Introduction
Despite advances in surgical techniques, adjuvant
chemotherapy, radiotherapy and immune therapy in the past decades,
cancer is still the second leading cause of death worldwide
alongside heart diseases (1). In
particular, gastric cancer (GC), with the highest prevalence in
Eastern Asia (including Japan, Korea and China) worldwide, has a
5-year survival rate of less than 30% (1,2).
Therefore, the discovery of novel and more effective therapeutic
targets against cancer appears to be of central importance.
In 1956, Otto Warburg observed that malignant cells
undergo a higher rate of glycolysis for energy in contrast to
healthy cells (3). Based on this
characteristic of cancer cells,
2′-[18F]-fluoro-2′-deoxy-D-glucose positron emission
tomography (18F-FDG PET) has been extensively used to
diagnose and assess various cancers including GC, and the changes
in 18F-FDG uptake can also reflect early treatment
response (4,5). In addition, this glycolytic metabolic
characteristic of cancer cells also suggests that targeting
glycometabolism may be an effective way to selectively inhibit
cancer cells (6). Moreover, this
dysregulated metabolism has been revealed to promote drug
resistance in malignant cells (7).
Recently, a number of molecules have been discovered
to suppress glycolysis in cancer cells. Various representative
drugs are iodoacetic acid (IAA), dichloroacetate (DCA),
2-deoxyglucose and 3-bromopyruvate (3-BrPA). Previous studies from
Pedersen and our team revealed that 3-BrPA decreased glycolysis by
suppressing mitochondrial hexokinase activity (8,9).
Similarily, sodium citrate (SCT), a member of the mitochondrial
tricarboxylic acid cycle, has also been revealed to inhibit
medullary thyroid cancer, leucocythemia, malignant pleural
mesothelioma and ovarian carcinoma growth (10–13).
It is generally known that the potential anticancer
ability of a new therapeutic approach is often evaluated by
activation of tumor cell apoptosis. There are 2 major signaling
pathways that induce apoptosis: the extrinsic (death receptor)
pathway mediated by procaspase-8, and the intrinsic (mitochondrial)
pathway which is triggered by the disruption of the mitochondrial
transmembrane potential, release of apoptogenic proteins such as
cytochrome c (Cyt-C) into the cytoplasm and subsequent
activation of caspase-9 (14–16).
The 2 signaling pathways converge at the activation of caspase-3, a
key mediator in the execution apoptosis (17). Furthermore, the proteins in the
Bcl-2 family such as pro-apoptotic Bax and anti-apoptotic Bcl-2,
modulate the mitochondrial membrane potential thus regulating
apoptotic signaling (18). In
addition, as a member of the IAP family, survivin is abundantly
expressed in the majority of human cancers, and is associated with
tumor proliferation and treatment resistance (19). Survivin inhibits the activation of
caspases and is a target of anticancer drugs (20,21).
Moreover, reactive oxygen species (ROS) generated in and around
mitochondria, has been indicated to induce uncontrolled oxidative
stress and subsequent cell apoptosis (22).
The application of tumor animal models is extremely
important in pre-clinical studies of oncology (23), and traditional ectopic models were
restricted in their poor representation of the pathophysiological
milieu around tumors (24). Thus,
it is necessary to create practicable orthotopic xenograft tumor
models that mimic the biological characteristics of human tumors in
order to discover successful therapeutic strategies (25).
The results from our previous studies indicated that
the antitumor mechanisms of 3-BrPA and SCT warrant further
investigation (9,13). In the present study, we created a GC
orthotopic xenograft model in nude mice and intraperitoneally
injected 3-BrPA and SCT. The antitumor efficacy of 3-BrPA and SCT
was detected using a micro-PET/CT scanner. We also explored the
underlying mechanisms in the inhibition of tumors induced by 3-BrPA
and SCT. In the present study the results demonstrated that the
antitumor effect was associated with the generation of ROS,
upregulation of Bax, downregulation of Bcl-2 and survivin, release
of Cyt-C and activation of caspase-3 and −9 cascades.
Materials and methods
Cell line and reagents
Gastric carcinoma cell line SGC-7901 was purchased
from the Cell Bank of the Chinese Academy of Sciences (Shanghai,
China). The cell line was maintained in RPMI-1640 medium
supplemented with 10% fetal calf serum (Gibco, Carlsbad, CA, USA),
100 U/ml penicillin and 100 µg/ml streptomycin, in a humidified
atmosphere with 5% CO2 at 37°C.
3-BrPA, SCT and 5-fluorouracil (5-FU) were purchased
from Sigma-Aldrich (St. Louis, MO, USA), dissolved in medium and
phosphate-buffered saline (PBS) to create working solutions and
filtered and sterilized prior to treatment.
Cell proliferation assay
In order to determine the sensitivity of the
SGC-7901 cell line to 3-BrPA, SCT or 5-FU, ~2,000 cells were seeded
into 96-well plates and exposed to varying concentrations of the
drugs for 24 and 48 h. Then, the cells were incubated with 10 µl of
5 mg/ml 3-(4,5-demethylthiazol-2-yl)-2,5-diphenyltetrazonium
bromide (MTT) for a further 4-h incubation at 37°C. Dimethyl
sulfoxide (DMSO) was added to each well, and was shaken for 30 sec.
The absorbance at 490 nm was quantified using a microplate reader
(Bio-Rad, Hercules, CA, USA).
Hochest 33258 staining
To detect apoptosis in vitro, SGC-7901 cancer
cells were grown in 6-well plates and treated with 3-BrPA, SCT and
5-FU for 24 h, then stained with Hochest 33258 (Beyotime
Biotechnology, Jiangsu, China), and visualized under a fluorescence
microscope (Olympus, Tokyo, Japan) at an excitation wavelength of
350 nm and an emission wavelength of 460 nm.
Detection of intracellular ROS
level
The production of peroxides was assessed using the
ROS assay kit (Beyotime, Haimen, China). Briefly, SGC-7901 cancer
cells were treated with 3-BrPA, SCT and 5-FU for 4 h, incubated
with 10 µM DCFH-DA in serum-free medium at 37°C for 20 min.
Subsequently, the cells were imaged with a laser scanning confocal
microscope (Nikon A1; Nikon Corporation, Tokyo, Japan) at
excitation and emission wavelengths of 488 and 525 nm,
respectively. The fluorescence intensity was regarded as the
generation of ROS.
Establishment of a gastric carcinoma
orthotopic xenograft model
Four-week-old female BALB/c athymic nude mice,
weighing 20±2 g, were obtained from the Animal Experimental Center
of Guangxi Medical University (Guangxi, China) and fed under
specific pathogen-free conditions. All experimental procedures were
conducted in accordance with the internationally recognized
guidelines for conduct and animal welfare.
Mice were subcutaneously injected with 200 µl of
suspension (2×106 cells/ml) of the SGC-7901 cell line in
the back of axillary regions and sacrificed by cervical dislocation
after 14 days when the palpable tumor diameter reached 1.0 cm.
Tumor tissues were stripped and minced to 1–2 mm3, and
then transplanted into the next new group of mice for 6 sequential
generations. The sixth subcutaneously transplanted tumor was used
as the source of orthotopic transplantation.
Nude mice were anesthetized with sodium
pentobarbital (45 mg/kg of body weight) by intraperitoneal
injection, and an incision was made to carefully expose the
stomach. The seromuscular layer of greater curvature was punctured
with a needle to form a local concave niche where a tumor mass of
~1.0 mm3 with good conditioning was imbedded and then
the surface and greater omentum was covered with medical OB
glue.
After transplantion for 2 weeks, 96 mice were
randomly divided into the following 8 groups (n=12): control group
(PBS, 10 ml/kg), 5-FU group (5-FU, 10 mg/kg), 3-BrPA low-dose group
(3-BrPA-L, 1.85 mg/kg), 3-BrPA medium-dose group (3-BrPA-M, 2.23
mg/kg), 3-BrPA high-dose group (3-BrPA-H, 2.67 mg/kg), SCT low-dose
group (SCT-L, 7.5 mg/kg), SCT medium-dose group (SCT-M, 15 mg/kg),
and SCT high-dose group (SCT-H, 30 mg/kg). Nude mice were daily
intraperitoneally injected with corresponding drugs or PBS (10
ml/kg), respectively. For 4 weeks the physical, stool and abdominal
conditions of nude mice were observed throughout this experiment.
After a 4-week treatment, half of the nude mice in each group were
imaged with a micro-PET/CT scanner and then sacrificed by cervical
dislocation. The tumor tissues and organs were stripped for
subsequent studies. The remaining mice were continually treated
until their death in order to evaluate the life prolonging
rate.
Small-animal 18F-FDG PET/CT
scanning
A small-animal PET/CT scanner (Inveon; Siemens
Medical Solutions, Knoxville, TN, USA) was used for in vivo
imaging. The SGC-7901 tumor-bearing mice were anesthetized with
isoflurane (2% in 98% oxygen), kept at 38°C and the caudal vein was
injected with 18F-FDG (5.55 MBp, 150 µCi) 40 min prior
to imaging. A PET scan for 15 min was conducted on the mice
followed by a 5 min CT scan. Three-dimensional regions of interest
(ROI) were drawn around the gastric tumors and the standardized
uptake value was measured as the percentage of injected
radioactivity dose/gram (% ID/g).
Hematoxylin and eosin and
immunohistochemistry (IHC) staining
The harvested tumor tissues, livers and kidneys were
embedded in paraffin, sectioned at 5-µm thickness and stained with
hematoxylin and eosin (H&E) for examination by light
microscopy.
In addition, immunohistochemical (IHC) staining was
performed using antibodies for cleaved caspase-9 (1:100 dilution)
and cleaved caspase-3 (1:400 dilution) (both from Cell Signaling
Technology, Danvers, MA, USA). The expression of cleaved caspase-9
and cleaved caspase-3 were determined based on the cytoplasmic and
nuclear staining. The results were scored according to the mean
density (MD) by Image-Pro Plus 6.0 and evaluated by 2
pathologists.
Lactate, ATP and glycolytic enzyme
assay
To detect the inhibitory effect on glycolysis of
3-BrPA and SCT in vivo, the lactate production, ATP content
and the glycolytic enzyme activity (HK, PFK-1 and PK) were assessed
using assay kits (Jiancheng, Nanjing, China). According to the
manufacturer's instructions, the absorbance at 340 nm was assessed
spectrophotometrically (UV-2450; Shimadzu, Kyoto, Japan) and the
results were calibrated with cellular protein concentration.
Western blot analysis
Total proteins were extracted from orthotopic
tumors, electrophoresed in sodium dodecyl sulphate-polyacrylamide
gel electrophoresis (SDS-PAGE), and then transferred onto a
nitrocellulose filter membrane (Millipore, Billerica, MA, USA).
Non-specific binding was blocked with 5% skimmed milk for 2 h at
room temperature and incubation followed with specific primary
antibodies at 4°C overnight. The primary antibodies used were
rabbit antibodies against Bax, Bcl-2, Cyt-C, survivin and GAPDH
(1:1,000 dilution; Cell Signaling Technology). The anti-rabbit IgG
DyLight 800-conjugated antibody was incubated at room temperature
for 90 min (1:1,000 dilution; Cell Signaling Technology). The
protein bands were visualized and quantified using the Odyssey
system (LI-COR Biosciences, Lincoln, NE, USA).
Results
Drug sensitivity of cell lines
As shown in Table I,
the IC50 values of 3-BrPA, SCT or 5-FU in the SGC-7901
GC cell line were 10.23±2.37 µg/ml and 7.19±1.05 mg/ml at 24 h,
6.15±1.24 µg/ml and 4.12±0.87 mg/ml at 48 h, 0.38±0.05 and
0.25±0.03 mmol/l at 48 h respectively.
 | Table I.IC50 values of 3-BrPA, SCT
and 5-FU in the SGC-7901 cancer cell line in vitro. |
Table I.
IC50 values of 3-BrPA, SCT
and 5-FU in the SGC-7901 cancer cell line in vitro.
| Groups | 3-BrPA (µg/ml) | SCT (mg/ml) | 5-FU (mmol/l) |
|---|
| 24 h | 10.23±2.37 | 7.19±1.05 | 0.38±0.05 |
| 48 h | 6.15±1.24 | 4.12±0.87 | 0.25±0.03 |
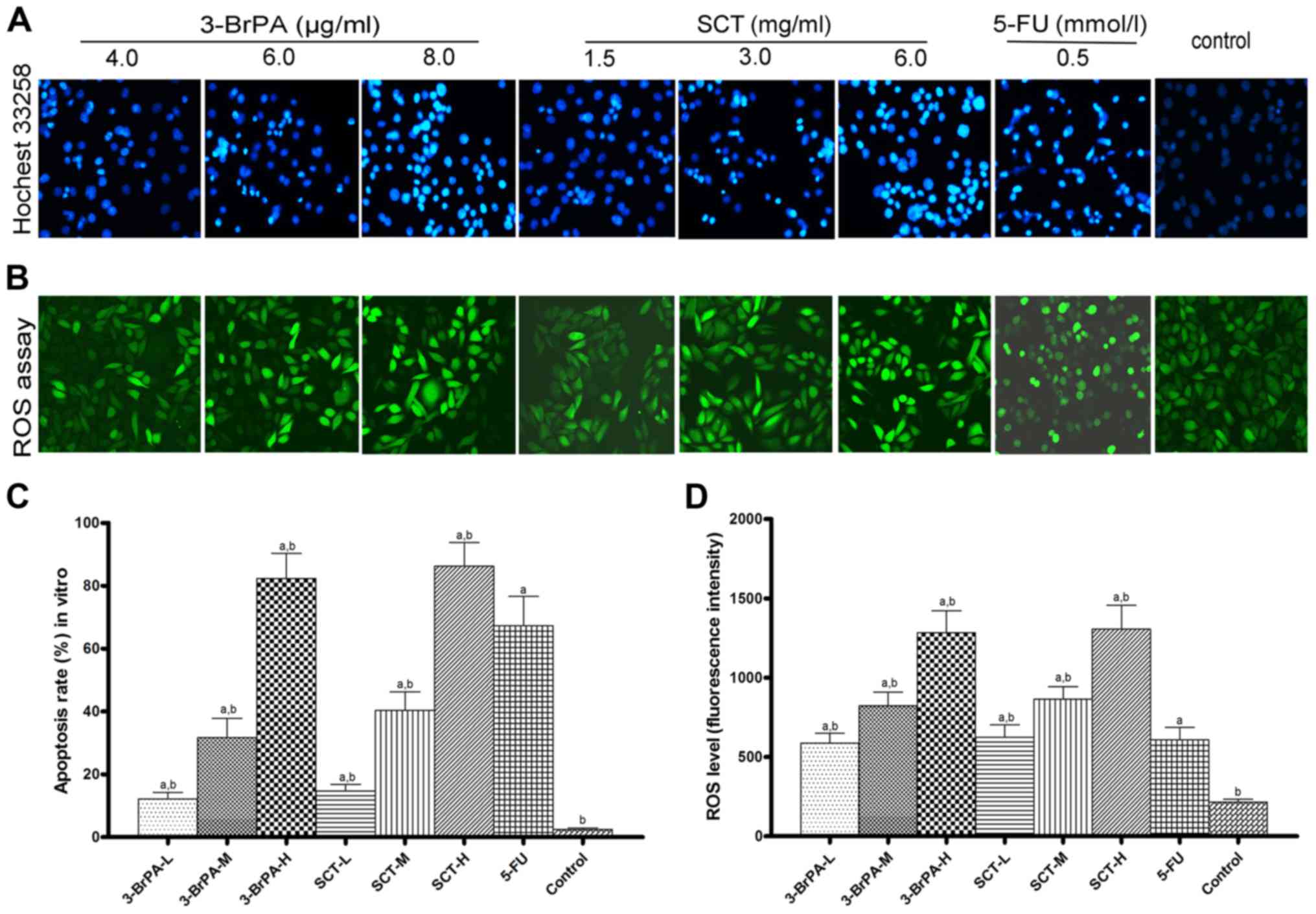
Apoptosis is assessed by Hochest 33258
staining
The apoptotic cells, characterized by chromatin
condensation and fragmentation, were observed after exposure to
3-BrPA and SCT for 24 h (Fig. 1A and
C). The percentage of apoptotic cells increased along with the
concentrations of 3-BrPA and SCT. The results strongly indicated
that 3-BrPA and SCT induced SGC-7901 cell apoptosis in
vitro.
ROS is generated after 3-BrPA and SCT
treatment
Intracellular ROS generation was evaluated using
intracellular peroxide-dependent oxidation of DCFH-DA to form
fluorescent DCF and detected with laser scanning confocal
microscope after treatment with 3-BrPA and SCT for 4 h (Fig. 1B). ROS production was significantly
increased upon treatment with 3-BrPA, SCT and 5-FU compared with
the control (Fig. 1D; p<0.05).
Furthermore, the fluorescence intensity increased along with the
dose of 3-BrPA and SCT.
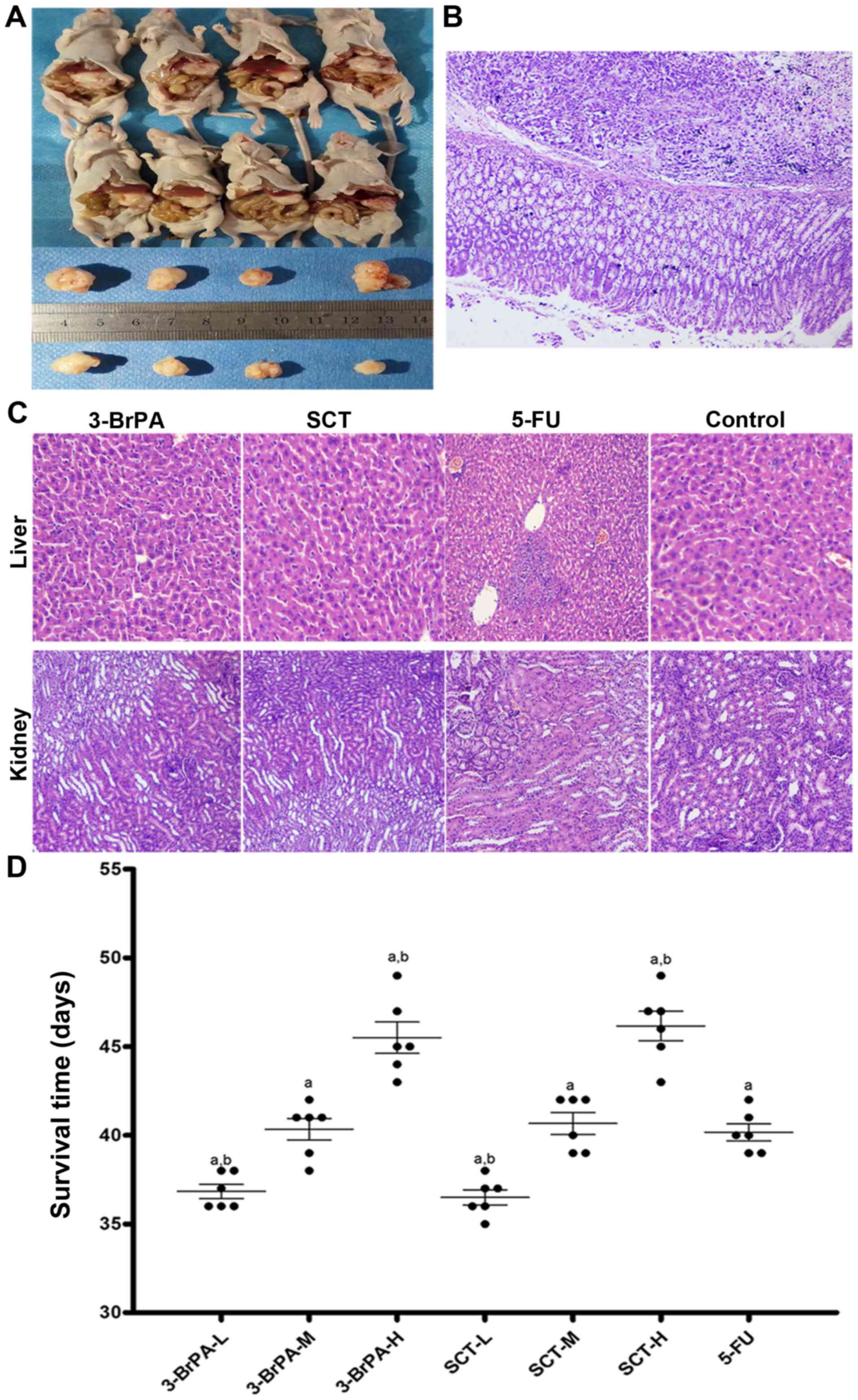
Effect of 3-BrPA and SCT on gastric
orthotopic xenograft tumors
Gastric orthotopic xenograft models were
successfully built (Fig. 2A and B),
and the effect of 3-BrPA and SCT on tumor growth and survival time
in mice were investigated. The orthotopic xenograft tumors were
identified by touch through the abdominal wall 10 days after
transplantation and the tumor formation rate was ~95% and the
mortality rate was 4%. The mice administered by intraperitoneal
injection with 3-BrPA and SCT exhibited tumor growth inhibition
compared with the control group (Table
II) (p<0.05). Moreover, as shown in Fig. 2D, the survival time of mice
increased in the 3-BrPA and SCT groups (p<0.05). The results
demonstrated that 3-BrPA and SCT suppressed tumor growth and
prolonged tumor-bearing mice survival time.
 | Table II.Results of nude mice weights, tumor
volumes, tumor weights, and the inhibitory rate of tumors (%). |
Table II.
Results of nude mice weights, tumor
volumes, tumor weights, and the inhibitory rate of tumors (%).
| Group | Nude mice (n) | Tumor volume
(mm3) | Tumor weight
(g) | Inhibitory rate of
volume (%) | Inhibitory rate of
weight (%) |
|---|
| 3-BrPA-L | 12 | 810.41±72.51 | 0.80±0.05 |
29.70±6.29a,b |
30.23±3.43a,b |
| 3-BrPA-M | 12 | 730.08±60.10 | 0.70±0.04 |
36.67±5.21a,b |
39.43±5.09a,b |
| 3-BrPA-H | 12 | 643.03±77.76 | 0.62±0.05 |
44.22±6.75a |
46.86±5.96a |
| SCT-L | 12 | 799.78±64.79 | 0.79±0.06 |
30.62±5.62a,b |
29.18±3.14a,b |
| SCT-M | 12 | 708.60±86.93 | 0.69±0.07 |
38.53±7.54a,b |
38.02±4.65a,b |
| SCT-H | 12 | 615.72±57.00 | 0.60±0.03 |
46.59±4.94a |
45.62±4.82a |
| 5-FU | 12 | 605.48±61.53 | 0.60±0.05 |
47.48±5.34a |
47.04±5.07a |
| Control | 12 | 1152.77±88.99 | 1.13±0.08 | 0.00b | 0.00b |
Histology assessment
In order to determine whether 3-BrPA and SCT had a
toxic effect on mice, the histopathological changes in the liver
and kidneys were observed by H&E staining. As shown in Fig. 2C, histological examination did not
reveal any apparent abnormalities, indicating the safety of the
3-BrPA and SCT doses used in the present study. However, slight
vacuolar changes and spotty necrosis in hepatocytes, hydropic
changes and leukocyte infiltration in kidney cells were observed in
the 5-FU-treated mice.
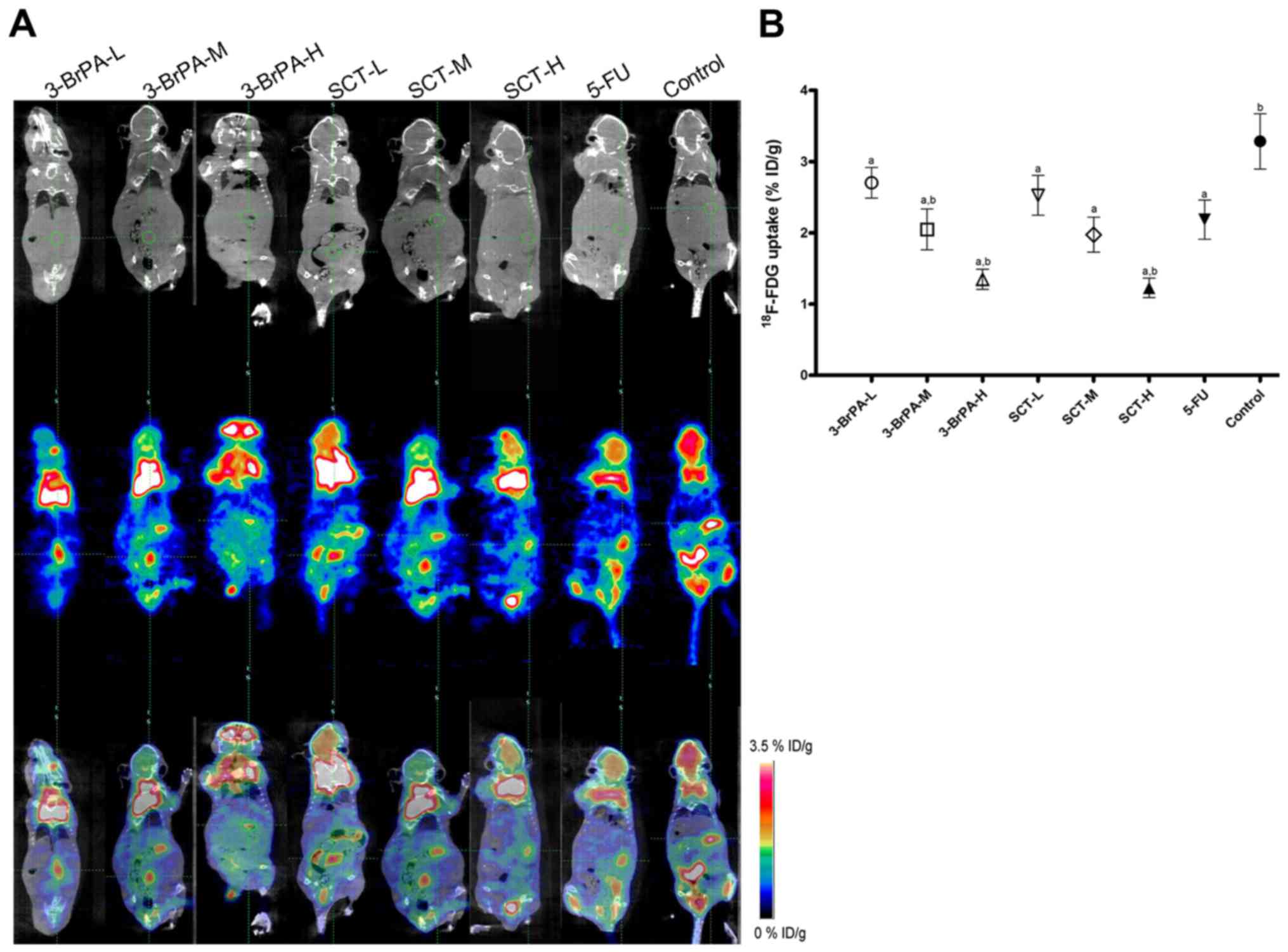
3-BrPA and SCT suppresses glycolytic
activity in tumors as determined by micro-PET/CT imaging
PET/CT with 18F-FDG is a non-invasive
approach to detect the glycolytic activity, and the radiotracer
accumulation (% ID/g) in tumor areas, reflecting the intensity of
tumor glucose metabolism. In the present study, we observed a
significantly decreased radioactivity uptake in the mice treated
with 3-BrPA and SCT (Fig. 3)
(p<0.05), which may have resulted from the inhibition of PFK-1
and HK activities. Notably, a decrease of radiotracer accumulation
was also observed in the 5-FU-treated group, which may be
associated with the decrease of HK activity.
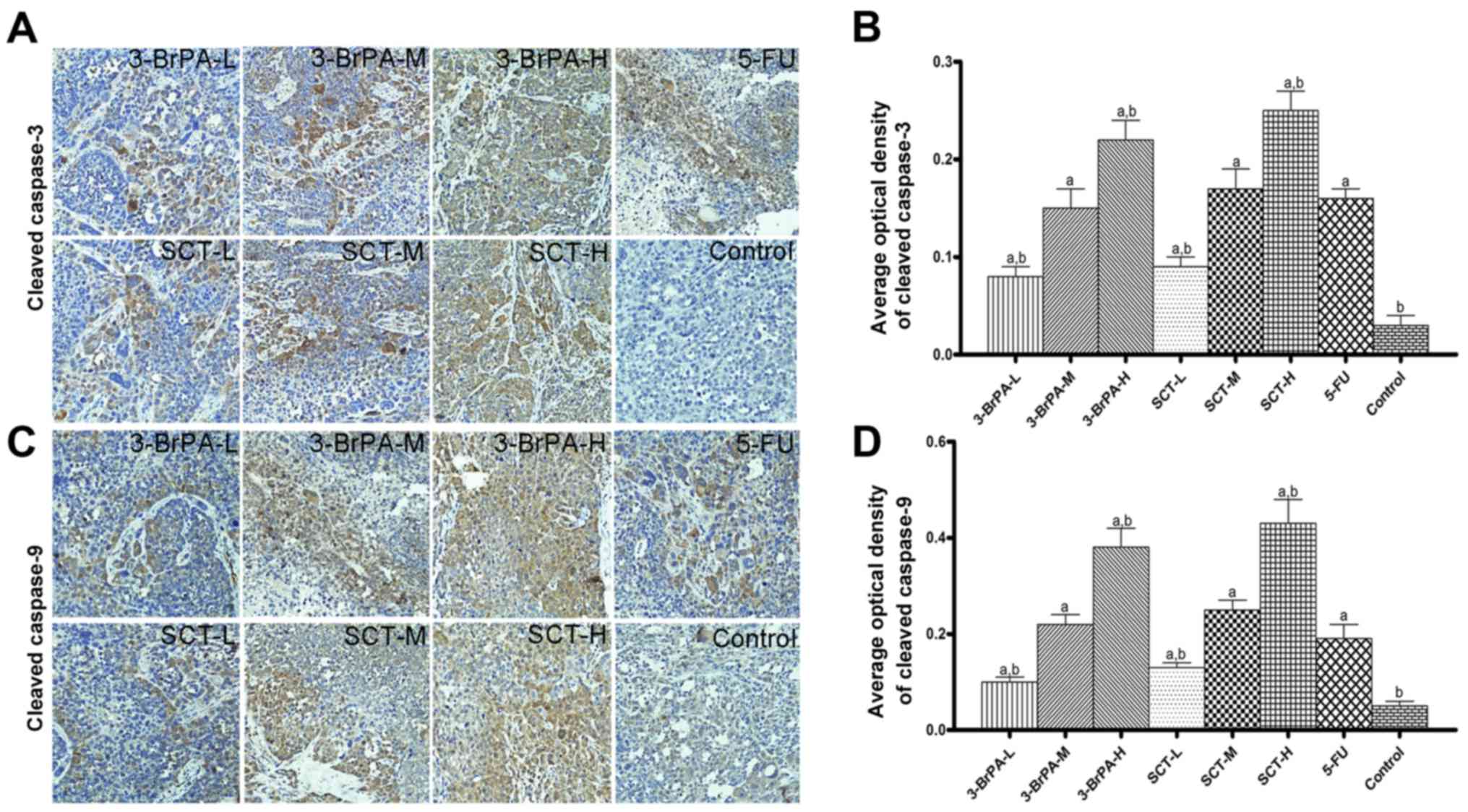
3-BrPA and SCT activate caspase
cascades
Activation of caspase-3 and −9 is a key downstream
event involved in the initiation and execution of apoptosis. From
the IHC analysis (Fig. 4), the
tumors from mice treated with 3-BrPA and SCT exhibited increased
levels of cleaved caspase-3 and −9 in a dose-dependent manner,
suggesting that the mechanism of apoptosis induced by 3-BrPA and
SCT is related to caspase activation (p<0.05). Similarly, a
significant increase in the expression of cleaved caspase-3 and −9
was also found in tumors with the 5-FU treatment.
Effects of 3-BrPA and SCT on Bcl-2,
Cyt-C, Bax and survivin expression
To further investigate the possible underlying
mechanism responsible for executing 3-BrPA and SCT induced
apoptosis, we analyzed the expression of Bcl-2, Bax, Cyt-C and
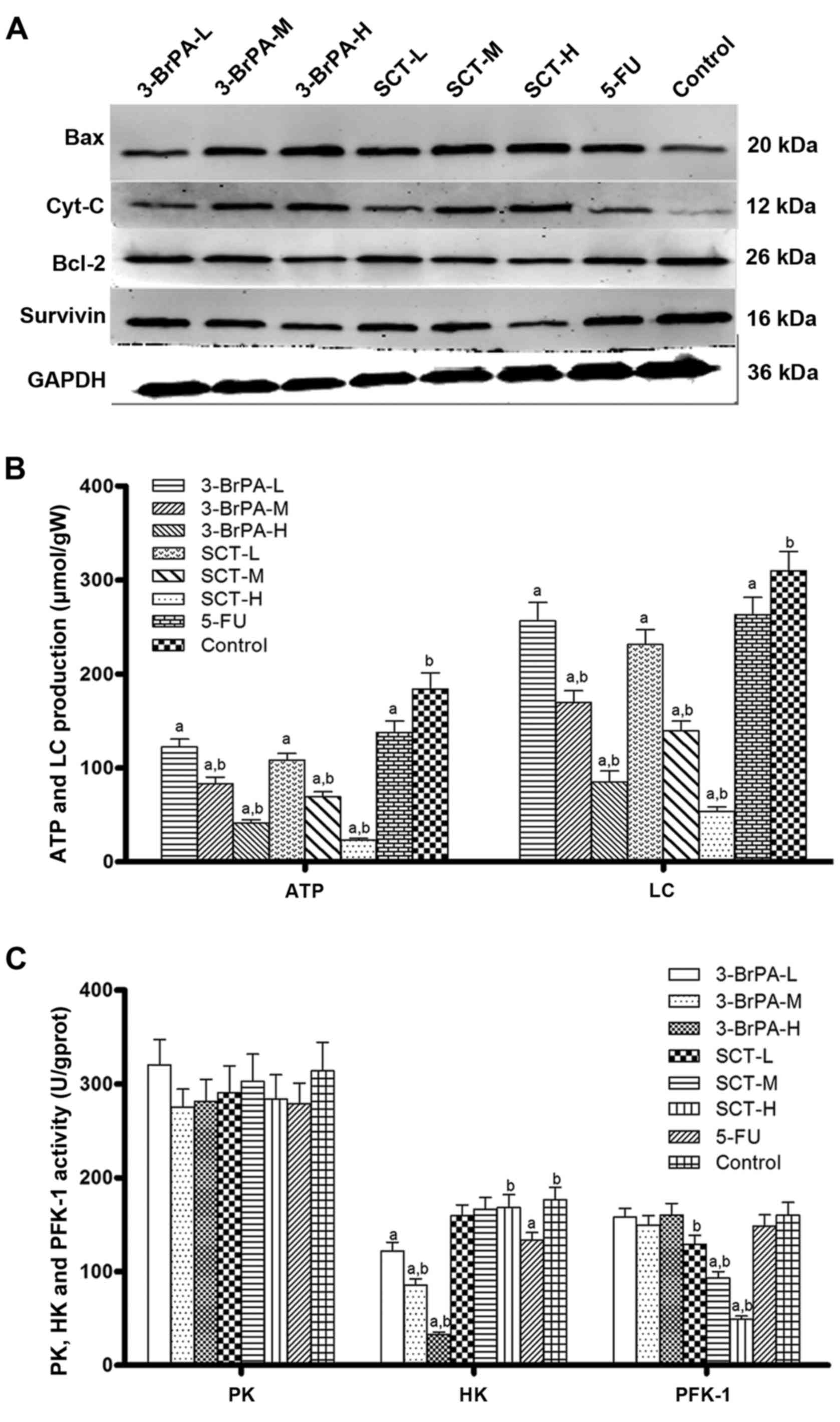
survivin using western blot analysis (Fig. 5A). The 3-BrPA and SCT-treated groups
exhibited an increased expression of pro-apoptotic factors, Bax and
Cyt-C, and a decreased expression of anti-apoptotic factors, Bcl-2
and survivin, in a concentration-dependent manner (p<0.05).
However, with 5-FU treatment, Bax and Cyt-C were also upregulated
while Bcl-2 and survivin were downregulated (p<0.05). These
results from western blot and IHC analyses indicated that the
mechanism of induced apoptosis by 3-BrPA and SCT may be mediated by
suppressing survivin and activating the mitochondria-dependent
pathway.
3-BrPA and SCT inhibit the activities
of glycolytic enzymes to decrease lactate and ATP production
To further explore the potential mechanisms involved
in the regulation of glucose metabolism of 3-BrPA and SCT in
vivo, the activities of glycolytic enzymes (PK, HK and PFK-1),
lactate and ATP production in tumor tissues was assessed (Fig. 5B and C). The data revealed that
lactate and ATP production was decreased in the 3-BrPA-treated
group by suppression of HK activity, and it was also decreased in
the SCT-treated group by inhibition of PFK-1 activity. Lactate and
ATP production in the 5-FU-treated group was significantly lower in
contrast to the PBS group (p<0.05). Moreover, the activity of HK
and PFK-1 was suppressed by 3-BrPA and SCT in a
concentration-dependent manner, respectively.
Discussion
As discovered by Warburg 80 years ago, most
malignant cells preferentially utilize glycolysis rather than
oxidative phosphorylation for energy production even in the
presence of oxygen (3) although the
energy generation of glycolysis is far less efficient than
oxidative phosphorylation (2 ATPs/glucose in glycolysis, but 36
ATPs from oxidative phosphorylation). However, increased glycolysis
not only provides faster ATP production, but also greater lactic
acid for tumor cell proliferation and invasion and may promote
their resistance to chemoradiotherapy (3,7,26). Due
to the altered metabolism in malignant cells, targeting glycolysis
may provide a novel approach in the treatment of cancer.
A variety of agents have been explored to
selectively suppress glycolysis. Some representative examples are
DCA, 2-deoxyglucose, IAA and 3-BrPA. DCA activates the pyruvate
dehydrogenase complex by targeting pyruvate dehydrogenase kinase
(27). 2-Deoxyglucose can
non-competitively inhibit hexokinase II to block glycolysis
(28). IAA is reported to inhibit
glyceraldehyde-3-phosphate dehydrogenase and 6-phosphogluconate
dehydrogenase (29). However, the
exact underlying molecular mechanisms of these drugs are still
under further investigation. Previous studies from Pedersen and our
team had revealed that 3-BrPA decreased the glycolysis rate by
suppressing mitochondrial hexokinase activity (9,10).
Similarly, SCT has also been revealed to inhibit medullary thyroid
cancer, leucocythemia, malignant pleural mesothelioma and ovarian
carcinoma growth (10–13). On account of their potential ability
in the treatment of malignant tumors, we employed 3-BrPA and SCT in
the present study and attempted to reveal their underlying
antitumor mechanisms. As shown in Fig.
5B and C, 3-BrPA and SCT decreased lactate and ATP production
by targeting HK and PFK-1, respectively. The decrease of ATP and
lactate may contribute to decrease the energy supply and disrupt
the acidic microenvironment, resulting in tumor growth inhibition
and apoptosis induction (Figs. 2
and 4).
In addition, based on the high glycolytic rates in
malignant cancer, PET imaging with 18F-FDG has been
extensively used for clinical detection of tumors (30). Moreover, small animal PET/CT imaging
has been frequently utilized to detect, monitor metastasis and
evaluate therapeutic response in animal experiments (31,32).
Due to the aforementioned rationale, in the present study,
micro-PET/CT was employed to detect the accumulation of
18F-FDG in gastric orthotopic xenografts to reflect
their metabolic activity and viability. As shown in Fig. 3, the 18F-FDG uptake
decreased in the 3-BrPA, SCT and 5-FU-treated groups. The changes
in radioactivity uptake were significantly correlated with the
inhibition of HK and PFK-1 activities (Fig. 5C). In view of these results we
suggest that a decrease in the uptake of 18F-FDG
reflects the glycolysis inhibition of tumors.
Apoptosis, known as a programmed cell death, is
frequently utilized to evaluate the potential anticancer ability of
a new therapeutic approach (33).
To understand the effect on the apoptosis of 3-BrPA and SCT, we
employed Hoechst 33258 staining. From the results of the Hoechst
33258 staining, we found that apoptosis was induced by 3-BrPA and
SCT in a time- and concentration-dependent manner in vitro
(Fig. 1A and C). In addition, the
results of a TUNEL staining experiment in our previous study also
indicated that 3-BrPA and SCT promote tumor cell apoptosis in a
dose-dependent manner in vivo (9). Moreover, the typical morphological
features of cell apoptosis were observed by TEM (9), and further demonstrated that 3-BrPA
and SCT could induce apoptosis with intraperitoneal
administration.
It is well known that there are 2 major signaling
pathways that trigger apoptosis in mammalian cells: the extrinsic
(death receptor) pathway and the intrinsic (mitochondrial) pathway.
The extrinsic pathway can immediately activate caspase-8 by death
receptors on the cell surface. However, in the intrinsic pathway,
the Bcl-2 family modulates the mitochondrial membrane potential to
regulate mitochondrial apoptotic signaling (18). Pro-apoptotic Bax promotes
permeabilization of the outer mitochondrial membrane, induces the
release of apoptogenic proteins such as cytochrome c (Cyt-C)
into the cytoplasm and subsequent activation of caspase-9 (14–16).
Conversely, anti-apoptotic Bcl-2 can negatively regulate the
activity of Bax to inhibit apoptosis. Thus, the expression of Bax,
Bcl-2 and Cyt-C proteins in tumors was assessed by western blot
analyses. Our results indicated that when treated with increasing
levels of 3-BrPA and SCT, the expression of the Bax and Cyt-C
proteins gradually increased, while the expression of the Bcl-2
protein gradually decreased (Fig.
5A).
Moreover, the Cyt-C release from the mitochondria
into the cytosol, forms apoptosomes with Apaf-1 and caspase-9,
which ultimately activates caspase-3 (34). Activated caspase-9 could be regarded
as an indicator of the mitochondrial-mediated apoptotic pathway and
activated caspase-3 is the intersection of the extrinsic and
intrinsic pathways. Both play critical roles in the execution of
apoptosis (17). In the present
study, the expression levels of cleaved caspase-9 and −3 were
assessed by IHC staining. As shown in Fig. 4, 3-BrPA and SCT expedited the
activation of caspase-9 and −3 in a concentration-dependent
manner.
It is believed that the generation of ROS can cause
uncontrolled oxidative stress, alter the mitochondrial membrane
potential and activate caspase-9 to induce subsequent cell
apoptosis (22,35,36).
Increasing levels of ROS generated in the SGC-7901 cells were
observed after a dose-increase of 3-BrPA and SCT treatment
(Fig. 1B and D). Combined with the
increased expression of Cyt-C, cleaved caspase-9 and −3 in tumor
tissues, we speculated that ROS acted as upstream signaling
molecules and played an important role in the induction of
apoptosis.
In addition, as a member of the IAP family, survivin
is abundantly expressed in the majority of human cancers, and
numerous studies have revealed that it acts as a critical inhibitor
of apoptosis to promote tumor proliferation and treatment
resistance (19,37). Furthermore, survivin can inhibit the
activation of caspases by directly binding to caspase-9 and −3
(20,21). As shown in Fig. 5A, a marked decrease of survivin in
tumors was observed after treatment, which resulted in the
upregulation of cleaved caspase-9 and −3. Thus, one of the
antitumor mechanisms of 3-BrPA and SCT may rely on the inhibition
of survivin to induce apoptosis.
To the best of our knowledge, this is the first
demonstration that intraperitoneal injection of 3-BrPA and SCT
leads to glycolysis restraint in gastric orthotopic xenografts and
that this method is non-invasive and detectable by
18F-FDG PET/CT. The results encourage the application of
18F-FDG PET/CT to detect glycometabolism changes in
tumors after treatment, as a considerable strategy to evaluate
therapeutic response in pre-clinical research of malignant tumors.
Furthermore, the present study revealed the underlying antitumor
mechanisms of 3-BrPA and SCT, by suppressing the activities of
glycolytic enzymes to decrease ATP and lactate production;
increasing generation of ROS, downregulating the expression of
survivin and inducing mitochondrial-mediated apoptosis. In
addition, no significant pathological alterations in the liver and
kidneys were observed after treatment, indicating the low toxicity
of 3-BrPA and SCT. Collectively, our findings highlight the
potential of intraperitoneal injection of 3-BrPA and SCT as a safe,
novel and attractive strategy in gastric cancer treatment.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81260366), and The
Guangxi Scientific Research and Technology Development Project.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heiden MG Vander, Cantley LC and Thompson
CB: Understanding the Warburg effect: The metabolic requirements of
cell proliferation. Science. 324:1029–1033. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park MJ, Lee WJ, Lim HK, Park KW, Choi JY
and Kim BT: Detecting recurrence of gastric cancer: The value of
FDG PET/CT. Abdom Imaging. 34:441–447. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weber WA and Wieder H: Monitoring
chemotherapy and radiotherapy of solid tumors. Eur J Nucl Med Mol
Imaging. 33 Suppl 1:S27–S37. 2006. View Article : Google Scholar
|
|
6
|
Najafov A and Alessi DR: Uncoupling the
Warburg effect from cancer. Proc Natl Acad Sci USA. 107:pp.
19135–19136. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhao Y, Butler EB and Tan M: Targeting
cellular metabolism to improve cancer therapeutics. Cell Death Dis.
4:e5322013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pedersen PL: Warburg, me and Hexokinase 2:
Multiple discoveries of key molecular events underlying one of
cancers' most common phenotypes, the ‘Warburg Effect’, i.e.,
elevated glycolysis in the presence of oxygen. J Bioenerg Biomembr.
39:211–222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang TA, Zhang XD, Guo XY, Xian SL and Lu
YF: 3-Bromopyruvate and sodium citrate target glycolysis, suppress
survivin, and induce mitochondrial-mediated apoptosis in gastric
cancer cells and inhibit gastric orthotopic transplantation tumor
growth. Oncol Rep. 35:1287–1296. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kruspig B, Nilchian A, Orrenius S,
Zhivotovsky B and Gogvadze V: Citrate kills tumor cells through
activation of apical caspases. Cell Mol Life Sci. 69:4229–4237.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lincet H, Kafara P, Giffard F,
Abeilard-Lemoisson E, Duval M, Louis MH, Poulain L and Icard P:
Inhibition of Mcl-1 expression by citrate enhances the effect of
Bcl-xL inhibitors on human ovarian carcinoma cells. J Ovarian Res.
6:722013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Halabe Bucay A: Hypothesis
proved&citric acid (citrate) does improve cancer: A case of a
patient suffering from medullary thyroid cancer. Med Hypotheses.
73:2712009. View Article : Google Scholar
|
|
13
|
Lu Y, Zhang X, Zhang H, Lan J, Huang G,
Varin E, Lincet H, Poulain L and Icard P: Citrate induces apoptotic
cell death: A promising way to treat gastric carcinoma? Anticancer
Res. 31:797–805. 2011.PubMed/NCBI
|
|
14
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Slee EA, Adrain C and Martin SJ:
Executioner caspase-3, −6, and −7 perform distinct, non-redundant
roles during the demolition phase of apoptosis. J Biol Chem.
276:7320–7326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Taylor RC, Cullen SP and Martin SJ:
Apoptosis: Controlled demolition at the cellular level. Nat Rev Mol
Cell Biol. 9:231–241. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: Key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shin S, Sung BJ, Cho YS, Kim HJ, Ha NC,
Hwang JI, Chung CW, Jung YK and Oh BH: An anti-apoptotic protein
human survivin is a direct inhibitor of caspase-3 and −7.
Biochemistry. 40:1117–1123. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
22
|
Orrenius S, Gogvadze V and Zhivotovsky B:
Mitochondrial oxidative stress: Implications for cell death. Annu
Rev Pharmacol Toxicol. 47:143–183. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
ille EA Sausv and Burger AM: Contributions
of human tumor xenografts to anti cancer drug development. Cancer
Res. 66:3351–3354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnson JI, Decker S, Zaharevitz D,
Rubinstein LV, Venditti JM, Schepartz S, Kalyandrug S, Christian M,
Arbuck S, Hollingshead M, et al: Relationships between drug
activity in NCI preclinical in vitro and in vivo models and early
clinical trials. Br J Cancer. 84:1424–1431. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bibby MC: Orthotopic models of cancer for
preclinical drug evaluation: Advantages and disadvantages. Eur J
Cancer. 40:852–857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Solaini G, Sgarbi G and Baracca A:
Oxidative phosphorylation in cancer cells. Biochim Biophys Acta.
1807:534–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bonnet S, Archer SL, Allalunis-Turner J,
Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta
L, Bonnet S, et al: A mitochondria-K+ channel axis is suppressed in
cancer and its normalization promotes apoptosis and inhibits cancer
growth. Cancer Cell. 11:37–51. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Singh D, Banerji AK, Dwarakanath BS,
Tripathi RP, Gupta JP, Mathew TL, Ravindranath T and Jain V:
Optimizing cancer radiotherapy with 2-deoxy-d-glucose dose
escalation studies in patients with glioblastoma multiforme.
Strahlenther Onkol. 181:507–514. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fahim FA, Esmat AY, Mady EA and Ibrahim
EK: Antitumor activities of iodoacetate and dimethylsulphoxide
against solid Ehrlich carcinoma growth in mice. Biol Res.
36:253–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Buerkle A and Weber WA: Imaging of tumor
glucose utilization with positron emission tomography. Cancer
Metastasis Rev. 27:545–554. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bradbury MS, Hambardzumyan D, Zanzonico
PB, Schwartz J, Cai S, Burnazi EM, Longo V, Larson SM and Holland
EC: Dynamic small-animal PET imaging of tumor proliferation with
3′-deoxy-3′-18F-fluorothymidine in a genetically engineered mouse
model of high-grade gliomas. J Nucl Med. 49:422–429. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walter MA, Hildebrandt IJ, Hacke K, Kesner
AL, Kelly O, Lawson GW, Phelps ME, Czernin J, Weber WA and Schiestl
RH: Small-animal PET/CT for monitoring the development and response
to chemotherapy of thymic lymphoma in Trp53-/- mice. J Nucl Med.
51:1285–1292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Talib WH and Mahasneh AM:
Antiproliferative activity of plant extracts used against cancer in
traditional medicine. Sci Pharm. 78:33–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Launay S, Hermine O, Fontenay M, Kroemer
G, Solary E and Garrido C: Vital functions for lethal caspases.
Oncogene. 24:5137–5148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gupta S, Yel L, Kim D, Kim C, Chiplunkar S
and Gollapudi S: Arsenic trioxide induces apoptosis in peripheral
blood T lymphocyte subsets by inducing oxidative stress: A role of
Bcl-2. Mol Cancer Ther. 2:711–719. 2003.PubMed/NCBI
|
|
36
|
Fan TJ, Han LH, Cong RS and Liang J:
Caspase family proteases and apoptosis. Acta Biochim Biophys Sin.
37:719–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Church DN and Talbot DC: Survivin in solid
tumors: Rationale for development of inhibitors. Curr Oncol Rep.
14:120–128. 2012. View Article : Google Scholar : PubMed/NCBI
|