Introduction
There were ~986,000 cases of head and neck cancer
(HNC), and oesophageal cancer diagnosed worldwide in 2012. In the
same year, in the UK, there were 5,000 cases of HNC in males and
2,500 in females (1). In 2013,
there were 8,784 cases of oesophageal cancer, with a male:female
ratio of 2:1. This makes HNC and oesophageal cancer the 12th and
13th most common cancers in the UK (2). The ratio of male:female cases of HNC
in persons aged 50–60 years is ~3:1, however, the gender disparity
is reduced in the elderly population, with a male female ratio of
1.5:1 in individuals over 80 years of age (3). For oesophageal squamous cell carcinoma
(SCC) the male:female ratio is lower, at 1.1:1.
Understanding the differences between subgroups of
the population is important to ensure that policy makers can make
informed decisions regarding preventive measures. Smoking and
alcohol are the major risk factors for HNC and oesophageal SCC;
however, the differences in incidence between age categories cannot
be solely attributed to differences in smoking and alcohol
consumption (4). Hormones are known
to play an important role in several types of cancers, such as
breast, ovarian and uterine, endometrial, prostate, testis and
thyroid cancers.
Studies have been carried out on hormone-related
risk factors for squamous cancers, such as oesophageal, cervical
and lung cancer (5). While hormone
replacement therapy (HRT) is a known risk factor for certain
cancers (e.g. breast cancer), a recent meta-analysis found that the
use of HRT is protective against oesophageal SCC (6). Early-menopause has also been linked to
an increased risk of oesophageal SCC [risk ratio (RR), 1.32 (95%
CI, 1.11–1.56)/5 years younger at menopause] (7). Another meta-analysis suggested
decreased risk of lung cancer in never-smoker females who use HRT
[OR, 0.86 (95% CI, 0.75–0.99)]. This leads to the hypothesis that
hormone levels may be a potential risk factor for squamous cancers.
There is uncertainty surrounding the role of female hormones and
the risk of head and neck oesophageal SCC. However, to the best of
our knowledge, no systematic review has been conducted to address
this uncertainty. The aim of this systematic review was to address
the uncertainty surrounding the role of female hormones and the
risk of head and neck and oesophageal SCC by evaluating two
specific questions: i) is early menopause a risk factor for HNC or
oesophageal SCC and ii) is HRT protective against HNC or
oesophageal SCC?
Methods
In the present study, we combined head and neck, and
oesophageal SCC due to their histology and strong similarities in
their epidemiology and aetiology.
Search strategy
Electronic databases MEDLINE, Web of Science, EMBASE
and Cochrane were searched up until February 11, 2016. Search
strategies were developed using Medical Subject Heading (MeSH)
words such as: head and neck neoplasms, head and neck and
oesophageal cancer, esophageal neoplasms, HRT, female hormone,
early menopause and text words related to hormones and HNC or
(o)esophageal cancer. Only search terms describing the association
were used. Reference lists were also extensively searched and
relevant papers obtained.
Eligibility criteria
Randomized controlled trials (RCTs), controlled
(non-randomised) clinical trials (CCTs) or cluster trials,
prospective and retrospective comparative cohort studies,
case-control or nested case-control studies, and cross-sectional
studies, addressing the question of female hormones as a risk
factor for HNC or oesophageal SCC, were considered. Studies were
included when they: i) examined the general adult population (age
>18 years), specifically patients with at least 50 cases of
HNC/oesophageal SCC and healthy controls; ii) addressed the
question of HRT or reproductive factors (menopause) and
HNC/oesophageal SCC; iii) administered HRT as an intervention for
prevention of cancer or being therapeutically taken due to symptoms
of menopause; iv) collected data on age at menopause, smoking,
alcohol, age and socioeconomic status or educational attainment;
and v) reported odds ratios, risk ratios or incidence/prevalence of
HNC or oesophageal SCC defined using the World Health Organisation
(WHO) classification of diseases ICD-10 codes, C00-15 and
C30-31.
Cohort studies were only eligible when follow-up
time was at least 5 years; case series and case reports were
excluded. Only English language articles published in peer-reviewed
journals, from 1948 to 2016, were considered.
Data extraction
Titles and/or abstracts of studies retrieved using
the search strategy and those from additional sources were
independently screened by two review authors (C.M. and M.M.), to
identify studies that potentially met the inclusion criteria
outlined above. Studies combining HNC with oesophageal squamous
cell cancers were considered, but data were extracted to separate
HNC and oesophageal cancer where possible. Data were extracted in
all forms (e.g. dichotomous, continuous) as reported in the
included studies. The full texts of these potentially eligible
studies were independently assessed for eligibility by C.M. and
M.M. Any disagreement over the eligibility of particular studies
was resolved through discussion with a third reviewer (J.K.F.).
Risk of bias
The risk of bias was assessed using the Newcastle
Ottawa Scale which includes the following areas of evaluation of
the risk of bias: selection of patients, comparability of groups in
the study, methods for assessing outcomes, proof of exposure and
appropriate follow-up. Studies were categorised as low, medium,
high or unclear risk of bias, using a star-based scoring system. A
maximum of 9 stars are available; the higher the number of stars
the lower the risk of bias. For ease of interpretation, a score of
7 or greater is considered ‘low-risk’ of bias, 4–6 is ‘medium-risk’
and 3 or below is considered ‘high-risk’ of bias.
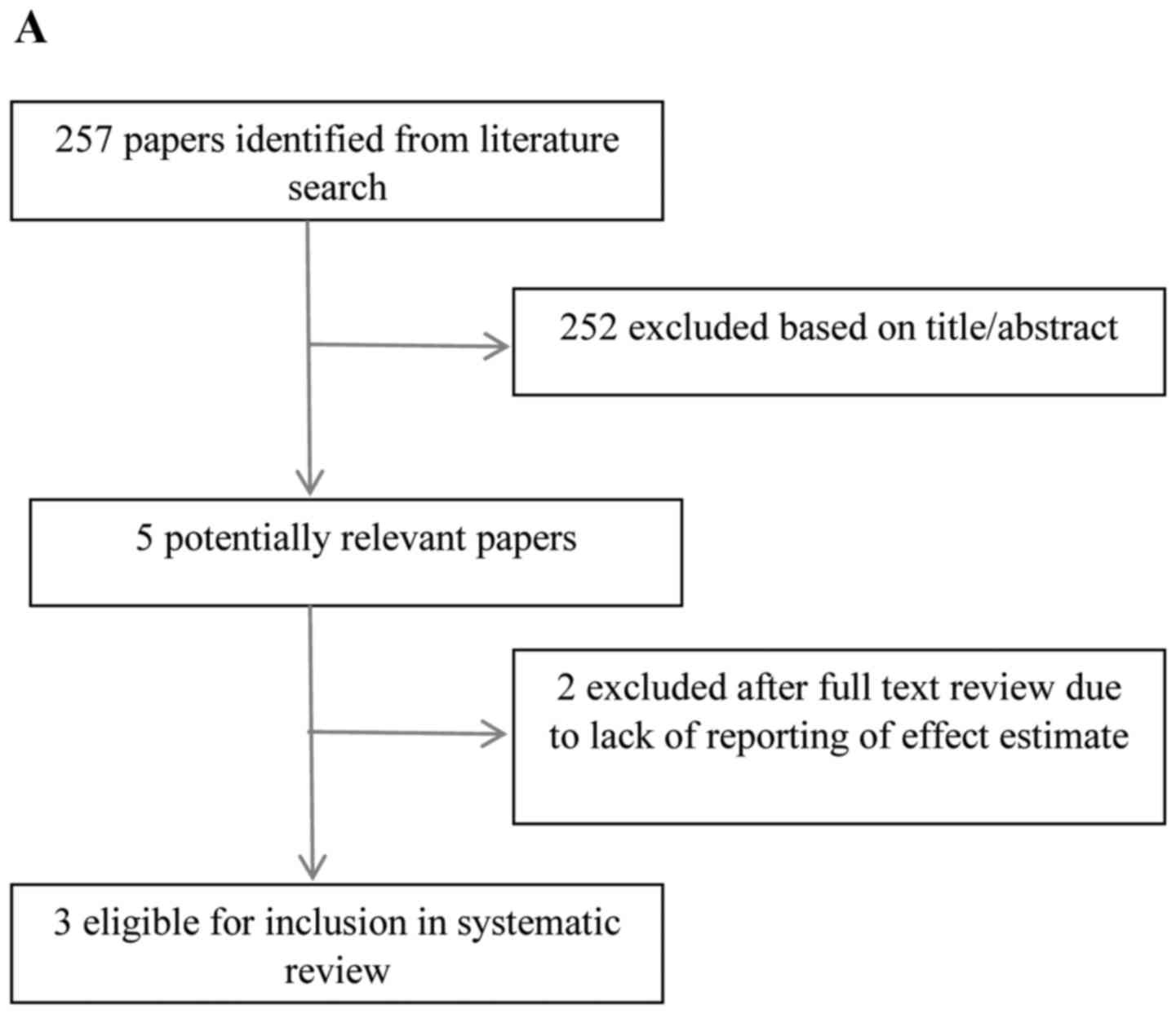
Search results
The search identified 13 potentially eligible
studies following the review of titles and abstracts of studies
identified from the initial search. One study considered HNC and
oesophageal cancer separately, therefore was included in both arms
of the review. Five papers were excluded, based on insufficient
number of cases (n=2), failure to report an effect
estimate/confidence intervals (n=2) and lack of categorisation by
histopathological subtype (n=1). Eight studies met the inclusion
criteria (4,7–13). The
literature search results and selection process are depicted in
Fig. 1A and B.
The systematic review includes two cohort studies
(one oesophageal/HNC and one oesophageal cancer only) (7,8) with
follow-up time of 7.5 and 9.1 years, respectively. Six case-control
studies (4 oesophageal cancer and 2 HNC) (4,9–13) were
also included. Studies covered the UK (7,13), USA
(4,8), European continent (9,14) and
China (11,12). The mean number of cases/study for
the HNC articles was 214 (range 149–297). For oesophageal cancer,
the average case number/study was 163 (range 56–578). A summary of
the demographic data for each study is presented in Table I. A summary of the findings
regarding use of HRT and risk of HNC/oesophageal SCC is shown in
Table IIA. Table IIB summarises the findings
regarding age at menopause.
 | Table I.Demographic characteristics of the
included studies. |
Table I.
Demographic characteristics of the
included studies.
| First author (year)
(ref.) | Country | Study type | Cancer type | Participant
demographics | Time period |
|---|
| Freedman (2010)
(8) | USA | Cohort |
HNC/oesophageal | NIH-AARP Diet and
Health Study cohort, aged 50–71 years (median follow-up 7.5
years) | 1995–2003 |
| Langevin (2011)
(4) | USA | Case-control | HNC | Cases of primary
HNC and complaint-free hospital controls at ENT Department,
University of Pittsburgh Medical Center | 2006–2010 |
| Bosetti (2000)
(9) |
Italy/Switzerland | Case-control | HNC | Cases of
histologically confirmed oral/ pharyngeal cancer age <75 years
attending hospitals in Italy/Switzerland and hospital controls with
acute, non-neoplastic conditions | 1984–1997 |
| Lindblad (2006)
(13) | UK | Nested
case-control | Oesophageal | UK General Practice
Research Database (UK GPRD) cohort aged 50–84 years | 1994–2001 |
| Gallus (2003)
(14) |
Italy/Switzerland | Case-control | Oesophageal | Cases aged <79
years with histologically confirmed oesophageal SCC admitted to
study hospitals; hospital controls admitted to the same hospitals
for acute, non-neoplastic conditions | 1984–1999 |
| Yu (2011) (11) | China | Case-control | Oesophageal | Cases of
histopathologically confirmed oesophageal SCC; hospital based
controls confirmed not to have oesophageal cancer | 2008–2010 |
| Chen (2011)
(12) | China | Case-control | Oesophageal | Cases of newly
diagnosed primary oesophageal cancer; hospital controls with no
history of cancer | 2004–2010 |
| Green (2012)
(7) | UK | Cohort | Oesophageal | Million Women Study
cohort (women aged 50–64 years) with mean 9.1 years follow-up | 1996–2008 |
 | Table II.The use of hormone replacement
therapy and risk of HNC/oesophageal SCC, and age at menopause and
risk of HNC/oesophageal SCC. |
Table II.
The use of hormone replacement
therapy and risk of HNC/oesophageal SCC, and age at menopause and
risk of HNC/oesophageal SCC.
| A, The use of
hormone replacement therapy and risk of HNC/oesophageal SCC |
|---|
|
|---|
| Outcome of
interest | First author (year)
(ref.) | Type of study | No. of cases |
| Relative effect (OR
or HR) | 95% CI | Risk of
Bias(Newcastle Ottawa Scale) |
|---|
| HNC | Freedman (2010)
(8) | Cohort | 297 |
| HR 0.78 | 0.61–0.99 | Low (8/9) |
| HNC | Langevin (2011)
(4) | Case-control | 149 |
| OR 0.47 | 0.20–1.08 | Medium (6/9) |
| HNC | Bosetti (2000)
(9) | Case-control | 195 |
| OR 0.88 | 0.45–1.72 | Medium-high
(4/9) |
| Oesophageal
SCC | Lindblad (2006)
(13) | Nested
case-control | 74 |
| OR 0.93 | 0.40–2.16 | Low (9/9) |
| Oesophageal
SCC | Gallus (2001)
(10) | Case-control | 114 |
| OR 0.32 | 0.09–1.13 | Medium-high
(4/9) |
| Oesophageal
SCC | Freedman (2010)
(8) | Cohort | 56 |
| HR 0.74 | 0.42–1.26 | Low (8/9) |
| Oesophageal
SCC | Yu (2011) (11) | Case-control | 88 |
| OR 0.94 | 0.53–1.70 | Medium-high
(4/9) |
|
| Bold text denotes a
statistically significant result. |
|
| B, Age at
menopause and risk of HNC/oesophageal SCC |
|
| Outcome of
interest | First author
(year) (ref.) | Type of
study | No. of
cases | Age
(years) | Relative effect
(OR or HR) | 95% CI | Risk of Bias
(Newcastle Ottawa Scale) |
|
| HNC | Freedman (2010)
(8) | Cohort | 297 | >55 | HR 0.92 | 0.50–1.71 | Low (8/9) |
| HNC | Bosetti (2000)
(9) | Case-control | 195 | >50 | OR 0.46 |
0.30–0.70 | Medium-high
(4/9) |
| Oesophageal
SCC | Gallus (2001)
(10) | Case-control | 114 | >50 | OR 0.43 |
0.22–0.83 | Medium-high
(4/9) |
| Oesophageal
SCC | Freedman (2010)
(8) | Cohort | 56 | Increasing age |
Ptrend=0.019 |
| Low (8/9) |
| Oesophageal
SCC | Yu (2011) (11) | Case-control | 88 | <45 | OR 2.27 |
1.03–4.97 | Medium-high
(4/9) |
| Oesophageal
SCC | Green (2012)
(7) | Cohort | 578 | Per 5 years
younger | RR 1.32 |
1.11–1.56 | Low (7/9) |
| Oesophageal
SCC | Chen (2011)
(12) | Case-control | 68 | >48 | OR 0.94 | 0.31–2.85 | Medium (5/10) |
Hormone replacement therapy (HRT)
HNC
Three papers (1 cohort study and 2 case-control
studies) addressed the question of the use of HRT and incidence of
HNC. Only the study by Freedman et al (8) was considered at ‘low-risk’ of bias,
with a Newcastle Ottawa score of 7/9 stars. This was a cohort study
conducted in the USA, using the NIH-AARP Diet and Health Cohort, of
125,887 women. Two hundred and ninety-seven cases of HNC were
identified with mean follow-up of 7.5 years. The risk of HNC was
22% lower for ever-users of HRT (HR, 0.78; 95% CI, 0.61–0.99);
44.1% of cases (n=127) had ever-used HRT compared to 54.6% of
controls (n=106,934). Further analysis by hysterectomy status
revealed that the risk reduction was greatest for women with an
intact uterus who were current users of HRT for >5 years (HR,
0.23; 95% CI, 0.09–0.57). Notably, use of HRT other than
oestrogen-alone or oestrogen-progesterone therapy conferred a
greater risk of HNC (HR, 2.31; 95% CI, 1.15–4.65); however, this
analysis was based on only 9 cases who used an alternative HRT. Two
case-control studies were considered at medium/high risk of bias;
they reported a non-significant reduction in the risk of HNC for
ever-users of HRT (4,9).
Oesophageal SCC
Four studies analysed HRT use and the risk of
oesophageal SCC. Two were considered low-risk of bias (8,13) and
two were medium/high risk of bias (10,11).
Although, all studies reported an effect estimate of <1 for
users of HRT, implying a protective effect, none of the results
were statistically significant.
Age at menopause
HNC
Two studies (8,9)
assessed the link between age at menopause and risk of HNC. Bosetti
et al (9) found a protective
effect of later age at menopause (>50 years), with an OR of 0.46
(95% CI, 0.30–0.70). Freedman et al (8) found no significant effect on the risk
of HNC with later age at menopause (>55 years).
Oesophageal SCC
Four out of 5 studies reported a significant effect
of age at menopause and risk of oesophageal SCC. The method of
reporting varied. Gallus et al (10) reported an OR of 0.43 (95% CI,
0.22–0.83) for age at menopause of >50 years vs. menopause at
age <45 years. Yu et al (11) reported an increased risk of
oesophageal SCC for women entering menopause at <45 years (OR,
2.27; 95% CI, 1.03–4.97) and for 45–49 years (OR, 2.16; 95% CI,
1.14–4.78) compared to menopause at age >50 years. Green et
al (7) reported increased risk
of oesophageal SCC for every 5 years younger a women was at the
time of menopause (RR, 1.32; 95% CI, 1.11–1.56). Although, Freedman
et al (8) found no
significant effect for individual age categories, they did observe
a significant trend (p=0.019) for a lower risk of oesophageal SCC
with older age at menopause. Chen et al (12) observed no significant effect for age
at menopause, although these authors classified older age at
menopause as >48 years.
Discussion
This systematic review has considered evidence from
8 studies investigating the risk of head and neck cancer (HNC) or
oesophageal squamous cell carcinoma (SCC) in relation to age at
menopause and use of hormone replacement therapy (HRT); 5 studies
investigated oesophageal SCC, 2 studies investigated HNC and 1
study included both cancers.
Early menopause
We found that earlier age at menopause is associated
with a higher risk of oesophageal cancer, based on 4 studies with a
total of 836 cases of oesophageal SCC.
Most women experience menopause between the ages of
45 and 55 years; the median age at menopause is 47.2 years,
according to a prospective cohort study of over 5,000 women
enrolled at the Royal College of GP's Oral Contraception study
(15). Menopause is considered to
be early in women aged 40–45 years (~5% of women) and premature in
women <40 years (~1% of women) (16).
Early menopause is more frequent in women with
certain genetic or autoimmune disorders, infections or a history of
chemotherapy/radiotherapy or surgery to remove the ovaries
(16).
Mean age of menopause for smokers is significantly
lower than non-smokers (45.6 vs. 46.9 years) (15). Women with early natural menopause
are more likely to be smokers, ever-users of oral contraceptive
medication, undergone tubal ligation, have at least one episode of
endometriosis and are less likely to use HRT. No association with
alcohol, BMI, physical activity or parity was reported (15). Diabetic women have also been found
to be at risk of early menopause (OR, 2.76; 95% CI, 1.32–5.66)
(17). In a pooled analysis of
case-control studies, diabetes diagnosed at age <50 years
conferred a greater risk of HNC (OR, 1.37; 95% CI, 1.07–1.74) when
analysing 6,448 cases of HNC and 13,747 controls (18), but no link with age at menopause was
considered in the present study.
A recent systematic review and meta-analysis of
oesophageal SCC and reproductive factors also found a protective
effect for older age at menopause (SRR=0.70; 95% CI, 0.51–0.95).
The authors concluded that ‘properly extending the time of
menstruation for pre-menopausal women is a possible way to reduce
the risk of oesophageal SCC’ (19).
A separate meta-analysis found that menopausal status was
associated with higher risk of oesophageal SCC (RR, 1.66; 95% CI,
1.12–2.48), but age at menopause was not significant for
Caucasians. In Asians, older age at menopause was found to confer
greater risk of oesophageal SCC (RR, 1.68; 95% CI, 1.05–2.70)
(6). This implies that ethnicity
and genetic background may influence the role of female hormones in
oesophageal carcinogenesis.
The effect of age at menopause has been explored in
several other cancer types. A meta-analysis of 8 studies of gastric
cancer and reproductive factors found a protective effect of longer
years of fertility (RR=0.74; 95% CI, 0.63–0.86) (20). Early menopause has also been found
to be a risk factor for bladder cancer (meta-analysis including 7
studies: RR=1.59; 95% CI, 1.31–1.92) (21). Two studies from Hungary, from the
same unit, found that the mean age at menopause was significantly
lower in females with oral cancer compared to controls (43.5 vs.
50.9 and 45.4 years vs. 51.3 years, respectively) (22,23).
These studies were excluded from this review due to the failure to
provide accurate effect estimates with confidence intervals. Other
recent meta-analyses found no significant effect of age at
menopause in relation to risk of brain tumours (24), pancreatic (25) or thyroid cancer (26). A large cohort study found no
association with age at menopause and risk of colorectal cancer
(27).
HRT
HRT is long-established in the management of the
symptoms of the menopause and has also been shown to reduce the
risk of osteoporotic fractures, cardiovascular disease, Alzheimers,
depression, stroke and colon cancer. Approximately 30% of UK women
used HRT in 2001–2002 (28).
Following this, a large US-based trial (Women's Health Initiative)
was prematurely halted due to concerns over the evidence of
increased risk of breast cancer, coronary heart disease, stroke and
pulmonary embolism among users of HRT (29). The UK-based Million Women Study
(MWS) also reported increased risk of breast cancer with HRT in
2003 (30). Following media
coverage of the results of these trials, use of HRT declined
steadily in the UK for the next 3–4 years. In 2005, only 10–11% of
menopausal women were using HRT (28). Further reports of a slight increased
risk of ovarian cancer for ever-users of HRT compounds enhanced
concerns over the safety of HRT (31).
However, concerns have been raised by some authors
surrounding the reporting of the WHI and MWS trial results, and the
fact that little coverage was given to the evidence of the reduced
incidence of osteoporotic fractures and colon cancer (32). Both trials recruited women aged over
50 years; therefore, the results cannot be applied to women who
undergo premature menopause (33).
HRT for women with premature menopause (primary ovarian
insufficiency), prescribed up to the age of natural menopause (~51
years), is endorsed by the British Menopause Society and NICE
guidelines. The NICE guidelines also recommend the development of a
collaborative ‘primary ovarian insufficiency’ registry to allow
data collection to clarify, among other factors, the long-term risk
of cancers in this group (34).
Despite reports of an increased risk of breast and
ovarian cancers in certain groups using HRT, there are many
observational studies reporting a reduced risk of other cancers in
HRT users. There are several reports of a reduced risk of colon
cancer with HRT (27,35,36).
Green et al reported a relative risk reduction of 17% (RR,
0.83; 95% CI, 0.79–0.88) in their meta-analysis of 30 studies of
colorectal cancer and HRT (36). In
a recent meta-analysis of oesophageal cancer and reproductive
factors, the authors reported a 33% relative risk reduction with
HRT use (SRR, 0.67; 95% CI, 0.56–0.81) (19). Similar results were reported for
reduced risk of gastric cancers (RR, 0.77; 95% CI, 0.64–0.92)
(20). Combined HRT (oestrogen and
progesterone) has also been shown to reduce the risk of lung
cancer, in a meta-analysis of 25 studies (RR, 0.83; 95% CI,
0.76–0.91) (37). HRT has not been
found to significantly reduce the risk of melanoma or pancreatic
cancer (25,38).
This systematic review did not find evidence of a
significant risk reduction for oesophageal cancer among users of
HRT, in contrast to the findings of the meta-analysis by Zhu et
al (19). However, all effect
estimates were <1 and the studies included contained only modest
numbers of oesophageal cancer cases. The Freedman et al
(8) cohort study included 297 cases
of HNC and was the only study to report a significant relative risk
reduction for HNC in users of HRT (HR, 0.78; 95% CI, 0.61–0.99).
Further large studies are needed to confirm the finding that HRT is
protective against HNC.
Confounding factors must be considered. Users of HRT
tend to be of higher socioeconomic status (SES) and have higher
levels of education. Both of these factors may reduce the risk of
HNC. To address this, only studies controlling for a measure of SES
or education were eligible for inclusion in this review. Freedman
et al (8) controlled for
education, alcohol, BMI, tobacco smoking, physical activity and
diet (fruit and vegetable intake), although residual confounding
factors could still be relevant.
Role of oestrogen deficiency
Females have been found to have survival advantage
for HNC, oesophageal, gastric and pancreatic cancer, as well as
cancers at 11 other sites. For all cancers combined, women have a
5% lower risk of death than men; for HNC, a 12% increased survival
is reported (39). This finding is
consistent across all European regions in the EUROCARE-4 cohort of
1.6 million population-based cancer cases (39). The advantage is most pronounced in
younger women and declines with age, with a marked decline beyond
the age of menopause. It is possible that female hormones play a
part in this 12% improvement in survival.
Oestrogen is known to promote cancer in
oestrogen-responsive tissues, such as breast, endometrium and
cervix; however, evidence from mouse models suggests that oestrogen
has an inhibitory role in oesophageal SCC growth (40). Oestrogen receptors have been found
in oesophageal SCC tissue samples and HNCs (40–42).
Oesophageal SCC cells with oestrogen receptors have been shown to
be inhibited by oestrogen exposure and this may initiate apoptosis
(43). If oestrogen is responsible
for inhibiting the growth of some cancer cells, oestrogen
deficiency could be considered a risk factor for certain cancers. A
women who undergoes premature menopause has less oestrogen exposure
over her lifetime and this may increase her risk of cancers such as
oesophageal SCC. However, oestrogen appears to have both
tumorigenic and antitumour properties, depending on the tissue and
presence of oestrogen receptors. HNC cell lines, from males and
females, have been found to contain oestrogen receptors, and
laboratory studies appear to show that oestrogen promotes the
growth of HNC cells (44,45). Further high quality basic science
studies are required to confirm the role of oestrogen in HNC and
oesophageal SCC.
The studies included in this review were all
assessed for risk of bias, using the Newcastle Ottawa Scale. Only 3
of the 8 studies included were low risk for bias. This does limit
the significance of our findings and is an indication of the need
for further, high quality, studies addressing the issue of female
hormones and squamous cancers.
We only included studies that controlled for
significant potential confounding factors, such as smoking and
socioeconomic status, however, the risk of residual confounding
factors remains. Smokers undergo menopause at a younger age than
non-smokers. We recommend that future studies should control for
this important confounder.
The rationale for combining HNC and oesophageal SCC
is based on the strong similarities in their epidemiology and
aetiology as mentioned earlier. We deliberately excluded
oesophageal adenocarcinomas as these cancers have quite different
aetiology. One of the studies included in our review by Bosetti
et al (9) failed to clarify
the histology of the oral/pharyngeal cancer cases included, which
introduces a potential source of bias. However, over 90% of oral
cancers and >80% of pharyngeal cancers are of squamous histology
(46), therefore the bias is
unlikely to be significant.
We found that earlier age at menopause is a risk
factor for oesophageal SCC, with women entering menopause at <45
years having double the risk of those entering menopause at age
>50 years. Similar, but less striking, results were observed for
HNC. HRT was found to reduce the risk of HNC/oesophageal SCC, but
the evidence is inconclusive. We used strict eligibility criteria
and only considered studies that controlled for other risk factors,
however, there is still the risk of residual bias. Consideration
should be given to collecting data on reproductive factors and
exposure to HRT, as routine practice, in future epidemiological and
clinical studies of these cancers. The concept of oestrogen
deficiency as a risk for HNC/oesophageal SCC warrants further
investigation in future laboratory and clinical studies.
Acknowledgements
Mrs. Caroline McCarthy is funded by NIHR (Academic
Clinical Fellowship in Oral Medicine). The present review presents
independent research funded by the National Institute for Health
Research (NIHR). The views expressed are those of the author(s) and
not necessarily those of the NHS, the NIHR or the Department of
Health.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McCarthy CE, Field JK, Rajlawat BP, Field
AE and Marcus MW: Trends and regional variation in the incidence of
head and neck cancers in England: 2002 to 2011. Int J Oncol.
47:204–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Statistics OfNCancer Registration
Statistics. England: 2013, 2015.
|
|
4
|
Langevin SM, Grandis JR and Taioli E:
Female hormonal and reproductive factors and head and neck squamous
cell carcinoma risk. Cancer Lett. 310:216–221. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bae JM: Modifiable risk factors of lung
cancer in ‘never-smoker’ women. Epidemiol Health. 37:e20150472015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang BJ, Zhang B, Yan SS, Li ZC, Jiang T,
Hua CJ, Lu L, Liu XZ, Zhang DH1, Zhang RS, et al: Hormonal and
reproductive factors and risk of esophageal cancer in women: A
meta-analysis. Dis Esophagus. 29:448–454. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Green J, Roddam A, Pirie K, Kirichek O,
Reeves G and Beral V: Million Women Study collaborators:
Reproductive factors and risk of oesophageal and gastric cancer in
the Million Women Study cohort. Br J Cancer. 106:210–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Freedman ND, Lacey JV Jr, Hollenbeck AR,
Leitzmann MF, Schatzkin A and Abnet CC: The association of
menstrual and reproductive factors with upper gastrointestinal
tract cancers in the NIH-AARP cohort. Cancer. 116:1572–1581. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bosetti C, Negri E, Franceschi S, Conti E,
Levi F, Tomei F and La Vecchia C: Risk factors for oral and
pharyngeal cancer in women: A study from Italy and Switzerland. Br
J Cancer. 82:204–207. 2000.PubMed/NCBI
|
|
10
|
Gallus S, Bosetti C, Franceschi S, Levi F,
Simonato L, Negri E and La Vecchia C: Oesophageal cancer in women:
Tobacco, alcohol, nutritional and hormonal factors. Br J Cancer.
85:341–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yu H, Liu G, Zhao P and Zhu L: Hormonal
and reproductive factors and risk of esophageal cancer in Chinese
postmenopausal women: A case-control study. Asian Pac J Cancer
Prev. 12:1953–1956. 2011.PubMed/NCBI
|
|
12
|
Chen ZH, Shao JL, Lin JR, Zhang X and Chen
Q: Reproductive factors and oesophageal cancer in Chinese women: A
case-control study. BMC Gastroenterol. 11:492011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindblad M, García Rodríguez LA, Chandanos
E and Lagergren J: Hormone replacement therapy and risks of
oesophageal and gastric adenocarcinomas. Br J Cancer. 94:136–141.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gallus S, Bosetti C, Franceschi S, Levi F,
Negri E and La Vecchia C: Laryngeal cancer in women: Tobacco,
alcohol, nutritional, and hormonal factors. Cancer Epidemiol
Biomarkers Prev. 12:514–517. 2003.PubMed/NCBI
|
|
15
|
Pokoradi AJ, Iversen L and Hannaford PC:
Factors associated with age of onset and type of menopause in a
cohort of UK women. Am J Obstet Gynecol. 205:34.e1–34.e13. 2011.
View Article : Google Scholar
|
|
16
|
Faubion SS, Kuhle CL, Shuster LT and Rocca
WA: Long-term health consequences of premature or early menopause
and considerations for management. Climacteric. 18:483–491. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Monterrosa-Castro A, Blümel JE,
Portela-Buelvas K, Mezones-Holguín E, Barón G, Bencosme A, Benítez
Z, Bravo LM, Calle A, Chedraui P, et al: Collaborative Group for
Research of the Climacteric in Latin America (REDLINC): Type II
diabetes mellitus and menopause: A multinational study.
Climacteric. 16:663–672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stott-Miller M, Chen C, Chuang SC, Lee YC,
Boccia S, Brenner H, Cadoni G, Dal Maso L, La Vecchia C, Lazarus P,
et al: History of diabetes and risk of head and neck cancer: A
pooled analysis from the International Head and Neck Cancer
Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev.
21:294–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu Y, Yue D, Yuan B, Zhu L and Lu M:
Reproductive factors are associated with oesophageal cancer risk:
Results from a meta-analysis of observational studies. Eur J Cancer
Prev. 26:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Camargo MC, Goto Y, Zabaleta J, Morgan DR,
Correa P and Rabkin CS: Sex hormones, hormonal interventions, and
gastric cancer risk: A meta-analysis. Cancer Epidemiol Biomarkers
Prev. 21:20–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dietrich K, Demidenko E, Schned A, Zens
MS, Heaney J and Karagas MR: Parity, early menopause and the
incidence of bladder cancer in women: A case-control study and
meta-analysis. Eur J Cancer. 47:592–599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suba Z: Gender-related hormonal risk
factors for oral cancer. Pathol Oncol Res. 13:195–202. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Takács D, Koppány F, Mihályi S and Suba Z:
Decreased oral cancer risk by moderate alcohol consumption in
non-smoker postmenopausal women. Oral Oncol. 47:537–540. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zong H, Xu H, Geng Z, Ma C, Ming X, Shang
M, Li K, He X, Du H, Zhao J, et al: Reproductive factors in
relation to risk of brain tumors in women: An updated meta-analysis
of 27 independent studies. Tumour Biol. 35:11579–11586. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang B, Lv J, Li Y, Yuan S, Wang Z and He
S: Relationship between female hormonal and menstrual factors and
pancreatic cancer: A meta-analysis of observational studies.
Medicine. 94:e1772015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Caini S, Gibelli B, Palli D, Saieva C,
Ruscica M and Gandini S: Menstrual and reproductive history and use
of exogenous sex hormones and risk of thyroid cancer among women: A
meta-analysis of prospective studies. Cancer Causes Control.
26:511–518. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arem H, Park Y, Felix AS, Zervoudakis A,
Brinton LA, Matthews CE and Gunter MJ: Reproductive and hormonal
factors and mortality among women with colorectal cancer in the
NIH-AARP Diet and Health Study. Br J Cancer. 113:562–568. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Menon U, Burnell M, Sharma A,
Gentry-Maharaj A, Fraser L, Ryan A, Parmar M, Hunter M and Jacobs
I: UKCTOCS Group: Decline in use of hormone therapy among
postmenopausal women in the United Kingdom. Menopause. 14:462–467.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rossouw JE, Anderson GL, Prentice RL,
LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA,
Howard BV, Johnson KC, et al: Writing Group for the Women's Health
Initiative Investigators: Risks and benefits of estrogen plus
progestin in healthy postmenopausal women: Principal results From
the Women's Health Initiative randomized controlled trial. JAMA.
288:321–333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Beral V, Banks E, Reeves G and Bull D:
Million Women Study Collaborators: Breast cancer and
hormone-replacement therapy in the Million Women Study. Lancet.
362:419–427. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beral V, Gaitskell K, Hermon C, Moser K,
Reeves G and Peto R: Collaborative Group On Epidemiological Studies
Of Ovarian Cancer: Menopausal hormone use and ovarian cancer risk:
Individual participant meta-analysis of 52 epidemiological studies.
Lancet. 385:1835–1842. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Studd J: ‘PROFOX’ - the post HRT
nightmare. Climacteric. 14:217–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ayres J: Premature menopause: Management
challenges after the Women's Health Initiative. J Br Menopause Soc.
11:1572005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
(NICE) NIfHaCE, . Menopause: Diagnosis and
management (NG23) 2015. NICE guideline. Published.
12–November;2015.simplenice.org.uk/guidance/ng23
|
|
35
|
Murphy N, Strickler HD, Stanczyk FZ, Xue
X, Wassertheil-Smoller S, Rohan TE, Ho GY, Anderson GL, Potter JD
and Gunter MJ: A prospective evaluation of endogenous sex hormone
levels and colorectal cancer risk in postmenopausal women. J Natl
Cancer Inst. 107:djv2102015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Green J, Czanner G, Reeves G, Watson J,
Wise L, Roddam A and Beral V: Menopausal hormone therapy and risk
of gastrointestinal cancer: Nested case-control study within a
prospective cohort, and meta-analysis. Int J Cancer. 130:2387–2396.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao Y, Gu X, Zhu J, Yuan D and Song Y:
Hormone replacement therapy in females can decrease the risk of
lung cancer: A meta-analysis. PLoS One. 8:e712362013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gandini S, Iodice S, Koomen E, Di Pietro
A, Sera F and Caini S: Hormonal and reproductive factors in
relation to melanoma in women: Current review and meta-analysis.
Eur J Cancer. 47:2607–2617. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Micheli A, Ciampichini R, Oberaigner W,
Ciccolallo L, de Vries E, Izarzugaza I, Zambon P, Gatta G and De
Angelis R: EUROCARE Working Group: The advantage of women in cancer
survival: An analysis of EUROCARE-4 data. Eur J Cancer.
45:1017–1027. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Utsumi Y, Nakamura T, Nagasue N, Kubota H
and Morikawa S: Role of estrogen receptors in the growth of human
esophageal carcinoma. Cancer. 64:88–93. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kalayarasan R, Ananthakrishnan N, Kate V
and Basu D: Estrogen and progesterone receptors in esophageal
carcinoma. Dis Esophagus. 21:298–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lukits J, Remenár E, Rásó E, Ladányi A,
Kásler M and Tímár J: Molecular identification, expression and
prognostic role of estrogen- and progesterone receptors in head and
neck cancer. Int J Oncol. 30:155–160. 2007.PubMed/NCBI
|
|
43
|
Chandanos E and Lagergren J: The mystery
of male dominance in oesophageal cancer and the potential
protective role of oestrogen. Eur J Cancer. 45:3149–3155. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Egloff AM, Rothstein ME, Seethala R,
Siegfried JM, Grandis JR and Stabile LP: Cross-talk between
estrogen receptor and epidermal growth factor receptor in head and
neck squamous cell carcinoma. Clin Cancer Res. 15:6529–6540. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chang YL, Hsu YK, Wu TF, Huang CM, Liou
LY, Chiu YW, Hsiao YH, Luo FJ and Yuan TC: Regulation of estrogen
receptor α function in oral squamous cell carcinoma cells by FAK
signaling. Endocr Relat Cancer. 21:555–565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Davies L and Welch HG: Epidemiology of
head and neck cancer in the United States. Otolaryngol Head Neck
Surg. 135:451–457. 2006. View Article : Google Scholar : PubMed/NCBI
|