Instruction
The estimated number of diagnosed esophageal
carcinoma is more than 450,000 per year worldwide, and the largest
number of cases are esophageal squamous cell carcinoma (ESCC)
(1,2). At present, it is considered that ESCC
aggresses and spreads by a variety of pathways, including direct
invasion, lymphatic and hematogenous metastasis, and the prognosis
for metastatic or recurrent ESCC are mostly poor. Due to the high
incidence and mortality rates, large susceptible population and
limited responses to current therapy causes 5-year survival rate to
remain 5–10% in China (3–5). Although the pathogenesis of ESCC is
unclear, it is crucial for the identification of specific
biomarkers for accurate evaluation of aggressiveness, elucidating
the relevant molecular mechanisms and early detection of tumor
recurrence.
Tumors require taking adequate signal factors for
unlimited proliferation and distant metastasis, like vascular
endothelial growth factor (VEGF), matrix metallopeptidases (MMPs),
and directed signaling pathway activation, that all lead to
malignance via neovascularization, matrix rearrangement and tumor
cell transformation (6,7). In fact, due to the rapid expansion of
cancerous cells and relatively hysteresis of blood density in the
solid tumor mass, the hypoxia and nutrient deficiency has been
identified as crucial incentives (8–12). The
altering of nutrients supply can be captured inevitably, but
whether it as an independent mechanism affects tumor progression
should be studied further. Previous reports showed that Ras protein
family members are involved in cellular signal transduction,
regulating diverse cell behavior (13,14).
In general, once Ras respond to a series of exterior and interior
signals, it will subsequently amplify or turn on other factors,
which ultimately regulate cell differentiation, growth, invasion,
angiogenesis and survival; however, the Ras mutations are rare in
tumors (15–17). Moreover, it was suggested that Ras
could be constitutively overactivated by various upstream signaling
elements, such as Ras guanyl nucleotide releasing peptides
(RasGRPs) (18–20). Among them, RasGRP3 promotes guanine
nucleotide exchange of H-Ras, R-Ras and Rap1 exerting oncogenic
effects; and its overexpression or active form phospho-RasGRP3 were
observed in numerous cancer types, regulating the proliferation,
survival and migration of human solid tumors and several
hematologic neoplasms (14,21–29).
In the present study, we investigated whether RasGRP3 also acts as
a responder within the tumor microenvironment especially under
nutrient stress (NS) in human ESCC.
Of note, the presence and activity of Ras induce
biological responses closely related with other signaling in cancer
progression. For instance, Ras activation leading to upregulation
of Notch1 and Notch-mediated oncogenesis requires Ras pathway
signaling. Notch signaling is an evolutionary conserved pathway and
decides the fate of various cells including cancer cells for a
lifetime (30). The interactions
between Notch receptors and matched ligands result in the release
of Notch intracellular domain (NICD) that translocates to the
nucleus and functions as a transcription factor (31). Aberrant Notch signaling has been
associated with various cancers; moreover, the high-level
expression of Notch1 suggests a poor prognosis for ESCC (32). In some cancer cells, the activation
of Notch signaling upregulates and stabilizes Snail under hypoxic
conditions or directly stimulates Slug through its promoter while
NICD indirectly represses the expression of E-cadherin by binding
to its promoter, which form an epithelial-mesenchymal transition
(EMT) phenotype (33–35). It is known that Notch pathway is
also involved in various steps of vascular development in normal
tissue and tumor angiogenesis; and tumor vascularity is associated
with aggressive phenotypes including EMT (36,37).
Recent evidence indicates that tumor cells with abnormal expression
of Notch signaling triggers angiogenesis, and the targeting
interruption, such as γ-secretase inhibitors, may provide effective
measures (38). Taken together,
Notch signaling as an initiator not only causes malignant
aggressiveness but also co-activates with other pathways such as
Ras, which might be critical for identifying potential therapeutic
targets or modalities.
In the present study, we characterized the
expression and functions of RasGRP3 in ESCC specimens and cells,
examined the role of RasGRP3 in the activation of angiogenesis and
EMT, and studied the Notch signaling pathway that mediated its
effects. We found that RasGRP3 was highly expressed in ESCC tumors,
involved in the cell migration, invasion of ESCC, regulation of
NICD activity and recruiting endothelial cells. In addition, we
identified the nutrient stress as a crucial condition, inducing the
expression of RasGRP3.
Materials and methods
Reagents
Penicillin-streptomycin, bull serum albumin (BSA),
crystal violet were purchased from Beyotime Institute of
Biotechnology (Haimen, China); trypsin was obtained from HyClone
Laboratories (Logan, UT, USA). Dulbeccos modified Eagles medium
(DMEM), fetal bovine serum (FBS) were from Gibco (Grand Island, NY,
USA). Matrigel was from Invitrogen (Carlsbad, CA, USA). Protease
inhibitors were from Merck Millipore (Darmstadt, Germany) and the
inhibitor for γ-secretase, DAPT was obtained from Shanghai Selleck
Chemicals Co., Ltd. (Shanghai, China).
Patients and specimens
The Ethics Committees at Affiliated Hospital of
Jiangsu University approved this study. From the specimen
repository of Pathology Department at Affiliated Hospital of
Jiangsu University, 70 patients (48 males and 22 females, from
January 2009 to December 2010) and 46 specimen (for co-localization
assay, 33 males and 13 females, from October 2015 to August 2016)
with ESCC during surgical resection were identified, written and
informed consents were provided by all participants. The main
criteria for prognostic evaluation were pathological
characteristics (e.g., tumor site, stage, histologic type and
size), clinical symptoms (e.g., degree of infiltration and lymph
node metastasis) and the 5-year survival rate. The above specific
standards were diagnosed as previously described (39). The characteristics of subjects
involved in this study are summarized in Table I.
 | Table I.Specimens assayed for RasGRP3
expression. |
Table I.
Specimens assayed for RasGRP3
expression.
| Clinicopathological
features | N (n=70) | RasGRP3 (mean ±
SD) | P-value |
|---|
| Sex |
|
| 0.578 |
|
Male | 48 | 2.87±1.05 |
|
|
Female | 22 | 2.63±1.21 |
|
| Age (years) |
|
| 0.625 |
|
≤60 | 9 | 3.01±1.34 |
|
|
≤65 | 36 | 2.97±1.46 |
|
|
>65 | 25 | 3.24±1.19 |
|
| Tumor location |
|
| 0.319 |
|
Upper/middle | 37 | 4.86±2.31 |
|
|
Lower | 33 | 3.37±1.18 |
|
|
Differentiation |
|
| 0.3426 |
|
Poor | 27 | 3.59±0.89 |
|
|
Moderately | 19 | 2.11±0.57 |
|
|
High | 24 | 3.46±0.62 |
|
| TNM stage |
|
| 0.251 |
| I–II | 41 | 4.62±1.03 |
|
|
III–IV | 29 | 4.07±1.59 |
|
| Lymphatic
metastasis |
|
| 0.012 |
| No | 26 | 1.28±0.90 |
|
|
Yes | 44 | 5.14±1.56 |
|
| Distant
metastasis |
|
| 0.0239 |
| No | 49 | 1.87±2.23 |
|
|
Yes | 21 | 4.96±1.27 |
|
| Recurrence |
|
| 0.037 |
| No | 40 | 3.22±0.93 |
|
|
Yes | 30 | 3.91±1.66 |
|
Histological analysis
Immunohistochemical staining was performed on
sections fixed and embedded in paraffin as previously described
(39). In brief, primary antibodies
were rabbit anti-RasGRP3 (Cell Signaling Technology, Beverly, MA,
USA, and 1:20 dilution), rabbit anti-phospho-RasGRP3 (Thr133)
(Abcam, Cambridge, UK, 1:100 dilution), goat anti-vimentin (Santa
Cruz Biotechnology, Santa Cruz CA, USA, 1:50 dilution), rabbit
anti-MMP9 and anti-NICD (Cell Signaling Technology, and 1:50
dilution), rabbit anti-VEGFR and anti-Ki-67 (Santa Cruz
Biotechnology, 1:50 dilution). Secondary antibodies were HRP
anti-mouse, anti-rabbit or anti-goat from R&D Systems Inc.
(Minneapolis, MN, USA) at 1:200. All antibodies were diluted in
0.5% BSA/PBS. HRP-antibodies were visualized using the DAB
Substrate kit from Abcam. All slides were under low magnification
(×100) to identify, and 4 different regions with the highest
vascularity were scanned and blindly analyzed by two investigators
using Olympus microscopes connected with a computer running
Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD,
USA). The evaluation criteria for RasGRP3 or other markers were
based on our published study: the area was entirely dark brown or
>75% brown score 3; the matrix >50% brown was 2 and >20%
of brown or light brown as 1 while other cases were not scored
(37,39). In this study, the average score
>10 as the strongly positive samples of RasGRP3, <10 but
>5 as moderate, <5 but >2 as weak samples, while <2 as
negative.
Microvessel density evaluation by
immunofluorescence co-localization
Tissue freshly prepared was submitted from the
confirmed ESCC for microvessel density (MVD) immunostaining.
Immunohistochemistry and immunofluorescence were applied in frozen
sections to evaluate expression and location for RasGRP3 and
CD31+ vascular endothelial cells. The primary antibody
used was anti-human RasGRP3. The slides were then incubated in HRP
anti-mouse followed by DAB Substrate kit. For each sample, after
antigen unmasking, the sections were blocked with 2% BSA in
phosphate-buffered saline (PBS) for 1 h at room temperature.
Sections were incubated overnight at 4°C with a secondary antibody
against CD31 (Santa Cruz Biotechnology, 1:50 in blocking, goat
anti-human), followed by incubation for 2 h with the fluorescence
labeling antibody FITC anti-goat (1:200; R&D Systems). After
washed by PBS, sections were viewed under a fluorescence microscope
(Olympus, Tokyo, Japan) and counted under ×200 magnification. Any
groups of cells that stained positive for CD31 with or without a
vessel lumen and distinct from adjacent structures of specimens
were counted as a single countable micro-vessel. The score of ≤10
vessels/x200 magnification was 0; >10 and ≤25 vessels was
considered as 1; while, the score of >25 vessels as 2. The
average of the values obtained by the two reviewers was reported as
a single value. The numbers of microvessels in the 4 regions were
averaged to give mean MVD score per tumor specimen.
Cell culture and nutrient stress
treatment
Human esophageal squamous cancer cells TE-1, TE-10,
ECA 109, KYSE-150 and esophageal epithelial cells, Het-1a
(Guangzhou Jennio Biotech Co., Ltd., Guangzhou, China), were grown
and maintained in DMEM supplemented 10% FBS. Human umbilical vein
endothelial cells (HUVEC-2) were purchased from Guangzhou Jennio
Biotech and were maintained in endothelial cell growth Basal
Medium-2 supplemented with 10% FBS. Simultaneously, cancer cells
were incubated in the growth medium only containing 1% FBS for more
than 1 month as the nutrient stress treatment. All cells were in a
humidified incubator containing 5% CO2 at 37°C.
RNA interference
Oligonucleotides for human RasGRP3 siRNA kit were
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China). The
kit contains predesigned two siRNAs targeting RasGRP3 gene to
ensure work efficiency (siRNA sense: GCU UAC UUC CUG AGA GCU ATT
and antisense: UAG CUC UCA GGA AGU AAG CTT; sense: GGU ACU GGA UUC
UGA AGU UTT and antisense: AAC UUC AGA AUC CAG UAC CTT). Cells were
transfected with RasGRP3 siRNA or non-specific siRNA (2 µg/well for
6-well culture plates) using the Opti-MEM (Roche, Mannheim,
Germany) plus Lipofectamine 2000 (Thermo Fisher Scientific Inc.,
Waltham, MA, USA) according to the instruction manual. After 24-h
post-transfection, the cells underwent different treatment
combinations for ELISA, western blot analysis and cell
proliferation assay.
Real-time quantitative PCR
analyses
RNA was extracted from ESCC and reverse-transcribed
into cDNA as described before. Real-Time PCR was performed using a
Biosystems (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Relative expression was calculated by the 2−ΔΔCt method
using GAPDH as an internal control. The sequences of PCR primers
(Invitrogen, Shanghai, China) were as follows: RasGRP3 (forward,
5′-CACGGTCATCAACAAGCACA-3′ and reverse 5′-CAGTGTTCGCAGAAGGTTGG-3′);
VEGF-A (forward, 5′-AGGAGGAGGGCAGAATCATCA-3′ and reverse,
5′-CTCATTGGATGGCAGTAGCT-3′); Hes1 (forward,
5′-GGTATTTCCCCAACACGCT-3′ and reverse, 5′-GGCAGACATTCTGGAAATGA-3′);
Snail1 (forward, 5′-CTTCTCCTCTACTTCAGTCTCTTCC-3′ and reverse,
5′-TGAGGTATTCCTTGTTGCAGTATTT-3′); Slug (forward,
5′-AACAGAGCATTTGCAGACAGGTC-3′ and reverse,
5′-GCTACACAGCAGCCAGATTCC-3′); GAPDH (forward,
5′-TCAACGGATTTGGTCGTATTG-3′ and reverse,
5′-TGGGTGGAATCATATTGGAAC-3′).
Western blot analysis
Cells were harvested and lysed in a RIPA buffer
(CWbiotech, Beijing, China) containing protease inhibitors. Total
protein was denatured and separated with 6–12% SDS-PAGE and further
transferred to polyvinylidene difluoride (PVDF) membrane (Thermo
Fisher Scientific). After blocking the membrane in 5% BSA dissolved
in TBST, membranes were incubated overnight at 4°C with primary
antibodies. Afterwards, membranes were incubated with an
HRP-conjugated secondary antibody (Cell Signaling Technology)
diluted 1:1,000. Target proteins were detected by Pierce ECL Plus
Substrate (Thermo Fisher Scientific) and scanned with FluorChem FC3
camera system (Protein-Simple, San Jose, CA, USA).
ELISA
The ELISA analysis of this study was focused on the
most important pro-angiogenic factor VEGF-A. The TE-1 cells after
nutrient stress treatment, or not, were seeded on 96-well plates
(Corning, Inc., Corning, NY, USA) and cultured in serum-free DMEM
overnight, then treated with or without RNA interference for 24, 48
and 72 h. Culture medium was assayed for VEGF concentration using
the anti-human VEGF Immunoassay kit (R&D Systems) according to
the manufacturers instructions. Briefly, 200 µl of control, sample
or standard was added to 50 µl assay diluent. After 2-h incubation
at room temperature, the samples were washed three times. Then, 200
µl VEGF conjugate was added for 2 h and the samples were washed
again. After incubation with 200 µl substrate solution for 20 min,
50 µl stop solution was added. The concentration of VEGF was
measured by the color intensity of solutions using a microplate
reader (BioTek Instruments, Inc., Winooski, VT, USA) at 450 and 570
nm, respectively. VEGF concentrations were obtained by comparing
the corresponding readings with those of the standard curve using
known concentrations of VEGF. Inhibitory rate of secreted VEGF (%)
= ([VEGF content in non-transfected group - VEGF content in
transfected group]/VEGF content in non-transfected group) ×
100.
Cell viability assay
Cell viability was detected by the Cell Counting kit
8 (CCK-8) assay according to the manufacturer's protocol (Biosharp,
Hefei, China). Briefly, cells were seeded in 96-well plates at a
density of 4×103 cells/ml, and allowed to adhere for 24
h. Measurement was carried out and cells were treated with the
indicated time intervals of si-RasGRP3, then incubated with CCK-8
solution for 3 h at 37°C. The optical density (OD) value was
measured at an absorbance wavelength of 450 nm in a microplate
reader. All experiments were performed in triplicate.
In vitro tube formation assay
A total of 30 µl of Matrigel was added to every well
in 96-well plate and incubated at 37°C for 30 min to solidify, and
3×104 HUVECs/well were seeded in medium from TE-1 cells.
The media were as follows: after treatment of nutrient stress, TE-1
cells were seeded on 24-well plates (5×104 cells/well)
for 24 h. Then, only replaced with a fresh medium or siRNA for
human RasGRP3 was transfected according to the above-described
method. The next day, the cultured media of different sources were
collected. After 12 h of HUVECs seeding, tube formation was
quantified using a microscope (Olympus). Images were analyzed under
an inverted microscope at ×200 magnification to determine the
formation and proportion of branch points and polyhedral closed
structures delimiting a lumen. Tube formations were counted from 6
random sites for each well and the experiment was repeated at least
three times independently.
Immunofluorescence staining
For immunofluorescence staining, the cells were
incubated with primary antibodies against E-cadherin and vimentin
(Cell Signaling Technology), followed by incubation with Cy3- or
FITC-conjugated secondary antibodies (BD Biosciences, San Jose, CA,
USA). For upright microscope, the cells grown on coverslips were
counterstained with DAPI and were imaged using a fluorescence
microscope (Olympus).
Scratch-induced migration assay
TE-1 cells (cultured in 1% FBS medium more than 1
month) were seeded and synchronized by fresh low serum medium for 8
h. Moreover, the cell monolayers were damaged with a micropipette
to create a linear wound 2 mm in width and were rinsed twice with
PBS. The indicated concentrations of siRNA were added and the cells
were incubated for 24 h. The scratch phase-contrast images were
obtained using a microscope (Olympus), and wound healing was
assessed at 5 randomly selected points by measuring the width of
the scratch. The results are expressed as the percentage inhibition
rate of migration compared with untreated cells using the Adobe
Photoshop CS5.
Transwell migration assay
Migration assays were performed as follows: TE-1 and
KYSE-150 cells cultured under nutrient stress were transfected with
siRNA-RasGRP3 for 24 h. Transwell inserts with a polycarbonate
membrane (pore size of 8 µm; Corning). A density ~1×105
of cells was suspended and then seeded in the upper chambers of
24-well plates with FBS-free medium, while medium containing 10%
FBS was deposited in the lower chambers. One-half of the wells with
the cells contained the inhibitor DAPT at a final concentration of
10 µM. After 24 h, cells that migrated were stained by 0.1% crystal
violet and imaged microscopically. Cells were enumerated and the
results are expressed as the percentage inhibition rate compared
with untreated cells. Each experiment was performed in
duplicate.
Statistical analysis
Data are expressed as mean ± standard deviation
(SD). The differences between groups were analyzed using the
Student's t-test when only two groups were compared; multiple
comparisons were done by either one-way analysis of variance
(ANOVA) or ANOVA on ranks. The association between RasGRP3 and the
clinicopathological features was assessed using the χ2
test. Recurrence-free survival (RFS) curves were calculated by the
Kaplan-Meier methods. P<0.05 was considered statistically
significant. These analyses were performed with the SPSS version
16.0.
Results
RasGRP3 is positively correlated with
MVD in human ESCC
RasGRP3 has previously shown to be upregulated in
human breast, prostate cancer, glioblastoma and melanoma (22,23,27–29),
so we also examined the expression of RasGRP3 in ESCC cells using
the western blot method. TE-1, TE-10, ECA 109 and KYSE-150 cells
detectably express endogenous RasGRP3 and less in Het-1a cells. As
mentioned, RasGRP3 is an activator of Ras proteins or pathway. To
examine whether RasGRP3 contributes to the activation of Ras
pathway in ESCC cells, we set a mimic environment of nutrient
deficiency and further examine the potential correlation among
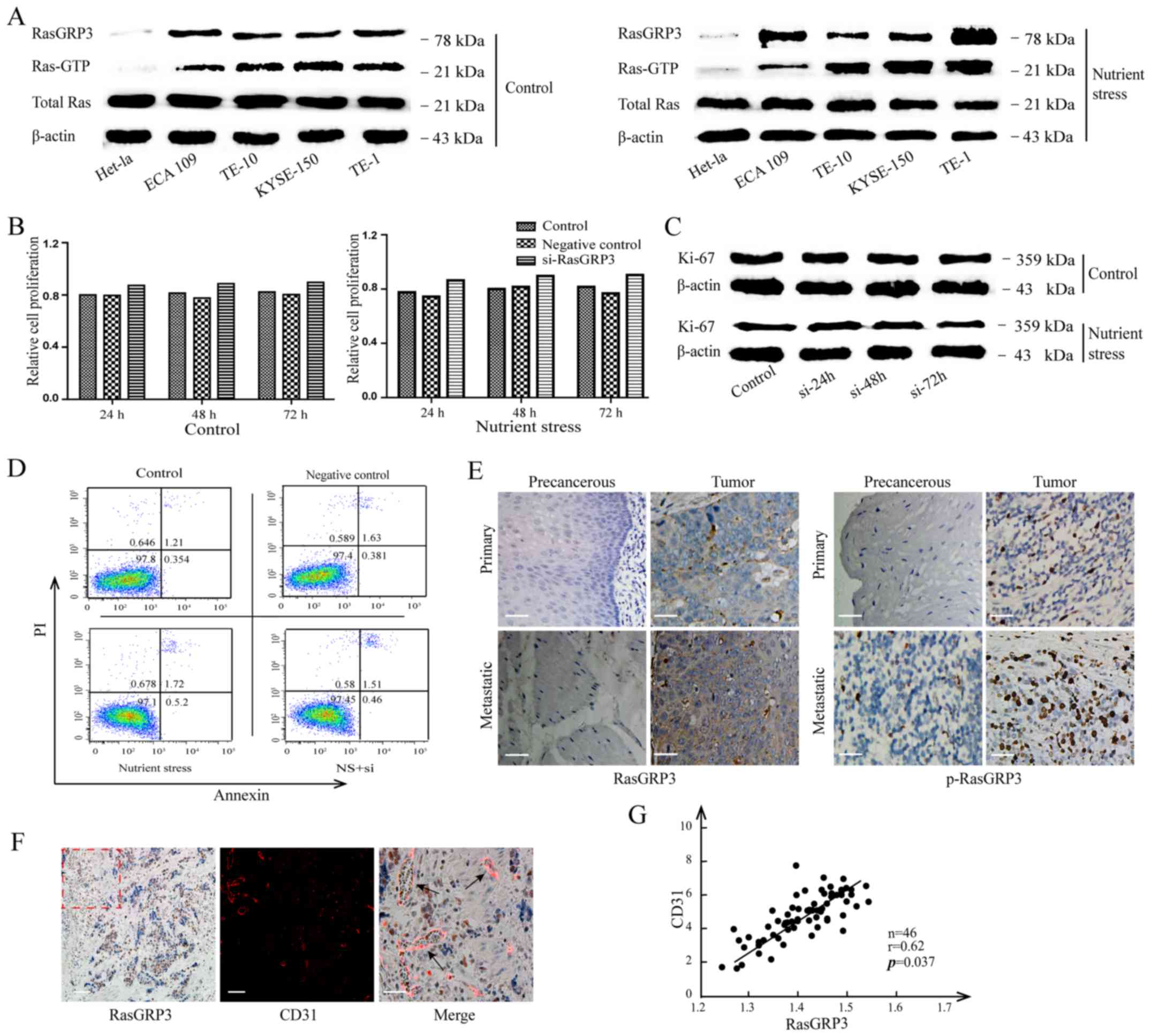
RasGRP3, Ras-GTP and total Ras. As shown in Fig. 1A, ESCC cells cultured under the
1%FBS-containing medium (more than one month) had a significantly
increased RasGRP3 and Ras-GTP, however, without any perceptible
change in the total Ras. A similar trend of variation was obtained
from TE-1 cells stripped of adequate nutrient for 24, 48 h and 7
days at a relatively short time (data not shown). Since RasGRP3
promotes the proliferation of multiple cancer cells, we also
examined the changes of Ki-67 and cell viability after
siRNA-RasGRP3 treatment; however, there was no significant change
between nutrient stress and normal culture (Fig. 1B and C). In order to avoid the
influence caused by apoptosis, the Annexin V-PI assay was also
conducted after different treatments, but the differences showed no
significance (P>0.05) (Fig. 1D).
Taken together, the upregulation of RasGRP3 was positively related
with nutrient deficiency. Moreover, we found that most of ESCC
tumors (reviewing from the paraffin sections of 70 patients)
strongly expressed RasGRP3, localized in the cytoplasm, compared to
normal adjacent tissues by immunohistochemistry (IHC) staining
(Fig. 1E). The intensity of
RasGRP3, which was analyzed according to the standard as described
in Materials and methods, was independent of patient age, sex,
differentiation, tumor location and TNM stage, whereas
significantly correlated to the lymphatic or distant metastatic
status and recurrence (Table I). In
addition, the average nuclear p-RasGRP3 of tumors were higher than
precancerous groups, but had no positive correlation with
metastasis or recurrence (all P>0.05) (data not shown). Because
of the deficiency of nutrient has been identified as a trigger to
angiogenesis, we also randomly selected and stained 46 frozen
specimens of ESCC with antibody CD31 to determine the integrated or
anomaly construct of blood vessels by immunofluorescence (IF). The
co-location analysis of IHC and IF revealed a positive correlation
between the intensity of RasGRP3 and the number of vessels in human
ESCC. As shown in Fig. 1F, which
displays a representative sample, we counted the number of vessels
per area of RasGRP3 histological sections. There was an average
vessel density of 7.6 in RasGRP3 strongly positive samples, 3.8 in
moderate ones and 2.4 for weak positive and 1.8 in negative
samples. Throughout the entire comparison process, the intensity of
RasGRP3 staining was positively correlative with the increased MVD
(r=0.62; P=0.037; Fig. 1G),
suggesting that RasGRP3 plays an important role in the regulation
of vascularity in ESCC tissues.
 | Figure 1.The correlation between RasGRP3 and
MVD in esophageal squamous cell carcinoma (ESCC). (A) The
expression of RasGRP3 was analyzed by western blot analysis,
Ras-GTP and total Ras of ESCC cells lines, TE-1, ECA 109, TE-10 and
KYSE-150 and normal esophageal epithelial cells, Het-1a cells under
nutrient stress or sufficiency. (B) The viability of TE-1 cells was
examined by CCK-8 assay after si-RasGRP3 treatment or not for 24,
48 and 72 h before or after nutrient stress treatment. (C) The
expression of Ki-67 in TE-1 cells with or without RasGRP3
interference was measured by immunoblotting under nutrient stress
or sufficiency. (D) The percentage of apoptotic cells was detected
by flow cytometry that labeled Annexin V-PI. (E) Representative
images of RasGRP3 and p-RasGRP3 expression in ESCC tumor and
precancerous tissue with or without metastasis (×200; scale bars,
50 µm). (F) The co-location images of IHC (RasGRP3) and IF (Cy3
labelled CD31) in ESCC tumors. Left and middle panel (scale bars,
100 µm). Right panels are magnifications of the area marked by
dashed lines (scale bars, 50 µm). (G) The relationship of RasGRP3
and MVD was assessed using Pearsons rank correlation coefficient.
All data derive from at least three independent experiments.
Significance level, P<0.05. |
Nutrient stress-instigated RasGRP3
participates in angiogenesis of ESCC
In order to further characterize the contribution of
RasGRP3, the factors involved in neovascularization and
rearrangement of endothelial cells were quantified. Firstly, the
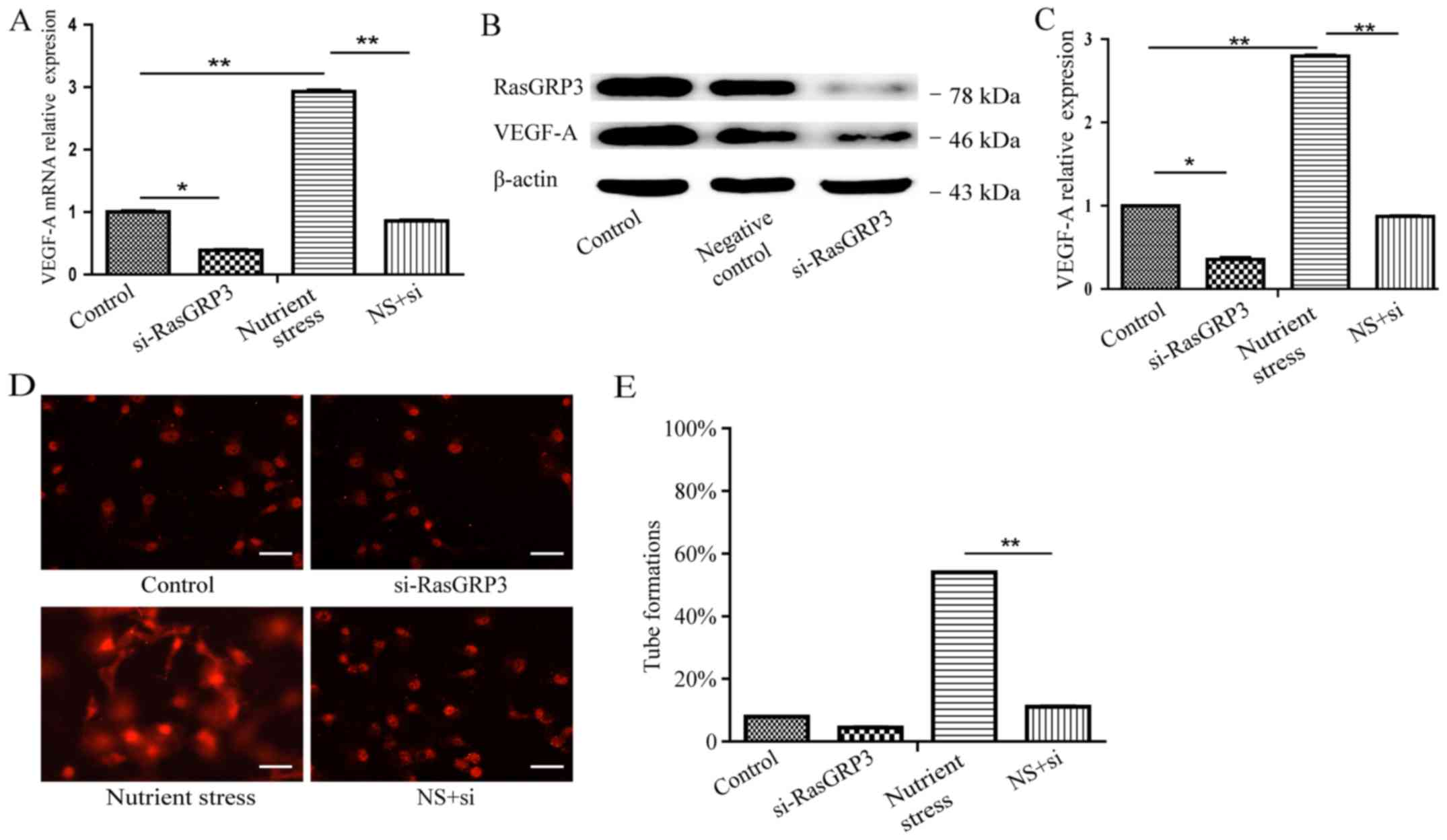
significantly increased VEGF-A mRNA under NS was shown to reduce
nearly 70% after siRNA-RasGRP3 treatment (24 h) (Fig. 2A). Immunoblotting assays showed that
the targeted interference at RasGRP3 decreased the expression of
VEGF-A protein as compared to TE-1 cells under nutrient deprivation
(Fig. 2B). Furthermore, ELISA assay
was used to measure the secreted VEGF-A from supernatant of ESCC
cells. The result showed that siRNA-RasGRP3 treatment reversed the
significant increase of VEGF-A caused by chronic low-serum
culturing (Fig. 2C). Given the role
that restriction of nutrient might regulate angiogenesis via
rearranging endothelial cells with the increased VEGF-A, we sought
to determine the effect of RasGRP3 on tube formation of HUVECs. As
shown in Fig. 2D, culture
supernatant from TE-1 cells under NS induced complete tube
formation, whereas cells after pre-knockdown of RasGRP3 suppressed
the formation of robust tube at 12 h (Fig. 2E; P<0.01). Taken together, these
data suggested that RasGRP3 as a crucial factor could mediate
angiogenic signaling under the nutrient stress.
RasGRP3 triggers the EMT and finally
metastasis of ESCC cells under nutrient stress
Although RasGRP3 contributes to the angiogenesis of
ESCC, the focus of this study so far has been on its role in
further malignant aggressiveness. Therefore, we tested whether
there were differences in EMT-related proteins due to the change of
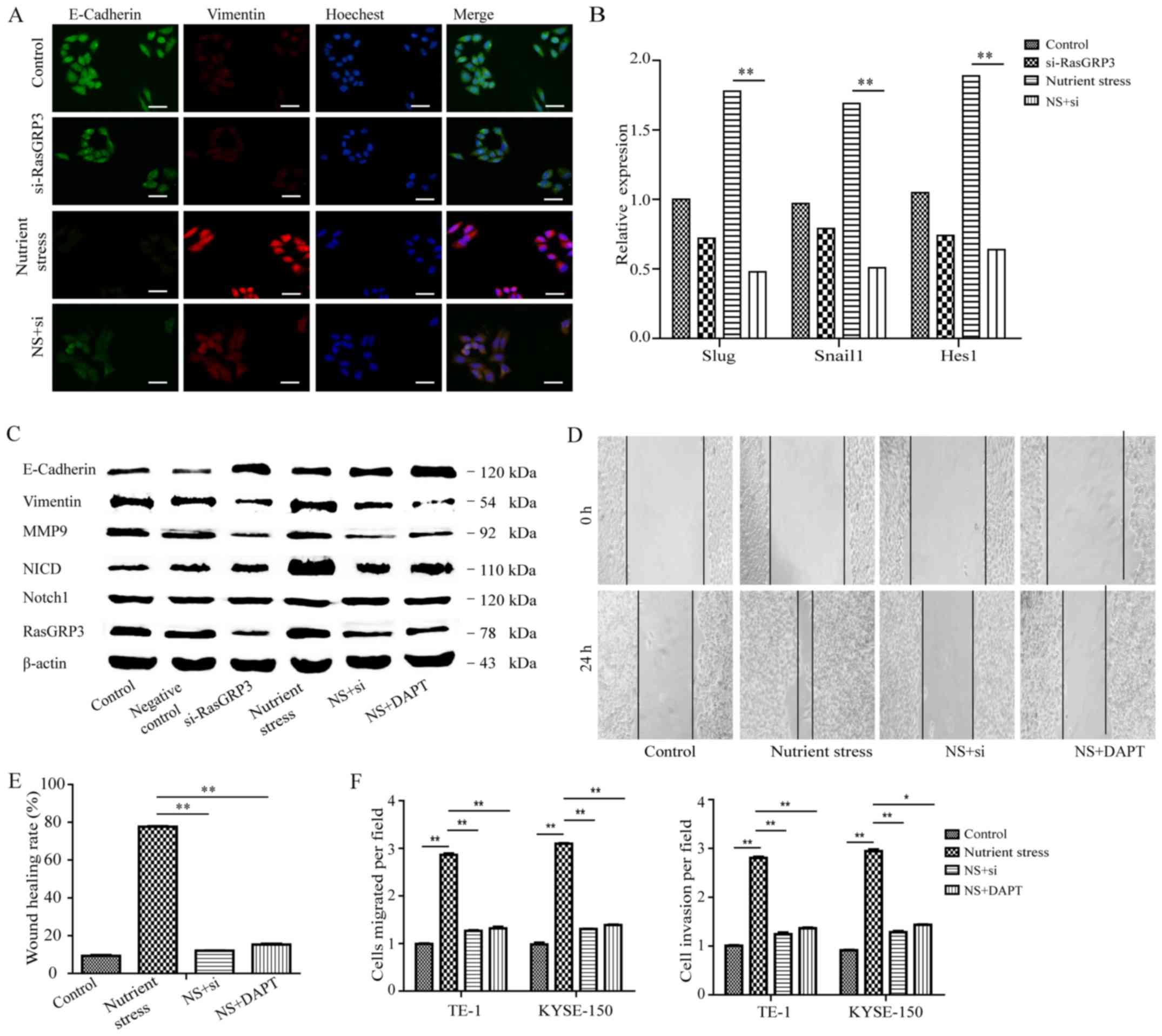
RasGRP3. Firstly, the figures based on immunofluorescence staining
of E-cadherin and vimentin indicated that there were significance
differences between NS treatment or not. However, the RNA
interference targeting RasGRP3 could inhibit the downregulation of
E-cadherin and upregulate vimentin caused by NS (Fig. 3A). To go a step further, we also
validated the activation of EMT by detecting the expression of
Snail1, Slug and Hes1. The expression of Snail 1, Slug and Hes1
mRNA in TE-1 cells under NS were downregulated to ~20% after
siRNA-RasGRP3 treatment (P<0.01; Fig. 3B). The cellular morphology
transformation and increased expression of MMPs are related to the
acquisition of invasiveness and metastasis in various cancer cells.
To investigate the effects of RasGRP3 on human ESCC cells invasion
and metastasis, we subsequently analyzed the expression of
E-cadherin, vimentin and MMP9 by western blot analysis (Fig. 3C). The decreased levels of vimentin
and MMP9 in combination with upregulation of E-cadherin after
siRNA-RasGRP3 treatment suggested that RasGRP3 regulated the
progress of EMT. In the present study, RasGRP3 accumulation also
upregulated the expression of NICD, whereas had no effect on Notch1
protein level (Fig. 3C). Then, we
found that suppression of the Notch signaling by DAPT (10 µM) or
siRNA-RasGRP3 decreased levels of vimentin, MMP9 and NICD induced
by NS (Fig. 3C). Moreover,
carcinoma cells produce a whole range of proteases that degrade the
extracellular matrix and basement membranes, which has been
regarded as initial steps in the process of invasion. Therefore, we
determined ESCC cells by scratch-induced migration assay (Fig. 3D). As shown in Fig. 3E, the TE-1 cells under NS migrated
more rapidly than that in control groups, and the final distance of
cells from the starting wound edge were increased by 58%
(P<0.05); however, the number was back to 12% for cells
transfected with interference fragment of RasGRP3. To further
investigate the impact of RasGRP3 on migration, Transwell assays
were performed. Approximately 3-fold increases of invasion were
observed under NS compared with control groups (P<0.01). In
keeping with above results, knockdown of RasGRP3 or DAPT treatment
showed a significant decrease of the abilities of migration and
invasion (Fig. 3E). Collectively,
these results indicated that RasGRP3 promotes metastasis dependent
on the activity of Notch pathway.
Association of RasGRP3 and
clinicopathological features with prognosis in human ESCC
samples
In order to address the diagnostic and prognostic
value of RasGRP3, precancerous or tumor serial sections of ESCC
were stained with VEGFR, Ki-67, vimentin, MMP9 and NICD antibody to
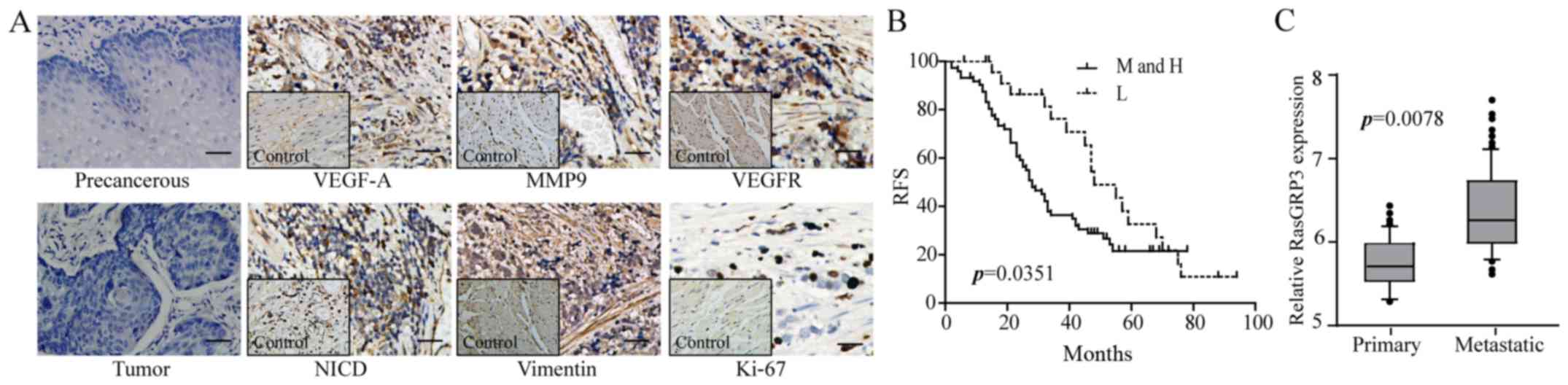
quantify the potential correlation. Among 70 patients with ESCC, 34
samples expressed with a high level of RasGRP3, 32 samples
expressed at moderate intensity, whereas 4 samples with lower or
negative staining. The expression of vimentin and MMP9 showed
moderate or high representation in 78% and 76.9% of RasGRP3 stable
positive samples (the 34 high and 32 moderate sections), with 11
and 14% in the remaining 4, respectively. NICD was strongly
positive in ~80% of RasGRP3 stable positive samples, compared with
6% in lower or negative sections. However, Ki-67 was densely
labeled among 54% of stable RasGRP3 positive samples and 50% of
remaining 4 samples. Furthermore, data showed no significant
differences of VEGFR among the samples (64% positive staining with
stable RasGRP3 and 67% for lower and negative expression) (Fig. 4A and Table II). In Kaplan-Meier survival
curves, patients with high and moderate expression of RasGRP3
showed a worse outcome, and the lower expression group had a longer
survival time (P=0.0351; Fig. 4B).
According to the log-rank method, the metastasis rates were 61.0%
for patients with moderate and high RasGRP3 expression, compared
with 29.8% with lower expression (P=0.0078; Fig. 4C). Thus, RasGRP3 appears to be a
strong marker of poor prognosis in patients of ESCC.
 | Figure 4.The relationship between
clinicopathological features, prognosis and levels of RasGRP3 in
human ESCC. (A) The representative IHC results show the expression
of VEGF-A, VEGFR, Ki-67, vimentin, MMP9 and NICD in ESCC tissues.
Left panels are negative controls, and the inset images are control
tissues (×200; scale bars, 50 µm). (B) Kaplan-Meier survival curves
of the 5-year RFS for ESCC patients stratified by RasGRP3
expression. M and H, the moderate and high expression of RasGRP3;
L, lower expression of RasGRP3. (C) Based on home interview and IHC
results of ESCC patients, the metastasis rate and effects of
RasGRP3 were analyzed by correlation analysis. Significance level,
P<0.05. |
 | Table II.Association between RasGRP3
expression and clinic-pathological features with prognosis. |
Table II.
Association between RasGRP3
expression and clinic-pathological features with prognosis.
|
| M and H | L and N | P-value |
|---|
| Vimentin | 78% | 11% | 0.028 |
| MMP9 | 76.9% | 14% | 0.019 |
| NICD | 80% | 6% | 0.000 |
| Ki-67 | 54% | 50% | 0.506 |
| VEGFR | 64% | 67% | 0.319 |
Discussion
It is evident that enough vessels are the mechanical
approach for tumor cell migration across vascular wall, restoration
of oxygen and nutritional provision (37). Therefore, an appropriate definition
to label blood supply along with cancer progression contributes to
diagnostic and guiding therapeutic treatment. Recent research has
shown that Ras is one of the important stress-response genes, and
its activation is attributed to upstream signaling such as tyrosine
kinase receptors, excessive activation of guanine nucleotide
exchange factors (GEFs) (13,14,16,17,22–29).
As mentioned, RasGRP, as a GEFs in various tissues and tumors, not
only have the structures for binding diacylglycerol (DAG) but also
modulates Ras pathway activity and even malignant transformation
processes. For instance, RasGRPs can function as oncogenes in
multiple cancers, and an elevated expression of RasGRP3 have been
found in Burkitt's lymphoma, B cell leukemia, breast, prostate
cancer, glioblastoma and melanoma, affecting the proliferation and
treatment-resistance (22–29). Here, we found that RasGRP3 and
p-RasGRP3 were expressed higher in ESCC specimens comparing to the
peritumoral normal tissues. Moreover, the treatment of nutrient
stress, whether at a short or long time, increased RasGRP3 of ESCC
cells, contributing to the activation of Ras-GTP. These results
suggest that RasGRP3 have the indicative function for malignant
aggressiveness. Note that an existing study have confirmed RasGRP3
can be upregulated by VEGF in angiogenesis and promoted cancer
multiplication, invasion and metastasis (40). VEGF, as one of the most potent
growth factors, mediating angiogenesis via recruiting and
stimulating the growth of endothelial cells, leading to increased
vascularity (41). Thus, in order
to refine the patient population of ESCC most likely to respond to
high RasGRP3, we performed MVD analyses using section from the
original esophagectomy blocks, and data based on pathologic
analysis showed a close relationship between MVD and RasGRP3. For
further confirmation, experiments in vitro have also
investigated RNA and protein of VEGF-A which were mediated by the
accumulation of RasGRP3 after chronic low-serum treatment. However,
another way for tumors to expand is by proliferation, dependent on
the well-known proliferation marker of Ki-67 and cell viability.
Our data have shown that pretreatment of ESCCs with siRNA-RasGRP3
do not suppress proliferation or induce apoptosis either with or
without nutrient deprivation. In patients who presented with high
levels of RasGRP3, we observed no consistent increases of Ki-67
also, surprisingly, no positive correlation to VEGFR, which has
been proved to cause new tubule formation, while the accumulated
RasGRP3 enhanced tube formation of HUVECs in vitro (41). Therefore, even though RasGRP3 has
been shown to take part in the angiogenesis, but as a complex
process taking several steps, the disparity and significance are
worthy to study further.
The reasons why ESCC caused recurrence, spreading
and metastasis remain unknown. We presumed that this could be
explained by abnormal microenvironment and signaling dysfunction.
It is well-known that cancer cells more easily undergo EMT during
penetration through vascular wall, causing an increase of
circulating cells and metastasis (42). At the cellular level, RasGRP3 showed
no effect on proliferation but amplified the process of mesenchymal
transition, downregulated the E-cadherin as well as increased the
expression of Snail1, Slug, vimentin and MMP9 in TE-1 cells.
Moreover, these effects were consistent with ESCC tumor samples,
showing that the strong expression and co-localization of RasGRP3
with vimentin and MMP9. Considering the risks and impacts of EMT in
ESCC, we performed a regression analysis and observed an earlier
relapse to lymph nodes and organs in patients who presented with
high levels of RasGRP3. A median survival of 32 months in patients
displaying steady RasGRP3 (strongly and moderate positive) compared
to patients more than five years who exhibited low or no RasGRP3.
Indeed, for the activation of RasGRP3 is likely a dynamic process,
the expression of p-RasGRP3 was not representative among ESCC with
different degrees, however, the abnormal RasGRPs activation
contributes to oncogenesis via affecting the regulation of multiple
downstream molecules (21–23,29).
To explore the mechanism underlying RasGRP3-induced angiogenesis
and metastasis in ESCC, we examined its effects on Notch signaling,
known to be activated by the Ras protein family, although Ras
mutations are not common in cancers (15,30).
Aberrant Notch signaling has been associated with the development
and progression of various cancers, high-level expression of Notch1
and its ligand Jagged1 are predictive of poor survival and
recurrence in ESCC (32,43). Consistent with this, we found that
the RasGRP3 was mostly localized in the cytoplasm whereas NICD
showed nuclear staining in ESCC tumors. As presented, the
downregulation of RasGRP3 caused a significant decrease of Hes-1 in
TE-1 cells. Of relevance, it was confirmed that modulation of
RasGRP3 contributes to Akt and ERK1/2 activation in prostate cancer
and melanoma cells, we are the first to demonstrate that RasGRP3
modulated the enrichment of NICD and contributed the activation of
Notch under nutrient stress (23).
Accordingly, we propose that RasGRP3 promoted angiogenesis,
migration and invasion of ESCC cells via upregulation of the Notch
pathway. The activation of Ras led to upregulation of NICD, and
Notch pathway mediated oncogenesis required Ras signals, suggesting
an association between these two pathways.
Thus, the nutrient stress is an independent factor,
and has an influence on tumour growth through biological targeting
of malignant aggressiveness. In the present study, we sought to
investigate the consequences of coordinate activation of Notch and
Ras pathways on the survival of ESCC patients, and the influence of
RasGRP3 on ESCCs. In conclusion, the γ-secretase inhibitor, DAPT,
was shown to have sufficient activity against aggressiveness in
esophageal squamous cell carcinoma, which provides a potential
treatment strategy for ESCC patients with metastasis under nutrient
stress.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (nos. 81572956 and 81370889),
and the Wu Jieping Medical Foundation (no. 320.6755.15022).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen MF, Yang YH, Lai CH, Chen PC and Chen
WC: Outcome of patients with esophageal cancer: A nationwide
analysis. Ann Surg Oncol. 20:3023–3030. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin Y, Totsuka Y, He Y, Kikuchi S, Qiao Y,
Ueda J, Wei W, Inoue M and Tanaka H: Epidemiology of esophageal
cancer in Japan and China. J Epidemiol. 23:233–242. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu C, Hu Z, He Z, Jia W, Wang F, Zhou Y,
Liu Z, Zhan Q, Liu Y, Yu D, et al: Genome-wide association study
identifies three new susceptibility loci for esophageal
squamous-cell carcinoma in Chinese populations. Nat Genet.
43:679–684. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Akakura N, Kobayashi M, Horiuchi I, Suzuki
A, Wang J, Chen J, Niizeki H, Kawamura K, Hosokawa M and Asaka M:
Constitutive expression of hypoxia-inducible factor-1alpha renders
pancreatic cancer cells resistant to apoptosis induced by hypoxia
and nutrient deprivation. Cancer Res. 61:6548–6554. 2001.PubMed/NCBI
|
|
7
|
Catalano V, Turdo A, Di Franco S, Dieli F,
Todaro M and Stassi G: Tumor and its microenvironment: A
synergistic interplay. Semin Cancer Biol. 23:522–532. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghosh R, Lipson KL, Sargent KE, Mercurio
AM, Hunt JS, Ron D and Urano F: Transcriptional regulation of
VEGF-A by the unfolded protein response pathway. PLoS One.
5:e95752010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horsman MR and Vaupel P:
Pathophysiological basis for the formation of the tumor
microenvironment. Front Oncol. 6:662016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kranenburg O, Gebbink MF and Voest EE:
Stimulation of angiogenesis by Ras proteins. Biochim Biophys Acta.
1654:23–37. 2004.PubMed/NCBI
|
|
11
|
Ojha R, Bhattacharyya S and Singh SK:
Autophagy in Cancer stem cells: A potential link between
chemoresistance, recurrence, and metastasis. Biores Open Access.
4:97–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu Y, Xia X and Pan H: Active autophagy in
the tumor microenvironment: A novel mechanism for cancer
metastasis. Oncol Lett. 5:411–416. 2013.PubMed/NCBI
|
|
13
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stone JC: Regulation of Ras in
lymphocytes: Get a GRP. Biochem Soc Trans. 34:858–861. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kolch W: Meaningful relationships: The
regulation of the Ras/Raf/MEK/ERK pathway by protein interactions.
Biochem J. 351:289–305. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zuber J, Tchernitsa OI, Hinzmann B,
Schmitz AC, Grips M, Hellriegel M, Sers C, Rosenthal A and Schäfer
R: A genome-wide survey of RAS transformation targets. Nat Genet.
24:144–152. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcia LC, Donadío LG, Mann E, Kolusheva
S, Kedei N, Lewin NE, Hill CS, Kelsey JS, Yang J, Esch TE, et al:
Synthesis, biological, and biophysical studies of
DAG-indololactones designed as selective activators of RasGRP.
Bioorg Med Chem. 22:3123–3140. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hennig A, Markwart R, Esparza-Franco MA,
Ladds G and Rubio I: Ras activation revisited: Role of GEF and GAP
systems. Biol Chem. 396:831–848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frau M, Feo F and Pascale RM: Pleiotropic
effects of methionine adenosyltransferases deregulation as
determinants of liver cancer progression and prognosis. J Hepatol.
59:830–841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee HK, Finniss S, Cazacu S, Xiang C,
Poisson LM, Blumberg PM and Brodie C: RasGRP3 regulates the
migration of glioma cells via interaction with Arp3. Oncotarget.
6:1850–1864. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nagy Z, Kovács I, Török M, Tóth D, Vereb
G, Buzás K, Juhász I, Blumberg PM, Bíró T and Czifra G: Function of
RasGRP3 in the formation and progression of human breast cancer.
Mol Cancer. 13:962014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Porpaczy E, Bilban M, Heinze G, Gruber M,
Vanura K, Schwarzinger I, Stilgenbauer S, Streubel B, Fonatsch C
and Jaeger U: Gene expression signature of chronic lymphocytic
leukaemia with Trisomy 12. Eur J Clin Invest. 39:568–575. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Teixeira C, Stang SL, Zheng Y, Beswick NS
and Stone JC: Integration of DAG signaling systems mediated by
PKC-dependent phosphorylation of RasGRP3. Blood. 102:1414–1420.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamagishi M, Katano H, Hishima T,
Shimoyama T, Ota Y, Nakano K, Ishida T, Okada S and Watanabe T:
Coordinated loss of microRNA group causes defenseless signaling in
malignant lymphoma. Sci Rep. 5:178682015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang D, Kedei N, Li L, Tao J, Velasquez
JF, Michalowski AM, Tóth BI, Marincsák R, Varga A, Bíró T, et al:
RasGRP3 contributes to formation and maintenance of the prostate
cancer phenotype. Cancer Res. 70:7905–7917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang D, Tao J, Li L, Kedei N, Tóth ZE,
Czap A, Velasquez JF, Mihova D, Michalowski AM, Yuspa SH, et al:
RasGRP3, a Ras activator, contributes to signaling and the
tumorigenic phenotype in human melanoma. Oncogene. 30:4590–4600.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng X, Hu Z, Wang Z, Tao J, Lu T, Yang C,
Lee B and Ye Z: Upregulation of RASGRP3 expression in prostate
cancer correlates with aggressive capabilities and predicts
biochemical recurrence after radical prostatectomy. Prostate Cancer
Prostatic Dis. 17:119–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weijzen S, Rizzo P, Braid M, Vaishnav R,
Jonkheer SM, Zlobin A, Osborne BA, Gottipati S, Aster JC, Hahn WC,
et al: Activation of Notch-1 signaling maintains the neoplastic
phenotype in human Ras-transformed cells. Nat Med. 8:979–986. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takebe N, Nguyen D and Yang SX: Targeting
notch signaling pathway in cancer: Clinical development advances
and challenges. Pharmacol Ther. 141:140–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ogawa R, Ishiguro H, Kimura M, Funahashi
H, Wakasugi T, Ando T, Shiozaki M and Takeyama H: NOTCH1 expression
predicts patient prognosis in esophageal squamous cell cancer.
European surgical research. Eur Surg Res. 51:101–107. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lim SOI, Gu JM, Kim MS, Kim HS, Park YN,
Park CK, Cho JW, Park YM and Jung G: Epigenetic changes induced by
reactive oxygen species in hepatocellular carcinoma: methylation of
the E-cadherin promoter. Gastroenterology. 135:2128–2140,
2140.e2121-2128. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sahlgren C, Gustafsson MV, Jin S,
Poellinger L and Lendahl U: Notch signaling mediates
hypoxia-induced tumor cell migration and invasion. Proc Natl Acad
Sci USA. 105:pp. 6392–6397. 2008; View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shao S and Zhao X, Zhang X, Luo M, Zuo X,
Huang S, Wang Y, Gu S and Zhao X: Notch1 signaling regulates the
epithelial-mesenchymal transition and invasion of breast cancer in
a Slug-dependent manner. Mol Cancer. 14:282015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Benedito R, Roca C, Sörensen I, Adams S,
Gossler A, Fruttiger M and Adams RH: The notch ligands Dll4 and
Jagged1 have opposing effects on angiogenesis. Cell. 137:1124–1135.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Noguera-Troise I, Daly C, Papadopoulos NJ,
Coetzee S, Boland P, Gale NW, Lin HC, Yancopoulos GD and Thurston
G: Blockade of Dll4 inhibits tumour growth by promoting
non-productive angiogenesis. Nature. 444:1032–1037. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Dorneburg C, Goß AV, Fischer M, Roels F,
Barth TF, Berthold F, Kappler R, Oswald F, Siveke JT, Molenaar JJ,
et al: γ-Secretase inhibitor I inhibits neuroblastoma cells, with
NOTCH and the proteasome among its targets. Oncotarget.
7:62799–62813. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen D, Li W, Liu S, Su Y, Han G, Xu C,
Liu H, Zheng T, Zhou Y and Mao C: Interleukin-23 promotes the
epithelial-mesenchymal transition of oesophageal carcinoma cells
via the Wnt/β-catenin pathway. Sci Rep. 5:86042015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roberts DM, Anderson AL, Hidaka M,
Swetenburg RL, Patterson C, Stanford WL and Bautch VL: A vascular
gene trap screen defines RasGRP3 as an angiogenesis-regulated gene
required for the endothelial response to phorbol esters. Mol Cell
Biol. 24:10515–10528. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng F and Wu G: The rejuvenated scenario
of epithelial-mesenchymal transition (EMT) and cancer metastasis.
Cancer Metastasis Rev. 31:455–467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yuan Y, Hu Y, Zhao Y and Chen L:
Expressions of Notch signaling-associated proteins in esophageal
squamous cell carcinoma. Zhonghua Wei Chang Wai Ke Za Zhi.
18:909–913. 2015.(In Chinese). PubMed/NCBI
|