Introduction
Autologous fat grafts are used to fill soft tissue
defects, and were reported to be performed as early as 1893. With
refinements in the technique, autologous fat transplantation has
become increasingly popular in cosmetic and reconstructive surgery
(1). After liposuction from an area
where fat is present in excess, such as the abdomen or thigh, the
fat can be grafted for an array of problems, including atrophic and
posttraumatic facial deficits, facial rhytids, scarring, and lip
and breast augmentation (2,3). Furthermore, adipose tissue has gained
significant importance for the complexity of its components and
functions over the past decade. The main subcomponents of adipose
tissue contain mature adipocytes and a stromal vascular fraction.
Recent biotechnological advancements have allowed for the
harvesting of adult stem cells from the stromal vascular fraction
(3). Using a patient's own stem
cells avoids ethical issues associated with the use of human
embryonic or bone marrow stem cells, and reduces the chance of
tissue rejection. In addition, it is less invasive and much easier
to obtain adipose-derived stem cells (ASCs) than performing a bone
marrow extraction. ASCs have been proven to efficiently allow
stimulation of cell-differentiation, immune-modulation, peripheral
nerve repair and angiogenesis (4).
In a recent study, ASCs have become powerful tools to improve wound
healing and attenuate scar formation by promoting cell migration,
angiogenesis and a possible regenerative rather than fibrotic
microenvironment at the wound site (5).
Breast asymmetry after breast conservation surgery
is challenging for plastic surgeons. Autologous fat grafts with or
without skin flap reconstruction seems to be a good choice to fill
defects and improve cosmetic outcome of breast conservation surgery
(6). However, oncological safety is
a major concern in cell therapy-related issues; especially in ASCs
since they secrete cytokines, chemokines and growth factors, which
may promote cancer cell growth, metastasis, and angiogenesis.
Multiple studies have discussed the interaction between lung, colon
and breast cancer cells, ASCs and fat grafting (7–12).
Although autologous fat grafting is not associated with an
increased risk of tumor recurrence or distant metastasis of
patients with breast cancer in clinical studies (3,13,14),
some studies have reported that adipocytes or ASCs may stimulate
cancer cell growth or metastasis (9–12).
Patients with head and neck cancer usually have
aesthetic and functional problems after excision and reconstructive
surgery, such as chin deformity, scar contracture, and drooling.
Autologous fat injection is a good method to rebuild postoperative
soft tissue volume insufficiency and scar contracture for these
patients. Similar to breast cancer patients, carcinogenesis of
autologous fat grafting is a critical issue. ASCs may induce
tyrosine kinase receptor signaling pathways to promote tumor growth
or chemoresistance (8,15). However, the underlying interaction
between ASCs and head and neck squamous cell carcinoma remains
unclear. In the present study, we aimed to investigate the
biological effects and molecular mechanisms of the crosstalk
between ASCs and oral cancer cells in vitro.
Materials and methods
Chemicals and reagents
Dimethyl sulfoxide (DMSO), potassium phosphate,
trypan blue, propidium iodide (PI), Triton X-100, Tris-HCl and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Dulbecco's modified Eagle's medium (DMEM), 1.5 mM L-glutamine, 10%
fetal bovine serum (FBS), trypsin-EDTA, penicillin G, and
streptomycin were obtained from Gibco BRL (Grand Island, NY, USA).
Antibodies against β-actin (GTX109639, 1:5,000), protein kinase B
(Akt; GTX128414, 1:500), phospho-Akt (GTX128414, 1:500),
extracellular regulated kinases (ERK1/2; GTX59618, 1:500),
phospho-ERK1/2 (GTX59568, 1:500), Bcl-2-associated death promoter
(BAD; GTX130108, 1:1,000), phospho-BAD (GTX50136, 1:500),
insulin-like growth factor 1 receptor (IGF1R; GTX83064, 1:1,000),
phospho-IGF1R (GTX55013, 1:500), epidermal growth factor receptor
(EGFR; GTX100448, 1:5,000), phospho-EGFR (GTX61353, 1:500),
caspase-3 (GTX110543, 1:1,000), caspase-9 (GTX112888, 1:1,000), and
all peroxidase-conjugated secondary antibodies were purchased from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA).
Cell culture
CAL-27 and SCC-25, human tongue squamous cell
carcinoma cell lines, were obtained from the Food Industry Research
and Development Institute (Hsinchu, Taiwan). Cisplatin-resistant
CAL-27 (CAR) cells were kindly provided by Dr Jai-Sing Yang. CAR
cells were generated from the CAL-27 cell line by sub-culturing in
increasing cisplatin concentrations from 10 to 80 µM by 10 cycles
of 1 passage, as previously described (16). All cells were cultured in 75
cm2 tissue culture flasks with 90% DMEM with 1.5 mM
L-glutamine adjusted to 1.5 mg/l sodium bicarbonate, supplemented
with 10% FBS and 1% penicillin-streptomycin (100 U/ml penicillin G
and 100 mg/ml streptomycin), and maintained at 37°C in a 5%
CO2 atmosphere.
Adipose tissue-derived stem cell (ASC)
preparation
This study was approved by the Institutional Review
Board of Taipei Veterans General Hospital (VGHIRB no.
2016-06-005A). The board is organized and operates according to the
International Conference on Harmonisation (ICH)/WHO Good Clinical
Practice (GCP) and applicable laws and regulations. Briefly,
lipo-aspirate was obtained from the liposuction waste of three
volunteers. The fat was immersed in phosphate-buffered saline (PBS)
solution and centrifuged to remove excessive fluid. The washed fat
tissue was mixed with DMEM, collagenase (1 mg/ml),
N-acetylcysteine (2 mM), and l-ascorbic acid-2 phosphate
(0.2 mM) and cultured at 37°C for 1 h. After centrifugation to
remove excessive collagenase, the pellet was cultured with DMEM
(10% FBS), N-acetylcysteine (2 mM), and l-ascorbic acid-2
phosphate (0.2 mM) under 5% CO2 overnight. The flask was
washed with PBS to remove unattached cells, and the medium was
changed every other day until confluence. Cells were sub-cultured
and characterized by flow cytometry after positive surface staining
for CD29 and CD90, but not for CD11B, CD31 or CD45. Before
experimental use, ASCs were tested for their ability to
differentiate into various mesenchymal lineages, including
adipocytes, osteoblasts and chondrocytes (data not shown).
Preparation of ASC conditioned medium
(ASC-CM)
To prepare ASC-CM, ASCs (1×106) were
cultured in T75 flasks for 2–3 days. Culture medium was removed at
cell confluence, and the cells were washed twice with PBS. Fresh
DMEM supplemented with 10% FBS was added and incubation was carried
out for 1 day. After incubation, the culture medium was collected
and centrifuged to remove the cells.
Cell viability assay
To evaluate the cytotoxic effect of cisplatin on
CAL-27, SCC-25, and CAR cells, an MTT
[3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide]
assay was used. Briefly, the cells were placed in a 96-well cell
culture plate at an initial concentration of 5×104
cells/ml, treated with cisplatin at different concentrations (5,
10, 15 and 20 µM) or with 0.1% DMSO for 24 h, and cultured in DMEM
or ASC-CM. Each concentration was repeated three times. After a
24-h incubation period, MTT solution (5 mg/ml, 100 µl/well) was
added to the cells for 4 h. Subsequently, the growth medium was
removed, and the formazan crystals formed by oxidation of the MTT
solution were dissolved with DMSO in isopropanol and measured
spectrophotometrically at 490 nm. The cell survival ratio was
expressed as a percentage of the control. Viability assays were
performed in triplicate from three independent experiments.
Phase-contrast microscopy of
morphological changes
CAL-27 cells were cultured in 6-well plates at a
density of 2.5×105 cells/well/ml before being treated
with 20 µM of cisplatin or with 0.1% DMSO for 24 h, and cultured in
DMEM or ASC-CM. After treatment for 24 h, morphological changes
were determined by a phase-contrast microscope.
Wound healing assay
CAL-27 and CAR cells were placed in a 6-well tissue
culture plate for 24 h and grown to 80–90% confluence. Individual
wells were scratched with a micro-pipette tip to create a denuded
zone of constant width. Cells were then cultured in serum-low DMEM
or ASC-CM for 24 h. Cells were photographed using phase-contrast
microscopy (×100). Wound healing assay were performed in triplicate
from three independent experiments.
DNA content by flow cytometry
CAL-27 cells (1×105) were plated in
24-well plates and cultured in DMEM or ASC-CM with 20 µM cisplatin
for 24 h. Cells were harvested and then fixed in 70% ethanol at
24°C overnight. Cells were incubated with PI buffer. The
distribution of the cell cycle and the sub-G1 population (apoptotic
cells) were detected using a flow cytometric method. BD
Paint-A-Gate™ was used for analysis of the flow cytometry (BD
Biosciences, San Jose, CA, USA).
Western blot analysis
Briefly, CAL-27 cells were plated in a T75 flask at
an initial concentration of 5×106 cells, and cultured in
DMEM or ASC-CM with 20 µM cisplatin for 24 h. The cells were
harvested and total protein was collected. Samples were
electrophoresed by 10% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto nitrocellulose
membranes (Invitrogen Life Technologies, Carlsbad, CA, USA). The
membranes were blocked with PBST solution (0.1% Tween-20 in PBS)
plus 5% powdered non-fat milk for 1 h, and incubated overnight at
4°C with the primary antibody diluted in blocking solution (0.1%
Tween-20 in PBS plus 5% powdered non-fat milk). Subsequently, the
membranes were washed with PBST three times for 10 min and
incubated with the appropriate alkaline HRP-conjugated secondary
IgG antibody (horseradish peroxidase-conjugated goat anti-rabbit
and goat anti-mouse) for 1 h at 24°C and then washed three times
again. Bands were detected by enhanced chemiluminescence with ECL
reagents (Amersham Pharmacia, Buckinghamshire, UK) and exposed on
X-OMAT AR film (Eastman Kodak, Rochester, NY, USA). Auto-radiograms
were scanned on a UMAX PowerLook Scanner (UMAX Technologies,
Fremont, CA, USA) with Photoshop software (Adobe Systems, Seattle,
WA, USA). Western blot analysis was performed in triplicate from
three independent experiments.
Statistical analysis
All data are expressed as mean ± SEM from at least
three separate experiments. Statistical calculations of the data
were performed using a one-way ANOVA. P<0.05 was considered to
indicate a statistically significant difference.
Patient data collection
We conducted a retrospective review using data drawn
from electronic medical records retained by our medical
institution, Taipei Veterans General Hospital. Between January 2000
and December 2014, patients who underwent fat grafting for scar
revision after head and neck cancer surgical resection were
included in the data collection and analysis.
Results
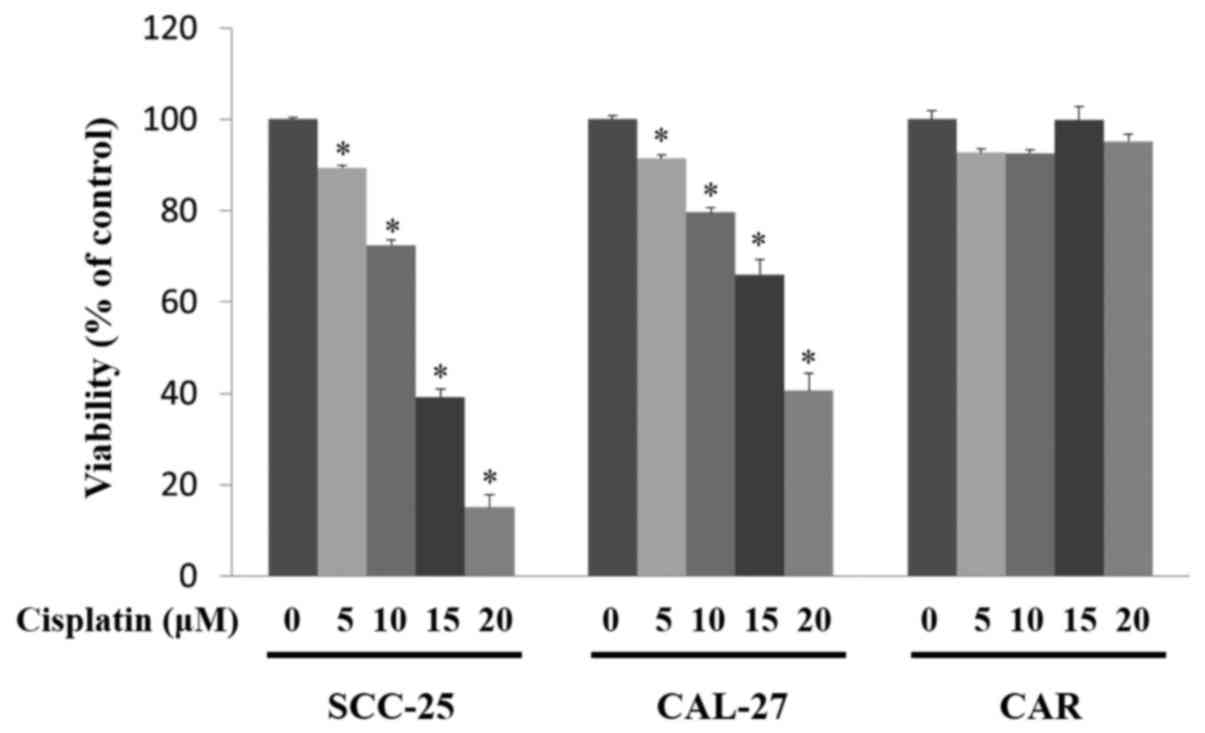
Cisplatin reduces the viability of
CAL-27 and SCC-25 cells, but does not have an effect on the CAR
cells
To determine the cytotoxic effects of cisplatin on
SCC-25, CAL-27 and CAR cells, an MTT assay was performed. As shown
in Fig. 1, cisplatin treatment
significantly decreased the viability of the SCC-25 and CAL-27
cells in a concentration-dependent manner (P<0.05). Cisplatin
was also found to induce the formation of apoptotic bodies in the
SCC-25 and CAL-27 cells after 10–20 µM cisplatin challenge (data
not shown). Importantly, there was no cytotoxic effect or
morphological characteristic change in the cisplatin-treated CAR
cells.
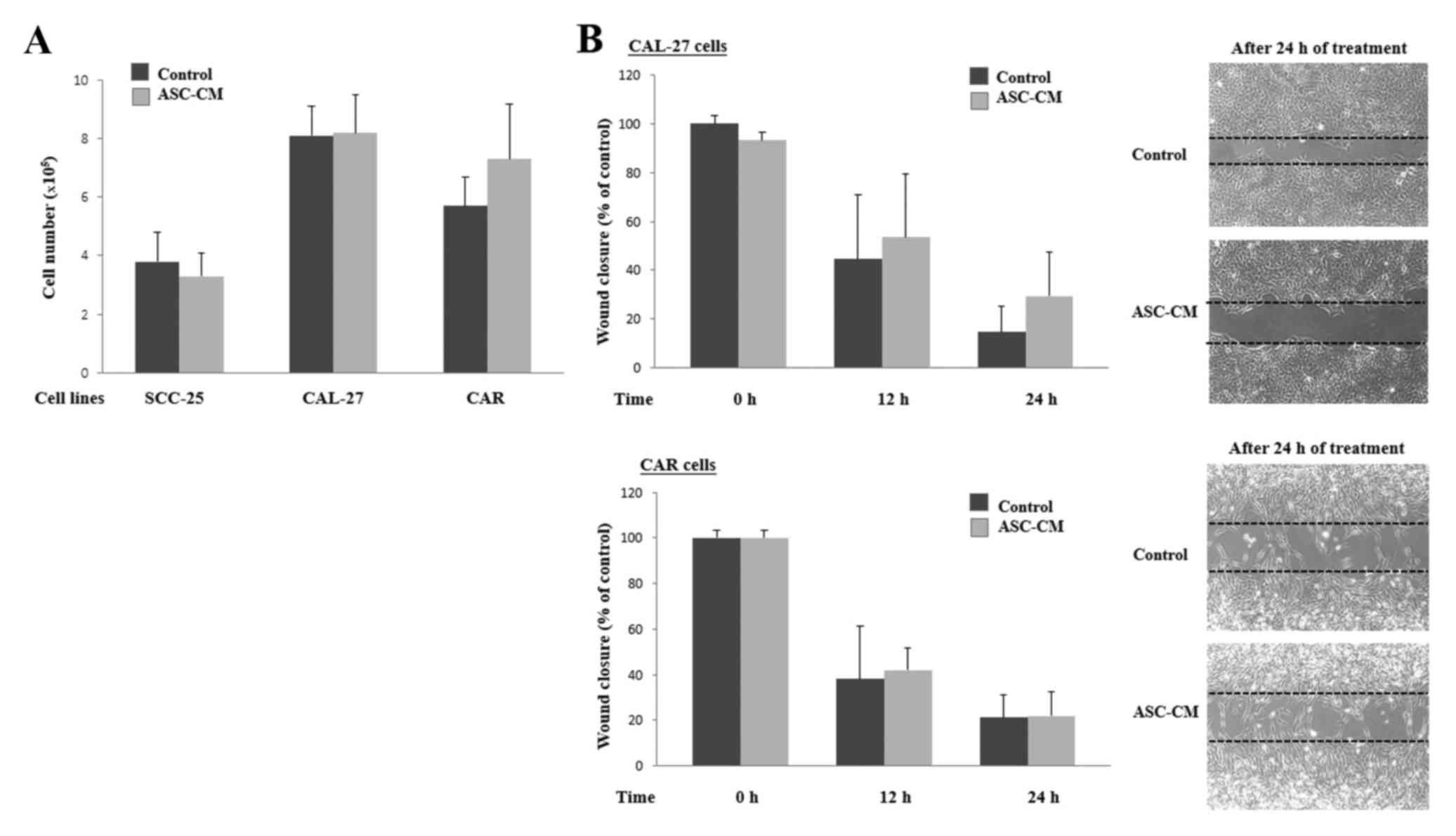
Adipose tissue-derived stem cell
conditioned medium (ASC-CM) treatment does not significantly
increase human tongue cancer cell viability, invasion and migration
abilities
To determine the cell activity of human tongue
cancer cells following ASC-CM treatment, viable cells were counted
using a hemocytometer after cell culture in DMEM or ASC-CM for 24
h. As shown in Fig. 2A, no
significant difference in viability of the SCC-25, CAL-27, and CAR
cells was noted between the DMEM and ASC-CM treatment group. We
subsequently performed a wound healing assay. As shown in Fig. 2B, our results also failed to reveal
a significant difference in the migration ability of the cancer
cells between cells cultured in DMEM and ASC-CM.
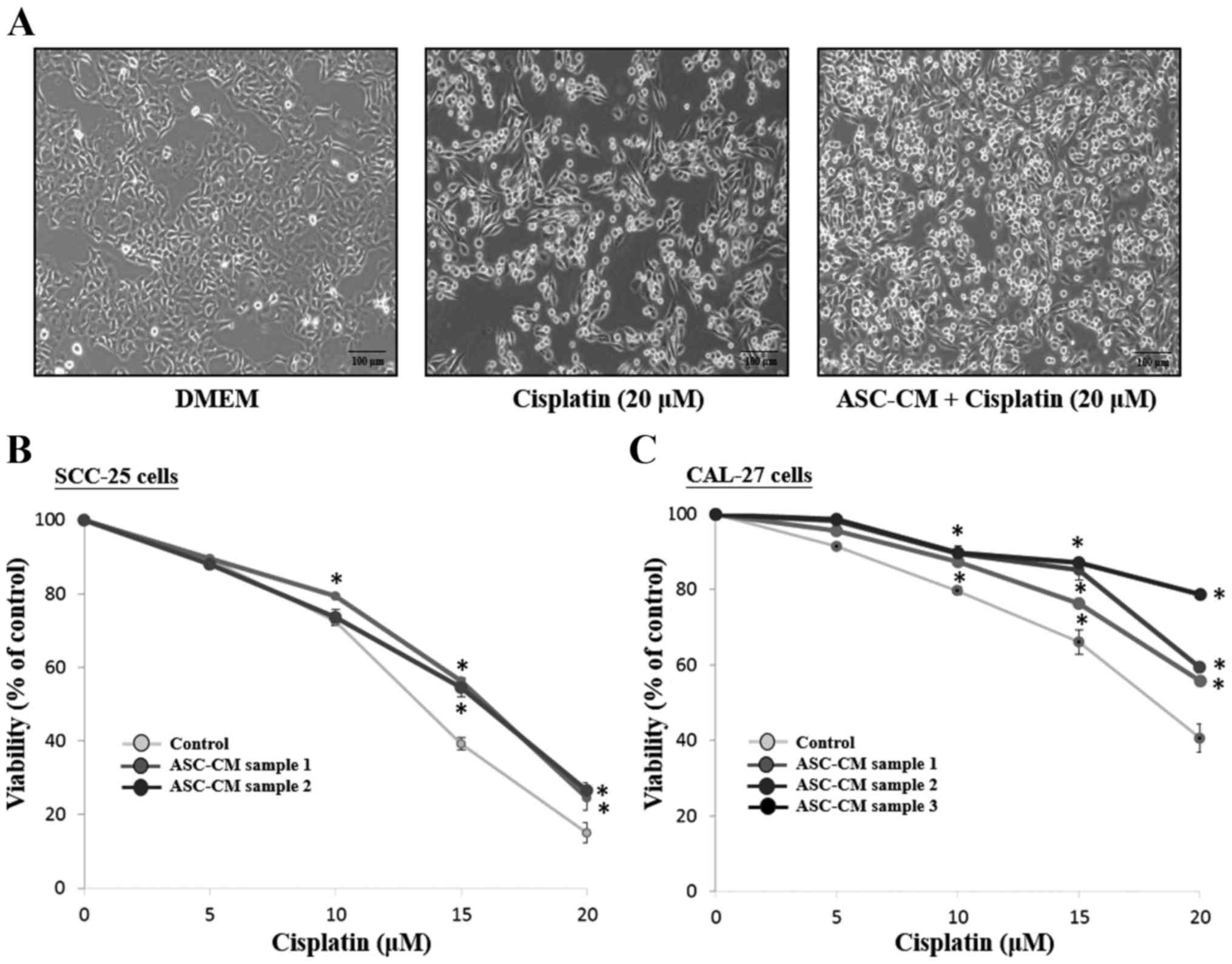
ASC-CM increases the cell viability of
the SCC-25 and CAL-27 cells treated with cisplatin
Phase-contrast microscopy was used to detect the
morphological differences between DMEM (control) and ASC-CM groups.
As shown in Fig. 3A, CAL-27 cells
grew well and spread with a flattened morphology after a 24-h
culture. The number of viable cells was decreased and some detached
from the surface and contained some debris in the cisplatin-treated
CAL-27 cells. However, an increased number of viable cells in the
cisplatin-treated CAL-27 cells was observed in the presence of
ASC-CM compared to the control group. To detect the growth
inhibitory effect of ASC-CM on cisplatin-treated SCC-25 and CAL-27
cells, a cell survival assay by MTT was performed. As shown in
Fig. 3, ASC-CM from three different
patients increased viable cells in the cisplatin-treated SCC-25
(Fig. 3B) and CAL-27 cells
(Fig. 3C) (P<0.05).
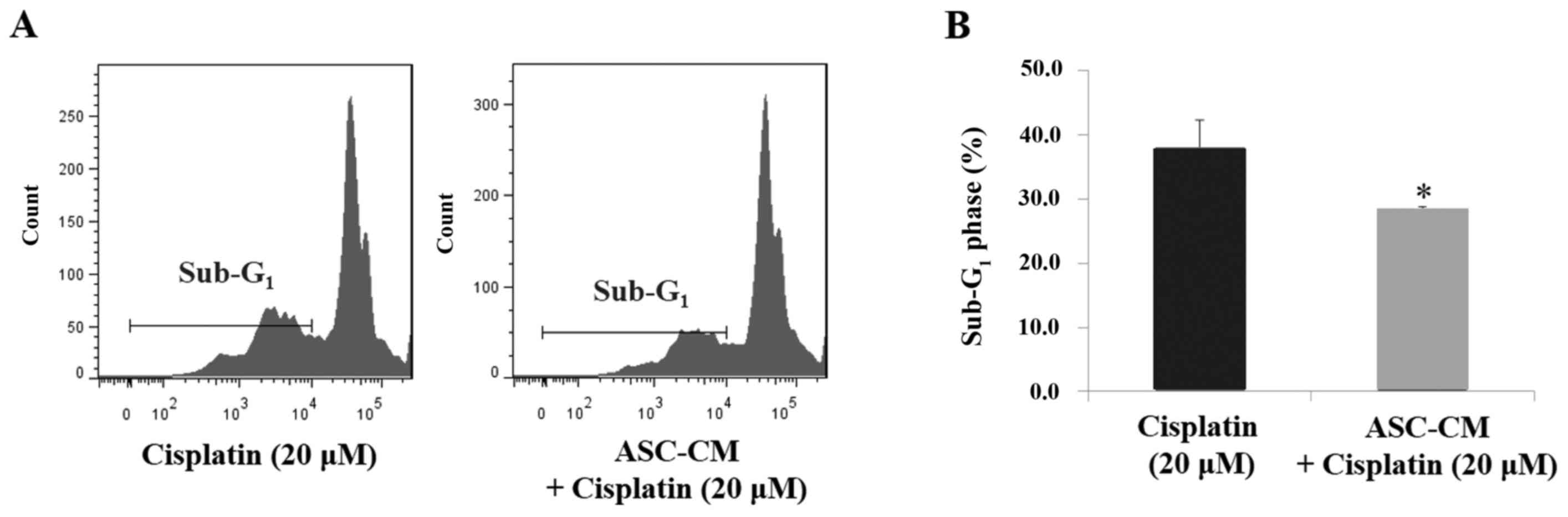
ASC-CM reduces cisplatin-induced
CAL-27 cell apoptosis
To further evaluate the apoptotic process in
cisplatin-treated CAL-27 cells under ASC-CM, flow cytometry was
used to exam the DNA content of the apoptotic population (sub-G1
phase). As shown in Fig. 4, ASC-CM
significantly reduced the sub-G1 phase cell population in the
cisplatin-treated CAL-27 cells (38.1±4.2% in DMEM and 28.4±0.4% in
ASC-CM, P<0.05).
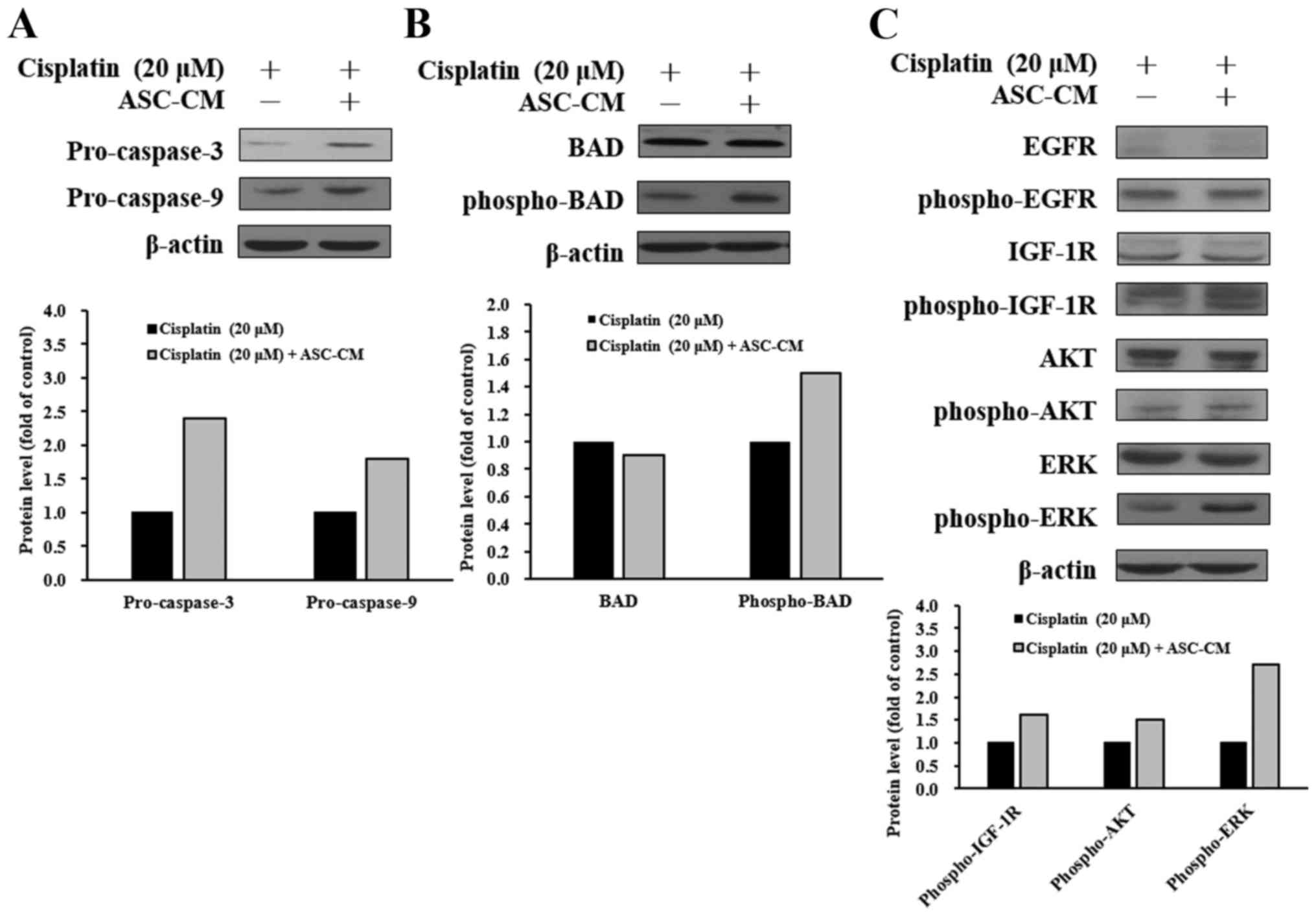
ASC-CM modulates caspase-3, caspase-9,
and IGF-1R signaling-related protein levels in cisplatin-treated
CAL-27 cells
To elucidate the possible molecular signaling
pathways in cisplatin-treated CAL-27 cells with ASC-CM treatment,
the protein levels in the tyrosine kinase signaling and apoptotic
pathways were evaluated by western blotting. As shown in Fig. 5, ASC-CM caused an increase in
protein levels of pro-caspase-3, pro-caspase-9 (Fig. 5A), and phospho-BAD (Fig. 5B) in the cisplatin-treated CAL-27
cells. To further investigate cell signaling, upstream-associated
proteins were also studied. ASC-CM caused an increase in protein
expressions of phospho-IGF-1R, phospho-AKT, and phospho-ERK
(Fig. 5C). These results suggest
that ASC-CM attenuated cisplatin-triggered apoptosis in CAL-27
cells through the IGF-1R/AKT/ERK signaling pathway.
Patients who underwent fat grafting
for scar revision after head and neck cancer surgical
resection
In the retrospective review, there were 10 patients
with head and neck squamous cell carcinoma who underwent fat
grafting for scar revision after head and neck cancer surgical
resection between January 2000 and December 2014. Patient
demographics, cancer type, cancer staging after initially surgical
resection, follow-up period, and outcome are summarized in Table I. Patient 1 died of
radiotherapy-induced sarcoma. There was no local recurrence in all
patients during the follow-up period.
 | Table I.The demographic data of 10 patients
with head and neck cancers who underwent fat grafting
procedures.a |
Table I.
The demographic data of 10 patients
with head and neck cancers who underwent fat grafting
procedures.a
| Patient | Age/sex | Cancer type | Staging | Follow-up | Outcome |
|---|
| 1 | 44/M | Buccal SCC | pT4aN0M0, IVA | 1.9 years | Died from
sarcoma |
| 2 | 48/M | Tongue SCC | Data missing | >5 years | Disease-free |
| 3 | 46/M | Lower gingival
SCC | pT4aN2bM0,IVA | >5 years | Disease-free |
| 4 | 53/M | Hypopharyngeal
SCC | Data missing | >5 years | Disease-free |
| 5 | 49/M | Buccal SCC | pT1N2bM0, IVA | 3.8 years | Disease-free |
| 6 | 41/M | Buccal SCC | pT4aN2bM, IVA | 4.2 years | Disease-free |
| 7 | 85/M | Buccal SCC | pT1N0M0, I | 4.8 years | Disease-free |
| 8 | 51/F | Buccal SCC | pT2N2bM0, IVA | 4.4 years | Disease-free |
| 9 | 50/M | Buccal SCC | pT4aN1M0, IVA | 3.7 years | Disease-free |
| 10 | 51/M | Buccal SCC | pT3N2bM0, IVA | 2.2 years | Disease-free |
Discussion
With recent advances in techniques and procedures,
the frequency of autologous fat grafting has dramatically
increased, not only for cosmetic purposes, but also for revision of
deformity and reshaping after head and neck cancer and breast
cancer surgery. Adipose tissue-derived stem cells (ASCs), a
critical component of adipose tissue, has become one of the most
popular adult stem cell populations in the field of stem cell
research and tissue regeneration (17). However, carcinogenesis is a major
concern for cell therapy-related issues. The role of ASCs in
clinical applications in breast cancer patients has been disputed
intensively in the past decade (14). To the best of our understanding, we
investigated the interaction between tongue cancer cells and ASCs,
and we found that ASC-CM promoted the chemoresistance of
cisplatin-treated CAL-27 and SCC-25 cells.
The clinical effects of the ASC secretome in
multiple, biologically relevant scenarios have been widely analyzed
and documented, including immunomodulation, angiogenesis, wound
healing and tissue regeneration (18). Numerous adipokines have been
discovered that could potentially promote breast, lung and colon
cancer growth and metastasis (7–12,19).
Linkov et al reported that ASC-CM increased endometrial
adenocarcinoma proliferation (19).
Kim et al demonstrated that ASC-CM enhanced the migration of
ovarian cancer cells via activation of the JAK2/STAT3 signaling
pathway (20). However,
Skelhorne-Gross et al reported that stromal adipocyte PPARγ
protects against breast tumorigenesis (21). Zhang et al and Meleshina
et al reported that mesenchymal stem cells suppress tumor
growth and metastasis by modulating the immune system in mice
(22,23). Ryu et al demonstrated that
ASC-CM expresses IFN-β and suppresses the growth of human breast
cancer cells (24). Our results
revealed that there was no significant difference in cell growth
and cell migration in tongue cancer cells with the addition of
condition medium from ASC.
The ASC secretome was also reported to be associated
with drug resistance in cancer cells. Nowicka et al reported
that adipose stem cells increase ovarian cancer proliferation,
migration and chemoresistance (25). Sun et al demonstrated that
IGF-1 from ASCs promotes radioresistance of breast cancer cells
(15). IGF-1R is a receptor
tyrosine kinase, which is involved in tumor progression and
promotes cancer cell proliferation, metastasis and chemoresistance
(26). Inhibition of IGF-1R could
sensitize breast cancer cells to chemotherapy by inhibiting both
MAPK and AKT signaling pathways. Abnormal activation of the AKT and
ERK signaling cascade stimulates tumor cell growth, proliferation,
survival and resistance to drug-induced apoptosis (27). Our data demonstrated that ASC-CM
inhibited cell death through activation of IGF-1R and ERK/AKT
signaling in cisplatin-treated CAL-27 cells (Fig. 5).
Apoptosis is a type of programmed cell death. Two
major pathways are involved in apoptotic cell death: the intrinsic
(mitochondrial-mediated) and extrinsic (death receptor) pathways.
In the intrinsic pathway, regulatory protein is mediated by Bcl-2
family proteins. In apoptotic stimuli, the level of Bax (a
pro-apoptotic protein) is increased, which is followed by binding
to Bcl-2 (a pro-survival protein) and release of Bax/Bak molecules.
Free Bax and Bak lead to cytochrome c release from the
mitochondria to the cytoplasm. Released cytochrome c
activates the caspase-9 and caspase-3 cascade to induce apoptosis
(28,29). It was reported that ASC-CM is rich
in growth factors, such as IGF-1 and EGF, and these growth factors
have an anti-apoptotic function (18). Both ERK and PI-3K pathways provide
survival signaling that may neutralize pro-apoptotic Bcl-2 family
proteins. ERK induces Bax/Bcl-2 heterodimerization by directly
phosphorylating Bcl-2. Regulation of apoptosis by PI-3K is
primarily mediated through AKT, which in turn regulates BAD and
caspase-9. Phosphorylation of BAD enhances cell survival by
inducing cytoplasmic sequestration of Bad through formation of the
Bad/14-3-3 protein complex (21,30).
In the present study, treatment with condition medium from ASC
inhibited cell apoptosis in cisplatin-treated CAL-27 and SCC-25
cells. Furthermore, addition of ASC-CM also resulted in an increase
in protein levels of pro-caspase-3, pro-caspase-9 (Fig. 5A), and phospho-BAD (Fig. 5B) in cisplatin-treated CAL-27 cells.
There was a limitation to the results, as we did not routinely
perform the western blotting experiments in triplicate.
The ASC secretome could potentially promote tumor
initiation and growth, but clinical studies have failed to point
out a significant increase in the local recurrence rate of patients
who receive autologous fat grafting after breast cancer surgery
(14,18). We retrospectively reviewed 10
patients who underwent fat grafting for scar revision after head
and neck cancer surgical resection between January 2000 and
December 2014 at our institution. Only one patient died of
radiation-induced sarcoma. There was no local recurrence in all
patients during the follow-up period.
In conclusion, there was no significant difference
in cell growth and cell migration between tongue cancer cells with
or without addition of condition medium from ASC. Our study
revealed that ASC-CM enhanced anti-apoptotic effects through the
IGF-1R/AKT/ERK signaling pathway in cisplatin-treated tongue cancer
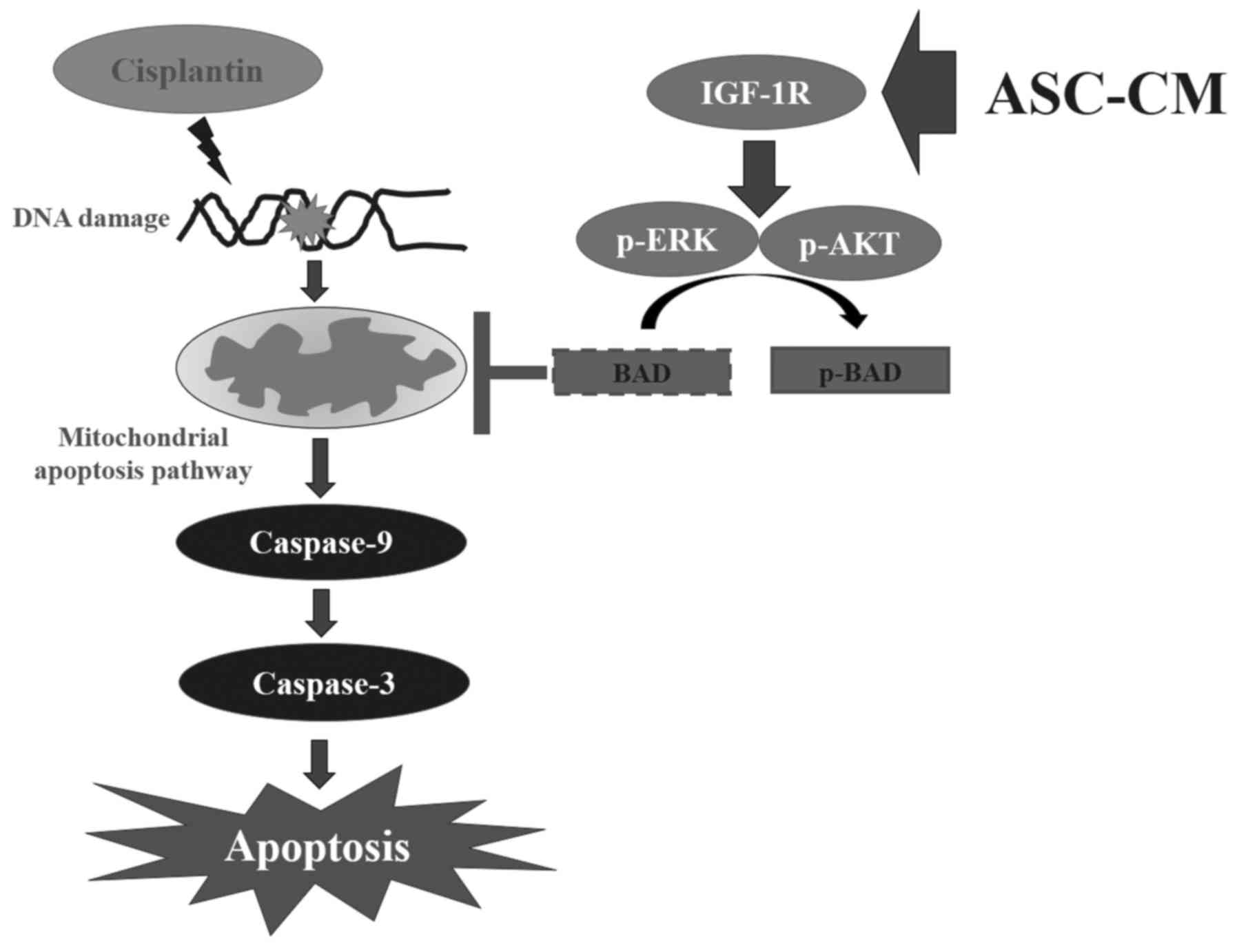
cells. The proposed signal pathways are shown in Fig. 6. This finding provides new insight
into the molecular mechanism of condition medium in tongue cancer
cells and for its potential therapeutic application. Furthermore,
our findings should be taken into consideration when performing fat
grafting to the head and neck area. Further investigation to
elucidate the function of ASCs in oral cancer is warranted.
Acknowledgements
This study was supported by a grant from the Taipei
Veterans General Hospital, Taipei, Taiwan (V106A-008).
Glossary
Abbreviations
Abbreviations:
|
ASC-CM
|
adipose-derived stem cell conditioned
medium
|
|
BAD
|
Bcl-2-associated death promoter
|
|
CAR
|
cisplatin-resistant CAL-27 cells
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DMSO
|
dimethyl sulfoxide
|
|
EGFR
|
epidermal growth factor receptor
|
|
ERK
|
extracellular regulated kinases
|
|
IGF-1R
|
insulin-like growth factor 1
receptor
|
|
PBS
|
phosphate-buffered saline
|
|
PI
|
propidium iodide
|
|
BCT
|
breast conservation treatment
|
References
|
1
|
Kim J-E and Sykes JM: Hyaluronic acid
fillers: History and overview. Facial Plast Surg. 27:523–528. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Delay E, Garson S, Tousson G and Sinna R:
Fat injection to the breast: Technique, results, and indications
based on 880 procedures over 10 years. Aesthet Surg J. 29:360–376.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brenelli F, Rietjens M, De Lorenzi F,
Pinto-Neto A, Rossetto F, Martella S, Rodrigues JR and Barbalho D:
Oncological safety of autologous fat grafting after breast
conservative treatment: A prospective evaluation. Breast J.
20:159–165. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsuji W, Rubin JP and Marra KG:
Adipose-derived stem cells: Implications in tissue regeneration.
World J Stem Cells. 6:312–321. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Upadhyay RK: Role of regeneration in
tissue repairing and therapies. J Tissue Eng Regen Med. 4:1–30.
2015. View Article : Google Scholar
|
|
6
|
Cardoso MJ, Oliveira H and Cardoso J:
Assessing cosmetic results after breast conserving surgery. J Surg
Oncol. 110:37–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen D, Liu S, Ma H, Liang X, Ma H, Yan X,
Yang B, Wei J and Liu X: Paracrine factors from adipose-mesenchymal
stem cells enhance metastatic capacity through Wnt signaling
pathway in a colon cancer cell co-culture model. Cancer Cell Int.
15:422015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sahin E, Baycu C, Koparal AT, Burukoglu
Donmez D and Bektur E: Resveratrol reduces IL-6 and VEGF secretion
from co-cultured A549 lung cancer cells and adipose-derived
mesenchymal stem cells. Tumour Biol. 37:7573–7582. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Manabe Y, Toda S, Miyazaki K and Sugihara
H: Mature adipocytes, but not preadipocytes, promote the growth of
breast carcinoma cells in collagen gel matrix culture through
cancer-stromal cell interactions. J Pathol. 201:221–228. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Iyengar P, Combs TP, Shah SJ, Gouon-Evans
V, Pollard JW, Albanese C, Flanagan L, Tenniswood MP, Guha C,
Lisanti MP, et al: Adipocyte-secreted factors synergistically
promote mammary tumorigenesis through induction of anti-apoptotic
transcriptional programs and proto-oncogene stabilization.
Oncogene. 22:6408–6423. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rowan BG, Gimble JM, Sheng M, Anbalagan M,
Jones RK, Frazier TP, Asher M, Lacayo EA, Friedlander PL, Kutner R,
et al: Human adipose tissue-derived stromal/stem cells promote
migration and early metastasis of triple negative breast cancer
xenografts. PLoS One. 9:e895952014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chamras H, Bagga D, Elstner E, Setoodeh K,
Koeffler HP and Heber D: Preadipocytes stimulate breast cancer cell
growth. Nutr Cancer. 32:59–63. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Petit JY, Lohsiriwat V, Clough KB, Sarfati
I, Ihrai T, Rietjens M, Veronesi P, Rossetto F, Scevola A and Delay
E: The oncologic outcome and immediate surgical complications of
lipofilling in breast cancer patients: A multicenter study -
Milan-Paris-Lyon experience of 646 lipofilling procedures. Plast
Reconstr Surg. 128:341–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Charvet HJ, Orbay H, Wong MS and Sahar DE:
The oncologic safety of breast fat grafting and contradictions
between basic science and clinical studies: A systematic review of
the recent literature. Ann Plast Surg. 75:471–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang HY, Qu RM, Lin XS, Liu TX, Sun QQ,
Yang C, Li XH, Lu W, Hu XF, Dai JX, et al: IGF-1 from
adipose-derived mesenchymal stem cells promotes radioresistance of
breast cancer cells. Asian Pac J Cancer Prev. 15:10115–10119. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yuan CH, Horng CT, Lee CF, Chiang NN, Tsai
FJ, Lu CC, Chiang JH, Hsu YM, Yang JS and Chen FA: Epigallocatechin
gallate sensitizes cisplatin-resistant oral cancer CAR cell
apoptosis and autophagy through stimulating AKT/STAT3 pathway and
suppressing multidrug resistance 1 signaling. Environ Toxicol.
32:845–855. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Raposio E, Caruana G, Bonomini S and
Libondi G: A novel and effective strategy for the isolation of
adipose-derived stem cells: Minimally manipulated adipose-derived
stem cells for more rapid and safe stem cell therapy. Plast
Reconstr Surg. 133:1406–1409. 2014.PubMed/NCBI
|
|
18
|
Kapur SK and Katz AJ: Review of the
adipose derived stem cell secretome. Biochimie. 95:2222–2228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Linkov F, Kokai L, Edwards R, Sheikh MA,
Freese KE, Marra KG and Rubin JP: The role of adipose-derived stem
cells in endometrial cancer proliferation. Scand J Clin Lab Invest
Suppl. 244:54–58. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim B, Kim HS, Kim S, Haegeman G, Tsang
BK, Dhanasekaran DN and Song YS: Adipose stromal cells from
visceral and subcutaneous fat facilitate migration of ovarian
cancer cells via IL-6/JAK2/STAT3 pathway. Cancer Treat. 49:338–349.
2017. View Article : Google Scholar
|
|
21
|
Skelhorne-Gross G, Reid AL, Apostoli AJ,
Di Lena MA, Rubino RE, Peterson NT, Schneider M, SenGupta SK,
Gonzalez FJ and Nicol CJ: Stromal adipocyte PPARγ protects against
breast tumorigenesis. Carcinogenesis. 33:1412–1420. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang L, Su XS, Ye JS, Wang YY, Guan Z and
Yin YF: Bone marrow mesenchymal stem cells suppress metastatic
tumor development in mouse by modulating immune system. Stem Cell
Res Ther. 6:452015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meleshina AV, Cherkasova EI, Shirmanova
MV, Klementieva NV, Kiseleva EV, Snopova LВ, Prodanets NN and
Zagaynova EV: Influence of mesenchymal stem cells on metastasis
development in mice in vivo. Stem Cell Res Ther. 6:152015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryu H, Oh J-E, Rhee K-J, Baik SK, Kim J,
Kang SJ, Sohn JH, Choi E, Shin HC, Kim YM, et al: Adipose
tissue-derived mesenchymal stem cells cultured at high density
express IFN-β and suppress the growth of MCF-7 human breast cancer
cells. Cancer Lett. 352:220–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nowicka A, Marini FC, Solley TN, Elizondo
PB, Zhang Y, Sharp HJ, Broaddus R, Kolonin M, Mok SC, Thompson MS,
et al: Human omental-derived adipose stem cells increase ovarian
cancer proliferation, migration, and chemoresistance. PLoS One.
8:e818592013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chong KY, Subramanian A, Mokbel K and
Sharma AK: The prognostic significance of the insulin-like growth
factor-1 ligand and receptor expression in breast cancer tissue. J
Surg Oncol. 104:228–235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang C-H, Lee C-Y, Lu C-C, Tsai FJ, Hsu
YM, Tsao JW, Juan YN, Chiu HY, Yang JS and Wang CC:
Resveratrol-induced autophagy and apoptosis in cisplatin-resistant
human oral cancer CAR cells: A key role of AMPK and Akt/mTOR
signaling. Int J Oncol. 50:873–882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
King YA, Chiu YJ, Chen HP, Kuo DH, Lu CC
and Yang JS: Endoplasmic reticulum stress contributes to arsenic
trioxide-induced intrinsic apoptosis in human umbilical and bone
marrow mesenchymal stem cells. Environ Toxicol. 31:314–328. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu C-C, Chen H-P, Chiang J-H, Jin YA, Kuo
SC, Wu TS, Hour MJ, Yang JS and Chiu YJ: Quinazoline analog HMJ-30
inhibits angiogenesis: Involvement of endothelial cell apoptosis
through ROS-JNK-mediated death receptor 5 signaling. Oncol Rep.
32:597–606. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chiu YJ, Hour MJ, Lu CC, Chung JG, Kuo SC,
Huang WW, Chen HJ, Jin YA and Yang JS: Novel quinazoline HMJ-30
induces U-2 OS human osteogenic sarcoma cell apoptosis through
induction of oxidative stress and upregulation of ATM/p53 signaling
pathway. J Orthop Res. 29:1448–1456. 2011. View Article : Google Scholar : PubMed/NCBI
|