Introduction
Laryngeal squamous cell carcinoma (LSCC) is a common
type of malignant tumor of the head and neck and is the second most
common reason for cancer-related deaths and poor prognosis among
malignancies in the respiratory tract (1). Current treatments, including surgical
resection, radiation therapy and chemotherapy, have already had a
good curative effect on early stage cases, but are less effective
in more advanced cases (2,3). Although LSCC patients have to bear
complete or partial loss of vocal function due to laryngectomy, and
many patients have to endure a tracheal cannula throughout their
lives due to a total laryngectomy, an increased local recurrence
rate and lower OS rates have been witnessed over the past decades
(4). Therefore, a comprehensive
understanding of the molecular mechanisms of LSCC progression and
findings of novel molecular markers for the diagnosis and
prognostic assessment of LSCC are in urgent demand.
Solute carrier family 7, membrane 11 (SLC7A11) or
xCT, is a type of amino-acid transporter which is responsible for
transporting cystine into cells in exchange for glutamate at a
ratio of 1:1 (5). Amino-acid
transporters are necessary for tumor cell growth and proliferation.
In addition, SLC7A11-mediated cystine transport for GSH synthesis
plays a key role in the prevention of oxidative stress signaling
that is strongly associated with cell proliferation and tumor
growth (6). Oxidative stress is
mainly generated in cancer cells due to their relatively high
metabolic rate (7,8). Thus, the activity of SLC7A11-mediated
cystine uptake for GSH synthesis in cancer cells is strongly
associated with cell proliferation and tumor growth (6,9).
SLC7A11 has been demonstrated to be involved in a variety of human
carcinomas, including glioma, breast, ovarian, colon, pancreatic,
gastric and esophageal cancer (6,10–16).
Although these studies suggest that the modulation of SLC7A11
activity is an intriguing strategy for the diagnosis, prognosis and
therapy of cancer, the detailed mechanisms by which SLC7A11
regulates the proliferation in human LSCC have not been fully
investigated. Furthermore, the clinicopathological impact of
SLC7A11 expression on LSCC has remained elusive to date.
To determine whether SLC7A11 can be used as a
biomarker in the diagnosis and prognosis of LSCC as well as the
role of SLC7A11 in LSCC, we examined the expression of SLC7A11,
Ki-67 and p53 in paraffin-embedded laryngeal squamous carcinoma
tissues by immunohistochemistry (IHC), and investigated the
statistical relationships of the aforementioned three proteins.
Then, we analyzed the relationship of SLC7A11 expression levels
with clinicopathological characteristics such as OS and
recurrence-free survival (RFS) rates in LSCC patients. SLC7A11 mRNA
expression was further examined using RT-PCR in LSCC tissues and in
para-cancerous tissues. Moreover, the human laryngeal cancer cell
line Hep-2 and UMCC-5 were used to assess the effects of SLC7A11 in
the cell proliferation process and the cell cycle. Our results
indicate that SLC7A11 plays an important role in the tumor
progression of LSCC.
Materials and methods
Tissue samples and patients
All specimens were obtained from the Beijing Tongren
Hospital, including paraffin-embedded and fresh tissues. Cases
(327) of paraffin-embedded laryngeal squamous carcinoma tissues
(including a recurrence group of 116 cases and a non recurrence
group of 211 cases) as well as 108 cases of paraffin-embedded
laryngeal atypical hyperplasia tissues and 62 cases of vocal polyp
tissues, which were collected between August 2000 and November 2012
for immunohistochemical analysis. Another 30 cases of fresh tumor
tissues and para-cancerous tissues, which were used for RT-PCR
analysis, were randomly collected from LSCC patients between June
2013 and August 2014 and frozen at −80°C until RNA extraction.
Para-cancerous tissues were obtained directly after surgery from
the surgery safety margin (0.5–1.0 cm beyond the tumor). All
patients were confirmed clinically and histologically by
laryngoscopy, CT, MRI and pathologic biopsy. The patient
eligibility criteria were as follows: no synchronous or
metachronous cancers (in addition to the LSCC) and no preoperative
chemotherapy or radiation therapy. We excluded the patients with
non-curative resected tumors or non-consecutive data. All patients
had signed written informed consent. Ethical approval was provided
by the Medical Ethics Committee of Beijing Tongren Hospital and was
consistent with the Declaration of Helsinki.
The main experimental reagents
The anti-SLC7A11 antibody produced in rabbits was
purchased from Abcam Biotechnology (Cambridge, UK) (1:500).
Monoclonal anti-Ki-67 and monoclonal anti-p53 antibodies, and an
IHC kit were purchased from Zhongshan Golden Bridge Biotechnology
(Beijing, China). The rabbit or mouse negative control serum were
purchased from Sigma Chemicals (St. Louis, MO, USA). RNeasy Mini
kit and MTT assay were purchased from Beyotime (Shanghai, China).
SLC7A11 short hairpin RNA (shRNA) and control shRNA lentiviruses
were designed and synthesized by Hanbio Biotechnology (Shanghai,
China). Antibiotic solution (104 U penicillin, 10 mg streptomycin,
25 µg amphotericin B), dimethyl sulfoxide (DMSO), and propidium
iodide (PI) were purchased from Sigma Chemicals. Fetal bovine serum
(FBS) and Dulbecco's modified Eagle's medium (DMEM) were purchased
from Gibco (Cambrex, MD, USA). Polyvinylidene difluoride (PVDF)
membranes were purchased from Millipore (Bedford, MA, USA).
IHC
The archival paraffin-embedded tissue blocks were
cut into 4-µm thick tissue sections. The sections were
deparaffinized with xylene and rehydrated by a graded ethanol
series. Blocking of endogenous peroxidases was accomplished by
incubating sections in 3% hydrogen peroxide for 10 min. Antigen
retrieval was performed using Heat Processor Solution (pH 6)
(Zhongshan Biotechnology, Beijing, China) at 125°C for 5 min by a
pressure cooker, and then sections were cooled to room temperature.
Goat blood serum (10%) was used to prevent non-specific binding.
Then, the sections were incubated with SLC7A11 purified polyclonal
antibody, monoclonal anti-Ki-67 and monoclonal anti-p53 overnight
at 4°C, followed by incubation with the secondary antibody.
Immunostaining was performed using two-step diaminobenzidine
visualization. Sections were counterstained with hematoxylin,
rinsed in water, dehydrated in ascending concentrations of ethanol
followed by clearance with xylene, and cover slipped permanently
for light microscopy. Negative controls were carried out with the
same procedure using the rabbit or mouse negative control serum
instead of the primary antibody. Repeatedly validated esophageal
adenocarcinoma specimens with positive staining of SLC7A11 served
as positive controls. Liver cancer and pulmonary squamous cell
carcinoma tissues were applied as the positive control to indicate
Ki-67 and p53 expression, respectively.
Evaluation of immunohistochemical
variables
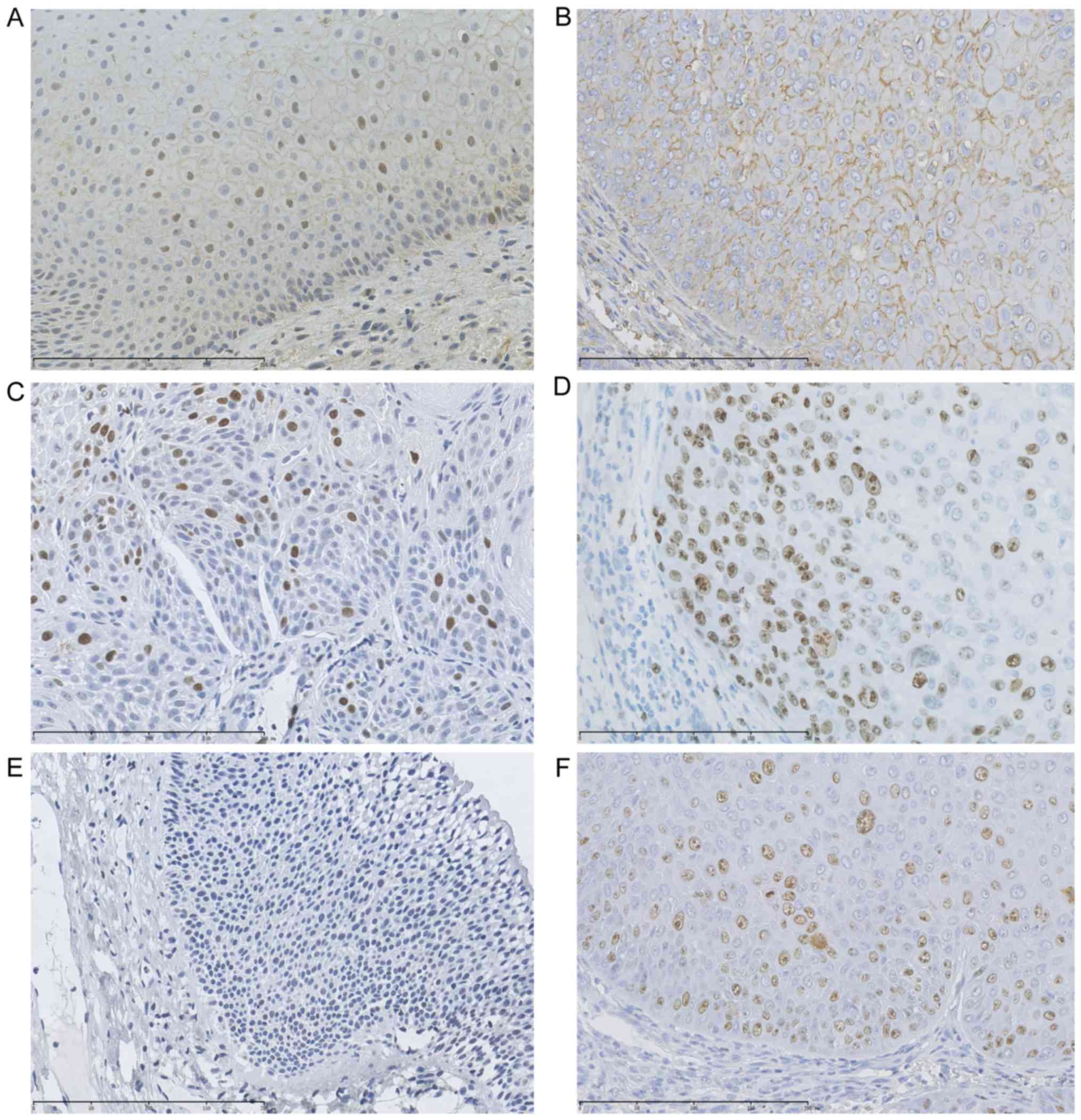
By IHC analysis of 327 laryngeal tissues, we found
that the positive staining of the SLC7A11 protein expression was
chiefly located in the membrane of the parabasal cell layer and
epithelial cells, and the positive staining of p53 and Ki-67
proteins were mainly located in the nuclei of epithelial cells
(Fig. 1). Two independent
investigators who were blinded to all immunohistochemical outcomes,
adopted a semi-quantitative system with the staining intensity and
proportion to scored all specimens. Five microscopic fields in
tumor tissues (original magnification, ×400) were randomly
selected. According to the color of the staining,
immunohistochemical results were divided into no staining (with the
same color as the background), low staining (with a color slightly
stronger than the background), middle staining (with a color
markedly stronger than the background), and high staining. Each
rate got a score of 0, 1, 2 and 3, respectively. According to the
percentage of the positive cells in the field, <10%, between
10–25%, 26–75% and >76% were scored as 0, 1, 2 and 3,
respectively. Then, these two values were combined to determine the
protein expression of each group (the final score 0–2 represented
negative ‘−’ staining, the final score 3–7 represented positive ‘+’
staining). The counting method is shown in (Table I).
 | Table I.Immunohistochemical staining and
scoring. |
Table I.
Immunohistochemical staining and
scoring.
| Proportion of
positive tumor cells (%) | Score 1 | Average intensity
of positive tumors | Score 2 | Final score (score
1 + score 2) | Grading |
|---|
| <10 | 0 | Negative | 0 | 0 | Negative |
| 10–25 | 1 | Pale yellow | 1 | 1–2 | Weak positive |
| 26–75 | 2 | Yellow | 2 | 3–5 | Positive |
| >76 | 3 | Dark yellow or | 3 | 6–7 | Strong
positive |
|
|
| Dark brown | 4 |
|
|
RT-PCR
Thirty cases of fresh LSCC and the corresponding
pericarcinomatous tissues were collected, and three cases were
separately randomized into a single sample. The SLC7A11 gene mRNA
sequences were acquired from the NCBI database to design an RT-PCR
primer. The sequences of the primer pairs were: forward,
5′-CATCTCTCCTAAGGGCGTGC-3′ and reverse, 5′-CCCACGAGAGAAAAAGTCG-3′.
Total RNA was extracted using TRIzol reagent, and reverse
transcribed to complementary DNA (cDNA) using an RT-PCR kit
(Gibicol) according to the manufacturers recommendations. PCR
reactions were incubated at 94°C for 3 min, followed by 30 cycles
of 94°C for 30 sec, 57°C for 40 sec, 70°C for 50 sec, and then a
final extension at 72°C for 10 min. GAPDH was used as an internal
control using the relative quantification method. All assays were
performed in triplicate.
Cell culture
The human LSCC cell line UMCC-5 was gifted by the
Cell Bank of the University of Michigan (Ann Arbor, MI, USA). The
human LSCC cell line Hep-2 was purchased from the China
Infrastructure of Cell Line Resources. All cells were maintained in
DMEM supplemented with 10% FBS and penicillin/streptomycin at 37°C
in a humidified atmosphere of 5% CO2. The cells were
split twice weekly and cells in the logarithmic growth phase were
used for experiments.
Construction of SLC7A11-shRNA
lentivirus and establishment of stable transfectants
SLC7A11-shRNAs (shRNA1, shRNA2 and shRNA3) and a
negative control-shRNA (NC-shRNA) lentivirus were designed and
synthesized by Hanbio Biotechnology. The oligonucleotide sequences
of SLC7A11-shRNA1 were: 5′-GAGTCTGGGTGGAACTCCTCATAAT-3′; the
oligonucleotide sequences of SLC7A11-shRNA2 were:
5′-CCCTGGAGTTATGCAGCTAAT-3′; the oligonucleotide sequences of
SLC7A11-shRNA3 were: 5′-GGTGTGTTTGCTGTCTCCAGGTTAT-3′; the
oligonucleotide of control-shRNA was: 5′-TTCTCCGAACGTGTCACGTAA-3′.
Hep-2 and UMCC-5 cells were cultured in growth medium to reach
confluency of 60–80%, and then, were respectively transfected with
50 nmol/l SLC7A11-shRNA1 or SLC7A11-shRNA2 or SLC7A11-shRNA3 or
NC-shRNA using Lipofectamine 2000 according to the manufacturer's
instructions (10). At 48–96 h
after transfection, the cells were collected for the following
assays. Luciferase-reporter assays, RT-PCR and western blotting
were used to illustrate the transfection efficiency of sh-SLC7A11
in UMCC-5 and Hep-2 cells. Independent triplicate wells were used
for each vector.
Western blot analysis
UMCC-5 and Hep-2 cells were cultured and infected
with recombinant lentivirus for four to five days, and then the
proteins of collected cells were lysed with 2X SDS sample buffer
[100 mM Tris-HCl (pH 6.8), 10 mM EDTA, 4% SDS, 10% glycine].
Protein (20 µg) was loaded onto a 10% SDS-PAGE and transferred to a
PVDF membrane. Next, antibodies against SLC7A11 (1:200; Abcam) or
GAPDH (1:300; Bioworld Technology, Minneapolis, MN, USA) were used
as the primary antibodies. The detection was performed using an ECL
kit. Bands on X-ray films were quantified with an ImageQuant
densitometric scanner (Molecular Dynamics, Sunnyvale, CA, USA).
Each experiment was repeated three times.
Flow cytometric assay
The Hep-2 and UMCC-5 cells were harvested 72 h after
transfection with either SLC7A11-shRNA or control-shRNA. After
trypsinization, the supernatant was discarded and the cells were
collected and washed with phosphate-buffered saline (PBS), and then
fixed with 1 ml ice-cold 70% alcohol. Following incubation at 4°C
for at least 12 h, the cells were washed with PBS and stained with
50 µl/ml PI (Sigma) solution and supplemented with 100 µl/ml RNase
in PBS, and incubated sequentially in the dark at room temperature
for 30 min. The cell cycle analysis of samples were analyzed
immediately using flow cytometry (FACSCalibur; BD Biosciences,
Franklin Lakes, NJ, USA) according to the manufacturer's protocol
as previously described (17). Each
experiment was performed in triplicate and repeated three times.
The percentage of cells in the G0/G1, S and G2/M phases were
determined using CellQuest software (version 3.3; BD
Biosciences).
MTT cell proliferation
Cell proliferation was determined by MTT assay after
0, 24, 48, 72 and 96 h of lentiviral transfection. Cells were
seeded into 96-well culture plates at a concentration of 3,000
cells/well and each well was incubated with 20 µl of MTT solution
for 4 h at 37°C and 5% CO2. Subsequently, the medium was
aspirated, and the resulting purple formazan crystals were
dissolved in DMSO (18). The
absorbance at 490 nm was determined using a microplate reader.
Experiments were performed in triplicate using an MTT assay
following the manufacturer's instructions. Cell viability was
expressed as a percentage of the control culture and the
IC50 values were calculated from non-linear regression
using the program GraphPad Prism 5.0 software (GraphPad Software,.
Inc., La Jolla, CA, USA).
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software. The Chi-square or Fisher's exact tests were used to
evaluate the association between the expression levels of SLC7A11
and the clinicopathological characteristics. The
Wilcoxon-Mann-Whitney test was used to evaluate the messenger RNA
levels of SLC7A11 in laryngeal carcinoma and the adjacent tissues.
The Spearman rank correlation was used to evaluate the correlation
between SLC7A11, p53 and Ki-67. Survival curves were constructed by
the Kaplan-Meier method, and the survival differences were examined
using the log-rank test. A multivariate analysis of factors
influencing survival was performed using the Cox proportional
hazards model. The Students t-test was used to evaluate the
differences between the SLC7A11 knockdown and the control cells.
P<0.05 was considered to indicate a statistically significant
result.
Results
Association of the immunohistochemical
expression of SLC7A11, Ki-67 and p53 with clinicopathological
features
The IHC results confirmed that the protein
expression levels of SLC7A11 were higher in the LSCC tissues than
in the vocal polyp and laryngeal atypical hyperplasia tissues
according to the Chi-square test (χ2=8.558, P=0.003;
χ2=4.324, P=0.038) (Table
II and Fig. 1). When comparing
the difference in the expression levels SLC7A11 according to T
stages of LSCC, we found that there were significant differences
between T1-2 and T3-4. However, no statistical difference was found
between LSCC patients with different ages, sex, lymph node and
distant metastases, or tumor differentiation (Table II). Therefore, these data clearly
indicated that SLC7A11 was increased in LSCC tissues and revealed
that it may be a diagnostic marker for LSCC.
 | Table II.Relationship between the expression
of SLC7A11 and the clinicopathological parameters in laryngeal
cancer patients. |
Table II.
Relationship between the expression
of SLC7A11 and the clinicopathological parameters in laryngeal
cancer patients.
|
Clinico-pathological parameters | No. of cases | SLC7A11
immunostaining high expression frequency (%) | χ2 | P-value |
|---|
| Vocal polyp | 62 | 21 (33.9) | 8.558 | 0.003a |
| Laryngeal atypical
hyperplasia | 108 | 46 (42.6) | 4.324 | 0.038b |
| LSCC | 327 | 177 (54.1) |
|
|
| Age (years) |
|
|
|
|
|
≤65 | 184 | 107 (58.1) | 2.744 | 0.098 |
|
>65 | 143 | 70 (48.9) |
|
|
| Sex |
|
|
|
|
|
Male | 318 | 175 (55.0) | 3.794 | 0.292 |
|
Female |
9 | 2 (28.6) |
|
|
| T stage |
|
|
|
|
|
T1-T2 | 207 | 99 (47.8) | 9.023 | 0.003 |
|
T3-T4 | 120 | 78 (65.0) |
|
|
| Lymph node
metastasis |
|
|
|
|
| N0 | 237 | 121 (51.1) | 1.031 | 0.31 |
|
NΧ | 90 | 56 (62.2) |
|
|
| Distant
metastasis |
|
|
|
|
| M0 | 292 | 159 (54.5) | 0.115 | 0.734 |
|
MΧ | 35 | 18 (51.4) |
|
|
| Tumor
differentiation |
|
|
|
|
| Highly
differentiated | 160 | 96 (60.0) | 4.368 | 0.113 |
|
Moderately differentiated | 125 | 61 (48.8) |
|
|
| Poorly
differentiated | 42 | 20 (47.6) |
|
|
In order to investigate which SLC7A11-related
proteins may be involved in the proliferation of LSCC, Ki-67 and
p53 protein expression levels were evaluated by immunochemistry.
The protein expression levels of Ki-67 and p53 were also higher in
the LSCC tissues than in the vocal polyp and laryngeal atypical
hyperplasia tissues (Tables III
and IV). The Spearman rank
correlation analysis revealed that the expression of SLC7A11 had a
positive correlation with the expression of p53 and Ki-67,
respectively (Table V). Meanwhile,
the expression of Ki-67 and p53 also had a positive correlation
with each other (Table V). These
data demonstrated that Ki-67, p53 and SLC7A11 in LSCC may influence
each other.
 | Table III.Relationship between the expression
of Ki-67 and the clinicopathological parameters in laryngeal cancer
patients. |
Table III.
Relationship between the expression
of Ki-67 and the clinicopathological parameters in laryngeal cancer
patients.
|
Clinico-pathological parameters | No. of cases | Ki-67
immunostaining high expression frequency (%) | χ2 | P-value |
|---|
| Vocal polyp | 62 | 17 (27.4) | 8.190 | 0.004a |
| Laryngeal atypical
hyperplasia | 108 | 38 (35.2) | 4.670 | 0.031b |
| LSCC | 327 | 154 (47.1) |
|
|
| Age (years) |
|
|
|
|
|
≤65 | 184 | 85 (46.2) | 1.595 | 0.207 |
|
>65 | 143 | 69 (48.2) |
|
|
| Sex |
|
|
|
|
|
Male | 318 | 151 (47.5) | 0.703 | 0.402 |
|
Female |
9 | 3 (33.3) |
|
|
| T stage |
|
|
|
|
|
T0-T2 | 207 | 86 (41.5) | 6.971 | 0.08 |
|
T3-T4 | 120 | 61 (56.7) |
|
|
| Lymph node
metastasis |
|
|
|
|
| N0 | 237 | 117 (49.4) | 1.784 | 0.182 |
|
NΧ | 90 | 37 (41.1) |
|
|
| Distant
metastasis |
|
|
|
|
| M0 | 292 | 131 (44.9) | 0.173 | 0.677 |
| MΧ | 35 | 17 (48.6) |
|
|
| Tumor
differentiation |
|
|
|
|
| Highly
differentiated | 160 | 81 (50.6) | 1.584 | 0.453 |
|
Moderately differentiated | 125 | 55 (44.0) |
|
|
| Poorly
differentiated | 42 | 18 (42.9) |
|
|
 | Table IV.Relationship between the expression
of p53 and the clinicopathological parameters in laryngeal cancer
patients. |
Table IV.
Relationship between the expression
of p53 and the clinicopathological parameters in laryngeal cancer
patients.
|
Clinico-pathological parameters | No. of cases | p53 immunostaining
high expression frequency (%) | χ2 | P-value |
|---|
| Vocal polyp | 62 | 9 (14.5) | 5.028 | 0.025a |
| Laryngeal atypical
hyperplasia | 108 | 24 (22.2) | 4.747 | 0.029b |
| LSCC | 327 | 92 (28.1) |
|
|
| Age (years) |
|
|
|
|
|
≤65 | 184 | 59 (32.1) | 3.215 | 0.073 |
|
>65 | 143 | 33 (23.1) |
|
|
| Sex |
|
|
|
|
|
Male | 318 | 91 (28.6) | 0.602 | 0.438 |
|
Female |
9 | 1 (11.1) |
|
|
| T stage |
|
|
|
|
|
T0-T2 | 207 | 51 (24.6) | 3.411 | 0.065 |
|
T3-T4 | 120 | 41 (34.2) |
|
|
| Lymph node
metastasis |
|
|
|
|
| N0 | 237 | 71 (29.9) | 1.416 | 0.234 |
|
NΧ | 90 | 21 (23.3) |
|
|
| Distant
metastasis |
|
|
|
|
| M0 | 292 | 85 (29.1) | 1.283 | 0.257 |
|
MΧ | 35 | 7 (20.0) |
|
|
| Tumor
differentiation |
|
|
|
|
| Highly
differentiated | 160 | 40 (25.0) | 2.866 | 0.239 |
|
Moderately differentiated | 125 | 36 (28.8) |
|
|
| Poorly
differentiated | 42 | 16 (38.1) |
|
|
 | Table V.The correlation of SLC7A11 with Ki-67
and p53 expression in laryngeal cancer. |
Table V.
The correlation of SLC7A11 with Ki-67
and p53 expression in laryngeal cancer.
|
| SLC7A11 | p53 |
|---|
| SLC7A11 |
|
|
|
Correlation coefficient | 1 | 0.171a |
| Sig.
(two-tailed) |
| 0.002 |
| N | 327 | 327 |
| Ki-67 |
|
|
|
Correlation coefficient | 0.155a | 0.180a |
| Sig.
(two-tailed) | 0.005 | 0.001 |
| N | 327 | 327 |
SLC7A11 gene mRNA expression levels in
clinical tissue samples detected by real-time PCR
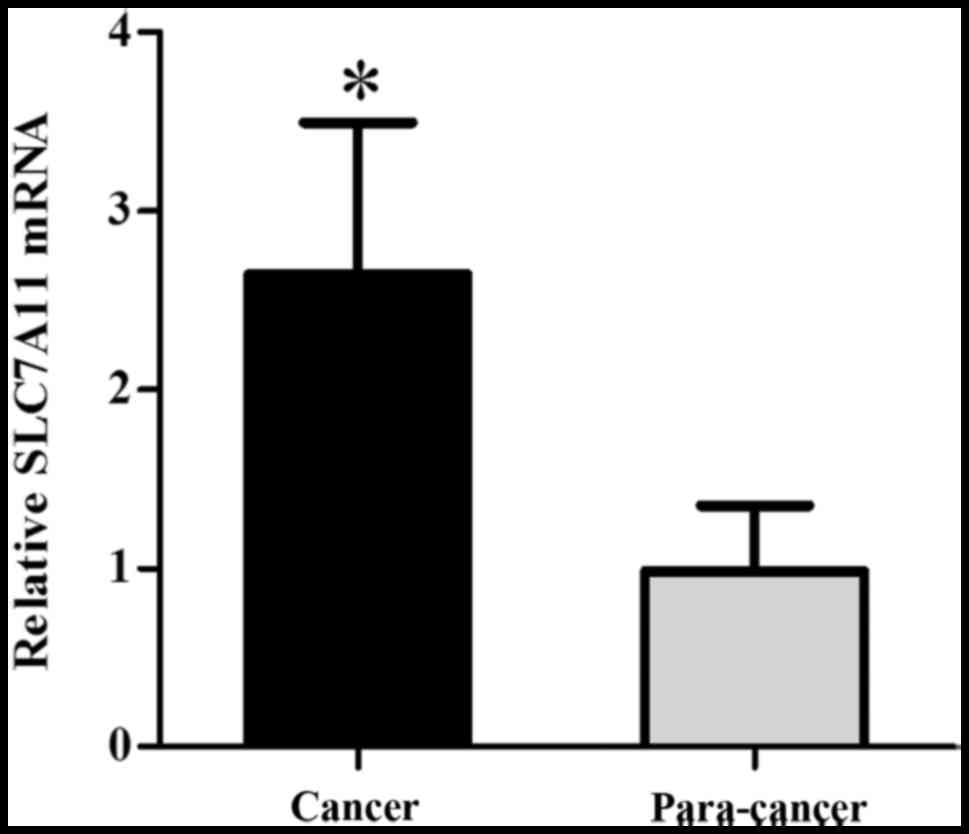
SLC7A11 mRNA levels were further assessed by
qRT-PCR. As shown in Fig. 2, the
levels of SLC7A11 mRNA transcription were significantly higher in
the carcinomatous tissue than in the pericarcinomatous tissue
(Fig. 2). GAPDH was regarded as an
internal reference. For the homogenization process, each sample was
analyzed by the ΔΔCt value. ΔCt = SLC7A11 mRNA copy number/GAPDH
mRNA copy number (n=30) (P=0.021, P<0.05). The graph was
generated by GraphPad Prism software.
SLC7A11 is a prognostic factor for
LSCC
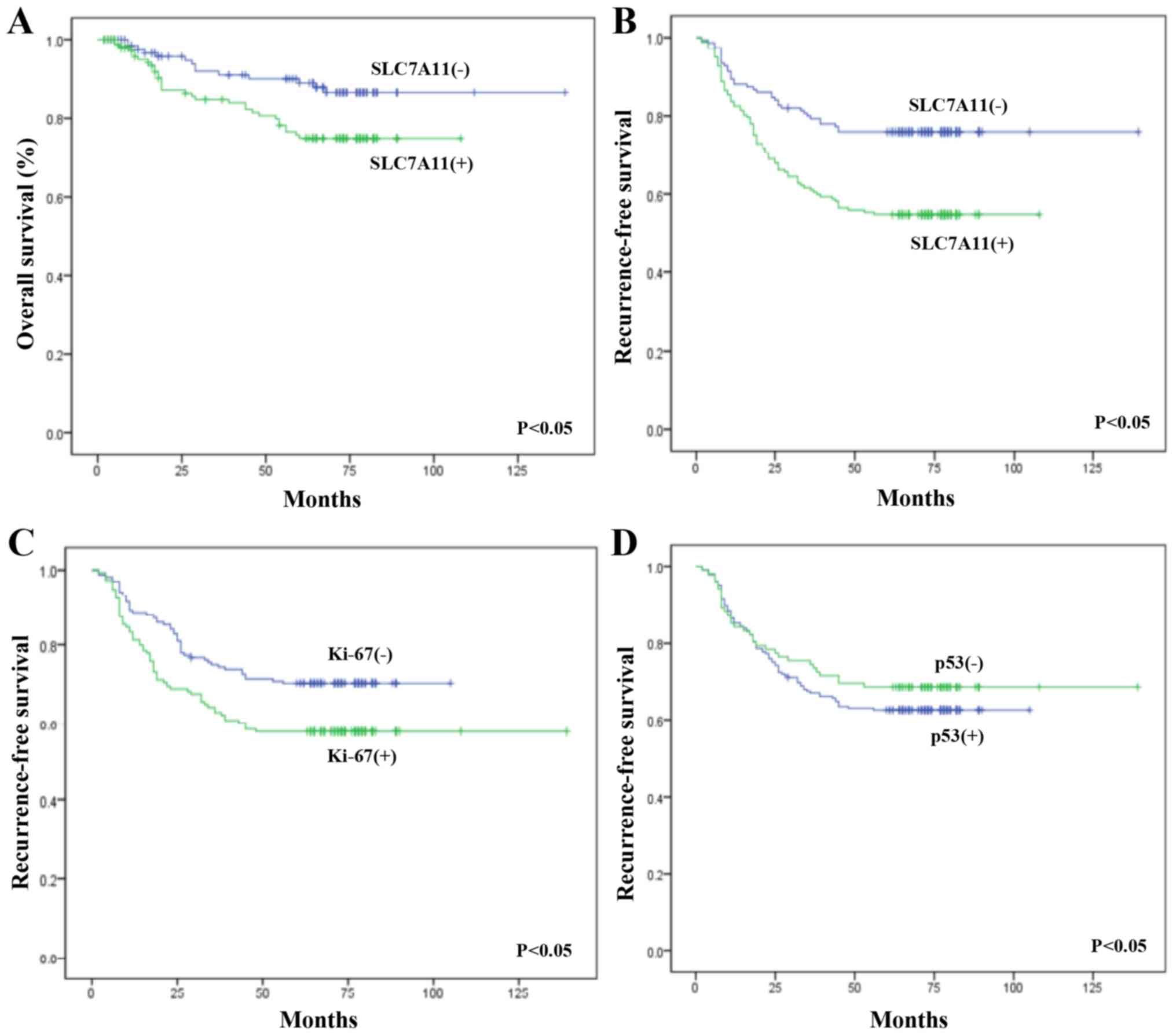
To investigate whether SLC7A11 was a prognostic
factor for LSCC, Cox regression and Kaplan-Meier analysis were
performed to evaluate OS and RFS. Cox regression analysis confirmed
that the expression levels of SLC7A11, Ki-67 and T stage were
prognostic factors for laryngeal cancer (Table VI). The OS was significantly worse
in patients with SLC7A11-positive tumors than that in patients with
SLC7A11-negative tumors (P<0.05). Furthermore, Kaplan-Meier
analysis confirmed that the SLC7A11 expression rate was 62.1%
(72/116) and 49.7% (105/211) in the recurrent and non-recurrent
groups of the postoperative LSCC patients, respectively, with a
statistically significant difference (P<0.05). Meanwhile, the
expression rate of Ki-67 in these two groups was 56.0% (65/116) and
42.2% (89/211), respectively, and this difference was also
statistically significant (P<0.05). The positive rates of p53
expression were 28.4% (33/116) and 27.9% (59/211) in the recurrent
and non-recurrent groups of the postoperative LSCC patients,
respectively, with no statistically significant difference
(P>0.05) (Table VII; Fig. 3).
 | Table VI.Multivariate analyses by Cox
regression for recurrence-free survival of the postoperative LSCC
patients. |
Table VI.
Multivariate analyses by Cox
regression for recurrence-free survival of the postoperative LSCC
patients.
|
|
|
| 95% CI for Exp
(B) |
|---|
|
|
|
|
|
|---|
|
Characteristics | Sig. | Exp (B) | Lower | Upper |
|---|
| Sex | 0.056 | 2.451 | 0.978 | 6.139 |
| Age (years) | 0.566 | 0.904 | 0.64 | 1.277 |
| T stage | 0.017 | 0.647 | 0.453 | 0.926 |
|
Differentiation | 0.958 | 0.993 | 0.771 | 1.279 |
| p53 | 0.115 | 0.714 | 0.470 | 1.085 |
| Ki-67 | 0.020 | 1.624 | 1.080 | 2.442 |
| SLC7A11 | 0.006 | 1.822 | 1.187 | 2.799 |
 | Table VII.Relationship between the expression
of SLC7A11, Ki-67 and p53, and the prognosis of the postoperative
LSCC patients. |
Table VII.
Relationship between the expression
of SLC7A11, Ki-67 and p53, and the prognosis of the postoperative
LSCC patients.
|
|
| SLC7A11 |
| Ki-67 |
| p53 |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Prognosis | Total no. | + | − | P-value | + | − | P-value | + | − | P-value |
|---|
| Recurrence | 116 | 72 | 44 | 0.03 | 65 | 51 | 0.016 | 33 | 83 | 0.925 |
| Non-recurrence | 211 | 105 | 106 |
| 89 | 122 |
| 59 | 152 |
|
| Total number | 327 | 177 | 150 |
| 154 | 173 |
| 92 | 235 |
|
SLC7A11 expression is suppressed by
SLC7A11-shRNAs in UMCC-5 and Hep-2 cells
To observe the role of SLC7A11 on the growth of LSCC
cells, we used SLC7A11-shRNAs (shRNA1, shRNA2 and shRNA3) to
suppress the expression of SLC7A11 in UMCC-5 and Hep-2 cells.
First, in order to show that the virus could be successfully
transfected into the UMCC-5 and Hep-2 cells, we used
SLC7A11-sh-RNA1 for luciferase experiments. As expected, the
luciferase activity was significantly higher in the SLC7A11-shRNA1
transfection group than that in the control group (untransfected
virus group) (P<0.05) (Fig. 4A),
indicating the high efficiency and stability of the transfection.
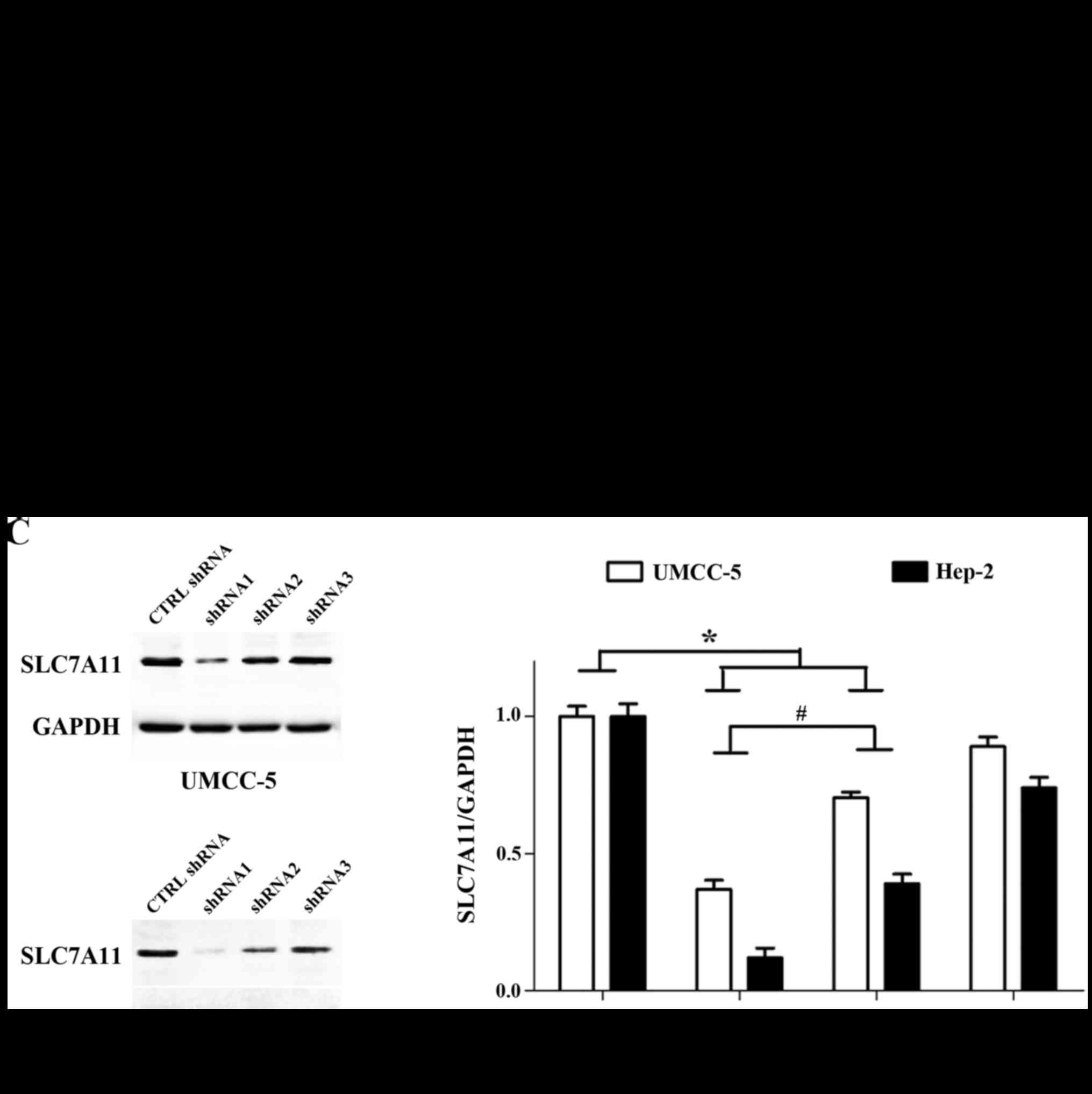
Furthermore, in order to demonstrate which of the three shRNAs
(shRNA1, shRNA2 and shRNA3) had the most efficient function, we
used PCR and western blotting. The mRNA levels of SLC7A11 were
decreased in the SLC7A11-shRNA1 and SLC7A11-shRNA2 groups, more
than that in the SLC7A11-shRNA3 and the sh-CTRL groups by RT-PCR
(Fig. 4B). The protein levels of
SLC7A11 were determined by western blot assays and normalized for
GAPDH. Compared with the SLC7A11-shRNA3 and the sh-CTRL groups, the
protein levels of SLC7A11 were decreased in the SLC7A11-shRNA1 and
SLC7A11-shRNA2 groups (Fig. 4C).
The quantified intensity of protein bands is shown as the ratio of
SLC7A11/GAPDH for the different groups (n=3). Values are expressed
as the mean ± SD, P<0.05 (compared with the sh-CTRL group)
(Fig. 4C). Finally, the mRNA and
protein levels of SLC7A11 were significantly inhibited in the
SLC7A11-shRNA1 group compared with the SLC7A11-shRNA2 group
(P<0.05; Fig. 4B and C), and
thus we selected SLC7A11-shRNA1 for the next related experimental
research.
The effect of SLC7A11 knockdown on the
proliferation of UMCC-5 and Hep-2 cells by MTT assay
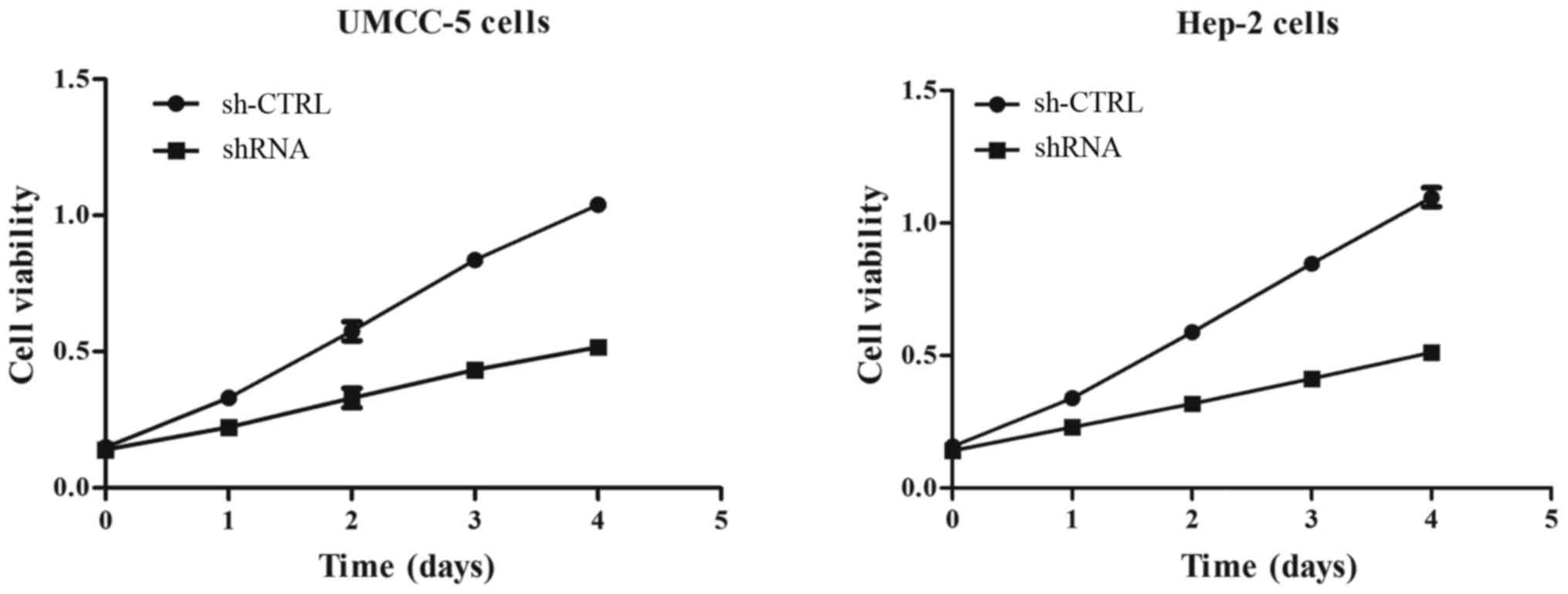
The MTT assay revealed that downregulation of
SLC7A11 markedly inhibited the growth of UMCC-5 and Hep-2 cells.
The MTT assay indicated that cell proliferation was decreased more
in the sh-RNA group than that in the sh-CTRL group (Fig. 5).
SLC7A11 controls the cell cycle
progression of LSCC cells
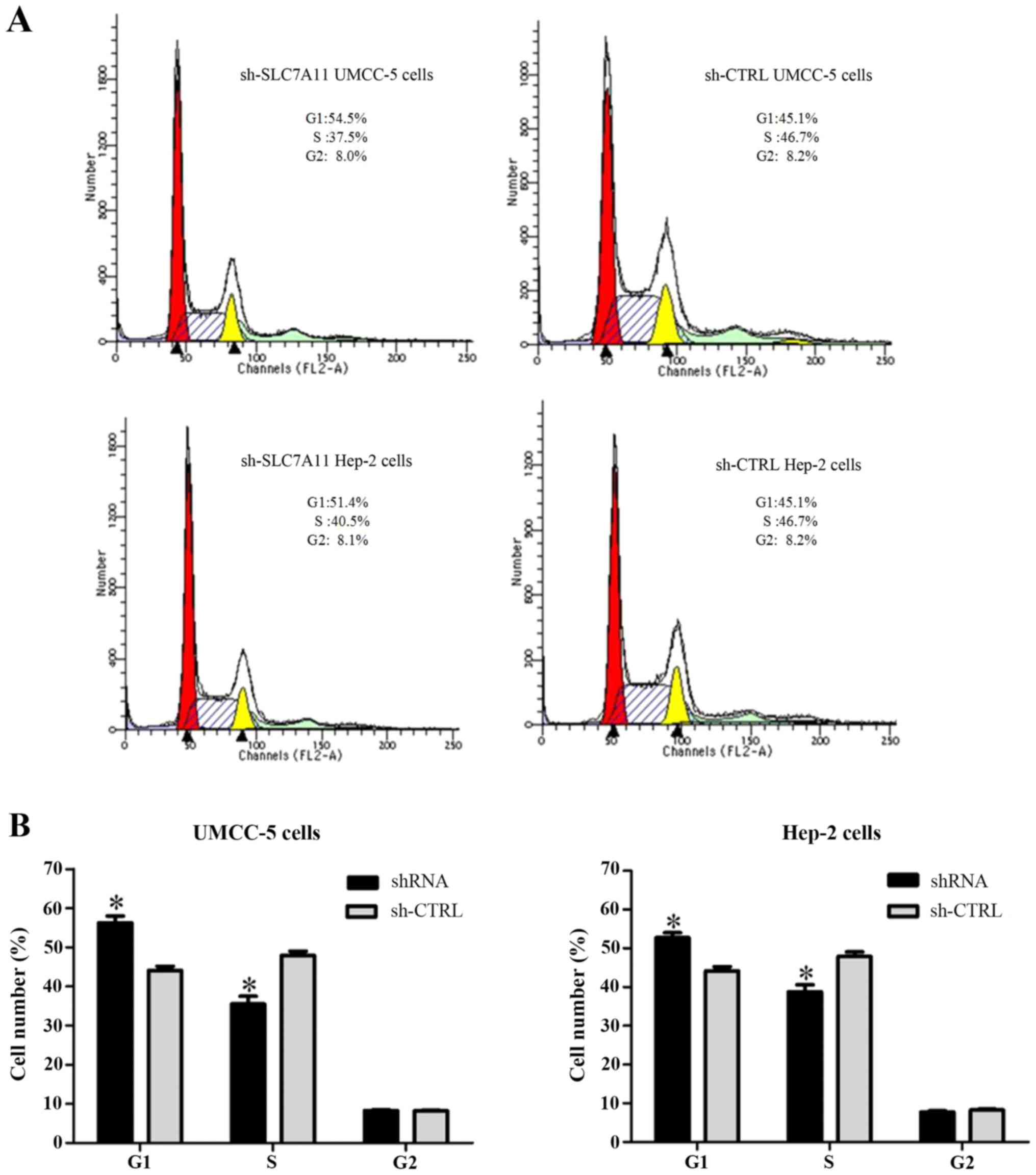
To investigate the mechanism underlying the
promotion of cell proliferation of SLC7A11, we conducted knockdown
experiments with SLC7A11-shRNA in UMCC-5 and Hep-2 cells, and
analyzed the effects of SLC7A11 on cell cycle progression. At 48 h
after the shRNA transfection, the cells in the G1 phase were
increased more in the shRNA-infected UMCC-5 and Hep-2 cells than in
the sh-CTRL-infected UMCC-5 and Hep-2 cells (Fig. 6).
Discussion
System Xc- is a type of amino-acid
transporter which is composed of a heavy subunit termed 4F2hc
(SLC3A2) and a light chain subunit xCT (SLC7A11). Whereas SLC3A2 is
a subunit common to several amino acid transporter systems, SLC7A11
is unique and responsible for importing the amino acid cystine into
cells at a ratio of 1:1 counter-transport of glutamate (5). SLC7A11-mediated cystine transport for
GSH synthesis plays a key role in the prevention of oxidative
stress signaling that is strongly associated with cell
proliferation and tumor growth (6).
Notably, oxidative stress leads to the oxidative modification of
proteins, lipids and DNAs and is thus thought to play an important
role in many diseases. Furthermore, oxidative stress is
particularly generated in cancer cells due to their relatively high
metabolism (7). Thus, the activity
of SLC7A11 is strongly associated with cancer cell proliferation
and tumor growth (19).
Previous studies have detected that SLC7A11 was
overexpressed in some types of solid tumor tissues or cells,
associated with poor prognosis, and served as a biomarker for
diagnosis (20,21). Robert et al (11) suggested that SLC7A11-overexpressing
tumors grew faster and shortened overall survival (OS). Guo et
al (22) demonstrated that
SLC7A11 expression was often increased in hepatocellular carcinoma
(HCC) and was associated with poor prognosis in HCC patients. In
agreement with previous studies, our results demonstrated that the
expression of the SLC7A11 protein was increased in LSCC tissues as
determined by immunochemistry and the mRNA expression levels of
SLC7A11 in LSCC were also higher than that in pericarcinomatous
tissue by RT-qPCR. Furthermore, The Kaplan-Meier plots indicated
that LSCC patients with SLC7A11-positive expression were associated
with poorer OS, and that the SLC7A11-positive tumors belonging to
high-risk group were associated with poorer RFS. These results
demonstrated a close association between SLC7A11 expression and
undesirable prognosis, and suggested that SLC7A11 may be an
important marker to predict prognosis in LSCC.
Ki-67, the cell proliferation-associated antigen of
antibody, has been explored as a prognostic or predictive marker in
many malignant diseases (23).
Pezzilli et al (23)
suggested that the Ki-67 index was an precise and well-studied
prognostic factor for pancreatic neuroendocrine neoplasms (PNENs)
and it can be utilized for selecting patient candidates for
surgical and particularly for non-surgical treatment. Shiozaki
et al (10) demonstrated
that SLC7A11-positive expression was positively correlated with the
Ki-67 labeling index in human esophageal squamous cell carcinoma
(ESCC). Our results from immunohistochemical examination revealed
that the expression of Ki-67 was significantly higher in LSCC
compared with those in precancerous lesions, and was positively
correlated with the expression of SLC7A11. The log-rank test
suggested that SLC7A11 (+)/Ki-67 (+) expression was correlated with
RFS in postoperative LSCC patients. These results demonstrated that
there was a close association between SLC7A11 expression and Ki-67
expression, and that SLC7A11-positive expression combined with
Ki-67-positive expression had a dismal prognosis in LSCC.
p53 is an important tumor suppressor gene, which is
the most commonly mutated gene in human cancers (24), and is stimulated by cellular stress
such as ionizing radiation, hypoxia, carcinogens and oxidative
stress. Although p53 mediated cell cycle arrest, senescence and
apoptosis serve as critical barriers to cancer development. Recent
studies revealed that other unconventional activities of p53 are
also crucial for its tumor-suppressive function (25–27).
Jiang et al (28) revealed
that p53 regulates a type of cell death dubbed ferroptosis which
belongs to a type of metabolic activity. By suppressing the
expression of SLC7A11, p53 inhibits cystine uptake and sensitizes
cells to ferroptosis, a non-apoptotic form of cell death (28,29).
In the present study, Spearman analysis revealed that the
expression of p53 had a positive correlation with the expression of
SLC7A11. We wondered whether SLC7A11 participates in the
ferroptosis of LSCC and if the role of SLC7A11 can be regulated by
p53 as well. Since ferroptosis can inhibit the growth of tumors
(30,31), our studies provide a research
foundation to further explore and provide new insight into cancer
therapy in LSCC.
On the basis of the results of the present study, we
speculate that SLC7A11 may play important roles in tumorigenesis
and tumor progression of LSCC. As RNA interference (RNAi) has been
widely used as an experimental tool in studying gene function, to
better explore the involvement of SLC7A11 in proliferation and
progression of LSCC, RNAi targeting of SLC7A11 was used to
knockdown the expression of SLC7A11 in in vitro experiments.
The dysregulated cell cycle control of normal epithelial cells
leading to uncontrolled proliferation is one of the major features
of tumor progression. When the cells cease proliferation, the cell
cycle may be arrested at the G1 check-points that assess cell size,
extracellular growth signals, and DNA integrity (32,33).
In the present results, silencing of SLC7A11 significantly
inhibited G1 to S phase transition of Hep-2 and UMCC-5 cell lines,
indicating that SLC7A11 shRNA inhibits cellular proliferation by
inducing G1 phase cell cycle arrest. Similar results have been
reported in human esophageal squamous cell carcinoma (10). By knockdown of SLC7A11 using siRNA,
Shiozaki et al (10)
observed that the expression of SLC7A11 affected the G1/S
checkpoint and impacted the growth inhibition of ESCC cells.
Furthermore, in the present study, the cell viability was assessed
with the MTT method at 24, 48, 72 and 96 h after lentiviral
transfection. We discovered that downregulation of SLC7A11
significantly inhibited the growth of Hep-2 and UMCC-5 cell lines.
In conclusion, SLC7A11 may play an important role in tumor growth
by regulating cell cycle progression and proliferation in the LSCC
cell lines UMCC-5 and Hep-2.
In conclusion, to the best of our knowledge, this is
the first study to demonstrate that SLC7A11 expression levels are
upregulated in tumor tissues of LSCC patients. The expression
levels of SLC7A11 can be used to distinguish LSCC from
precancerosis or laryngeal benign tumors, and the overexpression of
SLC7A11 in LSCC is correlated with laryngeal cancer recurrence and
poor survival after surgery. Moreover, a functional study revealed
that SLC7A11 plays a carcinogenic role in LSCC. In conclusion, the
present study revealed that SLC7A11 may be a candidate novel
molecular target for the diagnosis and prognosis in human LSCC, and
targeting SLC7A11 may have clinical significance for the treatment
of LSCC.
Acknowledgements
The present study was supported in part by the
National Natural Science Foundation Of China (no. 81502493) and the
Capital Health Research and Development of Special Project (no.
2014-2-2053).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Graboyes EM, Townsend ME, Kallogjeri D,
Piccirillo JF and Nussenbaum B: Evaluation of quality metrics for
surgically treated laryngeal squamous cell carcinoma. JAMA
Otolaryngol Head Neck Surg. 142:1154–1163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marioni G, Marchese-Ragona R, Cartei G,
Marchese F and Staffieri A: Current opinion in diagnosis and
treatment of laryngeal carcinoma. Cancer Treat Rev. 32:504–515.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mantsopoulos K, Psychogios G, Bohr C, Zenk
J, Kapsreiter M, Waldfahrer F and Iro H: Primary surgical treatment
of T3 glottic carcinoma: Long-term results and decision-making
aspects. Laryngoscope. 122:2723–2727. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim JC and Donaldson PJ: Focus on
molecules: The cystine/glutamate exchanger (System xc).
Exp Eye Res. 92:162–163. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lewerenz J, Hewett SJ, Huang Y, Lambros M,
Gout PW, Kalivas PW, Massie A, Smolders I, Methner A, Pergande M,
et al: The cystine/glutamate antiporter system xc in
health and disease: From molecular mechanisms to novel therapeutic
opportunities. Antioxid Redox Signal. 18:522–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Estrela JM, Ortega A and Obrador E:
Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci.
43:143–181. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koizume S and Miyagi Y: Lipid droplets: A
key cellular organelle associated with cancer cell survival under
normoxia and hypoxia. Int J Mol Sci. 17:1430–1453. 2016. View Article : Google Scholar :
|
|
9
|
Bhutia YD, Babu E, Ramachandran S and
Ganapathy V: Amino Acid transporters in cancer and their relevance
to ‘glutamine addiction’: Novel targets for the design of a new
class of anticancer drugs. Cancer Res. 75:1782–1788. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiozaki A, Iitaka D, Ichikawa D,
Nakashima S, Fujiwara H, Okamoto K, Kubota T, Komatsu S, Kosuga T,
Takeshita H, et al: xCT, component of cysteine/glutamate
transporter, as an independent prognostic factor in human
esophageal squamous cell carcinoma. J Gastroenterol. 49:853–863.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Robert SM, Buckingham SC, Campbell SL,
Robel S, Holt KT, Ogunrinu-Babarinde T, Warren PP, White DM, Reid
MA, Eschbacher JM, et al: SLC7A11 expression is associated with
seizures and predicts poor survival in patients with malignant
glioma. Sci Transl Med. 7:289ra862015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okuno S, Sato H, Kuriyama-Matsumura K,
Tamba M, Wang H, Sohda S, Hamada H, Yoshikawa H, Kondo T and Bannai
S: Role of cystine transport in intracellular glutathione level and
cisplatin resistance in human ovarian cancer cell lines. Br J
Cancer. 88:951–956. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lewerenz J, Maher P and Methner A:
Regulation of xCT expression and system xc function in
neuronal cells. Amino Acids. 42:171–179. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lo M, Ling V, Low C, Wang YZ and Gout PW:
Potential use of the anti-inflammatory drug, sulfasalazine, for
targeted therapy of pancreatic cancer. Curr Oncol. 17:9–16.
2010.PubMed/NCBI
|
|
15
|
Timmerman LA, Holton T, Yuneva M, Louie
RJ, Padró M, Daemen A, Hu M, Chan DA, Ethier SP, van 't Veer LJ, et
al: Glutamine sensitivity analysis identifies the xCT antiporter as
a common triple-negative breast tumor therapeutic target. Cancer
Cell. 24:450–465. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma MZ, Chen G, Wang P, Lu WH, Zhu CF, Song
M, Yang J, Wen S, Xu RH, Hu Y, et al: Xc inhibitor sulfasalazine
sensitizes colorectal cancer to cisplatin by a GSH-dependent
mechanism. Cancer Lett. 368:88–96. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang R, Guo Y, Ma H, Feng L, Wang Q, Chen
X, Lian M, Wang H and Fang J: Tumor necrosis factor superfamily
member 13 is a novel biomarker for diagnosis and prognosis and
promotes cancer cell proliferation in laryngeal squamous cell
carcinoma. Tumour Biol. 37:2635–2645. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stefanowicz-Hajduk J, Sparzak-Stefanowska
B, Krauze-Baranowska M and Ochocka JR: Securinine from Phyllanthus
glaucus induces cell cycle arrest and apoptosis in human cervical
cancer HeLa cells. PLoS One. 11:e01653722016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Anderson CL, Iyer SS, Ziegler TR and Jones
DP: Control of extracellular cysteine/cystine redox state by HT-29
cells is independent of cellular glutathione. Am J Physiol Regul
Integr Comp Physiol. 293:R1069–R1075. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lo M, Wang YZ and Gout PW: The
xc cystine/glutamate antiporter: A potential target for
therapy of cancer and other diseases. J Cell Physiol. 215:593–602.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gout PW, Buckley AR, Simms CR and
Bruchovsky N: Sulfasalazine, a potent suppressor of lymphoma growth
by inhibition of the xc cystine transporter: A new
action for an old drug. Leukemia. 15:1633–1640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo W, Zhao Y, Zhang Z, Tan N, Zhao F, Ge
C, Liang L, Jia D, Chen T, Yao M, et al: Disruption of xCT inhibits
cell growth via the ROS/autophagy pathway in hepatocellular
carcinoma. Cancer Lett. 312:55–61. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pezzilli R, Partelli S, Cannizzaro R,
Pagano N, Crippa S, Pagnanelli M and Falconi M: Ki-67 prognostic
and therapeutic decision driven marker for pancreatic
neuroendocrine neoplasms (PNENs): A systematic review. Adv Med Sci.
61:147–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheah PL and Looi LM: p53: an overview of
over two decades of study. Malays J Pathol. 23:9–16.
2001.PubMed/NCBI
|
|
25
|
Wang SJ and Gu W: To be, or not to be:
Functional dilemma of p53 metabolic regulation. Curr Opin Oncol.
26:78–85. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bieging KT and Attardi LD: Deconstructing
p53 transcriptional networks in tumor suppression. Trends Cell
Biol. 22:97–106. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brady CA, Jiang D, Mello SS, Johnson TM,
Jarvis LA, Kozak MM, Broz D Kenzelmann, Basak S, Park EJ,
McLaughlin ME, et al: Distinct p53 transcriptional programs dictate
acute DNA-damage responses and tumor suppression. Cell.
145:571–583. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang L, Kon N, Li T, Wang SJ, Su T,
Hibshoosh H, Baer R and Gu W: Ferroptosis as a p53-mediated
activity during tumour suppression. Nature. 520:57–62. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao JY and Dixon SJ: Mechanisms of
ferroptosis. Cell Mol Life Sci. 73:2195–2209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS,
et al: Pharmacological inhibition of cystine-glutamate exchange
induces endoplasmic reticulum stress and ferroptosis. Elife.
3:e025232014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu H, Guo P, Xie X, Wang Y and Chen G:
Ferroptosis, a new form of cell death, and its relationships with
tumourous diseases. J Cell Mol Med. 21:648–657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Golubnitschaja O: Cell cycle checkpoints:
The role and evaluation for early diagnosis of senescence,
cardiovascular, cancer, and neurodegenerative diseases. Amino
Acids. 32:359–371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Visconti R, Monica R Della and Grieco D:
Cell cycle checkpoint in cancer: a therapeutically targetable
double-edged sword. J Exp Clin Cancer Res. 35:1532016. View Article : Google Scholar : PubMed/NCBI
|