Introduction
Gap junctions (GJs), principally composed of
connexins (Cxs), are important for communication between tumor
cells and stromal cells. Increased GJ coupling has been reported to
hinder metastatic potential in a number of animal tumor models,
including breast cancer and melanoma (1). Although many studies sustain the
viewpoint that Cxs are tumor suppressors, recent evidence suggests
that, in some tumor types, they may promote certain stages of tumor
progression through junctional and non-junctional pathways
(2). Cx26, but not Cx40 or Cx43,
was shown to suppress tumorigenic features in cervical cancer
(CaCx) HeLa cells, even though all three Cxs increased GJ
intracellular communication (GJIC) (3). Downregulation in Cx32 results in the
proliferation and metastasis of hepatocellular carcinoma (HCC), and
the restoration of Cx32 expression may be a prospective strategy
for the treatment of HCC (4).
However, accumulation of cytoplasmic Cx32 can increase the
self-renewal of cancer stem cells (CSCs) to expand the CSC
population in HCC (5).
Tumor necrosis factor α (TNFα) activates caspase-8
in the extrinsic pathway of apoptosis. Aberrant secretion of TNFα
facilitates a number of human diseases and has been implicated in
tumor development and inflammation (6). In particular, TNFα polymorphisms have
been associated with cervical cancer (7). Persistent high-risk human
papillomavirus (HPV) infection gives rise to
inflammation-associated CaCx progression. HPV-negative head and
neck cancers express epidermal growth factor receptor (EGFR) to a
high level, and a monoclonal antibody for EGFR (cetuximab) is
currently the only targeted therapy that has improved survival in
patients with this disease (8). To
prevent the retention of HPV within cells, TNFα-induced apoptosis
is a key defense strategy (9);
however, the function of Cx32 proteins in this mechanism of
extrinsically triggered cell death remains unknown.
Our previous study demonstrated that Cx32 regulated
EGFR expression and exerted a pro-tumor effect in CaCx (10). Cx32 can suppress endogenous
apoptosis induced by streptonigrin in CaCx. However, the role of
Cx32 in TNFα-induced extrinsic apoptosis is unclear. A high CaCx
systemic inflammation score has been correlated with more advanced
FIGO stages and poor tumor differentiation (11). In the present study, we investigated
whether Cx32 was a key regulator of tumor growth associated with
TNFα-related inflammation.
Materials and methods
Materials
Dimethyl sulfoxide (DMSO), 18α-glycyrrhetinic acid
(18α-GA), 2-aminoethoxydiphenyl-borate (2-APB), anti-β-tubulin and
anti-β-actin mouse IgG primary antibodies, and secondary antibodies
were purchased from Sigma-Aldrich (St. Louis, MO, USA). Anti-Cx32
antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). Antibodies against Cox2, EGFR, p-ERK1/2
(Thr202/Tyr204), STAT3, p-STAT3 (Tyr705), TNFα and survivin were
obtained from Cell Signaling Technology, Inc. (Danvers, MA, USA).
TNFα was obtained from PeproTech, (Rocky Hill, NJ, USA). Hygromycin
B, G418 and doxycycline (Dox) were obtained from Calbiochem (San
Diego, CA, USA). An Annexin V-FITC apoptosis detection kit was
purchased from BioTool, LLC (Houston, TX, USA). Cycloheximide (CHX)
was purchased from Beijing Dingguo Changsheng Biotechnology, Co.,
Ltd. (Beijing, China). Lipofectamine™ 2000 was purchased from Gibco
(Carlsbad, CA, USA). Calcein-AM (acetoxymethyl ester) was obtained
from Invitrogen (Carlsbad, CA, USA). All other reagents were
purchased from Sigma-Aldrich unless stated otherwise.
Clinical tissue specimens
The clinical cervical carcinoma and para-carcinoma
tissue samples were obtained from the Affiliated Tumor Hospital of
Xinjiang Medical University (Urumqi, China). Cervical tissue
samples (n=15) were resected during surgery. The use of these
clinical samples was approved by the ethics committee of Xinjiang
Medical University Affiliated Tumour Hospital.
Cell lines and cell culture
The C-33A cell line was acquired from the American
Type Culture Collection (ATCC; Manassas, VA, USA). C-33A cells were
cultured in minimum essential medium supplemented with 10% fetal
bovine serum (FBS). Cx32 under the control of a bidirectional
tetracycline-inducible promoter was stably transfected into HeLa
cells (HeLa-Cx32) for subsequent induction of Cx32 expression via
incubation with Dox (1 µg/ml) for ~48 h (12). Prior to treatment with Dox, 100
µg/ml G418 sulfate and 200 µg/ml hygromycin B were added to the
medium [Dulbeccos modified Eagles medium (DMEM) supplemented with
10% FBS] to select for stably transfected cells. Besides
high-density culturing, we also use a low-density culture method,
and the HeLa-Cx32 cells were seeded into 150-mm dishes. At this
low-density, there was a lack of cell-cell contacts, which
prevented the formation of GJs and enabled investigation into the
non-junctional function of Cx32 in apoptosis.
GJ functional assay
GJIC function was evaluated using a ‘parachute’ dye
coupling assay, as described by Goldberg et al (13) and Koreen et al (12). The experiment was devided into
control group, Dox group, Dox+18α-GA group and Dox+2APB group. In
this assay, donor and receiver HeLa-Cx32 cells were grown to
confluence in 12-well plates. After the cells were cultured to
confluence, donor cells were labeled with 5 µM calcein-AM for 30
min at 37°C. The donor cells were then rinsed, trypsinized and
seeded onto the receiver cells at a 1:150 donor/receiver ratio. The
donor cells were incubated at 4 h at 37°C to allow attachment to
the monolayer of receiver cells and the formation of GJs. Cells
were subsequently observed under a fluorescence microscope (Olympus
IX71; Olympus Corp., Tokyo, Japan). Donor cells can be labeled and
observed by strong calcein-AM staining. Calcein-AM from donor cells
can be intracellularly transferred into receiver cells when GJIC is
present. The level of GJIC was measured as the average number of
receiver cells containing Calcein-AM per donor cell observed by
fluorescence microscopy.
Cx32 siRNA and EGFR interference
assay
After growing C-33A cells to 30–50% confluence, 50
nM of non-specific (NS) siRNA (negative control) or Cx32-siRNA
(Guangzhou RiboBio, Co., Ltd., Guangzhou, China) and Lipofectamine™
2000 were mixed and added to cells. Lipofectamine™ 2000 was used to
transfect cells according to the manufacturers protocol. After
incubation with the siRNAs for 48 h, further experiments were
conducted in the cells.
For the subsequent knockdown of EGFR, the following
EGFR siRNAs were synthesized: siEGFR_1, 5′-GGCTGGTTATGTCCTCATT-3′;
siEGFR_2, 5′-CCTTAGCAGTCTTATCTAA-3′; and siEGFR_3,
5′-GGAACTGGATATTCTGAAA-3′. Among them, siEGFR_1 was determined to
be the most effective by western blot analysis, and was selected
for an EGFR targeting assay.
For the subsequent knockdown of Cx32, the following
Cx32 siRNAs were synthesized: siCx32_1, 5′-CCGGCATTCTACTGCCATT-3′;
siCx32_2, 5′-GGCTCACCAGCAACACATA-3′; and siCx32_3,
5′-GCAACAGCGTTTGCTATGA-3′. Among them, siCx32_3 was chosen for
further experiments after confirmation by western blot
analysis.
Apoptosis assay
Approximately 1–2×105 HeLa-Cx32
cells/well were seeded into 6-well plates. After adherence, the
cells were incubated with Dox or DMSO for 48 h. TNFα (100 ng/ml)
was then added to the HeLa-Cx32 cells for 24 h, and cells were
incubated with CHX (0.1 or 1 µg/ml) or afatinib (1.25 µM) except
EGFR siRNA group. The cells were washed twice with
phosphate-buffered saline (PBS) and trypsinized, terminated by
medium and harvested. After the cells were centrifuged at 1,000 rcf
for 5 min at room temperature and resuspended with PBS twice,
binding buffer and Annexin V-FITC with propidium iodide (PI) were
used to stain cells for 15 min away from light at room temperature.
Subsequently, the cells were immediately analyzed with a flow
cytometer. Expo32 software was used to determine the rate of early
apoptosis.
Western blotting
Homogenized tissue samples (50–100 mg) or cells were
rinsed with PBS and treated with ice-cold lysis-buffer [1 mM
β-glycerophosphate, 2.5 mM sodium pyrophosphate, 20 mM Tris-HCl (pH
7.4), 1% Triton X-100, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM
Na3VO4 and 1:1,000 protease inhibitors) for
at least 30 min. After scraping, collection and ultrasonication,
the lysate solutions were centrifuged at 12,000 rcf for 30 min at
4°C, and the supernatant was retained. A BCA protein assay kit
(Thermo Fisher Scientific, Waltham, MA, USA) was used to measure
protein concentration. For western blotting, equal amounts (20 µg)
of protein were prepared and separated by SDS-PAGE and transferred
to PVDF membranes. The membranes were blocked with 5% (w/v) skimmed
dry milk in wash buffer [TBS and 0.05% Tween-20 (TBST)] for 1 h.
The membranes were then incubated with monoclonal antibodies
against Cx32 (1:1,000), EGFR (1:1,000), p-ERK (1:1,000), STAT3
(1:1,000), p-STAT3 (1:1,000), Cox-2 (1:1,000), survivin (1:1,000),
TNFα (1:1,000), β-actin (1:10,000) and β-tubulin (1:10,000)
overnight at 4°C. HRP-conjugated secondary antibodies were applied
to the membranes for 1–2 h at room temperature, and the membrane
was then washed with TBST. Immunoreactive bands on the membrane
were visualized using Western Lightning chemiluminescence reagents
(Thermo Fisher Scientific). β-tubulin and β-actin were used as
control markers. The control bands were set as ‘100’ and the fold
changes in each samples ratio to the control bands were used as the
finalized data.
Statistical analysis
All experiments were repeated at least three times.
The data were presented as the mean ± standard error (SE) and
analyzed using the SPSS 16.0 software. Statistical significance
(P<0.05) was analyzed by one-way ANOVA (>2 groups) or a
Student's t-test (2 groups). Western blot data was analyzed with
the ImageJ software. Histograms or scatter diagrams were
constructed with the Prism software. P<0.05 indicates a
significant difference.
Results
Inducible Cx32 expression model in
HeLa cells (HeLa-Cx32) confirmed by western blot analysis
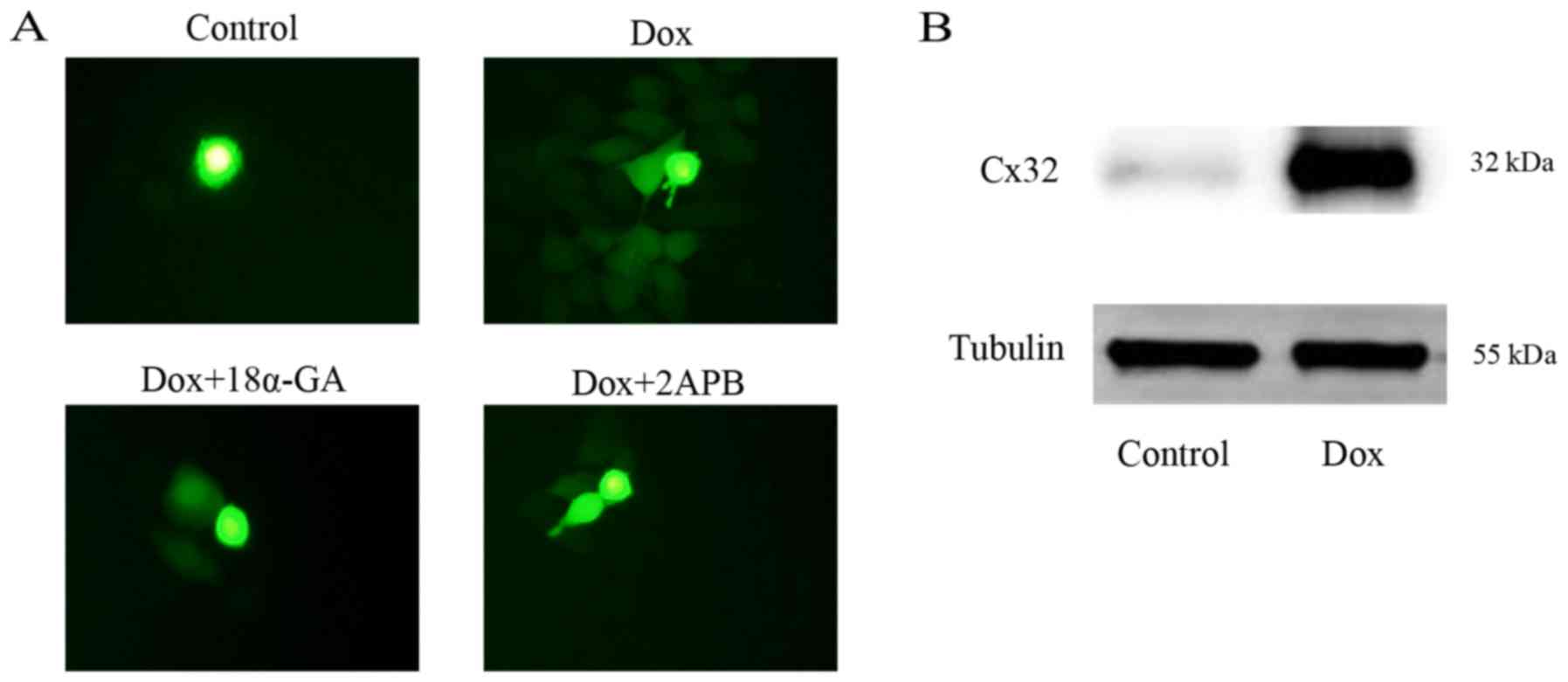
The GJ function of the HeLa-Cx32 cell model was
assessed with a parachute assay, as depicted in Fig. 1A. Results of the control group, Dox
group, Dox+18α-GA group and Dox+2APB group are displayed in the
figure. The results showed that after induction with Dox, GJs were
formed, and also indicated that 18α-GA and 2APB could effectively
suppress GJ function. The drug concentrations of 10 µM 18α-GA and
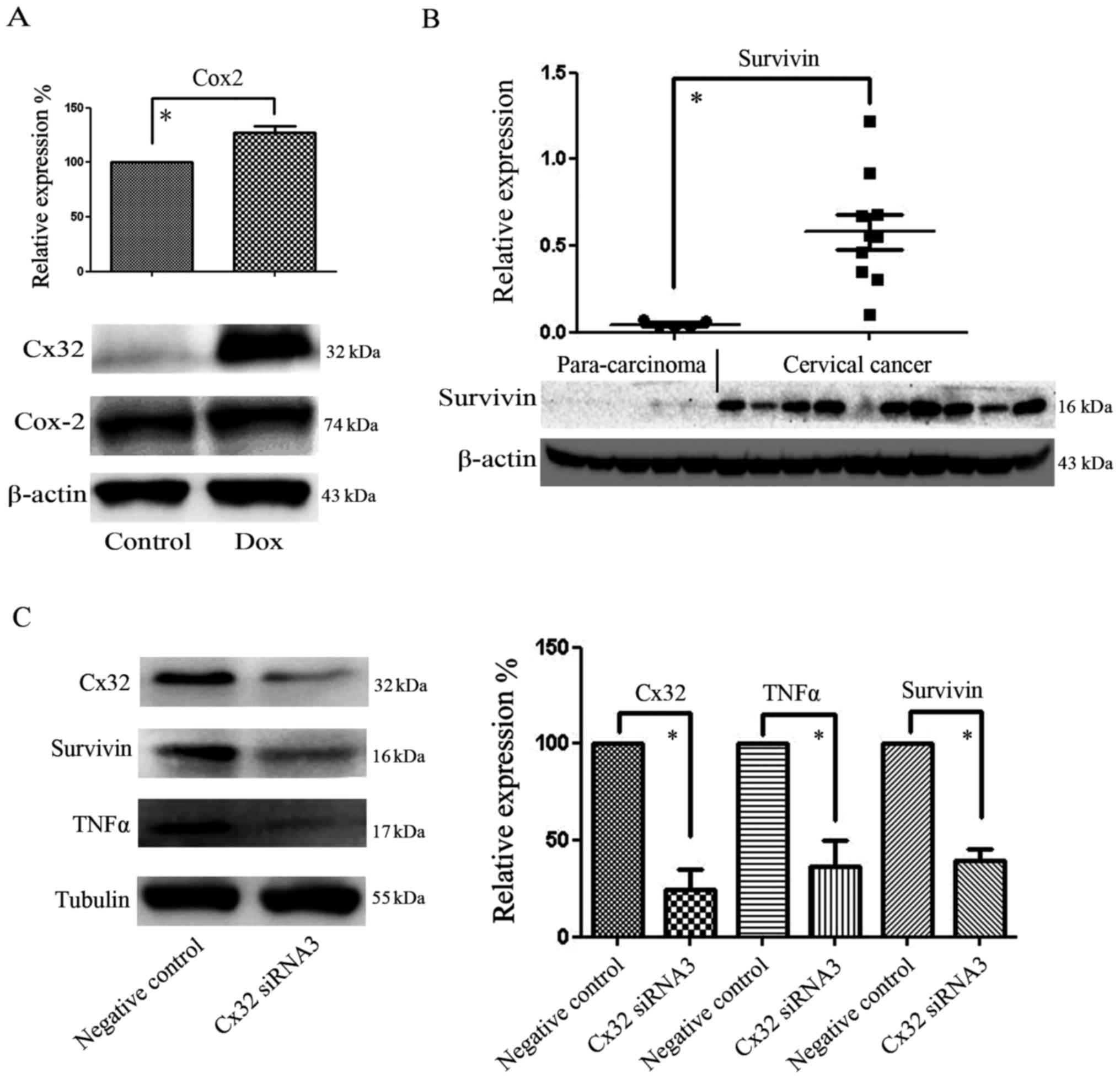
50 µM 2APB were selected according to our former study (10). As shown in Fig. 1B, western blotting indicated that
Dox induced high-level expression of Cx32, and thus this cell model
was used in subsequent experiments.
Cx32 expression and EGFR-related
signal molecules in the different HeLa-Cx32 cell groups
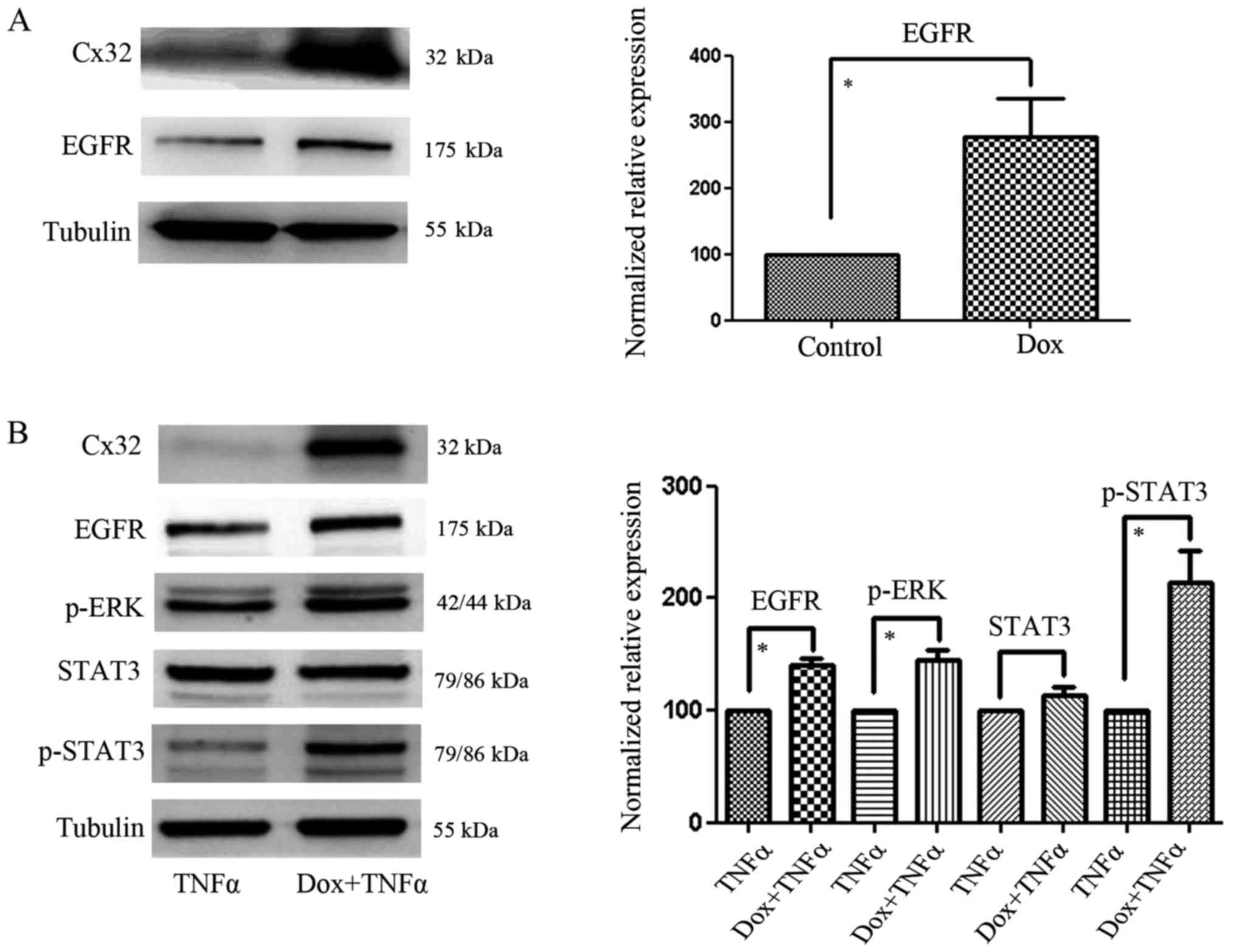
As shown in Fig. 2A,
a low-density culture was established to prevent GJ formation and
the expression of EGFR was subsequently detected. We found that
Cx32 could modulate EGFR expression without GJ formation.
Furthermore, after incubation with TNFα for 24 h, the high
expression levels of Cx32 in HeLa-Cx32 cells (induced by Dox) could
promote the expression of EGFR, p-STAT3 and p-ERK, without changing
the expression of total STAT3 (Fig.
2B). These results indicated that Cx32 could upregulate EGFR
not only in the absence of GJ formation, but also after treatment
with TNFα.
Cx32 inhibits apoptosis induced by
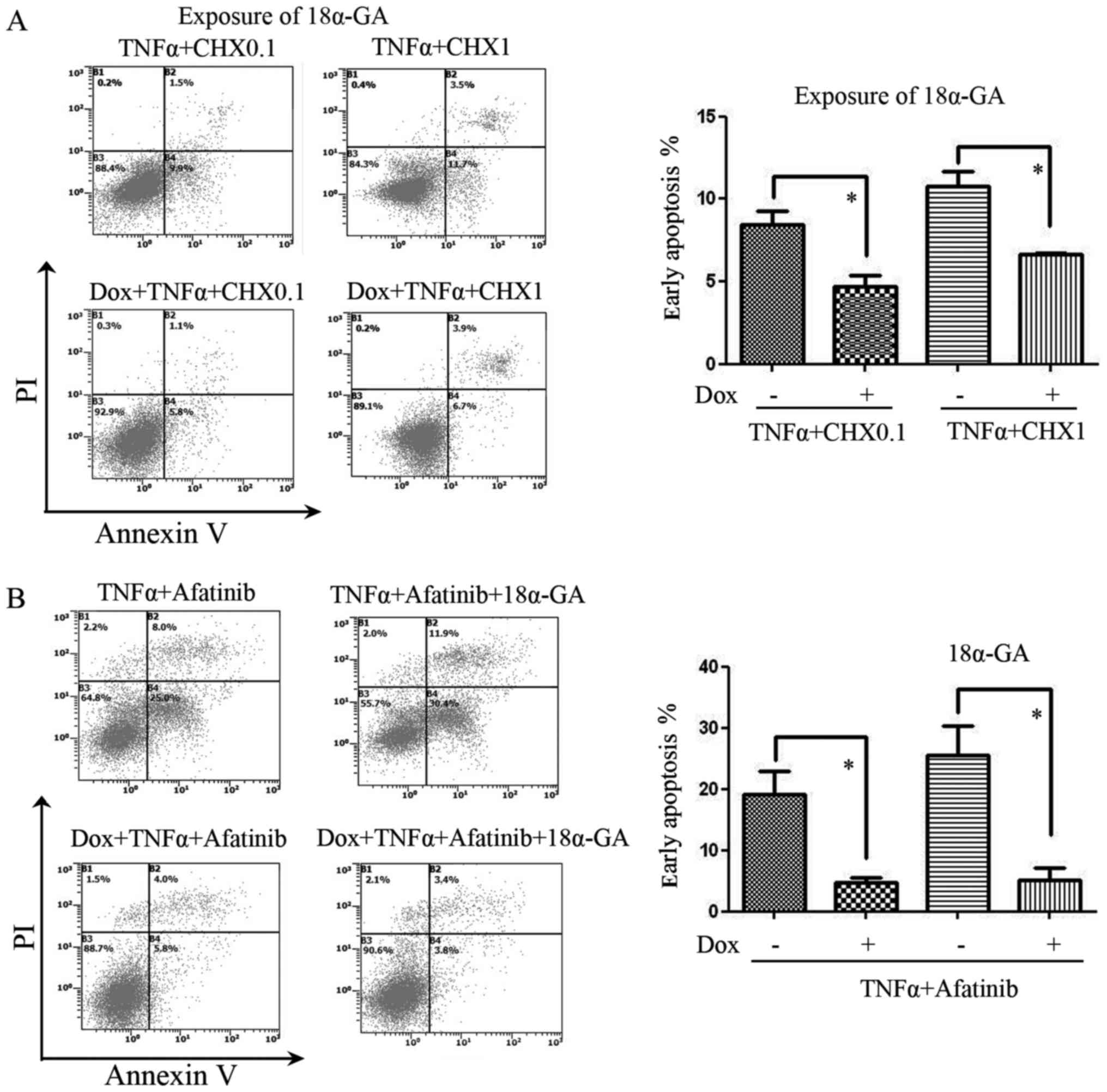
TNFα plus CHX or afatinib in HeLa-Cx32 cells
According to the reported ability of TNFα to induce
apoptosis (14), we used TNFα (50
ng/ml) + CHX (0.1 or 1 µg/ml) to induce apoptosis, as depicted in
Fig. 3A. The results showed that
apoptosis induced by TNFα and CHX co-treatment was inhibited by
Cx32 upregulation following treatment with the GJ inhibitor 18α-GA.
Afatinib (1.25 µM) was also used in co-treatment with TNFα to
inhibit EGFR. The results showed that, with or without GJ
inhibition by 18α-GA, the anti-apoptotic effect of Cx32 against
TNFα + afatinib was present (Fig.
3B).
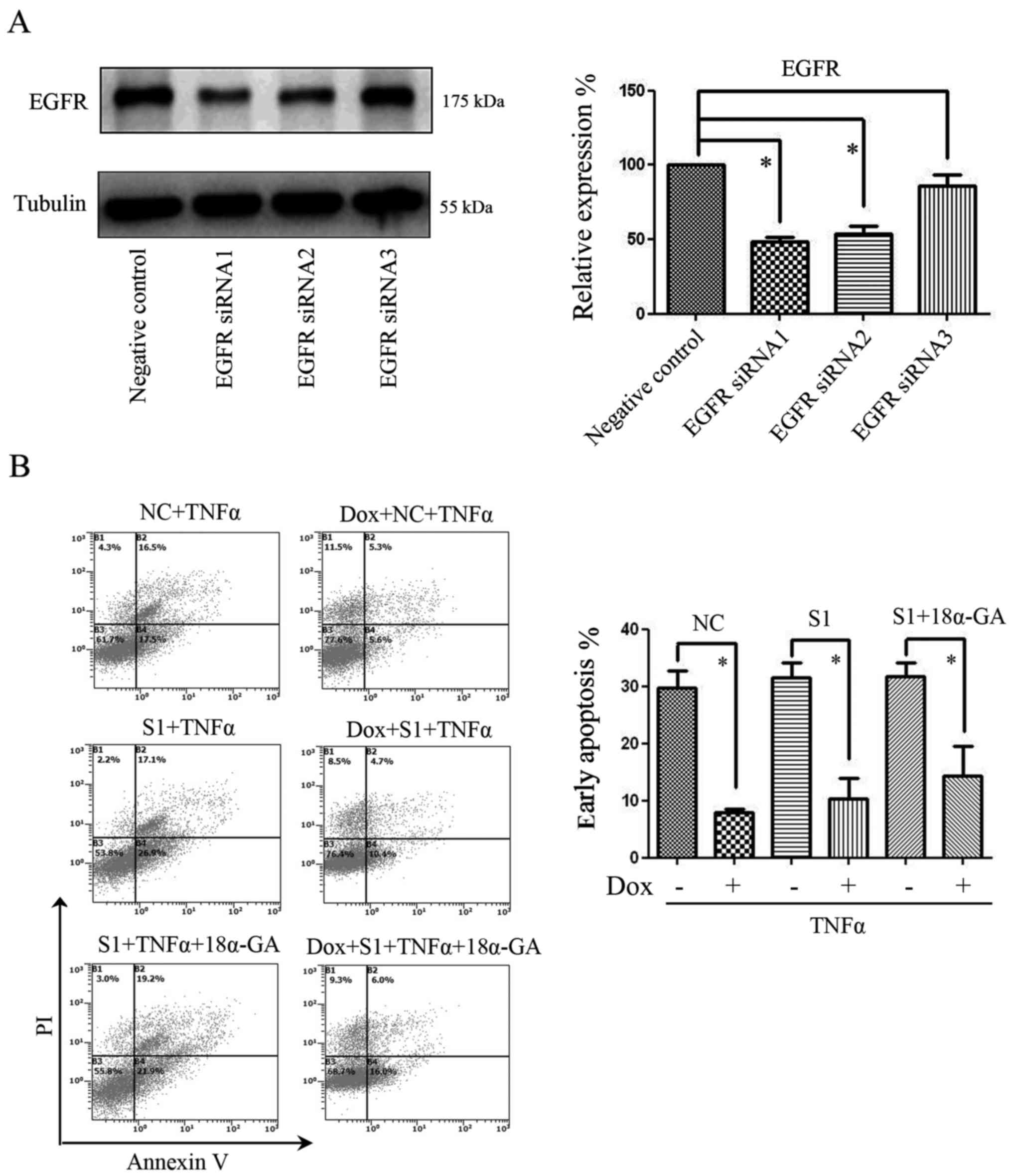
Effect of Cx32 on the apoptosis of
HeLa-Cx32 cells after transfection with EGFR siRNA
Due to the multi-functionality of EGFR inhibitors,
we used siRNA to specifically reduce EGFR expression in our further
experiments. Using an NS siRNA as a negative control, three EGFR
siRNAs (S1, S2 and S3) were used to knock down EGFR expression.
Among these, EGFR siRNA1 (S1) was determined to be the most
efficient fragment, and thus was used in our further experiments
(Fig. 4A). The results showed that
with or without GJ inhibition by 18α-GA, the anti-apoptotic effect
of Cx32 against TNFα was present, even after transfection with EGFR
siRNA (Fig. 4B).
Cx32 expression correlates with the
expression of Cox-2, survivin and TNFα in cervical cancer
cells
Cox-2 is an important factor for the prognosis of
CaCx. After the upregulation of Cx32 was induced with Dox, we found
that Cox-2 was also upregulated (Fig.
5A). Our previous study demonstrated that Cx32 expression was
higher in CaCx than in normal cervical tissue. Compared with
para-carcinoma tissue exhibiting low Cx32 expression, the
expression of survivin in CaCx was markedly increased and coincided
with Cx32 variation (Fig. 5B).
Furthermore, in the CaCx cell line C-33A, after knockdown of Cx32
with siRNA, the expression levels of survivin and TNFα were found
to be reduced (Fig. 5C). As the
expression levels of TNFα, EGFR and survivin have been associated
with anti-apoptotic processes and the tumor micro-environment,
these results suggest that Cx32 may serve as a tumor enhancer in
CaCx.
Discussion
Previous results have indicated that GJs may enhance
the bystander effect in tumor cells (15). However, through the upregulation of
death receptor 5 and downregulation of Cx43, carbenoxolone (an
inhibitor of GJIC) has been found to enhance TRAIL-induced
apoptosis (16). In contrast to the
effects of Cx26, irradiated HeLa cells expressing Cx32 have
previously showed enhanced survival capacity and greater metabolic
activity relative to control cells (17,18).
However, whether the promotion of GJIC involving Cx32 can increase
or decrease the efficacy of antitumor drugs is not well defined
(2). As the effect of GJs on
apoptosis is complex, the present study focused on the
non-junctional function of Cx32 in the apoptosis of CaCx cells.
Cx proteins may act as signaling effectors and
activate the canonical mitochondrial apoptotic pathway,
independently of their functional roles within GJs or hemichannels
(19). Among the Cx proteins, Cx43
is the most widely studied. In a series of breast cancer samples,
elevated levels of Cx43 were found to serve as positive prognostic
markers, while elevated levels of Cx30 were shown to be negative
prognostic markers (20). Thus,
regarding the impacts of Cx proteins on tumors, Cx family members
and the host tissues should be considered and assessed.
Additionally, Cx mutations and aberrancies in their distribution
require evaluation. For instance, aberrant trafficking of a
Leu89Pro Cx32 mutant was found to be associated with X-linked
dominant Charcot-Marie-Tooth disease (21). Strong Cx43 expression has been
detected in the inner mitochondrial membrane within cardiomyocytes
(22). According to our previous
study, Cx32 is also expressed in the nucleus of CaCx tissue
(10), and thus the present study
further explored the function of Cx32 in CaCx cells.
Through activation of PKC-δ, EGF can protect CaCx
ME180S cells from apoptosis induced by TNFα (23). EGFR and ErbB2 are important
mediators of TNFα-regulated anti-apoptotic signals in intestinal
epithelial cells (24). To
determine whether the anti-apoptotic function of Cx32 was related
to EGFR signaling, we used afatinib and siRNA to inhibit the
function and expression of EGFR, respectively. However, the results
showed that the effect of Cx32 on extrinsic apoptosis was not
significantly altered after EGFR inhibition. In addition to
afatinib, erlotinib can also be used to inhibit EGFR. The process
of cell autophagy may serve as a protective mechanism against EGFR
inhibitors, as inhibition of autophagy has been found to enhance
the sensitivity of erlotinib in EGFR-mutated non-small cell lung
cancer (25). Whether the resistive
effects of Cx32 against afatinib and TNFα in the present study are
related to autophagy is unclear and warrants further
investigation.
A previous study documented that STAT3 inhibition
markedly increased the sensitivity of HPV-related cancer to
TRAIL-based therapy (26). Notably,
it has been reported that EGFR inhibition may be enhanced by
inhibition of the STAT3 pathway; compared with the inhibition of
each pathway alone, combined blockade of both the EGFR and STAT3
pathways was more effective against human ovarian cancer in
vitro and in vivo (27).
Our results showed that in cell groups treated with TNFα, p-STAT3
expression was higher in the Cx32 high-expression group, which may
explain the resistance of Cx32 to EGFR inhibition. A previous study
reported that inhibition of the EGFR oncogene induced formation of
an EGFR-TRAF2-RIP1-IKK complex, which stimulated an NF-κB-mediated
transcriptional survival program (28). In addition, TNFα regulates NF-κB
signaling, and thus whether NF-κB signaling is a key factor in the
resistance of Cx32 to EGFR inhibition requires further
investigation. Additionally, a previous study found that
TNFα-induced NF-κB activation was not blocked by EGFR or Src
inhibition, suggesting that TNFα may exert both EGFR-dependent and
-independent effects (29).
Overexpression of survivin is involved in drug
resistance in cancer cells, and reduces patient survival rate after
chemotherapy and radiotherapy. Antagonism of survivin function
renders cancer cells sensitive to the pro-apoptotic affects of
TNFα, indicating that survivin blocks the extrinsic pathway of
apoptosis (30). Our data showed
that survivin expression was reduced in C-33A cells after knockdown
of Cx32 with siRNA. This result demonstrates the potential
relationship between Cx32 and survivin, which may account for the
anti-apoptotic effect of Cx32 in CaCx cells.
Several studies have shown that TNFα, Fas and TRAIL
serve as critical factors in the tumor environment (31). Abnormal secretion of TNFα
contributes to a number of human diseases, and has been implicated
in tumor development and inflammation (6). Notably, in endotoxemic mice,
inhibition of EGFR activation decreased the production of TNFα in
the myocardium (32). As our
western blot results showed that Cx32 expression was correlated
with both EGFR and TNFα expression, it is possible that the
relationship between EGFR and TNFα is co-regulated by Cx32. One
speculation is that Cx32 effects the tumor micro-environment by
changing the expression of TNFα, EGFR, survivin and associated
factors, although this requires further investigation.
In conclusion, high expression of Cx32 appeared to
produce anti-apoptotic effects independently of GJs, and also
modulated the expression levels of TNFα, Cox-2 and survivin, which
may alter the tumor micro-environment in CaCx.
Acknowledgements
The present study was supported in part by the
National Natural Science Foundation of China (grant no. 81473234),
the Fundamental Research Funds for the Central Universities (grant
nos. 16ykjc01 and 16ykzd11), the Medical Science Foundation of
Guangdong Province (grant no. A2015168), and the grant for
Development of Science and Technology from the Department of
Science and Technology of Guangzhou (grant no. 201704020170).
References
|
1
|
Mao XY, Li QQ, Gao YF, Zhou HH, Liu ZQ and
Jin WL: Gap junction as an intercellular glue: Emerging roles in
cancer EMT and metastasis. Cancer Lett. 381:133–137. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aasen T, Mesnil M, Naus CC, Lampe PD and
Laird DW: Gap junctions and cancer: Communicating for 50 years. Nat
Rev Cancer. 16:775–788. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mesnil M, Krutovskikh V, Piccoli C,
Elfgang C, Traub O, Willecke K and Yamasaki H: Negative growth
control of HeLa cells by connexin genes: Connexin species
specificity. Cancer Res. 55:629–639. 1995.PubMed/NCBI
|
|
4
|
Zhao B, Zhao W, Wang Y, Xu Y, Xu J, Tang
K, Zhang S, Yin Z, Wu Q and Wang X: Connexin32 regulates hepatoma
cell metastasis and proliferation via the p53 and Akt pathways.
Oncotarget. 6:10116–10133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kawasaki Y, Omori Y, Li Q, Nishikawa Y,
Yoshioka T, Yoshida M, Ishikawa K and Enomoto K: Cytoplasmic
accumulation of connexin32 expands cancer stem cell population in
human HuH7 hepatoma cells by enhancing its self-renewal. Int J
Cancer. 128:51–62. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen Y, Zou Z, Wu Z, Zhao Z, Luo X, Xie C
and Liang Y: TNF-α-induced programmed cell death in the
pathogenesis of acquired aplastic anemia. Expert Rev Hematol.
8:515–526. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barbisan G, Pérez LO, Contreras A and
Golijow CD: TNF-α and IL-10 promoter polymorphisms, HPV infection,
and cervical cancer risk. Tumour Biol. 33:1549–1556. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burtness B, Bauman JE and Galloway T:
Novel targets in HPV-negative head and neck cancer: Overcoming
resistance to EGFR inhibition. Lancet Oncol. 14:e302–e309. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gaud G, Guillemot D, Jacob Y, Favre M and
Vuillier F: EVER2 protein binds TRADD to promote TNF-α-induced
apoptosis. Cell Death Dis. 4:e4992013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao Y, Lai Y, Ge H, Guo Y, Feng X, Song
J, Wang Q, Fan L, Peng Y, Cao M, et al: Non-junctional Cx32
mediates anti-apoptotic and pro-tumor effects via epidermal growth
factor receptor in human cervical cancer cells. Cell Death Dis.
8:e27732017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng RR, Huang M, Jin C, Wang HC, Yu JT,
Zeng LC, Zheng FY and Lin F: Cervical cancer systemic inflammation
score: A novel predictor of prognosis. Oncotarget. 7:15230–15242.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koreen IV, Elsayed WA, Liu YJ and Harris
AL: Tetracycline-regulated expression enables purification and
functional analysis of recombinant connexin channels from mammalian
cells. Biochem J. 383:111–119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goldberg GS, Bechberger JF and Naus CC: A
pre-loading method of evaluating gap junctional communication by
fluorescent dye transfer. Biotechniques. 18:490–497.
1995.PubMed/NCBI
|
|
14
|
Qi Z, Shen L, Zhou H, Jiang Y, Lan L, Luo
L and Yin Z: Phosphorylation of heat shock protein 27 antagonizes
TNF-α induced HeLa cell apoptosis via regulating TAK1
ubiquitination and activation of p38 and ERK signaling. Cell
Signal. 26:1616–1625. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Y, Tao L, Fan L, Peng Y, Yang K,
Zhao Y, Song Q and Wang Q: Different gap junction-propagated
effects on cisplatin transfer result in opposite responses to
cisplatin in normal cells versus tumor cells. Sci Rep. 5:125632015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yulyana Y, Endaya BB, Ng WH, Guo CM, Hui
KM, Lam PY and Ho IA: Carbenoxolone enhances TRAIL-induced
apoptosis through the upregulation of death receptor 5 and
inhibition of gap junction intercellular communication in human
glioma. Stem Cells Dev. 22:1870–1882. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Autsavapromporn N, De Toledo SM, Jay-Gerin
JP, Harris AL and Azzam EI: Human cell responses to ionizing
radiation are differentially affected by the expressed connexins. J
Radiat Res (Tokyo). 54:251–259. 2013. View Article : Google Scholar
|
|
18
|
Zhao Y, de Toledo SM, Hu G, Hei TK and
Azzam EI: Connexins and cyclooxygenase-2 crosstalk in the
expression of radiation-induced bystander effects. Br J Cancer.
111:125–131. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carette D, Gilleron J, Chevallier D,
Segretain D and Pointis G: Connexin a check-point component of cell
apoptosis in normal and physiopathological conditions. Biochimie.
101:1–9. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Teleki I, Szasz AM, Maros ME, Gyorffy B,
Kulka J, Meggyeshazi N, Kiszner G, Balla P, Samu A and Krenacs T:
Correlations of differentially expressed gap junction connexins
Cx26, Cx30, Cx32, Cx43 and Cx46 with breast cancer progression and
prognosis. PLoS One. 9:e1125412014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Da Y, Wang W, Liu Z, Chen H, Di L, Previch
L and Chen Z: Aberrant trafficking of a Leu89Pro connexin32 mutant
associated with X-linked dominant Charcot-Marie-Tooth disease.
Neurol Res. 38:897–902. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miro-Casas E, Ruiz-Meana M, Agullo E,
Stahlhofen S, Rodríguez-Sinovas A, Cabestrero A, Jorge I, Torre I,
Vazquez J, Boengler K, et al: Connexin43 in cardiomyocyte
mitochondria contributes to mitochondrial potassium uptake.
Cardiovasc Res. 83:747–756. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Akca H, Akan SY, Yanikoglu A and Ozes ON:
Suppression of TNF-alpha mediated apoptosis by EGF in TNF-alpha
sensitive human cervical carcinoma cell line. Growth Factors.
21:31–39. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamaoka T, Yan F, Cao H, Hobbs SS, Dise
RS, Tong W and Polk DB: Transactivation of EGF receptor and ErbB2
protects intestinal epithelial cells from TNF-induced apoptosis.
Proc Natl Acad Sci USA. 105:pp. 11772–11777. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li YY, Lam SK, Mak JC, Zheng CY and Ho JC:
Erlotinib-induced autophagy in epidermal growth factor receptor
mutated non-small cell lung cancer. Lung Cancer. 81:354–361. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakamura H, Taguchi A, Kawana K, Kawata A,
Yoshida M, Fujimoto A, Ogishima J, Sato M, Inoue T, Nishida H, et
al: STAT3 activity regulates sensitivity to tumor necrosis
factor-related apoptosis-inducing ligand-induced apoptosis in
cervical cancer cells. Int J Oncol. 49:2155–2162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wen W, Wu J, Liu L, Tian Y, Buettner R,
Hsieh MY, Horne D, Dellinger TH, Han ES, Jove R, et al: Synergistic
anti-tumor effect of combined inhibition of EGFR and JAK/STAT3
pathways in human ovarian cancer. Mol Cancer. 14:1002015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Blakely CM, Pazarentzos E, Olivas V,
Asthana S, Yan JJ, Tan I, Hrustanovic G, Chan E, Lin L, Neel DS, et
al: NF-κB-activating complex engaged in response to EGFR oncogene
inhibition drives tumor cell survival and residual disease in lung
cancer. Cell Rep. 11:98–110. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kakiashvili E, Dan Q, Vandermeer M, Zhang
Y, Waheed F, Pham M and Szászi K: The epidermal growth factor
receptor mediates tumor necrosis factor-alpha-induced activation of
the ERK/GEF-H1/RhoA pathway in tubular epithelium. J Biol Chem.
286:9268–9279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cheung CH, Sun X, Kanwar JR, Bai JZ, Cheng
L and Krissansen GW: A cell-permeable dominant-negative survivin
protein induces apoptosis and sensitizes prostate cancer cells to
TNF-α therapy. Cancer Cell Int. 10:362010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cullen SP and Martin SJ: Fas and TRAIL
‘death receptors’ as initiators of inflammation: Implications for
cancer. Semin Cell Dev Biol. 39:26–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sun X, Liang J, Yao X, Lu C, Zhong T, Hong
X, Wang X, Xu W, Gu M and Tang J: The activation of EGFR promotes
myocardial tumor necrosis factor-α production and cardiac failure
in endotoxemia. Oncotarget. 6:35478–35495. 2015.PubMed/NCBI
|