Introduction
Glioblastoma (GBM) is among the least curable types
of cancer with a median survival of approximately 15 months
(1–3). Because of the high infiltrative
character of GBM, tumor cells cannot be completely removed through
surgical resection or irradiation (4,5). Thus,
temozolomide (TMZ), which is one of the most important agents of
current standard adjuvant chemotherapy for GBM, aims to abolish
these residual tumor cells (6–8).
However, resistance to temozolomide often develops quickly and
results in tumor recurrence and poor outcome (9–11).
Therefore, understanding the mechanisms of TMZ resistant in GBM
cells is essential for the GBM treatment.
O-mannose-b-1,2-N-acetylglucosaminyltransferase
(PomGnT1), a glycosyltransferase that participates in the formation
of GlcNAc-β1,2-Man glycan, is causally related to muscle-eye-brain
disease (MEB), a congenital muscular dystrophy (12). Our earlier studies showed that
PomGnT1 expression in GBM tissues was closely associated with poor
prognosis in GBM patients and PomGnT1 promoted GBM progression via
activation of β-catenin (13,14).
The pathological function of PomGnT1 in GBM is in control of cell
adhesion and migration abilities (15–17).
However, the function and underlying mechanisms of PomGnT1 in TMZ
resistance in GBM have not been reported.
Epithelial-to-mesenchymal transition (EMT) is a
process initially observed in embryonic development in which cells
lose epithelial characteristics and gain mesenchymal properties to
increase motility and invasion, and this process is also important
in tumor progression and metastasis (18–20).
Recent studies found that EMT was closely related to
chemo-resistance (21–23). Blocking the EMT pathway abrogated
resistance to anti-folate chemotherapy in lung cancer (24,25).
In the present study, we determined the expression
level of PomGnT1 in TMZ-resistant GBM cells, and explored the
biological function and potential mechanism of PomGnT1 in TMZ
resistant GBM cells.
Materials and methods
Materials
TMZ and all other reagents were provided by
Sigma-Aldrich (St. Louis, MO, USA). TMZ was diluted in dimethyl
sulfoxide (DMSO; Beijing Solarbio Science and Technology, Co.,
Ltd., Beijing, China) to a stock solution of 200 mM TMZ.
Immediately before use in cell culture, the stock was diluted in
media. Antibodies used for western blot analyses were obtained from
Cell Signaling Technology (Danvers, MA, USA).
Cells and cell culture
Human glioma cell lines U87-MG and U251-MG were
obtained from the American Type Culture Collection (ATCC; Manassas,
VA, USA). TMZ-resistant cell lines U87-TMZ and U251-TMZ were
obtained by culturing U87-MG or U251-MG cells with gradually
increased doses (2–20 µM) of TMZ for 4 months. All the cells were
cultured in Dulbeccos modified Eagles medium (DMEM) medium with 10%
fetal bovine serum (FBS; Gibco, Vienna, Austria), 100 units/ml
penicillin and 100 µg/ml streptomycin. All the cells were cultured
at 37°C in an atmosphere comprising 95% air and 5%
CO2.
Real-time-PCR (RT-PCR)
Cellular RNA was isolated by TRIzol reagent
according to the manufacturers instructions. Briefly, the DNA was
removed from the samples using DNase treatment (DNA-free kit;
Ambion-Applied Biosystems, Carlsbad, CA, USA) and cDNA was
synthesized from the purified RNA using Moloney murine leukemia
virus reverse transcription kit (Promega, Madison, WI, USA).
Primers for human PomGnT1 were: F, 5-GCCAAGTTTGCTGTGGTT CTGG-3 and
R, 5-CTGGTCATTCCAGGCAGAGATG-3. Actin primer sets were used to
produce a normalization control. Real-time PCR was carried out in
triplicate with the SYBR-Green PCR Master Mix (Applied Biosystems,
Foster City, CA, USA) and a 7900HT Fast Real-Time PCR machine
(Applied Biosystems).
Western blot analysis
RIPA buffer in the presence of protease inhibitor
cocktail and phosphorylation inhibitor cocktail were used to
extract total protein. Appropriate mount protein was loaded into
8–10% SDS-polyacrylamide gel and transferred onto a nitrocellulose
membrane (Millipore, Billerica, MA, USA). Primary antibodies were
incubated overnight and secondary antibodies were incubated for 1 h
at the appropriate dilutions. The signal was observed and developed
with Kodak film by exposure to enhanced chemiluminescence (ECL)
plus western blotting detection reagents (Amersham Biosciences,
Piscataway, NJ, USA). Western blot analysis was performed with
antibodies against PomGnT1, TCF8, vimentin, β-catenin, Slug and
actin was used as control.
Lentivirus mediated shRNA gene
knockdown PomGnT1
The stable knockdown PomGnT1 cell lines were
generated by transduction a lentiviral-mediated expression siRNA
specific target of PomGnT1. Lentivirus containing the
PomGnT1-specific shRNAs (shRNA sequences targeting PomGnT1 was:
SH1: 5-GCCATTGAGCTCAGCAGAAGA-3; SH2: 5-GCA TCCAGCATACTCCCATCA-3;
SH3: 5-AGGAGGAGCTTG AGCCCAA-3; SH4: 5-GGAGAAAGATGATGACTTC-3) were
purchased from Shanghai Hanyu Biotechnology Co., Ltd. (Shanghai,
China). The PomGnT1-specific shRNA lentiviral particles infected
U87-TR and U251-TR cells with 10 µg/ml polybrene for 12 h.
Afterwards, the medium containing viral particles was removed and
replaced with fresh medium. One ‘non-target’ construct containing
an shRNA sequence that did not target any known human gene was
transduced separately into U87-TMZ or U251-TMZ cells to serve as a
scrambled negative control. After 72 h, the cells were harvested,
and the knockdown efficiency was tested by real-time PCR and
western blot analysis. Transduced cell lines were named U87-TMZ
PomGnT1-KD and U87-TMZ NC, and U251-TMZ PomGnT1-KD and U251-TMZ
NC.
Lentivirus mediated overexpression of
PomGnT1
The stable overexpression of PomGnT1 cell lines were
generated by transduction a lentiviral-mediated overexpression
PomGnT1. An oligonucleotide coding for PomGnT1 (NM_017739) was
cloned into the plasmid murine stem cell virus (pMSCV)-puro
retroviral vector (Clontech Laboratories, Inc., Mountain View, CA,
USA) with which 293T cells were transfected along with packaging
plasmids pMD.env and pMD.gag.-pol by Shanghai Hanyu Biotechnology.
The produced retroviruses were used to infect U87-MG and U251-MG
cells, which were further selected in the presence of 1.0 mg/ml
puromycin to establish a cell line with stable PomGnT1
overexpression (U87-PomGnT1-OE or U251-PomGnT1-OE). Cells infected
with empty vector (EV) pMSCV-puro derived retroviruses were used as
control (U87-NC or U251-NC). After 72 h, the cells were harvested
and the overexpression efficiency was tested by real-time PCR and
western blot analysis.
Growth inhibition studies
TMZ-sensitive and -resistant glioma cells were
plated in 96-well white plates at 5×103 cells/well, and
TMZ (ranging from 200 to 1012.5 µM) was added and incubated for 48
h. A Cell Counting kit-8 (CCK-8; Dojindo Laboratories, Kumamoto,
Japan) was used to assess cell viability. Briefly, CCK-8 reagent
was added (10 µl/well), cells were maintained for 2 h, and the
absorbance was read under a microplate reader (SpectraMax M5;
Molecular Devices, Sunnyvale, CA, USA) at 450 nm. All half-maximal
inhibitory concentration (IC50) values were determined
using GraphPad Prism 5 software.
Flow cytometric analysis
Apoptosis was determined by translocation of
phosphatidylserine to the cell surface using an Annexin V-FITC and
PI apoptosis detection kit (BD Biosciences, San Diego, CA, USA).
TMZ-sensitive and -resistant cells were plated in 6-well plates at
a density of 5×105 cells/well and treated with TMZ for
48 h. The cells were then harvested and washed twice in cold
phosphate-buffered saline (PBS) and resuspended in Annexin V-FITC
and PI for 30 min in the dark. Cell apoptosis was analyzed by using
the CellQuest software on a FACSAria flow cytometer (BD
Biosciences). Fluorescence was detected with an excitation
wavelength of 480 nm.
Statistical analysis
For quantitative data, all results are expressed as
the mean ± SD. Statistical significance between the groups was
determined using the Students t-test using the SPSS 18.0 (SPSS,
Inc., Chicago, IL, USA). Each experiment was repeated at least
three times. P<0.01 was considered statistically
significant.
Results
Upregulated PomGnT1 expression in
TMZ-resistant GBM cells
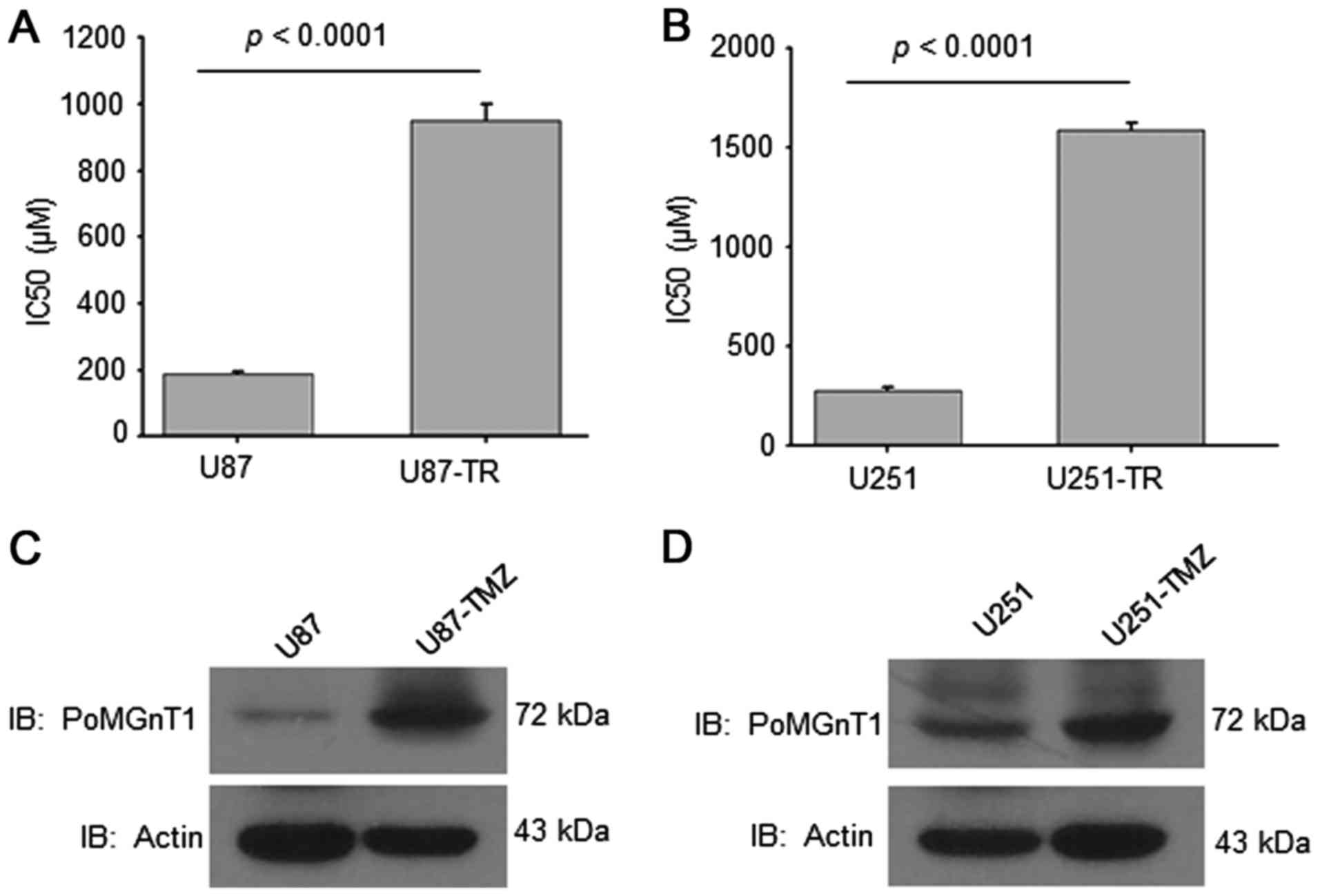
TMZ is the primary and most promising therapeutic
drug for GBM. In order to establish TMZ-resistant GBM cell lines,
we cultured U87-MG and U251-MG cells with gradually increased doses
(2–20 µM) of TMZ for 4 months. The IC50 analysis results
of U87-TMZ, U251-TMZ resistant cells and parental cells showed that
the IC50 value of both TMZ-resistant cells was increased
almost 5-fold (Fig. 1A and B). We
further examined the expression of PomGnT1 in parental
TMZ-sensitive (U87-MG and U251-MG) and TMZ-resistant (U87-TMZ and
U251-TMZ) cells. As shown in Fig. 1C
and D, PomGnT1 expression was significantly elevated in
TMZ-resistant cells compared with matched parental sensitive cells.
These data suggest that PomGnT1 expression might be associated with
TMZ-resistance in GBM cells.
Suppression of PomGnT1 decreased
IC50 values for TMZ and enhanced apoptosis of
TMZ-resistant GBM cells
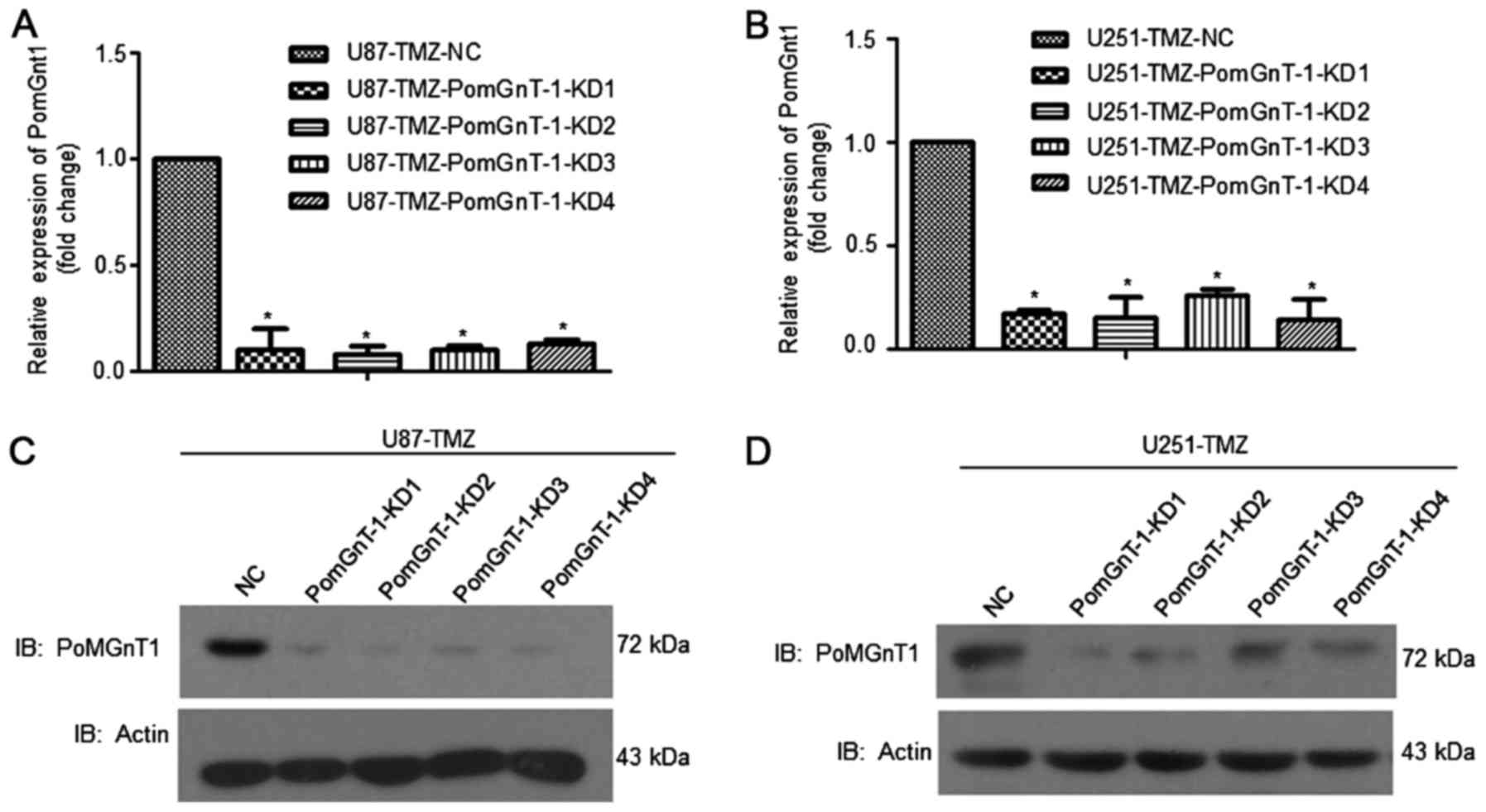
Using lentivirus mediated shRNA gene knockdown
system, we knocked down PomGnT1 expression in U87-TMZ and U251-TMZ
cells. From the mRNA level, >90% of PomGnT1 expression was
decreased. The protein level of PomGnT1 was also decreased
suggesting the high efficiency of shRNA knockdown system (Fig. 2). Cells were passaged upon reaching
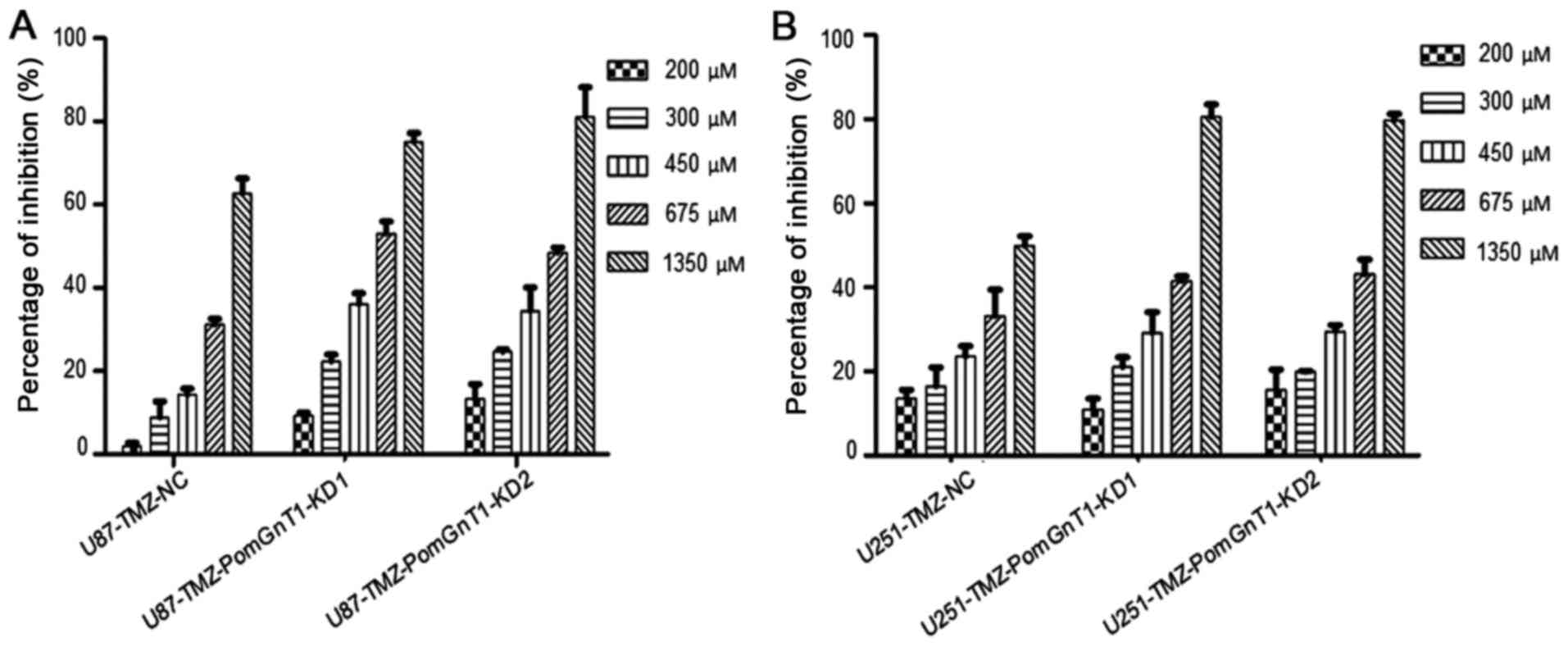
80% confluence. Then, we treated PomGnT1 knocked-down U87-TMZ and
U251-TMZ cells with TMZ (200–1350 µM) for 48 h and detected the
change of cell viability. As shown in Fig. 3, knockdown of PomGnT1 significantly
reduced the viability of TMZ-resistant glioma cell lines, in a
dose-dependent manner. The IC50 value of TMZ for
U87-resistant cells was 1033 µM and for U251-resistant cells was
1308 µM. However, the IC50 value of TMZ for U87-TMZ and
U251-TMZ was decreased significantly after knockdown of PomGnT1
(Table I). Hence, there was a
significant decrease in IC50 value for TMZ-resistant GBM
cells with knockdown of PomGnT1 compared with matched parental
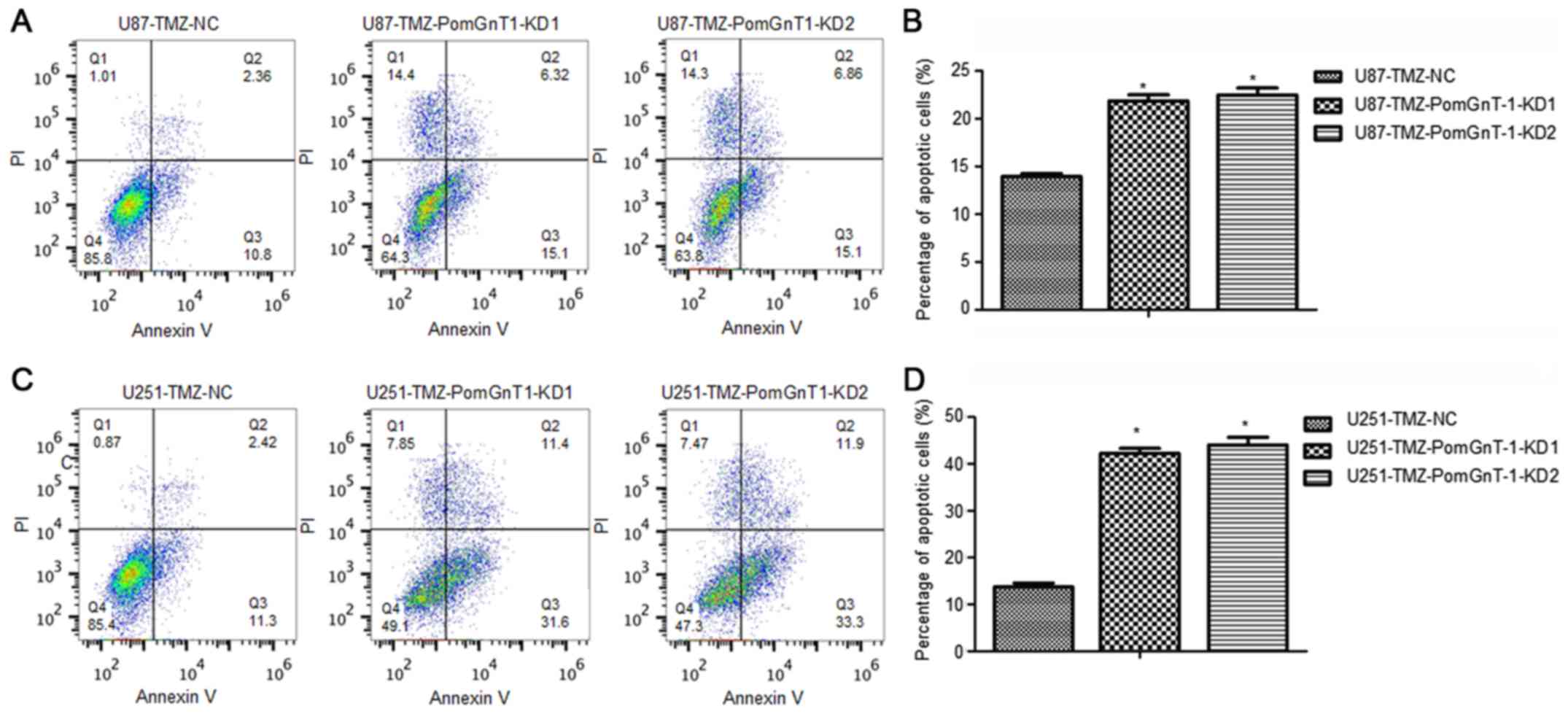
negative cells. Through the flow cytometric analysis, we found that
knockdown of PomGnT1 significantly induced apoptosis in
TMZ-resistant GBM cells after treated with TMZ compared with
matched parental negative cells (Fig.
4). These results indicated that loss of PomGnT1 suppressed
TMZ-resistant GBM cell survival mainly by induction of apoptosis
after treated with TMZ.
 | Table I.IC50 values of U87-TMZ and
U251-TMZ cells in response to TMZ. |
Table I.
IC50 values of U87-TMZ and
U251-TMZ cells in response to TMZ.
|
| U87-TMZ | U251-TMZ |
|---|
|
|
|
|
|---|
|
| NC | PomGnT1-KD1 | PomGnT1-KD2 | NC | PomGnT1-KD1 | PomGnT1-KD2 |
|---|
| IC50
(µM) | 1022.00±11.53 |
643.67±7.77a |
632.00±10.54a | 1322.67±16.17 |
714.33±13.20a |
704.00±12.29a |
Overexpression of PomGnT1 increases
IC50 values for TMZ and reduced apoptosis of TMZ
sensitive cells
Using lentivirus mediated gene overexpression
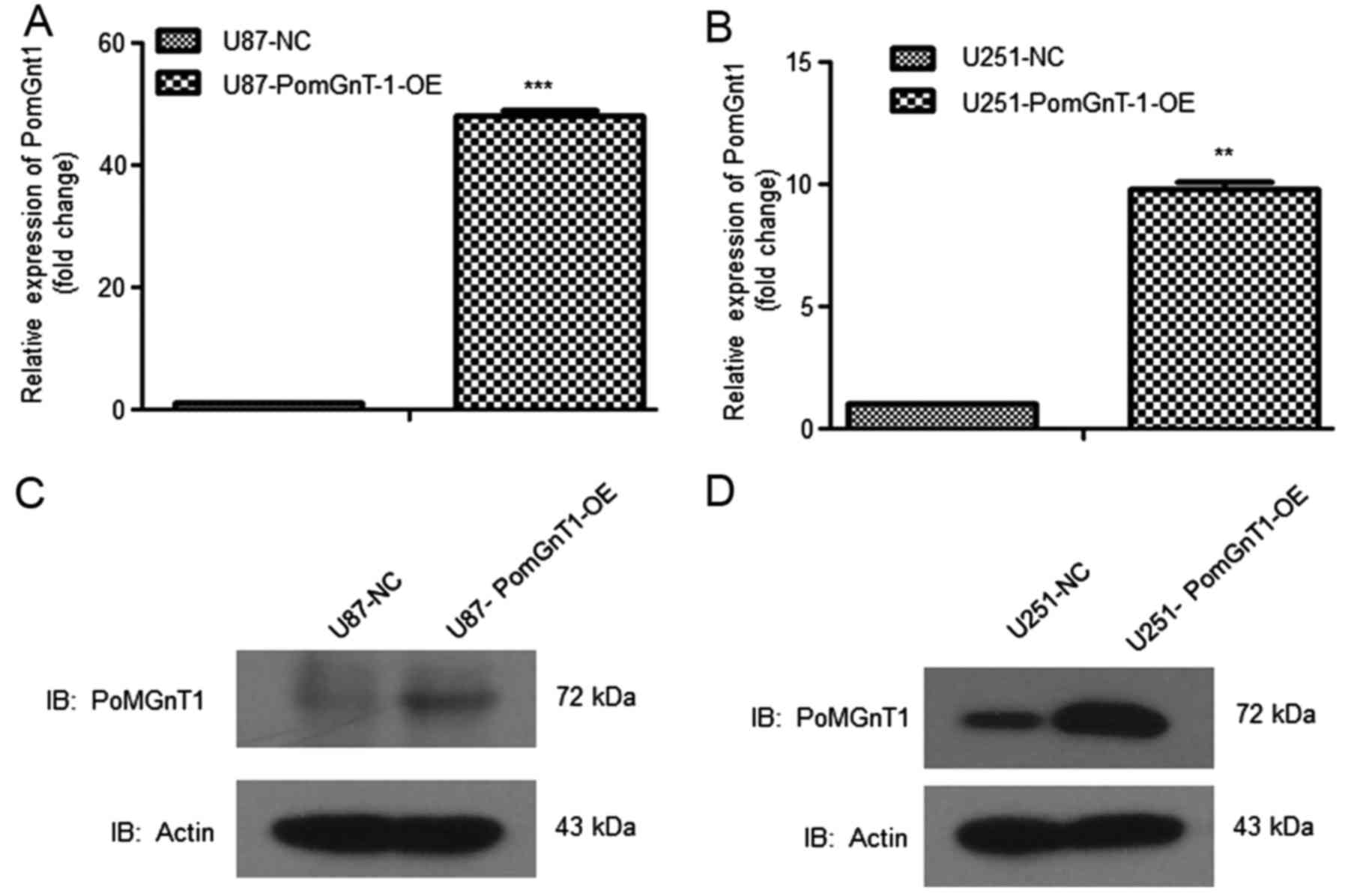
system, we overexpressed PomGnT1 in U87 and U251 cells. From the
mRNA level, PomGnT1 expression was increased >10-fold (Fig. 5A). The protein level of PomGnT1 was
also increased suggesting the high efficiency of gene
overexpression system (Fig. 5B).
Then, we treated PomGnT1 overexpressed U87-MG and U251-MG cells
with TMZ (100–1350 µM) for 48 h and detected the change of cell
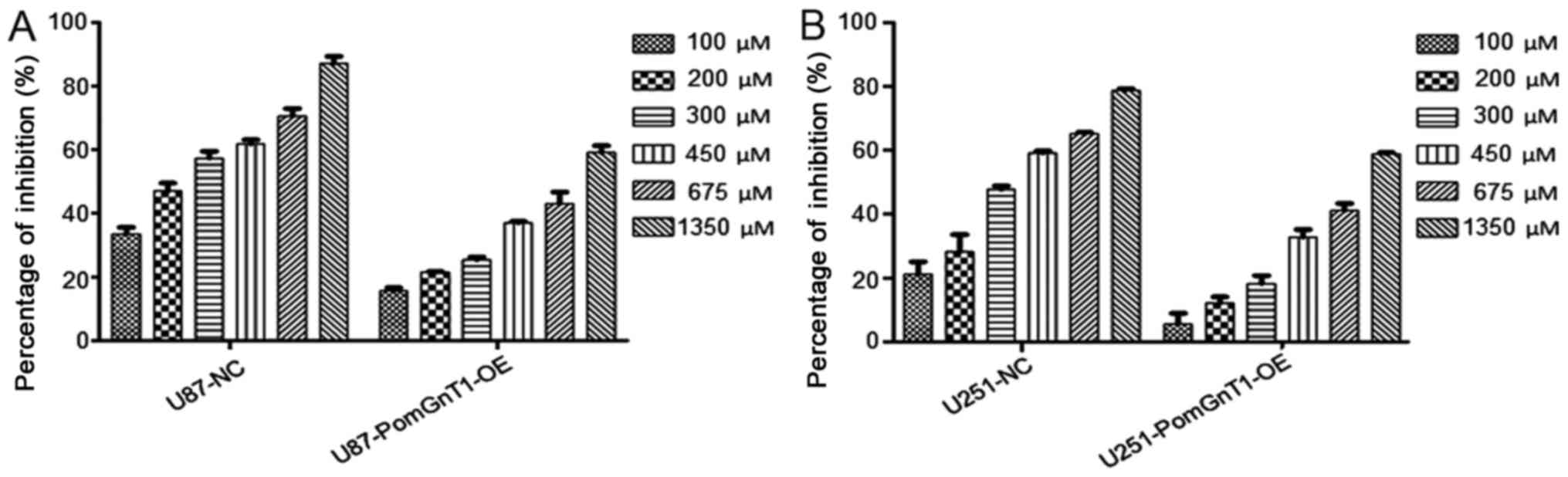
viability. As shown in Fig. 6,
overexpression of PomGnT1 increased the viability of TMZ-sensitive
lines, in a dose-dependent manner. The IC50 value of TMZ
for U87-MG was 227.4 µM and for U251-MG was 364.1 µM. However, the
IC50 value of TMZ for U87-MG and U251-MG was increased
significantly after overexpression of PomGnT1 (Table II). Hence, there was a significant
increase in IC50 value for TMZ-sensitive GBM cells with
overexpression of PomGnT1 compared with matched parental negative
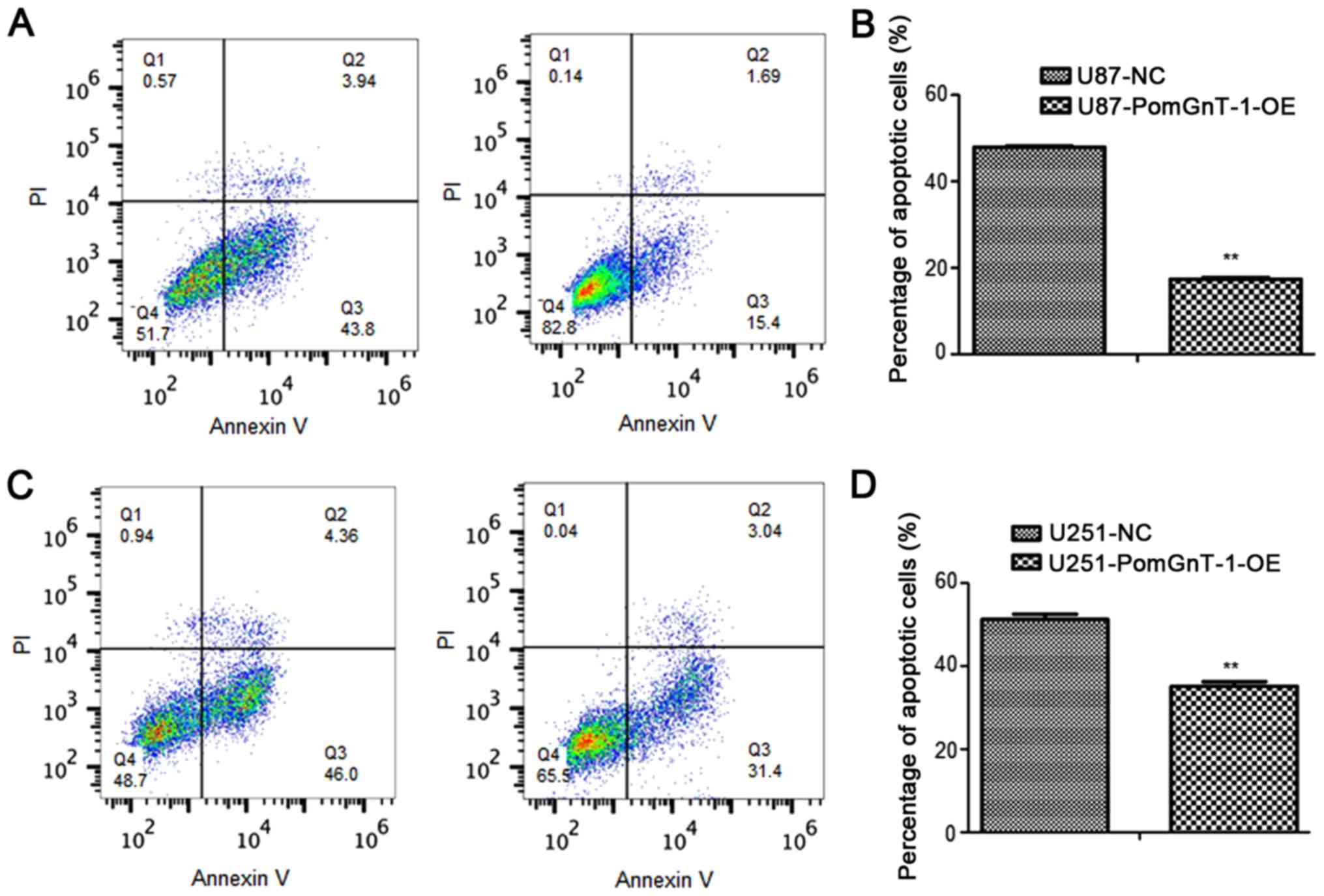
cells. Through the flow cytometric analysis, we found that the
percentage of apoptotic cells were decreased in TMZ-sensitive
PomGnT1 overexpressed glioma cells after treated with TMZ compared
with matched parental negative cells (Fig. 7). These results highlighted the
importance of PomGnT1 in the progression of TMZ in treatment of
TMZ-sensitive glioma cells.
 | Table II.IC50 values of U87 and
U251 cells in response to TMZ. |
Table II.
IC50 values of U87 and
U251 cells in response to TMZ.
|
| U87 | U251 |
|---|
|
|
|
|
|---|
|
| NC | PomGnT1-OE | NC | PomGnT1-OE |
|---|
| IC50
(µM) | 223.13±9.73 |
906.37±7.75a | 361.33±10.26 |
953.67±14.84a |
PomGnT1 regulates the expression of
EMT makers in TMZ-resistant GBM cells
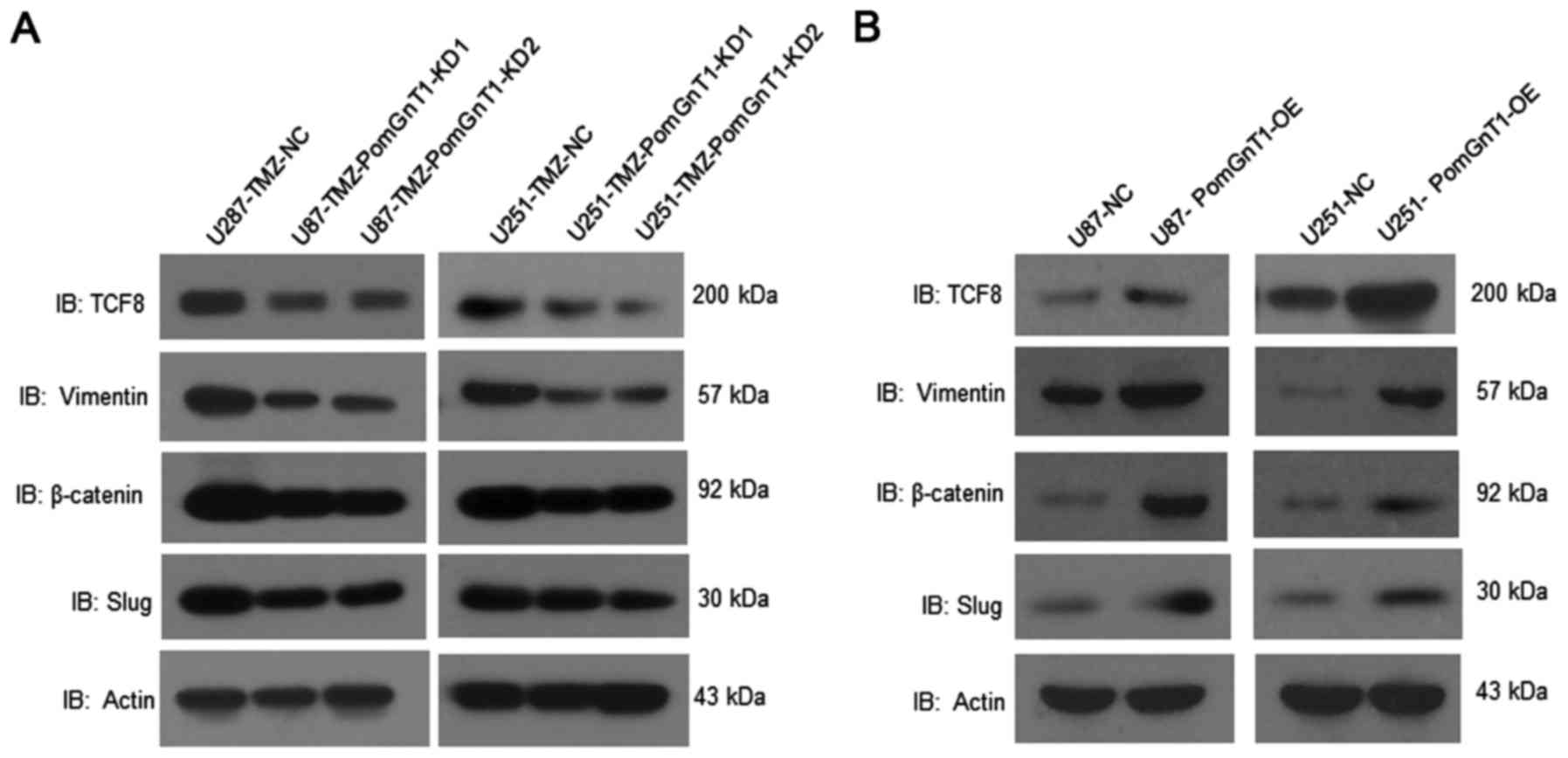
To find the underlying molecular changes regulated
by PomGnT1 in TMZ-resistant GBM cells, we observed the effect of
PomGnT1 on TCF8, vimentin, β-catenin, Slug expression in
TMZ-resistant and TMZ-sensitive GBM cells after treated with TMZ.
As shown in Fig. 8, knockdown of
PomGnT1 significantly inhibited expression of TCF8, vimentin,
β-catenin and Slug in protein level in TMZ-resistant cells compared
with matched negative control cells. Whereas, overexpression of
PomGnT1 significantly elevated expression of TCF8, vimentin,
β-catenin and Slug in protein level in TMZ-sensitive cells compared
with matched negative control cells. These results indicate that
PomGnT1-enhanced temozolomide resistance might partially be through
activating epithelial-mesenchymal transition signaling in
glioblastoma.
Discussion
The most challenging obstacle in the treatment of
GBM is tumor recurrence (26–28).
To date, because the recurring tumors are usually TMZ-resistant,
there are only a few options for recurrent GBM and even these
therapies have limited success (29,30).
Hence, strategies to neutralize and overcome chemo-resistance
require thorough understanding of the diverse concepts, and are a
significant unmet need in GBM therapy (31,32).
The present study explored the possibility of PomGnT1 in the
chemo-resistance of TMZ in GBM cells. Elevated expression of
PomGnT1 was detected in TMZ-resistant GBM cells compared to
TMZ-sensitive GBM cells, indicating that PomGnT1 might be a new
biomarker for TMZ treatment.
Moreover, knockdown of PomGnT1 in both TMZ resistant
GBM cells decreased IC50 values for TMZ and enhanced
apoptosis of GBM cells. On the contrary, overexpression of PomGnT1
in parental sensitive GBM cells increased IC50 values
for TMZ and reduced apoptosis of GBM cells. The study revealed
novel functions of PomGnT1 and indicated that PomGnT1 might be one
of the major determinants conferring TMZ resistant properties in
GBM.
We suggest that PomGnT1 influences the TMZ
resistance as follows: Protein levels of EMT markers including
TCF8, vimentin, β-catenin and Slug were changed with PomGnT1
expression, suggesting that PomGnT1 might be regulating TMZ
resistance via modulating the EMT pathway. Cadherins, which are
associated with cytoskeleton, epithelial-mesenchymal-transition as
well as chemotherapy, have been found as substrates of PomGnT1
(33–35), indicating that PomGnT1 might
influence TMZ resistance through the glycosylation modification of
cadherins.
Taken together, our data revealed the importance of
PomGnT1 in TMZ resistance in GBM and found a mechanistic pathway of
PomGnT1 mediated EMT signaling. These studies demonstrate that
PomGnT1 may be the focus of future research for treatment of
recurrent TMZ-resistant GBM.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (no. 81502146), the Shanghai Municipal
Commission of Health and Family Planning (no. 20154Y0067) and the
Shanghai Jiaotong University Affiliated First People's Hospital
(no. 06N1503016).
References
|
1
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schwartzbaum JA, Fisher JL, Aldape KD and
Wrensch M: Epidemiology and molecular pathology of glioma. Nat Clin
Pract Neurol. 2:494–503. 2016. View Article : Google Scholar
|
|
4
|
Wilson CB: Glioblastoma: The past, the
present, and the future. Clin Neurosurg. 38:32–48. 1992.PubMed/NCBI
|
|
5
|
Glas M, Rath BH, Simon M, Reinartz R,
Schramme A, Trageser D, Eisenreich R, Leinhaas A, Keller M,
Schildhaus HU, et al: Residual tumor cells are unique cellular
targets in glioblastoma. Ann Neurol. 68:264–269. 2010.PubMed/NCBI
|
|
6
|
Grzmil M, Seebacher J, Hess D, Behe M,
Schibli R, Moncayo G, Frank S and Hemmings BA: Inhibition of MNK
pathways enhances cancer cell response to chemotherapy with
temozolomide and targeted radionuclide therapy. Cell Signal.
28:1412–1421. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lorenzetti M, Motta F, Campanella R, Bauer
D, Assi A, Arienta C, Gaini SM and Caroli M: Adjuvant temozolomide
chemotherapy for treatment of papillary tumor of the pineal region.
World Neurosurg. 76:160–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pace A, Vidiri A, Galiè E, Carosi M,
Telera S, Cianciulli AM, Canalini P, Giannarelli D, Jandolo B and
Carapella CM: Temozolomide chemotherapy for progressive low-grade
glioma: Clinical benefits and radiological response. Ann Oncol.
14:1722–1726. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarkaria JN, Kitange GJ, James CD, Plummer
R, Calvert H, Weller M and Wick W: Mechanisms of chemoresistance to
alkylating agents in malignant glioma. Clin Cancer Res.
14:2900–2908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Segal MS, Goldstein MM and Attinger EO:
The use of noscapine (narcotine) as an antitussive agent. Dis
Chest. 32:305–309. 1957. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
St-Coeur PD, Poitras JJ, Cuperlovic-Culf
M, Touaibia M and Morin P Jr: Investigating a signature of
temozolomide resistance in GBM cell lines using metabolomics. J
Neurooncol. 125:91–102. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Akasaka-Manya K, Manya H, Kobayashi K,
Toda T and Endo T: Structure-function analysis of human protein
O-linked mannose beta1,2-N-acetylglucosaminyltransferase 1,
POMGnT1. Biochem Biophys Res Commun. 320:39–44. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lan J, Guo P, Chen M, Wu B, Mao Q and Qiu
Y: O-linked mannose β-1,2-N-acetylglucosaminyltransferase 1
correlated with the malignancy in glioma. J Craniofac Surg.
24:1441–1446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lan J, Guo P, Lin Y, Mao Q, Guo L, Ge J,
Li X, Jiang J, Lin X and Qiu Y: Role of glycosyltransferase PomGnT1
in glioblastoma progression. Neuro-oncol. 17:211–222. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Abbott KL, Troupe K, Lee I and Pierce M:
Integrin-dependent neuroblastoma cell adhesion and migration on
laminin is regulated by expression levels of two enzymes in the
O-mannosyl-linked glycosylation pathway, PomGnT1 and GnT-Vb. Exp
Cell Res. 312:2837–2850. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abbott KL, Troupe K, Matthews RT and
Pierce M: GnT-Vb expression increases O-mannosyl-linked HNK-1
epitope leading to changes in neuronal cell adhesion and migration.
Glycobiology. 16:1111. 2006.
|
|
17
|
Miller MR, Ma D, Schappet J, Breheny P,
Mott SL, Bannick N, Askeland E, Brown J and Henry MD:
Downregulation of dystroglycan glycosyltransferases LARGE2 and ISPD
associate with increased mortality in clear cell renal cell
carcinoma. Mol Cancer. 14:1412015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeisberg M and Neilson EG: Biomarkers for
epithelial-mesenchymal transitions. J Clin Invest. 119:1429–1437.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Makker A and Goel MM: Tumor progression,
metastasis and modulators of EMT in endometrioid endometrial
carcinoma: An update. Endocr Relat Cancer. 23:R85–R111. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shen Y, Zhou J, Li Y, Ye F, Wan X, Lu W,
Xie X and Cheng X: miR-375 mediated acquired chemo-resistance in
cervical cancer by facilitating EMT. PLoS One. 9:e1092992014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Toge M, Yokoyama S, Kato S, Sakurai H,
Senda K, Doki Y, Hayakawa Y, Yoshimura N and Saiki I: Critical
contribution of MCL-1 in EMT-associated chemo-resistance in A549
non-small cell lung cancer. Int J Oncol. 46:1844–1848. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zou C, Lu Y, Mizokami A, Keller ET, Pienta
KJ and Zhang J: CCL2 and interleukin-6 regulate EMT-mediated
chemo-resistance in prostate cancer. Cancer Res. 72 Suppl:Abst
841–841. 2012. View Article : Google Scholar
|
|
24
|
Liang SQ, Marti TM, Dorn P, Froment L,
Hall SR, Berezowska S, Kocher G, Schmid RA and Peng RW: Blocking
the epithelial-to-mesenchymal transition pathway abrogates
resistance to anti-folate chemotherapy in lung cancer. Cell Death
Dis. 6:e18242015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang S, Marti TM, Dorn P, Froment L, Hall
S, Berezowska S, Kocher G, Schmid RA and Peng R: 18P
Epithelial-to-mesenchymal transition (EMT) is required for
resistance to anti-folate chemotherapy in lung cancer. J Thorac
Oncol. 11:(Suppl) S632016. View Article : Google Scholar
|
|
26
|
Stupp R, Hegi ME, Van den Bent MJ, Mason
WP, Weller M, Mirimanoff RO and Cairncross JG; European
Organisation for Research and Treatment of Cancer Brain Tumor and
Radiotherapy Groups, ; National Cancer Institute of Canada Clinical
Trials Group, : Changing paradigms: an update on the
multidisciplinary management of malignant glioma. Oncologist.
11:165–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dehdashti AR, Hegi ME, Regli L, Pica A and
Stupp R: New trends in the medical management of glioblastoma
multiforme: The role of temozolomide chemotherapy. Neurosurg Focus.
20:E62006.PubMed/NCBI
|
|
28
|
Lu C and Shervington A: Chemoresistance in
gliomas. Mol Cell Biochem. 312:71–80. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kroemer G, Galluzzi L, Vandenabeele P,
Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS,
Golstein P, Green DR, et al: Nomenclature Committee on Cell Death
2009: Classification of cell death: Recommendations of the
Nomenclature Committee on Cell Death 2009. Cell Death Differ.
16:3–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vega EA, Graner MW and Sampson JH:
Combating immunosuppression in glioma. Future Oncol. 4:433–442.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhu Z, Du S, Ding F, Guo S, Ying G and Yan
Z: Ursolic acid attenuates temozolomide resistance in glioblastoma
cells by downregulating O6-methylguanine-DNA
methyltransferase (MGMT) expression. Am J Transl Res. 8:3299–3308.
2016.PubMed/NCBI
|
|
32
|
Bobola MS, Kolstoe DD, Blank A,
Chamberlain MC and Silber JR: Repair of 3-methyladenine and abasic
sites by base excision repair mediates glioblastoma resistance to
temozolomide. Front Oncol. 2:1762012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
McConkey DJ, Choi W, Marquis L, Martin F,
Williams MB, Shah J, Svatek R, Das A, Adam L, Kamat A, et al: Role
of epithelial-to-mesenchymal transition (EMT) in drug sensitivity
and metastasis in bladder cancer. Cancer Metastasis Rev.
28:335–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
She X, Yu Z, Cui Y, Lei Q, Wang Z, Xu G,
Luo Z, Li G and Wu M: miR-181 subunits enhance the chemosensitivity
of temozolomide by Rap1B-mediated cytoskeleton remodeling in
glioblastoma cells. Med Oncol. 31:8922014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vester-Christensen MB, Halim A, Joshi HJ,
Steentoft C, Bennett EP, Levery SB, Vakhrushev SY and Clausen H:
Mining the O-mannose glycoproteome reveals cadherins as major
O-mannosylated glycoproteins. Proc Natl Acad Sci USA. 110:pp.
21018–21023. 2013; View Article : Google Scholar : PubMed/NCBI
|