Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer in males and the second in females worldwide
(1). The incidence rates of CRC are
rapidly increasing in several areas including Eastern European
countries and most parts of Asia (2,3).
However, the biological and molecular mechanisms underlying CRC
development remain largely unclear. Zinc finger protein 278
(ZNF278), also named POZ/BTB and AT-hook-containing zinc finger
protein (PATZ), is a transcription factor with seven C2H2-type zinc
fingers (4). ZNF278 belongs to the
Krueppel C2H2-type zinc finger protein family. It is a novel zinc
finger protein, which is ubiquitously distributed in human tissues.
Although the physiological role of ZNF278 is not clear, some
experimental evidence suggests that it is a potential transcription
suppressor (4,5). Rearrangement of ZNF278 was involved in
small round cell sarcoma (6).
Indeed, our previous study revealed that ZNF278 expression was
increased in 53% of CRC tissues compared to corresponding
non-cancerous tissues. The functional study revealed that ZNF278
promoted cell growth and its knockdown suppressed cell
proliferation, indicating that ZNF278 could be a potential
proto-oncogene in CRC (7). However,
the molecular mechanism of ZNF278 in CRC remains unclear. In the
present study, we analyzed the expression of cyclin D1 and E1 by
ZNF278-siRNA transfection. Knockdown of ZNF278 induced cell cycle
arrest in CRC cell lines. The present study revealed that depletion
of ZNF278 may decrease the proliferation of CRC cells via
inhibition of the ERK/MAPK pathway.
Materials and methods
Cell culture and PD98059
treatment
The human CRC cell line HT29 was maintained in
McCoy's 5A medium and the CRC cell line SW1116 in RPMI-1640 medium
(both from Gibco, Gaithersburg, MD, USA) supplemented with 10 %
fetal bovine serum at 37°C in a 5% CO2 incubator. For
treatment with PD98059 (Cell Signaling Technology, Danvers, MA,
USA), the cells were incubated with 20 µM PD98059 for 48 h before
harvesting for assessments.
Real-time PCR for four different
ZNF278 variants
Total RNA was isolated using TRIzol reagent
(Invitrogen/Gibco-BRL, Carlsbad, CA, USA). Total RNA (1 µg) was
reverse transcribed using the PrimeScript RT reagent kit (Perfect
Real-Time; Takara, Tokyo, Japan) to detect relative mRNAs. Relative
quantitative data were obtained using the comparative Ct method on
an Applied Biosystem 7900 quantitative PCR system (Applied
Biosystems, Foster City, CA) according to the manufacturer's
protocol. The specific primers for ZNF278 variants were as follows:
variant 1 (GenBank accession no. NM_032050) F,
5′-GCAGTATCTGTAACCGAGAAGGC-3′ and R, 5′-ATTTCCCTTCAGGCCCCATG-3′;
variant 2 (NM_032051) F, 5′-GAGGGTTGACAGTGGAAGGG-3′ and R,
5′-ATTTGGGGGCTCTGACATGG-3′; variant 3 (NM_032052) F,
5′-GCAGTATCTGTAACCGAGGTCTC-3′ and R, 5′-CGGACATGCACCTTCTGGAT-3′;
variant 4 (NM_014323) F, 5′-AAAACCCACCACGGTGTTCC-3′ and R,
5′-CATTTCCCTTCAGGCCCCAT-3′. The Ct values obtained from different
samples were compared using the 2−ΔΔCt method. GAPDH
served as an internal reference gene and the results were presented
as the ratio of copies of target genes to GAPDH.
Construction of expression vectors and
transfection
To construct the wild-type ZNF278 (GenBank accession
no. NM_032050) expression vector, a PCR-generated full length
ZNF278 cDNA was inserted into the EcoRI-HindIII sites
of the expression vector CMV-MCS-3FLAG-SV40-Neomycin. Nested PCR
was carried out to amplify the full-length ZNF278 cDNA. The
following primers were used: F1, 5′-CGGCGCACCTGCGAGACTACAGA-3′ and
R1, 5′-TCCCAGCAGTCCCCAGATGGTTGT-3′ for the first PCR; and F2,
5′-CCCAAGCTTCCATGGAGCGGGTGAAC-3′ and R2,
5′-CCGGAATTCTTTCCCTTCAGGCCCCAT-3′ for the second PCR. Before
transfection, 5×105 HT29 and SW1116 cells were seeded in
6 cm wells. The cells were transfected with 2 µg of either ZNF278
or control plasmid, using FuGENE HD transfection reagent (Promega,
Madison, WI, USA), in accordance with the manufacturer's
instructions. The cells were collected for measurements 48 h after
transfection.
RNA interference and transient
transfections
All transfections were performed using DharmaconFECT
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), according to
the manufacturer's instructions. ZNF278 small interfering RNA
(siRNA) (sense, 5′-GCGCCGAUAUAAUGCUCUUTT-3′ and antisense,
5′-AAGAGCAUUAUAUCGGCGCGG-3′); and negative control siRNA (sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′) were designed and synthesized by
GenePharma (Shanghai, China). ERK2 siRNA was sense,
5′-CACUUGUCAAGAAGCGUUAdTdT-3′ and antisense,
5′-CACUUGUCAAGAAGCGUUAdTdT-3′. Non-specific siRNAs (GenePharma)
were used as the negative controls. The siRNA was complexed with
the transfection reagent in a serum- and antibiotic-free medium for
6 h. After applying the transfection reagents, the cellular medium
was replaced with the serum-containing maintenance medium, and the
cells were incubated for 48 h. Gene expression silencing was
confirmed by western blotting analysis.
Western blot analysis
Western blotting was performed according to the
standard protocols, as previously described (8). The primary antibodies used in the
present study were as follows: anti-ZNF278 (1:1,000; ab126903;
Abcam, Cambridge, UK); anti-p-MEK1/2 (1:2,000; CST9121s);
anti-p-ERK1/2 (CST4370s), anti-ERK1/2 (CST9102s), anti-MEK1/2
(CST9122s), anti-cyclin E1 (CST4129s), anti-cyclin D1 (CST2978s)
and anti-p21 (CST2947T) (1:1,000) (all from Cell Signaling
Technology). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH;
KC-5G5; KangChen, Shanghai, China) was used as a loading control.
The blots were analyzed using ImageJ 1.43 software and protein
expression was normalized to GAPDH and relative to the control.
Cell viability assay
Cell viability was assessed by a tetrazolium salt
(WST-8)-based colorimetric assay called Cell Counting Kit-8 (CCK-8;
Dojindo, Kumamoto, Japan). Briefly, 5×103 cells were
transfected with ERK2 siRNA or negative control siRNA after 24 h of
culture in 96-well plates. At the specified time-points, 10 µl of
CCK-8 solution was added to each well and the cells were incubated
for 1 h. Then, cell viability was determined by measuring the
absorbance values at 450 nm using a microplate reader. Data are
expressed as the percentage of viable cells, calculated as:
relative viability (%) = [A450 (treated) - A450 (blank)]/[A450
(control) - A450 (blank)] × 100%.
Statistical analysis
Data are expressed as the mean ± SEM. Differences
between two groups were compared using the Student's t-test. All
experiments were repeated at least three times. P-values <0.05
were considered statistically significant.
Results
Effect of ZNF278 siRNA and plasmid
transfection on the cell cycle in CRC cells
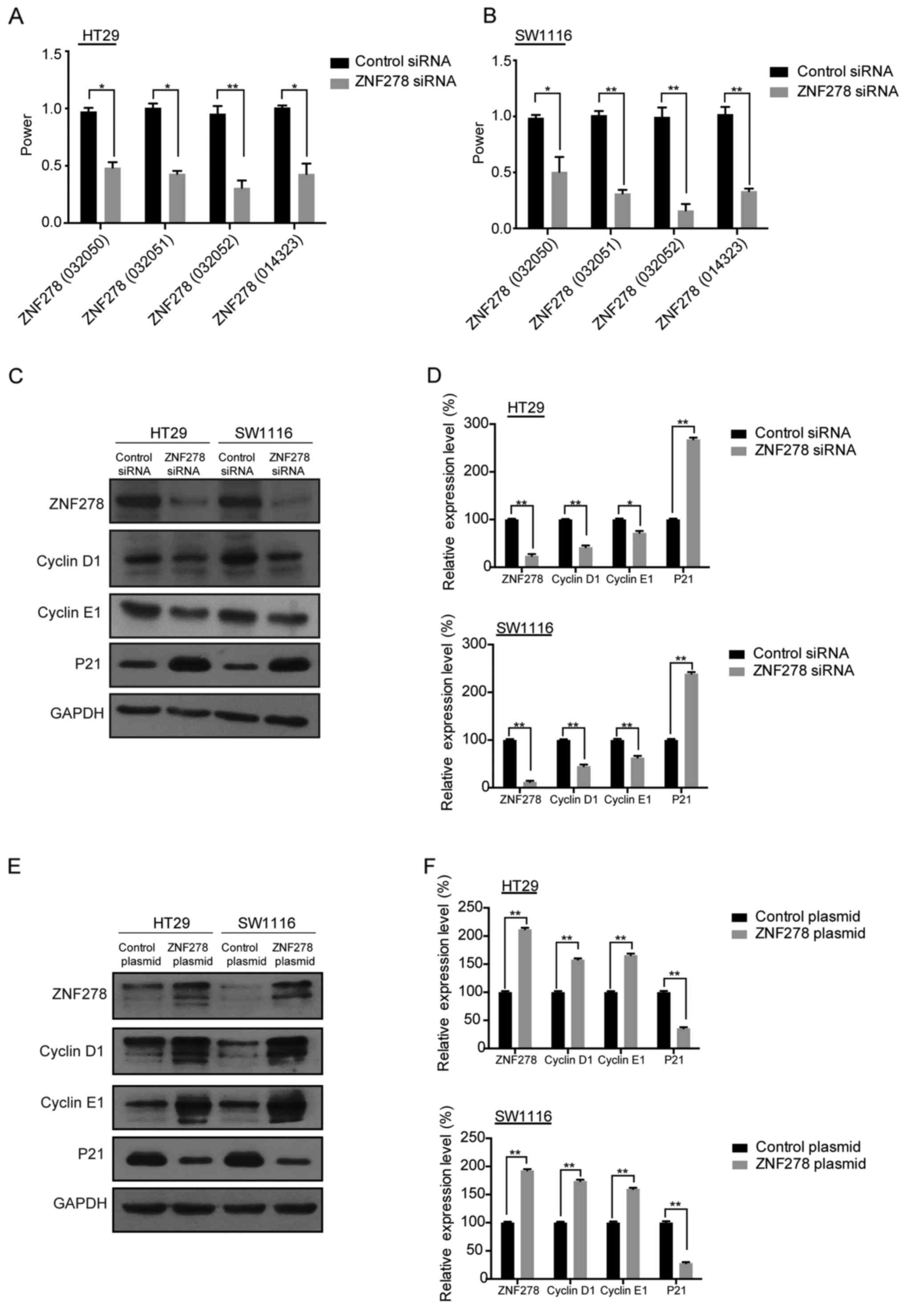
To confirm which variants of ZNF278 were decreased
after ZNF278-siRNA transfection, four pairs of specific primers
were designed and synthesized. It was identified that all four
ZNF278 variants were downregulated by ZNF278-siRNA transfection
(Fig. 1A and B). As a previous
study revealed overexpression of ZNF278 in the cells transfected
with the pcDNA3.1-ZNF278 plasmid significantly increased the
percentage of the S phase cells and decreased the percentage of the
G0/G1 phase cells. Knockdown of ZNF278 expression significantly
blocked the cell cycle at the G0/G1 phase (7). The cell cycle analysis revealed that
G1 phase arrest was detected in SW1116 cells after depletion of
ZNF278, suggesting that knockdown of ZNF278 induced cell cycle
arrest. Furthermore, we examined the protein expression of cyclin
E1 and D1 and p21, the three key genes involved in cell cycle
progression (9,10). Expression of cyclin E1 and cyclin D1
were significantly decreased and p21 was increased after
transfection of ZNF278 siRNA compared with the control siRNA in
HT29 and SW1116 cells (Fig. 1C and
D). Conversely, upregulation of cyclin E1 and D1 and
downregultion of p21 were detected by ZNF278 plasmid transfection
(Fig. 1E and F). The aforementioned
data indicated that ZNF278 was involved in the regulation of CRC
cell cycle progression.
ZNF278 siRNA decreases the
proliferation of CRC cells via inhibition of the ERK/MAPK
pathway
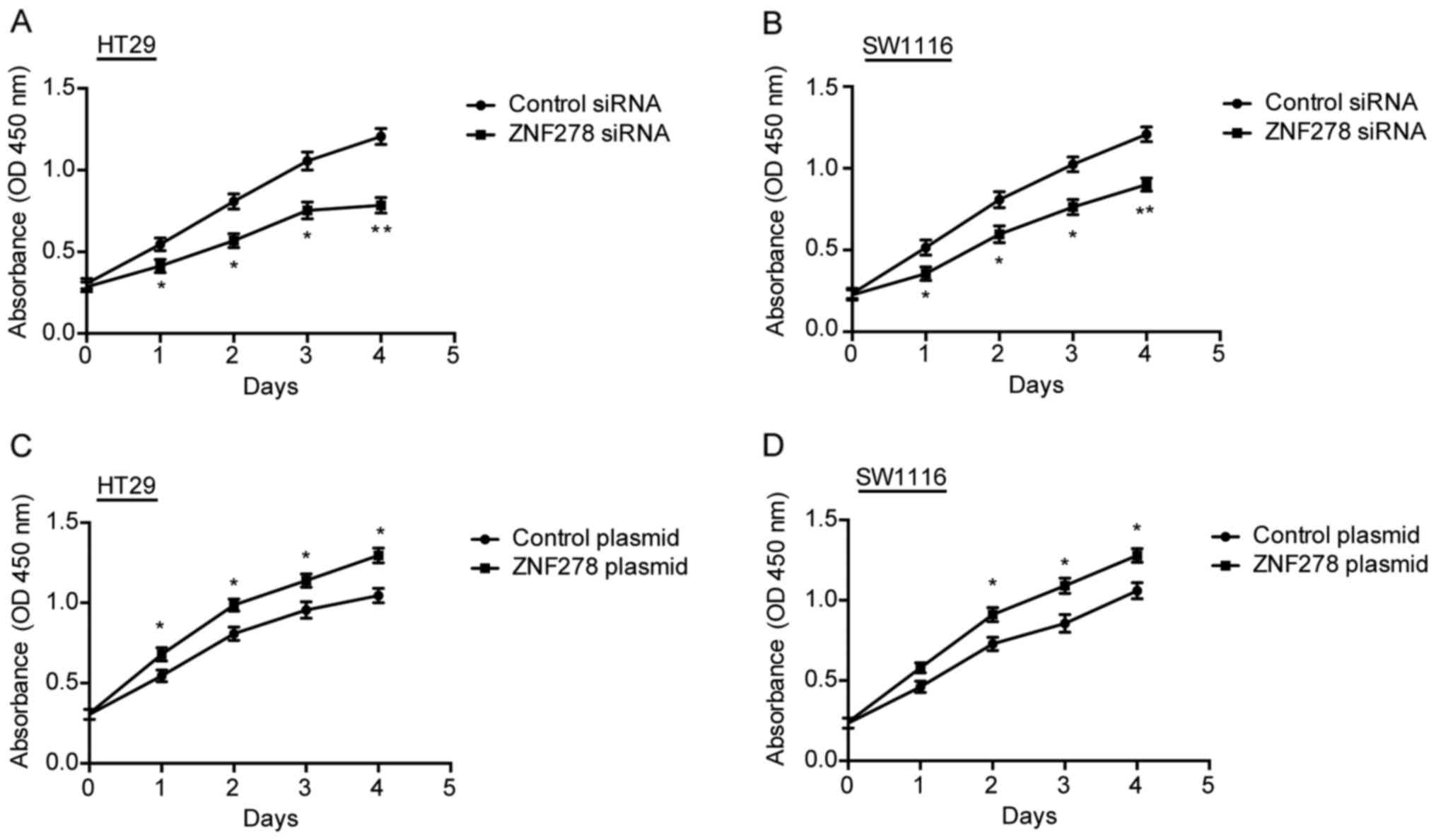
We previously demonstrated that transfection of the
SW1116 cells with pcDNA3.1-ZNF278 promoted cell growth, and
ZNF278-siRNA transfection resulted in a significant inhibition of
cell growth (7). In the present
study, we further ensured this result in HT29 and SW1116 CRC cells
using the CCK-8 assay. Knockdown of ZNF278 significantly inhibited
cell proliferation compared to the control siRNA-transfected cells
in both HT29 (Fig. 2A) and SW1116
(Fig. 2B) cells. Conversely,
ectopic overexpression of ZNF278 accelerated cell proliferation in
HT29 (Fig. 2C) and SW1116 cells
(Fig. 2D). To elucidate the
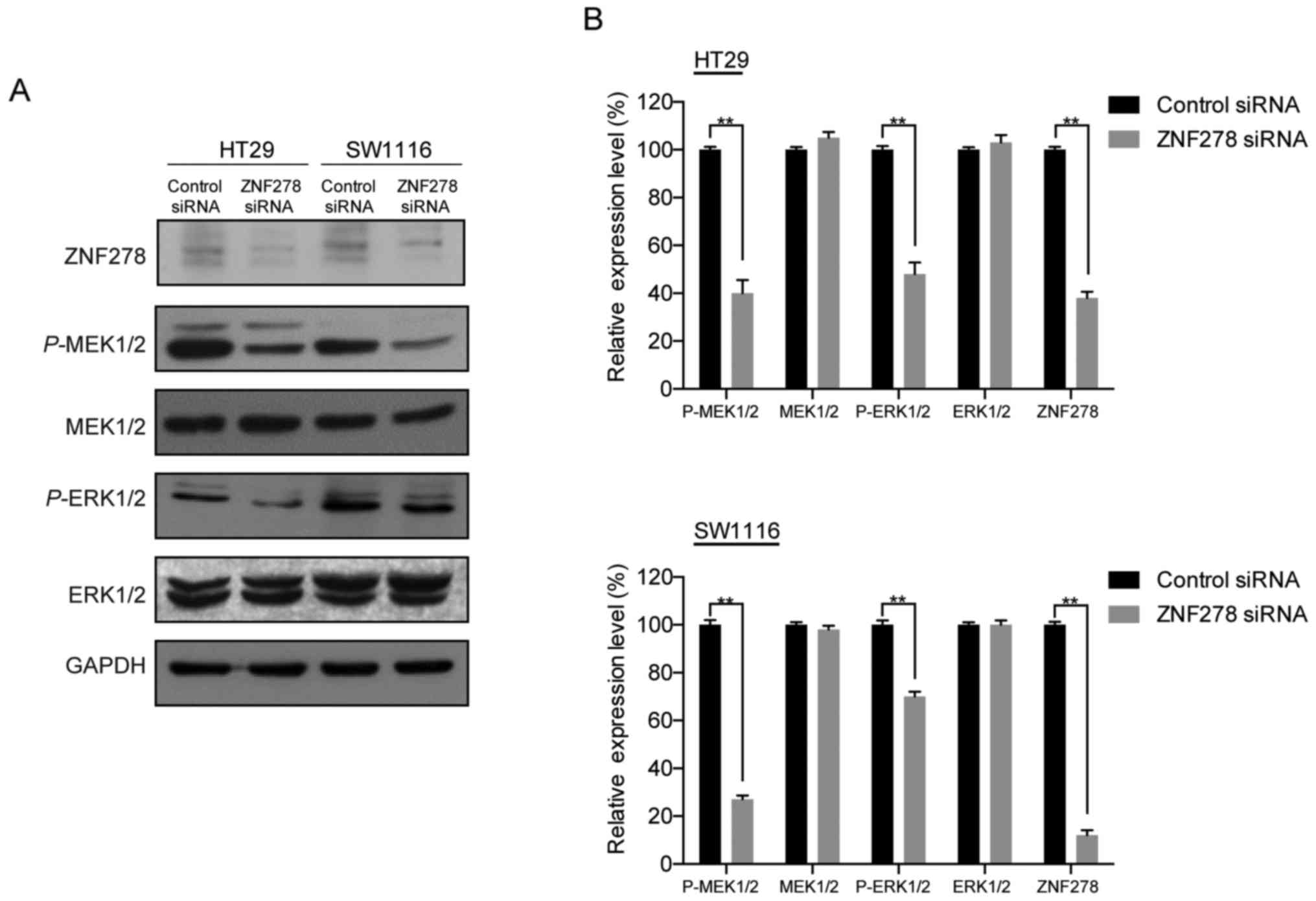
molecular mechanisms by which ZNF278-modulated CRC cell
proliferation, we investigated the effects of knockdown of ZNF278
on the extracellular signal-regulated kinase/mitogen-activated
protein kinase (ERK/MAPK) pathway, which is frequently aberrantly
activated in human cancers and contributes to cell proliferation
and survival (11,12). Western blotting revealed that
transfection with ZNF278 siRNA significantly decreased ERK1/2 and
MEK1/2 phosphorylation in both HT29 and SW1116 cells. However, no
detectable changes in the total levels of ERK1/2 and MEK1/2 were
observed (Fig. 3A and B). In
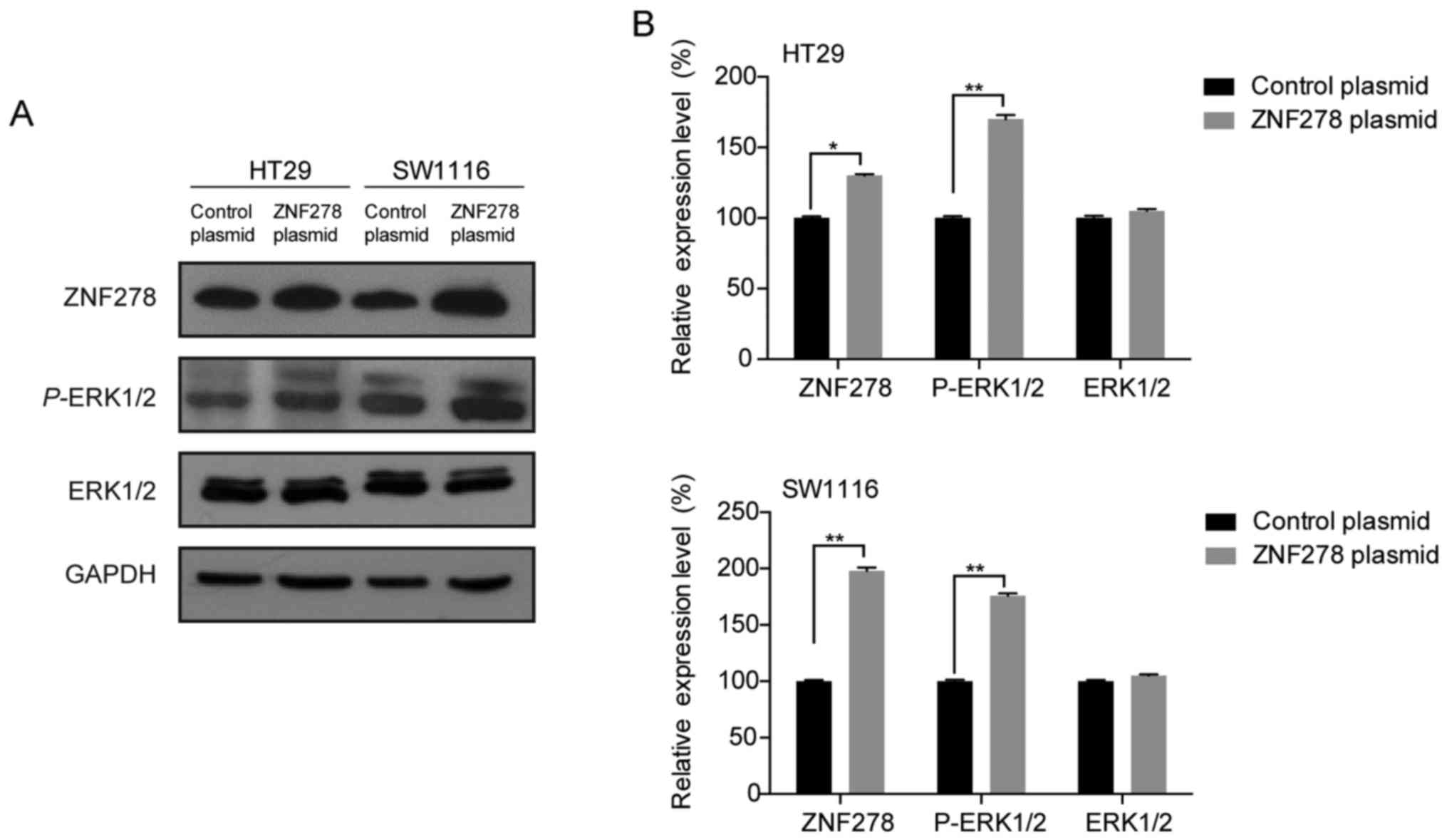
contrast, ectopic overexpression of ZNF278 induced activation of
ERK1/2 and promoted cell proliferation in HT29 and SW1116 cells
(Fig. 4A and B). Furthermore,
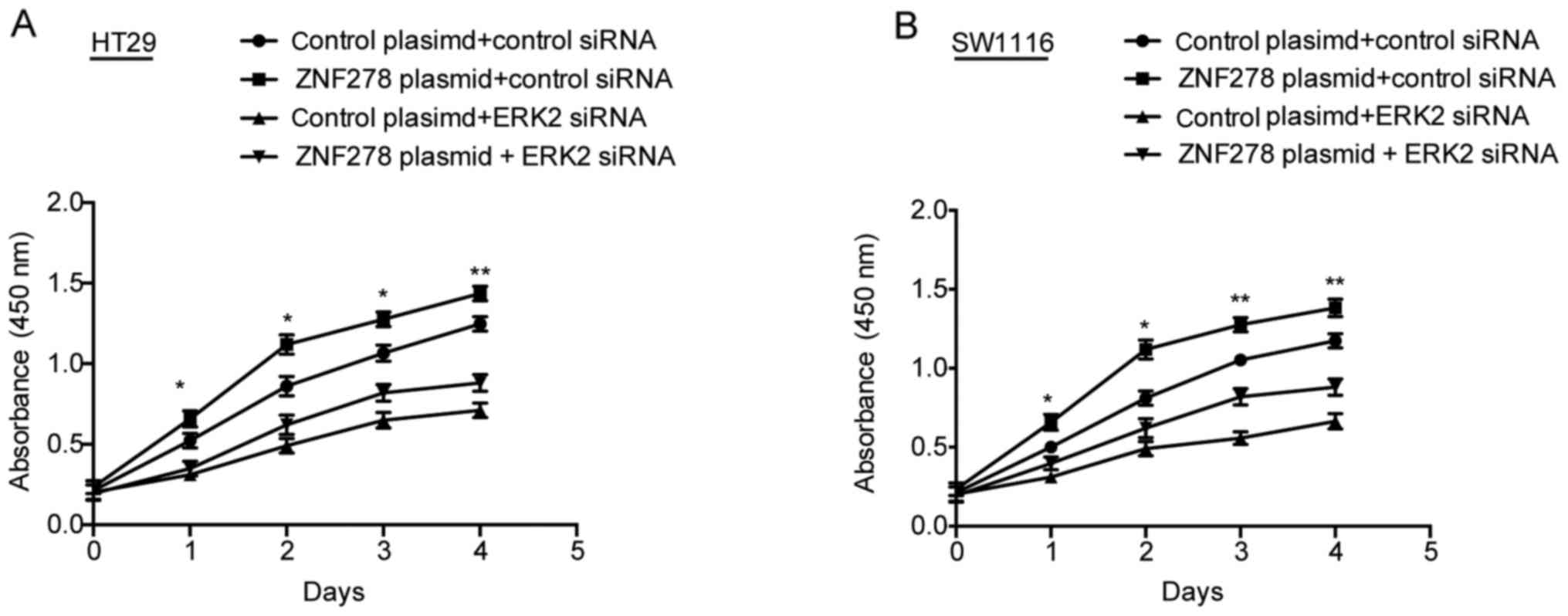
ZNF278 overexpression-induced CRC cell proliferation was markedly
blocked by ERK2-siRNA transfection (Fig. 5). Thus, these data suggested that
the ERK/MAPK pathway may participate in ZNF278-induced cell
proliferation in CRC cells.
The ERK/MAPK pathway may not regulate
the expression of ZNF278
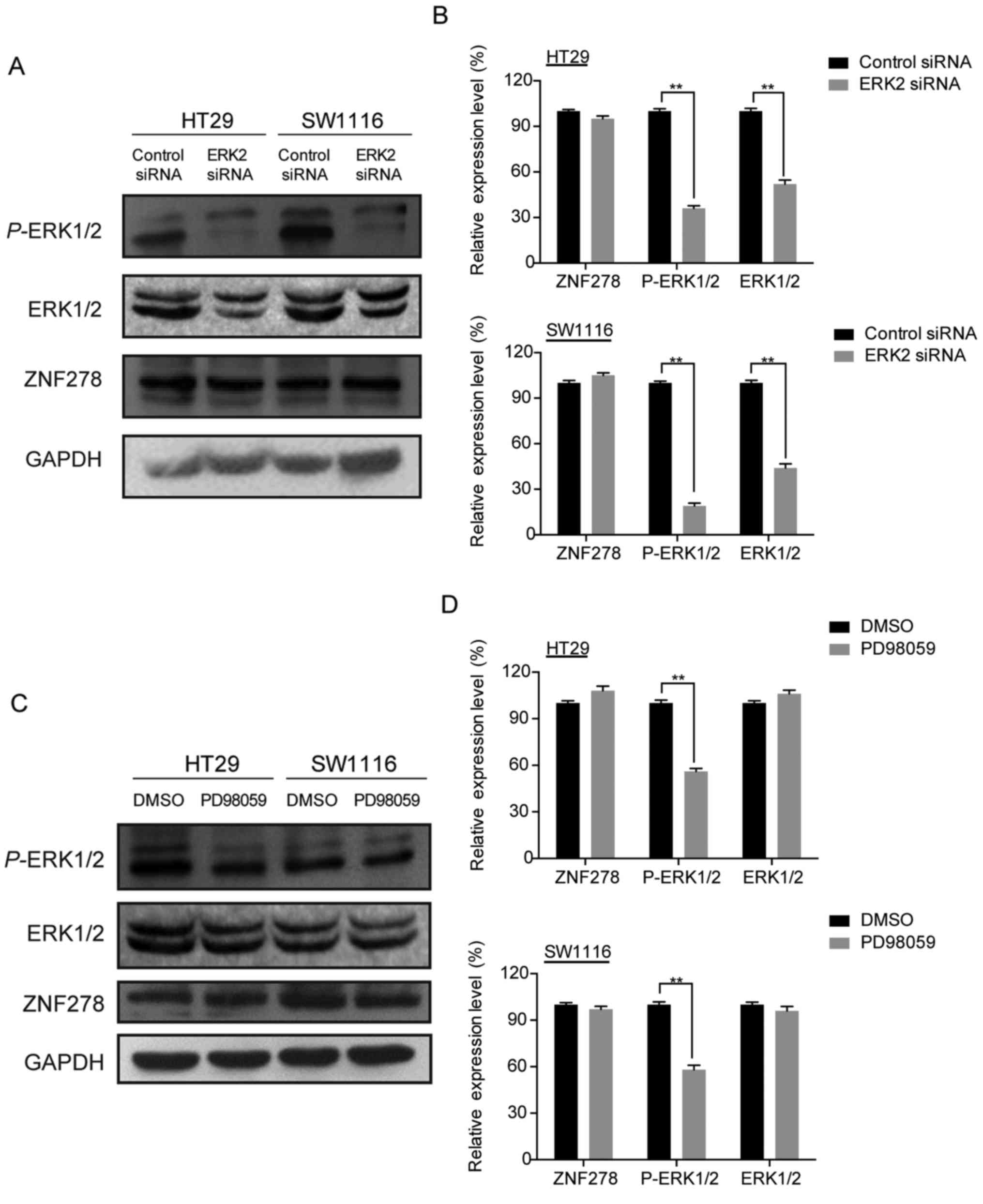
We demonstrated that downregulation of ZNF278
inhibited the ERK/MAPK pathway. To investigate whether reciprocal
regulation of ZNF278 by the ERK-MAPK pathway occurs, we assessed
the expression of ZNF278 in HT29 and SW1116 cells treated with
PD98059 (a MEK1 inhibitor) or ERK2 siRNA using western blotting
(13,14). Neither PD98059 nor ERK2 siRNA
significantly affected the expression of ZNF278 in either HT29 or
SW1116 cells (Fig. 6), which
indicated that the ERK/MAPK signaling pathway may not participate
in the regulation of ZNF278 expression.
Discussion
Colorectal cancer (CRC) is a common malignant tumor
worldwide, with the incidence increasing in Asian countries
(15) Aberrant gene expression is
involved in colorectal carcinogenesis (16,17).
The ZNF278 protein contains an AT-hook DNA-binding motif that
usually binds to other DNA-binding structures to play an important
role in chromatin modeling and transcription regulation. Its Poz
domain is thought to function as a site for protein-protein
interaction and is required for transcriptional suppression
(4,5). ZNF278 belongs to the C2H2-type zinc
finger protein family. Various studies have supported that
C2H2-type zinc finger proteins regulate cell proliferation, growth,
differentiation, and carcinogenesis (18–21).
The ZNF278 protein has typical features of a transcription factor.
Recently, a study indicated a critical role of ZNF278 in the
control of cell growth and embryonic development (22). Based on previous research, ZNF278
was considered to be an important factor in the physiological
state. It has been confirmed that aberrant expression of ZNF278 was
related to the development of some diseases, but its cancer-related
function as an oncogene or tumor-suppressor remains unclear. In has
been reported that, the rearrangement of the ZNF278 gene was
detected in small round cell sarcoma (6). Moreover, ZNF278 could be a molecular
biomarker and potential target for therapeutic strategies in human
testicular germ cell tumors (23).
Another study revealed that ZNF278 could be a potential
tumor-suppressor in thyroid cancer progression (24). We speculated that ZNF278 plays a
role as an oncogene or a tumor-suppressor depending on the
different molecules interacting with ZNF278 in different tissue
cells. ZNF278 may enhance apoptosis or cell survival depending on
the different cellular context.
In the previous study, we investigated the possible
roles of ZNF278 in colon carcinogenesis (7). We examined the ZNF278 expression level
in CRC tissues and corresponding non-cancerous tissues, and found
that the ZNF278 expression was significantly higher in cancer
tissues compared to the non-cancerous tissues. This revealed that
the upregulation of ZNF278 expression may contribute to colorectal
tumor carcinogenesis. In order to identify the function of ZNF278,
we constructed a wild-type ZNF278 expression vector and transfected
to the CRC cell line SW1116. We also performed transient
transfection in SW1116 cells with ZNF278 siRNA. In addition, we
studied the effects of ZNF278 on the biological functions of CRC
cells with regard to the overexpression and knockdown of ZNF278.
The data revealed that ZNF278 increased CRC cell proliferation, and
that the knockdown of ZNF278 suppressed cell proliferation and
arrested the cell cycle (7). Some
previous studies also focused on the effect of ZNF278 knockdown on
cell cycle and apoptosis. Ow et al reported that ZNF278
knockdown largely decreased upregulation of apoptotic genes and
downregulation of the cell cycle and cellular metabolism genes in
embryonic stem cells (ESCs) (25).
Tritz et al found that human glioma cells increased their
sensitivity to apoptotic stimuli in response to ZNF278 treatment
(26). In particular, ZNF278 is a
type of zinc finger protein and contains domains involved in
DNA-binding and protein-protein interactions (27). It is possible that ZNF278 is
involved in some important signaling pathways or regulates the
transcription of other important genes. In addition, recent studies
revealed that ZNF278 may play biological functions by suppressing
the p53 pathway (28–30). It is still unclear whether ZNF278 is
involved in other signaling pathways.
In the present study, we investigated the molecular
mechanisms by which downregulation of ZNF278 arrested the cell
cycle and decreased proliferation of CRC cells. It has been
demonstrated that the ERK/MAPK pathway is one of the most important
signal transduction pathways, and several key growth factors and
proto-oncogenes promote tumor growth by activating this signaling
cascade (31–35). Therefore, we examined the effects of
ZNF278 on the ERK/MAPK pathway. Knockdown of ZNF278 significantly
decreased ERK1/2 and MEK1/2 phosphorylation in HT29 and SW1116
cells; however, no detectable changes in total ERK1/2 and MEK1/2
protein expression were observed. In contrast, ectopic
overexpression of ZNF278 increased the phosphorylation of ERK1/2
and promoted HT29 and SW1116 cell proliferation. Moreover, ERK2
siRNA significantly abolished ZNF278 overexpression-induced HT29
and SW1116 cell proliferation. However, the direct link between
ZNF278 and the ERK/MAPK pathway warrants further investigation in a
future study.
In summary, we found that knockdown of ZNF278 could
arrest the CRC cell cycle by decreasing the expression levels of
cyclin D1 and E1 and increasing in the expression level of p21.
Furthermore, we demonstrated that knockdown of ZNF278 decreased the
cell proliferation of CRC cells via inhibition of the ERK/MAPK
pathway. Therefore, ZNF278 may serve as a diagnostic molecular
marker or potential therapeutic target for CRC treatment.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation (nos. 81421001, 81530072 and
81320108024) to J.-Y.F., and the National Natural Science
Foundation (no. 81001070) to D.-F.S.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pero R, Lembo F, Palmieri EA, Vitiello C,
Fedele M, Fusco A, Bruni CB and Chiariotti L: PATZ attenuates the
RNF4-mediated enhancement of androgen receptor-dependent
transcription. J Biol Chem. 277:3280–3285. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fedele M, Benvenuto G, Pero R, Majello B,
Battista S, Lembo F, Vollono E, Day PM, Santoro M, Lania L, et al:
A novel member of the BTB/POZ family, PATZ, associates with the
RNF4 RING finger protein and acts as a transcriptional repressor. J
Biol Chem. 275:7894–7901. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mastrangelo T, Modena P, Tornielli S,
Bullrich F, Testi MA, Mezzelani A, Radice P, Azzarelli A, Pilotti
S, Croce CM, et al: A novel zinc finger gene is fused to EWS
in small round cell tumor. Oncogene. 19:3799–3804. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tian X, Sun D, Zhang Y, Zhao S, Xiong H
and Fang J: Zinc finger protein 278, a potential oncogene in human
colorectal cancer. Acta Biochim Biophys Sin. 40:289–296. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu R, Wang X, Chen ZF, Sun DF, Tian XQ and
Fang JY: Inhibition of the extracellular signal-regulated
kinase/mitogen-activated protein kinase pathway decreases DNA
methylation in colon cancer cells. J Biol Chem. 282:12249–12259.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ye X, Nalepa G, Welcker M, Kessler BM,
Spooner E, Qin J, Elledge SJ, Clurman BE and Harper JW: Recognition
of phosphodegron motifs in human cyclin E by the SCFFbw7
ubiquitin ligase. J Biol Chem. 279:50110–50119. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong SK, Yoon S, Moelling C, Arthan D and
Park JI: Noncatalytic function of ERK1/2 can promote
Raf/MEK/ERK-mediated growth arrest signaling. J Biol Chem.
284:33006–33018. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Crews CM, Alessandrini A and Erikson RL:
The primary structure of MEK, a protein kinase that phosphorylates
the ERK gene product. Science. 258:478–480. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cowley S, Paterson H, Kemp P and Marshall
CJ: Activation of MAP kinase kinase is necessary and sufficient for
PC12 differentiation and for transformation of NIH 3T3 cells. Cell.
77:841–852. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sung JJ, Lau JY, Goh KL and Leung WK: Asia
Pacific Working Group on Colorectal Cancer: Increasing incidence of
colorectal cancer in Asia: Implications for screening. Lancet
Oncol. 6:871–876. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fodde R: The APC gene in colorectal
cancer. Eur J Cancer. 38:867–871. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rochlitz CF, Herrmann R and de Kant E:
Overexpression and amplification of c-myc during progression of
human colorectal cancer. Oncology. 53:448–454. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Abdollahi A, Pisarcik D, Roberts D,
Weinstein J, Cairns P and Hamilton TC: LOT1
(PLAGL1/ZAC1), the candidate tumor suppressor gene at
chromosome 6q24-25, is epigenetically regulated in cancer. J Biol
Chem. 278:6041–6049. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pourquié O: Developmental biology. A macho
way to make muscles. Nature. 409:679–680. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Purandare SM, Ware SM, Kwan KM, Gebbia M,
Bassi MT, Deng JM, Vogel H, Behringer RR, Belmont JW and Casey B: A
complex syndrome of left-right axis, central nervous system and
axial skeleton defects in Zic3 mutant mice. Development.
129:2293–2302. 2002.PubMed/NCBI
|
|
21
|
Yang JJ: A novel zinc finger protein,
ZZaPK, interacts with ZAK and stimulates the ZAK-expressing cells
re-entering the cell cycle. Biochem Biophys Res Commun. 301:71–77.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Valentino T, Palmieri D, Vitiello M,
Simeone A, Palma G, Arra C, Chieffi P, Chiariotti L, Fusco A and
Fedele M: Embryonic defects and growth alteration in mice with
homozygous disruption of the Patz1 gene. J Cell Physiol.
228:646–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chieffi P and Chieffi S: Molecular
biomarkers as potential targets for therapeutic strategies in human
testicular germ cell tumors: An overview. J Cell Physiol.
228:1641–1646. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chiappetta G, Valentino T, Vitiello M,
Pasquinelli R, Monaco M, Palma G, Sepe R, Luciano A, Pallante P,
Palmieri D, et al: PATZ1 acts as a tumor suppressor in thyroid
cancer via targeting p53-dependent genes involved in EMT and cell
migration. Oncotarget. 6:5310–5323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ow JR, Ma H, Jean A, Goh Z, Lee YH, Chong
YM, Soong R, Fu XY, Yang H and Wu Q: Patz1 regulates embryonic stem
cell identity. Stem Cells Dev. 23:1062–1073. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tritz R, Mueller BM, Hickey MJ, Lin AH,
Gomez GG, Hadwiger P, Sah DW, Muldoon L, Neuwelt EA and Kruse CA:
siRNA Down-regulation of the PATZ1 gene in human glioma cells
increases their sensitivity to apoptotic stimuli. Cancer Ther.
6:865–876. 2008.PubMed/NCBI
|
|
27
|
Pero R, Palmieri D, Angrisano T, Valentino
T, Federico A, Franco R, Lembo F, Klein-Szanto AJ, Del Vecchio L,
Montanaro D, et al: POZ-, AT-hook-, and zinc finger-containing
protein (PATZ) interacts with human oncogene B cell lymphoma 6
(BCL6) and is required for its negative autoregulation. J Biol
Chem. 287:18308–18317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keskin N, Deniz E, Eryilmaz J, Un M, Batur
T, Ersahin T, Atalay Cetin R, Sakaguchi S, Ellmeier W and Erman B:
PATZ1 is a DNA damage-responsive transcription factor that inhibits
p53 function. Mol Cell Biol. 35:1741–1753. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Valentino T, Palmieri D, Vitiello M,
Pierantoni GM, Fusco A and Fedele M: PATZ1 interacts with p53 and
regulates expression of p53-target genes enhancing apoptosis or
cell survival based on the cellular context. Cell Death Dis.
4:e9632013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cho JH, Kim MJ, Kim KJ and Kim JR: POZ/BTB
and AT-hook-containing zinc finger protein 1 (PATZ1) inhibits
endothelial cell senescence through a p53 dependent pathway. Cell
Death Differ. 19:703–712. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan L, Gu H, Li J, Xu M, Liu T, Shen Y,
Chen B and Zhang G: RKIP and 14-3-3ε exert an opposite effect on
human gastric cancer cells SGC7901 by regulating the ERK/MAPK
pathway differently. Dig Dis Sci. 58:389–396. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Luca A, Maiello MR, D'Alessio A,
Pergameno M and Normanno N: The RAS/RAF/MEK/ERK and the PI3K/AKT
signalling pathways: Role in cancer pathogenesis and implications
for therapeutic approaches. Expert Opin Ther Targets. 16 Suppl
2:S17–S27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Levidou G, Saetta AA, Gigelou F, Karlou M,
Papanastasiou P, Stamatelli A, Kavantzas N, Michalopoulos NV,
Agrogiannis G, Patsouris E, et al: ERK/pERK expression and B-raf
mutations in colon adenocarcinomas: Correlation with
clinicopathological characteristics. World J Surg Oncol. 10:472012.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|