Introduction
Obesity is a potential risk factor for the
development of various types of cancer. In this regard, the results
of a population study suggest that obesity also increases the risk
of prostate cancer (PC) development, in particular its aggressive
forms (1–8). Both overweight and obesity are closely
related to the prevalence of the metabolic syndrome (9,10). In
the context of obesity, special attention is paid now to the role
of biologically active peptides involved in the regulation of
energy homeostasis. Of these, the most important are leptin and
ghrelin (11,12). These peptides originate from adipose
tissue or the stomach, respectively and both regulate energy
homeostasis, mainly at the level of the hypothalamus. Both peptides
are involved in the regulation of body weight, food intake and
energy homeostasis and their mutual interaction is known as a
‘ghrelin-leptin tango’ (13–16).
It is worth emphasizing that in the regulation of energy
homeostasis, leptin exerts a long-term effect, while ghrelin is
rather a fast-acting signal. In light of the above mentioned ‘tango
dancers’, we aimed to investigate leptin, whose blood levels are
elevated in obese individuals. In addition, in many tissues, this
cytokine exerts a significant mitogenic effect, inter alia,
in the prostate.
Studies on leptin-deficient mouse led the group of
Friedman to the discovery of a leptin (17). They also identified the leptin gene
(OB) which encodes the synthesis of proleptin consisting of
166 amino acids. The mature form of circulating leptin is composed
of 146-amino acid residues (18,19).
The leptin gene is almost exclusively expressed in adipose tissue.
This hormone exerts biological effects through the leptin receptor
(OB-R) (20). As described by them,
the receptor is the so-called long leptin receptor, which exerts
full biological activity. Subsequently Cioffi et al
(21) identified three variants of
the short leptin receptor, which they called huB219.1, huB219.2 and
huB219.3.
In the early days of research, leptin receptor
isoforms were determined by letters, respectively Ob-Ra, Ob-Rb,
Ob-Rc, Ob-Rd, Ob-Re and Ob-Rf (reviewed in ref. 22). This nomenclature is still used in
relation to the leptin receptor in rats, in the prostate of which
such isoforms are present (23).
However, with regard to the human leptin receptor isoforms, this
nomenclature has totally changed. Current data indicate that there
are six isoforms of the human leptin receptor (Table I). It should be emphasized that the
transcript corresponding to the soluble leptin isoform was not
demonstrated in humans (24,25).
Therefore, it has been suggested that in humans this receptor
originates from proteolytic cleavage of ectodomains of the
remaining receptor isoforms. Isoforms of the leptin receptor are
ubiquitously expressed and show a tissue-specific distribution
(21,26,27).
 | Table I.Human leptin receptor isoforms -
actual nomenclature and original terminology (Genebank, accession
January 2017). |
Table I.
Human leptin receptor isoforms -
actual nomenclature and original terminology (Genebank, accession
January 2017).
| Actual
terminology |
|---|
|
|---|
| LEPR var 1 | LEPR var 2 | LEPR var 3 | LEPR var 4 | LEPR var 5 | LEPR var 6 |
|---|
| Homo
sapiens | Homo
sapiens | Homo
sapiens | Homo
sapiens | Homo
sapiens | Homo
sapiens |
| leptin
receptor | leptin
receptor | leptin
receptor | leptin
receptor | leptin
receptor | leptin
receptor |
| (LEPR),
transcript | (LEPR),
transcript | (LEPR),
transcript | (LEPR),
transcript | (LEPR),
transcript | (LEPR),
transcript |
| variant 1,
mRNA. | variant 2,
mRNA. | variant 3,
mRNA. | variant 4,
mRNA. | variant 5,
mRNA. | variant 6,
mRNA. |
| NM_002303.5 | NM_001003680.3 | NM_001003679.3 | NM_001198687.1 | NM_001198688.1 | NM_001198689 |
| 4,161 bp mRNA | 3,079 bp mRNA | 5,142 bp mRNA | 3,044 bp mRNA | 2,935 bp mRNA | 5,107 bp mRNA |
| 6-Oct-2016 | 6-Oct-2016 | 6-Oct-2016 | 6-Oct-2016 | 7-Oct-2016 | 7-Oct-2016 |
|
| Original
terminology |
|
| LEPR var
1 | LEPR var
2 | LEPR var
3 | LEPR var
4 | LEPR var
5 | LEPR var
6 |
|
| OB-R |
|
| Human B219/OB | Human B219/OB | Human B219/OB |
|
|
|
| receptor
isoform | receptor
isoform | receptor
isoform |
|
|
|
| HuB219.1 | HuB219.2 | HuB219.3 |
|
|
|
| precursor mRNA,
complete cds. | precursor mRNA,
complete cds. | precursor mRNA,
complete cds. |
|
|
|
| HSU52912 | HSU52913 | HSU52914 |
|
|
|
| 2,991 bp mRNA | 2,880 bp mRNA | 2,877 bp mRNA |
| Tartaglia et
al (20) | First listed
(2004), as variant 3 | First listed
(2004), as variant 2 | 5-Jun-1996 Cioffi
et al (21) | PRI 5-Jun-1996
Cioffi et al (21) | PRI 5-Jun-1996
Cioffi et al (21) |
Expression of leptin receptor isoforms is also found
in the human prostate and in human normal prostate and cancer
prostate cell lines. As early as 1996, Cioffi et al, by
means of RT-PCR, demonstrated high expression of the B219/obr gene
in the human prostate (21).
Subsequently, by means of immunohistochemistry, leptin receptors
were found in epithelial cells in both normal and malignant
prostate glands (28). In benign
prostatic hyperplasia, expression of OB-R was found in 2 out of 5
studied cases; HuB219.1 was present in all cases and HuB219.2 and
HuB219.3 isoforms were noted in 3 and 4 cases, respectively
(29). Leptin receptor isoforms are
also expressed in prostate cancer cell lines. Two functional
receptor isoforms (huOB-R and huB219.3) were found in DU145, PC-3,
and LNCaP-FGC cell lines (30).
Furthermore, expression of huOb-Ra and huOb-Rb was reported in
DU145 and PC3 cell lines (31).
Multiple alignment of the DNA sequences of leptin receptor gene
isoforms revealed that in these studies huOB-R and huOb-Rb are the
same isoforms (comparison of data from Genebank), while huOb-Ra =
huB219.3 = variant 6. Current leptin receptor gene structure
indicates that it might be impossible to design specific primers
only for variant 6; therefore we suggest that authors determine the
total expression of leptin receptor variants 3 and/or 6.
Experimental data indicate that leptin exerts a
multidirectional effect on human prostate cancer cells. Available
data suggest that in different human normal prostate and prostate
cancer cell lines, leptin may influence cellular differentiation,
proliferation, apoptosis, migration, invasion, metastatic potential
and release of growth factors. Previous studies have been usually
carried out on a small number of cell lines and often provide
controversial results. Therefore, the objective of the present
study was to investigate the expression of leptin receptor isoforms
in human normal prostate and prostate cancer cell lines, to
investigate the effect of leptin on the expression level of these
receptors and to study the effect of leptin on the proliferation
rate of these cells.
Materials and methods
Cell culture
The human prostate cell lines, prostate epithelial
cells (PrEC, cat. no. CC-2555), prostate stromal cells (PrSC, cat.
no. CC-2508) and prostate smooth muscle cells (PrSMC, cat. no.
CC-2587) were purchased from Lonza (Walkersville, MD, USA). Each
cell line was grown under specific conditions, including special
medium in accordance with the recommendations of the provider.
Prostate epithelial PrEC cells were grown using the Prostate
Epithelial Cell Medium BulletKit™ (PrEGM) containing bovine
pituitary extract (BPE), hydrocortisone, hEGF, epinephrine,
transferrin, insulin, retinoic acid, triiodothyronine, and GA-1000
(gentamycin) (Lonza). Prostate stromal PrSC cells were grown using
the Stromal Cell Medium BulletKit (SCGM) containing growth
supplements [hFGF-B, insulin, fetal bovine serum (FBS), GA-1000
(Lonza)]. Prostate smooth muscle PrSMC cells were grown using the
Smooth Muscle Cell Medium BulletKit (SmGM-2) containing growth
supplements [hEGF, insulin, hFGF-B, FBS and GA-1000 (Lonza)].
Prostate carcinoma cell lines, LNCaP [LNCaP clone FGC
(ATCC® CRL-1740D™)], DU145 (ATCC HTB-81™) and PC3 (ATCC
CRL-1435™) were purchased from ATCC (American Type Culture
Collection, Manassas, VA, USA). LNCaP and DU145 cells were grown in
RPMI-1640 medium (1X) + GlutaMAX + HEPES (Gibco Life Technologies,
Carlsbad, CA, USA). PC3 cells were grown in F-12K medium
commercially available from ATCC. All cell lines were cultured in
Falcon flasks in a humidified incubator at 37°C in 5%
CO2. After seeding on the first day, the culture medium
was replaced with a new one and then changed every second day. All
cell lines were cultured for 3–4 days but LNCaP cells for 7–8 days,
and subsequently the cells were transferred into culture wells
(32,33).
Leptin receptor isoform
expression
To study the effects of leptin on leptin receptor
isoform expression, cells were transferred into 6-well plates
(Nunc). First, the cells were cultured for 24 h in specific medium
as mentioned above. Subsequently cells were incubated for another
96 h in starved medium - without FBS, but with HEPES, glucose and
antibiotics (LNCaP, DU145, PC3 cell lines). Normal prostate cell
lines, PrEC, PrSMC, PrSC, were incubated in a medium with special
FBS, charcoal stripped (Gibco Life Technologies). From day 3 of
culture, new medium with leptin at concentrations
1×10−6, 1×10−8 and 1×10−10 M was
added, and the cells were incubated for at least 2 days (without
changing medium). These concentrations are within the range of
leptin concentrations in human blood. The serum leptin
concentration in healthy human blood is 6.6–20 ng/ml, while in
obese individuals it is 30–80 ng/ml. The concentration of
1×10−10 M corresponds to a concentration of 1.6 ng/ml,
and 1×10−8 M to a concentration of 160 ng/ml. Thus, the
leptin concentrations used in the incubation medium (excluding
10−6 M concentration) are similar to serum leptin levels
in healthy and obese subjects.
RNA isolation
At the end of culture, the cells were washed two
times using PBS, and then the total RNA was extracted using TRI
Reagent (Sigma-Aldrich, Poznan, Poland). Medium from each well was
collected into tubes and frozen for ELISA research. Total RNA was
purified on columns (NucleoSpin Total RNA Isolation, Qiagen GmbH,
Hilden, Germany) (33–36). The amount of total RNA was
determined by optical density at 260 nm and its purity was
estimated by a 260/280 nm absorption ratio (higher than 1.8)
(NanoDrop spectrophotometer, Thermo Fisher Scientific, Waltham, MA,
USA).
Reverse transcription
Reverse transcription was performed using reverse
transcriptase from the Transcriptor First Strand cDNA Synthesis kit
(Roche Diagnostics Corp., Indianapolis, IN, USA) with Oligo(dT) as
primers at a temperature of 55°C for 40 min (Thermocycler UNO II,
Biometra GmbH, Göttingen, Germany). Total RNA (1 µg) was used for a
single RT reaction. The RTs were carried out in standard final
volumes (20 µl). After RT, each cDNA containing sample was diluted
with 100 µl of RNase-free water.
qPCR
Primers were designed by Primer 3 software
(Whitehead Institute for Biomedical Research, Cambridge, MA, USA)
and purchased from the Laboratory of DNA Sequencing and
Oligonucleotide Synthesis, Institute of Biochemistry and
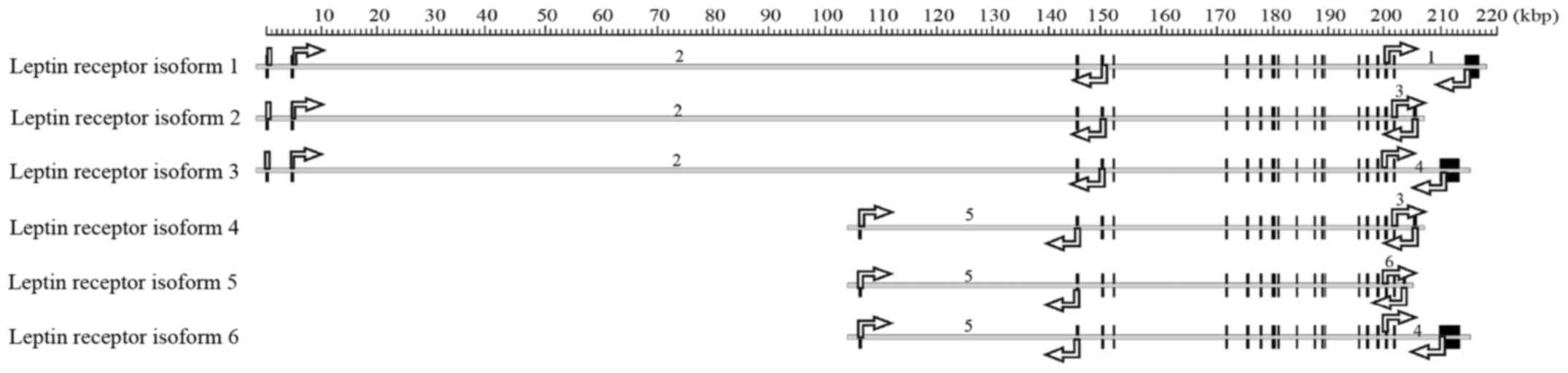
Biophysics, Polish Academy of Sciences, Warsaw (Fig. 1, Table
II). qPCR was performed by means of the Lightcycler 2.0
instrument (Roche Diagnostics Corp., 4.05 software version). Using
the above mentioned primers, SYBR Green detection system was
applied. Every 20 µl of reaction mixtures contained 2 µl template
cDNA (standard or control), 0.5 µM of each specific primer, and a
previously determined optimum MgCl2 concentration (3.5
µM for each reaction). According to the Roche LightCycler FastStart
DNA Master SYBR-Green 1 kit procedure for qPCR, 1–5 µl of the RT
reaction product (cDNA) can be used in 20 µl or 50 µl final
reaction volume. LightCyclerFastStart DNA Master SYBR-Green I mix
program (Roche Diagnostics Corp.) was used. The real-time PCR
program included 10 min of a denaturation step to activate the
Taq DNA polymerase, followed by a three-step amplification
program: denaturation at 95°C for 10 sec, annealing at 56°C for 5
sec, and extension at 72°C for 5 sec. Assays were run for a maximal
45 cycles. Specificity of the reaction products was checked by the
determination of melting points (0.1°C/sec transition rate). HPRT
was used as a standard for controlling RT-PCR reaction quality as
well as the reference gene in prostate cell lines. The primers used
exhibited a positive reaction in human organs, as demonstrated in
our previous study (37).
 | Table II.PCR analyses of leptin receptor
isoforms. |
Table II.
PCR analyses of leptin receptor
isoforms.
| [Position on
Fig. 1], Genebank accession number
of transcript variant | Primer | Primer sequence
(5′-3′) | Position | PCR product size
(bp) |
|---|
| [1] NM_002303.5
transcript variant 1 | S |
CAGAGTGATGCAGGTTTATATG | 2694–2715 | 208 |
|
| A |
CTGATGCTGTATGCTTGATAA | 2901–2881 |
|
| [2] NM_002303.5,
NM_001003680.3, NM_001003679.3 transcript variant 1–3 | S |
CGGGCGTTAAAGCTCTCGT | 78–96 | 391 |
|
| A |
GCTCACTCCGAAAGCAACAGT | 468–448 |
|
| [3] NM_001003680.3,
NM_001198687.1 transcript variant 2,4 | S |
GCCAGTAATTATTTCCTCTTCC | 2723–2744 | 198 |
|
| A |
CCCTGGGTACTTGAGATTAG | 2920–2901 |
|
|
|
|
| (in relation to
NM_001003680.3) |
|
| [4] NM_001003679.3,
NM_001198689.1 transcript variant 3,6 | S |
TATGTAATTGTGCCAGTAA | 2712–2730 | 200 |
|
| A |
ACATTGGGTTCATCTGTAGTG | 2911–2891 |
|
|
|
|
| (in relation to
NM_001003679.3) |
|
| [5] NM_001198687.1,
NM_001198688.1, NM_001198689.1 transcript variant 4–6 | S |
CTGCCTTCGGTCGAGTTGGA | 98–117 | 207 |
|
| A |
CAGCAGGCAAAAGGAAGTAGTCA | 304–282 |
|
| [6] NM_001198688.1
transcript variant 5 | S |
TCCCCATTGAGAAGTACCAGT | 2555–2575 | 300 |
|
| A |
CACCCAGTAGTTCCTTTGTGC | 2854–2834 |
|
| NM_000194.2 | S |
GCCATCACATTGTAGCCCTC | 343–362 | 172 |
| Homo sapiens
hypoxanthine phosphoribosyltransferase 1 | A |
ACTTTTATGTCCCCTGTTGACT | 514–493 |
|
Proliferation assay (in vitro culture
using a real-time cell analyzer - RTCA)
The study used an electrical impedance-based
technique, the real-time cell analyzer (RTCA, Roche Applied
Science, Penzberg, Germany). The system consists of an RTCA
analyzer, an RTCA SP station and RTCA software. As described by the
manufacturer, the main read-out of the RTPA is a dimensionless
parameter ‘Cell Index’. This index is derived as a relative change
in measured electrical impedance to represent cell status. From
RTCA software, the slope values of the proliferation rates were
extracted at times of exponential growth of the control groups (no
leptin addition), as indicated in the appropriate figures.
Six human normal prostate and prostate cancer cell
lines were cultured in E-Plate 48 of RTCA for at least 112 h. After
the first 24 h, cells were cultured in starved medium (medium
without growth factors) or in special FBS, charcoal stripped medium
(see above). At 48 h of culture, leptin at concentrations
1×10−6, 1×10−8 and 1×10−10 M was
added and cells were incubated for at least 48 h. Cell index was
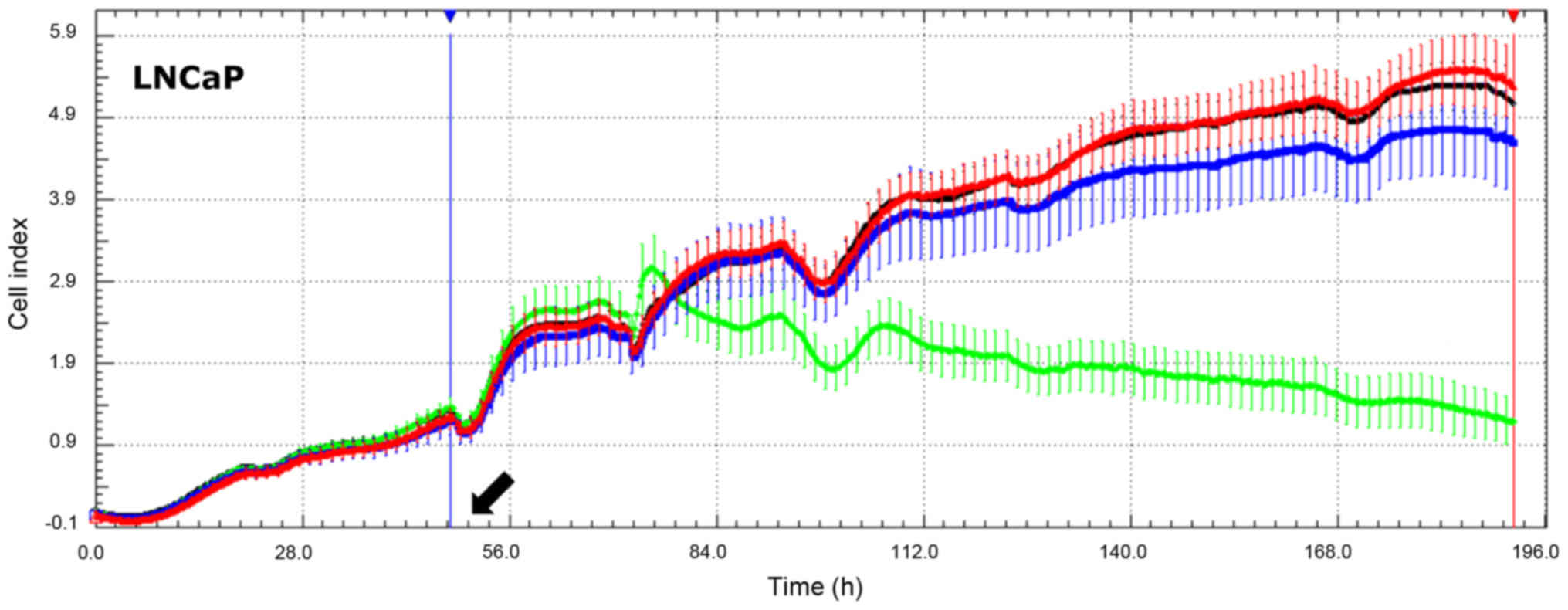
recorded every 15 min and an exemplary chart plot is shown in
Fig. 6. Subsequently this cell
index was normalized (normalized cell index). To improve the
readability of figures, on the normalized diagrams, points are
marked every 45 min. From RTCA software, the slope values of the
proliferation rates of cultured human normal prostate and prostate
cancer cell lines were extracted from each well. Each experiment
was repeated at least three times.
Statistical analysis
Where applicable, data presented are means ± SEM.
Statistical analysis of the data was performed using one-way
analysis of variance (ANOVA) followed by Tukey's HSD test.
Calculation was made by means of R environment with multcomp
library. Results were considered as statistically significant when
P values from ANOVA were <0.05. In such cases, post-hoc Tukey's
HSD test was performed. In this case, the threshold of statistical
significance was set again to 0.05. In the figures, results of the
Tukey's HSD test are marked by letters. Groups sharing the same
letter are not significantly different according to Tukey's HSD
test.
Results
Expression of leptin receptor
isoforms
As summarized in Table
III, expression of the leptin receptor variant 1 was not
detected in LNCaP and PrSMC cell lines, but it was found in the
remaining cell lines. On the other hand, in all examined cell
lines, isoforms 1–3, and 2 and 4 of the leptin receptor were found.
The expression of isoforms 3 and 6 of the leptin receptor was
observed in PC3, PrEC, PrSMC and PrSC cell lines, but not in LNCaP
and DU145 cells. Expression of the leptin receptor isoforms 4–6 and
5 was not demonstrated in any of the tested cell lines. Analysis of
the data showed that from the amplification product of variants 2
and 4, the expression of variant 4 may be ruled out. It follows
that all tested cell lines expressed variant 2 of the leptin
receptor. In turn, in lines PC3, PrEC, PrSMC and PrSC from the
amplification product of variant 3 and 6 of the leptin receptor,
isoform 6 can be excluded. Therefore, observed expression is caused
exclusively by isoform 3 of the leptin receptor. It should be noted
that in samples obtained from human adrenal we found expression of
all isoforms of the leptin receptor. This proves the correctness of
the primers used in the present study.
 | Table III.Analysis of expression of the leptin
receptor isoforms in the tested cell lines of the human
prostate. |
Table III.
Analysis of expression of the leptin
receptor isoforms in the tested cell lines of the human
prostate.
| Neoplastic prostate
cells |
|---|
|
|---|
| Cell line
(identified transcript variant) | LNCaP (var 2) | DU145 (var 1,
2) | PC3 (var 1, 2,
3) |
|---|
|
|
|
|
|---|
| Leptin
concentration (M)→ |
10−6 |
10−8 |
10−10 |
10−6 |
10−8 |
10−10 |
10−6 |
10−8 |
10−10 |
|---|
| Leptin receptor
isoform expression↓ |
|
|
|
|
|
|
|
|
|
| var
1 | – | – | – | = | = | = | = | = | = |
| var
1–3 | = | = | = | = | = | = | = | = | = |
| var 2,
4 | = | = | = | = | ▼ | ▼ | = | = | = |
| var 3,
6 | – | – | – | – | – | – | = | = | = |
| Proliferation
rate | ▼ | = | = | ▲ | = | = | = | = | = |
|
| Normal prostate
cells |
|
| Cell line
(identified transcript variant) | PrEC (var 1, 2,
3) | PrSMC (var 2,
3) | PrSC (var 1, 2,
3) |
|
|
|
|
| Leptin
concentration (M)→ |
10−6 |
10−8 |
10−10 |
10−6 |
10−8 |
10−10 |
10−6 |
10−8 |
10−10 |
|
| Leptin receptor
isoform expression↓ |
|
|
|
|
|
|
|
|
|
| var
1 | = | = | = | – | – | – | = | = | ▲ |
| var
1–3 | = | = | = | = | = | = | = | = | = |
| var 2,
4 | = | ▼ | ▼ | = | = | = | = | = | ▲ |
| var 3,
6 | = | = | = | = | ▼ | = | = | = | ▲ |
| Proliferation
rate | = | = | = | = | = | = | = | ▲ | ▲ |
We also conducted experiments on the effects of
leptin on the expression of its receptor isoforms in all tested
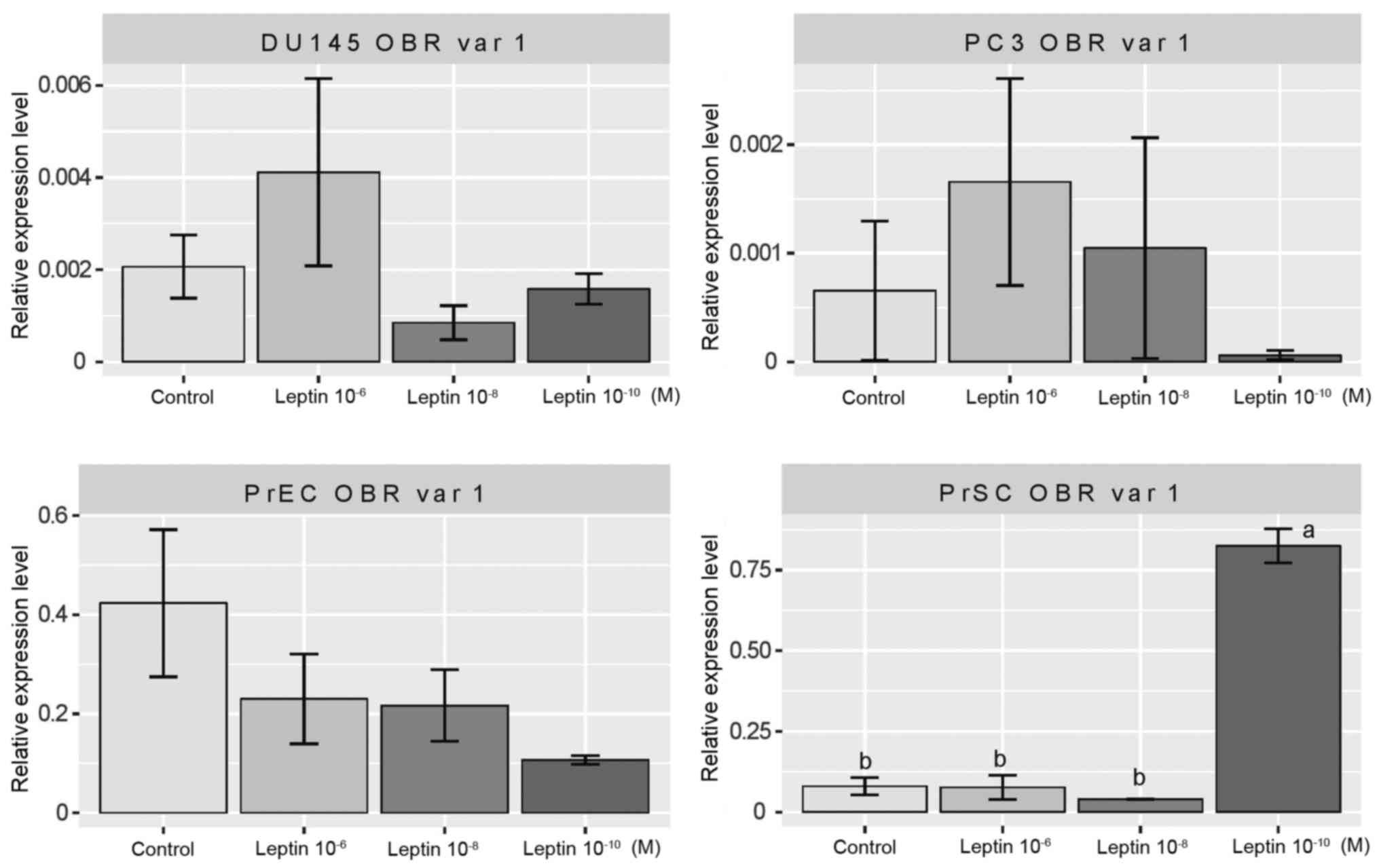
cell lines. As is apparent from Fig.
2 at a wide range of concentrations, leptin did not change the
expression of leptin receptor variant 1 in DU145, PrEC and PC3 cell
lines. In contrast, in the PrSC cell line, leptin at
10−10 M significantly increased the expression of the
gene of interest, while higher concentrations of the cytokine did
not exert such an effect.
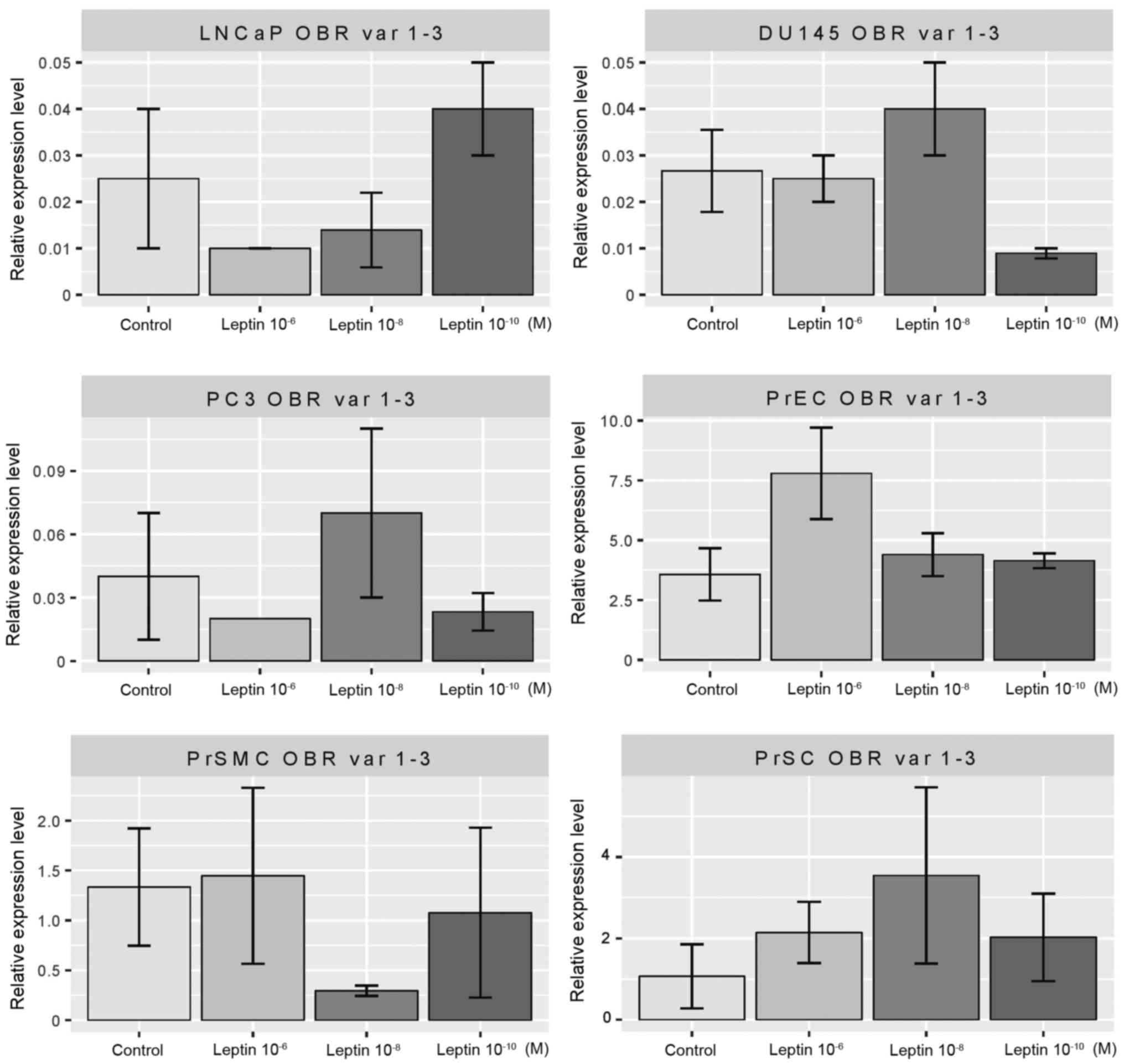
In the prostate cell lines tested, LNCaP, PC3,
DU145, PrEC, PrSMC and PrSC, leptin did not change the expression
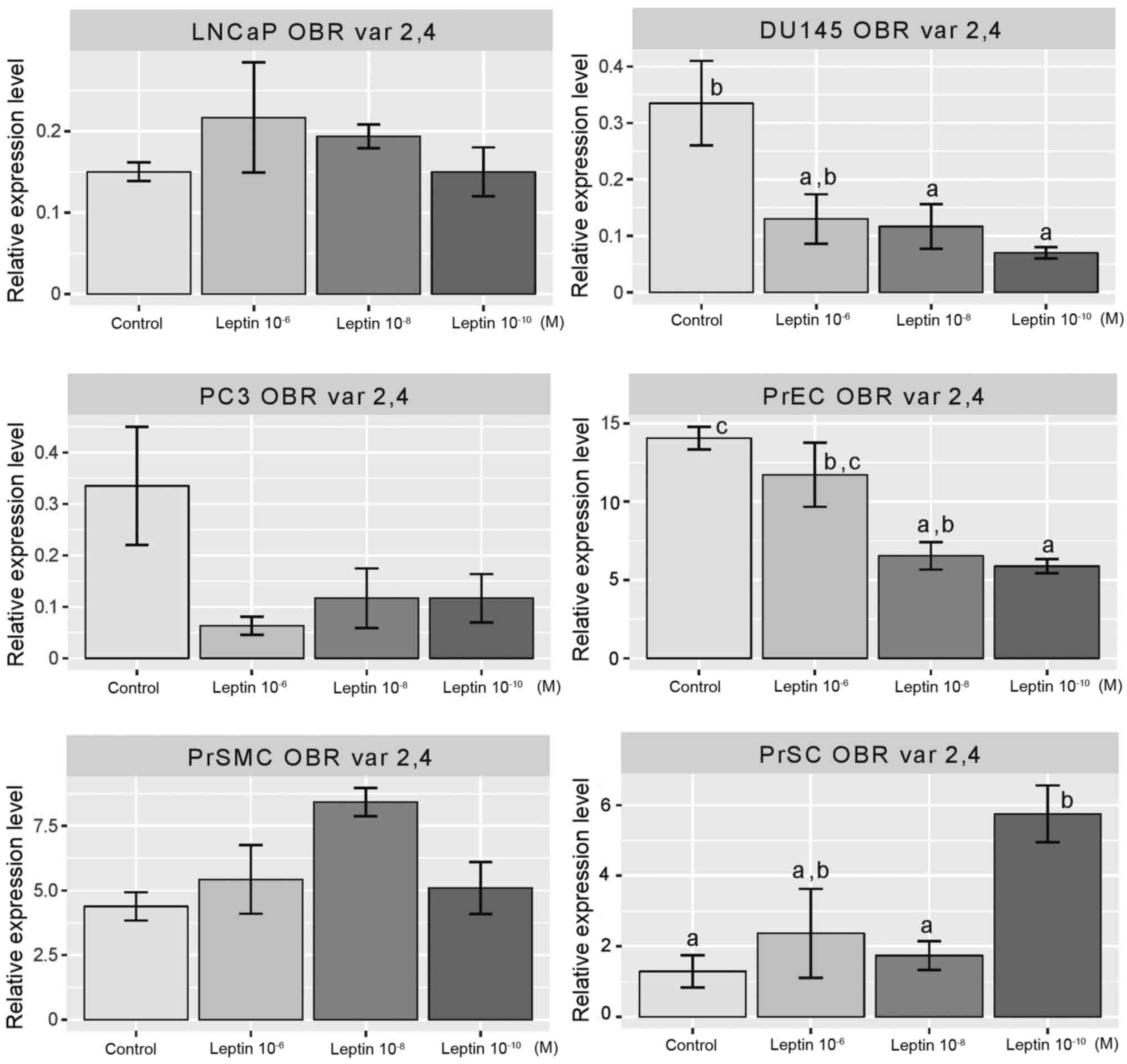
levels of variants 1–3 of the leptin receptor isoforms (Fig. 3). Concerning variants 2 and 4 of the
leptin receptor isoforms, the applied cytokine did not alter their
expression in the LNCaP, PC3 and PrSMC cell lines (Fig. 4). In the DU145 (at 10−8
and 10−10 M) and PrEC cells (at concentrations of
10−8 and 10−10 M) leptin inhibited expression
of the receptor isoforms tested. In contrast, in the PrSC cells (at
10−10 M), this cytokine substantially enhanced
expression of the studied genes.
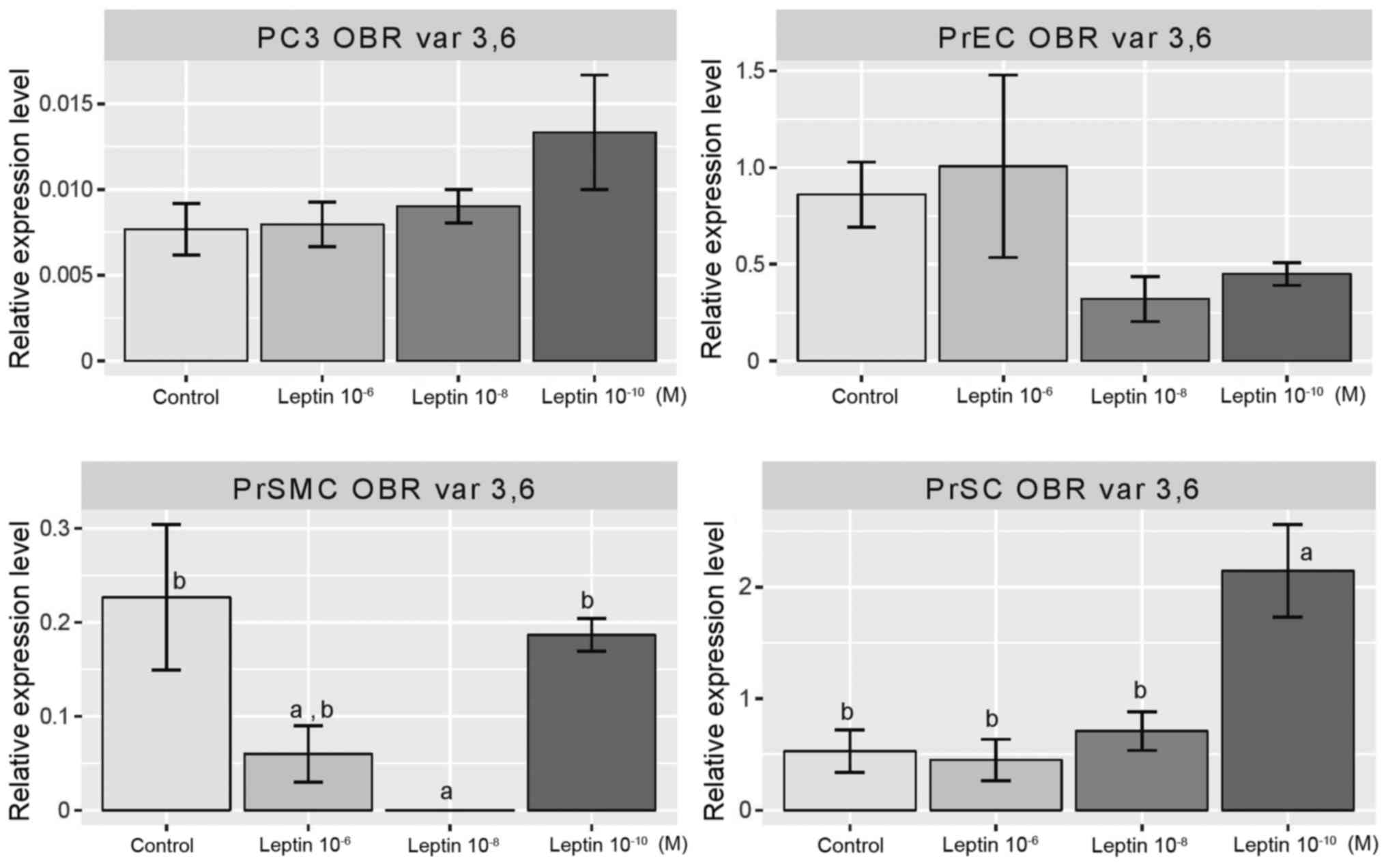
Leptin did not affect the expression level of
variants 3 and 6 of its receptor in the PC3 and PrEC cell lines
(Fig. 5). However, in PrSMC cells
leptin at concentrations of 10−6 and 10−8 M
inhibited the expression of the genes of interest. In contrast, in
the PrSC cell line, this cytokine at 10−10 M
significantly increased the level of expression of the receptor
isoforms tested.
Proliferation assay. In this study, we were
interested in using the RTCA platform to monitor the effects of
leptin on proliferation of human normal prostate and prostate
cancer cell lines. As documented in Fig. 7, in the DU145 cell line leptin at a
concentration 1×10−6 M stimulated the proliferation
rate, while lower leptin concentrations were ineffective. In
contrast, in the LNCaP cell line, the same leptin concentration
exerted an inhibitory effect on proliferative activity, and again
lower leptin concentrations did not change the studied parameter.
For all leptin concentrations, the proliferation rate of PC3 cells
remained unchanged. Likewise, at all concentrations, leptin did not
affect the proliferation of PrEC or PrSMC cell lines. In contrast,
leptin at concentrations 1×10−8 and 1×10−10 M
notably stimulated proliferative activity of the PrSC (prostate
stromal cell) cell line.
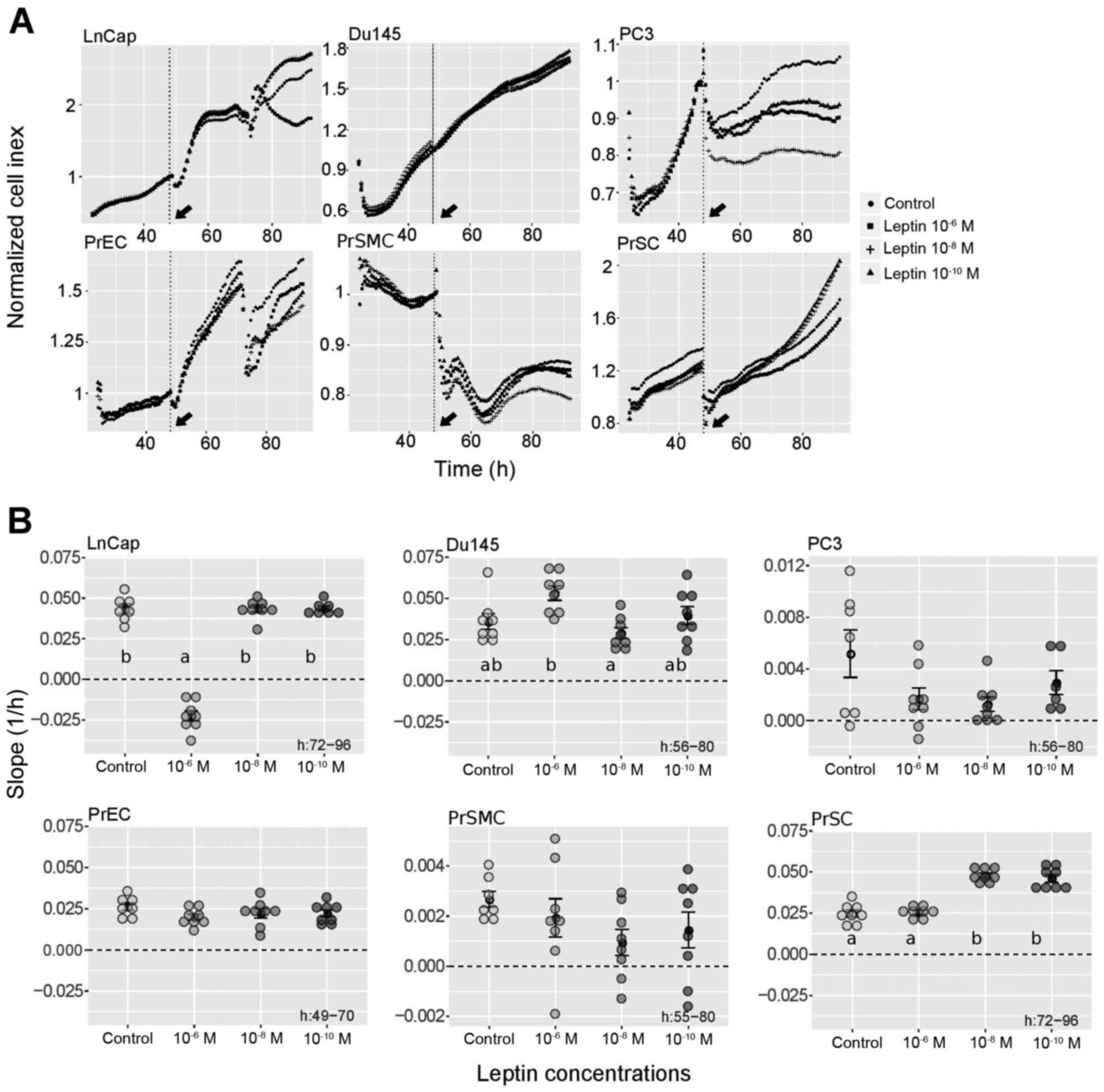 | Figure 7.Effects of leptin on the
proliferative activity of cultured human normal prostate and
prostate cancer cell lines. (A) Normalized RTCA chart plot (cell
index, recorded every 15 min) in which points are marked every 45
min. (B) Effects of leptin on slope values of proliferation rates
of cultured human normal prostate and prostate cancer cell lines.
The human prostate cancer cell lines: DU145, LNCaP, PC3. The human
normal prostate cell lines: PrEC (prostate epithelial cells), PrSC
(prostate stromal cells) and PrSMC (prostate smooth muscle cells).
At 48 h of culture, leptin at concentrations 1×10−6,
1×10−8 and 1×10−10 M was added - black
arrows. Cells were incubated for at least 70 h. From RTCA software,
the slope values of proliferation rates were extracted at times of
exponential growth of the control (no leptin addition) groups, as
indicated with appropriate figures (h). (B) Each circle represents
one cell culture. Means ± SEM are shown. There were no
statistically significant differences between group means as
determined by one-way ANOVA for PC3 (P=0.0806), PrEC (P=0.308) and
PrSMC (P=0.283) cell lines. In the remaining cell lines, groups
sharing the same letter were not significantly different according
to Tukey's HSD test. |
Discussion
In light of the controversial data concerning the
influence of leptin on human normal prostate and prostate cancer
cell lines, we performed a comprehensive and systematic study on
LNCaP, DU145, PC3, PrEC, PrSMC and PrSC cell lines. In the first
instance, we examined the expression of leptin receptor isoforms in
the tested cell lines and the response of transcription variants of
the leptin receptor to leptin. Furthermore, we assessed the effects
of leptin on the proliferation rate of the studied cells.
Only scanty data are available on the expression of
leptin receptor in human normal prostate and prostate cancer cell
lines. As mentioned earlier, two functional leptin receptor
isoforms (huOB-R and huB219.3) were found in DU145, PC3, and
LNCaP-FGC cell lines (30).
Furthermore, expression of huOb-Ra and huOb-Rb was reported in
DU145 and PC3 lines (31). In these
studies, identical primers were used and described as a primers for
huB219.3 (30) and OB-Ra (31). In their studies, expression of these
leptin receptor isoforms was found in DU145, PC3, and LNCaP cell
lines. Comparing the binding of these primers to the current
isoforms of the leptin receptor gene (Fig. 1) indicates that these primers
amplify variants 3 and/or 6 of receptor isoforms (as described in
the present study). Furthermore, the primer for huOB-Rb applied by
Somasundar et al (31)
amplifies only a fragment of the single exon specific to isoform 1.
Therefore this design did not reduce the risk of false-positive
amplification from genomic DNA. On the other hand, in a study by
Onuma et al (30), exons
spinning primers designed for huObR bound to specific exon of
variant 1 of the leptin receptor. They found this expression in the
LNCaP cell line; however, in the present study we were not able to
confirm their finding. Onuma et al (30) also studied expression of the isoform
huB219.1 of the leptin receptor gene. Applied primers are specific
for variants 2 and 4. Expression of these variants was very high in
LNCaP cells. In the present study, application of the various
primers revealed that in the LNCaP cell line expression of only
variant 2 of the leptin receptor gene was present (Table III). Furthermore, in the study by
Onuma et al (30), the
primers used for demonstration of huB219.2 were highly specific for
variant 5 of the leptin receptor gene. On the basis of agarose gel
electrophoresis data they found low expression levels of this
isoform in the LNCaP cell line, the highest expression in DU145 and
no expression in PC3 cells. In the present study by means of qPCR,
we were not able to demonstrate expression of isoform 5 in all
studied cell lines. However, as pointed above, a positive reaction
for this isoform was found in human adrenal glands. Furthermore, in
the study of Onuma et al (30) huB219.3 expression was found in DU145
and PC3 cells, and only very low expression was noted in the LNCaP
cells. The same primers in a study by Somasundar et al
(31), described as specific for
huOb-Ra, provided transcripts in DU145 and PC3 cells. However, in
relation to actual nomenclature, applied primers may demonstrate
variants 3 and/or 6 of leptin receptor isoforms. Our study did not
reveal expression of isoform 6 in all analyzed cell lines;
therefore, observed expression in their study may be connected with
variant 3 of the leptin receptor.
Analysis of the literature indicates that only one
publication contains data on the relationship between leptin and
leptin receptor expression in human prostate cancer cell lines. By
densitometry of agarose separated PCR products Somasundar et
al (31) demonstrated that
during 48 h of culture in serum-free medium, leptin did not alter
the expression of the huOB-Ra isoform in DU145 cells, while in the
PC3 cell line expression of this isoform was significantly
increased. As it follows from the above analysis, their primers for
huOB-Ra recognized variants 3 and/or 6 of leptin receptor isoforms.
In the case of the huOB-Rb isoform, leptin did not change
expression of this gene in the DU145 cells and notably increased it
in the PC3 cell line. According to the recent nomenclature, huOB-Rb
is called variant 1 of the leptin receptor gene. In our study we
observed a differential effect of leptin on the expression of
leptin receptor isoforms in the studied cells. Observed effects
were dependent mainly on leptin concentrations. In our study,
leptin stimulated expression of leptin receptor variant 1 only in
the PrSC cell line (at a concentration of 10−10 M).
Expression levels of variants 2 and 4 of the leptin receptor were
suppressed by leptin in the DU145 and PrEC cell lines and opposite
effects were observed in the PrSC cells. As far as leptin variants
3 and 6 are concerned, leptin inhibited their expression in PrSMC
cells and stimulated it in PrSC cells. Thus, our study showed that
among the tested cell lines, leptin exerted the highest stimulatory
effect on the expression of its receptor isoforms in PrSC cells. In
the other cell lines, the effects of leptin on the expression of
its receptor isoforms did not show any regularity. Recently, Noda
et al (38) demonstrated
that long-term exposure to leptin (28 days) increased ObR (leptin
receptor) expression in the LNCaP, DU145 and PC3 cell lines. In
this study, ObR was demonstrated by western blot analysis with
polyclonal rabbit antibodies against human ObR (Sigma-Aldrich).
However, these data are not sufficient to identify the specific
variant of the leptin receptor.
In addition, data concerning the effects of leptin
on the proliferation of human prostate cancer cell lines are
controversial. By means of 3H-thymidine incorporation
and MTT assay, Onuma et al (30) demonstrated that in serum-free medium
leptin stimulated cell proliferation specifically in
androgen-independent DU145 and PC3 prostate cancer cells but not in
androgen-dependent LNCaP-FGC cells, although both cell types
express functional leptin receptor isoforms. Similar results (by
means of MTT assay) were observed in DU145 and PC3 cells cultured
in FBS-containing culture medium (31,39).
By means of MTT assay, the stimulatory effect of leptin on the
growth of DU145 cells was also observed by Bub et al
(40). Recently Noda et al
(38) demonstrated that a 48-h
exposure to leptin did not affect the growth of LNCaP, DU145 and
PC3, cells as assessed by WST-8 assay. However, after a 28-day
incubation with leptin, a notable increase in growth in all tested
cell lines was found.
In the present study, using the RTCA platform, we
examined the effects of leptin on the proliferation of LNCaP,
DU145, PC3, PrEC, PrSMC and PrSC cell lines in the exponential
phase of growth. As documented by changes in the proliferation
slope, leptin inhibited the proliferation of LNCaP cells,
stimulated growth of PrSC cells and had no effect on DU145, PC3,
PrEC and PrSMC cells. A markedly pronounced stimulatory effect of
leptin on proliferative activity was observed in the PrSC cell
line. In the available literature, we did not encounter data
concerning the effect of leptin on the proliferation of human
normal prostate cell lines. Therefore, this observation appears to
be extremely important, as it may explain the increased incidence
of benign prostatic hyperplasia in obese patients with elevated
blood leptin levels.
Considering the relationships between mitogenic
action of leptin and expression of leptin receptor isoforms in
DU145 and PC3 cell lines, Somasundar et al (31) suggested that mitogenic leptin
effects are not a consequence of altered receptor isoform
expression. It should be emphasized that in our study, the level of
expression of leptin receptor isoforms was examined 48 h after
leptin administration, while the ‘proliferation slope’ was
evaluated after exposure of cells to leptin during the exponential
growth phase (see Fig. 7B for
details). In this situation, changes in the expression level of
leptin receptor isoforms can not be ruled out [Noda et al
(38)]. Thus, our data on the
degree of expression of the leptin receptor isoforms analyzed
reflect the condition after 48 h after leptin administration, which
does not exclude changes in their expression level during
subsequent incubation periods (corresponding to the proliferation
slope). Our results also indicated no correlation between the level
of expression of individual leptin receptor isoforms and the
mitogenic effect of leptin protein. The analysis of our data
suggest, however, that in prostatic cells which are deficient in
leptin receptor variant 1, leptin rather inhibits the mitogenic
activity while in cells expressing this variant rather stimulates
this activity or does not change this parameter.
Thus, the present study demonstrated that in all
tested human normal prostate and prostate cancer cell lines (LNCaP,
DU145, PC3, PrEC, PrSMC and PrSC) transcription variants 4, 5 and 6
of the leptin receptor were not expressed. Leptin receptor
transcription variants 1, 2 and 3 showed differential expression,
all of them present in the PC3, PrEC and PrSC cell lines. Our data
also suggest that the stimulating effects of leptin on
proliferative activity of the studied cell lines require the
expression of leptin receptor variant 1 in the affected cells.
Acknowledgements
The study was supported by grant no.
2011/03/N/NZ3/06095 from the National Science Center, Krakow,
Poland.
References
|
1
|
Bergström A, Pisani P, Tenet V, Wolk A and
Adami HO: Overweight as an avoidable cause of cancer in Europe. Int
J Cancer. 91:421–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hsing AW, Chua S Jr, Gao YT, Gentzschein
E, Chang L, Deng J and Stanczyk FZ: Prostate cancer risk and serum
levels of insulin and leptin: A population-based study. J Natl
Cancer Inst. 93:783–789. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray GA: The underlying basis for obesity:
Relationship to cancer. J Nutr. 132 Suppl:S3451–S3455. 2002.
|
|
4
|
Calle EE, Rodriguez C, Walker-Thurmond K
and Thun MJ: Overweight, obesity, and mortality from cancer in a
prospectively studied cohort of U.S. adults. N Engl J Med.
348:1625–1638. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allott EH, Masko EM and Freedland SJ:
Obesity and prostate cancer: Weighing the evidence. Eur Urol.
63:800–809. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hoda MR, Mohammed N, Theil G, Fischer K
and Fornara P: Obesity and prostate cancer. Role of adipocytokines
and clinical implications. Urologe A. 51:1253–1260. 2012.(In
German).
|
|
7
|
Hoda MR, Theil G, Mohammed N, Fischer K
and Fornara P: The adipocyte-derived hormone leptin has
proliferative actions on androgen-resistant prostate cancer cells
linking obesity to advanced stages of prostate cancer. J Oncol.
2012:2803862012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao Y and Giovannucci E: Obesity and
prostate cancer. Recent Results Cancer Res. 208:137–153. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patel SB, Reams GP, Spear RM, Freeman RH
and Villarreal D: Leptin: Linking obesity, the metabolic syndrome,
and cardiovascular disease. Curr Hypertens Rep. 10:131–137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaur J: A comprehensive review on
metabolic syndrome. Cardiol Res Pract. 2014:9431622014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mantzoros CS: The role of leptin in human
obesity and disease: A review of current evidence. Ann Intern Med.
130:671–680. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Klok MD, Jakobsdottir S and Drent ML: The
role of leptin and ghrelin in the regulation of food intake and
body weight in humans: A review. Obes Rev. 8:21–34. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Halaas JL, Gajiwala KS, Maffei M, Cohen
SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK and Friedman JM:
Weight-reducing effects of the plasma protein encoded by the obese
gene. Science. 269:543–546. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cummings DE and Foster KE: Ghrelin-leptin
tango in body-weight regulation. Gastroenterology. 124:1532–1535.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Konturek PC, Konturek JW,
Cześnikiewicz-Guzik M, Brzozowski T, Sito E and Konturek SJ:
Neuro-hormonal control of foodintake: Basic mechanisms and clinical
implications. J Physiol Pharmacol. 56 Suppl 6:5–25. 2005.
|
|
16
|
Perry B and Wang Y: Appetite regulation
and weight control: The role of gut hormones. Nutr Diabetes.
2:e262012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Proenca R, Maffei M, Barone M,
Leopold L and Friedman JM: Positional cloning of the mouse obese
gene and its human homologue. Nature. 372:425–432. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Maffei M, Halaas J, Ravussin E, Pratley
RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al:
Leptin levels in human and rodent: Measurement of plasma leptin and
ob RNA in obese and weight-reduced subjects. Nat Med. 1:1155–1161.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Friedman JM and Halaas JL: Leptin and the
regulation of body weight in mammals. Nature. 395:763–770. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tartaglia LA, Dembski M, Weng X, Deng N,
Culpepper J, Devos R, Richards GJ, Campfield LA, Clark FT, Deeds J,
et al: Identification and expression cloning of a leptin receptor,
OB-R. Cell. 83:1263–1271. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cioffi JA, Shafer AW, Zupancic TJ,
Smith-Gbur J, Mikhail A, Platika D and Snodgrass HR: Novel B219/OB
receptor isoforms: Possible role of leptin in hematopoiesis and
reproduction. Nat Med. 2:585–589. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahima RS and Flier JS: Leptin. Annu Rev
Physiol. 62:413–437. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Malendowicz W, Rucinski M, Macchi C,
Spinazzi R, Ziolkowska A, Nussdorfer GG and Kwias Z: Leptin and
leptin receptors in the prostate and seminal vesicles of the adult
rat. Int J Mol Med. 18:615–618. 2006.PubMed/NCBI
|
|
24
|
Maamra M, Bidlingmaier M, Postel-Vinay MC,
Wu Z, Strasburger CJ and Ross RJ: Generation of human soluble
leptin receptor by proteolytic cleavage of membrane-anchored
receptors. Endocrinology. 142:4389–4393. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ge H, Huang L, Pourbahrami T and Li C:
Generation of soluble leptin receptor by ectodomain shedding of
membrane-spanning receptors in vitro and in vivo. J Biol Chem.
277:45898–45903. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schultz SC and Widmaier EP: Leptin
receptorsLeptin. Castracane VD and Henson MC: 25. Springer;
Endocrine Updates, Heidelberg: pp. 11–31. 2007, View Article : Google Scholar
|
|
27
|
Wada N, Hirako S, Takenoya F, Kageyama H,
Okabe M and Shioda S: Leptin and its receptors. J Chem Neuroanat
61–62. 191–199. 2014. View Article : Google Scholar
|
|
28
|
Stattin P, Söderberg S, Hallmans G, Bylund
A, Kaaks R, Stenman UH, Bergh A and Olsson T: Leptin is associated
with increased prostate cancer risk: A nested case-referent study.
J Clin Endocrinol Metab. 86:1341–1345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Malendowicz W and Kwias Z: Leptin receptor
isoforms in benign prostatic hyperplasia (BPH). BPH and prostate
cancer - no association between plasma concentrations of leptin and
prostate specific antigen (PSA). Cent European J Urol. 62:96–100.
2009. View Article : Google Scholar
|
|
30
|
Onuma M, Bub JD, Rummel TL and Iwamoto Y:
Prostate cancer cell-adipocyte interaction: Leptin mediates
androgen-independent prostate cancer cell proliferation through
c-Jun NH2-terminal kinase. J Biol Chem. 278:42660–42667. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Somasundar P, Frankenberry KA, Skinner H,
Vedula G, McFadden DW, Riggs D, Jackson B, Vangilder R, Hileman SM
and Vona-Davis LC: Prostate cancer cell proliferation is influenced
by leptin. J Surg Res. 118:71–82. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paschke L, Rucinski M, Ziolkowska A,
Zemleduch T, Malendowicz W, Kwias Z and Malendowicz LK: ZFP91 - a
newly described gene potentially involved in prostate pathology.
Pathol Oncol Res. 20:453–459. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Szyszka M, Paschke L, Tyczewska M,
Rucinski M, Grabowska P and Malendowicz LK: Lack of expression of
preproorexin and orexin receptors genes in human normal and
prostate cancer cell lines. Folia Histochem Cytobiol. 53:333–341.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rucinski M, Albertin G, Spinazzi R,
Ziolkowska A, Nussdorfer GG and Malendowicz LK: Cerebellin in the
rat adrenal gland: Gene expression and effects of CER and
(des-Ser1)CER on the secretion and growth of cultured
adrenocortical cells. Int J Mol Med. 15:411–415. 2005.PubMed/NCBI
|
|
35
|
Rucinski M, Ziolkowska A, Tyczewska M and
Malendowicz LK: Expression of prepro-ghrelin and related receptor
genes in the rat adrenal gland and evidences that ghrelin exerts a
potent stimulating effect on corticosterone secretion by cultured
rat adrenocortical cells. Peptides. 30:1448–1455. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tyczewska M, Rucinski M, Ziolkowska A,
Szyszka M, Trejter M, Hochol-Molenda A, Nowak KW and Malendowicz
LK: Enucleation-induced rat adrenal gland regeneration: Expression
profile of selected genes involved in control of adrenocortical
cell proliferation. Int J Endocrinol. 2014:1303592014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Markowska A, Belloni AS, Rucinski M,
Parenti AR, Nardelli GB, Drews K, Nussdorfer GG and Malendowicz LK:
Leptin and leptin receptor expression in the myometrium and uterine
myomas: Is leptin involved in tumor development? Int J Oncol.
27:1505–1509. 2005.PubMed/NCBI
|
|
38
|
Noda T, Kikugawa T, Tanji N, Miura N, Asai
S, Higashiyama S and Yokoyama M: Long-term exposure to leptin
enhances the growth of prostate cancer cells. Int J Oncol.
46:1535–1542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Somasundar P, Yu AK, Vona-Davis L and
McFadden DW: Differential effects of leptin on cancer in vitro. J
Surg Res. 113:50–55. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bub JD, Miyazaki T and Iwamoto Y:
Adiponectin as a growth inhibitor in prostate cancer cells. Biochem
Biophys Res Commun. 340:1158–1166. 2006. View Article : Google Scholar : PubMed/NCBI
|