Introduction
Liver kinase B1 (LKB1), also known as
serine/threonine kinase 11 (STK11), plays critical roles in cell
growth, differentiation, polarity and migration (1,2). LKB1
signaling controls energy metabolism and tissue homeostasis, and
deletion of the LKB1 gene is embryonic-lethal (3). LKB1 signaling is also highly involved
in human diseases. Germ-line mutations in LKB1 are associated with
the predisposition of Peutz-Jeghers syndrome (4). Loss of LKB1 expression by either
somatic mutations or promoter hypermethylation is frequently
identified in sporadic cancers including lung cancer (1). Disruption of LKB1 gene function
promotes tumor progression in multiple animal tumor models
(1). As such, LKB1 is considered as
a tumor suppressor in general. Mechanistically, LKB1 regulates
cellular events by targeting multiple critical signaling pathways,
including AMPK/mTOR, p53 and PTEN/Akt (5).
Accumulating evidence has demonstrated that
extracellular vesicles, such as exosomes and microvesicles, carry
and transmit cellular molecules and signals, and mediate cell-cell
communications (6). In cancers,
this process is shown to be important for modulating the tumor
microenvironment, in which tumor cells and tumor-associated cells
intercommunicate to control tumor progression (7). Exosomes secreted by cancer cells can
target both tumor cells (autocrine actions) and other types of
cells associated with tumors (paracrine actions). Of the molecules
contained in exosomes, microRNAs (miRNAs) have received the most
attention due to their diverse and critical roles in tumor
progression and their highly potential diagnostic and therapeutic
applications in cancer treatment (8). Notably, while intracellular LKB1
signaling has been well-studied, its roles in extracellular
vesicle-mediated cell signaling remain unclear. In the present
study, we found that restoration of LKB1 in LKB1-deficient H460 and
A549 lung cancer cells markedly enhanced motility and increased
secretion of exosomes. Importantly, in comparison with those from
H460 cells with LKB1 deficiency, exosomes secreted by H460 cells
with restoration of LKB1 had highly increased ability to promote
cancer cell migration. Mechanistically, restoration of LKB1 in H460
cells inhibited cellular expression and exosomal secretion of
migration-suppressing miRNAs, including miR-125a, miR-126 and
let7b.
Materials and methods
Generation of a construct for
lentiviral expression of human LKB1 (pCDH-LKB1)
The pCDNA3-Flag-LKB1 construct was a gift from Dr
Lewis Cantley (Addgene, plasmid #8590; Cambridge, MA, USA).
pCDH-LKB1 was generated by inserting the Flag-LKB1 fragment
released from pCDNA3-Flag-LKB1 into a lentiviral expression vector
pCDH-CMV-MCS-EF1-Puro (System Biosciences, Mountain View, CA, USA)
by EcoRI digestion. The resulting clone was verified by DNA
sequencing.
Cell culture
Cell lines 293T, H460 and A549 were purchased from
the American Type Culture Collection (ATCC; Manassas, VA, USA).
293T cells were cultured in Dulbecco's modified Eagles medium
supplemented with 10% fetal bovine serum (FBS). H460 and A549 cell
lines were maintained in RPMI-1640 medium supplemented with 10%
FBS. All the culture media and supplements were purchased from
Invitrogen (Carlsbad, CA, USA).
Generation of H460 and A549 cell pools
stably expressing LKB1 by lentiviral transduction
Production of pseudolentiviral particles and stable
cell pools by lentiviral transduction was performed by following
the manufacturer's instructions (System Biosciences).
Pseudolentiviruses were produced in 293T cells by co-transfecting
pCDH-LKB1 (or pCDH-CMV-MCS-EF1-Puro control vector) and pPACK
packaging plasmid mix (System Biosciences) using FuGENE HD reagent
(Roche Applied Biosciences, San Diego, CA, USA). Pseudoviral
particles were harvested 48 h post-transfection and concentrated
using PEG-it™ Virus Precipitation Solution following the
manufacturer's instructions (System Biosciences). H460 or A549 lung
cancer cells were transduced with the prepared lentiviruses in the
presence of Polybrene (5 µg/ml) in culture media. Two days
post-transduction, the cells were split and selected by puromycin
(1 µg/ml) for 10 days for obtaining stable cell pools.
Western blotting
Cells were lysed with EBC lysis buffer [50 mM Tris,
pH 7.5, 150 mM NaCl, 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride
(PMSF), 1 mM complete protease inhibitors (Roche Diagnostics,
Mannheim, Germany), 10 mM NaF, 1 mM sodium orthovanadate]. Total
cell lysates were cleared by centrifugation at 13,000 rpm for 10
min at 4°C. The supernatant (protein lysate) was added with 5X
Laemmli sample buffer and boiled at 95°C for 5 min for denaturing
the proteins. Protein samples were resolved on 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and were
transferred to nitrocellulose membranes. Western blotting was
performed by first blocking nitrocellulose membranes with 5%
non-fat milk in PBS-T buffer for 30 min, followed by overnight
incubation with the primary antibody at 4°C and 1 h incubation with
appropriate secondary antibody at room temperature. The western
blotting was visualized by enhanced chemiluminescent (ECL) reagent
(Pierce, Rockford, IL, USA). Primary antibodies used in western
blotting were: anti-LKB1 (Cell Signaling Technology, Danvers, MA,
USA) and anti-actin (Sigma-Aldrich, St. Louis, MO, USA).
Cell morphology analysis
H460 and A549 stable cell pools were plated into a
6-well plate at a density of 1×105 cells/well. When cell
density reached ~80% confluence, cells were photographed using a
phase-contrast microscope/camera system (Carl Zeiss, Jena, Germany)
at a magnification of ×40.
Cell migration assay
Cell migration was analyzed using a modified
two-chamber Transwell system (BD Biosciences, San Jose, CA, USA)
following the procedures previously described (9). Briefly, cells were detached by
trypsin-EDTA, washed once with 1X phosphate-buffered saline (PBS),
and then resuspended in serum-free medium. Complete culture media
(0.5 ml) was added to each bottom well. Cells (1×105)
were added to each Transwell insert and allowed to migrate for 16 h
in a 37°C cell incubator. Cells in the upper surface of the
Transwell were removed using cotton swabs. Migrated cells that had
attached to the undersurface were fixed with 4% paraformaldehyde
for 10 min and stained with crystal violet solution (0.5% in water)
for 10 min. Cells were counted under microscopy at a magnification
of ×100.
Cell colony formation and MTS cell
proliferation assays
Cell colony formation assay was performed using
Millipore transformation assay kit (Millipore, Bedford, MA, USA)
following the manufacturer's protocol. Briefly, a base agar layer
containing 0.8% agarose was first prepared in a 24-well plate. Cell
solution (0.25 ml) resuspended in complete culture media containing
0.4% agarose was then added to the top of the base agar layer
(1,200 cells/well). Cells were incubated for 2–3 weeks at 37°C with
5% CO2 and with medium exchange every 3 days. Colonies
were photographed using a Primovert microscope (Carl Zeiss, Jena,
Germany) equipped with a Motic camera/software system. Colonies
were then quantified either by staining with 0.5 ml of cell stain
solution overnight and counting, or by adding cell quantification
solution (0.08 ml/well) and measuring absorbance at 490 nm using
Synergy H1 microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA).
Cell proliferation was determined using the
CellTiter 96 AQueous One Solution Cell Proliferation Assay kit
(Promega, Madison, WI, USA), following the manufacturer's
instructions.
Exosome release and isolation
For induction of exosome production and release,
H460 cells were cultured in complete media until 80–90% confluence,
and then washed 3 times with PBS and cultured for 24 h in
serum-free media. The conditioned media were collected and
centrifuged at 300 xg for 10 min to remove dead cells. The
supernatant was centrifuged at 2,000 × g for 20 min to remove cell
debris, followed by centrifugation at 20,000 × g for 30 min. The
supernatant was then collected and exosomes were pelleted by
centrifugation at 169,000 × g for 90 min. The exosome pellet was
resuspended in 1X PBS and analyzed using an NanoSight NS300
instrument (Malvern Instruments, Amesbury, UK) according to the
manufacturer's instructions. The size distribution and
concentration of the secreted vesicles were recorded. Meantime,
cells left on the culture plate were trypsinized and counted by
Countless II (Life Science). The exosomes released/cell were then
determined.
Exosome uptake test
Exosomes were labeled with the red fluorescent dye
PKH26 (Sigma-Aldrich) according to the manufacturer's
recommendation. Briefly, isolated exosomes were resuspended in 2 ml
Diluent C solution containing 1 µl PKH26. After 5 min of
incubation, 2 ml of 1% bovine serum albumin was added to stop the
labeling reaction. The exosome suspension was centrifuged at
169,000 × g for 90 min to pellet the labeled exosomes, followed by
resuspension in 1 ml of complete medium. Resuspended exosomes were
counted, and then added to H460 cells at a dose of 100
exosomes/receipt cell for 18 h of incubation.
4′,6-Diamidino-2-phenylindone (DAPI; Invitrogen) was used for
nuclear staining. Cell images were captured using an inverted
microscope (EVOS; NY).
RNA extraction and RT-qPCR
Total RNA in cells or isolated exosomes was
extracted using mirVana miRNA Isolation kit (Ambion, Austin, TX,
USA) and reverse transcribed to cDNA using TaqMan Advanced MicroRNA
cDNA Synthesis kit (Thermo Fisher Scientific Waltham, MA, USA).
qPCR was carried out using TaqMan Advanced MicroRNA Assay kit
(Thermo Fisher Scientific) on the Applied Biosystems 7500 (Applied
Biosystems, Foster City, CA, USA) following the manufacturer's
instructions. The relative levels of let7b, miR-125a or miR-126
were normalized to that of hsa-miR-191-5p (for cellular samples) or
that of U6 (for exosome samples).
Statistical analysis
Results are expressed as mean ± SEM. Statistical
significance was determined by a two-sided Student's t-test.
P<0.05 was considered statistically significant.
Results
Restoration of LKB1 in LKB1-deficient
lung cancer cells markedly promotes cell motility
In an attempt to investigate the role of LKB1 in
exosome secretion and the related cellular signaling, we stably
restored LKB1 expression in LKB1-dificient H460 lung cancer cells
(10) by lentiviral transduction
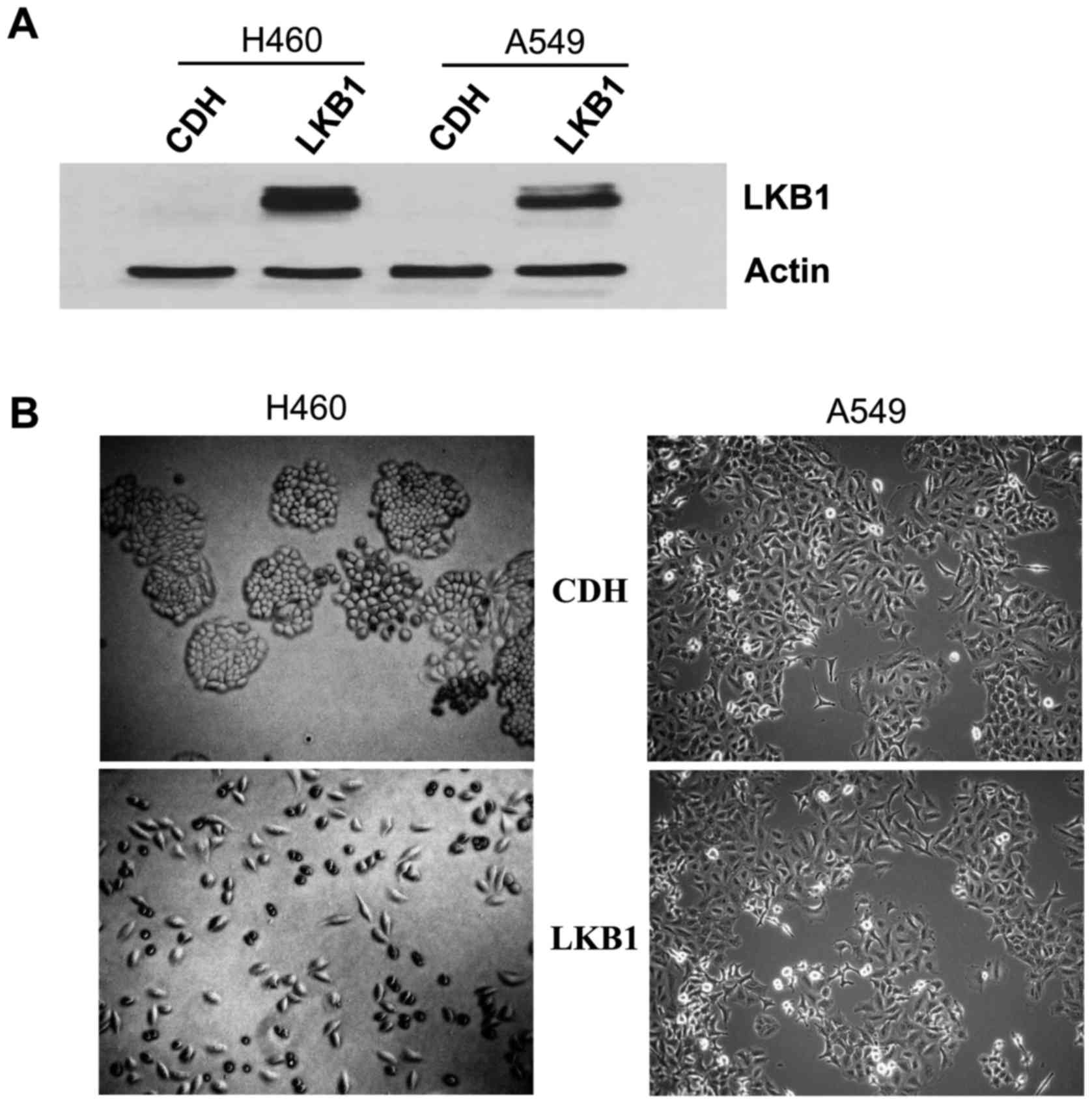
(Fig. 1A). We noted that
restoration of LKB1 led to a marked change in cell morphology in
the plastic plate (left panels, Fig.
1B). While H460 cells with stable expression of the lentiviral
vector (H460CDH) were similar to the parental H460 cells
that exhibited a round shape and were clustered, H460 cells with
stable expression of LKB1 (H460LKB1) had an elongated
shape and were well-dispersed (Fig.
1B), implying the increased motility of the H460LKB1
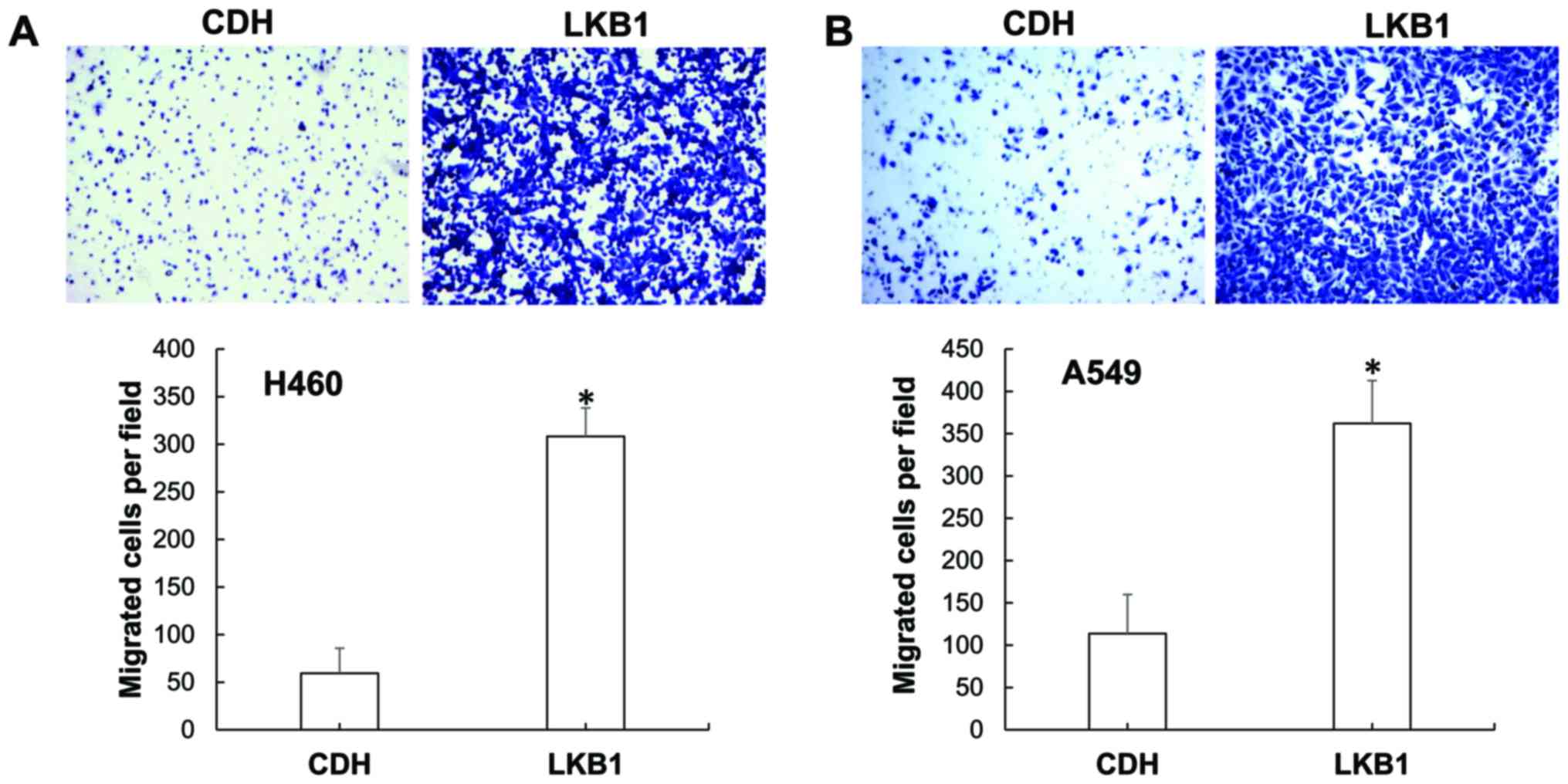
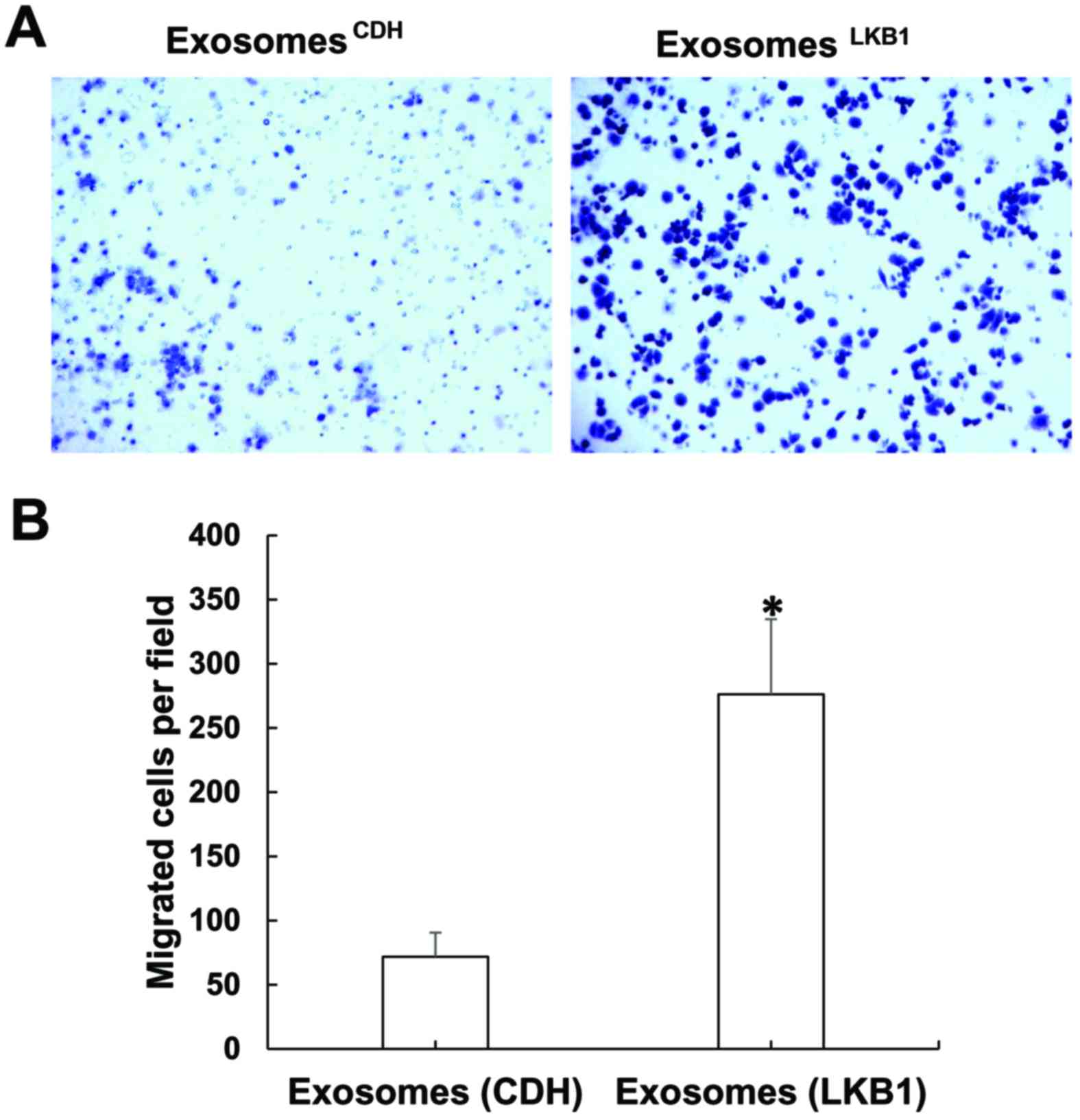
cells. Indeed, compared with the H460CDH cells,
H460LKB1 cells exhibited greatly increased migration
ability (Fig. 2A). We then decided
to confirm this finding using another LKB1-deficient lung cancer
cell line: A549. While restoration of LKB1 in A549 cells did not
cause an obvious change in cell morphology (right panels, Fig. 1B), it did greatly increase A549 cell
migration (Fig. 2B). As LKB1 is
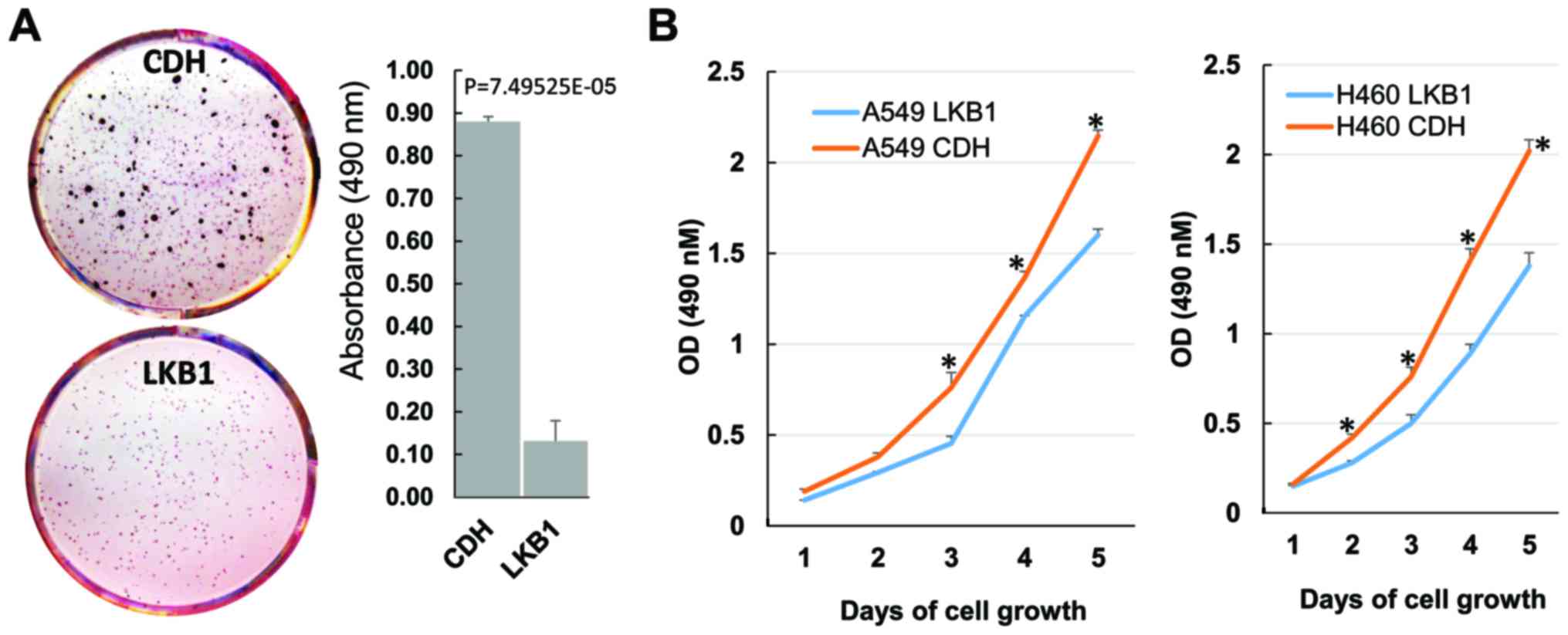
known to suppress tumor cell growth, we examined the effects of
LKB1 restoration on lung cancer cell growth by performing soft-agar
colony formation assay. As expected, LKB1 restoration greatly
suppressed H460 colony formation (Fig.
3A). A549 cells did not show measurable colony formation in our
experiments. We therefore conducted MTS cell proliferation assay
for this cell line. LKB1 restoration significantly decreased A549
cell proliferation (Fig. 3B). In
accordance with its effect on colony formation, LKB1 restoration
also significantly decreased H460 cell proliferation (Fig. 3B).
LKB1 greatly enhances the release of
exosomes by H460 lung cancer cells
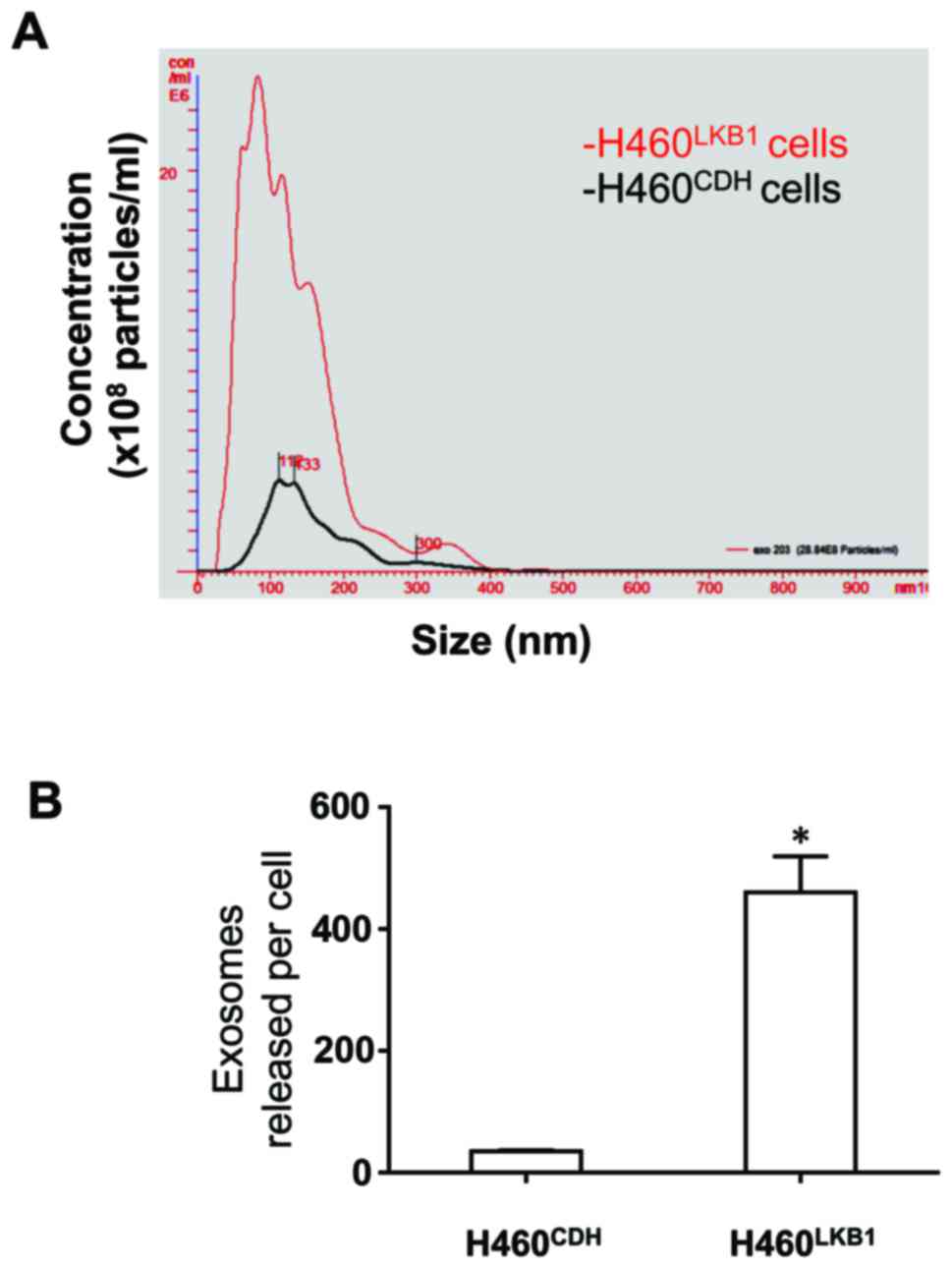
We then analyzed the effect of LKB1 expression on
the secretion of exosomes by H460 cells, which was the original aim
of the present study. We isolated extracellular vesicles from
conditioned media and analyzed them using NanoSight NS300
instrument. As shown in Fig. 4,
LKB1 greatly increased the release of exosomes by H460 cells.
Exosomes secreted by H460-LKB1 cells
have enhanced ability to promote cell migration
Exosomes have been shown to be involved in cell
migration (11), which can be
either promoting or inhibiting, depending on the contents contained
in the extracellular vesicles. We therefore tested the difference
in exosomes from either H460CDH or H460LKB1
cells in impacting motility of receipt cells. As exosomes can
target both tumor cells and tumor-associated cells in both distant
sites and local areas (7), we
simply used H460 parental cells for testing the differential
effects of these exosomes on cell motility of receipt cells. As
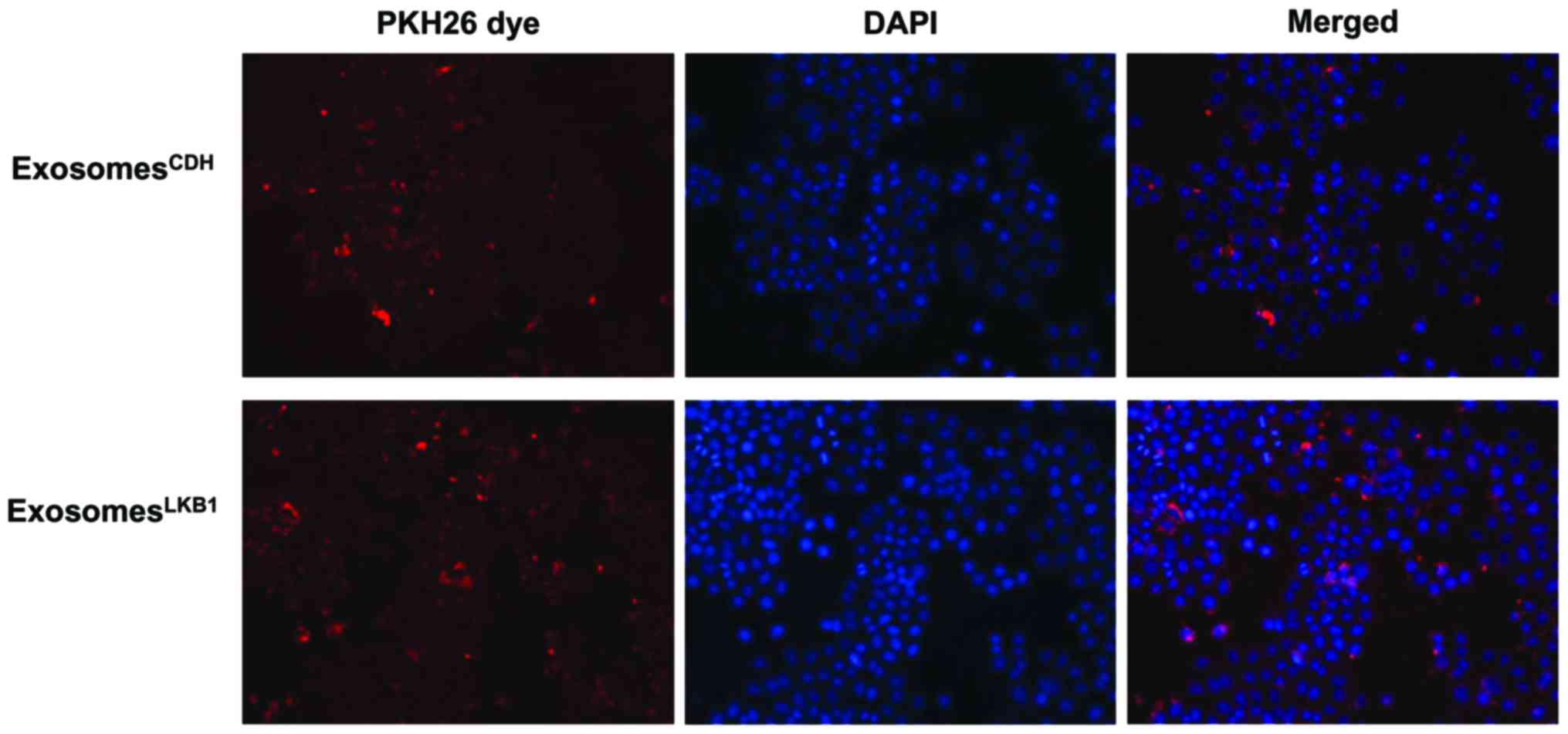
shown in Fig. 5, exosomes isolated
from the conditioned media of either H460CDH or
H460LKB1 were readily taken up by H460 cells. Notably,
in comparison with those that received H460CDH exosomes,
cells that received H460LKB1 exosomes had much higher
motility (Fig. 6). These results
suggest that restoration of LKB1 in H460 cells not only stimulated
the secretion of exosomes, but also changed the feature of exosomes
towards an increase in migration-promoting ability.
LKB1 decreases the levels of
migration-suppressing miRNAs both in cells and in exosomes
We then investigated how restoration of LKB1
promotes H460 lung cell migration and why exosomes from
H460LKB1 cells have higher activity in promoting
migration of receipt cells. As miRNAs are the essential components
in exosomes, we examined the effect of LKB1 restoration on the
levels of miRNAs both in cells and in exosomes. We focused on
let7b, miR-125a and miR-126, all of which are known to regulate
cell migration and be secreted in exosomes (12–16).
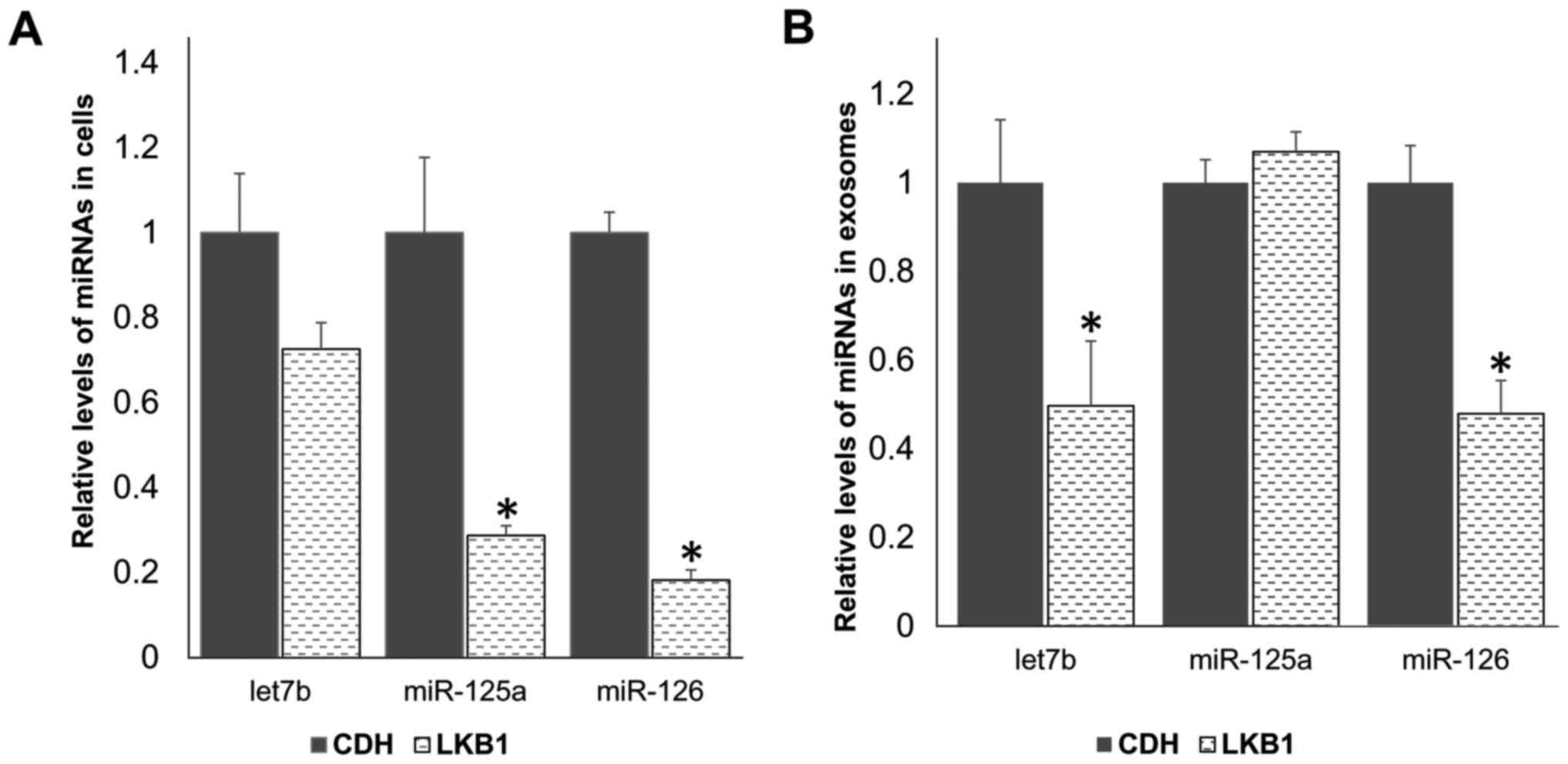
As shown in Fig. 7, LKB1
restoration caused a significant decrease in the cellular levels of
both miR-125a and miR-126, but had no significant effect on the
cellular level of let7b. In exosomes, the levels of let7b and
miR-126 were significantly reduced by LKB1 restoration, whereas the
level of miR-125a was not significantly changed. These results
suggest that LKB1 promotes cell migration, at least partly through
downregulating the levels of migration-suppressing miRNAs both in
cells and in exosomes.
Discussion
LKB1 is a critical factor in controlling cell
polarity and morphology (17,18),
both of which are implicated in cell migration. Indeed, LKB1
promotes the migration of neuron (19,20)
and endothelial cells (21). The
role of LKB1 in cancer cell migration, however, has been elusive.
While some studies have shown that LKB1 inhibits cancer cell
migration and tumor metastasis (22–25),
others have reported that LKB1 positively regulates the signaling
of PAKs at the cell membrane region and stimulates the formation of
protrusive membrane structures (lamellipodia) (26), suggesting a motility-stimulating
role for LKB1. Indeed, two recent studies showed that LKB1
re-expression enhanced directional migration of the H157 lung
cancer cell line and HeLa cervical cancer cell line, both of which
lack LKB1 expression (27,28). The present study corroborated the
stimulating effect of LKB1 restoration in motility of
LKB1-deficient lung cancer cells. In addition, the present study
revealed a new mechanism by which LKB1 promotes cancer cell
migration, i.e., downregulating the levels of migration-suppressing
microRNAs (miRNAs) in both cells and exosomes. Notably, although
LKB1 restoration enhanced motility of both H460 and A549 cells, it
changed H460 cells from a round epithelial shape to an elongated
mesenchymal-like shape, but had little effect on A549 cell
morphology. These results suggest that LKB1 promotes the motility
of H460 and A549 cells probably by different mechanisms. LKB1 may
stimulate epithelial-mesenchymal transition in H460 cells on the
basis of the morphological change aforementioned, whereas in A549
cells, it may increase directional migration, a similar effect for
LKB1 restoration reported in H157 cells (27). While the underlying molecular
mechanisms remain to be elucidated, it is interesting to note that
H460 is a cell line derived from large cell lung carcinoma, whereas
both A549 and H157 are non-small cell lung adenocarcinoma cell
lines. Taken together, the exact role of LKB1 in cell migration is
likely dependent upon cell type and the physiological and
pathological conditions.
While LKB1 is well-known for regulating multiple
critical signaling pathways, such as AMPK/mTOR and p53 pathways,
little is known concerning the involvement of LKB1 in
exosome-mediated cellular signaling. The present study demonstrates
that LKB1 greatly stimulates the release of exosomes by H460 cells.
Our finding concerning LKB1 in stimulating exosome release is new,
but may not be surprising, given that LKB1 signaling is shown to be
involved in protein trafficking and secretion (2,29).
Notably, LKB1 was shown to interact with Rab7 GTPase and regulates
RAB7-mediated protein trafficking (30). Rab7 is involved in exosome secretion
of endothelial (31) and melanoma
cells (32). In addition, LKB1 is
known to be implicated in insulin secretion although the underlying
mechanisms remain to be elucidated (33). Moreover, LKB1 was reported to
regulate microtubule-dependent trafficking of ABCB11 in hepatocytes
(34). Intriguingly, microtubule
affinity-regulating kinases (MARKs) are downstream targets of LKB1
and play essential roles in microtubule skeleton organization
(35,36). As such, it may be important to
investigate in future research whether Rab7 and/or MARK proteins
are involved in LKB1-stimulated exosome release in H460 lung cancer
cells.
Another interesting finding from the present study
is that exosomes from H460 cells with LKB1 restoration, in
comparison with those released by LKB1-deficient H460 cells, have
highly increased ability in promoting migration of receipt cells.
This is likely due to the decreased levels of tumor-suppressing
miRNAs (e.g., let7b and miRNA-126) in exosomes from H460 cells upon
LKB1 restoration. Taken together, the present study revealed a new
role for LKB1 in promoting cell motility, at least partly by
downregulating migration-suppressing miRNA expression and exosome
secretion. One of the proposed therapeutic strategies for treating
lung cancer with loss of LKB1 function is to restore LKB1
expression and/or signaling. However, our findings suggest that
this strategy may be a double-edged sword with a suppressing role
in tumor cell growth and possibly a promoting role in cancer cell
invasiveness.
Acknowledgements
The present study was supported by a start-up fund
of Wright State University and by NCI 1R01CA193264-01 to W.W.L.
References
|
1
|
Korsse SE, Peppelenbosch MP and van Veelen
W: Targeting LKB1 signaling in cancer. Biochim Biophys Acta.
1835:194–210. 2013.PubMed/NCBI
|
|
2
|
Gao Y, Ge G and Ji H: LKB1 in lung
cancerigenesis: A serine/threonine kinase as tumor suppressor.
Protein Cell. 2:99–107. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ylikorkala A, Rossi DJ, Korsisaari N,
Luukko K, Alitalo K, Henkemeyer M and Mäkelä TP: Vascular
abnormalities and deregulation of VEGF in Lkb1-deficient mice.
Science. 293:1323–1326. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hemminki A, Markie D, Tomlinson I,
Avizienyte E, Roth S, Loukola A, Bignell G, Warren W, Aminoff M,
Höglund P, et al: A serine/threonine kinase gene defective in
Peutz-Jeghers syndrome. Nature. 391:184–187. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marcus AI and Zhou W: LKB1 regulated
pathways in lung cancer invasion and metastasis. J Thorac Oncol.
5:1883–1886. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gangoda L, Boukouris S, Liem M, Kalra H
and Mathivanan S: Extracellular vesicles including exosomes are
mediators of signal transduction: Are they protective or
pathogenic? Proteomics. 15:260–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vader P, Breakefield XO and Wood MJ:
Extracellular vesicles: Emerging targets for cancer therapy. Trends
Mol Med. 20:385–393. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao L, Liu W, Xiao J and Cao B: The role
of exosomes and ‘exosomal shuttle microRNA’ in tumorigenesis and
drug resistance. Cancer Lett. 356:339–346. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Elkhadragy L, Chen M, Miller K, Yang MH
and Long W: A regulatory BMI1/let-7i/ERK3 pathway controls the
motility of head and neck cancer cells. Mol Oncol. 11:194–207.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Blanco R, Iwakawa R, Tang M, Kohno T,
Angulo B, Pio R, Montuenga LM, Minna JD, Yokota J and
Sanchez-Cespedes M: A gene-alteration profile of human lung cancer
cell lines. Hum Mutat. 30:1199–1206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weidle UH, Birzele F, Kollmorgen G and
Rüger R: The multiple roles of exosomes in metastasis. Cancer
Genomics Proteomics. 14:1–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu G, Drescher KM and Chen XM: Exosomal
miRNAs: Biological properties and therapeutic potential. Front
Genet. 3:562012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spolverini A, Fuchs G, Bublik DR and Oren
M: let-7b and let-7c microRNAs promote histone H2B ubiquitylation
and inhibit cell migration by targeting multiple components of the
H2B deubiquitylation machinery. Oncogene. Jun 12–2017.(Epub ahead
of print). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang L, Huang Q, Zhang S, Zhang Q, Chang
J, Qiu X and Wang E: Hsa-miR-125a-3p and hsa-miR-125a-5p are
downregulated in non-small cell lung cancer and have inverse
effects on invasion and migration of lung cancer cells. BMC Cancer.
10:3182010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang J, Yan F, Zhao Q, Zhan F, Wang R,
Wang L, Zhang Y and Huang X: Circulating exosomal miR-125a-3p as a
novel biomarker for early-stage colon cancer. Sci Rep. 7:41502017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ebrahimi F, Gopalan V, Smith RA and Lam
AK: miR-126 in human cancers: Clinical roles and current
perspectives. Exp Mol Pathol. 96:98–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Martin SG and St Johnston D: A role for
Drosophila LKB1 in anterior-posterior axis formation and epithelial
polarity. Nature. 421:379–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baas AF, Kuipers J, van der Wel NN, Batlle
E, Koerten HK, Peters PJ and Clevers HC: Complete polarization of
single intestinal epithelial cells upon activation of LKB1 by
STRAD. Cell. 116:457–466. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Asada N, Sanada K and Fukada Y: LKB1
regulates neuronal migration and neuronal differentiation in the
developing neocortex through centrosomal positioning. J Neurosci.
27:11769–11775. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Asada N and Sanada K: LKB1-mediated
spatial control of GSK3beta and adenomatous polyposis coli
contributes to centrosomal forward movement and neuronal migration
in the developing neocortex. J Neurosci. 30:8852–8865. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ohashi K, Ouchi N, Higuchi A, Shaw RJ and
Walsh K: LKB1 deficiency in Tie2-Cre-expressing cells impairs
ischemia-induced angiogenesis. J Biol Chem. 285:22291–22298. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li J, Liu J, Li P, Mao X, Li W, Yang J and
Liu P: Loss of LKB1 disrupts breast epithelial cell polarity and
promotes breast cancer metastasis and invasion. J Exp Clin Cancer
Res. 33:702014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chan KT, Asokan SB, King SJ, Bo T, Dubose
ES, Liu W, Berginski ME, Simon JM, Davis IJ, Gomez SM, et al: LKB1
loss in melanoma disrupts directional migration toward
extracellular matrix cues. J Cell Biol. 207:299–315. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji H, Ramsey MR, Hayes DN, Fan C, McNamara
K, Kozlowski P, Torrice C, Wu MC, Shimamura T, Perera SA, et al:
LKB1 modulates lung cancer differentiation and metastasis. Nature.
448:807–810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Roy BC, Kohno T, Iwakawa R, Moriguchi T,
Kiyono T, Morishita K, Sanchez-Cespedes M, Akiyama T and Yokota J:
Involvement of LKB1 in epithelial-mesenchymal transition (EMT) of
human lung cancer cells. Lung Cancer. 70:136–145. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Schafer-Hales K, Khuri FR, Zhou
W, Vertino PM and Marcus AI: The tumor suppressor LKB1 regulates
lung cancer cell polarity by mediating cdc42 recruitment and
activity. Cancer Res. 68:740–748. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Konen J, Wilkinson S, Lee B, Fu H, Zhou W,
Jiang Y and Marcus AI: LKB1 kinase-dependent and -independent
defects disrupt polarity and adhesion signaling to drive collagen
remodeling during invasion. Mol Biol Cell. 27:1069–1084. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wilkinson S, Hou Y, Zoine JT, Saltz J,
Zhang C, Chen Z, Cooper LA and Marcus AI: Coordinated cell motility
is regulated by a combination of LKB1 farnesylation and kinase
activity. Sci Rep. 7:409292017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gan RY and Li HB: Recent progress on liver
kinase B1 (LKB1): Expression, regulation, downstream signaling and
cancer suppressive function. Int J Mol Sci. 15:16698–16718. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okon IS, Coughlan KA, Zhang C, Moriasi C,
Ding Y, Song P, Zhang W, Li G and Zou MH: Protein kinase LKB1
promotes RAB7-mediated neuropilin-1 degradation to inhibit
angiogenesis. J Clin Invest. 124:4590–4602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jaé N, McEwan DG, Manavski Y, Boon RA and
Dimmeler S: Rab7a and Rab27b control secretion of endothelial
microRNA through extracellular vesicles. FEBS Lett. 589:3182–3188.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Peinado H, Alečković M, Lavotshkin S,
Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M,
Williams C, García-Santos G, Ghajar C, et al: Melanoma exosomes
educate bone marrow progenitor cells toward a pro-metastatic
phenotype through MET. Nat Med. 18:883–891. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Granot Z, Swisa A, Magenheim J,
Stolovich-Rain M, Fujimoto W, Manduchi E, Miki T, Lennerz JK,
Stoeckert CJ Jr, Meyuhas O, et al: LKB1 regulates pancreatic beta
cell size, polarity, and function. Cell Metab. 10:296–308. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Homolya L, Fu D, Sengupta P, Jarnik M,
Gillet JP, Vitale-Cross L, Gutkind JS, Lippincott-Schwartz J and
Arias IM: LKB1/AMPK and PKA control ABCB11 trafficking and
polarization in hepatocytes. PLoS One. 9:e919212014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Drewes G, Ebneth A and Mandelkow EM: MAPs,
MARKs and microtubule dynamics. Trends Biochem Sci. 23:307–311.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kojima Y, Miyoshi H, Clevers HC, Oshima M,
Aoki M and Taketo MM: Suppression of tubulin polymerization by the
LKB1-microtubule-associated protein/microtubule affinity-regulating
kinase signaling. J Biol Chem. 282:23532–23540. 2007. View Article : Google Scholar : PubMed/NCBI
|