Introduction
Glioblastoma, the most common primary brain tumor in
adults, is rapidly fatal. The current standard of care for newly
diagnosed glioblastoma is surgical resection to a feasible extent,
followed by adjuvant radiotherapy (1,2).
Current therapeutic strategies against glioblastoma (GBM) have
failed to prevent disease progression and recurrence effectively
(3,4). The part played by molecular imaging
(MI) in the development of novel therapies has gained increasing
attraction in recent years (5). The
combination of anatomical (MRI or CT) with metabolic (PET or SPECT)
imaging techniques might provide even more detailed information on
response to drug treatment compared with single modality imaging
(5,6).
Epidermal growth factor receptor (EGFR) is a
cell-surface receptor that plays a key role in signaling pathways
regulating cell proliferation, angiogenesis, and tumor metastases
(7,8). EGFR is one of the four members of the
EGFR family. EGFR is widely overexpressed in several tumor types
including breast cancer, melanoma, and brain glioblastoma, making
this receptor an attractive candidate for anticancer therapy
(7,9). EGFR is also an attractive drug target
because it is widely expressed in many cancers and has
well-documented oncogenic activities. Anticancer therapies
targeting EGFR have been studied since the 1980s. To date, several
antibodies and small-molecule inhibitors are directed against EGFR.
These antibodies and inhibitors are actively being developed by
biotechnology and pharmaceutical companies as cancer therapeutics,
such as C225 (cetuximab), EMD 72000 (matuzumab), gefitinib,
erlotinib, and lapatinib. EGFR expression level is related to
disease development and prognosis (10,11).
Some studies showed that treatment of locoregionally advanced head
and neck cancer with concomitant high-dose radiotherapy plus
cetuximab improves locoregional control and reduces mortality
without increasing the common toxic effects associated with
radiotherapy to the head and neck (12). Traditional EGFR-targeted
nanoparticle carriers play a role in the treatment of malignancy
but do not provide direct guidance in the diagnosis and evaluation
of the prognosis of malignant tumor.
In the current study, radioiodine-labeled anti-EGFR
binding nanoparticles were constructed for treatment and imaging of
glioblastoma in vitro and in vivo.
Materials and methods
Materials
Monoclonal anti-human EGFR antibody was obtained
from Cetuximab, Merck KGaA, Germany. 131I was provided
by the Beijing Atomic Gaoke Limited by Share Ltd. The nanoparticles
were obtained from the Institute of Nanobiotechnology, School of
Materials Science and Engineering, Tianjin Key Laboratory of
Composites and Functional Materials, Tianjin University.
Self-assembly of amphiphilic BSA-PCL
conjugate; (9,13–15)
The amphiphilic BSA-PCL conjugate was synthesized as
previously described (16), and the
nanosized self-assembly of the amphiphilic BSA-PCL conjugate was
obtained via emulsion-solvent evaporation method. Briefly, 4 mg of
BSA-PCL conjugate was dissolved in 4 ml of phosphate buffer (PB,
0.1 M, pH 7.4) at room temperature and sonicated in a bath
sonicator for 10 min. During the ultrasonic treatment process, 2 ml
of dichloromethane was slowly injected into the PB via syringe.
Dichloromethane was then evaporated with a vacuum rotary
evaporator, and self-assembly of the cetuximab-decorated BSA-PCL
conjugate was prepared with the same process. The obtained PB
solutions of the self-assemblies of the BSA-PCL and
cetuximab-decorated BSA-PCL conjugates were stored at 4°C.
Cell culture
U251 and U87 human glioblastoma cells were purchased
from Cell Resource Center, Institute of Basic Medical Sciences,
Chinese Academy of Medical Sciences/Peking Union Medical College
(Beijing, China). The U251 and U87 cells which overexpressed EGFR
were cultured as a monolayer in DMEM media supplemented with 100
U/ml of penicillin, 100 M/ml streptomycin, and 10% FBS in a
humidified atmosphere containing 5% CO2 at 37°C.
131I-labeling of
nanoparticles; (17)
We used the well-established direct labeling method
for anti-EGFR-nanoparticles EGFR-BSA-PCL and BSA-PCL (18,19).
EGFR-BSA-PCL and BSA-PCL were labeled with 131I (Beijing
Atomic Hi-Tech Co., Ltd.) using chloramine-T method. The same
amounts of EGFR-BSA-PCL or BSA-PCL were diluted in PB to a total
volume of 100 µl (1 mg/ml), and ~37 MBq 131I was added.
Up to 100 µl of chloramine-T (5 mg/ml in PB) was added. After 60
sec of incubation, complete oscillation using an oscillator was
simultaneously needed. The reaction was stopped by adding 100 µl of
sodium metabisulfite (5 mg/ml in PB), and complete oscillation for
60 sec was also needed. To separate the labeled EGFR-BSA-PCL or
BSA-PCL from the low-molecular-weight compounds, centrifugation
method was adopted to remove the small molecules. Our group used a
centrifuge tube (Amicon® Pro Purification System, Merck
Millipore). The specific radioactivity was approximately 333 and
296 MBq/mg for EGFR-BSA-PCL and BSA-PCL, respectively. The ratio of
radioiodine label was also calculated.
Confocal microscopy; (10,15,20)
As previously mentioned, cells were planted in the
confocal small dish (5×103 cells/dish). Using the above
steps, EGFR-BSA-PCL and BSA-PCL (marked with FITC) were added to
each dish and incubated another 4 and 12 h, respectively. After
incubating the rejected nanoparticles, the cells were washed twice
or thrice repeatedly with sterile PBS and fixed with 0.5 ml of
paraformaldehyde. Extracellular localization of fluorescent
EGFR-labeled nanoparticles was then periodically visualized and
recorded using Laser Scanning Confocal Microscope (Leica TCS SP2;
Leica, Munich, Germany).
Flow cytometry; (21)
Flow cytometry was used to evaluate the binding of
EGFR-BSA-PCL and BSA-PCL to target cells (EGFR+: U251
and U87). U251 and U87 cells were seeded into a cell-culture flask
(5×106 cells) and incubated for 24 h. Up to 1 ml each of
EGFR-BSA-PCL and BSA-PCL (1 mg/ml, marked with FITC) was added, and
the cells were incubated for 4 or 12 h. After incubation, the
supernatant was discarded, and the plates were washed two to four
times in PBS and then used for trypsinization. After
centrifugation, 250 µl of the cell suspension was obtained, and
another 250 µl of paraformaldehyde was added. The cells were then
analyzed by a BD FACSCalibur flow cytometry (BD Biosciences,
Franklin Lakes, NJ, USA) using 490 nm laser source.
MTT cytotoxicity assay; (20,22)
MTT assays were conducted to evaluate the
cytotoxicity of 131I-labeled EGFR-BSA-PCL and BSA-PCL.
U251 and U87 cells were seeded into 96-well microplates with
1×104 cells/well. The 131I-EGFR-BSA-PCL,
131I-BSA-PCL, EGFR-BSA-PCL, or BSA-PCL under different
concentration gradients were added and then incubated for another 4
h. After removing all the nanoparticles, 20 µl of MTT reagent
[3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide;
Sigma] was added (5 mg/ml) into each well. The plates were
incubated for 4 h, and the MTT-containing medium was removed and
replaced with DMSO. The amount of the blue formazan compound
indicated the number of living cells and was determined using a
spectrophotometer (492 nm). Finally, after gently shaking the
microplates for 10 min to dissolve the formazan, each sample with
six replicates (n=6) was analyzed on a BioTek ELX 800 microplate
reader (BioTek Instruments, Inc., Winooski, VT, USA) at λ=492
nm.
Radionuclide uptake in vitro;
(23,24)
Briefly, the cells (105/well) were plated
in triplicates in 96-well plates. Subsequently, 0.37–3.7 MBq
131I-EGFR-BSA-PCL and 131I-BSA-PCL were added
into each well for 4 h. The medium was then completely removed, and
the cells were quickly washed twice with ice-cold PBS. Up to 200 µl
of 10% FBS-DMEM was added into each well. A γ-counter (LKB Gamma
1261; LKB Instruments, Mt. Waverley, Australia) was used to
calculate the counts per minute (CPM) after 24 h.
Experimentation on animals; (25)
This study was carried out in strict accordance with
the recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health and China Regulations
For the Administration of Affairs Concerning Experimental Animals.
The protocol was approved by the Committee on the Ethics of Animal
Experiments of the Tianjin Medical University General Hospital. All
surgery was performed under sodium pentobarbital anesthesia, and
all efforts were made to minimize suffering.
Four-week-old BALB/c nude female mice were obtained
from the PLA Military Academy of Medical Sciences Laboratory Animal
Center. The mice were normally bred and maintained under
specific-pathogen-free conditions with constant temperature and
humidity range. The nude mice (n=27) were inoculated with U87 in
the subcutaneous tissue upper back. A total volume of 50 µl was
inoculated in the subcutaneous tissue upper back of the mice using
a syringe. The U87 cells were slowly injected, and the syringe was
kept in for 1–2 min before being retracted to avoid cells from
ascending through the injection canal. Based on preliminary
studies, the total span of the experiments was set to 21 days to
ensure sufficient tumor development. However, the mice developed
signs of considerable tumor burden, which is defined as loss of
>20% of the mice initial body weight, and the diameter of the
tumor deposit was >1 cm. After 21 days, the animals were divided
into three experimental groups with three mice each: groups A, B,
and C were treated by intratumoral injection with 74 MBq (370
MBq/ml) 131I-EGFR-BSA-PCL, 74 MBq (370 MBq/ml)
131I-BSA-PCL, and 74 MBq (370 MBq/ml) 131I,
respectively.
At 4, 24, and 72 h after intratumoral injection,
single-photon emission computed tomography (SPECT) (Discovery VH;
GE Healthcare, Milwaukee, WI, USA) imaging was previously performed
(26). To reduce the exposure of
salivary and thyroid glands to unwanted radiation, all the mice
were administered with 1% sodium perchlorate solution via their
drinking water for 4 h before the experiment.
Statistical analysis
All experiments were performed in triplicate unless
otherwise indicated. Statistical analysis was performed using SPSS
software (SPSS 15.0). Results are presented as mean ± SD.
Statistical significance was examined using Student's t-test.
P<0.05 was considered statistically significant. For different
experimental groups, we compared the difference between different
drugs at the same medication time and at the same dose of the drug;
for the same group comparison, we compared the efficacy of the same
drug in different administration times, and compared the efficacy
of the same medication time at different doses.
Results
Internalization of EGFR-BSA-PCL and
BSA-PCL
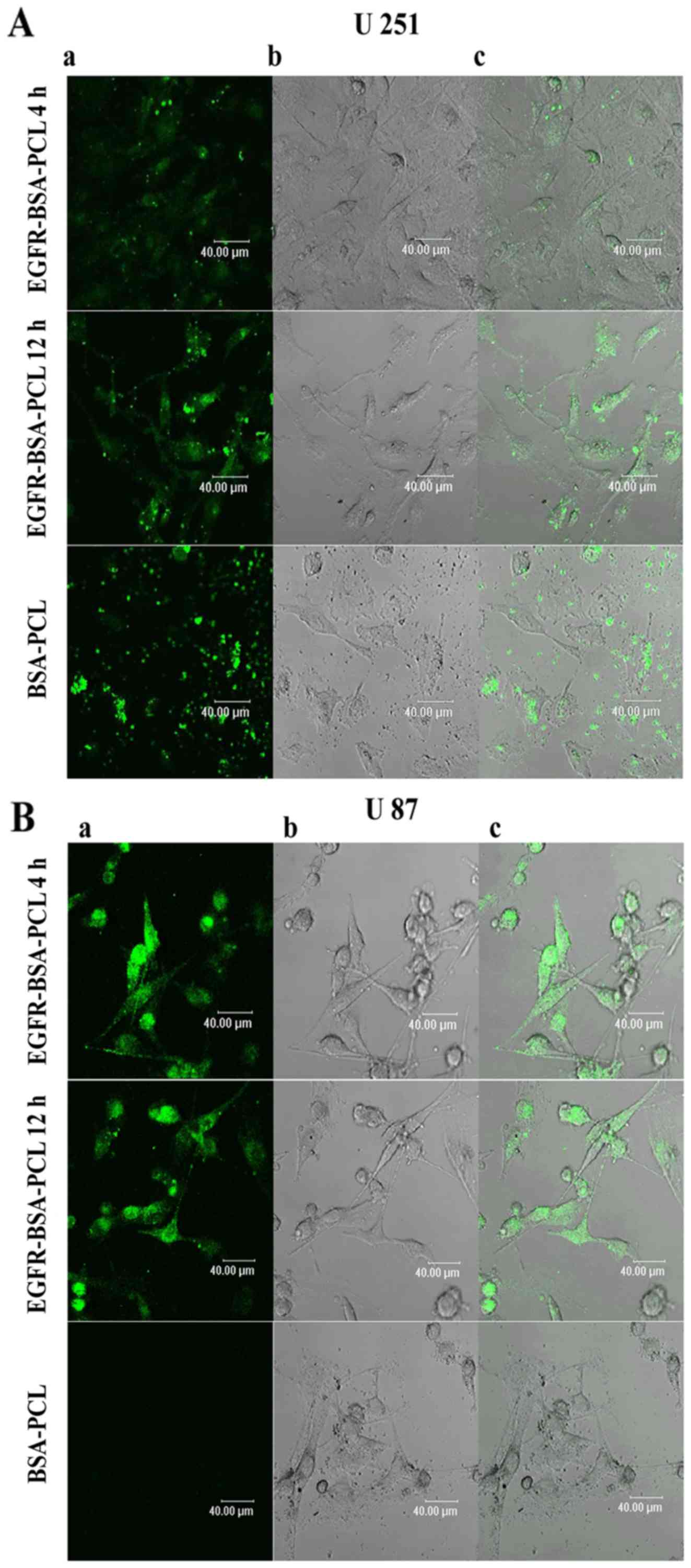
Confocal microscopy was used to analyze the binding
and internalization of targeted and non-targeted BSA-PCL. Specific
fluorescence staining on U251 and U87 cells was shown upon
incubation with EGFR-BSA-PCL and BSA-PCL after 4 or 12 h at 37°C
(Fig. 1). The fluorescence
intensity of the EGFR-BSA-PCL group was stronger than that of the
BSA-PCL group in both U251 and U87 cells regardless of similar
conditions at 37°C for 4 or 12 h of incubation.
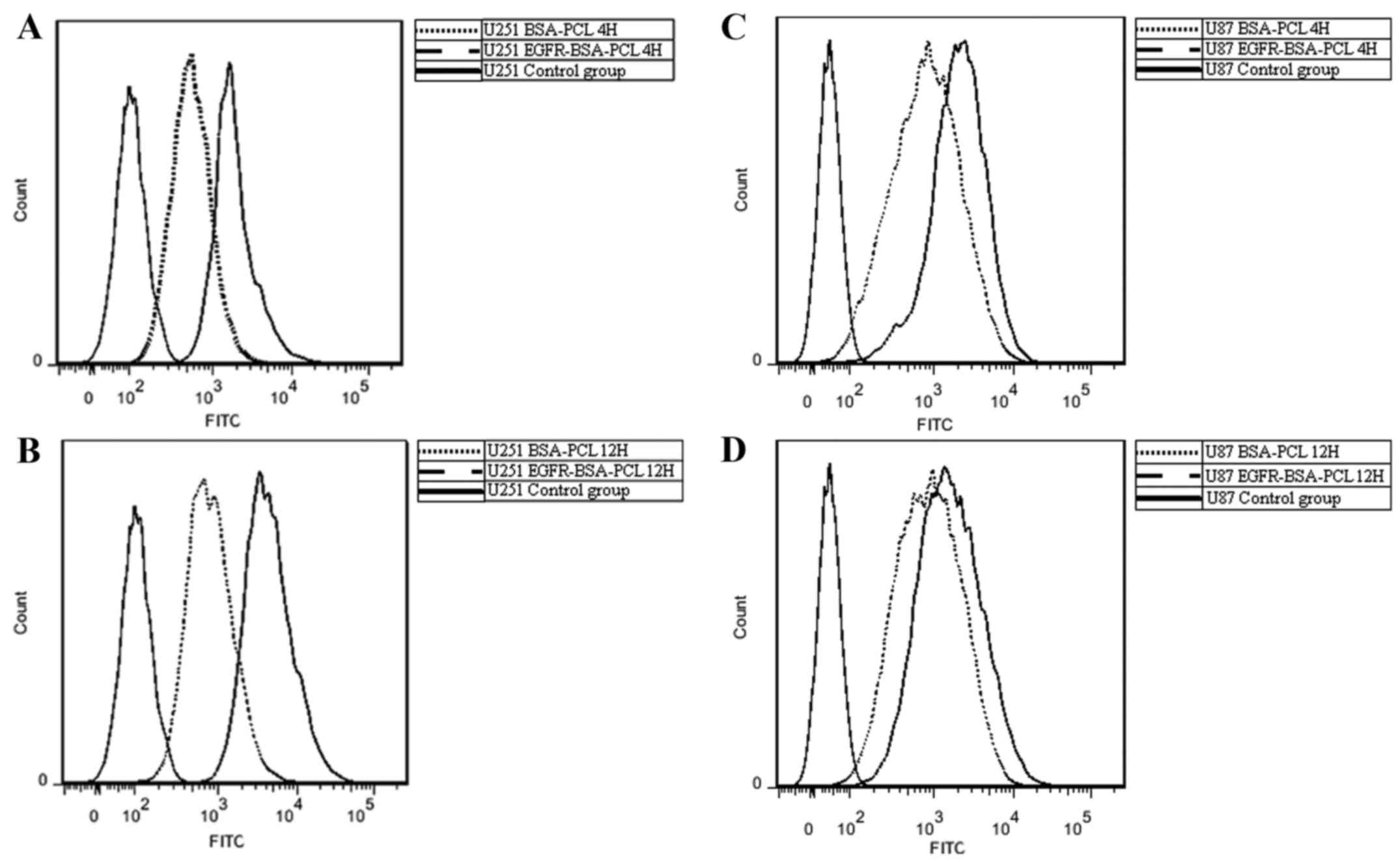
Flow cytometry binding analyses of
EGFR-BSA-PCL and BSA-PCL
The binding activity of the EGFR-BSA-PCL and BSA-PCL
conjugates was further confirmed by flow cytometry using the U251
and U87 cells that express EGFR. FCM analyses revealed results
consistent with those observed in the fluorescence microscopy
analyses. Hence, the binding and uptake of EGFR-BSA-PCL were
significantly higher than those of BSA-PCL in both the U87 and U251
cells after 4 or 12 h of incubation at 37°C. The uptake of
EGFR-BSA-PCL was 70.5 and 59.2% in the U251 and U87 cells,
respectively, after 4 h of incubation. The uptake of EGFR-BSA-PCL
was 76.0 and 60.0% in the U251 and U87 cells, respectively, after
12 h of incubation (Fig. 2). These
findings implied that EGFR-BSA-PCL had special binding efficiency
in the U251 and U87 cells which expressed EGFR.
Cellular binding and uptake of the two different
131I-labeled BSA-PCLs were evaluated by confocal
fluorescence microscopy and flow cytometry in the U251 and U87 cell
lines. The targeting efficiency of 131I-EGFR-BSA-PCL was
considerably higher than that of 131I-BSA-PCL,
EGFR-BSA-PCL, or naked BSA-PCL in both U251 and U87 cell lines.
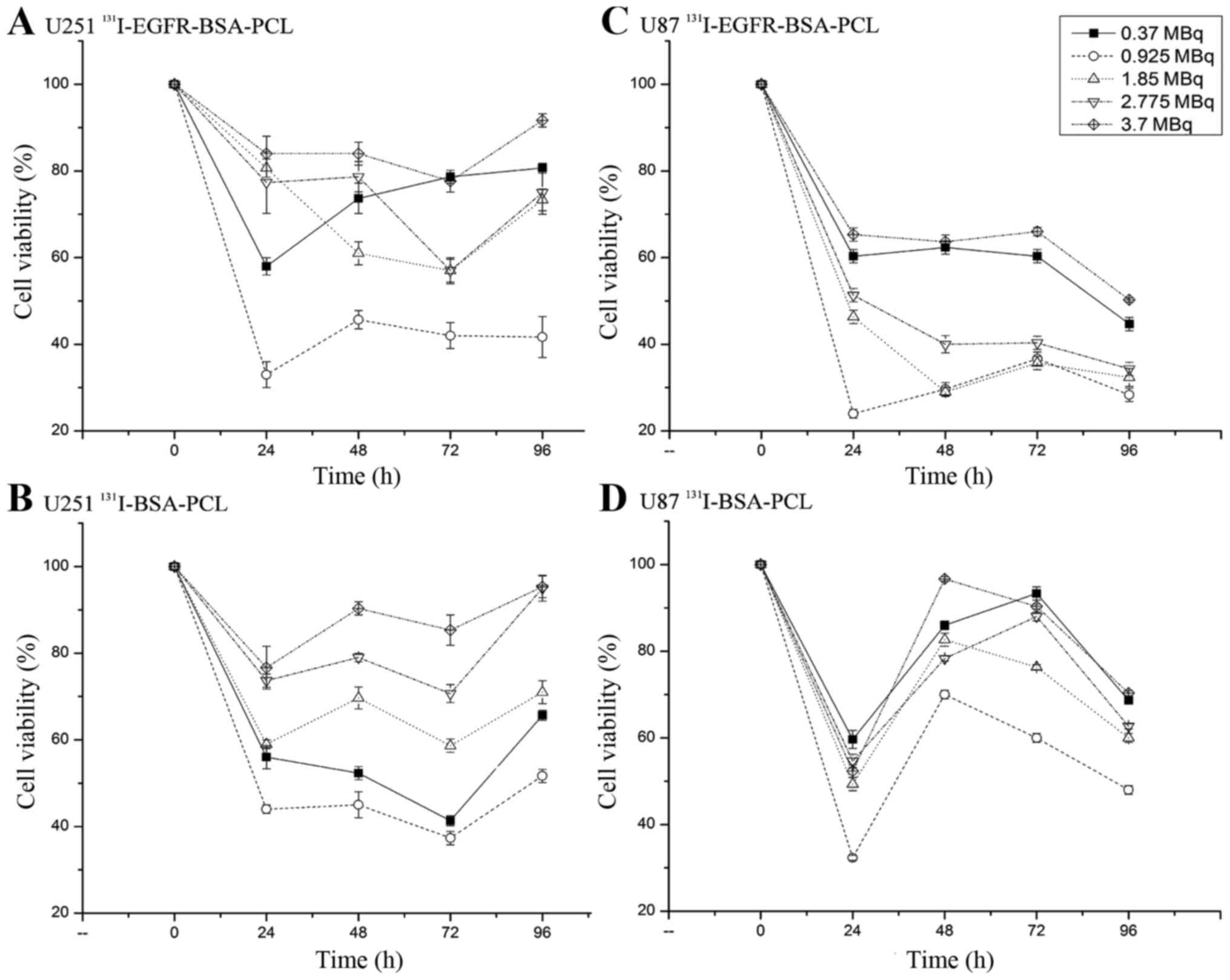
Analysis of cell viability determined by MTT cytotoxicity
experiment; (7,27–29).
Cytotoxicity was determined using MTT assay as previously reported.
Time- and dose-dependent MTT studies were performed (Fig. 3, Table
I). The data showed that 0.925 MBq 131I-EGFR-BSA-PCL
or 131I-BSA-PCL had the strongest inhibition effect on
tumor cells at 24, 48, 72 and 96 h. Experiments also showed that
131I-EGFR-BSA-PCL had better tumor inhibition effect
than 131I-BSA-PCL with extended time. After incubating
the nanoparticles for 4 h, the cell viability of the U251 and U87
cells was determined after 24, 48, 72 and 96 h, respectively. The
cells incubated with 131I or cetuximab alone have no
effect on the survival of glioma cells (data not shown).
 | Table I.After incubated with nanoparticles 4
h, the U251 and U87 cell viability in 96 h. |
Table I.
After incubated with nanoparticles 4
h, the U251 and U87 cell viability in 96 h.
|
| U251 | U87 |
|---|
|
|
|
|
|---|
| Treatment |
131I-EGFR-BSA-PCL (%) |
131I-BSA-PCL (%) |
131I-EGFR-BSA-PCL (%) |
131I-BSA-PCL (%) |
|---|
| 0.37 MBq | 80.67±1.15 | 65.67±1.16 | 44.67±1.53 | 68.67±0.58 |
| 0.925 MBq | 41.67±4.73 | 51.67±1.53 | 28.33±1.53 | 48.00±1.00 |
| 1.85 MBq | 73.33±2.52 | 71.00±2.65 | 32.33±2.08 | 60.00±1.00 |
| 2.775 MBq | 75.00±5.00 | 95.00±3.00 | 34.33±1.53 | 62.67±0.58 |
| 3.7 MBq | 91.67±1.53 | 95.33±2.52 | 50.33±0.58 | 70.33±0.58 |
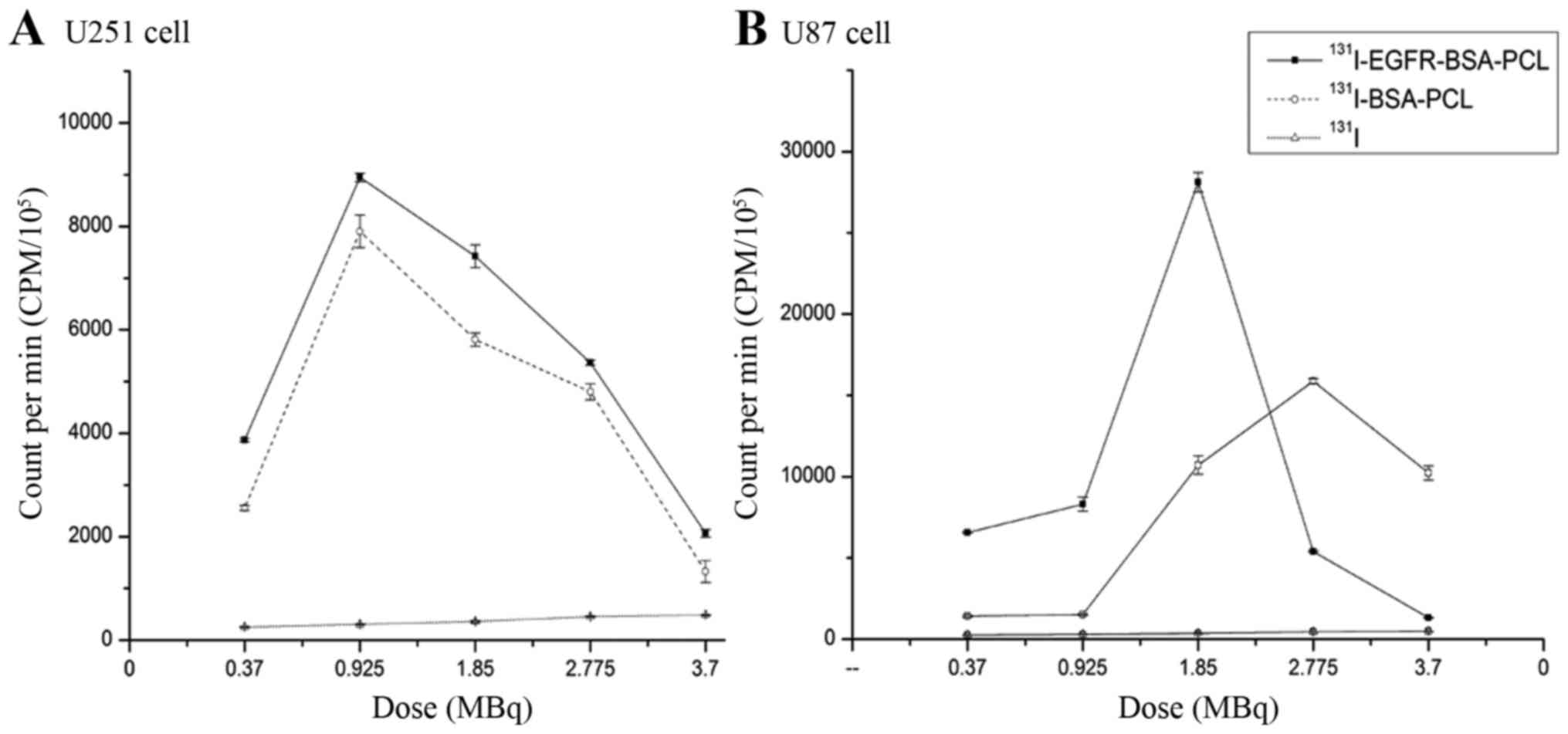
Radioiodine uptake of
131I-labeled nanoparticles in vitro
Confocal microscopy, flow cytometry, and MTT
analyses revealed that both 131I-EGFR-BSA-PCL and
131I-BSA-PCL could inhibit cell growth. However, the
inhibition effect of 131I-EGFR-BSA-PCL was better than
that of 131I-BSA-PCL in both U251 and U87 cells.
Radionuclide uptake results were consistent with the CPM of
131I-EGFR-BSA-PCL, which was always higher than
131I-BSA-PCL in both U251 and U87 cells. After 4 h
incubation with 131I-EGFR-BSA-PCL and
131I-BSA-PCL, the radioiodine uptake was distributed in
a parabola fashion in the U251 and U87 cells (Fig. 4). When radioiodine uptake reached
the peak, the CPM decreased as the dose of 131I-labeled
nanoparticles increased. In the U251 cells, 0.925 MBq
131I-EGFR-BSA-PCL and 1.85 MBq 131I-BSA-PCL
had the highest CPM. In the U87 cells, 1.85 MBq
131I-EGFR-BSA-PCL and 2.775 MBq 131I-BSA-PCL
had the highest CPM.
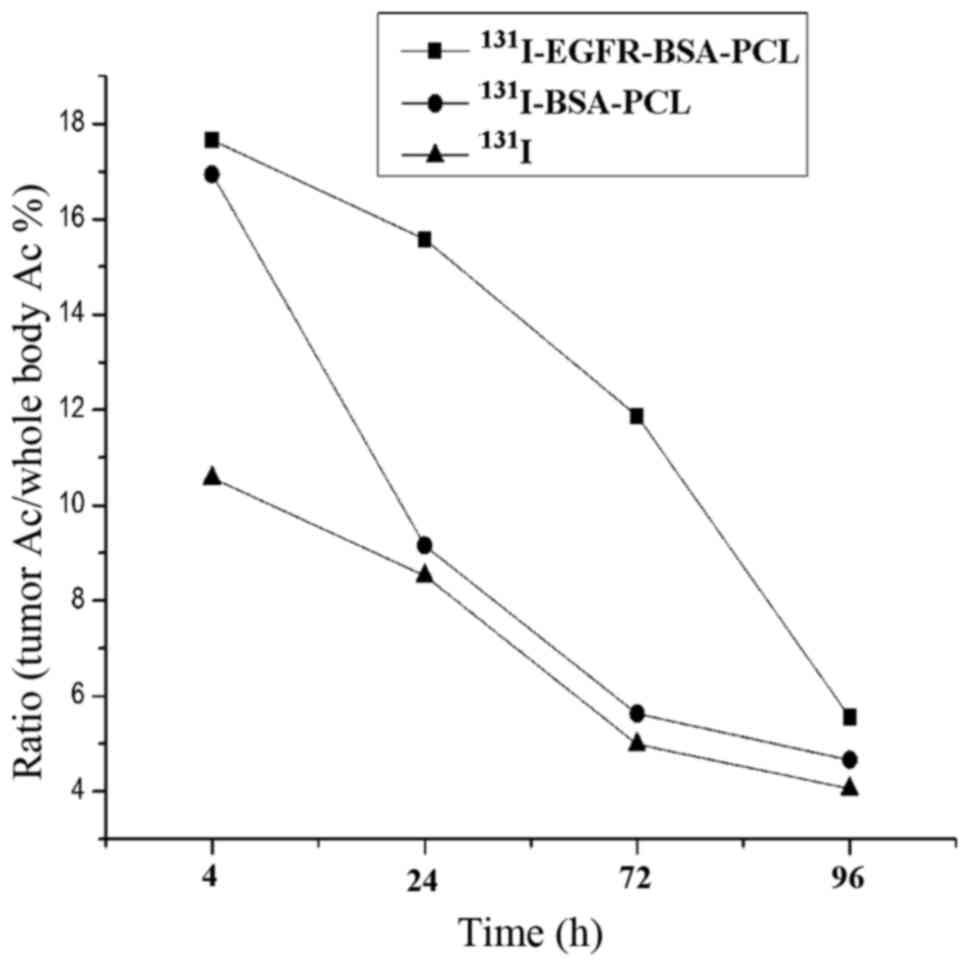
SPECT imaging: Biodistribution in
animal experiments; (17,21,22)
The biodistribution of 131I-EGFR-BSA-PCL,
131I-BSA-PCL, and 131I in mice bearing U87
tumor xenografts at 4, 24, and 72 h and the ratio of radioactivity
counts at different time points are shown in Fig. 5.
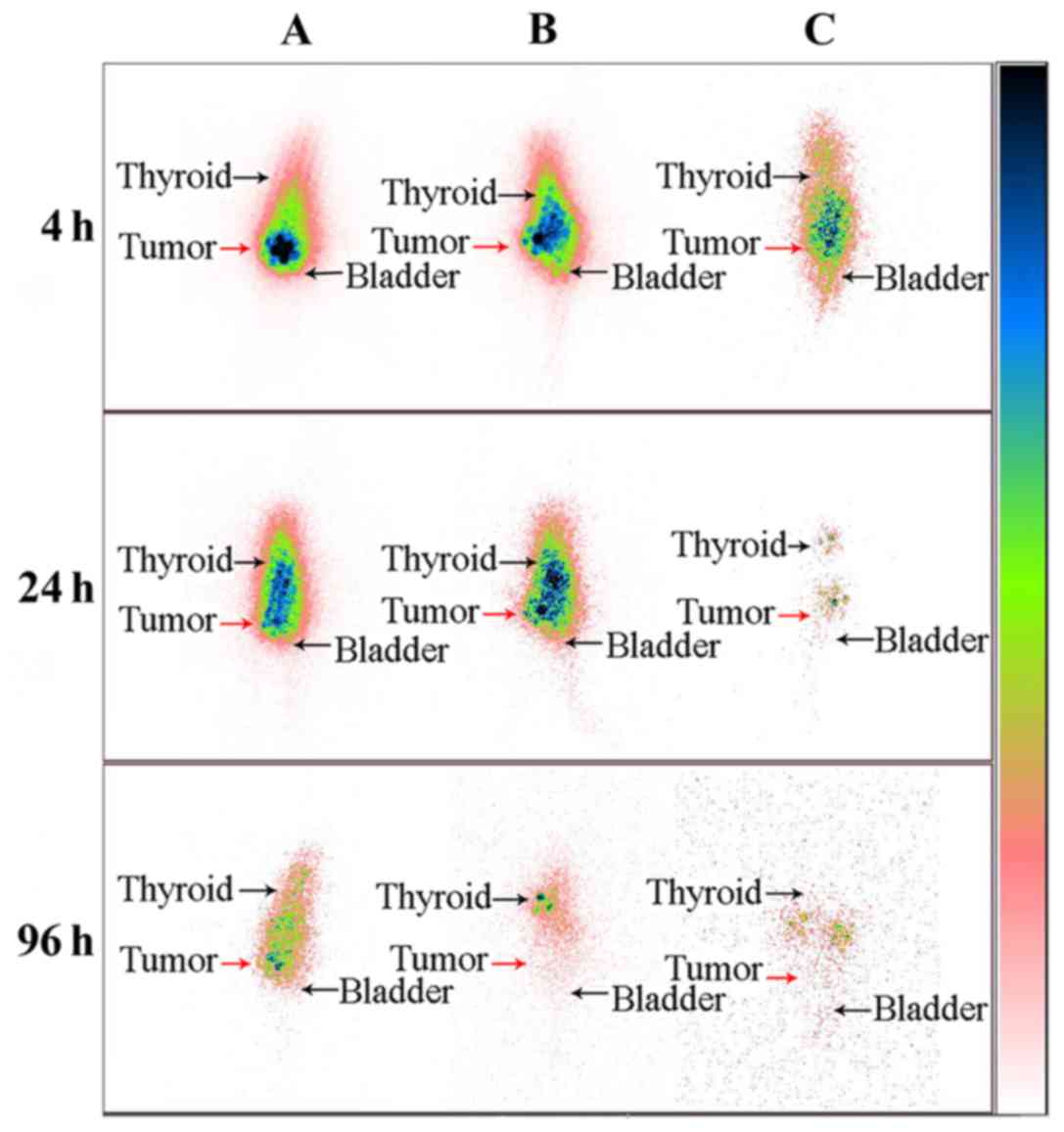
SPECT images showed that after the injection of the
drug, the development of the tumor site was the most clear
(Fig. 6). This finding indicated
that the drugs are mainly gathered at the tumor site, and with
time, the development of the tumor tissues gradually fade; the
other parts of the nude mice develop gradually, indicating that the
drug entered through the bloodstream to other parts of the body and
the retention time of 131I-EGFR-BSA-PCL in the tumor
tissues was longer than that of 131I-BSA-PCL and
131I. The ratio of radioactivity count with tumor tissue
and nude body at 4 h after injection was the highest, followed by a
decreasing trend. In the ratio of radioactive counts at different
time points, 131I-EGFR-BSA-PCL group was higher than the
131I-BSA-PCL and 131I groups, and the
decreasing trend was slower, indicating that the residence time of
131I-EGFR-BSA-PCL in the tumor was the longest.
Discussion
EGFR overexpression or overactivation is commonly
observed in GBM tumors (40–70% of patients) (30,31).
EGFR overexpression has been correlated with treatment resistance,
as well as poor survival and prognosis (22,32).
Given the advantage of this feature of EGFR, anti-EGFR was
connected to the surface of nanoparticles, thereby targeting the
combination of nanoparticles to the surface of glioma cells and
simultaneously increasing the number of nanoparticles on the cell
surface or with internal retention time. The use of the EGFR
tyrosine kinase inhibitor cetuximab showed no measurable responses
(33). In vitro studies
revealed that EGFR-bound nanoparticles are easier to combine with
the GMB cells, and the rate of combination was higher. Cell
viability was determined by MTT assays as described above. The data
showed that both 131I-EGFR-BSA-PCL and
131I-BSA-PCL could inhibit the growth of U251 and U87
glioma cells. After 4 h incubation with nanoparticles, the
inhibition effect of 131I-EGFR-BSA-PCL was better than
that of 131I-BSA-PCL on tumor cells. The 0.925 MBq
131I-EGFR-BSA-PCL or 131I-BSA-PCL also had
the strongest inhibition effect on tumor cells at 24, 48, 72, and
96 h.
In the radioiodine uptake experiments, when the
concentration of 131I-EGFR-BSA-PCL achieved the maximum
lethal dose, further increase of the 131I-EGFR-BSA-PCL
concentration did not increase the killing effect to the glioma
cell. The 0.925 MBq 131I-EGFR-BSA-PCL and
131I-BSA-PCL had the strongest inhibitory effect on cell
growth.
Given that the radiation dose of nanoparticles
reached 0.925 MBq, the inhibitory effect on tumor cells would
decrease as the nanoparticles increased. This finding may be
ascribed to the blunt inhibition phenomena, which often occur in
the nuclide treatment. A dosage more than the maximum lethal dose
of 131I-labeled nanoparticles kills the capability of
glioma cells to increase; this inhibition usually occurs in thyroid
cells (34). Some studies also
showed that positive EGFR immunoreactivity predicted poor
radiographically assessed radiation response. Significant
relationships were noted among the EGFR scores (35,36).
Previous studies also suggested that the observed relative
radioresistance of some GMs is associated with overexpression of
EGFR (36). However, the results
cannot explain the weakened inhibition effect of glioma cells when
131I-labeled nanoparticles exceed the maximum lethal
dose.
Overall, the results raise the possibility that GBM
with EGFR overexpression may derive the most significant benefit,
in terms of tumor regression, if treated with
131I-EGFR-BSA-PCL. This regimen represents new
therapeutic and diagnostic approach options for most patients with
GBM and provides a foundation for additional studies directed
toward further improvement in the outcome of this disease.
In conclusion, the findings of the present study
suggested that 131I-EGFR-BSA-PCL leading radioiodine
therapy for U251 and U87 cells had a good effect on in vitro
and in vivo experiments. The 131I-EGFR-BSA-PCL
may provide a new method for glioblastoma treatment.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (to J.T.) (no. 81171372) (to
W.L.) (no. 81301244), the Tianjin Research Program of Application
Foundation and Advanced Technology (to J.T.) (no. 12JCZDJC26000),
National Key Clinical Speciatly Project of China and the Young
incubation funding (TMUGH funding no. 2015040) of Tianjin Medical
University General Hospital.
References
|
1
|
Oike T, Suzuki Y, Sugawara K, Shirai K,
Noda SE, Tamaki T, Nagaishi M, Yokoo H, Nakazato Y and Nakano T:
Radiotherapy plus concomitant adjuvant temozolomide for
glioblastoma: Japanese mono-institutional results. PLoS One.
8:e789432013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bouras A, Kaluzova M and Hadjipanayis CG:
Radiosensitivity enhancement of radioresistant glioblastoma by
epidermal growth factor receptor antibody-conjugated iron-oxide
nanoparticles. J Neurooncol. 124:13–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lun X, Wells JC, Grinshtein N, King JC,
Hao X, Dang NH, Wang X, Aman A, Uehling D, Datti A, et al:
Disulfiram when combined with copper enhances the therapeutic
effects of temozolomide for the treatment of glioblastoma. Clin
Cancer Res. 22:3860–3875. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okura H, Smith CA and Rutka JT: Gene
therapy for malignant glioma. Mol Cell Ther. 2:212014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jarzabek MA, Sweeney KJ, Evans RL, Jacobs
AH, Stupp R, O'Brien D, Berger MS, Prehn JH and Byrne AT: Molecular
imaging in the development of a novel treatment paradigm for
glioblastoma (GBM): An integrated multidisciplinary commentary.
Drug Discov Today. 18:1052–1066. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Macher-Goeppinger S, Penzel R, Roth W,
Dienemann H, Thomas M, Schnabel PA, Schirmacher P and Bläker H:
Expression and mutation analysis of EGFR, c-KIT, and β-catenin in
pulmonary blastoma. J Clin Pathol. 64:349–353. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kao H-W, Lin Y-Y, Chen C-C, Chi KH, Tien
DC, Hsia CC, Lin MH and Wang HE: Evaluation of EGFR-targeted
radioimmuno-gold-nanoparticles as a theranostic agent in a tumor
animal model. Bioorg Med Chem Lett. 23:3180–3185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang Y, Wang W, Zheng K, Jiang L, Zou Y,
Su X, Chen J, Zhang W and Liu W: EGFR mutations in non-small cell
lung cancer: An audit from West China University Hospital. Expert
Rev Mol Diagn. 16:915–919. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brinkman AM, Chen G, Wang Y, Hedman CJ,
Sherer NM, Havighurst TC, Gong S and Xu W: Aminoflavone-loaded
EGFR-targeted unimolecular micelle nanoparticles exhibit
anti-cancer effects in triple negative breast cancer. Biomaterials.
101:20–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen CL, Hu GY, Mei Q, Qiu H, Long GX and
Hu GQ: Epidermal growth factor receptor-targeted ultra-small
superparamagnetic iron oxide particles for magnetic resonance
molecular imaging of lung cancer cells in vitro. Chin Med J (Engl).
125:2322–2328. 2012.PubMed/NCBI
|
|
11
|
Chakravarti A, Dicker A and Mehta M: The
contribution of epidermal growth factor receptor (EGFR) signaling
pathway to radioresistance in human gliomas: A review of
preclinical and correlative clinical data. Int J Radiat Oncol Biol
Phys. 58:927–931. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu Z, Dong C, Wang X, Wang H, Li W, Tan J
and Chang J: Self-assembled biodegradable protein-polymer vesicle
as a tumor-targeted nanocarrier. ACS Appl Mater Interfaces.
6:2393–2400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su X-Y, Liu P-D, Wu H and Gu N:
Enhancement of radiosensitization by metal-based nanoparticles in
cancer radiation therapy. Cancer Biol Med. 11:86–91.
2014.PubMed/NCBI
|
|
15
|
Akbari B, Farajnia S, Zarghami N, Mahdieh
N, Rahmati M, Khosroshahi SA and Rahbarnia L: Design, expression
and evaluation of a novel humanized single chain antibody against
epidermal growth factor receptor (EGFR). Protein Expr Purif.
127:8–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu S, Shi F, Chen L and Su X: Bovine
serum albumin coated CuInS2 quantum dots as a near-infrared
fluorescence probe for 2,4,6-trinitrophenol detection. Talanta.
116:870–875. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Behray M, Webster CA, Pereira S, Ghosh P,
Krishnamurthy S, Al-Jamal WT and Chao Y: Synthesis of diagnostic
silicon nanoparticles for targeted delivery of thiourea to
epidermal growth factor receptor-expressing cancer cells. ACS Appl
Mater Interfaces. 8:8908–8917. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sundberg ÅL, Blomquist E, Carlsson J,
Steffen A-C and Gedda L: Cellular retention of radioactivity and
increased radiation dose. Model experiments with EGF-dextran. Nucl
Med Biol. 30:303–315. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nordberg E, Friedman M, Göstring L, Adams
GP, Brismar H, Nilsson FY, Ståhl S, Glimelius B and Carlsson J:
Cellular studies of binding, internalization and retention of a
radiolabeled EGFR-binding affibody molecule. Nucl Med Biol.
34:609–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sheng R, Luo T, Zhu Y, Li H, Sun J, Chen
S, Sun W and Cao A: The intracellular plasmid DNA localization of
cationic reducible cholesterol-disulfide lipids. Biomaterials.
32:3507–3519. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mortensen JH, Jeppesen M, Pilgaard L,
Agger R, Duroux M, Zachar V and Moos T: Targeted antiepidermal
growth factor receptor (Cetuximab) immunoliposomes enhance cellular
uptake in vitro and exhibit increased accumulation in an
intracranial model of glioblastoma multiforme. J Drug Deliv.
2013:2092052013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Verreault M, Weppler SA, Stegeman A,
Warburton C, Strutt D, Masin D and Bally MB: Combined RNAi-mediated
suppression of Rictor and EGFR resulted in complete tumor
regression in an orthotopic glioblastoma tumor model. PLoS One.
8:e595972013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weiss SJ, Philp NJ and Grollman EF: Iodide
transport in a continuous line of cultured cells from rat thyroid.
Endocrinology. 114:1090–1098. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Petrich T, Helmeke H-J, Meyer GJ, Knapp WH
and Pötter E: Establishment of radioactive astatine and iodine
uptake in cancer cell lines expressing the human sodium/iodide
symporter. Eur J Nucl Med Mol Imaging. 29:842–854. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen J, Wu H, Han D and Xie C: Using
anti-VEGF McAb and magnetic nanoparticles as double-targeting
vector for the radioimmunotherapy of liver cancer. Cancer Lett.
231:169–175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milane L, Duan ZF and Amiji M:
Pharmacokinetics and biodistribution of lonidamine/paclitaxel
loaded, EGFR-targeted nanoparticles in an orthotopic animal model
of multi-drug resistant breast cancer. Nanomedicine (Lond).
7:435–444. 2011. View Article : Google Scholar
|
|
27
|
Dua P and Gude RP: Antiproliferative and
antiproteolytic activity of pentoxifylline in cultures of B16F10
melanoma cells. Cancer Chemother Pharmacol. 58:195–202. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goel PN and Gude RP: Unravelling the
antimetastatic potential of pentoxifylline, a methylxanthine
derivative in human MDA-MB-231 breast cancer cells. Mol Cell
Biochem. 358:141–151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jain DS, Athawale RB, Bajaj AN, Shrikhande
SS, Goel PN, Nikam Y and Gude RP: Unraveling the cytotoxic
potential of Temozolomide loaded into PLGA nanoparticles. Daru.
22:182014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohgaki H and Kleihues P: Genetic pathways
to primary and secondary glioblastoma. Am J Pathol. 170:1445–1453.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nicholas MK, Lukas RV, Jafri NF, Faoro L
and Salgia R: Epidermal growth factor receptor - mediated signal
transduction in the development and therapy of gliomas. Clin Cancer
Res. 12:7261–7270. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang PH, Xu AM and White FM: Oncogenic
EGFR signaling networks in glioma. Sci Signal. 2:re62009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Herbst RS, Johnson DH, Mininberg E,
Carbone DP, Henderson T, Kim ES, Blumenschein G Jr, Lee JJ, Liu DD,
Truong MT, et al: Phase I/II trial evaluating the anti-vascular
endothelial growth factor monoclonal antibody bevacizumab in
combination with the HER-1/epidermal growth factor receptor
tyrosine kinase inhibitor erlotinib for patients with recurrent
non-small-cell lung cancer. J Clin Oncol. 23:2544–2555. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schaefers MM, Breshears LM, Anderson MJ,
Lin YC, Grill AE, Panyam J, Southern PJ, Schlievert PM and Peterson
ML: Epithelial proinflammatory response and curcumin-mediated
protection from staphylococcal toxic shock syndrome toxin-1. PLoS
One. 7:e328132012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mukohara T, Engelman JA, Hanna NH, Yeap
BY, Kobayashi S, Lindeman N, Halmos B, Pearlberg J, Tsuchihashi Z,
Cantley LC, et al: Differential effects of gefitinib and cetuximab
on non-small-cell lung cancers bearing epidermal growth factor
receptor mutations. J Natl Cancer Inst. 97:1185–1194. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Barker FG II, Simmons ML, Chang SM, Prados
MD, Larson DA, Sneed PK, Wara WM, Berger MS, Chen P, Israel MA, et
al: EGFR overexpression and radiation response in glioblastoma
multiforme. Int J Radiat Oncol Biol Phys. 51:410–418. 2001.
View Article : Google Scholar : PubMed/NCBI
|