of
ceramides). With our study, the longer time interval selected out
these cells (as they died within 1 week), where final analysis
involved only cells that survived radiation.
To confirm the upregulation of ASAH1 and Sph-1P as a
mechanism of radioresistance, we performed western blotting and IHC
on GBM cell lines and patient GBM tissues. IHC staining of both U87
and U87-10gy cells with humanized anti-Sph-1P revealed increased
levels of Sph-1P in irradiated U87-10gy (Fig. 3). Similar to the western blot data,
ASAH1 IHC analysis of four different sets of data from the same
patient (pre- and post-radiation GBM specimens) confirmed the
upregulation of ASAH1 in post-radiation samples, ranging from 1.5-
to 60-fold higher in staining intensity as assessed by ImageJ
(Fig. 4). This finding was further
supported by data showing a significantly lower Allred median ASAH1
staining score for non-irradiated GBMs in comparison to radiated
GBM samples (Fig. 8). Consistent
with previous data (13), we showed
that irradiated GBMs also have a higher protein expression of CD133
than non-irradiated GBMs. Given the concomitant high expression
level of ASAH1 in irradiated GBMs, this raises the possibility that
CD133+ cells or CSCs are the cells that survive
radiation and overexpress ASAH1, as shown in western blot and IHC
studies (Fig. 4A). These results
indicate that the U87-10gy cell line is a potential,
clinically-relevant model to study recurrent GBMs, especially in
studies that target the sphingolipid metabolism pathway.
ASAH1 was shown in this study to be secreted into
the extracellular space (Figs. 4,
5, and 8), which is consistent with other reports
that document secretion of Sph-1P into the extracellular space as
well (21,26,28,29).
Consequently, cancer cells with increased secretion of ASAH1 and
Sph-1P create a tumor microenvironment that favors cancer survival
by virtue of the ASAH1 and Sph-1P known tumor-promoting functions
(21,27,29–31).
In support of this microenvironment theory, we demonstrated that
media from SJGBM2-10gy cells, which secreted a high amount of
ASAH1, promoted 50% more cell growth than media from SJGBM2 cells
that contained a lower amount of secreted ASAH1 (Fig. 5). In addition, staining of
irradiated GBMs also demonstrated significant ASAH1 staining in the
extracellular space, suggesting that irradiated GBMs also secrete
ASAH1 into the extracellular space (Figs. 4 and 8). The presence of tumor promoters ASAH1
and Sph-1P outside the intracellular space provides a unique
opportunity to target these molecules with antibodies. Employing
this strategy, we found that treatment of U87-10gy cells with
anti-ASAH1 antibody reduced cell growth by 50% (Fig. 6). A similar 50% reduction in cell
growth was observed in U87-10gy treated with the humanized
anti-Sph-1P antibody (Fig. 6). This
reduction in cell growth is likely attributed to the ability of
antibodies to disrupt the roles that ASAH1 and Sph-1 have in the
promotion of cell growth and survival (3,21,28,29,31–33).
The benefit of an anti-ASAH1 antibody was clearly displayed in a
serum autoantibody profiling study of patients with melanoma
(34).
This study found that melanoma patients who
developed auto anti-ASAH1 antibody were protected from lymph node
metastasis. The study even suggested that upregulation of auto
anti-ASAH1 antibody may play an important preventative role in
melanoma metastasis, and the loss of this antibody may result in
melanoma progression (34). With
regards to the benefit of anti-Sph-1P antibody, multiple animal
studies have shown that anti-Sph-1P antibody can neutralize the
ability of Sph-1P to induce cell proliferation, promote
angiogenesis, and protect tumor cells from apoptosis in several
tumor cell lines (16,35). In addition, Sph-1P has also been
shown to be an important player in promoting malignancy in GBMs.
Results from a previous study indicated that GBM malignancy is
associated with an increased drive of the pathway that converts
ceramide to Sph-1P (36). To
explore the potential therapeutic benefit of inhibiting ASAH1
activity, we examined the effect of carmofur on cell survival.
Carmofur decreased U87, SJGBM2, U87-10gy and SJGBM-10gy cell
viability with IC50 values of 37, 50, 28, and 21 µM,
respectively (Fig. 7). Irradiated
cells are more sensitive to carmofur than their non-irradiated
counterparts, possibly due to higher expression levels of ASAH1 in
the former (Fig. 7). Treatment of
U87-10gy cells with carmofur increased ceramide levels (Fig. 7).
In conclusion, GBM is a highly malignant tumor.
Radiation is a mainstay treatment option. Despite aggressive
management, the pathology inevitably recurs and/or progresses. This
study provides an explanation as to why radiation treatments of GBM
often have limited success, mainly due to the upregulation of ASAH1
and Sph-1P, leading to resistance to radiation. This study
identifies ASAH1 and Sph-1P as excellent drug targets that can be
taken advantage of to improve outcome. Inhibition of ASAH1 and
Sph-1P, with antibodies, small molecule drugs (carmofur), or a
combination of both, could represent an innovative clinical
approach for treating GBMs, especially for ASAH1-overexpressed
recurrent GBMs.
Acknowledgements
This study was funded by the Musella Foundation
Grant and Department of Neurosurgery Larson Endowment Grant.
References
|
1
|
Mao C and Obeid LM: Ceramidases:
Regulators of cellular responses mediated by ceramide, sphingosine,
and sphingosine-1-phosphate. Biochim Biophys Acta. 1781:424–434.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vethakanraj HS, Babu TA, Sudarsanan GB,
Duraisamy PK and Kumar Ashok S: Targeting ceramide metabolic
pathway induces apoptosis in human breast cancer cell lines.
Biochem Biophys Res Commun. 464:833–839. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu X, Elojeimy S, Turner LS, Mahdy AE,
Zeidan YH, Bielawska A, Bielawski J, Dong JY, El-Zawahry AM, Guo
GW, et al: Acid ceramidase inhibition: a novel target for cancer
therapy. Front Biosci. 13:2293–2298. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Seelan RS, Qian C, Yokomizo A, Bostwick
DG, Smith DI and Liu W: Human acid ceramidase is overexpressed but
not mutated in prostate cancer. Genes Chromosomes Cancer.
29:137–146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roh JL, Park JY, Kim EH and Jang HJ:
Targeting acid ceramidase sensitises head and neck cancer to
cisplatin. Eur J Cancer. 52:163–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Realini N, Palese F, Pizzirani D, Pontis
S, Basit A, Bach A, Ganesan A and Piomelli D: Acid ceramidase in
melanoma: Expression, localization and effects of pharmacological
inhibition. J Biol Chem. 291:2422–2434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Morales A, París R, Villanueva A, Llacuna
L, García-Ruiz C and Fernández-Checa JC: Pharmacological inhibition
or small interfering RNA targeting acid ceramidase sensitizes
hepatoma cells to chemotherapy and reduces tumor growth in vivo.
Oncogene. 26:905–916. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morad SA, Messner MC, Levin JC,
Abdelmageed N, Park H, Merrill AH Jr and Cabot MC: Potential role
of acid ceramidase in conversion of cytostatic to cytotoxic
end-point in pancreatic cancer cells. Cancer Chemother Pharmacol.
71:635–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: European Organisation for Research and Treatment of
Cancer Brain Tumor and Radiotherapy Groups; National Cancer
Institute of Canada Clinical Trials Group: Radiotherapy plus
concomitant and adjuvant temozolomide for glioblastoma. N Engl J
Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iwamoto FM, Abrey LE, Beal K, Gutin PH,
Rosenblum MK, Reuter VE, DeAngelis LM and Lassman AB: Patterns of
relapse and prognosis after bevacizumab failure in recurrent
glioblastoma. Neurology. 73:1200–1206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wong ET, Hess KR, Gleason MJ, Jaeckle KA,
Kyritsis AP, Prados MD, Levin VA and Yung WK: Outcomes and
prognostic factors in recurrent glioma patients enrolled onto phase
II clinical trials. J Clin Oncol. 17:2572–2578. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lamborn KR, Yung WK, Chang SM, Wen PY,
Cloughesy TF, DeAngelis LM, Robins HI, Lieberman FS, Fine HA, Fink
KL, et al: North American Brain Tumor Consortium: Progression-free
survival: An important end point in evaluating therapy for
recurrent high-grade gliomas. Neuro Oncol. 10:162–170. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mahdy AE, Cheng JC, Li J, Elojeimy S,
Meacham WD, Turner LS, Bai A, Gault CR, McPherson AS, Garcia N, et
al: Acid ceramidase upregulation in prostate cancer cells confers
resistance to radiation: AC inhibition, a potential
radiosensitizer. Mol Ther. 17:430–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng JC, Bai A, Beckham TH, Marrison ST,
Yount CL, Young K, Lu P, Bartlett AM, Wu BX, Keane BJ, et al:
Radiation-induced acid ceramidase confers prostate cancer
resistance and tumor relapse. J Clin Invest. 123:4344–4358. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Visentin B, Vekich JA, Sibbald BJ, Cavalli
AL, Moreno KM, Matteo RG, Garland WA, Lu Y, Yu S, Hall HS, et al:
Validation of an anti-sphingosine-1-phosphate antibody as a
potential therapeutic in reducing growth, invasion, and
angiogenesis in multiple tumor lineages. Cancer Cell. 9:225–238.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O'Brien N, Jones ST, Williams DG,
Cunningham HB, Moreno K, Visentin B, Gentile A, Vekich J,
Shestowsky W, Hiraiwa M, et al: Production and characterization of
monoclonal anti-sphingosine-1-phosphate antibodies. J Lipid Res.
50:2245–2257. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
19
|
Bielawski J, Szulc ZM, Hannun YA and
Bielawska A: Simultaneous quantitative analysis of bioactive
sphingolipids by high-performance liquid chromatography-tandem mass
spectrometry. Methods. 39:82–91. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Visentin B, Reynolds G and Sabbadini R:
Immunohistochemical detection of sphingosine-1-phosphate and
sphingosine kinase-1 in human tissue samples. Methods Mol Biol.
874:55–67. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogretmen B and Hannun YA: Biologically
active sphingolipids in cancer pathogenesis and treatment. Nat Rev
Cancer. 4:604–616. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Realini N, Solorzano C, Pagliuca C,
Pizzirani D, Armirotti A, Luciani R, Costi MP, Bandiera T and
Piomelli D: Discovery of highly potent acid ceramidase inhibitors
with in vitro tumor chemosensitizing activity. Sci Rep. 3:10352013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lacroix M, Abi-Said D, Fourney DR,
Gokaslan ZL, Shi W, DeMonte F, Lang FF, McCutcheon IE, Hassenbusch
SJ, Holland E, et al: A multivariate analysis of 416 patients with
glioblastoma multiforme: Prognosis, extent of resection, and
survival. J Neurosurg. 95:190–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ballman KV, Buckner JC, Brown PD, Giannini
C, Flynn PJ, LaPlant BR and Jaeckle KA: The relationship between
six-month progression-free survival and 12-month overall survival
end points for phase II trials in patients with glioblastoma
multiforme. Neuro-oncol. 9:29–38. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hou LC, Veeravagu A, Hsu AR and Tse VC:
Recurrent glioblastoma multiforme: A review of natural history and
management options. Neurosurg Focus. 20:E52006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hannun YA and Obeid LM: Principles of
bioactive lipid signalling: Lessons from sphingolipids. Nat Rev Mol
Cell Biol. 9:139–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Park JH and Schuchman EH: Acid ceramidase
and human disease. Biochim Biophys Acta. 1758:2133–2138. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ségui B, Andrieu-Abadie N, Jaffrézou JP,
Benoist H and Levade T: Sphingolipids as modulators of cancer cell
death: Potential therapeutic targets. Biochim Biophys Acta.
1758:2104–2120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Young N and Van Brocklyn JR: Roles of
sphingosine-1-phosphate (S1P) receptors in malignant behavior of
glioma cells. Differential effects of S1P2 on cell migration and
invasiveness. Exp Cell Res. 313:1615–1627. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Johnson IR, Parkinson-Lawrence EJ, Butler
LM and Brooks DA: Prostate cell lines as models for biomarker
discovery: Performance of current markers and the search for new
biomarkers. Prostate. 74:547–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Young N, Pearl DK and Van Brocklyn JR:
Sphingosine-1-phosphate regulates glioblastoma cell invasiveness
through the urokinase plasminogen activator system and CCN1/Cyr61.
Mol Cancer Res. 7:23–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pettus BJ, Chalfant CE and Hannun YA:
Ceramide in apoptosis: An overview and current perspectives.
Biochim Biophys Acta. 1585:114–125. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zeidan YH, Jenkins RW, Korman JB, Liu X,
Obeid LM, Norris JS and Hannun YA: Molecular targeting of acid
ceramidase: Implications to cancer therapy. Curr Drug Targets.
9:653–661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu Y, He J, Xie X, Su G, Teitz-Tennenbaum
S, Sabel MS and Lubman DM: Serum autoantibody profiling using a
natural glycoprotein microarray for the prognosis of early
melanoma. J Proteome Res. 9:6044–6051. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Wang X, Bullock AJ, Callea M,
Shah H, Song J, Moreno K, Visentin B, Deutschman D, Alsop DC, et
al: Anti-S1P antibody as a novel therapeutic strategy for VEGFR
TKI-resistant renal cancer. Clin Cancer Res. 21:1925–1934. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Abuhusain HJ, Matin A, Qiao Q, Shen H,
Kain N, Day BW, Stringer BW, Daniels B, Laaksonen MA, Teo C, et al:
A metabolic shift favoring sphingosine 1-phosphate at the expense
of ceramide controls glioblastoma angiogenesis. J Biol Chem.
288:37355–37364. 2013. View Article : Google Scholar : PubMed/NCBI
|