Introduction
Fibronectin (FN) is one of the most abundant
adhesive glycoproteins of the extracellular matrix and acts as a
substrate for cell adhesion during wound healing and metastasis
(1). The complex of integrin and FN
can have modulating effects on various signaling pathways including
focal adhesion kinase (FAK), signal transducer and activator of
transcription-3 (STAT-3), and mitogen-activated protein kinase
(MAPK) pathways that promote cell invasion and metastasis in
multiple cancer cells (2–4). The induction of FN by HER2
overexpression triggers cell adhesion and invasion capacities in
breast cancer cells (5). In
addition, excessive FN expression is linked to poor metastatic-free
survival and overall survival in breast cancer patients (6,7).
The tumor suppressor protein p53 is a transcription
factor that can trigger cell cycle arrest, replicative senescence,
or apoptosis (8). The induction of
p53 expression by a wide variety of cellular stresses such as
hypoxia, UV, and cytotoxic drugs is involved in accelerated
cellular senescence as well as G1/S and G2/M cell cycle checkpoint
activation (9–11). The TP53 gene encoding p53 is
the most frequently inactivated gene in human malignancy, and
somatic missense mutations of p53 are found in ~50% of human
cancers (12). Mutations in the
TP53 gene result in loss of wild-type p53 tumor suppressor
functions (13). Wild-type p53
upregulates p21, the negative regulator of cyclin-dependent kinases
(cdks), whereas mutant p53 enhances cdk1 and increases cell
proliferation and survival (13).
The effect of p53 status on FN in breast cancer
cells is not fully understood. In this study, we investigated the
role of p53 on FN expression in p53 wild-type and mutant human
breast cancer cells. Specifically, we examined how p53 expression
is regulated by the tumor promoter TPA and whether the alteration
of p53 expression by TPA affects FN expression. We found that the
level of wild-type p53 expression regulated the basal and
TPA-induced FN expression levels in breast cancer cells.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM) was
purchased from Thermo Scientific (Hemel Hempstead, UK). Fetal
bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA).
Phenol red-free DMEM, penicillin (100 U/ml), and 100 mg/ml
streptomycin were purchased from Life Technologies (Rockville, MD,
USA). MG132 was purchased from Sigma-Aldrich (St. Louis, MO, USA),
UO126, SP600125, and Stattic were purchased from Tocris
(Ellisville, MO, USA). RITA (p53 activator III), secondary
horseradish peroxidase (HRP)-conjugated antibodies, and mouse
monoclonal anti-β-actin antibody were purchased from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-fibronectin was
purchased from Abcam (Cambridge, UK). Antibodies against total (t)
and phospho (p) ERK, JNK, and STAT3 were purchased from Cell
Signaling Technology (Beverly, MA, USA). ECL Western Blotting
Detection reagents (West-Q Chemiluminescent Substrate Plus kit)
were obtained from Genedepot (Barker, TX, USA).
Cell culture
MCF7 (p53 wild-type), Hs578T (p53 mutant V157F), and
BT549 (p53 mutant R249S) human breast cancer cells were grown in a
humidified atmosphere of 95% air and 5% CO2 at 37°C in
DMEM supplemented with 10% FBS, 2 mM glutamine, 100 IU/ml
penicillin, and 100 µg/ml streptomycin. Each cell line was
maintained in culture medium without FBS for 24 h before
experiments.
Western blotting
To determine the expression of secreted FN in
culture media, we analyzed equal aliquots of conditioned culture
media from equal numbers of cells. Cell lysates were prepared to
detect total (t) and phosphor (p)-ERK, -JNK, and -STAT3, FN, p53,
and β-actin expression. Equal amounts of protein (50 µg) were
boiled for 5 min in Laemmli sample buffer and then electrophoresed
in 10% sodium dodecyl sulfate polyacrylamide (SDS-PAGE) gels.
Separated proteins were transferred to polyvinylidene fluoride
(PVDF) membranes, and the membranes were blocked with 10% skim milk
in Tris-buffered saline (TBS) containing 0.01% Tween-20 (TBS/T) for
15 min. Blots were washed three times in TBS/T and then incubated
with antibodies against t- or p-ERK, p-JNK, and p-STAT3, FN, p53,
and β-actin in TBS/T buffer at 4°C overnight. Blots were washed
three times in TBS/T and subsequently incubated with secondary
HRP-conjugated antibodies (1:2,000 dilutions) in TBS/T buffer.
After 1-h incubation at room temperature (RT), blots were washed
three times in TBS/T, and positive immunoreactive proteins were
detected using the West-Q Chemiluminescent Substrate Plus kit.
Real-time PCR
Total RNA was extracted from cells using TRIzol
reagent (Invitrogen, Carlsbad, CA, USA) according to the
manufacturer's protocol. Isolated RNA samples were then used for
RT-PCR. Samples of total RNA (1 µg) were reverse transcribed into
cDNA in 20-µl reaction volumes using a first-strand cDNA synthesis
kit for RT-PCR, according to the manufacturer's instructions (MBI
Fermentas, Hanover, MD, USA). Gene expression levels were
quantified by real-time PCR using a SensiMix SYBR kit (Bioline
Ltd., London, UK) and 100 ng of cDNA per reaction. The primer
sequences used for this analysis were as follows: human p53
(forward, 5′-GGCCCACTTCACCGTACTAA-3′; reverse,
5′-AAGCGAGACCCAGTCTCAAA-3′); human FN (forward,
5′-CCACCCCCATAAGGCATAGG-3′; reverse,
5′-GTAGGGGTCAAAGCACGAGTCATC-3′), and GAPDH as an internal control
(forward, 5′-ATTGTTGCCATCAATGACCC-3′; reverse,
5′-AGTAGAGGCAGGGATGATGT-3′). An annealing temperature of 60°C was
used for all primers. PCR was performed in a standard 384-well
plate format with an ABI 7900HT real-time PCR detection system
(Foster City, CA, USA). For data analysis, the raw threshold cycle
(CT) value was first normalized to the
housekeeping gene for each sample to obtain a
∆CT. The normalized ∆CT was
then calibrated to control cell samples and obtain
∆∆CT values.
Adenovirus induction
Adenovirus expressing Lac Z and human p53 cDNA
(Ad-p53) was a gift from Dr Hyunil Ha (Korea Institute of Oriental
Medicine, Daejeon, Korea). Recombinant adenovirus expressing human
p53 was reproduced in 293A cells. Expression of this construct was
confirmed by western blotting. Each construct was transfected into
Hs578T and BT549 cells for 24 h and incubated for 24 h in fresh
culture media. Ad-p53-overexpressing Hs578T and BT549 cells were
further incubated for 24 h in serum-free culture media, and then
cell lysates and culture medium were harvested for analysis of FN
and p53 expression.
Statistical analysis
Statistical significance was determined using
Student's t-test. Results are presented as means ± SEM. All quoted
P-values are two-tailed, and differences were considered
statistically significant when the P-value was <0.05.
Statistical analyses were performed using Microsoft Excel.
Results
Fibronectin expression is regulated by
p53 in breast cancer cells
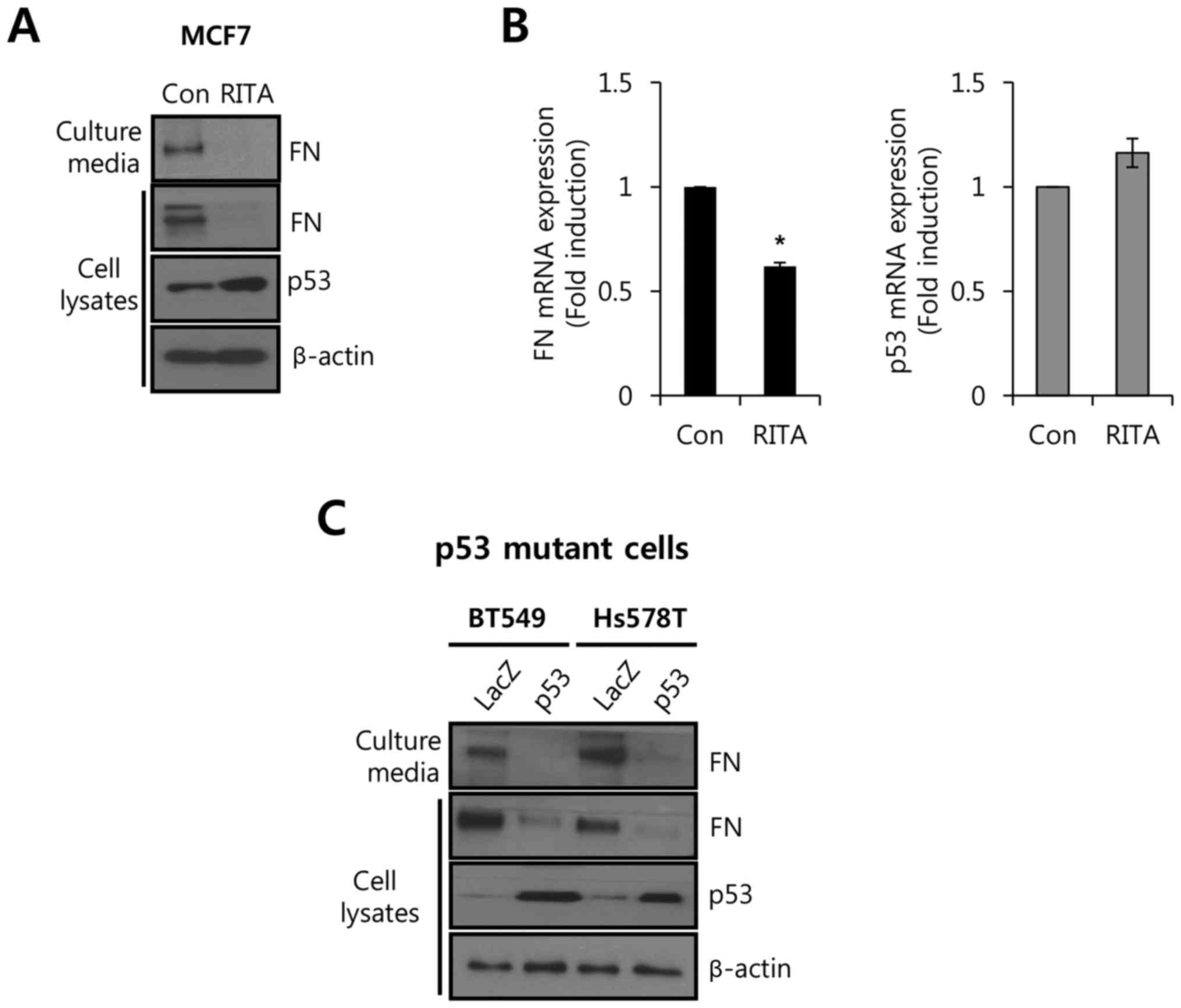
As shown in Table I,
the p53 status of breast cancer cell lines was determined from the
database http://p53.free.fr/Database/Cancer_cell_lines/Breast_cancer.html,
and p53 wild-type breast cancer cells (MCF7) and p53-mutant breast
cancer cells (Hs578T and BT549) were selected to verify the
relationship between p53 and FN expression. First, we treated MCF7
breast cancer cells with wild-type p53 and the p53 activator III
RITA to disrupt the MDM2-p53 complex. After 24 h, we harvested cell
culture media and whole cell lysates for analysis of FN and p53
expression. As shown in Fig. 1A,
the level of FN protein expression was decreased by RITA treatment
in both culture media and cell lysates. However, the basal level of
p53 protein expression was slightly increased (Fig. 1A). Under the same conditions, we
analyzed the levels of FN and p53 mRNA expression. As expected, the
level of FN mRNA expression was decreased 0.62±0.02-fold by RITA
relative to the control (Fig.
1B).
 | Table I.p53 status in breast cancer cells. |
Table I.
p53 status in breast cancer cells.
| Codon position | Normal (amino
acid) | Mutant (amino
acid) | Cell name |
|---|
| – | – | – | MCF7 |
| 157 | GTC (Val) | TTC (Phe) | Hs578T |
| 249 | AGG (Arg) | AGC (Ser) | BT549 |
Next, we transfected p53-mutant breast cancer cells
BT549 and Hs578T with adenovirus expressing wild-type p53. As shown
in Fig. 1C, the level of FN protein
expression was significantly decreased by p53 overexpression in
both BT549 and Hs578T cells. These results demonstrated that
wild-type p53 expression directly influenced FN expression in
breast cancer cells.
TPA reciprocally regulated the levels
of FN and p53 expression in breast cancer cells
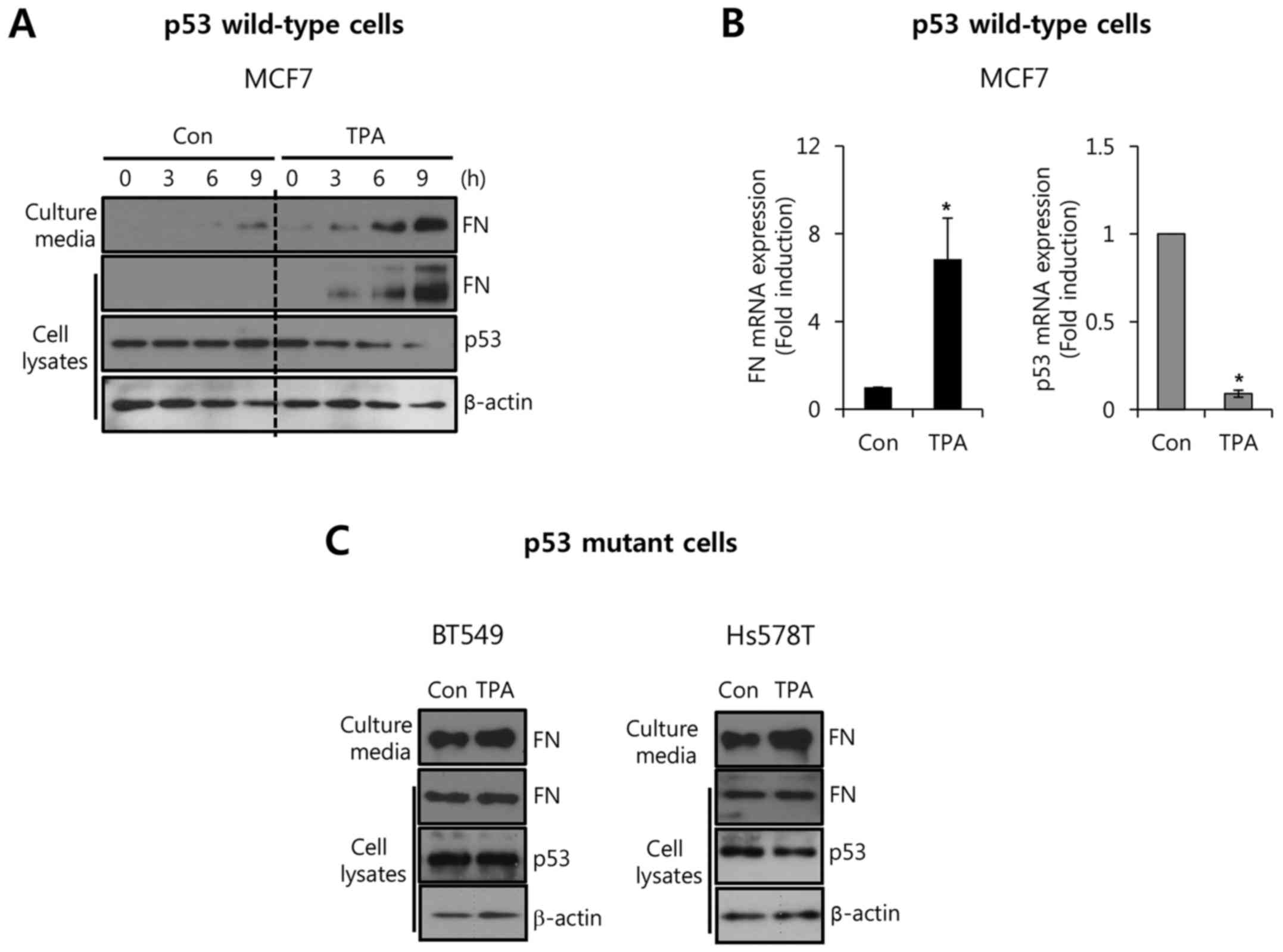
To verify the relationship between p53 and FN
expression, we treated breast cancer cells with 10 nM TPA for the
indicated time. As shown in Fig.
2A, basal FN expression in MCF7 cells was increased by TPA
treatment in a time-dependent manner. In contrast, the level of p53
expression was decreased from 3 h after TPA treatment (Fig. 2A). TPA also upregulated FN
expression in ZR75-1 cells with wild-type p53 (data not shown). In
addition, the level of FN mRNA expression was increased by TPA,
whereas the level of p53 mRNA expression was decreased (Fig. 2B). The level of FN mRNA expression
was significantly increased 6.84±1.89-fold by treatment with 10 nM
TPA compared with the control level (Fig. 2B). As expected, the level of the p53
mRNA expression decreased 0.09±0.02-fold compared with the control
after treatment with 10 nM TPA (Fig.
2B). However, TPA did not affect the level of FN or p53
expression in p53-mutant breast cancer cells (Fig. 2C). These results demonstrated that
TPA reciprocally regulated the levels of FN and p53 expression in
breast cancer cells.
Upregulation of FN and downregulation
of p53 by TPA are mediated through an MEK/ERK-dependent
pathway
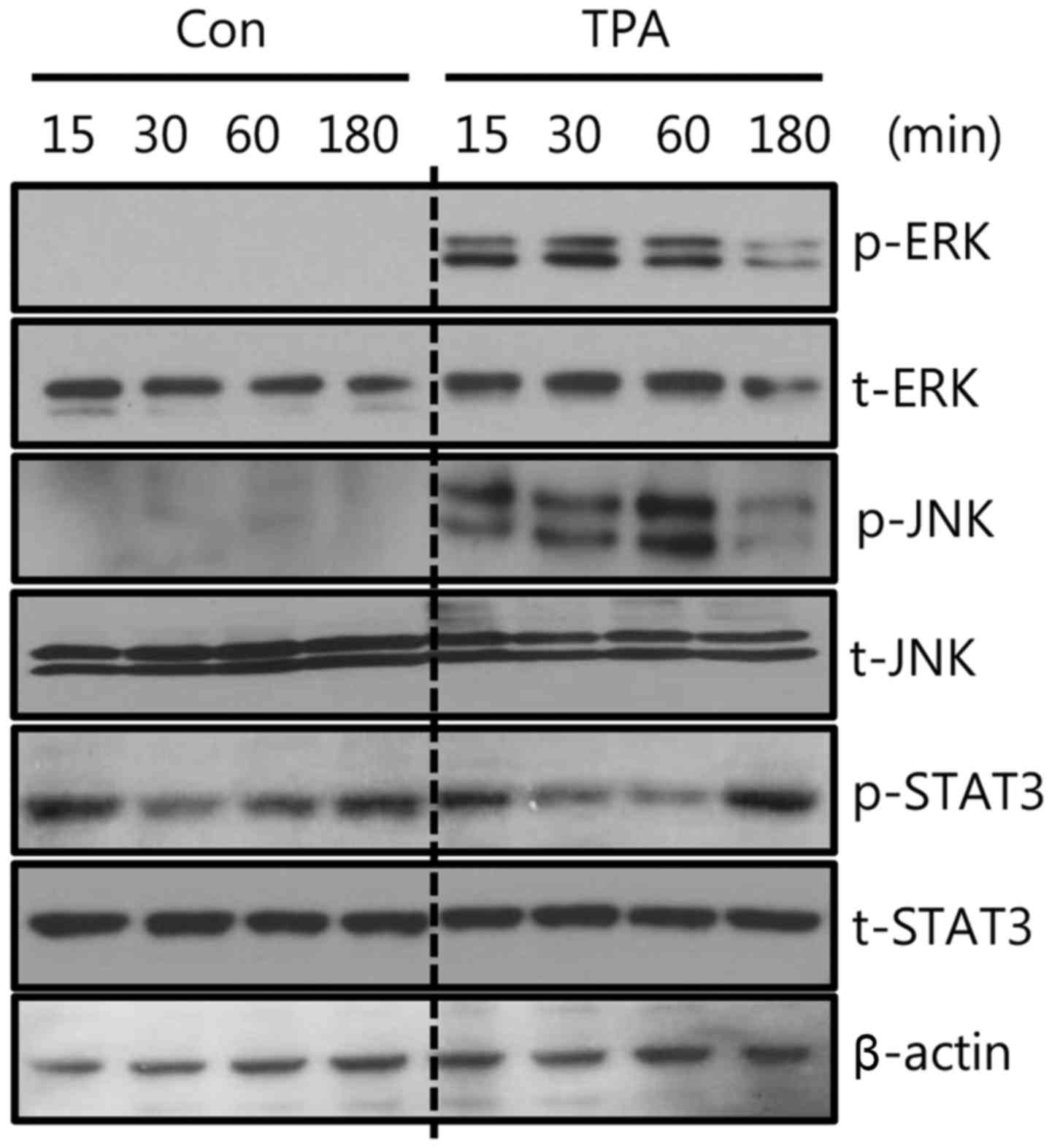
To verify the regulatory mechanism of FN and p53
expression by TPA treatment, we examined the effect of TPA on the
phosphorylation of signaling molecules ERK, JNK, and STAT3. As
shown in Fig. 3, the
phosphorylation of ERK and JNK was significantly increased by TPA
treatment, whereas STAT3 phosphorylation was not affected.
Next, we investigated whether the MEK/ERK pathway is
directly associated with the TPA-induced FN upregulation and p53
downregulation. After pretreatment with specific inhibitors for 30
min, the cells were treated with 10 nM TPA for 24 h. As shown in
Fig. 4A, TPA-induced FN
upregulation and p53 downregulation were reversed by the
MEK1/2-specific inhibitor UO126 at the level of protein expression.
Under the same conditions, the TPA-induced FN mRNA expression was
also decreased by UO126. The level of FN mRNA increased
3.95±0.34-fold relative to the control level after treatment with
10 nM TPA treatment, and this effect was suppressed to
1.39±0.06-fold by treatment with 5 µM UO126 (Fig. 4B). In contrast, p53 mRNA expression
was decreased to 0.89±0.01-fold relative to the control level by
TPA, and this TPA-induced downregulation of p53 mRNA was reversed
to 1.34±0.22-fold relative to the control by treatment with 5 µM
UO126 (Fig. 4B). However, JNK or
STAT3 signaling pathway did not affect TPA-induced FN expression
(Fig. 4). These results
demonstrated that the upregulation of FN and downregulation of p53
by TPA are mediated by the MEK/ERK-dependent pathway.
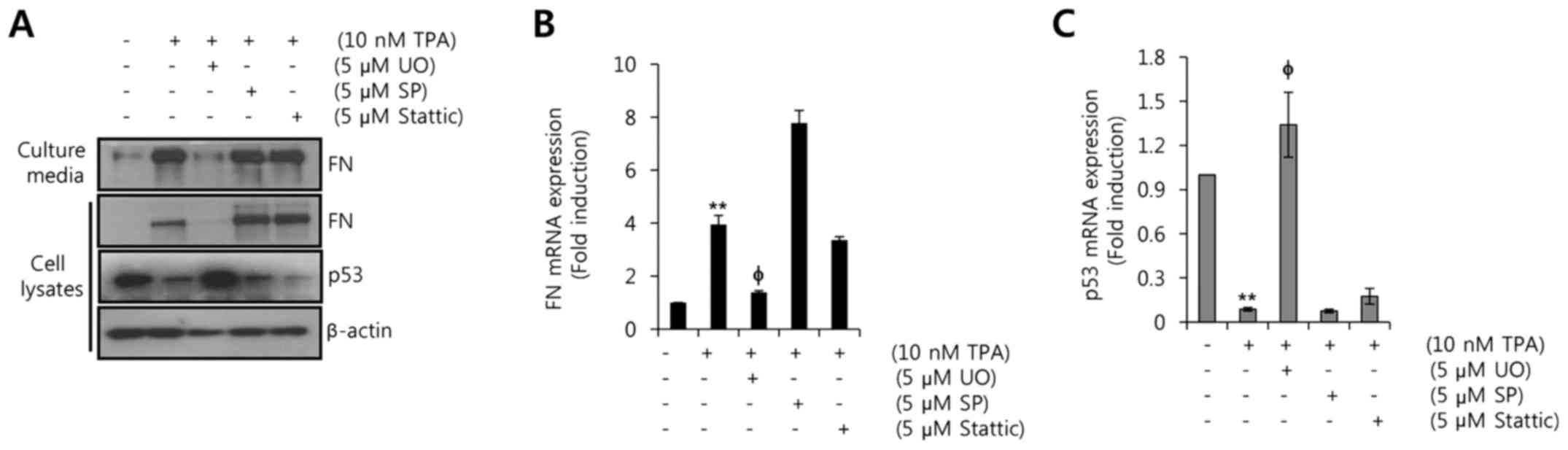 | Figure 4.Upregulation of FN and downregulation
of p53 by TPA are mediated by the MEK/ERK-dependent pathway. After
serum starvation for 24 h, MCF7 cells were pretreated with 5 µM UO,
SP, and Stattic, respectively, for 30 min and then treated with 10
nM TPA for 24 h. (A) Levels of FN, p53, and β-actin protein
expression were analyzed by western blotting. (B and C) Levels of
FN and p53 mRNA expression were analyzed by real-time PCR. The
results are representative of three independent experiments. Values
shown are mean ± SEM. **P<0.01 vs. control;
φP<0.05 vs. TPA-treated cells. Con, control; UO,
UO126; SP, SP600125. |
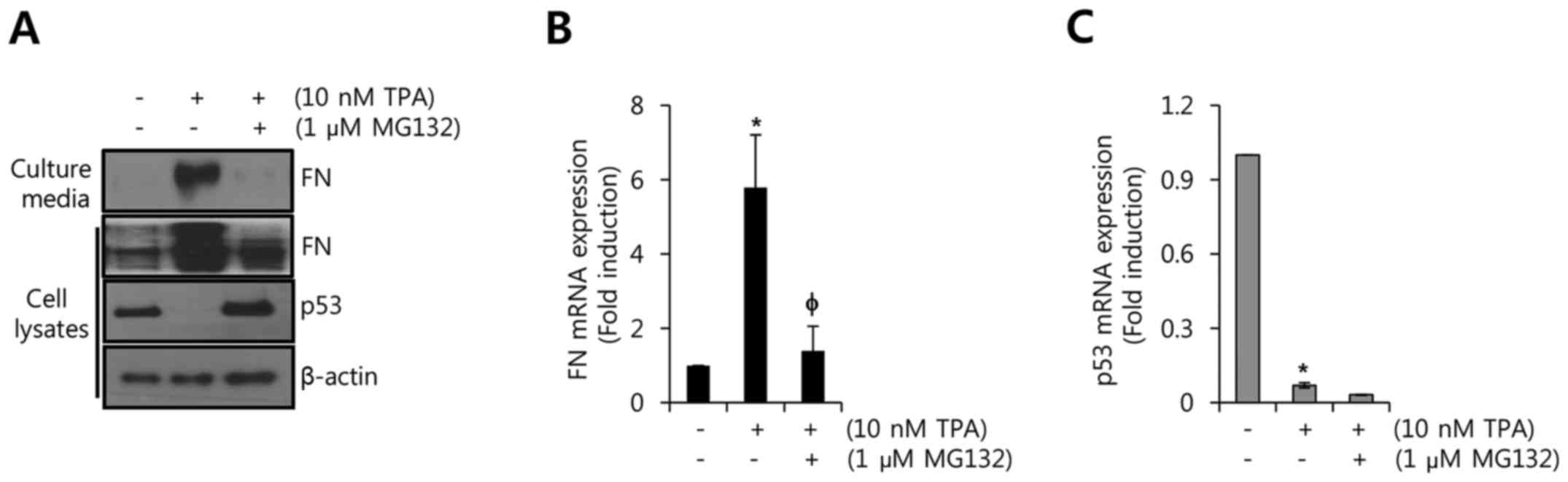
Degradation of p53 by TPA upregulates
the level of FN expression in MCF7 cells with wild-type p53
In a previous study, TPA stimulated the
ubiquitination and degradation of p53 through downregulation of
PKC-δ (14). We therefore
investigated whether TPA-induced FN expression is regulated by the
degradation of p53 in MCF7 cells. As shown in Fig. 5A, TPA-induced FN protein expression
was significantly decreased by MG132, a proteasomal inhibitor.
Conversely, the reduction of p53 protein by TPA was reversed by
MG132 (Fig. 5A). Furthermore, we
investigated the levels of FN and p53 mRNA expression after
treatment with TPA and/or MG132. As expected, TPA-induced FN mRNA
expression was decreased to 1.40±0.67-fold relative to the control
(Fig. 5B). However, the TPA-induced
reduction of p53 mRNA expression was not recovered by MG132
(Fig. 5C). Based on our results, we
conclude that TPA triggers the degradation and transcriptional
suppression of p53. In addition, the alteration of p53 level by TPA
is associated with the level of FN expression in p53 wild-type
breast cancer cells.
Discussion
FN is markedly upregulated in the malignant breast
cancer cells but not in non-malignant breast epithelial cells
(15,16). Abnormal FN expression in carcinoma
cells is associated with tumor aggressiveness and poor prognoses in
patients with invasive breast cancer (6,7). The
binding of FN/α5β1 integrin augments the invasiveness of breast and
ovarian cancer cells through c-Met/FAK/Src signaling pathway
(15,17). In addition, FN contributes to
endocrine resistance through interaction with β1 integrin and then
stimulates PI3K/Akt and MAPK/ERK1/2 signaling pathway in ER-α
positive breast cancer cells (18).
Recently, we also reported that the FN-induced adhesion and
invasion characteristics of breast cancer cells are suppressed by
an FN inhibitor (RGD tetrapeptide) (5). Here, we explored whether p53 regulates
FN expression in breast cancer cells.
p53 is a tumor suppressor gene that is stimulated by
cellular stress including hypoxia, ultraviolet radiation, and
oxidative stress (9–11). In addition, p53 is a known
transcription factor that induces the expression of various genes
associated with apoptosis, cell cycle arrest, and DNA repair
(19). RITA (reactivation of p53
and induction of tumor cell apoptosis) is a small molecule that
binds to the N-terminus of the p53 protein and thereby induces
conformational changes within the molecule that prevent its
association with MDM2 (20,21). RITA can activate p53 downstream
targets in both p53 wild-type and p53-mutant cells in a variety of
cancers such as breast, fibrosarcoma, colon, and lung carcinoma
(22–24). In accordance with these reports, we
found that activation of p53 by RITA stimulated the downregulation
of FN expression in breast cancer cells with wild-type p53. In
addition, the basal level of FN expression was decreased by
overexpression of wild-type p53 in p53-mutant breast cancer cells.
Therefore, we demonstrated that the level of wild-type p53
expression plays an important role in FN expression in breast
cancer cells.
Phorbol esters such as TPA are well-known tumor
promoters and augment tumor invasion and migration through the
induction of MMP-1 and MMP-9 (25–27).
TPA treatment is directly correlated with increase in p53 and PTEN
nuclear translocation (28). We
found that TPA time-dependently decreased the level of p53
expression in breast cancer cells with wild-type p53, but not in
p53-mutant breast cancer cells. Previously, Abbas et al
reported that the transcriptional regulation of p53 by TPA
treatment is mediated by protein kinase Cδ (14). In this study, we found that UO126 (a
specific MEK inhibitor) controls the downregulation of p53 and
upregulation of FN by TPA treatment in wild-type p53 cells. In
addition, we also observed that JNK or STAT3 signaling pathway
could not regulate TPA-induced FN expression. Therefore, our data
demonstrate that the transcriptional level of p53 in response to
TPA is mediated by an MEK/ERK-dependent pathway in p53 wild-type
breast cancer cells.
In response to DNA damage, p53 protein is
post-translationally modified, triggering its degradation through
the ubiquitin proteasome pathway (29). Rottlerin, a PKC-δ inhibitor, is able
to prevent p53 accumulation induced by proteasome inhibition in
ML-1 human thyroid cancer cells (14). Therefore, we investigated the effect
of the proteasome inhibitor MG132 on the TPA-induced reduction of
p53 and induction of FN expression in p53 wild-type breast cancer
cells. As expected, our results showed that TPA-induced p53
degradation was prevented by proteasome inhibition. In contrast,
TPA-induced FN expression was significantly decreased by MG132
treatment. These data demonstrate that the level of wild-type p53
expression is directly involved in FN expression in breast cancer
cells.
In conclusion, wild-type p53 expression directly
regulates the level of FN expression in breast cancer cells. Our
results showed that RITA suppresses the levels of FN mRNA and
protein expression. In addition, overexpression of wild-type p53
decreases FN expression in p53-mutant breast cancer cells. We also
showed that TPA triggers MEK/ERK-dependent p53 transcriptional
suppression and proteasomal-dependent degradation. Furthermore, the
TPA-induced downregulation of p53 is associated with the
upregulation of FN in p53 wild-type breast cancer cells. Therefore,
we demonstrate that wild-type p53 expression is directly associated
with the level of FN expression in breast cancer cells.
Acknowledgements
This study was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education (2016R1D1A1B01010508) and
by the National Research Foundation of Korea (NRF) Grant funded by
the Korean Government (MSIP) (2016R1A5A2945889).
References
|
1
|
Kornblihtt AR and Gutman A: Molecular
biology of the extracellular matrix proteins. Biol Rev Camb Philos
Soc. 63:465–507. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balanis N, Wendt MK, Schiemann BJ, Wang Z,
Schiemann WP and Carlin CR: Epithelial to mesenchymal transition
promotes breast cancer progression via a fibronectin-dependent
STAT3 signaling pathway. J Biol Chem. 288:17954–17967. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guan JL: Role of focal adhesion kinase in
integrin signaling. Int J Biochem Cell Biol. 29:1085–1096. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qian P, Zuo Z, Wu Z, Meng X, Li G, Wu Z,
Zhang W, Tan S, Pandey V, Yao Y, et al: Pivotal role of reduced
let-7g expression in breast cancer invasion and metastasis. Cancer
Res. 71:6463–6474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jeon M, Lee J, Nam SJ, Shin I, Lee JE and
Kim S: Induction of fibronectin by HER2 overexpression triggers
adhesion and invasion of breast cancer cells. Exp Cell Res.
333:116–126. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernandez-Garcia B, Eiró N, Marín L,
González-Reyes S, González LO, Lamelas ML and Vizoso FJ: Expression
and prognostic significance of fibronectin and matrix
metalloproteases in breast cancer metastasis. Histopathology.
64:512–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bae YK, Kim A, Kim MK, Choi JE, Kang SH
and Lee SJ: Fibronectin expression in carcinoma cells correlates
with tumor aggressiveness and poor clinical outcome in patients
with invasive breast cancer. Hum Pathol. 44:2028–2037. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woods DB and Vousden KH: Regulation of p53
function. Exp Cell Res. 264:56–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugrue MM, Shin DY, Lee SW and Aaronson
SA: Wild-type p53 triggers a rapid senescence program in human
tumor cells lacking functional p53. Proc Natl Acad Sci USA.
94:9648–9653. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Royds JA and Iacopetta B: p53 and disease:
When the guardian angel fails. Cell Death Differ. 13:1017–1026.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sur S, Pagliarini R, Bunz F, Rago C, Diaz
LA Jr, Kinzler KW, Vogelstein B and Papadopoulos N: A panel of
isogenic human cancer cells suggests a therapeutic approach for
cancers with inactivated p53. Proc Natl Acad Sci USA.
106:3964–3969. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Petitjean A, Mathe E, Kato S, Ishioka C,
Tavtigian SV, Hainaut P and Olivier M: Impact of mutant p53
functional properties on TP53 mutation patterns and tumor
phenotype: Lessons from recent developments in the IARC TP53
database. Hum Mutat. 28:622–629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Agostino S, Strano S, Emiliozzi V,
Zerbini V, Mottolese M, Sacchi A, Blandino G and Piaggio G: Gain of
function of mutant p53: The mutant p53/NF-Y protein complex reveals
an aberrant transcriptional mechanism of cell cycle regulation.
Cancer Cell. 10:191–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abbas T, White D, Hui L, Yoshida K, Foster
DA and Bargonetti J: Inhibition of human p53 basal transcription by
down-regulation of protein kinase Cdelta. J Biol Chem.
279:9970–9977. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nam JM, Onodera Y, Bissell MJ and Park CC:
Breast cancer cells in three-dimensional culture display an
enhanced radioresponse after coordinate targeting of integrin
alpha5beta1 and fibronectin. Cancer Res. 70:5238–5248. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang L, Cheng HC, Isom R, Chen CS, Levine
RA and Pauli BU: Protein kinase Cepsilon mediates polymeric
fibronectin assembly on the surface of blood-borne rat breast
cancer cells to promote pulmonary metastasis. J Biol Chem.
283:7616–7627. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mitra AK, Sawada K, Tiwari P, Mui K, Gwin
K and Lengyel E: Ligand-independent activation of c-Met by
fibronectin and α(5)β(1)-integrin regulates ovarian cancer invasion
and metastasis. Oncogene. 30:1566–1576. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pontiggia O, Sampayo R, Raffo D, Motter A,
Xu R, Bissell MJ, Joffé EB and Simian M: The tumor microenvironment
modulates tamoxifen resistance in breast cancer: A role for soluble
stromal factors and fibronectin through β1 integrin. Breast Cancer
Res Treat. 133:459–471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Issaeva N, Bozko P, Enge M, Protopopova M,
Verhoef LG, Masucci M, Pramanik A and Selivanova G: Small molecule
RITA binds to p53, blocks p53-HDM-2 interaction and activates p53
function in tumors. Nat Med. 10:1321–1328. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Espinoza-Fonseca LM: Targeting MDM2 by the
small molecule RITA: Towards the development of new multi-target
drugs against cancer. Theor Biol Med Model. 2:382005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Enge M, Bao W, Hedström E, Jackson SP,
Moumen A and Selivanova G: MDM2-dependent downregulation of p21 and
hnRNP K provides a switch between apoptosis and growth arrest
induced by pharmacologically activated p53. Cancer Cell.
15:171–183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grinkevich VV, Nikulenkov F, Shi Y, Enge
M, Bao W, Maljukova A, Gluch A, Kel A, Sangfelt O and Selivanova G:
Ablation of key oncogenic pathways by RITA-reactivated p53 is
required for efficient apoptosis. Cancer Cell. 15:441–453. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao CY, Grinkevich VV, Nikulenkov F, Bao
W and Selivanova G: Rescue of the apoptotic-inducing function of
mutant p53 by small molecule RITA. Cell Cycle. 9:1847–1855. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh SJ, Jung SP, Han J, Kim S, Kim JS, Nam
SJ, Lee JE and Kim JH: Silibinin inhibits TPA-induced cell
migration and MMP-9 expression in thyroid and breast cancer cells.
Oncol Rep. 29:1343–1348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoon JH, Choi YJ and Lee SG: Ginsenoside
Rh1 suppresses matrix metalloproteinase-1 expression through
inhibition of activator protein-1 and mitogen-activated protein
kinase signaling pathway in human hepatocellular carcinoma cells.
Eur J Pharmacol. 679:24–33. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kikkawa U, Takai Y, Tanaka Y, Miyake R and
Nishizuka Y: Protein kinase C as a possible receptor protein of
tumor-promoting phorbol esters. J Biol Chem. 258:11442–11445.
1983.PubMed/NCBI
|
|
28
|
Robbins D, Ponville J, Morris K and Zhao
Y: Involvement of PTEN in TPA-mediated p53-activation in mouse skin
epidermal JB6 cells. FEBS Lett. 586:4108–4113. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ashcroft M, Taya Y and Vousden KH: Stress
signals utilize multiple pathways to stabilize p53. Mol Cell Biol.
20:3224–3233. 2000. View Article : Google Scholar : PubMed/NCBI
|