Introduction
Prostate cancer (PCa) is one of the most common
cancers with indolent features in men (1). Distant bone metastasis is among the
most preferential sites of metastasis in several human tumors,
including breast cancer, prostate cancers, lung and kidney cancers
(2,3). Therefore, clarifying in depth the
molecular mechanisms underlying bone metastasis of PCa will
facilitate the development of novel therapeutic avenues in the
treatment of PCa.
TGF-β signaling is implicated in several
physiological processes, including inhibiting cell proliferation,
embryogenesis and bone remodeling (4). In cancers, TGF-β signaling has been
identified to function as oncogene or tumor-suppressor dependent on
the developmental stage and types of tumor (5,6).
Accumulating studies have shown that TGF-β signaling is essential
for the invasion and metastasis of tumor cells to bone in various
cancers, such as breast cancer and melanoma (7,8).
Importantly, TGF-β signaling has been demonstrated to play
important roles in the development of bone metastasis of prostate
cancer (9,10). Reportedly, therapy targeting TGF-β
significantly reduced the metastasis of tumor cells to bone
(9–11). Fournier and colleagues reported that
SD208, a small-molecule inhibitor of the kinase activity of TGFBR1,
significantly decreased the progression of PC-3 osteolytic
metastases (12), indicating TGF-β
signaling pathway is crucial for the bone metastasis of PCa cells.
However, the molecular mechanism contributing to constitutive
activation of TGF-β in PCa remains poorly known.
MicroRNAs (miRNAs) are a diverse group of small
non-coding RNAs composed of 19–25 nucleotides, which
mechanistically function by binding to the 3′-untranslated region
(3′-UTR) of downstream mRNAs, leading to mRNA degradation or
repression of translation (13,14). A
growing body of literature has demonstrated that miRNAs not only
play crucial roles in many biological processes including
proliferation, differentiation, cell cycle and apoptosis (13), but also regulate the progression and
metastasis in various types of tumors (15–19).
Furthermore, several miRNAs have been identified as critical
mediators in the bone metastasis of human cancer (14,20–22).
Our previous studies demonstrated that loss of wild-type P53 in
PC-3 cells resulted in downregulation of miR-145, which further
promoted bone metastasis of PCa via regulating several positive
regulators of EMT, including ZEB2, and HEF1 (23–25).
Therefore, these studies indicate that dysregulation of miRNAs
plays a pivotal role in the bone metastasis of PCa.
In this study, we found that miR-19a-3p expression
is dramatically decreased in bone metastatic PCa tissues and cells.
Moreover, upregulation of miR-19a-3p suppresses, while silencing
miR-19a-3p promotes invasion and migration in vitro.
Importantly, upregulating miR-19a-3p repressed the osteolytic bone
lesions in vivo. Our results further reveal that
upregulating miR-19a-3p inhibits TGF-β signaling via targeting
downstream effectors of TGF-β signaling SMAD2 and SMAD4,
suppressing invasion and migration of PCa cells. Therefore, our
results demonstrate that miR-19a-3p inhibits invasion and migration
of PCa cells via directly targeting SMDA2 and SMAD4, indicating
that miR-19a-3p play a tumor-suppressive role by suppressing
invasion and migration ability in bone metastasis of PCa.
Materials and methods
Cell lines and cell culture
Human RWPE-1, DU145, LNCaP, 22Rv1, PC-3 and VCaP
cells were obtained from the American Type Culture Collection
(ATCC) and cultured according to the manufacturer's instructions.
The C4-2B cell line was purchased from the MD Anderson Cancer
Center. RWPE-1 cells were grown in defined keratinocyte-SFM (1X)
(Invitrogen). LNCaP, 22Rv1, C4-2B and PC-3 cells were grown in
RPMI-1640 medium (Invitrogen) supplemented with 10% FBS
(Invitrogen), while DU145 and VCaP cells were grown in Dulbecco's
modified Eagle's medium (Invitrogen) supplemented with 10% FBS. All
cells were incubated at 37°C in a humidified atmosphere with 5%
CO2 and were routinely sub-cultured using 0.25% (w/v)
trypsin-ethylenediamine-tetraacetic acid solution.
Patients and tumor tissues
In total 121 archived PCa tissues, including 76
non-bone metastatic PCa tissues and 45 bone metastatic PCa tissues
were obtained during surgery or needle biopsy at The First People's
Hospital of Guangzhou City (Guangzhou, China). Patients were
diagnosed based on clinical and pathological evidence, and the
specimens were immediately snap-frozen and stored in liquid
nitrogen. For the use of these clinical materials for research
purposes, prior patients' consents and approval from the
Institutional Research Ethics Committee were obtained. The
clinicopathological features of the patients are summarized in
Table I. The median age (74-years),
serum PSA level (76.5 µg/ml) and Gleason grade (7) in all 121 PCa tissues was used as the
cut-off value for age, serum PSA level and Gleason grade,
respectively. The median of miR-19a-3p expression in all 121 PCa
tissues was used to stratify high and low expression of
miR-19a-3p.
 | Table I.The relationship between miR-19a-3p
expression and clinicopathological characteristics in 121 patients
with prostate cancer. |
Table I.
The relationship between miR-19a-3p
expression and clinicopathological characteristics in 121 patients
with prostate cancer.
|
|
| miR-19a-3p
expression |
|
|---|
|
|
|
|
|
|---|
| Parameters | No. of cases | Low | High | P-value |
|---|
| Age (years) |
|
|
|
|
|
≤74 | 54 | 28 | 26 | 0.081 |
|
>74 | 67 | 33 | 34 |
|
|
Differentiation |
|
|
|
|
|
Well/moderate | 53 | 20 | 33 |
0.014a |
|
Poor | 68 | 41 | 27 |
|
| Serum PSA |
|
|
|
|
|
<76.5 | 56 | 22 | 34 |
|
|
>76.5 | 65 | 39 | 26 |
0.023a |
| Gleason grade |
|
|
|
|
| ≤7 | 62 | 19 | 43 |
|
|
>7 | 59 | 42 | 17 |
<0.001a |
| Operation |
|
|
|
|
|
TURP | 42 | 22 | 20 |
|
| Needle
biopsy | 43 | 23 | 20 | 0.932 |
|
TURP+PP | 7 | 3 | 4 |
|
|
TURP+BO | 19 | 11 | 8 |
|
| BO | 10 | 6 | 4 |
|
| BM-status |
|
|
|
|
|
nBM | 76 | 31 | 45 |
|
| BM | 45 | 30 | 15 |
0.006a |
RNA extraction, reverse transcription,
and real-time PCR
Total RNA from tissues or cells were extracted using
TRIzol (Life Technologies) according to the manufacturer's
instructions. Messenger RNA (mRNA) and miRNA were reverse
transcribed of total mRNA using the Revert Aid First Strand cDNA
Synthesis kit (Thermo, USA) according to the manufacturer's
protocol. Complementary DNA (cDNA) was amplified and quantified on
ABI 7500HT system (Applied Biosystems, Foster City, CA, USA) using
SYBR Green I (Applied Biosystems). The primers used in the
reactions are listed in Table II.
Real-time PCR was performed according to a standard method, as
described previously (26). Primers
for U6 and miR-19a-3p (miRQ0000073-1-2) were synthesized and
purified by RiboBio (website for miR-19a-3p primer: http://www.ribobio.com/sitecn/product_info.aspx?id=200681)
(Guangzhou, China). U6 or glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) was used as endogenous controls for miRNA or mRNA
respective. Relative fold expressions were calculated with the
comparative threshold cycle (2−∆∆Ct) method.
 | Table II.The list of primers used in the
reactions for real-time RT-PCR. |
Table II.
The list of primers used in the
reactions for real-time RT-PCR.
|
| Real-time PCR
primer |
|---|
| SMAD2-up |
CACGCTAGGAAAACAGCCTC |
| SMAD2-dn |
TCGGAAGAGGAAGGAACAAA |
| SMAD4-up |
TTGATCCTTTGGAAACAGTGAA |
| SMAD4-dn |
GCCTTCCCACTCCCCTC |
| CTGF-up |
GCTACCACATTTCCTACCTAGAAATCA |
| CTGF-dn |
GACAGTCCGTCAAAACAGATTGTT |
| PTHRP-up |
ACTCGCTCTGCCTGGTTAGA |
| PTHRP-dn |
GGAGGTGTCAGACAGGTGGT |
| IL11-up |
TGAAGACTCGGCTGTGACC |
| IL11-dn |
CCTCACGGAAGGACTGTCTC |
| GAPDH-up |
ATTCCACCCATGGCAAATTC |
| GAPDH-dn |
TGGGATTTCCATTGATGACAAG |
Plasmid, small interfering RNA and
transfection
The human miR-19a-3p expression plasmid was
generated by cloning the genomic pre-miR-19a-3p gene into
retroviral transfer plasmid pMSCV-puro (Clontech Laboratories Inc.,
Tokyo, Japan) to generate plasmid pMSCV-miR-19a-3p.
pMSCV-miR-19a-3p was cotransfected with the pIK packaging plasmid
into 293FT cells using the standard calcium phosphate transfection
method, as previously described (18). Thirty-six hours after the
co-transfection, supernatants were collected and incubated with
cells to be infected for 24 h in the presence of polybrene (2.5
µg/ml). After infection, puromycin (1.5 µg/ml) was used to select
stably transduced cells over a 10-day period. The (CAGAC) 12/pGL3
TGF-β/Smad-responsive luciferase reporter plasmid and control
plasmids (Clontech) were used to examine the transcriptional
activity of TGF-β signaling quantitatively. The 3′-untranslated
region (3′-UTR) of the human SMAD2 and SMAD4 were PCR-amplified
from genomic DNA and cloned into pmirGLO luciferase reporter vector
(Promega, Madison, WI, USA). The miArrest plasmids for
anti-miR-19a-3p and negative control plasmids were constructed and
cloned into pH1 plasmids by GeneChem (Shanghai, China).
Anti-miR-19a-3p, small interfering RNA (siRNA) for SMAD2 (sc-37238)
and SMAD4 (sc-29484) knockdown (50 nmol/l) were obtained from Santa
Cruz (Dallas, TX, USA). The sequence of anti-miR-19a-3p is TCAG
TTTTGCATAGATTTGCACA. Transfection of siRNAs and plasmids was
performed using Lipofectamine 3000 (Life Technologies) according to
the manufacturer's instructions.
Invasion and migration assays
Migration and invasion were assayed using Transwell
chamber consisting of 8-µm membrane filter inserts (Corning) coated
with or without Matrigel (BD Biosciences) as described previously
(27). Briefly, the cells were
trypsinized and suspended in serum-free medium. Then
1.5×105 cells were added to the upper chamber, and lower
chamber was filled with the culture medium with 10% FBS. After
incubated for 24–48 h, cells passed through the coated membrane to
the lower surface, in which cells were fixed with 4%
paraformaldehyde and stained with hematoxylin. The cell count was
done under a microscope (×100).
In vivo model of PCa bone
metastasis
We used an intratibial injection model to detect
whether upregulation of miR-19a-3p could reduce the bone metastatic
capacity of PCa cells. Six male severe combined immunodeficient
(SCID) mice at 3–4 weeks old were purchased from HFK Bio-Technology
(Beijing, China). Each mouse was injected with stably selected
PC-3/miR-19a-3p cells into its right tibia and with PC-3/vector
cells into its left tibia as a matching control. The inoculation
procedure was performed as previously described (28). Bone lesions were analyzed by using
the following scoring standard based on X-ray examination (29): 0 (no lesion); 1 (minor lesions); 2
(small lesions); 3 (significant lesions with minor breaks in their
margins); 4 (significant lesions with major breaks in peripheral
lesions). The ethics approval statements for animal work were
provided by The Institutional Animal Care and Use Committee of Sun
Yat-Sen University Cancer Center. The ethics approval number for
animal work was L102012016110D.
Luciferase assay
Cells (4×104) were seeded in triplicate
in 24-well plates and cultured for 24 h. Cells were transfected
with 250 ng (CAGAC) 12/pGL3 reporter luciferase plasmid, or 100 ng
pmirGLO-PICK1-3′-UTR, luciferase plasmid, plus 5 ng pRL-TK
Renilla plasmid (Promega) using Lipofectamine 3000
(Invitrogen) according to the manufacturer's instructions.
Luciferase and Renilla signals were measured 36 h after
transfection using a Dual Luciferase Reporter assay kit (Promega)
according to the manufacturer's protocol.
RNA immunoprecipitation
Cells (5×105) were plated in 60-mm cell
culture dishes, proliferating to 60–80% confluence after 24 h of
culture, and the pIRESneo-FLAG/HA-Ago2 plasmid (10822; Addgene,
Cambridge, MA, USA) were cotransfected into cells using
Lipofectamine 3000, as previously described (30). After 48-h transfection, cells were
washed and lysed in radioimmunoprecipitation buffer (Sigma-Aldrich)
containing 10% proteinase inhibitor cocktail (Sigma-Aldrich) and 1
mM phenylmethylsulfonyl fluoride (Sigma-Aldrich). A fraction of the
whole cell lysate was used for RNA isolation, and the remaining
lysate was subjected to immunoprecipitation (IP) using an antibody
against Ago2 (Abcam) or immunoglobulin G (IgG) (Abcam). RNA from
whole cell lysates and RNA IP (RIP) fractions was extracted with
TRIzol (Life Technologies) according to the manufacturer's
instructions. The relative levels of mRNA were determined using
real-time RT-PCR as described above. The relative mRNA enrichment
in the RIP fractions was computed based on the ratio of relative
mRNA levels in the RIP fractions and the relative mRNA levels in
the whole cell lysates.
Western blotting
The proteins extracted from the cell lysates were
loaded with 50 µg in each lane, which was further separated by 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to polyvinylidene fluoride membranes (Millipore,
Billerica, MA, USA). Western blotting was performed according to a
standard method, as described previously (31). The membranes were probed with
antibodies against SMAD2 (no. 5339; dilution, 1:1,000), SMAD4 (no.
38454; dilution, 1:1,000), pSMAD2/3 (no. 9510; diilution, 1:1,000),
SMAD2/3 (no. 8685; dilution, 1:1,000) (Cell Signaling Technology,
Beverly, MA, USA) and p84 (no. PA5-27816; dilution: 1:1,000) from
Invitrogen overnight at 4°C, and then incubated with horseradish
peroxidase-conjugated secondary antibodies (Cell Signaling
Technology) for 1 h at room temperature. Immune complexes were
detected by enhanced chemiluminescence (Cell Signaling Technology).
α-tubulin (Cell Signaling Technology) was used to correct for
differences in protein loading from the control and experimental
groups.
Statistical analysis
All values are presented as means ± standard
deviation (SD). Significant differences were determined using SPSS
19.0 software (SPSS, Chicago, IL, USA). A paired Student's t-test
was used to analyze the paired control group (vector or NC) and
treatment group (miR-19a-3p or anti-miR-19a-3p) in vitro
experiment. One-way ANOVA was used to determine statistical
differences between multiple testing. An independent Student's
t-test was used to analyze the paired control group (vector) and
treatment group (miR-19a-3p) in vivo experiment. P<0.05
was considered statistically significant. All the experiments were
repeated three times.
Results
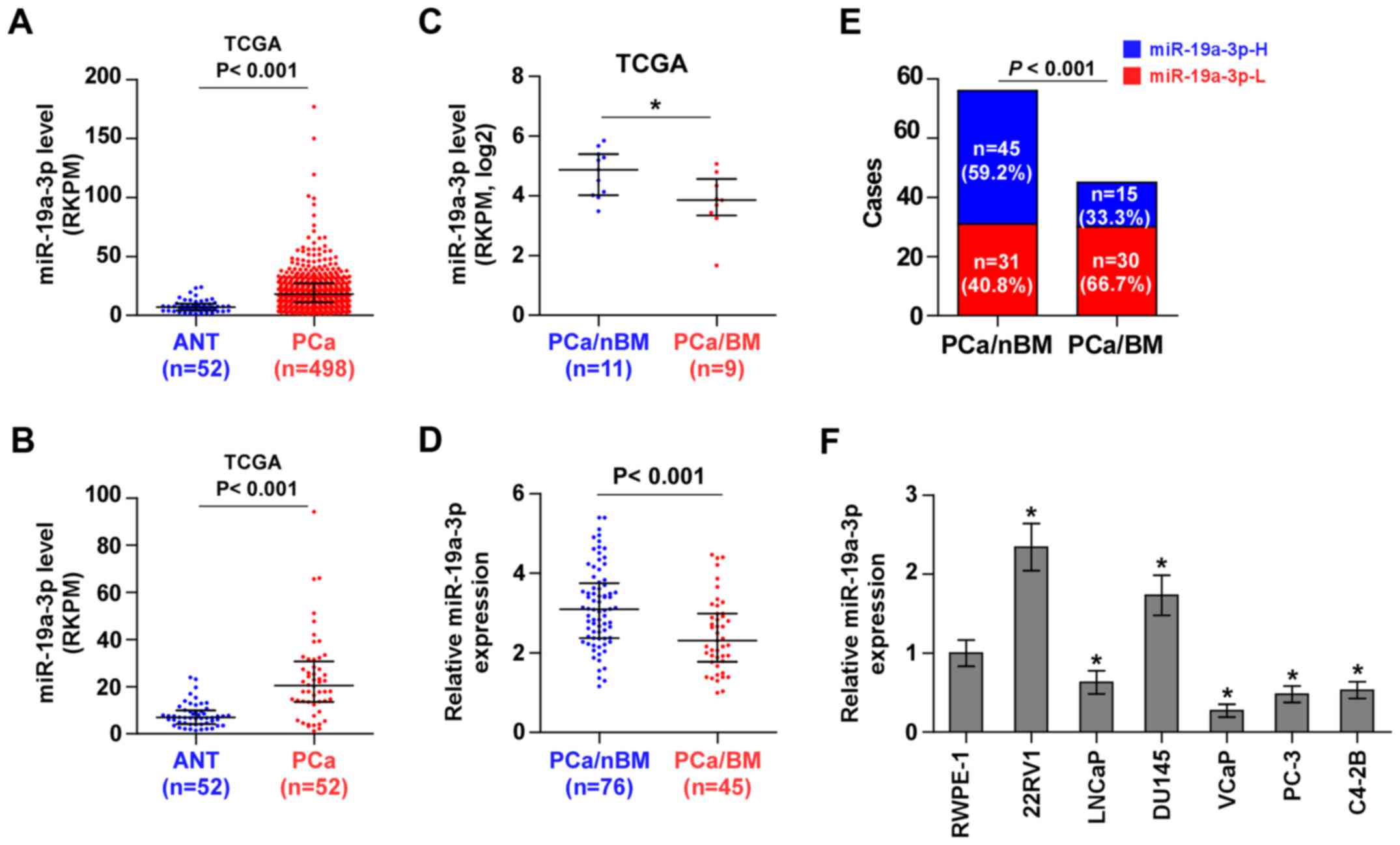
miR-19a-3p is downregulated in bone
metastatic PCa tissues and cell lines
To screen the aberrant miRNA expression between bone
metastatic PCa tissues and non-bone metastatic PCa tissues, we
first analyzed the miRNA sequencing dataset of PCa from The Cancer
Genome Atlas (TCGA) and found that miR-19a-3p expression was
dramatically overexpressed in 498 PCa tissues compared with 52 ANT
(Fig. 1A). Consistently, miR-19a-3p
expression was overexpressed in 52 paired PCa tissues compared with
the matched ANT (Fig. 1B).
Interestingly, we found that miR-19a-3p expression was
downregulated in bone metastatic PCa tissues compared with non-bone
metastatic PCa tissues (Fig. 1C).
To validate the miR-19a-3p expression in PCa tissues, real-time PCR
was performed on 76 non-bone metastatic PCa tissues and 45 bone
metastatic PCa tissues. As shown in Fig. 1D, miR-19a-3p expression was
downregulated in bone metastatic PCa tissues compared with non-bone
metastatic PCa tissues. The percentage of low miR-19a-3p expression
was higher in PCa tissues with bone metastasis compared to PCa
tissues without bone metastasis (Fig.
1E). We further examined miR-19a-3p expression in normal
prostate cells (RWPE-1) and 6 PCa cell lines. Consistent with
miR-19a-3p expression in PCa tissues, miR-19a-3p expression was
elevated in primary PCa cells 22RV1 and brain metastatic PCa DU145
cells, but was differentially decreased in bone metastatic PCa cell
lines (PC-3, C4-2B and VCaP) and lymph node metastatic cell line
LNCaP (Fig. 1F). Statistical
analysis of PCa tissue samples revealed that miR-19a-3p expression
inversely correlated with differentiation, serum PSA levels,
Gleason grade and bone metastasis status in PCa (Table I). Therefore, the published miRNA
datasets and our results indicated that downregulation of
miR-19a-3p may be implicated in bone metastasis of PCa.
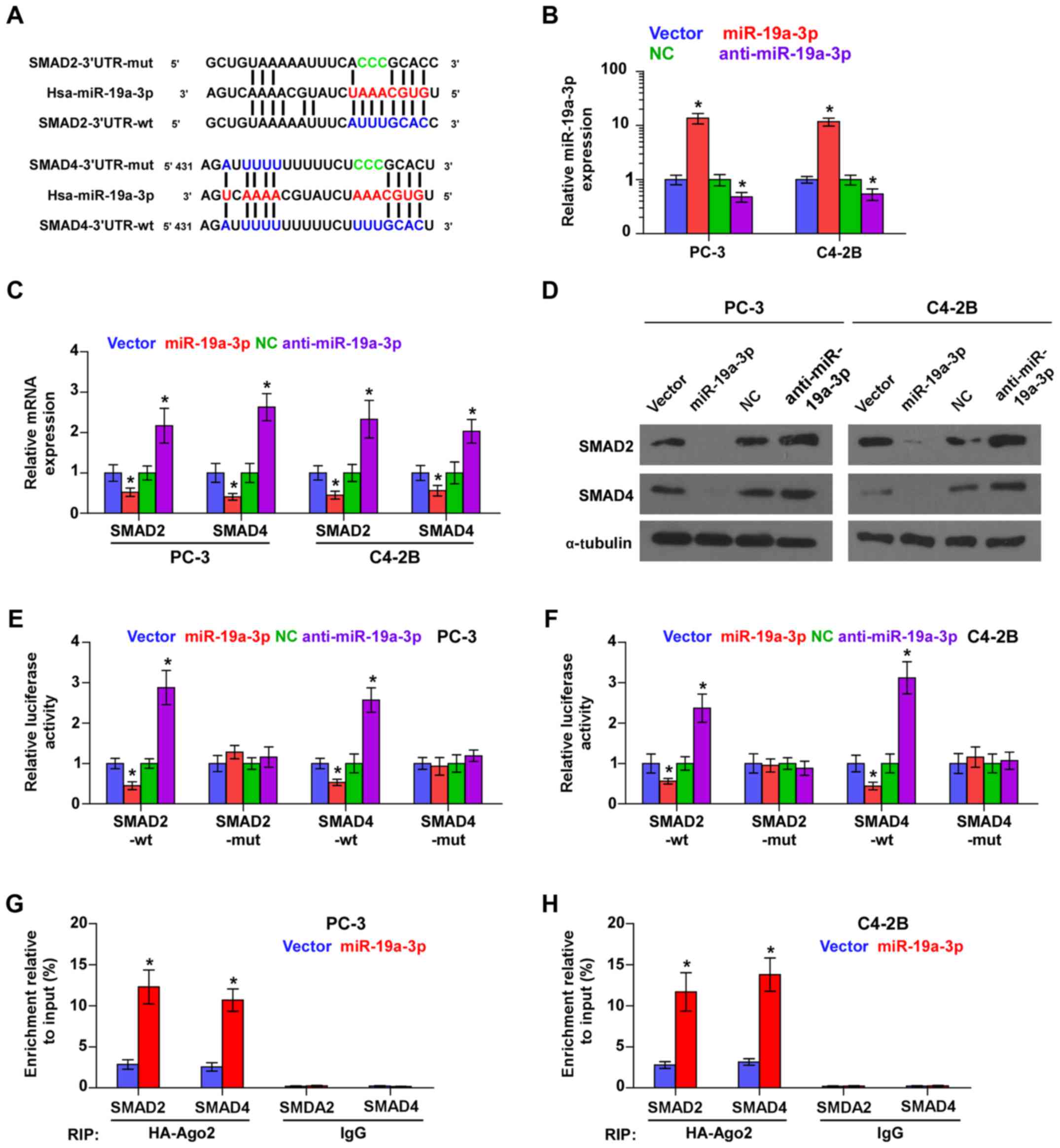
miR-19a-3p targets effectors of TGF-β
signaling SMAD2 and SMAD4
Using the publicly available algorithms TargetScan
and miRanda, we found that SMAD2 and SMAD4 are potential targets of
miR-19a-3p (Fig. 2A), both of which
are critical downstream effectors of TGF-β signaling (32). Then, we further exogenously
overexpressed miR-19a-3p and endogenously silenced miR-19a-3p via
virus transduction in PCa cells (Fig.
2B). Real time-PCR and western blot analysis showed that
miR-19a-3p overexpression reduced, while silencing miR-19a-3p
enhanced the mRNA and protein expression levels of SMAD2 and SMAD4
(Fig. 2C and D). To examine whether
miR-19a-3p-mediated SMAD2 and SMAD4 downregulation occurs through
miR-19a-3p-binding in the 3′-UTR of SMAD2 and SMAD4, the 3′-UTR of
SMAD2 and SMAD4 were cloned into pmirGLO luciferase reporter
vectors. As shown in Fig. 2E and F,
miR-19a-3p overexpression decreased, whereas anti-miR-19a-3p
increased, the luciferase reporter activity of SMAD2 and SMAD4, but
not by the mutant 3′-UTR of SMAD2 and SMAD4 within
miR-19a-3p-binding seed regions in PCa cells. Moreover,
microribonucleoprotein (miRNP) immunoprecipitation (IP) assay
showed a direct association of miR-19a-3p with SMAD2 and SMAD4
transcripts (Fig. 2G and G),
further elucidating the direct repressive effects of miR-19a-3p on
SMAD2 and SMAD4. Taken together, these results indicated that SMAD2
and SMAD4 are authentic targets of miR-19a-3p in PCa cells.
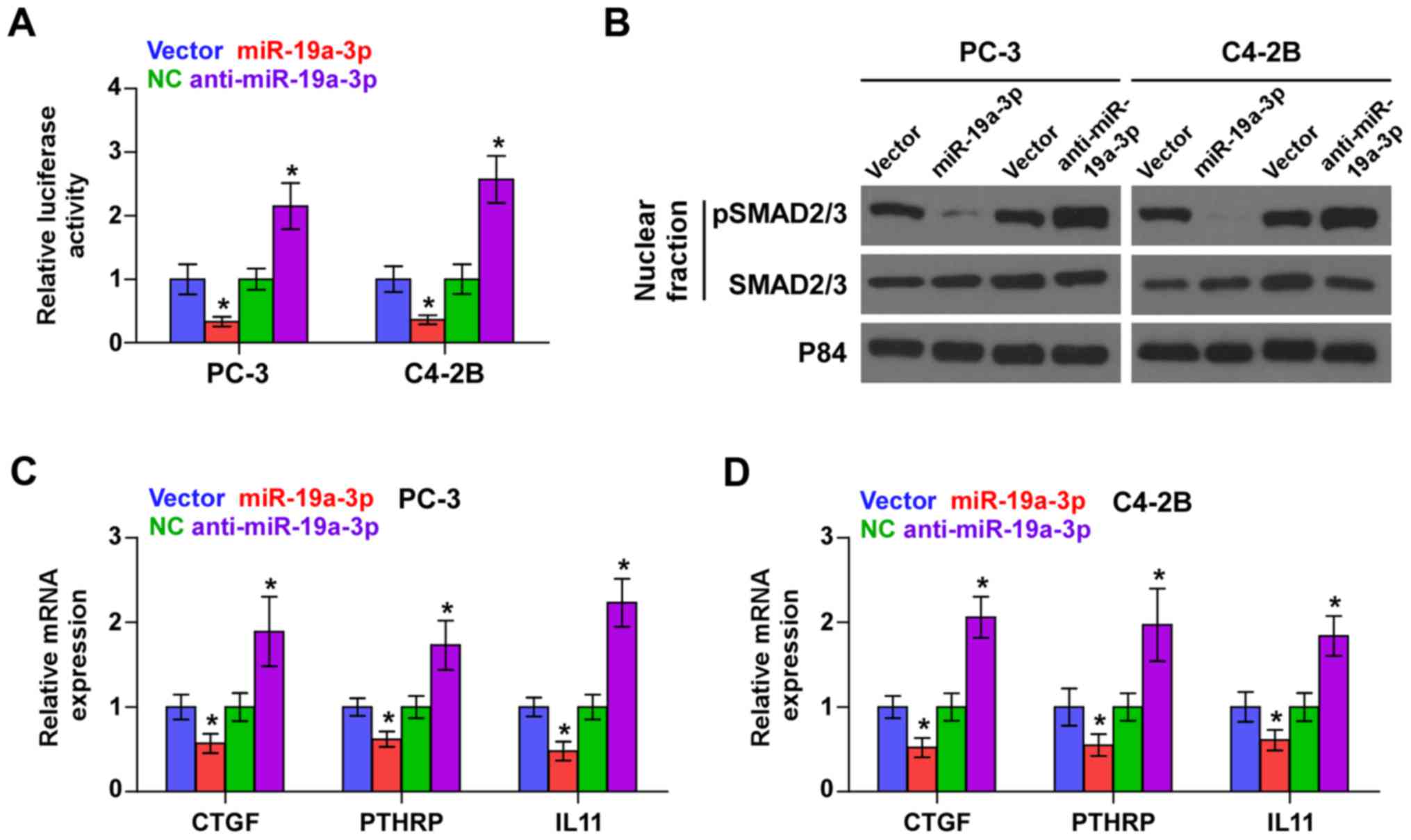
Downregulation of miR-19a-3p activates
TGF-β signaling in PCa cells
As SMAD2 and SMAD4 are critical effectors of TGF-β
signaling, we further investigated the effect of miR-19a-3p on
TGF-β signaling activity and found that miR-19a-3p overexpression
reduced, while silencing miR-19a-3p enhanced the transcriptional
activity of a TGF-β/Smad-responsive luciferase reporter plasmid
known as CAGA12 composed of 12 tandem copies of a Smad/DNA binding
element CAGAC sequence in PCa cells (Fig. 3A). Cellular fractionation and
western blot analysis revealed that upregulation of miR-19a-3p
decreased, while miR-19a-3p silencing increased pSMAD2/3 nuclear
translocation in PCa cells (Fig.
3B). Real-time PCR analysis showed that upregulating miR-19a-3p
decreased expression levels of multiple TGF-β signaling downstream
bone metastasis-related target genes including CTGF, PTHRP and IL11
in PCa cells. Conversely, silencing miR-19a-3p increased expression
of these downstream genes in PCa cells (Fig. 3C and D). Thus, these results
demonstrate that downregulation of miR-19a-3p activates TGF-β
signaling pathway in PCa cells.
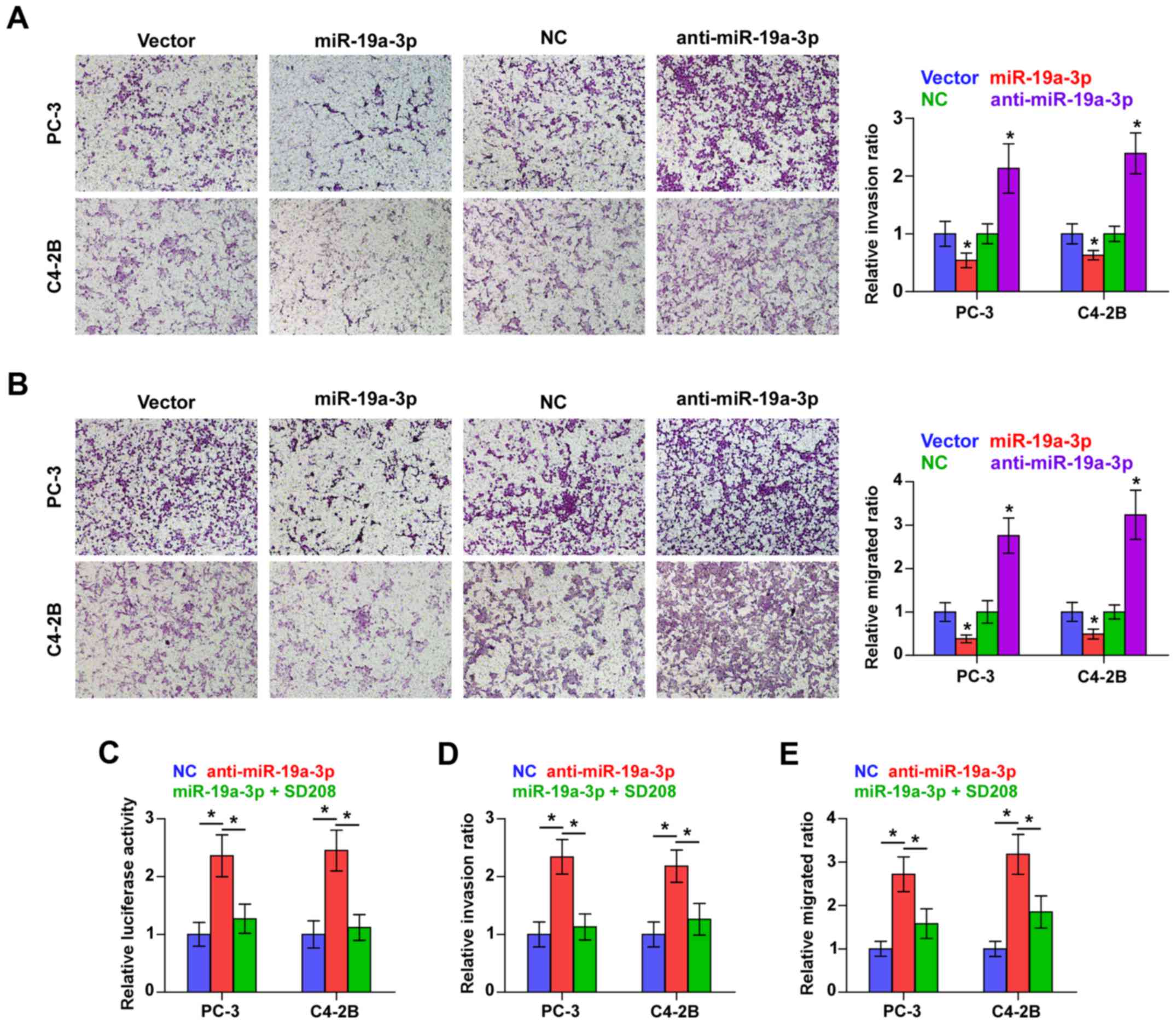
Downregulation of miR-19a-3p promotes
invasion and migration via activating TGF-β signaling pathway in
PCa cells
We examined whether miR-19a-3p is involved in the
invasion and migration PCa cells. Transwell-Matrigel invasion assay
was used to assess the invasive ability of PCa cells and the result
indicated that miR-19a-3p overexpression dramatically decreased,
while silencing miR-19a-3p increased, the invasive ability of PC-3
and C4-2B cells (Fig. 4A).
Furthermore, migration assay revealed that upregulating miR-19a-3p
decreased, while silencing miR-19a-3p increased the migration
capability of PCa cells (Fig. 4B).
These results indicated that miR-19a-3p inhibits invasion and
migration ability of PCa cells in vitro.
We further explored the functional significance of
TGF-β signaling in the stimulatory role of miR-19a-3p
downregulation in PCa cells using TGF-β signaling inhibitor SD208.
As shown in Fig. 4C, SD208
abrogated the TGF-β signaling activity enhanced by miR-19a-3p
downregulation in PCa cells. Furthermore, the stimulatory effects
of miR-19a-3p on invasion and migration of PCa cells were impaired
by SD208 respectively (Fig. 4D and
E). Therefore, these results imply that downregulation of
miR-19a-3p promotes invasion and migration via activating TGF-β
signaling pathway in PCa cells.
Upregulation of miR-19a-3p inhibits
bone lesions of PC-3 cells in vivo
To demonstrate further the effect of miR-19a-3p on
the development of PCa bone metastasis in vivo, an
intratibial injection mouse model was established. As shown in
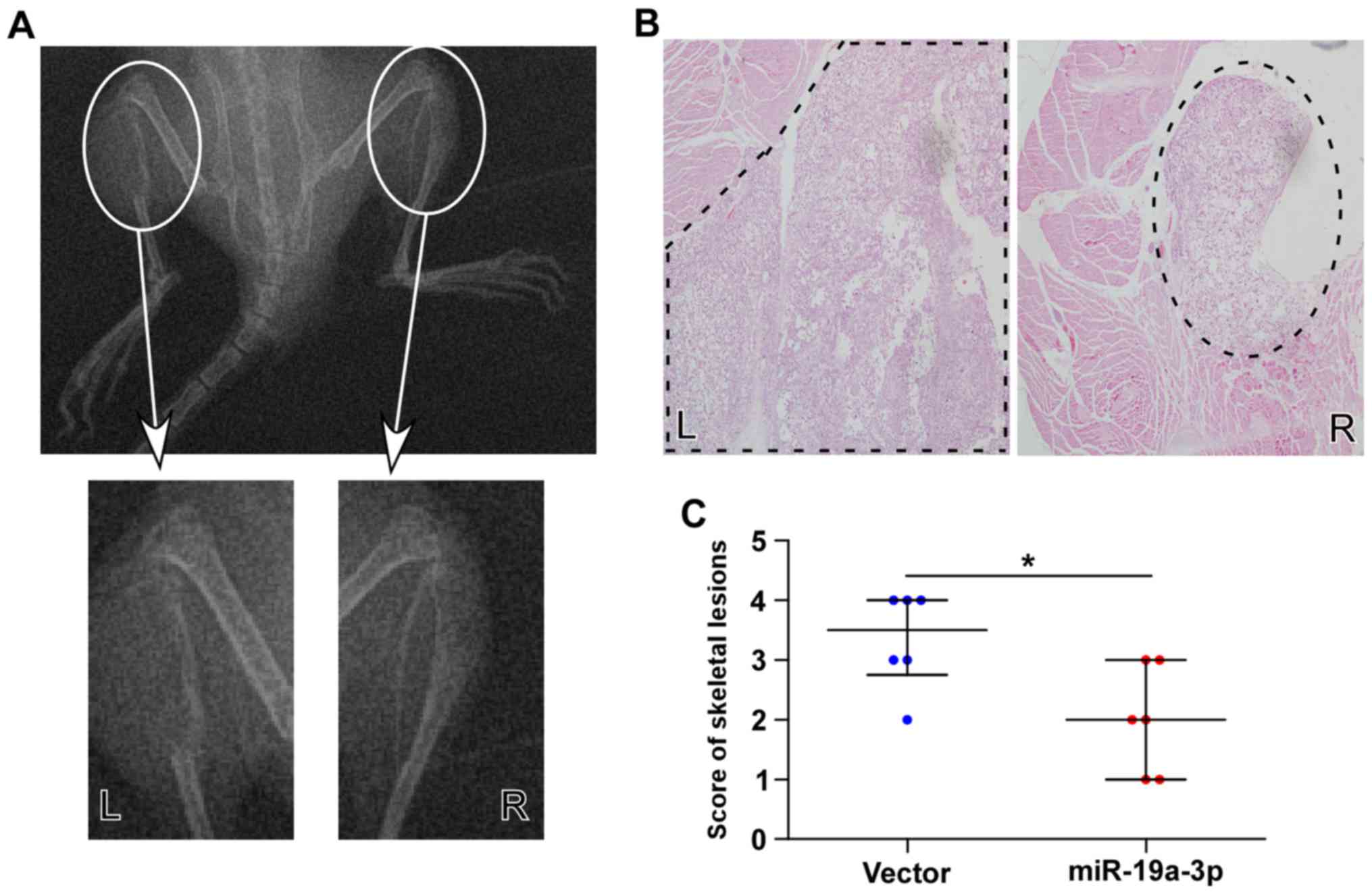
Fig. 5A, the skeletal lesions of
the animals in the left tibia injected with PC-3/vector cells were
significantly larger than those in the right tibia injected with
PC-3/miR-19a-3p cells, indicating that upregulating miR-19a-3p
inhibited the bone invasive abilities of PC-3 cells in vivo.
Histological analysis of H&E staining showed that the extent
and areas of skeletal lesions observed in the scoring of X-rays
were significantly smaller tumors and less bone invasion in mice
injected with PC-3/miR-19a-3p cells compared with PC-3/vector cells
(Fig. 5B and C). These observations
indicated that the upregulation of miR-19a-3p represses osteolytic
bone lesions of PCa cells in bone.
SMAD2 and SMAD4 mediate the
stimulatory effects of anti-miR-19a-3p on TGF-β signaling activity,
invasion and migration in PCa cells
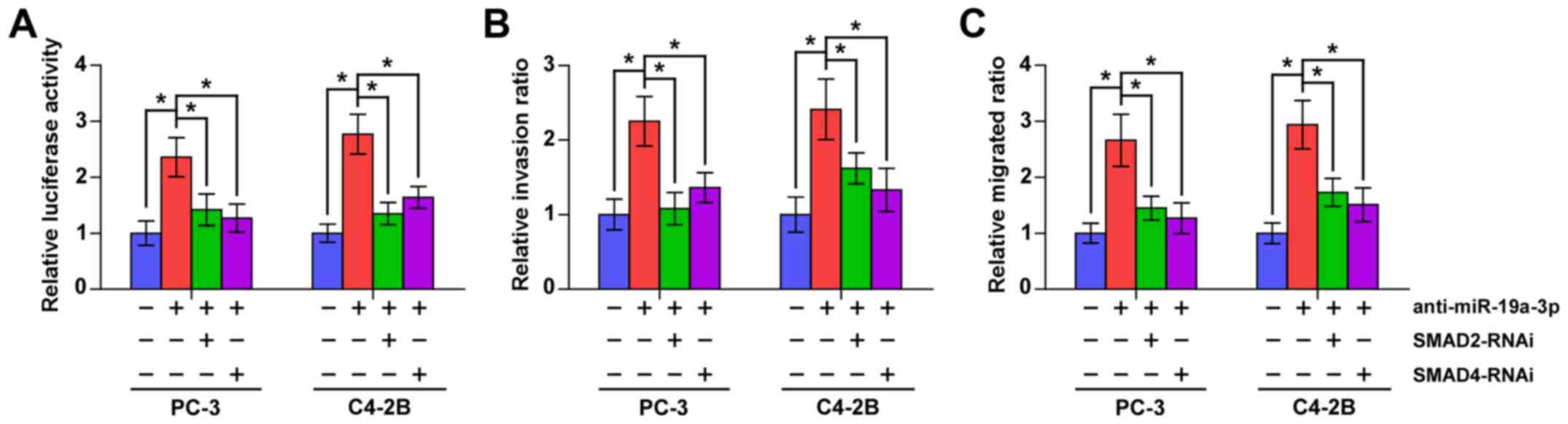
To further investigate whether SMAD2 and SMAD4
contribute to the TGF-β signaling activity, invasion and migration
abilities induced by miR-19a-3p downregulation in PCa cells, we
used RNA interference to knock down the SMAD2 and SMAD4 in
miR-19a-3p-downregulation in PCa cells. As shown in Fig. 6, individual silencing SMDA2 and
SMAD4 significantly attenuated the stimulatory effects of
anti-miR-19a-3p on TGF-β signaling activity, invasion and migration
abilities in PCa cells. These results indicated that SMAD2 and
SMAD4 mediate the stimulatory effects of anti-miR-19a-3p on TGF-β
signaling activity, invasion and migration in PCa cells.
Discussion
In the present study, our results indicate that
miR-19a-3p expression is markedly downregulated in bone metastatic
PCa tissues and cells, which is consistent with the results of
publicly available PCa datasets. Furthermore, upregulating
miR-19a-3p suppresses, while downregulating miR-19a-3p enhances
invasion and migration in vitro. Importantly, upregulating
miR-19a-3p represses the osteolytic bone lesions in vivo.
Our results further demonsrate that overexpression of miR-19a-3p
inhibits TGF-β signaling via targeting downstream effectors of
TGF-β signaling SMAD2 and SMAD4, which further suppresses invasion
and migration of PCa cells. In addition, the stimulatory effects of
downregulating miR-19a-3p on the TGF-β signaling activity, invasion
and migration abilities in PCa cells are reversed by individual
silencing of SMAD2 and SMAD4. Therefore, these results present our
improved understanding of miR-19a-3p-induced tumor suppressive role
in bone metastasis of PCa.
Accumulating studies indicate that constitutive
activation of TGF-β signaling is reported in bone metastasis of
several human cancers. Kang and colleagues reported that Smad4 was
essential for the induction of IL-11, a gene implicated in bone
metastasis in this mouse model system, which further contributed to
the formation of osteolytic bone metastases in breast cancer
(33). Moreover, a study by
Javelaud et al (7) revealed
that TGF-β promoted osteolytic bone metastases in melanoma cells by
stimulating the expression of prometastatic factors via the Smad
pathway. In PCa, Fournier and colleagues reported that upregulation
of the negative regulator of the TGF-β pathway PMEPA1 by TGF-β
signaling inhibited bone metastasis of PCa. Importantly, breaking
the negative feedback loop by PMEPA1 silencing promoted bone
metastases in vivo (12).
These studies demonstrate that TGF-β signaling plays an important
role in bone metastasis of cancer, including PCa. In the present
study, we found that ectopic expression of miR-19a-3p expression
suppressed activation of TGF-β signaling via directly repressing
SMAD2 and SMAD4 expression, which further inhibited the invasion
and migration in vitro and bone metastasis in vivo in
PCa cells. Therefore, our results present a novel mechanism
responsible for the inhibitory effects of miR-19a-3p on the
invasion, migration and bone metastasis in PCa cells.
miR-19a-3p has been reported to be upregulated in
multiple human cancers, including cutaneous squamous cell
carcinoma, colorectal cancer myeloma, follicular lymphoma, gastric
cancer and astrocytoma, which contributed to cancer cell
proliferation and metastasis via various mechanisms (34–39).
However, the expression of miR-19a-3p has also been reported to be
downregulated in breast cancer and non-melanoma skin cancer
(40–42). These findings suggested that
miR-19a-3p functions as an oncomir or tumor suppressive miRNA
depending on the tumor type. However, the expression level and
biological role of miR-19a-3p in bone metastasis of PCa remain
largely unknown. In this study, we found that miR-19a-3p expression
was downregulated in bone metastatic PCa tissues and cells compared
with non-bone metastatic PCa tissues and cell lines. Upregulating
miR-19a-3p suppressed the invasion, migration and osteolytic
capability of PCa cells via targeting downstream effectors of TGF-β
signaling, SMAD2 and SMDA4, leading to inactivation of TGF-β
signaling. Moreover, the stimulatory effects of miR-19a-3p
silencing on the invasion and migration in PCa cells were
attenuated by individual silencing of SMAD2 and SMAD4. Therefore,
our findings demonstrate that downregulation of miR-19a-3p promotes
invasion, migration and bone metastasis of PCa cells via activating
TGF-β signaling pathway. Furthermore, several studies have shown
that miR-19a-3p was identified in the serum of cancer patients,
including colorectal cancer and astrocytoma (35,39,43),
suggesting that miR-19a-3p may serve as a serum marker for the
diagnosis of cancer. However, whether miR-19a-3p may be used as a
serum marker for the diagnosis of PCa bone metastasis needs to be
further validated of in a larger series of studies.
In conclusion, our results demonstrate that
tumor-suppressive miR-19a-3p inhibits the invasion, migration and
osteolytic capability of PCa cells via targeting SMAD2 and SMDA4,
leading to inactivation of TGF-β signaling. Thus, improved
understanding of the specific role of downregulation of miR-19a-3p
in the bone metastasis of PCa will increase our knowledge of the
development of PCa bone metastasis, which will help to develop new
therapeutic strategies against PCa.
Acknowledgements
This study was supported by the grant from the
National Natural Science Foundation of China (nos. 81402227 and
81660362).
References
|
1
|
Nelson WG, De Marzo AM and Isaacs WB:
Prostate cancer. N Engl J Med. 349:366–381. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bubendorf L, Schöpfer A, Wagner U, Sauter
G, Moch H, Willi N, Gasser TC and Mihatsch MJ: Metastatic patterns
of prostate cancer: An autopsy study of 1,589 patients. Hum Pathol.
31:578–583. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ell B and Kang Y: SnapShot: Bone
metastasis. Cell. 151:690–690 e691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mohammad KS, Chen CG, Balooch G, Stebbins
E, McKenna CR, Davis H, Niewolna M, Peng XH, Nguyen DH,
Ionova-Martin SS, et al: Pharmacologic inhibition of the TGF-beta
type I receptor kinase has anabolic and anti-catabolic effects on
bone. PLoS One. 4:e52752009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel PM and Massagué J: Cytostatic and
apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev
Cancer. 3:807–821. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jakowlew SB: Transforming growth
factor-beta in cancer and metastasis. Cancer Metastasis Rev.
25:435–457. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Javelaud D, Mohammad KS, McKenna CR,
Fournier P, Luciani F, Niewolna M, André J, Delmas V, Larue L,
Guise TA, et al: Stable overexpression of Smad7 in human melanoma
cells impairs bone metastasis. Cancer Res. 67:2317–2324. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yin JJ, Selander K, Chirgwin JM, Dallas M,
Grubbs BG, Wieser R, Massagué J, Mundy GR and Guise TA: TGF-beta
signaling blockade inhibits PTHrP secretion by breast cancer cells
and bone metastases development. J Clin Invest. 103:197–206. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu Z, Gupta J, Zhang Z, Gerseny H, Berg A,
Chen YJ, Zhang Z, Du H, Brendler CB, Xiao X, et al: Systemic
delivery of oncolytic adenoviruses targeting transforming growth
factor-β inhibits established bone metastasis in a prostate cancer
mouse model. Hum Gene Ther. 23:871–882. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wan X, Li ZG, Yingling JM, Yang J,
Starbuck MW, Ravoori MK, Kundra V, Vazquez E and Navone NM: Effect
of transforming growth factor beta (TGF-β) receptor I kinase
inhibitor on prostate cancer bone growth. Bone. 50:695–703. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Juárez P and Guise TA: TGF-β in cancer and
bone: Implications for treatment of bone metastases. Bone.
48:23–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fournier PG, Juárez P, Jiang G, Clines GA,
Niewolna M, Kim HS, Walton HW, Peng XH, Liu Y, Mohammad KS, et al:
The TGF-β signaling regulator PMEPA1 suppresses prostate cancer
metastases to bone. Cancer Cell. 27:809–821. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ventura A and Jacks T: MicroRNAs and
cancer: Short RNAs go a long way. Cell. 136:586–591. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khew-Goodall Y and Goodall GJ:
Myc-modulated miR-9 makes more metastases. Nat Cell Biol.
12:209–211. 2010.PubMed/NCBI
|
|
15
|
Baranwal S and Alahari SK: miRNA control
of tumor cell invasion and metastasis. Int J Cancer. 126:1283–1290.
2010.PubMed/NCBI
|
|
16
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang X, Liu J, Zang D, Wu S, Liu A, Zhu
J, Wu G, Li J and Jiang L: Upregulation of miR-572
transcriptionally suppresses SOCS1 and p21 and contributes to human
ovarian cancer progression. Oncotarget. 6:15180–15193. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hahn WC, Dessain SK, Brooks MW, King JE,
Elenbaas B, Sabatini DM, DeCaprio JA and Weinberg RA: Enumeration
of the simian virus 40 early region elements necessary for human
cell transformation. Mol Cell Biol. 22:2111–2123. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren D, Lin B, Zhang X, Peng Y, Ye Z, Ma Y,
Liang Y, Cao L, Li X, Li R, et al: Maintenance of cancer stemness
by miR-196b-5p contributes to chemoresistance of colorectal cancer
cells via activating STAT3 signaling pathway. Oncotarget.
8:49807–49823. 2017.PubMed/NCBI
|
|
20
|
Ma L, Young J, Prabhala H, Pan E, Mestdagh
P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S,
et al: miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin
and cancer metastasis. Nat Cell Biol. 12:247–256. 2010.PubMed/NCBI
|
|
21
|
Colden M, Dar AA, Saini S, Dahiya PV,
Shahryari V, Yamamura S, Tanaka Y, Stein G, Dahiya R and Majid S:
MicroRNA-466 inhibits tumor growth and bone metastasis in prostate
cancer by direct regulation of osteogenic transcription factor
RUNX2. Cell Death Dis. 8:e25722017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Siu MK, Tsai YC, Chang YS, Yin JJ, Suau F,
Chen WY and Liu YN: Transforming growth factor-β promotes prostate
bone metastasis through induction of microRNA-96 and activation of
the mTOR pathway. Oncogene. 34:4767–4776. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren D, Wang M, Guo W, Zhao X, Tu X, Huang
S, Zou X and Peng X: Wild-type p53 suppresses the
epithelial-mesenchymal transition and stemness in PC-3 prostate
cancer cells by modulating miR-145. Int J Oncol. 42:1473–1481.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ren D, Wang M, Guo W, Huang S, Wang Z,
Zhao X, Du H, Song L and Peng X: Double-negative feedback loop
between ZEB2 and miR-145 regulates epithelial-mesenchymal
transition and stem cell properties in prostate cancer cells. Cell
Tissue Res. 358:763–778. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guo W, Ren D, Chen X, Tu X, Huang S, Wang
M, Song L, Zou X and Peng X: HEF1 promotes epithelial mesenchymal
transition and bone invasion in prostate cancer under the
regulation of microRNA-145. J Cell Biochem. 114:1606–1615. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang M, Ren D, Guo W, Huang S, Wang Z, Li
Q, Du H, Song L and Peng X: N-cadherin promotes
epithelial-mesenchymal transition and cancer stem cell-like traits
via ErbB signaling in prostate cancer cells. Int J Oncol.
48:595–606. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Ren D, Guo L, Wang L, Wu S, Lin
C, Ye L, Zhu J, Li J, Song L, et al: Thymosin beta 10 is a key
regulator of tumorigenesis and metastasis and a novel serum marker
in breast cancer. Breast Cancer Res. 19:152017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang S, Guo W, Tang Y, Ren D, Zou X and
Peng X: miR-143 and miR-145 inhibit stem cell characteristics of
PC-3 prostate cancer cells. Oncol Rep. 28:1831–1837. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang M, Burton DW, Geller J, Hillegonds
DJ, Hastings RH, Deftos LJ and Hoffman RM: The bisphosphonate
olpadronate inhibits skeletal prostate cancer progression in a
green fluorescent protein nude mouse model. Clin Cancer Res.
12:2602–2606. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Liu F, Lin B, Luo H, Liu M, Wu J, Li
C, Li R, Zhang X, Zhou K, et al: miR-150 inhibits proliferation and
tumorigenicity via retarding G1/S phase transition in
nasopharyngeal carcinoma. Int J Oncol. 50:1097–1108. 2017.
View Article : Google Scholar :
|
|
31
|
Wang M, Ren D, Guo W, Wang Z, Huang S, Du
H, Song L and Peng X: Loss of miR-100 enhances migration, invasion,
epithelial-mesenchymal transition and stemness properties in
prostate cancer cells through targeting Argonaute 2. Int J Oncol.
45:362–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cioffi M, Trabulo SM, Sanchez-Ripoll Y,
Miranda-Lorenzo I, Lonardo E, Dorado J, Vieira C Reis, Ramirez JC,
Hidalgo M, Aicher A, et al: The miR-17-92 cluster counteracts
quiescence and chemoresistance in a distinct subpopulation of
pancreatic cancer stem cells. Gut. 64:1936–1948. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kang Y, He W, Tulley S, Gupta GP,
Serganova I, Chen CR, Manova-Todorova K, Blasberg R, Gerald WL and
Massagué J: Breast cancer bone metastasis mediated by the Smad
tumor suppressor pathway. Proc Natl Acad Sci USA. 102:pp.
13909–13914. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sand M, Hessam S, Amur S, Skrygan M,
Bromba M, Stockfleth E, Gambichler T and Bechara FG: Expression of
oncogenic miR-17-92 and tumor suppressive miR-143-145 clusters in
basal cell carcinoma and cutaneous squamous cell carcinoma. J
Dermatol Sci. 86:142–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu M, Huang Z, Zhu D, Zhou X, Shan X, Qi
LW, Wu L, Cheng W, Zhu J, Zhang L, et al: A panel of microRNA
signature in serum for colorectal cancer diagnosis. Oncotarget.
8:17081–17091. 2017.PubMed/NCBI
|
|
36
|
Zhang X, Chen Y, Zhao P, Zang L, Zhang Z
and Wang X: MicroRNA-19a functions as an oncogene by regulating
PTEN/AKT/pAKT pathway in myeloma. Leuk Lymphoma. 58:932–940. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pan Y, Guo Y, Luo Y, Li H and Xu Y:
MicroRNA expression profiling of Chinese follicular lymphoma by
microarray: A preliminary study. Int Immunopharmacol. 39:41–47.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ibarrola-Villava M, Llorca-Cardeñosa MJ,
Tarazona N, Mongort C, Fleitas T, Perez-Fidalgo JA, Roselló S,
Navarro S, Ribas G and Cervantes A: Deregulation of ARID1A, CDH1,
cMET and PIK3CA and target-related microRNA expression in gastric
cancer. Oncotarget. 6:26935–26945. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zheng G, Du L, Yang X, Zhang X, Wang L,
Yang Y, Li J and Wang C: Serum microRNA panel as biomarkers for
early diagnosis of colorectal adenocarcinoma. Br J Cancer.
111:1985–1992. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu Q, Guo L, Jiang F, Li L, Li Z and Chen
F: Analysis of the miRNA-mRNA-lncRNA networks in ER+ and ER- breast
cancer cell lines. J Cell Mol Med. 19:2874–2887. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Balci S, Ayaz L, Gorur A, Yaroglu H
Yildirim, Akbayir S, Unal N Dogruer, Bulut B, Tursen U and Tamer L:
microRNA profiling for early detection of nonmelanoma skin cancer.
Clin Exp Dermatol. 41:346–351. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Leung CM, Chen TW, Li SC, Ho MR, Hu LY,
Liu WS, Wu TT, Hsu PC, Chang HT and Tsai KW: MicroRNA expression
profiles in human breast cancer cells after multifraction and
single-dose radiation treatment. Oncol Rep. 31:2147–2156. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhi F, Shao N, Wang R, Deng D, Xue L, Wang
Q, Zhang Y, Shi Y, Xia X, Wang S, et al: Identification of 9 serum
microRNAs as potential noninvasive biomarkers of human astrocytoma.
Neuro-oncol. 17:383–391. 2015. View Article : Google Scholar : PubMed/NCBI
|