Introduction
Human oral squamous cell carcinoma (HOSCC) is the
most common malignant neoplasm arising in the mucosa of the upper
aerodigestive tract. It is an aggressive tumor that is difficult to
treat with conventional therapies, including chemotherapy,
radiation and surgery. Since surgical treatment often profoundly
affects the quality of life and daily activities of patients with
HOSCC, new therapeutic strategies are necessary along with the
other conventional treatments. Over the last several decades,
epidemiological, preclinical and even early-phase clinical trials
have revealed that selected dietary constituents may be promising
for reducing the incidence of multiple cancers (1–3).
Considering the potential that some of these phytochemicals have
exhibited, it is essential to further identify and develop
promising new agents in the hope of creating a broad spectrum of
chemopreventive agents that can be used either alone or in
combination against cancer. Phytochemicals extracted from natural
sources, such as mangosteen, have recently been a focus of interest
as anticancer drugs that may exert fewer side effects in patients
with oral cancer.
Mangosteen (Garcinia mangostana L.) known as
the ‘queen of fruit’ in its native Thailand can be found in many
countries worldwide (4). For
centuries, people in South-East Asia have used the dried mangosteen
pericarp for medicinal purposes. It is used as an antiseptic, an
anti-inflammatory, an antiparasitic, an antipyretic, an analgesic,
as well as a treatment for skin rashes (5). Mangosteen fruit rind contains a high
concentration of xanthone, which is a type of polyphenol. Over 200
xanthones are currently known to exist in nature and approximately
50 of them are found in mangosteen (5). α-Mangostin has been identified as the
most abundant xanthone and has therefore received the most
attention for its health-promoting properties. It has recently been
demonstrated to induce cell-cycle arrest and apoptosis in various
types of human cancer cells (6–9). It
has also been reported to exert chemopreventive effects against
chemically-induced colon cancer by reducing the expression of c-Myc
(5).
Tumor necrosis factor (TNF)-related
apoptosis-inducing ligand (TRAIL), a member of the TNF superfamily,
selectively induces apoptosis through the death receptors (DRs) DR4
and/or DR5 in cancer cells (10,11).
As these receptors are expressed on the surface of cancer cells,
but not on the surface of normal cells, TRAIL is virtually inactive
against normal cells. However, the application of TRAIL in
anticancer treatment is limited because of its short half-life in
serum and the development of resistance to TRAIL-induced apoptosis
(12). On the other hand, it has
recently been reported that a combination of α-mangostin and TRAIL
synergistically suppresses the growth of TRAIL-resistant cancer
cells (13). Therefore, in the
present study, we investigated the degree to which α-mangostin can
inhibit the growth of human oral cancer cells through induction of
apoptosis and cell cycle arrest in vitro. In addition, we
examined the synergistic effects of α-mangostin in combination with
TRAIL against oral cancer cells.
Materials and methods
Reagents
Rabbit anti-human c-Myc monoclonal antibody (mAb
c-Myc) was purchased from Cell Signaling Technology (Danvers, MA,
USA) for immunoblot analysis and immunohistochemistry (IHC). Mouse
anti-human β-actin monoclonal antibody (mAb β-actin) and
α-mangostin were obtained from Sigma-Aldrich (St. Louis, MO, USA).
Recombinant human TRAIL/Apo2L (rhTRAIL/Apo2L) was purchased from
PeproTech (London, UK). α-Mangostin and rhTRAIL were used for the
stimulation of the cell lines. A Cytochrome c Apoptosis
Detection kit (including mouse anti-human cytochrome c
monoclonal antibody, mAb cytochrome c) was purchased from
PromoKine (Heidelberg, Germany).
Cell culture
The HOSCC cell lines (HSC-2, HSC-3, HSC-4, Ca9-22
and SAS) were obtained from the Japanese Cancer Research Resources
Bank (JCRB, Osaka, Japan) and were maintained in RPMI-1640 medium
supplemented with 10% heat-inactivated fetal bovine serum (FBS),
100 IU/ml penicillin and 100 µg/ml streptomycin. They were grown to
confluency in 25-cm2 culture flasks at 37°C in a
humidified 5% CO2 incubator until required.
RNA extraction and real-time
quantitative RT-PCR
Total RNA was extracted from monolayer HOSCC cells
(1×106 cells/ml) using the acid guanidinium
phenol-chloroform (AGPC) method as previously described (14). To ascertain the expression patterns
of the c-Myc gene in the HOSCC cell lines, especially in HSC-4 and
SAS that are derived from lymph node metastasis of tongue cancer,
after treatment with α-mangostin (20 µM), rhTRAIL (100 ng/ml), or
α-mangostin (20 µM) + rhTRAIL (100 ng/ml), real-time quantitative
RT-PCR (qRT-PCR) analyses were performed using a Bio-Rad iCycler
system (Bio-Rad, Tokyo, Japan) and an iScript One-Step RT-PCR kit
with SYBR Green I (Bio-Rad) according to the manufacturer's
instructions. Briefly, the mRNAs were reverse-transcribed into
cDNAs at 50°C for 10 min and then the reverse transcriptase (RT)
was inactivated at 95°C for 5 min. The PCR amplification was
performed for 45 cycles at 95°C for 10 sec and at 56°C for 30 sec,
followed by detection. The PCR primers were designed and
synthesized by Sigma-Aldrich (Ishikari, Japan) following special
design criteria for real-time PCR primers. The following primer
sequences were used in the PCR reactions: c-Myc forward,
CCAGCAGCGACTCTGAGGAG and reverse, TTGAGGACCAGTGGGCTGTG. Each sample
was tested in triplicate and for each reaction the corresponding
non-RT-treated mRNA sample was included as a negative control. The
relative level of c-Myc mRNA in each sample was normalized against
the mRNA level of GAPDH, a housekeeping gene. For GAPDH, the
forward primer was CAGCCTCAAGATCATCAGCA and the reverse primer was
ACAGTCTTCTGGGTGGCAGT. The results were analyzed using the Bio-Rad
iCycler Software 3.0 and Microsoft Excel 97 and expressed as the
fold induction in comparison to the amount of GAPDH mRNA (set at
1). The specificity of the PCR products was assessed on the basis
of melting curve data and agarose gel electrophoresis to determine
the product size and to establish that no by-products were
formed.
Immunoblot analysis
The monolayer cells (1×106 cells/ml) were
lysed in cell lysis buffer containing 50 mM Tris-HCl (pH 8.0), 150
mM NaCl, 0.02% sodium azide, 1% Triton X-100, 1 µg/ml aprotinin and
100 µg/ml phenylmethylsulfonyl fluoride (PMSF) and then scraped
from the dishes with a cell scraper (Nalge Nunc International,
Naperville, IL, USA). After 20 min on ice, the lysate was
centrifuged for 5 min at 15,000 rpm at 4°C and the soluble
supernatant fraction was used for western blot analysis. Protein
concentrations were assessed using the Bio-Rad protein assay
(Bio-Rad). For the detection of c-Myc and cytochrome c
proteins by gel electrophoresis, 10-µg of protein samples were
mixed with an equal volume of SDS-PAGE sample buffer and boiled for
5 min. The samples were loaded in lanes and separated on 10%
polyacrylamide gel and then the proteins were electroblotted onto
polyvinylidene difluoride (PVDF) membranes (Bio-Rad). Non-specific
binding sites on the membranes were blocked with 2% bovine serum
albumin in phosphate-buffered saline (BSA-PBS) for 60 min, followed
by incubation with anti-c-Myc mAb (1:1,000), or anti-cytochrome
c mAb (1:1,000) for 60 min at room temperature. The filters
were individually washed three times for 10 min in PBS containing
0.05% Tween-20 (PBT). After incubation with a secondary horseradish
peroxidase (HRP)-labeled goat anti-rabbit IgG (H+L) antibody
(1:25,000) or an HRP-labeled rabbit anti-mouse IgG (H+L) antibody
(1:25,000; GE Healthcare, Piscataway, NJ, USA) for 60 min at room
temperature, the filters were washed again three times for 10 min
in PBT. Finally, the membranes were immersed for 5 min in ECL Plus
western blotting reagent (GE Healthcare) and exposed for 2 min on
Hyperfilm ECL (GE Healthcare) to obtain autoradiographs.
Anti-β-actin mAb (1:5,000) was used as an internal control.
Cell viability assay
The assay is based on the cleavage of tetrazolium
salt WST-8 to formazan by cellular mitochondrial dehydrogenases,
the activity of which increases in proportion to the number of
viable cells. The formazan dye produced by viable cells was
quantified as an index of cell proliferation. Monolayer HSC-4 and
SAS cells (2×104 cells/100 µl/well) were incubated for
24 h on a 96-well plate. The cells were washed once with PBS and
incubated with α-mangostin (20 µM), rhTRAIL (100 ng/ml), or
α-mangostin (20 µM) + rhTRAIL (100 ng/ml) for 24 h in RPMI-1640
medium containing 10% FBS. Ten microliters of WST-8/ECS solution
(Dojindo Laboratories, Tokyo, Japan) was added to each well and
incubated with the cells for 4 h at 37°C in a humidified 5%
CO2 incubator. The cells were then shaken thoroughly for
1 min on a shaker. After shaking, the relative number of viable
cells was determined by assessing the absorbance of the dye
solution at 450 nm.
Apoptosis assay
The SAS cells were plated in white-walled 96-well
tissue culture plates at a density of 5×103 cells/well
in 100 µl of medium and allowed to adhere for 24 h. After
incubation of the cells with α-mangostin (20 µM), rhTRAIL (100
ng/ml), or α-mangostin (20 µM) + rhTRAIL (100 ng/ml) for 24 h, the
activities of caspase-3/7, −8 and −9 were determined using the
Caspase-Glo® 3/7, 8 and 9 assay (Promega, Madison, WI,
USA) according to the manufacturer's instructions. Briefly,
Caspase-Glo reagents were added to each well in a 50-µl volume and
incubated for 30 min before measurement of luminescence as relative
light units (RLUs) using a Veritas Microplate Luminometer
(Promega).
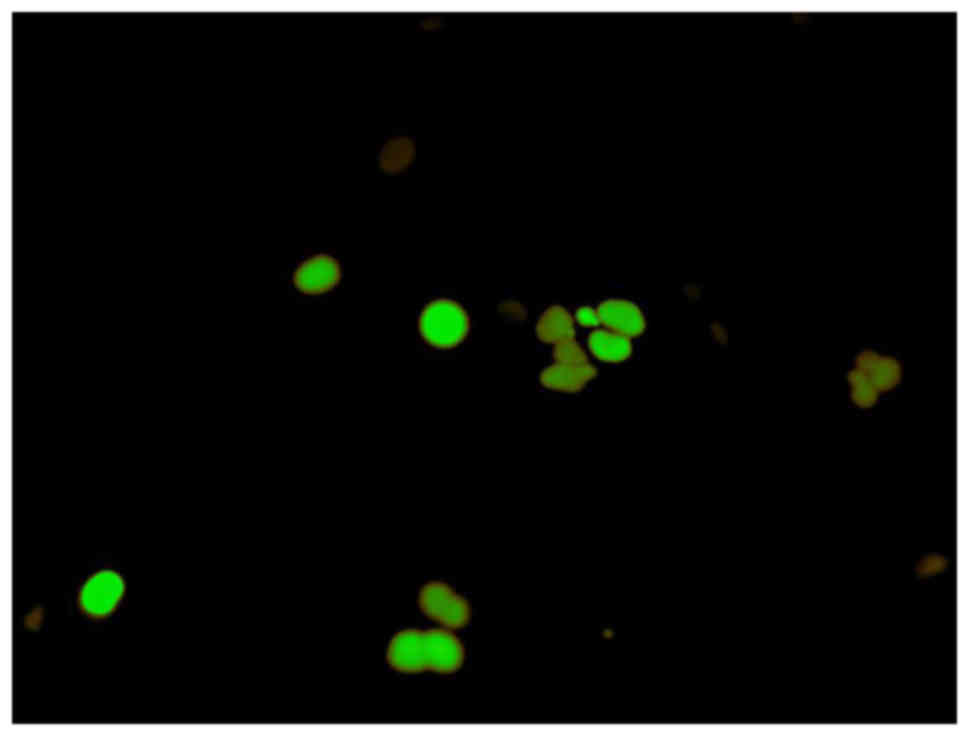
Analysis of cell cycle arrest
To determine the stage of the cell cycle at which
the SAS cells were arrested, they were treated with α-mangostin (20
µM) + rhTRAIL (100 ng/ml) for 24 h, and then examined using
Fluorescent Ubiquitination-based Cell Cycle Indicator (FUCCI).
FUCCI is a fluorescent cell cycle indicator that harnesses the
ubiquitination oscillators that control cell cycle transitions. The
original FUCCI probe was generated by fusing mAG (monomeric
Azami-Green) and mKO2 (monomeric Kusabira-Orange 2) to
the ubiquitination domains of human Geminin and Cdt1, respectively.
These two chimeric proteins, mAG-hGem and mKO2-hCdt1, accumulate
reciprocally in the nucleus of transfected cells during the cell
cycle, labeling the nuclei of the S/G2/M-phase cells green and
those in the G1 phase red. An mAG-hGem and an mKO2-hCdt1 expression
vector were purchased from Medical and Biological Laboratories
(Nagoya, Japan). To establish SAS cells transiently expressing mAG
or mKO2, the parent cells were transfected with each plasmid using
Lipofectamine (Invitrogen, Carlsbad, CA, USA) for 24 h. The
transfected cells were then treated with α-mangostin (20 µM) +
rhTRAIL (100 ng/ml) or PBS as a control for 24 h and observed by
fluorescence microscopy.
Cytochrome c detection
Cytochrome c was detected using a cytochrome
c apoptosis detection kit with an mAb cytochrome c
antibody according to the manufacturer's instructions. Briefly,
after induction of apoptosis in the SAS cells, the cells were
collected by centrifugation at 600 × g for 5 min at 4°C and washed
with 10 ml of ice-cold PBS. Subsequently the cells were resuspended
in 1.0 ml of 1× cytosol extraction buffer mix containing DTT and
protease inhibitors and incubated on ice for 10 min. The cells were
homogenized by 50 passes with a Dounce tissue grinder on ice. The
homogenate was transferred to a 1.5-ml microcentrifuge tube, and
centrifuged at 700 × g for 10 min at 4°C. The supernatant was
collected into a fresh 1.5-ml tube as the cytosolic fraction and
centrifuged at 10,000 × g for 30 min at 4°C. The pellet was
resuspended in 0.1 ml of mitochondrial extraction buffer mix
containing DTT and protease inhibitors, vortexed for 10 sec and
saved as the mitochondrial fraction. Ten micrograms each of the
cytosolic and mitochondrial fractions isolated from
apoptosis-uninduced and -induced cells were then subjected to 10%
SDS-PAGE, followed by standard western blotting and probed with
anti-cytochrome c mAb (1 µg/ml).
Primary tumor samples
Formalin-fixed, paraffin-embedded specimens were
obtained from 40 patients with squamous cell carcinoma (SCC)
treated at the Department of Oral and Maxillofacial Surgery, Meikai
University Hospital, Japan. The pathological diagnosis of oral
lesions was based on histological examination of hematoxylin and
eosin (H&E)-stained slides and made according to the WHO
classification (15). The
postsurgical TNM stage was determined according to the pTNM
pathological classification of the International Union Against
Cancer (UICC) (16). All specimens
were obtained by surgical biopsy. None of the patients had
undergone preoperative chemotherapy or radiotherapy. The labeling
index was defined as the percentage of tumor cells displaying
immunoreactivity and calculated by counting the number of
c-Myc-positive cells among 1,000 tumor cells in each section.
Tissue sections with <5% immunoreactive cells were defined as
negative and those with >5% immunoreactive cells were defined as
positive.
Immunohistochemical examination
For c-Myc staining, the sections were immersed in
absolute methanol containing 0.3% H2O2 for 20
min at room temperature to block endogenous peroxidase activity.
After washing, each section was immersed in 0.01 M citrate buffer
(pH 6.0) and heated in a microwave oven for 15 min as described by
Shi et al (17). After
washing with PBS (pH 7.4), the sections were incubated in 2%
BSA-PBS for 15 min at room temperature to block non-specific
reactions. Diluted anti-c-Myc mAb (1:50) was applied to each
section for 60 min at room temperature. After washing with PBS, the
slides were incubated with goat anti-rabbit IgG (H+L) for c-Myc
(1:200) for 30 min at room temperature. Diluted
streptavidin-peroxidase (1:1,000) was then applied to the sections
for 30 min. After washing, the sections were immersed for 10 min in
0.05% 3,3′-diaminobenzidine tetrahydrochloride in 0.05 M Tris-HCl
buffer (pH 7.6) containing 0.01% H2O2 and
then counterstained with Mayer's hematoxylin.
Ethical considerations
The study was approved by the Research Ethics
Committee of the Meikai University School of Dentistry, Saitama,
Japan (reference no. A0801).
Results
Detection of the c-Myc gene expression
in HOSCC cell lines
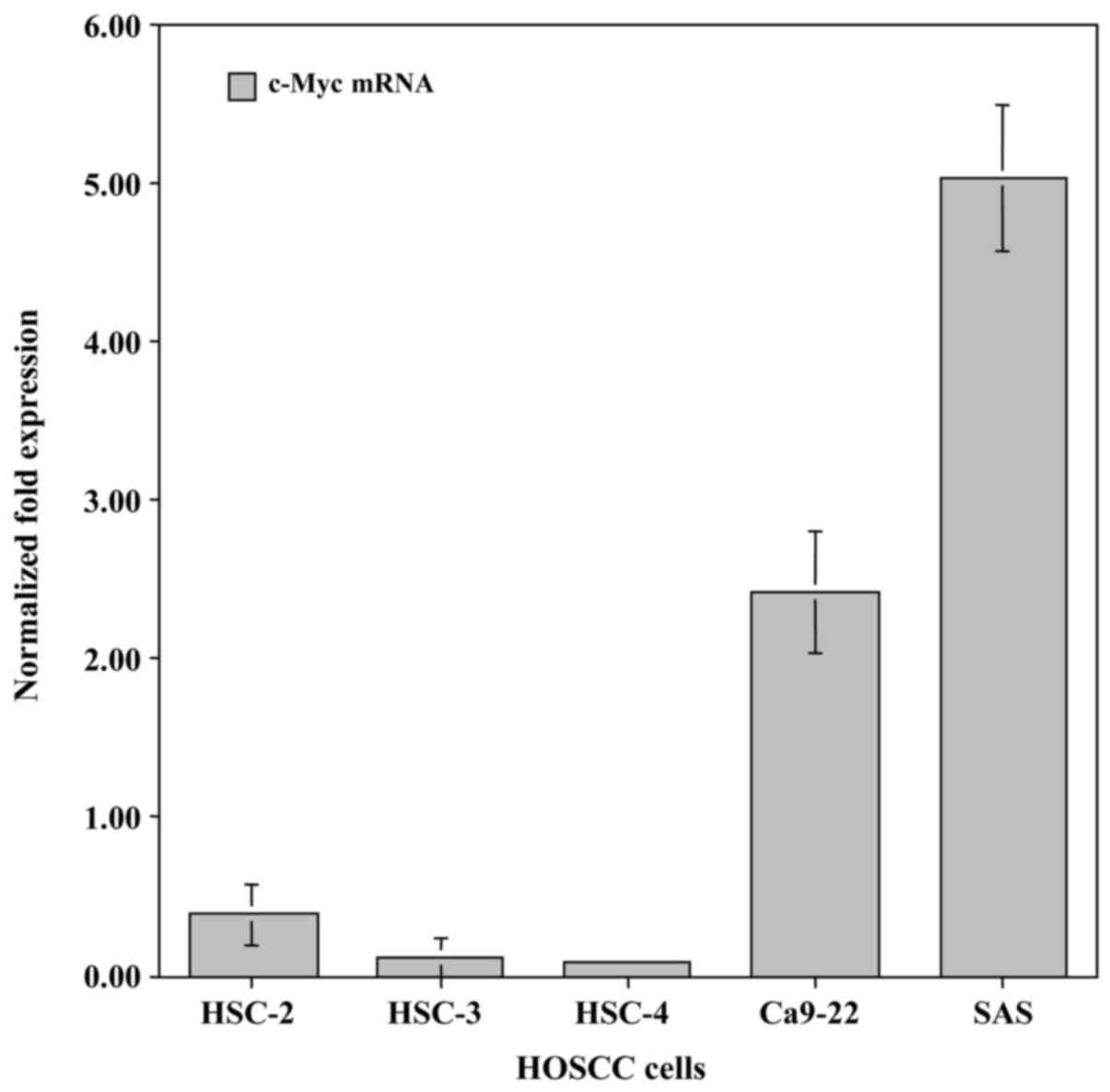
To examine differences in the expression of the
c-Myc gene in HOSCC cell lines (HSC-2, HSC-3, HSC-4, Ca9-22 and
SAS), qRT-PCR analysis was carried out using specifically designed
primer pairs. c-Myc mRNA was endogenously expressed in all of the
HOSCC cell lines. The highest level of expression was exhibited in
the SAS cells and lowest in the HSC-4 cells (Fig. 1).
Expression of the c-Myc protein in
HOSCC cell lines
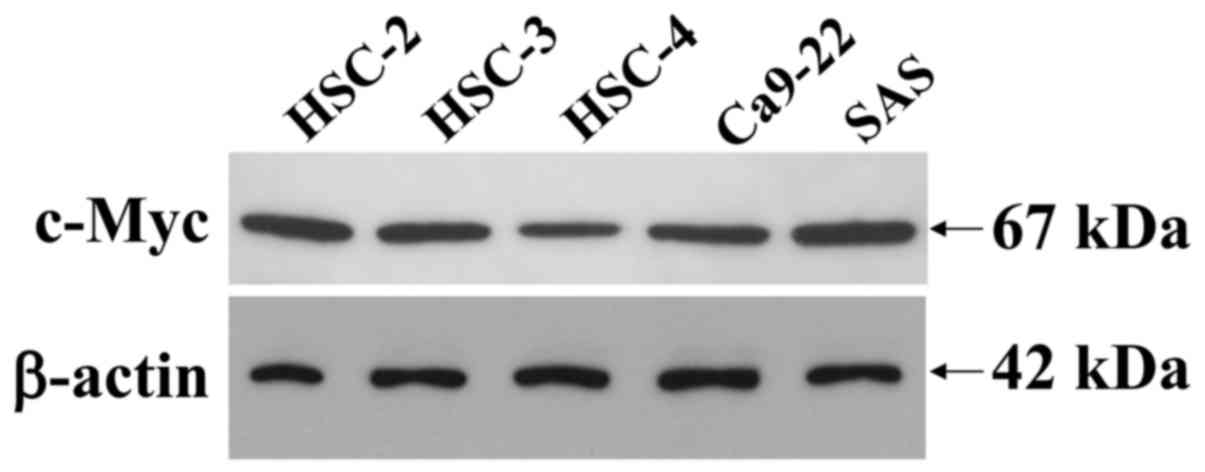
SDS-solubilized extracts of the HOSCC cell lines
were subjected to immunoblot analysis to determine the presence and
quantity of c-Myc protein expression. Immunoblot analysis revealed
that the c-Myc expressed in the cell lines was a 67-kDa peptide and
that it was clearly expressed in all of the HOSCC cell lines,
although the weakest expression was observed in the HSC-4 cells
(Fig. 2). The β-actin protein was
used as an internal control, to assess the integrity of the protein
extracted from the HOSCC cell lines, thus demonstrating that the
protein obtained was intact.
Growth inhibition of oral cancer cells
expressing c-Myc after combined treatment with α-mangostin and
rhTRAIL
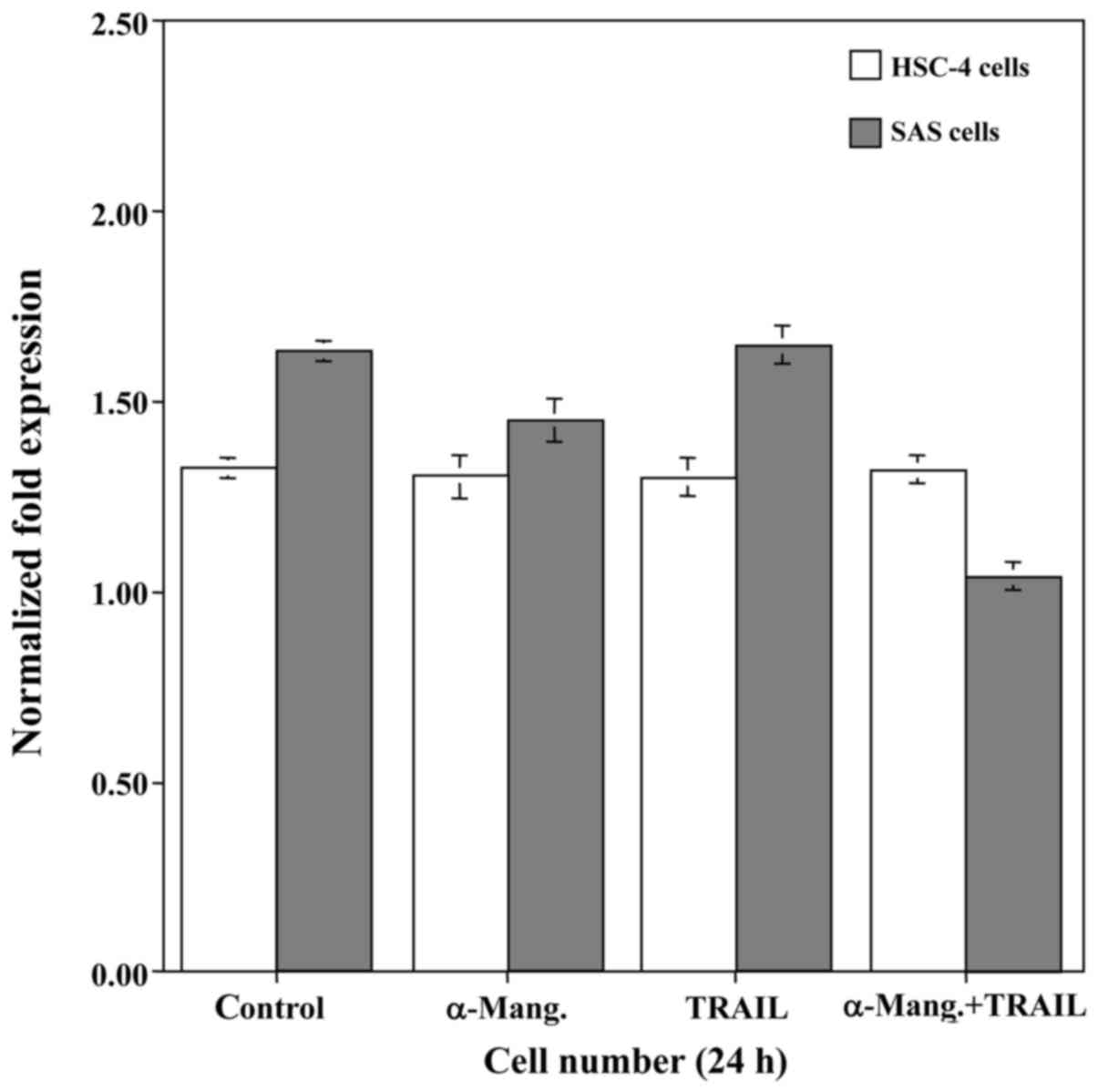
From the results of the qRT-PCR, we attempted to
determine whether α-mangostin induced cell death of SAS and HSC-4
cells by carrying out a cell viability assay (Fig. 3). The treatment with α-mangostin had
a slight cytocidal effect on the SAS cells, whereas the viability
of the HSC-4 cells was unaffected. Subsequently, to explore a
possible adjuvant compound that would sensitize oral cancer cells
to TRAIL-induced apoptosis, we examined the effect of rhTRAIL on
SAS and HSC-4 cells. However, this had no effect on the viability
of either cell line. Therefore, we examined the synergistic
anti-proliferative effect of α-mangostin (20 µM) and rhTRAIL (100
ng/ml) on SAS and HSC-4 cells by incubating them with both for 24
h. This combination treatment with α-mangostin and rhTRAIL
significantly suppressed the proliferative activity of the SAS
cells, but not that of the HSC-4 cells. Accordingly, we focused on
the SAS cells, which demonstrated high expression of c-Myc.
Induction of apoptosis in SAS cells by
combined treatment with α-mangostin and rhTRAIL
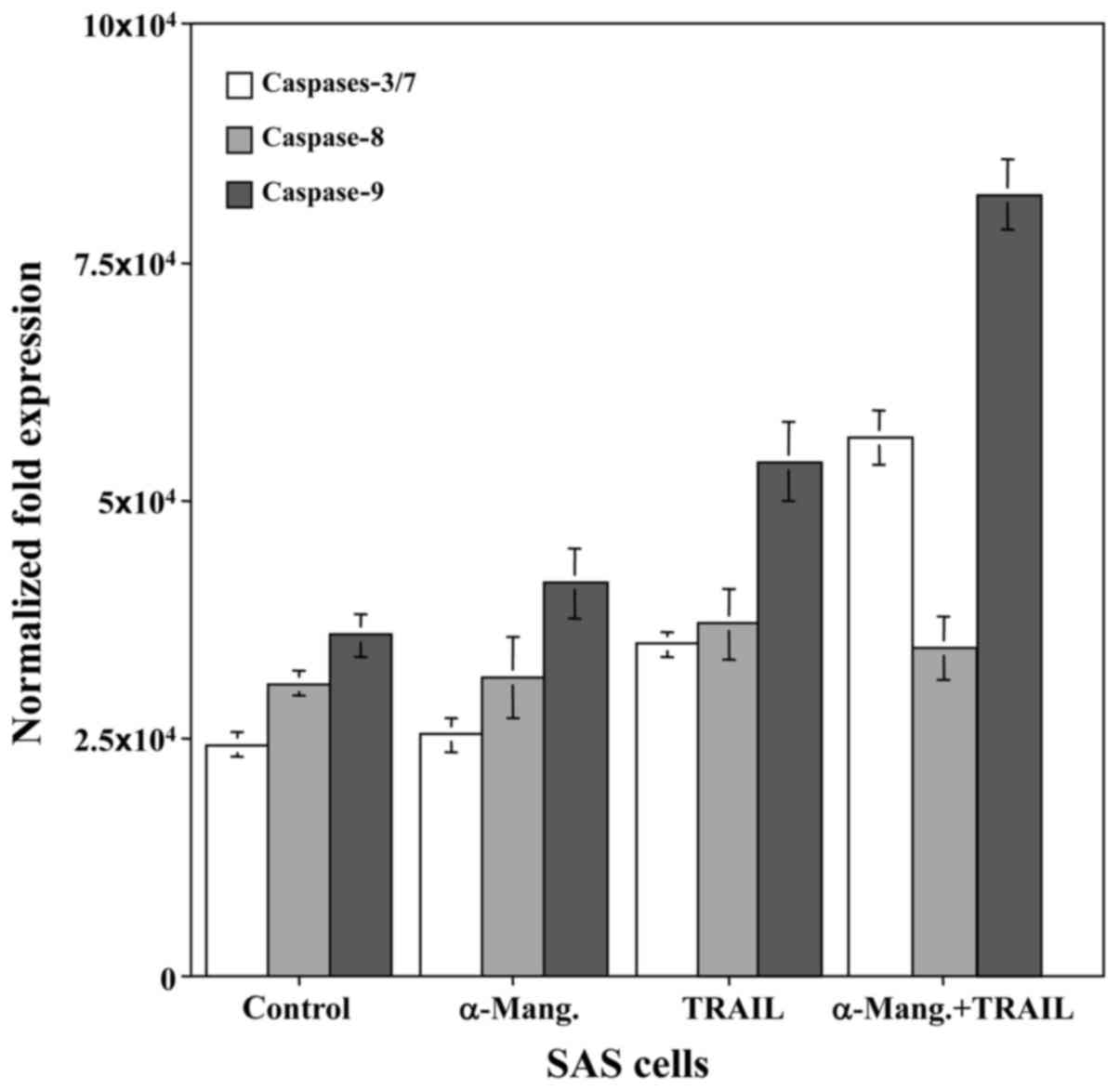
To determine whether caspase-dependent apoptotic
activity could be modulated by combined treatment with α-mangostin
and rhTRAIL, the SAS cells were treated with α-mangostin (20 µM)
and rhTRAIL (100 ng/ml), or left untreated as a control, for 24 h.
Subsequently, we examined the cleavage of procaspase to active
caspase-8, −9 and −3/7, markers of apoptotic activity, in SAS
cells. The levels of caspase-9 and −3/7 were increased in response
to α-mangostin and rhTRAIL, which revealed more than a two-fold
increase of activity at 24 h, in the SAS cells relative to the
control (Fig. 4). This finding
indicated that the combined treatment with α-mangostin and rhTRAIL
led to apoptosis of the SAS cells via activation of caspase-3/7 and
−9. We also used FUCCI to determine which stage of the cell cycle
arrest occurred. This revealed that treatment with both α-mangostin
and TRAIL induced S/G2/M-phase arrest and not G1-phase arrest, in
SAS cells (Fig. 5).
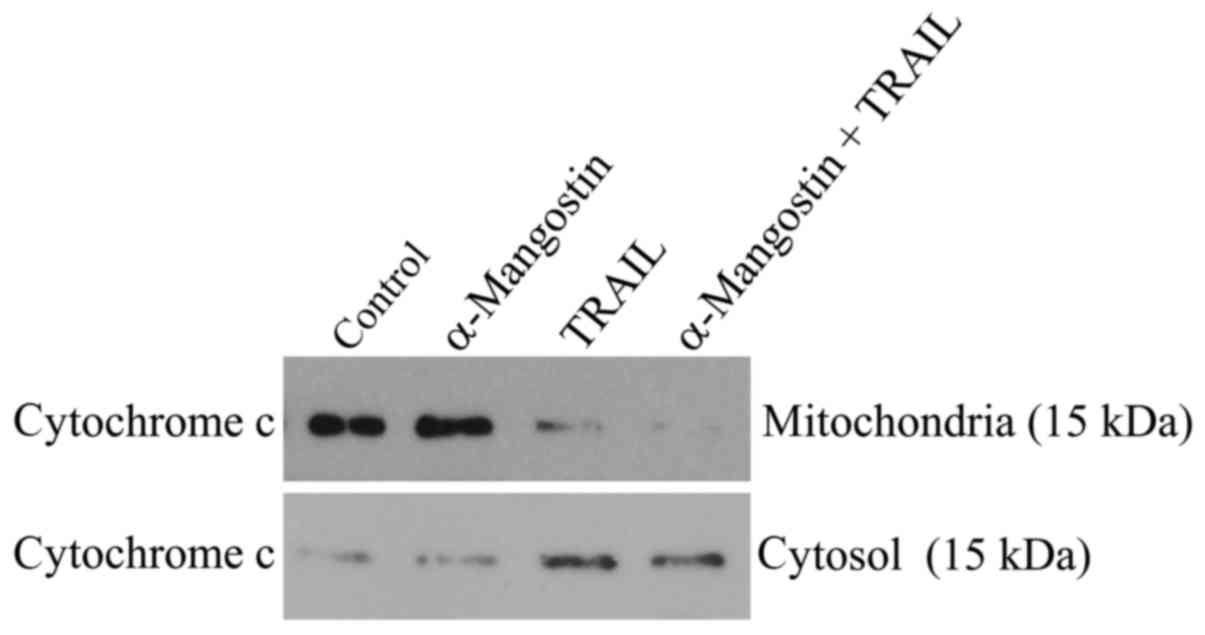
Release of cytochrome c from the
mitochondria into the cytosol
Release of cytochrome c from the mitochondria
is a critical step in the apoptosis cascade, since this activates
downstream caspases. To investigate the release of cytochrome
c in SAS cells treated with α-mangostin and rhTRAIL, we
conducted western blot analysis of both the cytosolic and
mitochondrial fractions. After treatment with α-mangostin and
rhTRAIL for 24 h, cytochrome c was detectable in the
cytosolic fraction at a markedly higher level than in the control
(Fig. 6). In contrast, cytochrome
c protein was decreased in the mitochondrial fraction,
indicating release of cytochrome c from the mitochondria
into the cytosol.
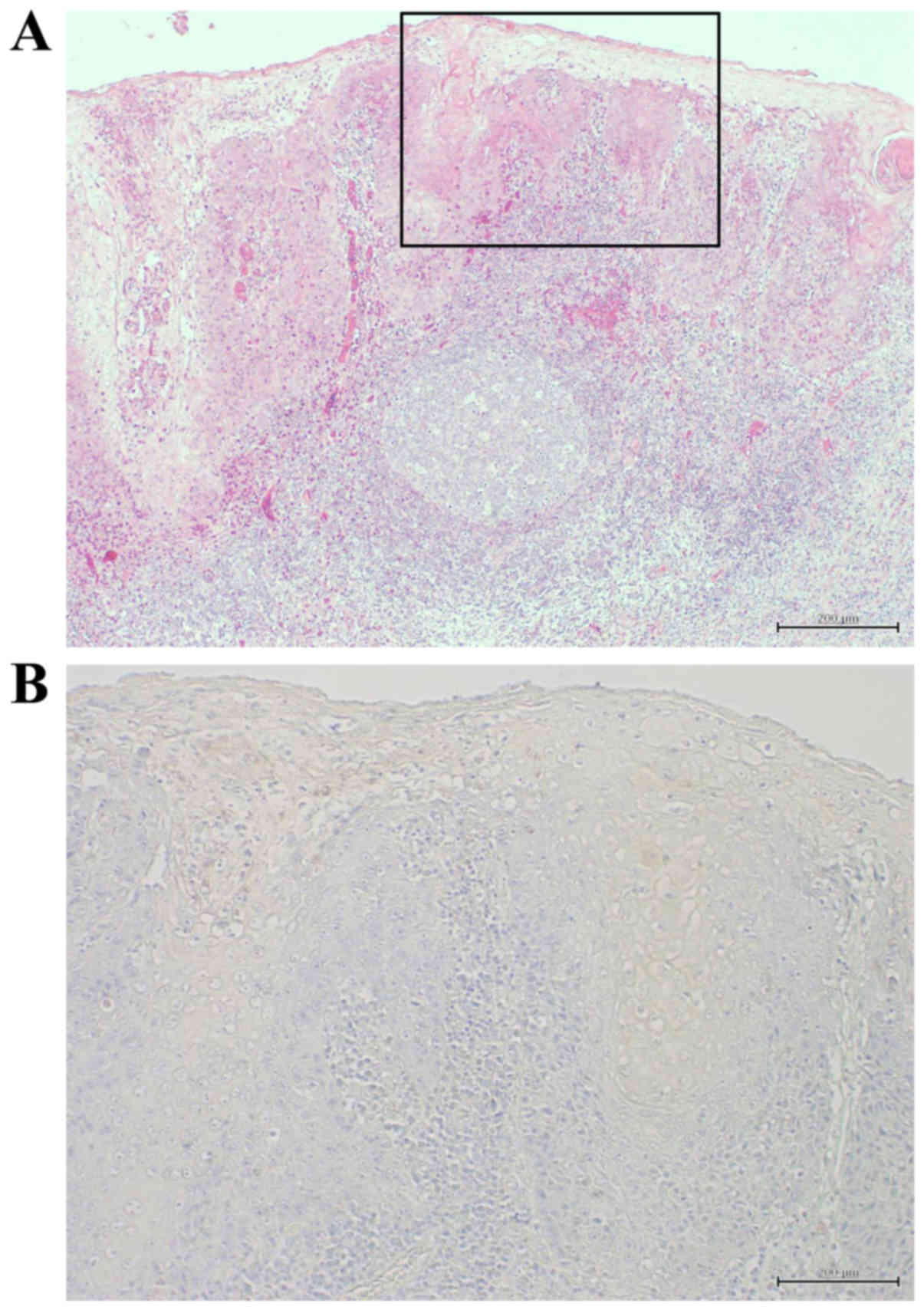
Immunohistochemical detection of c-Myc
and clinicopathological variables in HOSCC tissues
The correlations between the c-Myc expression and
the clinicopathological variables in HOSCC tissues are summarized
in Table I and Fig. 7A reveals an H&E-stained specimen
of well-differentiated HOSCC. Immunohistochemical detection of
c-Myc was carried out in 40 cases of HOSCC at various stages. There
were no correlations between the c-Myc expression and the
clinicopathological variables. Positivity for c-Myc was observed in
the cytoplasm of the tumor cells (Fig.
7B) in 16 of the 40 cases (40%).
 | Table I.Correlation between the expression of
c-Myc and clinicopathological variables in 40 cases of HOSCC. |
Table I.
Correlation between the expression of
c-Myc and clinicopathological variables in 40 cases of HOSCC.
| No. | Age (years) | Sex | Location | Differentiation | pTNM | Stage | IHC, c-Myc |
|---|
| 1 | 87 | M | Oral floor | Well | T2N2bM0 | IVA | + |
| 2 | 48 | F | Gingiva | Well | T4N0M0 | IVA | − |
| 3 | 56 | F | Buccal mucosa | Well | T2N0M0 | II | − |
| 4 | 75 | M | Tongue | Well | T4N2cM0 | IVA | + |
| 5 | 55 | M | Oral floor | Well | T2N0M0 | II | + |
| 6 | 54 | M | Tongue | Well | T4N2bM0 | IVA | − |
| 7 | 54 | M | Oral floor | Well | T2N2aM0 | IVA | − |
| 8 | 70 | M | Tongue | Well | T2N0M0 | II | − |
| 9 | 67 | M | Maxillary
gingiva | Well | T4N3M0 | IVB | − |
| 10 | 92 | M | Soft palate | Well | T2N0M0 | II | − |
| 11 | 66 | M | Tongue | Well | T2N0M0 | II | − |
| 12 | 87 | M | Tongue | Well | T2N0M0 | II | − |
| 13 | 48 | F | Tongue | Well | T1N0M0 | I | − |
| 14 | 85 | M | Tongue | Well | T2N1M0 | III | + |
| 15 | 56 | F | Tongue | Well | T1N2bM0 | IVA | + |
| 16 | 67 | M | Mandibular
gingiva | Well | T1N2bM0 | IVA | + |
| 17 | 55 | M | Tongue | Well | T1N0M0 | I | + |
| 18 | 85 | M | Buccal mucosa | Well | T1N0M0 | I | + |
| 19 | 50 | M | Mandibular
gingiva | Well | T3N1M0 | III | + |
| 20 | 67 | M | Mandibular
gingiva | Well | T4N0M0 | IVA | + |
| 21 | 79 | M | Buccal mucosa | Well | T2N0M0 | II | + |
| 22 | 54 | M | Buccal mucosa | Well | T1N0M0 | I | − |
| 23 | 54 | M | Mandibular
gingiva | Well | T4N1M0 | IVA | − |
| 24 | 62 | M | Mandibular
gingiva | Well | T1N0M0 | I | + |
| 25 | 60 | M | Maxillary
gingiva | Well | T1N0M0 | I | + |
| 26 | 79 | M | Mandibular
gingiva | Moderate | T4N2cM0 | IVA | − |
| 27 | 85 | F | Tongue | Moderate | T2N1M0 | III | − |
| 28 | 54 | M | Tongue | Moderate | T1N0M0 | I | − |
| 29 | 54 | M | Tongue | Moderate | T4N2bM0 | IVA | + |
| 30 | 75 | M | Mandibular
gingiva | Moderate | T1N0M0 | I | − |
| 31 | 66 | F | Mandibular
gingiva | Moderate | T1N0M0 | I | − |
| 32 | 65 | M | Mandibular
gingiva | Moderate | T2N0M0 | II | − |
| 33 | 88 | M | Mandibular
gingiva | Moderate | T2N0M0 | II | − |
| 34 | 79 | M | Mandibular
gingiva | Moderate | T2N0M0 | II | − |
| 35 | 54 | M | Buccal mucosa | Moderate | T2N0M0 | II | + |
| 36 | 64 | M | Buccal mucosa | Poor | T2N0M0 | II | − |
| 37 | 62 | M | Tongue | Poor | T2N1M0 | III | − |
| 38 | 60 | M | Tongue | Poor | T2N1M0 | III | − |
| 39 | 60 | M | Tongue | Poor | T1N0M0 | I | − |
| 40 | 68 | M | Maxillary
gingiva | Poor | T3N0M0 | III | + |
Discussion
Oral cancer accounts for ~3–5% of all cancers
(18,19) and the clinical outcome of HOSCC has
gradually improved. However, unlike cancers in other parts of the
body, damage to the facial region can cause psychological disorders
in patients and negatively affect their daily life, particularly in
terms of eating or speaking. Therefore, there is a great interest
in exploring potential treatments for oral cancer (20). Oral cancer poses a risk of invasion
to adjacent organs and is associated with a high likelihood of
metastatic recurrence. Therefore, combinations of surgical therapy,
radiation and chemotherapy are usually employed. However, these
treatments have low efficacy against cancer cell growth and
micrometastasis and are associated with severe adverse effects and
thus, the prognosis remains poor (21). It would therefore be valuable to
find naturally-derived substances that specifically target cancer
cells, exerting potent anticancer effects with limited side
effects.
A large number of natural products have already been
evaluated as potential chemopreventive or therapeutic agents, and
among them, paclitaxel, etoposide, camptothecin and vincristine
have also been applied as anticancer drugs. Among various
phytochemicals, polyphenols (including xanthones) hold considerable
promise as chemopreventive agents because of their antioxidative
and potential anticancer activities (22,23). A
previous study has demonstrated the potent antiproliferative
activity of four xanthones (α-mangostin, β-mangostin, γ-mangostin
and methoxy-β-mangostin) isolated from the pericarp of mangosteen,
against human leukemia HL60 cells (5). α-Mangostin in particular, has recently
been demonstrated to induce cell-cycle arrest and apoptosis in
various types of human cancer cells (6–9). It
has also been demonstrated to inhibit the invasion and migration of
mammary and prostate cancer cells, associated with the
downregulation of MMP-2 and MMP-9 (24,25).
Furthermore, α-mangostin has been shown to induce mitochondrial
dysfunction in human leukemia HL60 cells (7). However, the mechanisms responsible for
the apoptosis induced by α-mangostin in oral cancer cells remain
unknown. α-Mangostin has also been reported to exert
chemopreventive effects against chemically induced colon cancer via
reduction of the c-Myc expression (5).
Cytokines are a large family of secreted proteins
that bind to and signal through defined cell surface receptors on a
wide variety of target cells and play a pivotal role in the
establishment and maintenance of homeostasis. Many cytokines share
structural features and functions during development, immune
responses or inflammation. TRAIL/Apo2L has been identified as a
member of the TNF superfamily (10). TRAIL is a type 2 transmembrane
protein that functions in extracellular signaling through the DRs,
DR4 and/or DR5 (11). When
stimulated by this ligand, DRs recruit Fas-associated death domain
(FADD) protein and its initiator caspase-8, resulting in formation
of the death-inducing signaling complex (DISC). The recruited
caspase-8 undergoes autocatalytic cleavage and activation to
trigger the cascade that ultimately leads to apoptosis (26). With virtually no toxicity toward
normal cells, recombinant human TRAIL or agonistic antibodies
specifically targeting DR5 are currently being tested in several
clinical trials. However, their application for anticancer
treatment is limited because of their short half-life in serum and
the development of resistance to TRAIL-induced apoptosis (12). Moreover, it has been reported
(13) that α-mangostin functions as
a sensitizer of TRAIL-induced apoptosis and can become a possible
adjuvant compound for cytokine therapy to overcome TRAIL resistance
in cancer cells. Accordingly, we hypothesized that a combination
treatment with α-mangostin and TRAIL may induce apoptosis in cancer
cells, especially those expressing c-Myc.
In the present study, qRT-PCR and immunoblot
analysis were initially performed to confirm the expression of
c-Myc in five HOSCC cell lines. The results indicated that the
highest level of c-Myc expression was exhibited in SAS cells and
the lowest in HSC-4 cells. To investigate whether α-mangostin would
induce cell death in SAS and HSC-4 cells, a cell viability assay
was performed. Treatment with α-mangostin had a slightly cytocidal
effect on the SAS cells, whereas it had no effect on the viability
of the HSC-4 cells. Subsequently, to ascertain whether TRAIL would
be effective as a cytokine therapy against oral cancer, we examined
the effect of rhTRAIL on SAS and HSC-4 cells, however no effect on
the viability of either cell line was observed. Therefore, to
explore the possibility that α-mangostin may sensitize oral cancer
cells to TRAIL-induced apoptosis, a treatment with a combination of
α-mangostin and rhTRAIL was carried out. We found that this
significantly suppressed the proliferative activity of SAS cells,
but not that of HSC-4 cells. Subsequently, we focused on SAS cells,
which demonstrated high expression of c-Myc. To confirm whether the
reduction in the number of SAS cells was due to apoptosis, caspase
activities in the cells were analyzed and revealed that the
combination of α-mangostin and rhTRAIL led to the activation of
caspase-9 and −3/7. Furthermore, it was observed that the cell
cycle of the SAS cells was arrested at the S/G2/M phase during the
process of apoptotic cell death. There are two major pathways that
trigger apoptosis: the extrinsic and the intrinsic pathway. The
extrinsic, or death-receptor, pathway is mediated by a
death-inducing signaling complex comprised of a Fas-associated
death domain and procaspase-8, leading to activation of caspase-8
and subsequently the downstream caspases (27). The intrinsic, or mitochondrial,
pathway involves loss of mitochondrial membrane potential and
release of cytochrome c into the cytosol, where it binds to
Apaf-1, allowing the recruitment of caspase-9 and the formation of
an apoptosome complex, leading to caspase-3 activation and
execution of apoptosis (28). Most
microtubule inhibitors such as paclitaxel, doxetaxel, vincristine,
vinblastine and colchicine induce growth arrest, subsequent
inactivation of Bcl-2 and finally, apoptotic cell death through the
caspase-9-dependent pathway (29).
A previous study has demonstrated that α-mangostin activated
caspase-9 and −3, but not −8, in HL60 cells, indicating that
α-mangostin may mediate the mitochondrial pathway of apoptosis
(7). The findings of our present
study were similar: combination treatment with α-mangostin and
rhTRAIL led to activation of the proteolytic degradation products
of caspase-9 and −3/7. In contrast, the level of caspase-8 remained
the same, indicating that apoptosis was induced through the
caspase-dependent mitochondrial pathway. Furthermore, cytochrome
c release from mitochondria is a critical step in the
apoptotic cascade, since this activates downstream caspases. To
investigate the release of cytochrome c in SAS cells treated
with α-mangostin and rhTRAIL, we conducted western blot analysis of
both the cytosolic and mitochondrial fractions. After treatment
with α-mangostin and rhTRAIL, cytochrome c was detectable in
the cytosolic fraction of SAS cells at a markedly higher level than
in the control. In contrast, cytochrome c protein was
decreased in the mitochondrial fraction, indicating the release of
cytochrome c from mitochondria into the cytosol. If TRAIL
induced apoptotic death in SAS cells, caspase-8 would be activated
through the death-receptor pathway. However, in the present study
we found that caspase-9 was activated. In addition, release of
cytochrome c from the mitochondria into the cytosol was also
increased. Therefore, the mechanism responsible for apoptotic death
in SAS cells remained unclear. On the basis of the present
findings, we further hypothesized that TRAIL functioned as a
sensitizer of α-mangostin-induced apoptosis. Furthermore,
immunopositivity for the c-Myc protein was observed in 16 (40%) of
the 40 cases of HOSCC we examined and therefore a suitable approach
for investigation of cases that are c-Myc-negative remains to be
devised.
In conclusion, we revealed that combined treatment
of the SAS oral cancer cell line, which exhibits high expression of
c-Myc, with α-mangostin and TRAIL leads to reduction of tumor
growth. This antitumor effect, which involves induction of
apoptosis and cell cycle arrest in the S/G2/M phase, may have a
potential application as an immune-cytokine therapy against
TRAIL-resistant cells. Further investigations of the role of
α-mangostin will be required to fully elucidate the mechanism of
its anticancer effect and for the establishment of an
α-mangostin-based therapeutic strategy for oral cancer.
References
|
1
|
Johnson JJ: Carnosol: A promising
anti-cancer and anti-inflammatory agent. Cancer Lett. 305:1–7.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Johnson JJ, Bailey HH and Mukhtar H: Green
tea polyphenols for prostate cancer chemoprevention: A
translational perspective. Phytomedicine. 17:3–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Johnson JJ and Mukhtar H: Curcumin for
chemoprevention of colon cancer. Cancer Lett. 255:170–181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ji X, Avula B and Khan IA: Quantitative
and qualitative determination of six xanthones in Garcinia
mangostana L. by LC-PDA and LC-ESI-MS. J Pharm Biomed Anal.
43:1270–1276. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akao Y, Nakagawa Y, Iinuma M and Nozawa Y:
Anti-cancer effects of xanthones from pericarps of mangosteen. Int
J Mol Sci. 9:355–370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Matsumoto K, Akao Y, Ohguchi K, Ito T,
Tanaka T, Iinuma M and Nozawa Y: Xanthones induce cell-cycle arrest
and apoptosis in human colon cancer DLD-1 cells. Bioorg Med Chem.
13:6064–6069. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsumoto K, Akao Y, Yi H, Ohguchi K, Ito
T, Tanaka T, Kobayashi E, Iinuma M and Nozawa Y: Preferential
target is mitochondria in alpha-mangostin-induced apoptosis in
human leukemia HL60 cells. Bioorg Med Chem. 12:5799–5806. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Moongkarndi P, Kosem N, Kaslungka S,
Luanratana O, Pongpan N and Neungton N: Antiproliferation,
antioxidation and induction of apoptosis by Garcinia
mangostana (mangosteen) on SKBR3 human breast cancer cell line.
J Ethnopharmacol. 90:161–166. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakagawa Y, Iinuma M, Naoe T, Nozawa Y and
Akao Y: Characterized mechanism of alpha-mangostin-induced cell
death: Caspase-independent apoptosis with release of endonuclease-G
from mitochondria and increased miR-143 expression in human
colorectal cancer DLD-1 cells. Bioorg Med Chem. 15:5620–5628. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wiley SR, Schooley K, Smolak PJ, Din WS,
Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA,
et al: Identification and characterization of a new member of the
TNF family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Griffith TS, Stokes B, Kucaba TA, Earel JK
Jr, VanOosten RL, Brincks EL and Norian LA: TRAIL gene therapy:
From preclinical development to clinical application. Curr Gene
Ther. 9:9–19. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan K, Sun Y, Zhou T, McDonald J and Chen
Y: PARP-1 regulates resistance of pancreatic cancer to TRAIL
therapy. Clin Cancer Res. 19:4750–4759. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumazaki M, Shinohara H, Taniguchi K, Ueda
H, Nishi M, Ryo A and Akao Y: Understanding of tolerance in
TRAIL-induced apoptosis and cancelation of its machinery by
α-mangostin, a xanthone derivative. Oncotarget. 6:25828–25842.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukuda M, Horiuchi Y, Oku Y, Ishikawa M,
Suka N, Suzuki S, Kusama K and Sakashita H: Induction of apoptosis
in human salivary gland tumor cells by anti-NCAM antibody. Oncol
Rep. 14:1143–1149. 2005.PubMed/NCBI
|
|
15
|
Pindborg JJ, Reichart PA, Smith CJ and van
der Waal I: Histological Typing of Cancer and Precancer of the Oral
MucosaWorld Health Organization International Histological
Classification of Tumors. 2nd edition. Springer-Verlag Berlin
Heidelberg; New York: pp. 11–38. 1997
|
|
16
|
Sobin LH and Wittekind CH: International
Union Against Cancer (UICC) TNM Classification of Malignant Tumors.
6th edition. John Wiley and Sons, Inc.; New York: 1997
|
|
17
|
Shi SR, Key ME and Kalra KL: Antigen
retrieval in formalin-fixed, paraffin-embedded tissues: An
enhancement method for immunohistochemical staining based on
microwave oven heating of tissue sections. J Histochem Cytochem.
39:741–748. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Neville BW and Day TA: Oral cancer and
precancerous lesions. CA Cancer J Clin. 52:195–215. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Silverman S Jr: Early diagnosis of oral
cancer. Cancer. 62 Suppl 8:1796–1799. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim MY, Kim CS, Lee SH, Kim JW and Jang
HJ: A clinicostatistical analysis of oral cancer patients for
recent 8 years. J Korean Assoc Oral Maxillofac Surg. 33:660–668.
2007.
|
|
21
|
Lee EJ, Kim MJ and Myoung H: Change of the
invasiveness with selective Cox-2 inhibition in an oral squamous
cell carcinoma cell line, KB: Preliminary in vitro study. J Korean
Assoc Oral Maxillofac Surg. 33:103–108. 2007.
|
|
22
|
Sun J, Chu YF, Wu X and Liu RH:
Antioxidant and antiproliferative activities of common fruits. J
Agric Food Chem. 50:7449–7454. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Temple NJ and Gladwin KK: Fruit,
vegetables, and the prevention of cancer: Research challenges.
Nutrition. 19:467–470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hung SH, Shen KH, Wu CH, Liu CL and Shih
YW: Alpha-mangostin suppresses PC-3 human prostate carcinoma cell
metastasis by inhibiting matrix metalloproteinase-2/9 and
urokinase-plasminogen expression through the JNK signaling pathway.
J Agric Food Chem. 57:1291–1298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee YB, Ko KC, Shi MD, Liao YC, Chiang TA,
Wu PF, Shih YX and Shih YW: alpha-Mangostin, a novel dietary
xanthone, suppresses TPA-mediated MMP-2 and MMP-9 expressions
through the ERK signaling pathway in MCF-7 human breast
adenocarcinoma cells. J Food Sci. 75:H13–H23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim SY, Kim JH and Song JJ: c-Cbl
shRNA-expressing adenovirus sensitizes TRAIL-induced apoptosis in
prostate cancer DU-145 through increases of DR4/5. Cancer Gene
Ther. 20:82–87. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cho SG and Choi EJ: Apoptotic signaling
pathways: Caspases and stress-activated protein kinases. J Biochem
Mol Biol. 35:24–27. 2002.PubMed/NCBI
|
|
28
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang LG, Liu XM, Kreis W and Budman DR:
The effect of antimicrotubule agents on signal transduction
pathways of apoptosis: A review. Cancer Chemother Pharmacol.
44:355–361. 1999. View Article : Google Scholar : PubMed/NCBI
|