Introduction
A solid tumor is an abnormal mass of tissue
(excluding hematological malignancies) and can be classified as
benign (non-cancerous) and malignant (cancerous). Solid tumors are
classified according to the type of cells forming the tumor, where
localized solid tumors are either sarcoma, carcinoma, or lymphoma
(1). Solid tumor cancers directly
affect patient survival, particularly when tumors are malignant
(2). Improving the survival rate of
cancer patients requires suppression of the development and growth
of cancer (3). Although most tumor
treatments include surgery, radiation therapy and chemotherapy,
these methods are limited by tumor metastasis (4). Therefore, it is necessary to develop
chemotherapeutic methods using innocuous agents to complement
existing therapies.
Baicalein is a herbal medicine that has long been
used as a chemotherapeutic agent and an anti-inflammatory agent
(5,6). It is a flavonoid isolated from the
roots of S. baicalensis Georgi and is known to function by
inducing apoptosis, initiating autophagy, and causing cell cycle
arrest (7–10). Recent studies have shown that
baicalein inhibits carcinoma metastasis through inhibition of cell
motility and cell migration (11).
Tumor angiogenesis is necessary for tumor
progression, as the vascular network supplies oxygen and nutrients
and removes waste products (12,13).
Therefore, tumor growth and metastasis are dependent on
angiogenesis and lymphangiogenesis (14–16).
Recently, several studies found that baicalein inhibited
proliferation of smooth muscle cells and inhibited LPS-induced
angiogenesis in vascular endothelial cells (6,17).
Baicalein was also found to inhibit endothelial cell proliferation
by inhibiting VEGFR-2 phosphorylation and to decrease the activity
of MMP-2 in endothelial cells (18,19).
The anticancer effect of baicalein is mostly related to ERK
signaling and MMP-2 activation (7,11,20).
Previous studies have indicated that the anticancer
effect of baicalein may result from simultaneous action on tumor
cells and vascular endothelial cells. In the present study, we used
B16F10 cells, Lewis lung carcinoma (LLC) cells, and human umbilical
vein endothelial cells (HUVECs) to investigate the effect of
baicalein on these cells in vitro. In addition, an
experimental animal model was used to observe the growth rate and
metastasis of tumors and tumor vessel formation in vivo. Our
results confirmed that baicalein decreased both tumor metastasis
and the tumor growth rate, and inhibition of tumor growth was
mediated by baicalein-induced tumor cell death and suppression of
vasculature formation in the tumor. The purpose of the present
study was to investigate the effect of baicalein on tumor cells and
its impact on the formation of the tumor vascular network in order
to assess its potential as a safe and effective anticancer
agent.
Materials and methods
Mice
All animal experiments were performed with approval
from the Animal Care Committees of Chonbuk National University.
Specific pathogen-free C57BL/6 mice were purchased from the Samtako
Bio Korea Co., Ltd. (Osan, Korea). To confirm both the effects on
tumor cells and tumor angiogenesis, C57BL/6 mice were used in the
experiment based on a previous study (12). All mice were transferred to our
pathogen-free animal facilities and given ad libitum access
to a standard diet (PMI LabDiet) and water. Mice used for the
present study were 7 weeks old. All mice were divided into 4
groups: B16F10 injected, baicalein-treated after B16F10 injection,
LLC injected and baicalein treated after LLC injection.
Tumor model and baicalein
treatment
B16F10 melanoma cells were obtained from the Korean
Cell Line Bank and Lewis lung carcinoma LLC cells were obtained
from Bundang CHA Medical Center. To generate the tumor models,
suspended tumor cells (1ⅹ106 cells in 100 µl) were
subcutaneously (s.c.) injected into the dorsal flank of 7-week-old
male mice. After implantation, 1.5 mg/kg baicalein was
intraperitoneally (i.p.) injected at the same time every day for 2
weeks from day 7 after injection of the tumor cells. Tumor volume
and tumor growth rate were measured using previously reported
methods (12).
Histological analysis
The mice were sacrificed by cervical dislocation on
the indicated days. Tumor tissues and lymph nodes were fixed in 4%
paraformaldehyde (PFA) for 4 h, dehydrated in 20% sucrose solution
overnight, and embedded with tissue freezing medium (Leica,
Wetzlar, Germany). Frozen blocks were cut into 80-µm sections.
Samples were blocked with 5% donkey (or goat) serum in PBS-Tween-20
(PBST) (0.03% Triton X-100 in PBS), and then incubated for 4 h at
room temperature (RT; range, 20–27°C) with the following primary
antibodies: anti-CD31 (hamster, clone 2H8; Millipore Billerica, MA,
USA), FITC-conjugated anti-α-SMA (mouse, clone 1A4; Sigma-Aldrich,
St. Louis, MO, USA), anti-caspase-3 (rabbit polyclonal; R&D
Systems, Minneapolis, MN, USA), anti-LYVE-1 (rabbit polyclonal;
AngioBio, Del Mar, CA, USA), and anti-pan-cytokeratin (mouse, clone
AE1/AE3; Abcam, Cambridge, MA, USA). After 3–5 washes, the samples
were incubated for 2 h at RT with the following secondary
antibodies: Cy3-conjugated anti-hamster IgG (127-165-160),
Cy3-conjugated anti-mouse IgG (715-165-150), Cy3-conjugated
anti-rabbit IgG (711-165-152) and FITC-conjugated anti-rabbit IgG
(711-095-152) (Jackson ImmunoResearch, West Grove, PA, USA). Nuclei
were stained with 4′,6-diamidino-2-phenylindole (DAPI). The samples
were then mounted in fluorescent mounting medium (Dako,
Carpinteria, CA, USA) and immunofluorescent images were acquired
using a Zeiss LSM 510 confocal fluorescence microscope (Carl Zeiss,
Jena, Germany).
For hematoxylin and eosin (H&E) staining, lung
tissues were fixed overnight in 4% PFA. After tissue processing
using standard procedures, samples were embedded in paraffin and
cut into 5-µm sections followed by H&E staining.
Morphometric analysis
Blood vessel density measurements in the
intratumoral regions were analyzed using photographic analysis in
the ImageJ software (http://rsb.info.nih.gov/ij) and the LSM Image Browser
(Carl Zeiss). For blood vessel density, the CD31-positive area (per
random 0.44 mm2 area) was measured in the intratumoral
region. The range of α-SMA-positive mural cells was calculated as a
percentage of the corresponding positive fluorescence region along
the CD31-positive blood vessels in random 0.44 mm2
intratumoral regions.
Cell culture and reagents
HUVEC growth medium was purchased from Lonza Group
Ltd. (Basel, Switzerland). Cells were cultured in endothelial cell
growth medium (EGM-2 MV BulletKit) and used at passage 3–4 in all
experiments. B16F10 and LLC cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) (purchased from Gibco, Grand Island,
NY, USA) supplemented with 10% FBS (purchased from Atlas
Biologicals, Fort Collins, CO, USA), 100 U/ml penicillin and 100 µg
streptomycin (purchased from Sigma). All cells were incubated at
37°C with 5% CO2. Baicalein (purchased from Sigma) was
dissolved in dimethyl sulfoxide (DMSO) to create a stock solution
at 100 mM (for in vitro use) or 3 mg/ml (for in vivo
use), which was then diluted with autoclaved PBS buffer prior to
use.
Cell proliferation and cell viability
assay
Cell proliferation was detected by MTT assay and
cell viability was detected by Annexin V assay. The colorimetric
3–4,5-dimethylthiazol-2-yl-2,5-diphenyl tetrazolium bromide (MTT)
assay was performed to quantitate the effect of baicalein on
mitochondrial dysfunction. Briefly, 1ⅹ105 cells/well
were seeded in a 24-well microplate in a final volume of 300 µl,
incubated overnight, and treated with baicalein for 24 h. Following
treatment for 24 h, 30 µl of MTT (5 mg/ml in PBS) was added to the
cells and incubation was carried out for 2 h. The MTT solution was
then removed and replaced by 300 µl dimethyl sulfoxide (DMSO), and
the plates were shaken for 10 min. Then, 200 µl of the sample were
transferred to a 96-well microplate. The optical density was
determined at a wavelength of 570 nm. Apoptosis was assessed by a
commercial Annexin V assay (Santa Cruz Biotechnology) according to
the manufacturer's protocol. Annexin V content was determined by
measuring fluorescence at excitation 488 nm and emission at 525 nm
using a Guava EasyCyte HT system (Millipore).
Scratch wound healing assay
Tumor cells (B16F10, LLC) and HUVECs were grown on
6-well plates. Cultured cells were wounded by scraping with a 1,000
µl pipette tip. After wounding, the cells were gently washed twice
with PBS and incubated with 2% FBS-containing medium supplemented
with 100 µM baicalein for 24 h. Then, the wounded areas were
observed and photographed using a microscope (Nikon Eclipse TS100;
Nikon Corporation, Tokyo, Japan) at a magnification of ×10.
In vitro tube formation assay
Tube formation was assessed by an endothelial cell
tube formation assay (Corning Inc., Corning, NY, USA) according to
the manufacture's protocol. Tube number was counted in 3 random
fields using ImageJ software (http://rsb.info.nih.gov/ij).
Western blotting
B16F10 and LLC cells were homogenized in cold RIPA
buffer containing a protease inhibitor cocktail 18 h after
baicalein treatment. Each protein was separated by SDS-PAGE gel and
transferred to nitrocellulose membranes. After blocking with 5%
skim milk, the membranes were incubated with anti-caspase-3 (rabbit
monoclonal) and anti-cleaved caspase-3 (rabbit) and anti-PARP
(rabbit) (all from Cell Signaling Technology, Inc., Beverly, MA,
USA) and anti-β-actin antibody (mouse monoclonal; Sigma-Aldrich) in
blocking buffer overnight at 4°C, and then with HRP-conjugated
secondary antibody for 2 h at RT. Signals were developed with
enhanced chemiluminescence HRP substrate (Millipore) and detected
using the Fusion FX7 acquisition system (Vilbert Lourmat,
Eberhardzell, Germany).
Immunocytochemical analysis
Tumor cells (B16F10, LLC) were cultured on glass
slides, fixed with cold acetone and blocked using 5% donkey serum
in Tris-buffered saline with Tween-2 (TBST). The cells were
incubated with anti-caspase-3 (rabbit polyclonal; R&D Systems)
at 4°C. Cells were incubated with Cy3-conjugated anti-rabbit IgG
(Jackson ImmunoResearch, 711-165-152). Nuclei were stained with
DAPI. Then, the samples were mounted in fluorescent mounting medium
(Dako) and immunofluorescent images were acquired using a Zeiss
LSM510 confocal fluorescence microscope (Carl Zeiss).
Statistical analysis
Values are presented as mean ± standard deviation
(SD). Significant differences between means were determined by
unpaired Student t-tests or analysis of variance with one-way ANOVA
followed by the Student-Newman-Keuls test. Statistical significance
was set as <0.05 and indicated as *p<0.05 or **P<0.01 in
the figures and legends.
Results
Baicalein inhibits tumor growth via
activation of caspase-3
We administered baicalein to mice injected with
B16F10 melanoma or LLC carcinoma cells in order to examine its
anticancer effects on the two solid tumor models. As a result of
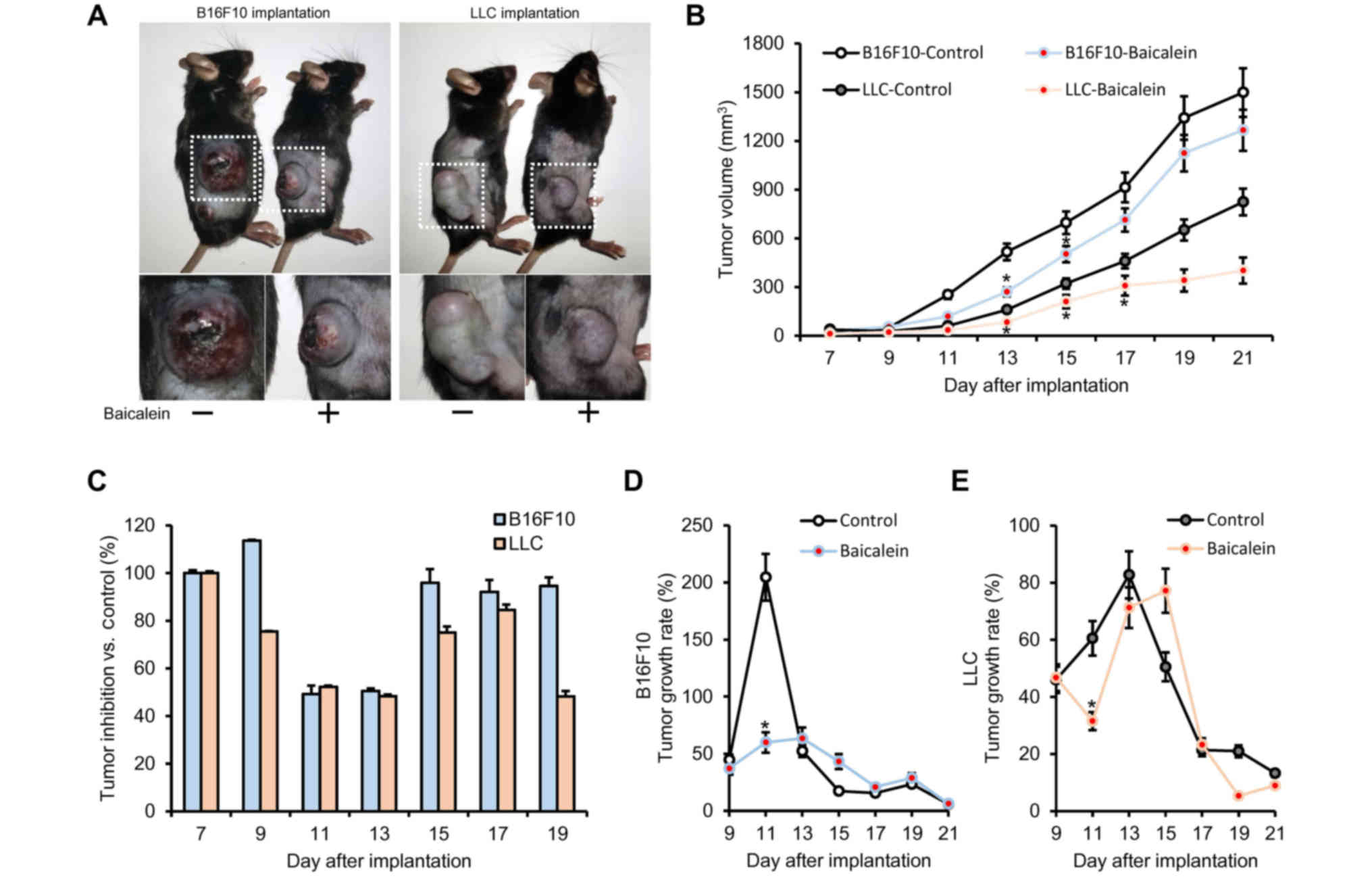
the baicalein treatment, tumor size and tumor volume were reduced
in each model compared to the untreated group (Fig. 1A and B). The maximum effect of
baicalein treatment was noted on day 11 (49.20% inhibition) for the
B16F10-implanted group and day 19 (48.25% inhibition) for the
LLC-implanted group (Fig. 1C).
Notably, the tumor growth rate was significantly reduced in the
baicalein-treated group compared to the untreated group, with the
most significant decrease observed 11 days after tumor implantation
(Fig. 1D and E). As shown in
Fig. 1D and E, tumor growth rates
in the B16F10- and LLC-injected mice decreased by 144 and 51%,
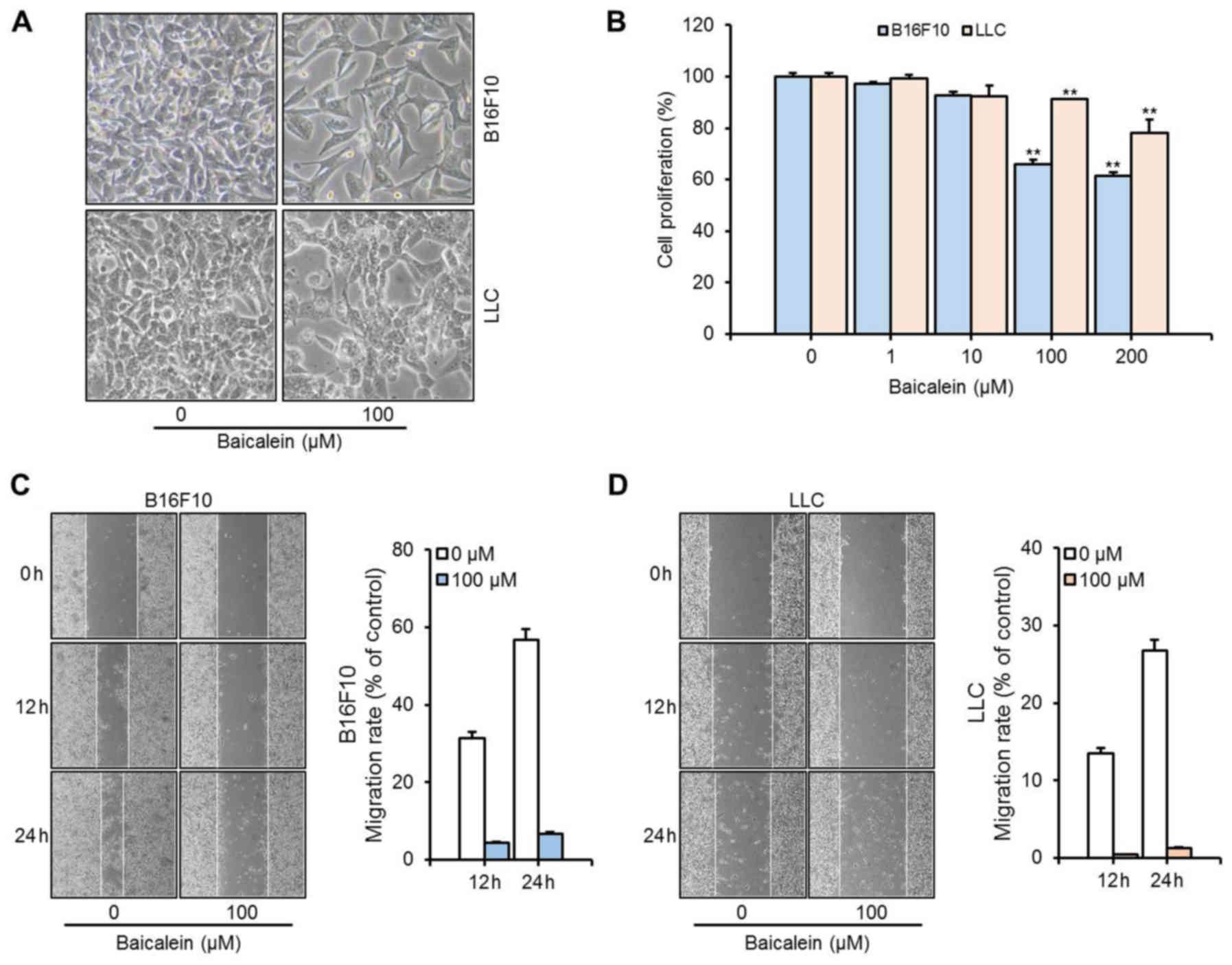
respectively, upon treatment with baicalein. In vitro
measurements of cell proliferation with B16F10 and LLC cells
revealed dose-dependent decreases by baicalein treatment (Fig. 2). Inhibition of cell proliferation
by baicalein was greater in the B16F10 cells than that in the LLC
cells at a given treatment concentration. Viability of B16F10 and
LLC cells was decreased upon baicalein treatment by 35 and 22%,
respectively. In addition, baicalein strongly inhibited tumor cell
migration (Fig. 2C and D). The
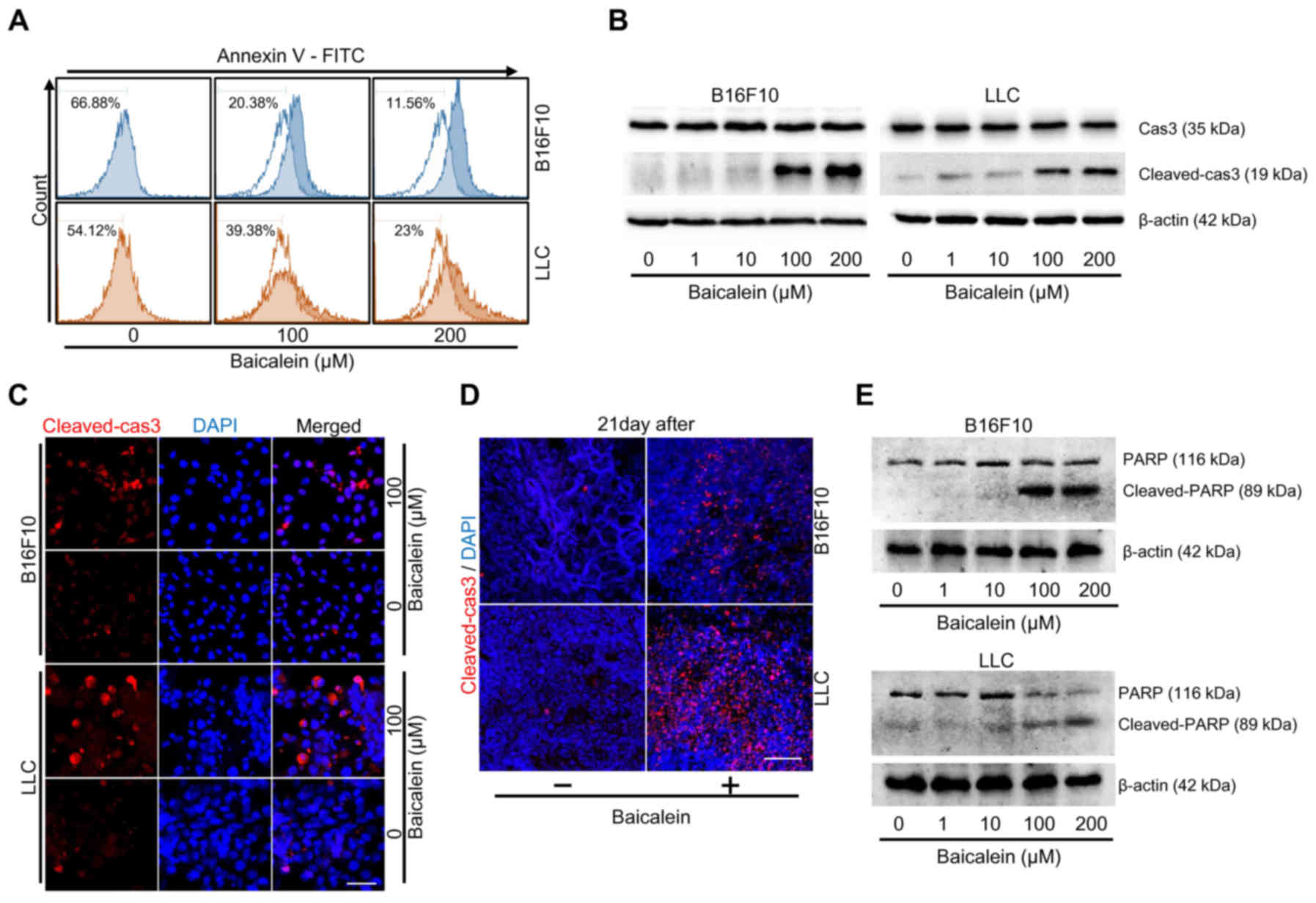
Annexin V assay was performed to assess whether baicalein induces
apoptosis of tumor cells. The results showed that cell viability
was reduced by 55% in the B16F10 cells and 31% in the LLC cells
(Fig. 3A). Western blot methods
were used to assess the effect of baicalein on the expression and
activation of caspase-3 (Cas3). Although Cas3 expression was not
significantly changed, Cas3 activation (cleaved-cas3) through
proteolytic cleavage was increased in a dose-dependent manner after
baicalein treatment (Fig. 3B). In
addition, Cas3 activation through cleavage was confirmed via
immunofluorescence staining in both in vitro tumor cells and
in vivo tumor tissue samples (Fig. 3C and D). To determine whether
apoptosis by baicalein was dependent on Cas3 activation, we
confirmed the expression of PARP. As a result, cleaved-PARP was
also increased by baicalein treatment (Fig. 3E). Therefore, these results
demonstrated that baicalein treatment induced apoptosis via Cas3
activation.
Baicalein inhibits vasculature
formation by directly affecting endothelial cells during tumor
angiogenesis
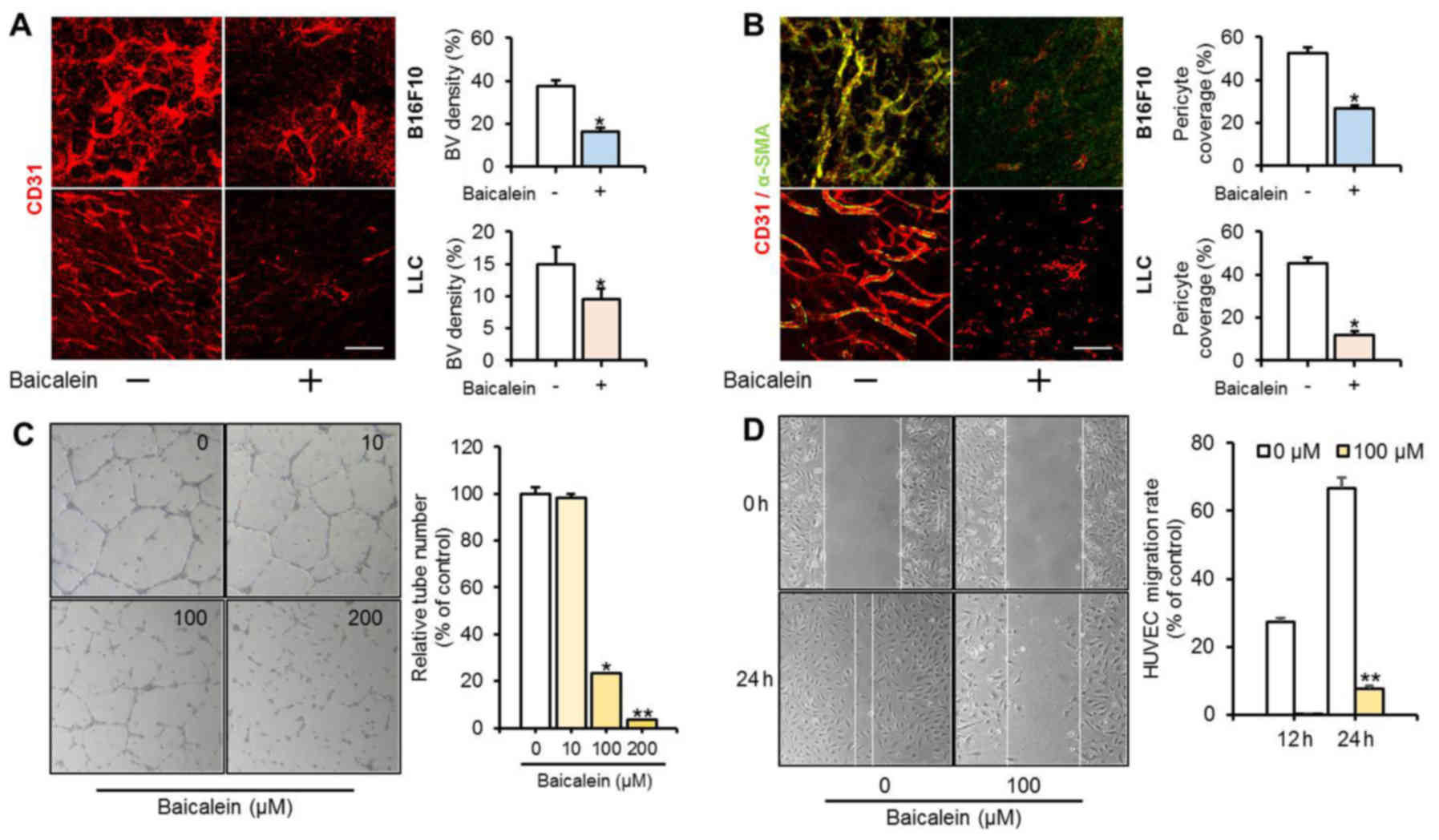
Tumor tissue was stained with CD31 (endothelial cell
specific) and α-SMA (mural cell specific) immunofluorescent markers
to confirm the effect of baicalein on tumor angiogenesis (Fig. 4A and B). The density of blood
vessels was markedly reduced by baicalein treatment in the tumor
models with B16F10 and LLC implanted cells. In mice injected with
B16F10 cells, intratumoral blood vessel density was 37.56% in the
untreated group and 21.24% in the baicalein-treated group. The mice
injected with LLC cells showed a pattern similar to the
B16F10-injected group, with intratumoral blood vessel densities of
14.88% in the untreated group and 9.51% in the baicalein-treated
group. α-SMA was expressed at very low levels. These results
indicate that baicalein treatment strongly inhibited vasculature
formation during tumor progression. HUVECs were treated with
baicalein and the effects on angiogenesis were examined in a tube
formation assay and a cell migration assay (Fig. 4C and D). Baicalein strongly
suppressed tube formation by almost 80% or more at concentrations
above 100 µM. A cell migration assay with 100 µM baicalein
significantly reduced migration of HUVECs. These results suggest
that baicalein directly affects vascular endothelial cells to
suppress angiogenesis.
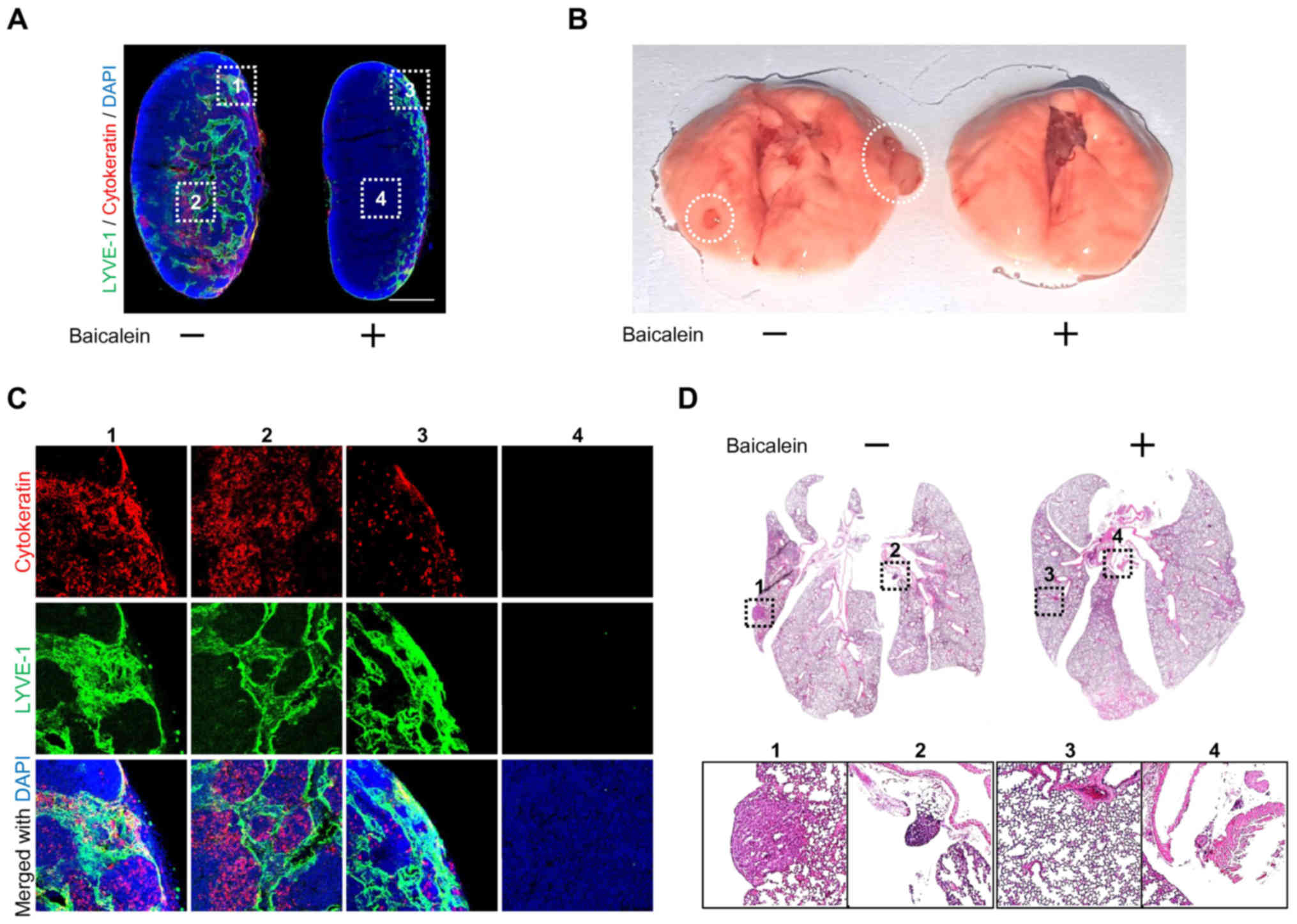
Baicalein delays tumor metastasis in
the LLC model
Our results showed that baicalein inhibited tumor
angiogenesis, while tumor cells are known to spread to other organs
via the tumor vasculature. Therefore, we used LLC-injected mouse
models to investigate the antimetastatic effect of baicalein
treatment. To confirm the effect of baicalein on metastasis, we
separated the inguinal lymph nodes and lungs of the LLC-implanted
mice and compared the control and baicalein treatment groups. As a
result, baicalein was found to delay tumor metastasis. In addition,
the lymph node of the baicalein-treated group showed a similar size
to the wild-type lymph node. As shown in Fig. 5A and C, the lymph nodes of the
baicalein-treated mice were smaller than those of the untreated
mice, in which the lymph nodes were hypertrophic. We determined the
LLC cells within the lymph nodes by immunohistochemistry using the
cytokeratin (LLC marker) and LYVE-1 (lymphatic marker) antibodies.
These experiments also showed that baicalein reduced LLC metastasis
to the lymph node (Fig. 5A and C).
In addition, it was confirmed that the LLC metastasis to the lungs
was reduced by baicalein treatment (Fig. 5B and D). These results showed that
baicalein can attenuate tumor cell metastasis by inhibiting
formation of the intratumoral vasculature.
Discussion
Baicalein is a natural flavonoid isolated from the
root of S.baicalensis Georgi that has long been used as a
chemotherapeutic agent due to its anticancer and anti-inflammatory
properties (7–9). Recent studies have also shown that
baicalein inhibits both mural cell proliferation and LPS-induced
angiogenesis in vascular endothelial cells (6). Despite considerable research on
baicalein, its effects on tumor progression and metastasis are not
yet clear. In the present study, we demonstrated the anticancer
effect of baicalein on the progression of tumors such as melanoma
and carcinoma. In order to elucidate the anticancer effect of
baicalein, we focused on the following 3 points: first, the effects
on tumor models and cells; second, the effects on the development
of tumor vasculature; and third, the effects on tumor
metastasis.
Our results showed that the tumor growth rate was
strongly inhibited by baicalein (Fig.
1) and tumor development was suppressed in the early stages of
tumor progression. As shown in Fig. 1D
and E, after 11 days of tumor implantation, tumor growth rates
were decreased in both the control and baicalein treatment groups.
However, in the treated group with baicalein at the early stage of
tumor progression, the tumor growth rates were relatively decreased
compared to the control group. During tumor progression, tumor
cells in the intratumoral core of the tumor tissues exhibit slow
proliferation, and growth rates are small (21). Nevertheless, on day 11 after tumor
implantation, tumor growth rates were markedly decreased by
baicalein treatment. However, the tumor growth rates after day 11
were similar in both the control and treatment groups. These
results indicated that the anticancer effect of baicalein is strong
at the early stage of tumor progression.
Previous studies have shown that baicalein induces
cell cycle arrest, leading to anticancer effects (7,20). The
anticancer effect of baicalein is thought to inhibit tumor cell
proliferation and induce apoptosis, thereby inhibiting tumor cell
progression (22,23). We examined the anticancer effect of
baicalein in B16F10 and LLC cells. Baicalein inhibited the
proliferation and migration of tumor cells, and although the
expression of caspase 3 was barely altered by baicalein treatment,
caspase 3 activation through proteolytic cleavage was markedly
increased (Fig. 3). These results
demonstrate that baicalein increased caspase 3 and PARP activation,
which induced apoptosis. Activation of caspase 3 was also observed
in tumor tissues in vivo, consistent with the in
vitro experiments (Fig. 3C and
D).
Tumor angiogenesis is an essential process for tumor
development and tumor cells utilize the tumor vasculature to
metastasize to other tissues (12,13).
Baicalein was found to inhibit the proliferation of vascular smooth
muscle cells (VSMCs) in cardiovascular disease (24). Notably, baicalein inhibited the
expression of VEGF and FGFR-2, leading to anticancer effects in
lung cancer (20). As the
expression of VEGF and VSMCs are closely related to angiogenesis
(25,26), we expected that baicalein may affect
the process of vasculature formation in tumors. We confirmed that
baicalein inhibited the expression of CD31 and α-SMA in tumor
tissues and strongly inhibited tube formation and cell migration in
vascular endothelial cells (HUVECs). The antimetastatic effect of
baicalein was verified in the lymph node and lung concurrently with
observations of tumor vasculature formation. Baicalein-mediated
disruption of tumor angiogenesis produced an expected delay in
tumor metastasis to both the lymph nodes and the lungs, as the
tumor vasculature provides one of the metastatic routes. These
results indicate that baicalein affects both tumor cells and
vascular endothelial cells in the tumor during tumor progression.
Notably, the anticancer effects of baicalein on tumor cells were
more strongly detected in B16F10 cells than LLC cells. However, the
in vivo experiment showed stronger anticancer effects in the
LLC-implanted mice. In addition, the tumor volume of LLC-implanted
mice was relatively smaller than that of the B16F10-implanted mice.
These results suggest that the anticancer effect of baicalein was
not efficiently acting on the tumor cells in the intratumoral core
of the tumor mass during tumor progression. To summarize our
results, the therapeutic effects of baicalein not only affect tumor
mass but also tumor angiogenesis (Table
I).
 | Table I.Effect of baicalein on tumor and
endothelial cells. |
Table I.
Effect of baicalein on tumor and
endothelial cells.
|
| Cell proliferation
(%) | Cell migration rate
(% of control) | Relative tube number
(% of control) |
|---|
|
|
|
|
|
|---|
|
|
|
| Control | Baicalein (100
µM) |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Type of cells | Control | Baicalein (100
µM) | 12 h | 24 h | 12 h | 24 h | Control | Baicalein (100
µM) |
|---|
| B16F10 | 100 |
65.86±1.66b | 31.40±2.01 | 56.81±2.57 | 4.28±1.82 |
6.63±1.99b | – | – |
| LLC | 100 |
91.24±0.03b | 13.46±0.72 | 26.8±1.34 | 0.42±0.02 |
1.24±0.05b | – | – |
| HUVECs | – | – | 27.18±1.45 | 66.59±3.48 | 0.23±0.01 |
7.74±0.43b | 99.99±2.75 |
23.21±1.59a |
Taken together, our results demonstrated the
anticancer effect of baicalein. Firstly, baicalein inhibited tumor
cell proliferation and induced apoptosis. Secondly, baicalein
inhibited tumor progression by disrupting intratumoral vasculature
formation. Thirdly, baicalein delayed tumor metastasis. Our results
strongly suggest that baicalein is a useful chemotherapeutic agent,
owing to its multifunctional effects on tumor cells and vascular
endothelial cells during tumor progression. Based on the present
study, we aim to identify the mechanisms related to
baicalein-induced tumor growth inhibition in future research.
Acknowledgements
The present study was supported by the Co-operative
Research Program for Agriculture, Science and Technology
Development (PJ012612012017) in the Rural Development
Administration and by the Korea Institute of Planning and
Evaluation for Technology in Food, Agriculture, Forestry and
Fisheries (IPET) through Agriculture, Food and Rural Affairs
Research Center Support Program, funded by the Ministry of
Agriculture, Food and Rural Affairs (MAFRA; 716002-7).
References
|
1
|
O'Neill AC, Jagannathan JP and Ramaiya NH:
Evolving cancer classification in the era of personalized medicine:
A primer for radiologists. Korean J Radiol. 18:6–17. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lechner MG, Karimi SS, Barry-Holson K,
Angell TE, Murphy KA, Church CH, Ohlfest JR, Hu P and Epstein AL:
Immunogenicity of murine solid tumor models as a defining feature
of in vivo behavior and response to immunotherapy. J Immunother.
36:477–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rolston KVI: Infections in cancer patients
with solid tumors: A review. Infect Dis Ther. 6:69–83. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu H, Dong Y, Gao Y, Du Z, Wang Y, Cheng
P, Chen A and Huang H: The fascinating effects of baicalein on
cancer: A review. Int J Mol Sci. 17:172016. View Article : Google Scholar
|
|
6
|
Lee W, Ku SK and Bae JS: Anti-inflammatory
effects of baicalin, baicalein, and wogonin in vitro and in vivo.
Inflammation. 38:110–125. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Song L, Cai L, Wei R, Hu H and
Jin W: Effects of baicalein on apoptosis, cell cycle arrest,
migration and invasion of osteosarcoma cells. Food Chem Toxicol.
53:325–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen YJ, Wu CS, Shieh JJ, Wu JH, Chen HY,
Chung TW, Chen YK and Lin CC: Baicalein triggers
mitochondria-mediated apoptosis and enhances the antileukemic
effect of vincristine in childhood acute lymphoblastic leukemia
CCRF-CEM cells. Evid Based Complement Alternat Med.
2013:1247472013.PubMed/NCBI
|
|
9
|
Wang Z, Jiang C, Chen W, Zhang G, Luo D,
Cao Y, Wu J, Ding Y and Liu B: Baicalein induces apoptosis and
autophagy via endoplasmic reticulum stress in hepatocellular
carcinoma cells. Biomed Res Int. 2014:7325162014.PubMed/NCBI
|
|
10
|
Wang YF, Li T, Tang ZH, Chang LL, Zhu H,
Chen XP, Wang YT and Lu JJ: Baicalein triggers autophagy and
inhibits the protein kinase B/mammalian target of rapamycin pathway
in hepatocellular carcinoma HepG2 cells. Phytother Res. 29:674–679.
2015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen K, Zhang S, Ji Y, Li J, An P, Ren H,
Liang R, Yang J and Li Z: Baicalein inhibits the invasion and
metastatic capabilities of hepatocellular carcinoma cells via
down-regulation of the ERK pathway. PLoS One. 8:e729272013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim C, Yang H, Fukushima Y, Saw PE, Lee J,
Park JS, Park I, Jung J, Kataoka H, Lee D, et al: Vascular RhoJ is
an effective and selective target for tumor angiogenesis and
vascular disruption. Cancer Cell. 25:102–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sundar SS and Ganesan TS: Role of
lymphangiogenesis in cancer. J Clin Oncol. 25:4298–4307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gomes FG, Nedel F, Alves AM, Nör JE and
Tarquinio SB: Tumor angiogenesis and lymphangiogenesis:
Tumor/endothelial crosstalk and cellular/microenvironmental
signaling mechanisms. Life Sci. 92:101–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Foubert P and Varner JA: Integrins in
tumor angiogenesis and lymphangiogenesis. Methods Mol Biol.
757:471–486. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dong LH, Wen JK, Miao SB, Jia Z, Hu HJ,
Sun RH, Wu Y and Han M: Baicalin inhibits PDGF-BB-stimulated
vascular smooth muscle cell proliferation through suppressing PDGFR
beta-ERK signaling and increase in p27 accumulation and prevents
injury-induced neointimal hyperplasia. Cell Res. 21:1276. 2011.
View Article : Google Scholar :
|
|
18
|
Ling Y, Chen Y, Chen P, Hui H, Song X, Lu
Z, Li C, Lu N and Guo Q: Baicalein potently suppresses angiogenesis
induced by vascular endothelial growth factor through the p53/Rb
signaling pathway leading to G1/S cell cycle arrest. Exp Biol Med.
236:851–858. 2011. View Article : Google Scholar
|
|
19
|
Huang Y, Miao Z, Hu Y, Yuan Y, Zhou Y, Wei
L, Zhao K, Guo Q and Lu N: Baicalein reduces angiogenesis in the
inflammatory microenvironment via inhibiting the expression of
AP-1. Oncotarget. 8:883–899. 2017.PubMed/NCBI
|
|
20
|
Cathcart MC, Useckaite Z, Drakeford C,
Semik V, Lysaght J, Gately K, O'Byrne KJ and Pidgeon GP:
Anti-cancer effects of baicalein in non-small cell lung cancer
in-vitro and in-vivo. BMC Cancer. 16:7072016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trédan O, Galmarini CM, Patel K and
Tannock IF: Drug resistance and the solid tumor microenvironment. J
Natl Cancer Inst. 99:1441–1454. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamashita S, Baba K, Makio A, Kumazoe M,
Huang Y, Lin IC, Bae J, Murata M, Yamada S and Tachibana H:
γ-Tocotrienol upregulates aryl hydrocarbon receptor expression and
enhances the anticancer effect of baicalein. Biochem Biophys Res
Commun. 473:801–807. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu TY, Gong W, Tan ZJ, Lu W, Wu XS, Weng
H, Ding Q, Shu YJ, Bao RF, Cao Y, et al: Baicalein inhibits
progression of gallbladder cancer cells by downregulating ZFX. PLoS
One. 10:e01148512015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong LH, Wen JK, Miao SB, Jia Z, Hu HJ,
Sun RH, Wu Y and Han M: Baicalin inhibits PDGF-BB-stimulated
vascular smooth muscle cell proliferation through suppressing
PDGFRβ-ERK signaling and increase in p27 accumulation and prevents
injury-induced neointimal hyperplasia. Cell Res. 20:1252–1262.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cébe-Suarez S, Zehnder-Fjällman A and
Ballmer-Hofer K: The role of VEGF receptors in angiogenesis;
complex partnerships. Cell Mol Life Sci. 63:601–615. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Appelmann I, Liersch R, Kessler T, Mesters
RM and Berdel WE: Angiogenesis inhibition in cancer therapy:
platelet-derived growth factor (PDGF) and vascular endothelial
growth factor (VEGF) and their receptors: biological functions and
role in malignancy. Recent Results Cancer Res. 180:51–81. 2010.
View Article : Google Scholar : PubMed/NCBI
|