Introduction
Modern lifestyle trends, such as a high-fat diet,
have rendered colon cancer as one of the most highly fatal tumors
worldwide (1). The multi-gene and
multi-step carcinogenic pathway, which is the traditional
adenoma-adenocarcinoma pathway, has been widely accepted. This
pathway has the morphological manifestations of intestinal
epithelial hyperplasia, adenoma and invasive carcinoma in
succession. It manifests as gradual superposition of staged
oncogene mutation activation and tumor-suppressor gene inactivation
at the molecular biological level. Key steps during colon cancer
genesis, such as transformation and evolution, are associated with
oncogene activation or tumor-suppressor gene inactivation (2). The former refers to the process where
normal cells transform into cancer cells under oncogene induction.
The latter represents cellular hyperplasia with gene instability
and the acquisition of all malignant heterogeneities of tumor
cells. As well known, early diagnosis is of crucial significance to
the therapeutic effectiveness in colon cancer. Therefore,
determining early lesions as well as the biomarkers during all
evolutionary stages of colon cancer is of vital importance
(3).
MicroRNAs (miRNAs) are non-coding RNA molecules with
a length of 18–22 nt. They regulate gene expression through
targeted binding of the 3 untranslated region (3′UTR) terminal of
mRNA molecules (4). In recent
years, numerous scholars have confirmed that miRNA molecules in
tumor tissue are novel markers for clinical tumor diagnosis.
Research has indicated that miRNAs can stably exist in serum
(5). Therefore, circulatory miRNAs
have attracted extensive attention as novel biomarkers (5). Moreover, their potential to serve as
disease diagnostic markers has been recognized (6).
The PI3K/Akt signal transduction pathway includes
PI3K, Akt and their downstream effector molecules (7). PI3K is an important intracellular
kinase, the excessive activation of which plays an important role
in tumor genesis. Akt is highly homologous to v-Akt, the virus
oncogene inducing leukemia in mice (8). It is a major effector molecule of
PI3K, the excessive activation of which can inhibit or activate its
downstream target proteins (8). In
this way, it may induce infinite cell proliferation through
multiple pathways (8). PI3K can be
activated through various pathways, thus producing an important
product, PI-3,4,5-P3 (PIP3). PIP3 thereby serves as an
intracellular second messenger to bind with Akt. Moreover, it can
phosphorylate Akt and changes its conformation (9). In the meantime, it prompts the
translocation of Akt from the cytoplasm to the cell membrane. This
can activate its downstream target protein and promote cell
survival by mediating growth factors and insulin (9).
Survivin is a new member of the anti-apoptosis
protein family discovered in 1997. It is specifically expressed in
human and mouse embryonic development tissues and most human tumor
cells (10). However, it can only
be observed in thymus, testicle and secretory endometrium in normal
adult tissue (10). Its major
function is to inhibit tumor cell apoptosis and promote tumor cell
proliferation (11). Moreover, it
is also related to tumor sensitivity to chemotherapy drugs and
prognosis (11). The tissue
specificity and function diversity of survivin has rendered it a
new ‘hotspot’ in the field of tumor research (12). The present study was designed to
assess the expression of miR-542-3p in human colon cancer and
investigate the possible molecular mechanisms underlying the
effects of miR-542-3p on the growth and invasion of colon cancer
cells.
Materials and methods
Patients with colon cancer
Serum samples of patients with colon cancer (n=12)
and healthy volunteers (n=6) were collected after centrifugation at
2,000 × g for 10 min at 4°C, and saved at −80°C. The study was
approved by the institutional medical Ethics Committee of The
Second Affiliated Hospital of Harbin Medical University.
RNA extraction and real-time PCR
TRIzol reagent (Life Technologies, Carlsbad, CA,
USA) was used to isolate total RNA from cultured cells or samples.
The PrimeScript™ RT Master Mix (Takara, Dalian, China) was used to
synthesize first-strand cDNA using 100 ng total RNA. miRNA
expression was determined by RT-PCR using Power SYBR-Green PCR
Master Mix (Carlsbad, CA, USA). The reactions were incubated at
95°C for 10 min, followed by 40 cycles of 95°C for 30 sec and 60°C
for 30 sec.
Cell culture and treatments
Human SW620 cells were purchased from the American
Type Culture Collection (ATCC; Manassas, VA, USA) and were
maintained in Dulbecco's modified Eagle's medium (DMEM) containing
10% fetal bovine serum (FBS) (both from Invitrogen, Carlsbad, CA,
USA) at 37°C with 5% CO2. miR-542-3p, anti-miR-542-3p
and negative mimics were synthesized by GenePharma (Shanghai,
China). SW620 cells were transfected with miR-542-3p,
anti-miR-542-3p and negative mimics using Lipofectamine 2000
(Invitrogen).
Cell viability assay
After transfection for 24, 48 and 72 h, SW620 cells
were stained using Cell Counting Kit-8 (Beyotime, Shanghai, China)
at 37°C for 1–2 h. Cell viability was examined by measuring
absorbance at 450 nm (Tecan Group Ltd., Männedorf,
Switzerland).
Apoptosis assay and caspase-3/−9
activity
After transfection for 48 h, SW620 cells were
harvested and stained with Annexin V-PE and propidium iodide using
an apoptosis kit (BD Pharmingen, Franklin Lakes, NJ) for 15 min in
darkness. The apoptotic rate was determined by a flow cytometer
(Beckman Coulter, Inc., Miami, FL, USA). Proteins were isolated
from cultured cells after transfection using lysis buffer and
measured using BCA assay (both from Beyotime). Proteins (5 µg) were
used to determine caspase-3/−9 activity using caspase-3/−9 activity
kits. Caspase-3/−9 activity were examined by measuring absorbance
at 405 nm (Tecan Group Ltd.).
Transwell invasion assay
After transfection for 24 h, SW620 cells
(2×105 cells/ml) were seeded in 24-well Transwells
coated with Matrigel (8-µm pore size; BD Biosciences, San Jose, CA,
USA) using DMEM containing 1% FBS. The cells remaining in the upper
chamber were removed by cotton swabs following 24 h of incubation
and cells in the lower surface were stained with hematoxylin after
the cells were fixed with formaldehyde solution.
Western blotting
Proteins were isolated from cultured cells after
transfection using lysis buffer (and measured using BCA (both from
Beyotime). Proteins (30–50 µg) were then subjected to sodium
dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) for
separation before being transferred onto a polyvinylidene
difluoride (PVDF) membrane (Millipore, Billerica, MA, USA). The
membrane was blocked with 5% milk-in Tris-buffered saline with
Tween-20 (TBST) for 1 h at 37°C and protein were detected by
incubation with Bax, PI3K and p-AKT, survivin and GAPDH (Cell
Signaling Technology, Inc., Danvers, MA, USA) antibodies overnight
at 37°C. The membranes were subsequently incubated with a goat
anti-rabbit horseradish peroxidase secondary antibody (1:5,000;
Cell Signaling Technology, Inc.) at 37°C for 1 h. Protein blots
were visualized with enhanced chemiluminescent substrate (Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
Statistical analyses
Data are presented as mean ± SD. Statistical
comparisons were performed by one-way ANOVA followed by Dunnett's
t-test. p<0.05 was considered to indicate a statistically
significant difference.
Results
miR-542-3p expression
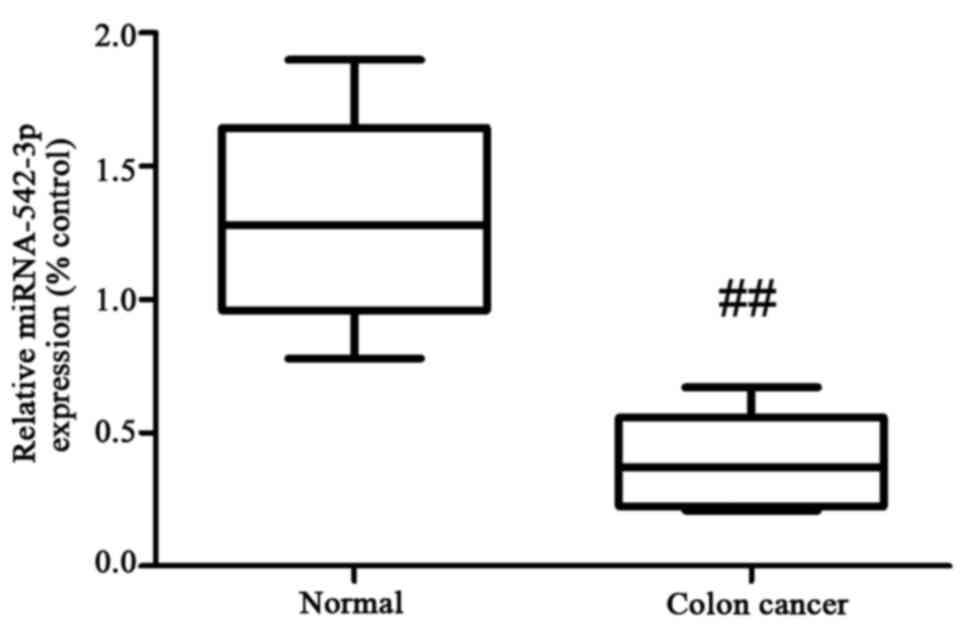
To determine the expression of miR-542-3p in
clinical serum samples, we collected 6 pairs of human colon cancer
samples and healthy controls. Our results showed that the
expression levels of miR-542-3p were downregulated in the serum
samples of the patients with colon cancer, compared with the levels
in the control group (Fig. 1).
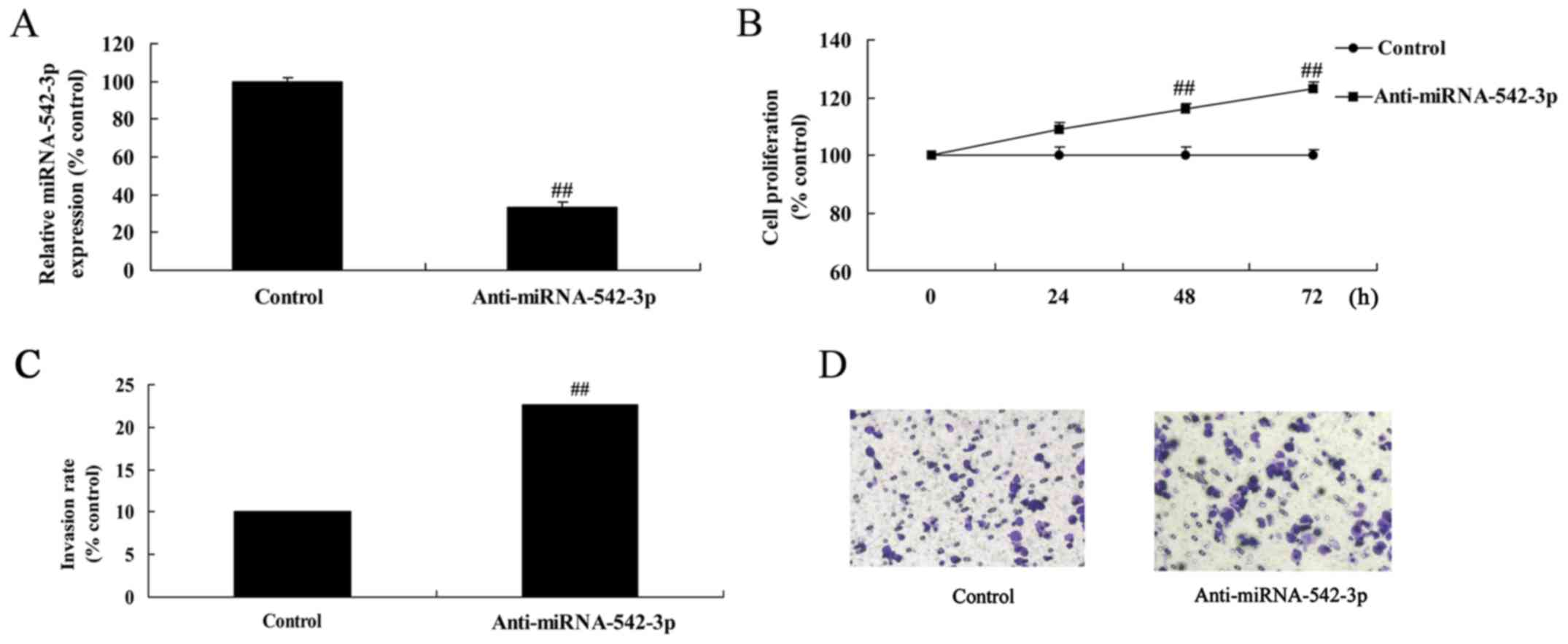
Silencing of miR-542-3p expression
promotes cell viability of colon cancer
Due to the downregulated expression of miR-542-3p in
human colon cancer serum samples, we speculated that miR-542-3p may
act as a tumor-suppressor in the development of colon cancer. The
expression of miR-542-3p in the anti-miR-542-3p-transfected cells
was lower than that in the control group (Fig. 2A). Silencing of miR-542-3p
expression significantly promoted cell viability (Fig. 2B) and invasion of colon cancer cells
compared with the control group (Fig.
2C and D).
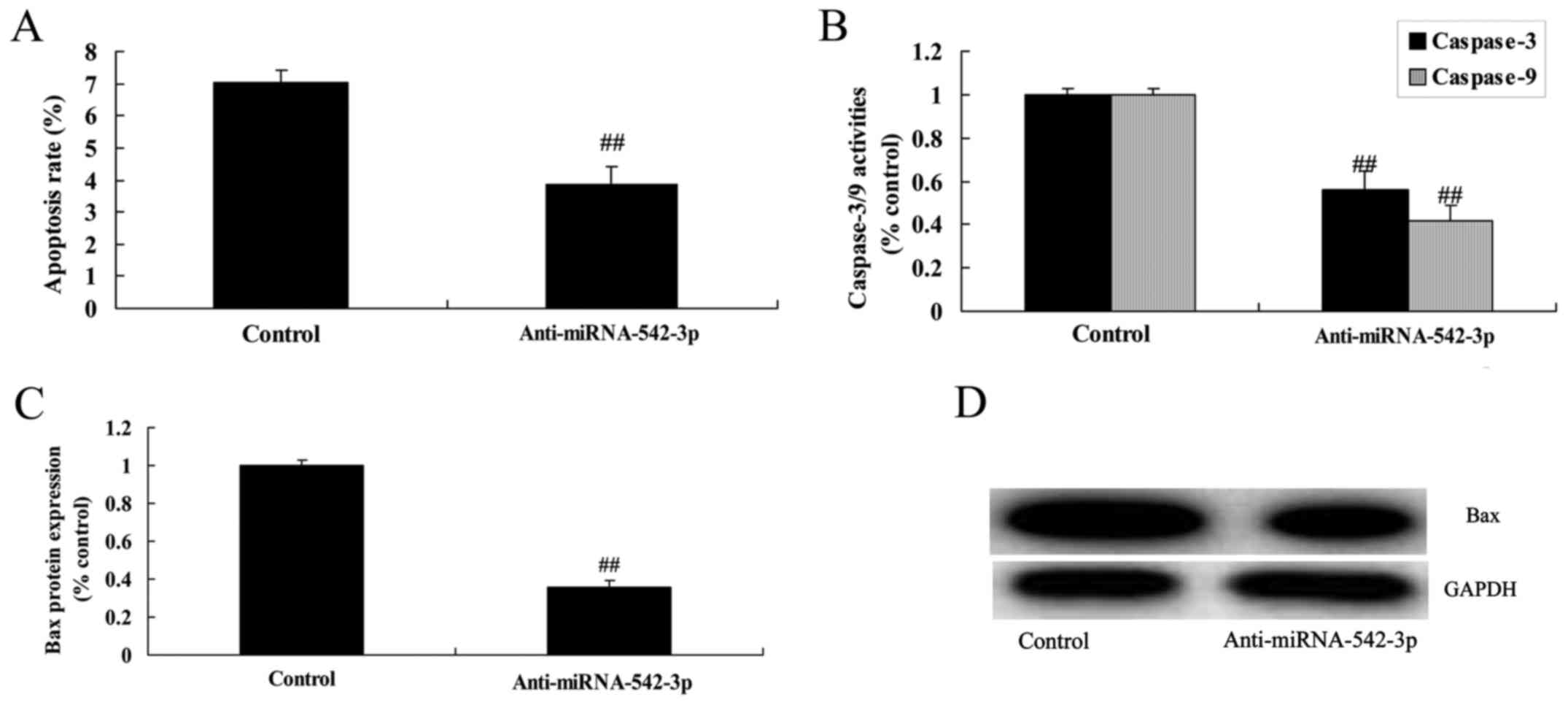
Silencing of miR-542-3p expression
inhibits the apoptosis of colon cancer cells
We demonstrated that the silencing of miR-542-3p
expression significantly inhibited apoptosis, caspase-3/−9
activities and Bax protein expression in the colon cancer cells
compared with the control group (Fig.
3).
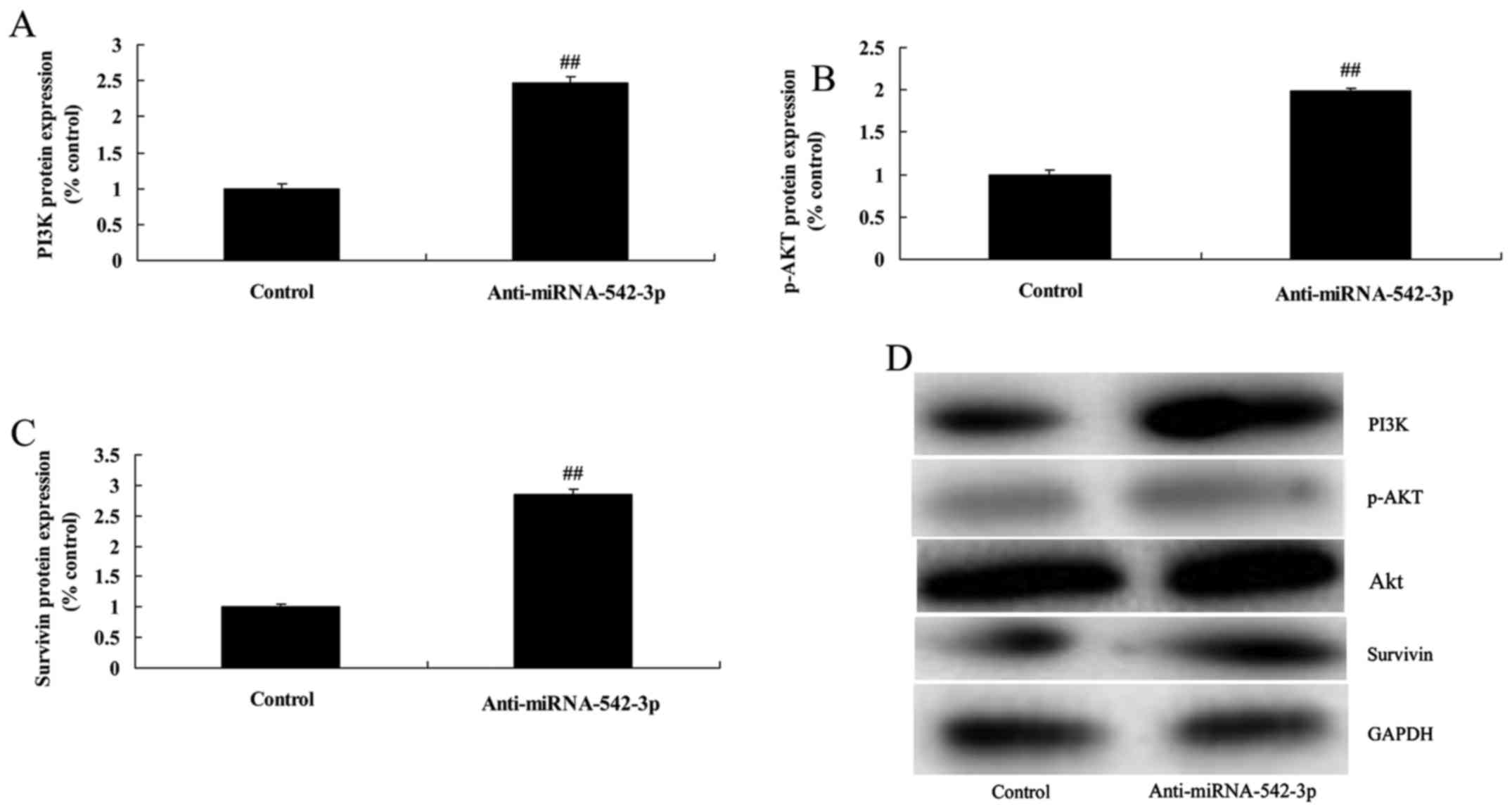
Silencing of miR-542-3p expression
induces PI3K/AKT/survivin signaling in colon cancer cells
Moreover, we explored the mechanism underlying the
miR-542-3p-mediated apoptosis of colon cancer cells. We analyzed
PI3K/AKT/survivin signaling. Silencing of miR-542-3p expression
significantly induced PI3K/AKT/survivin signaling in the colon
cancer cells, compared with the control group (Fig. 4).
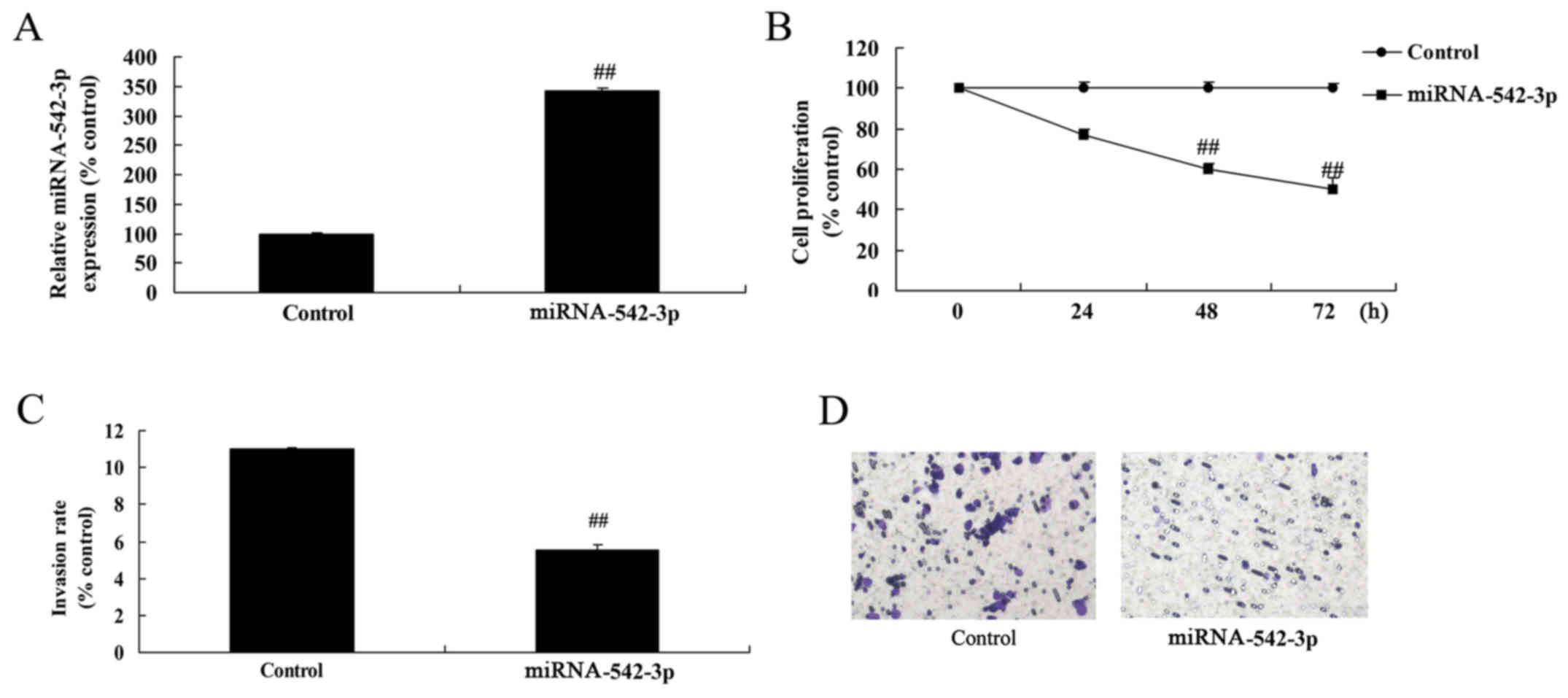
Overexpression of miR-542-3p inhibits
the viability of colon cancer cells
Next, we induced miR-542-3p expression to explore
the effect of miR-542-3p on the apoptosis of colon cancer cells. As
shown in Fig. 5A, miR-542-3p
expression in the cells transfected with miR-542-3p was higher than
that of the control group. Cell viability and invasion of the colon
cancer cells were significantly reduced by overexpression of
miR-542-3p, compared with the control group (Fig. 5B-D).
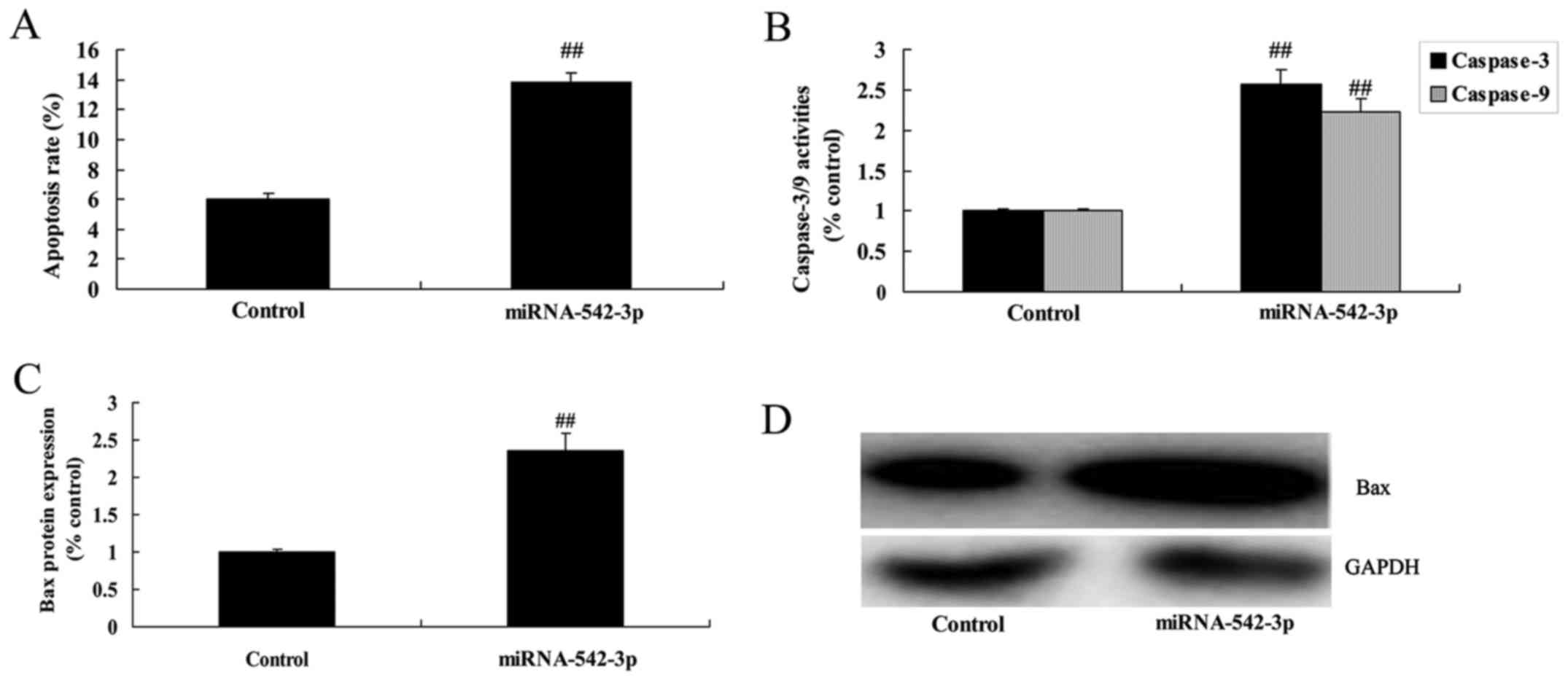
Overexpression of miR-542-3p promotes
the apoptosis of colon cancer cells
Overexpression of miR-542-3p significantly induced
apoptosis, caspase-3/−9 activity and Bax protein expression in the
colon cancer cells, compared with the control group (Fig. 6).
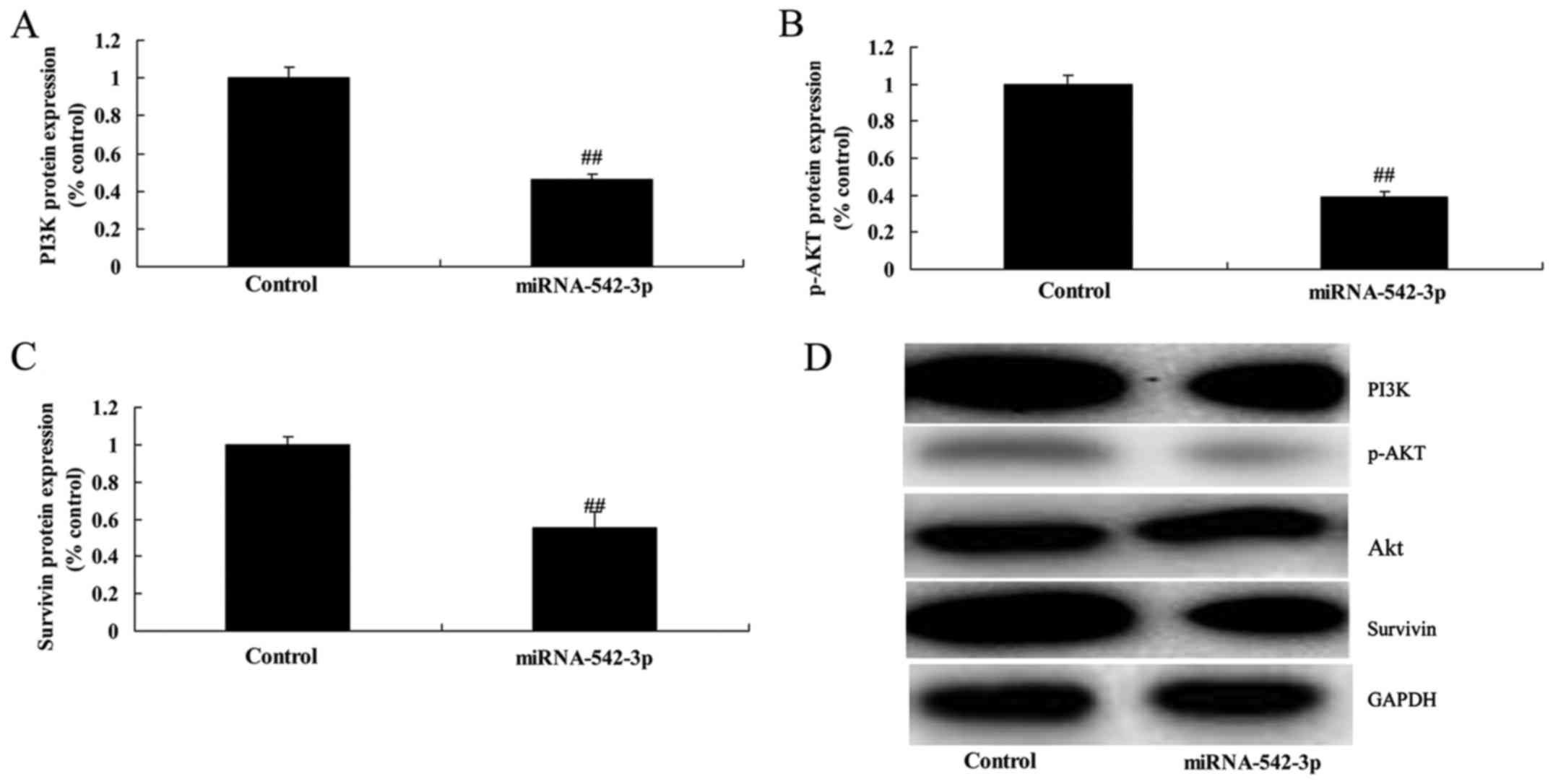
Overexpression of miR-542-3p
suppresses PI3K/AKT/survivin signaling in the colon cancer
cells
Overexpression of miR-542-3p significantly
suppressed PI3K/AKT/survivin signaling in the colon cancer cells,
compared with the control group (Fig.
7). There results showed that miR-542-3p regulates
PI3K/AKT/survivin signaling to affect the growth of colon cancer
cells.
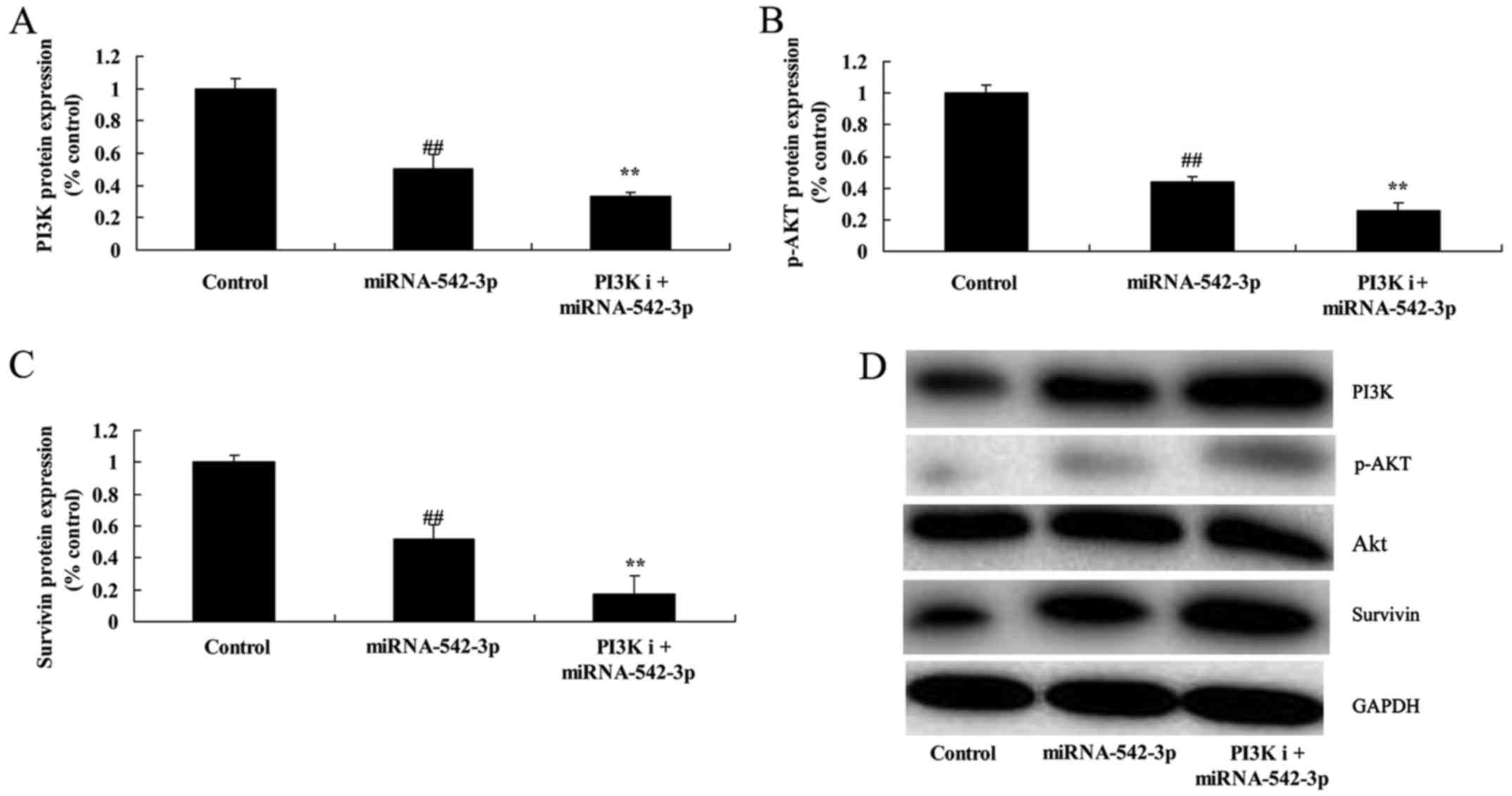
Inhibition of PI3K suppresses
PI3K/AKT/survivin signaling in the colon cancer cells following
miR-542-3p transfection
We performed bioinformatic analysis to identify the
role of PI3K in the function of miR-542-3p on the apoptosis of
colon cancer cells. As shown in Fig.
8, PI3K inhibitor (100 nM, LY294002), significantly suppressed
PI3K/AKT/survivin signaling in the colon cancer cells following
miR-542-3p transfection, when compared with the miR-542-3p group
(Fig. 8).
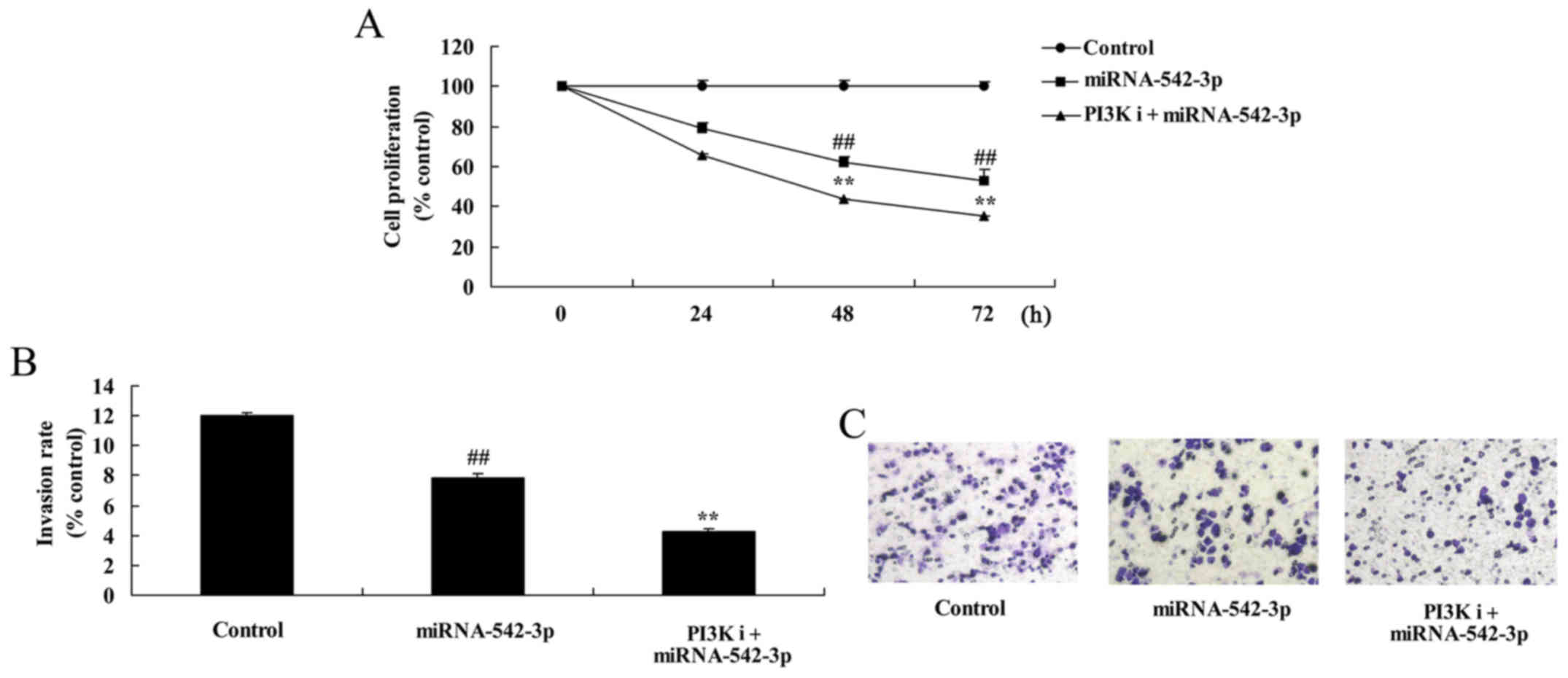
Inhibition of PI3K inhibits the growth
of colon cancer cells following miR-542-3p transfection
Cell viability and invasion of colon cancer cells
were significantly reduced by overexpression of miR-542-3p and
inhibition of PI3K, compared with the miR-542-3p group (Fig. 9).
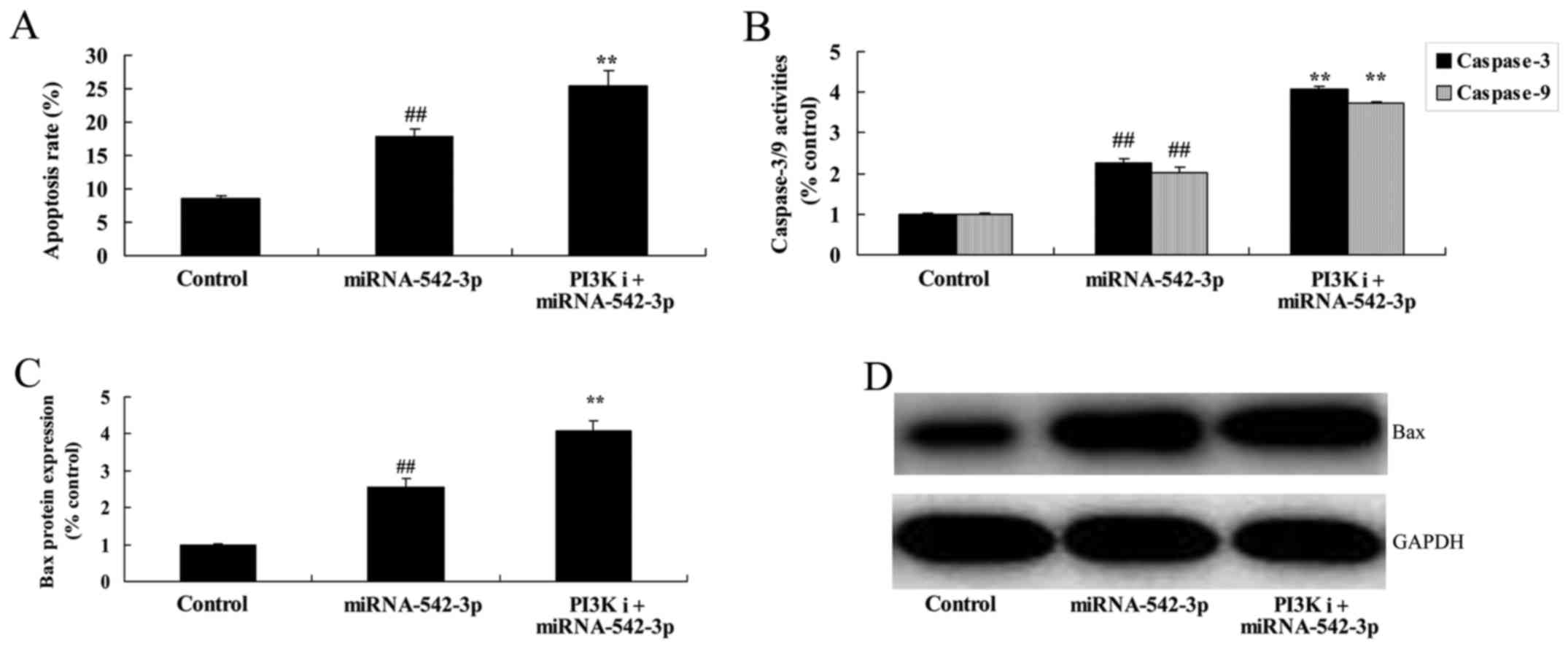
Inhibition of PI3K promotes the
apoptosis of colon cancer cells following miR-542-3p
transfection
Apoptosis, caspase-3 activity and Bax protein
expression in the colon cancer cells following miR-542-3p
transfection were significantly increased by the inhibition of
PI3K, compared with the miR-542-3p group (Fig. 10).
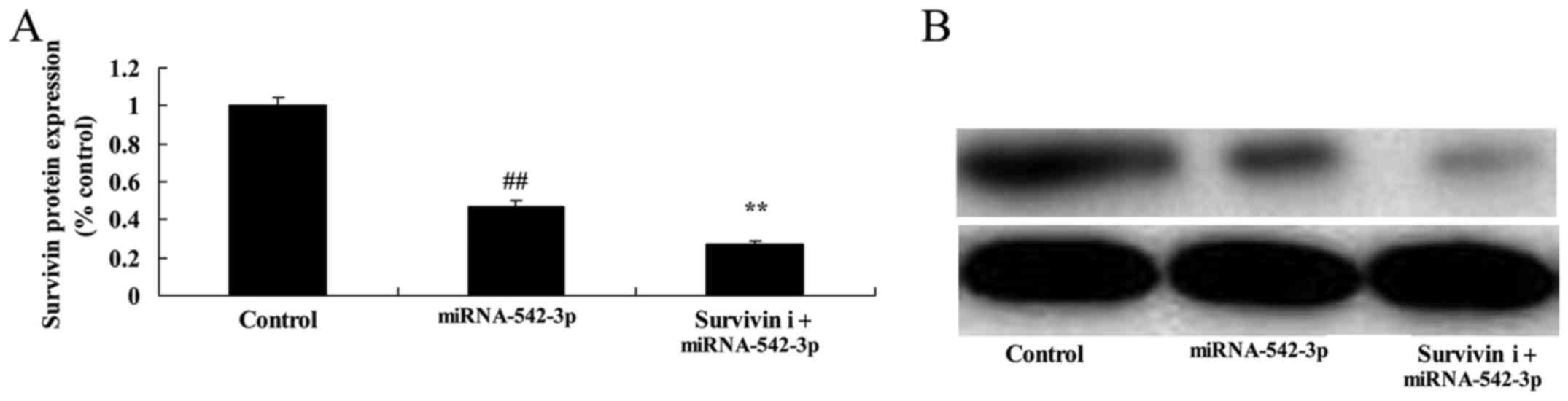
Inhibition of survivin suppresses the
survivin expression in the colon cancer cells
To investigate whether knockdown of survivin
influences the anticancer effects of miR-542-3p in colon cancer
cells, survivin inhibitor (YM155) was used to reduce survivin
expression in the colon cancer cells. As shown in Fig. 11, survivin inhibitor significantly
suppressed survivin signaling in the colon cancer cells transfected
with miR-542-3p, compared with the miR-542-3p group.
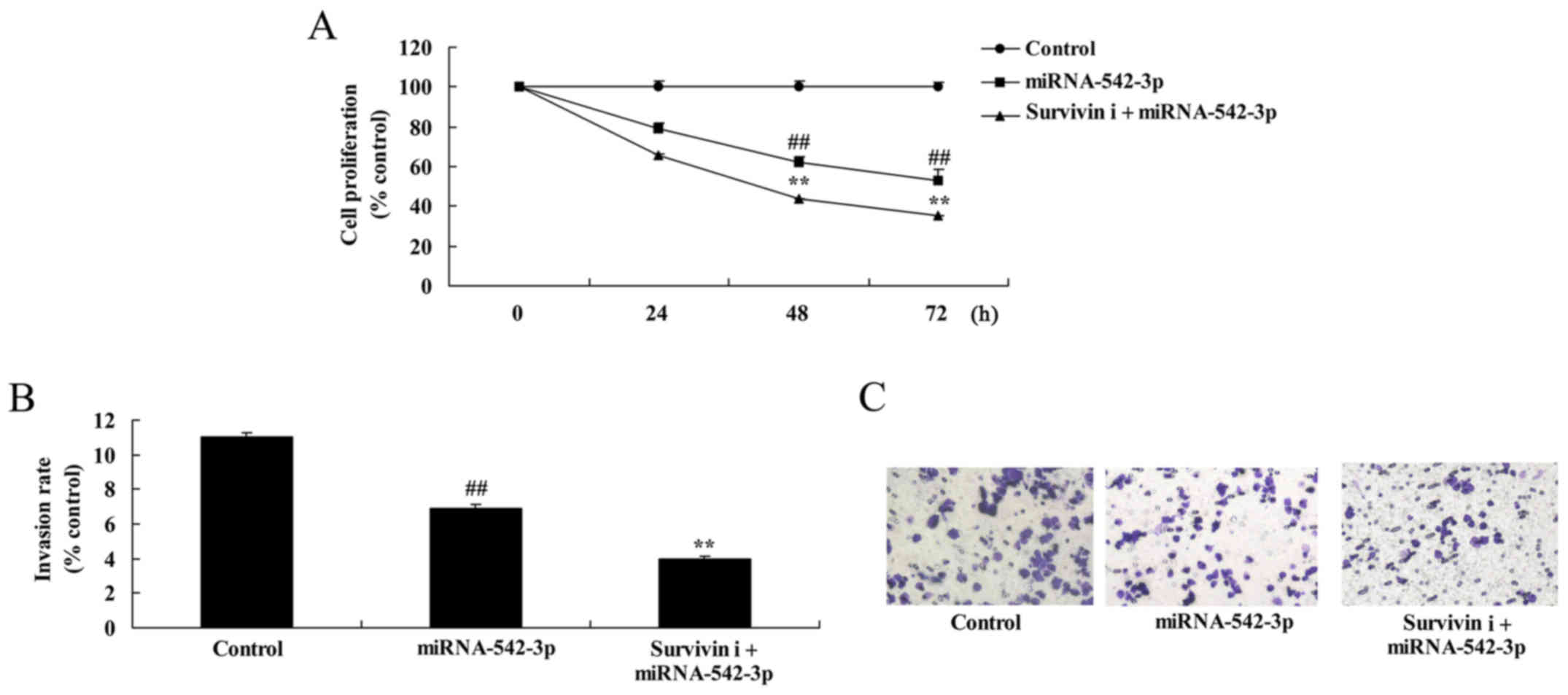
Inhibition of survivin suppresses the
growth and invasion of colon cancer cells following miR-542-3p
transfection
Fig. 12 shows that
inhibition of survivin significantly suppressed cell growth and
invasion of colon cancer cells following miR-542-3p transfection,
compared with the miR-542-3p group.
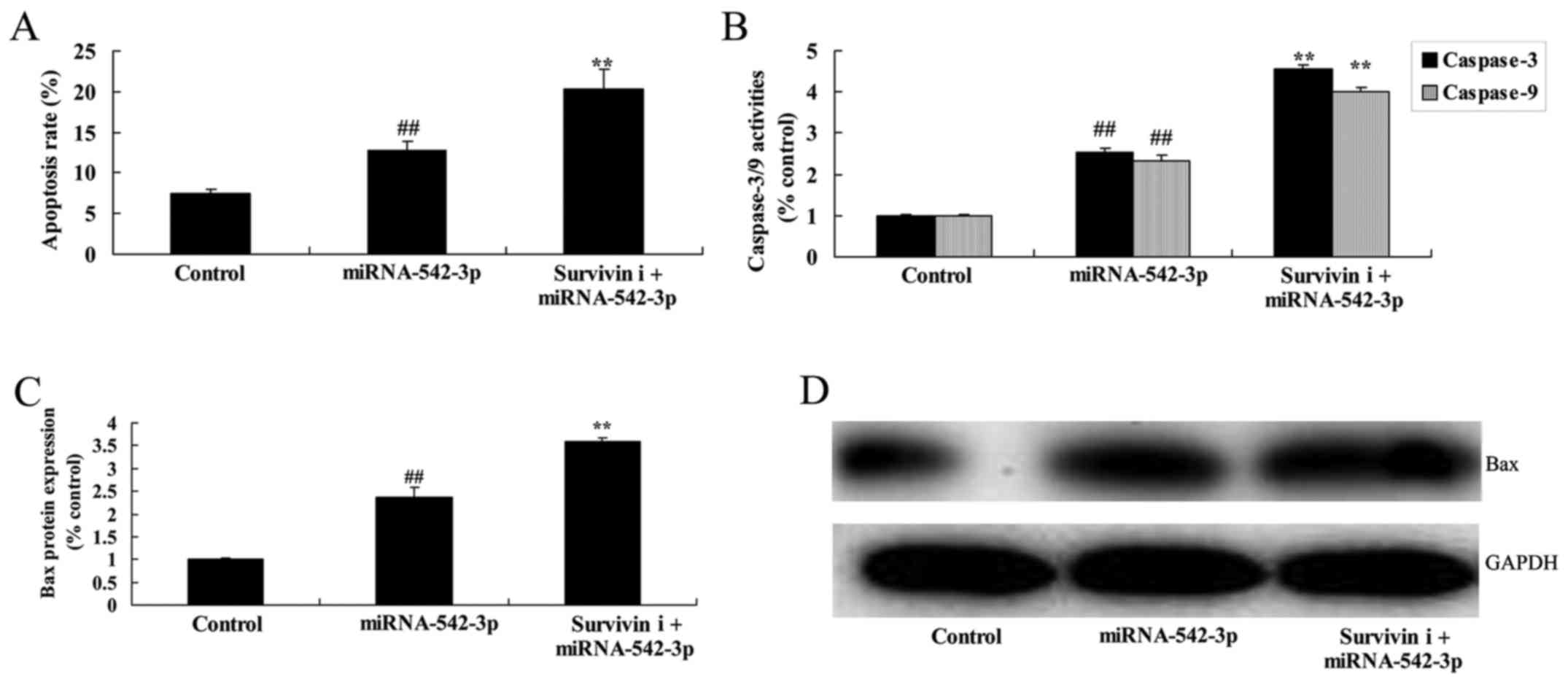
Inhibition of survivin promotes the
apoptosis of colon cancer cells following miR-542-3p
transfection
Finally, the inhibition of survivin significantly
promoted cell apoptosis, caspase-3/−9 activity and Bax protein
expression in the colon cancer cells following miR-542-3p
transfection (Fig. 13).
Discussion
Colon cancer is one of the most common digestive
system neoplasms (13). The gold
standard for the clinical diagnosis of colon cancer is electronic
colonoscopy diagnostic technology (14). Such technology requires specific
equipment, technical support, and high proficiency in the operation
of operators (1). Furthermore, at
the time of diagnosis it may have invaded into the human body.
Thus, it is associated with risks of complications of hemorrhage
and perforation (14). Therefore,
developing novel diagnostic methods is necessary to alleviate
patient suffering and reduce the risk of mortality (14). In the present study, we found that
expression levels of miR-542-3p in serum were downregulated in
patients with colon cancer.
Recent studies have found that deletion of the PTEN
gene is observed in malignant tumors, such as lung cancer,
endometrial cancer and breast cancer (15). This may result in the dysfunction of
the PI3K/Akt signaling pathway (15). Consequently, gene expression in this
pathway may become one of the important indices for predicting the
prognosis of malignant tumors. Meanwhile, small-molecule inhibitors
targeting this pathway may effectively suppress malignant tumor
cell growth and achieve gene therapy (16). The present study showed that the
silencing of miR-542-3p expression significantly promoted cell
viability, and inhibited apoptosis through suppression of PI3K and
p-AKT and survivin protein expression in human colon cancer. Kureel
et al showed that miR-542-3p suppresses osteoblast cell
proliferation and differentiation through BMP-7/PI3K-survivin
signaling in bone formation (17).
These results support our current findings and suggest a potential
mechanism for the tumor-suppressor role of miR-542-3p in human
colon cancer mediated through PI3K/AKT/survivin signals.
Excessive activation of PI3K can lead to that of
Akt. Thus, it may break the balance of the PI3K/Akt signaling
pathway (18). This may result in
excessive cell proliferation and give rise to carcinogenesis
(19). It is known that activated
PI3K can induce the genesis of multiple malignant tumors, such as
leukemia, prostate cancer and lymphoma (19). At the same time, it is verified in
experiments that excessive activation of PI3K can lead to the
genesis of colon cancer (19).
Evolution of PI3K protein in colon cancer shows an increasing
trend. In other words, it shows low or even negative expression in
normal large colorectal mucosa or polyps. However, it displays
significantly elevated expression in colon cancer tissues (20). The present study showed that PI3K
inhibitor (LY294002) suppressed the PI3K/AKT/survivin signal and
increased the anticancer effects of miR-542-3p on apoptosis in
colon cancer. Cai et al found that miR-542-3p suppressed the
tumor cell invasion of human astrocytoma via targeting the AKT
pathway (21).
Experiments in vitro indicate that survivin
can inhibit cell apoptosis by directly or indirectly interfering
with caspase function (22). Direct
action of survivin on caspases mainly represents the process of
caspase-3 and −7 activity suppression and cell apoptosis blocking
(22). Moreover, survivin
indirectly inhibits caspase through P21. Its mechanism is that
survivin forms the survivin-CDK4 complex with the cell cycle
control factor CDK4 (23). In this
way, P21 can be released from the CDK4 complex, which can further
bind with mitochondrial caspase-3. As a result, it may inhibit its
activity and prevent cell apoptosis. Survivin is expressed in the
G2/M phase of the cell cycle (23).
At the early stage of mitosis, survivin develops a specific
saturable reaction with mitotic spindle microtubule. However, it is
regulated by microtubule dynamics (24). The interaction between survivin and
the microtubule can be interfered with using microtubule
inhibitors. This results in the loss of anti-apoptotic activity of
survivin and the activation of caspase-3. Thus, it can induce cell
apoptosis (25). This result
indicates that survivin partly regulates mitosis through its action
on spindle microtubule. Thereby, it can exert an anti-apoptotic
effect (25). In the present study,
survivin inhibitor (YM155) suppressed the PI3K/AKT/survivin signal
and increased the anticancer effects of miR-542-3p on apoptosis in
colon cancer. Zhang et al reported that miR-542-3p
suppresses cell proliferation by post-transcriptionally regulating
survivin in bladder cancer cells (26).
In conclusion, the data in the present study
revealed that upregulation of miR-542-3p suppressed the growth and
invasion of colon cancer cells through PI3K/AKT/survivin signaling,
highlighting a novel therapeutic approach for the treatment of
colon cancer.
References
|
1
|
Floodeen H, Lindgren R, Hallböök O and
Matthiessen P: Evaluation of long-term anorectal function after low
anterior resection: A 5-year follow-up of a randomized multicenter
trial. Dis Colon Rectum. 57:1162–1168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hong YS, Nam BH, Kim KP, Kim JE, Park SJ,
Park YS, Park JO, Kim SY, Kim TY, Kim JH, et al: Oxaliplatin,
fluorouracil, and leucovorin versus fluorouracil and leucovorin as
adjuvant chemotherapy for locally advanced rectal cancer after
preoperative chemoradiotherapy (ADORE): An open-label, multicentre,
phase 2, randomised controlled trial. Lancet Oncol. 15:1245–1253.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reima H, Saar H, Innos K and Soplepmann J:
Methylene blue intra-arterial staining of resected colorectal
cancer specimens improves accuracy of nodal staging: A randomized
controlled trial. Eur J Surg Oncol. 42:1642–1646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu K, Yao H, Lei S, Xiong L, Qi H, Qian
K, Liu J, Wang P and Zhao H: The miR-124-p63 feedback loop
modulates colorectal cancer growth. Oncotarget. 8:29101–29115.
2017.PubMed/NCBI
|
|
5
|
Monzo M, Santasusagna S, Moreno I,
Martinez F, Hernández R, Muñoz C, Castellano JJ, Moreno J and
Navarro A: Exosomal microRNAs isolated from plasma of mesenteric
veins linked to liver metastases in resected patients with colon
cancer. Oncotarget. 8:30859–30869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu K, Zhao Z, Ma J, Chen J, Peng J, Yang S
and He Y: Deregulation of miR-193b affects the growth of colon
cancer cells via transforming growth factor-β and regulation of the
SMAD3 pathway. Oncol Lett. 13:2557–2562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nagappan A, Lee WS, Yun JW, Lu JN, Chang
SH, Jeong JH, Kim GS, Jung JM and Hong SC: Tetraarsenic hexoxide
induces G2/M arrest, apoptosis, and autophagy via PI3K/Akt
suppression and p38 MAPK activation in SW620 human colon cancer
cells. PLoS One. 12:e01745912017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang LL, Mu GG, Ding QS, Li YX, Shi YB,
Dai JF and Yu HG: Phosphatase and tensin homolog (PTEN) represses
colon cancer progression through inhibiting paxillin transcription
via PI3K/AKT/NF-κB pathway. J Biol Chem. 290:15018–15029. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gao Y, Xiao X, Zhang C, Yu W, Guo W, Zhang
Z, Li Z, Feng X, Hao J, Zhang K, et al: Melatonin synergizes the
chemotherapeutic effect of 5-fluorouracil in colon cancer by
suppressing PI3K/AKT and NF-κB/iNOS signaling pathways. J Pineal
Res. 62:e123802017. View Article : Google Scholar
|
|
10
|
Waligórska-Stachura J, Jankowska A, Waśko
R, Liebert W, Biczysko M, Czarnywojtek A, Baszko-Błaszyk D, Shimek
V and Ruchała M: Survivin - prognostic tumor biomarker in human
neoplasms - review. Ginekol Pol. 83:537–540. 2012.PubMed/NCBI
|
|
11
|
Hwang JS, Lee HC, Oh SC, Lee DH and Kwon
KH: Shogaol overcomes TRAIL resistance in colon cancer cells via
inhibiting of survivin. Tumour Biol. 36:8819–8829. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hsu YF, Sheu JR, Lin CH, Yang DS, Hsiao G,
Ou G, Chiu PT, Huang YH, Kuo WH and Hsu MJ: Trichostatin A and
sirtinol suppressed survivin expression through AMPK and p38MAPK in
HT29 colon cancer cells. Biochim Biophys Acta. 1820:104–115. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim CW, Baek JH, Choi GS, Yu CS, Kang SB,
Park WC, Lee BH, Kim HR, Oh JH, Kim JH, et al: The role of primary
tumor resection in colorectal cancer patients with asymptomatic,
synchronous unresectable metastasis: Study protocol for a
randomized controlled trial. Trials. 17:342016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Augestad KM, Norum J, Rose J and Lindsetmo
RO: A prospective analysis of false positive events in a National
Colon Cancer Surveillance Program. BMC Health Serv Res. 14:1372014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Chen L, Ko TC, Fields AP and
Thompson EA: Evi1 is a survival factor which conveys resistance to
both TGFbeta- and taxol-mediated cell death via PI3K/AKT. Oncogene.
25:3565–3575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tabusa H, Brooks T and Massey AJ:
Knockdown of PAK4 or PAK1 inhibits the proliferation of mutant KRAS
colon cancer cells independently of RAF/MEK/ERK and PI3K/AKT
signaling. Mol Cancer Res. 11:109–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kureel J, Dixit M, Tyagi AM, Mansoori MN,
Srivastava K, Raghuvanshi A, Maurya R, Trivedi R, Goel A and Singh
D: miR-542-3p suppresses osteoblast cell proliferation and
differentiation, targets BMP-7 signaling and inhibits bone
formation. Cell Death Dis. 5:e10502014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li XY and Wang X: The role of human
cervical cancer oncogene in cancer progression. Int J Clin Exp Med.
8:8363–8368. 2015.PubMed/NCBI
|
|
19
|
Jang HJ, Hong EM, Kim M, Kim JH, Jang J,
Park SW, Byun HW, Koh DH, Choi MH, Kae SH, et al: Simvastatin
induces heme oxygenase-1 via NF-E2-related factor 2 (Nrf2)
activation through ERK and PI3K/Akt pathway in colon cancer.
Oncotarget. 7:46219–46229. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moench R, Grimmig T, Kannen V, Tripathi S,
Faber M, Moll EM, Chandraker A, Lissner R, Germer CT, Waaga-Gasser
AM, et al: Exclusive inhibition of PI3K/Akt/mTOR signaling is not
sufficient to prevent PDGF-mediated effects on glycolysis and
proliferation in colorectal cancer. Oncotarget. 7:68749–68767.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cai J, Zhao J, Zhang N, Xu X, Li R, Yi Y,
Fang L, Zhang L, Li M, Wu J, et al: MicroRNA-542-3p suppresses
tumor cell invasion via targeting AKT pathway in human astrocytoma.
J Biol Chem. 290:24678–24688. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Lee MR, Choi E and Cho MY:
Clinicopathologic significance of survivin expression in relation
to CD133 expression in surgically resected stage II or III
colorectal cancer. J Pathol Transl Med. 51:17–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang R, Kang Y, Löhr CV, Fischer KA,
Bradford CS, Johnson G, Dashwood WM, Williams DE, Ho E and Dashwood
RH: Reciprocal regulation of BMF and BIRC5 (Survivin)
linked to Eomes overexpression in colorectal cancer. Cancer Lett.
381:341–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gao F, Zhang Y, Yang F, Wang P, Wang W, Su
Y and Luo W: survivin promotes the invasion of human colon
carcinoma cells by regulating the expression of MMP-7. Mol Med Rep.
9:825–830. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang H, Li S, Luo X, Song Z, Long X and
Zhu X: Knockdown of PARP6 or survivin promotes cell apoptosis and
inhibits cell invasion of colorectal adenocarcinoma cells. Oncol
Rep. 37:2245–2251. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang J, Wang S, Han F, Li J, Yu L, Zhou
P, Chen Z, Xue S, Dai C and Li Q: MicroRNA-542-3p suppresses
cellular proliferation of bladder cancer cells through
post-transcriptionally regulating survivin. Gene. 579:146–152.
2016. View Article : Google Scholar : PubMed/NCBI
|