Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor deriving from mesenchymal tissue, which is
characterized by the production of fusiform stromal cells of
osteoid tissue (1). It frequently
occurs in metaphysis of long bone in the adolescent, especially
around the knee joint, with the cause being unclear so far
(2). A majority of cases show
sporadic features without a definitely known genetic or
environmental factor (2). A
majority of cases show sporadic feature and have no definitely
known genetic or environmental factor. Various laboratories are now
probing into the possibility that tumor stem cells may be involved
in OS formation (3). Under normal
cell state, a part of genes participate in DNA repair and tumor
inhibitory pathways, which contributes to maintaining integrity of
cellular processes. Defects of these genes may participate in the
pathogenesis of OS (4).
MicroRNA is a kind of non-coding single-strand small
molecule, which specifically binds with its target gene and thus
regulates its expression (5). A
large amount of microRNAs have been discovered and shown to play
important roles in the genesis, differentiation, proliferation and
apoptosis of cells since the discovery of the first microRNA
(6). According to reports,
microRNAs regulate expression of human protein-coding genes, bind
with the 3′ untranslated region of target gene mRNA, and thus
result in mRNA degradation or translation inhibition (7). Apart from the silencing effect, some
microRNAs are verified to activate gene expression. MicroRNAs play
two distinct roles in various types of tumors, namely, tumor
suppressor gene and oncogene (8).
LRH1 is also named NR5A2, which is involved in the
differentiation of liver and pancreas during early embryonic
development (9). LRH1 regulates the
balance between cholesterol and bile acid, as well as the
production of steroid in adults. In the field of tumor research,
LRH1 is reported in literature to participate in the genesis and
development of breast cancer, gastric cancer, colon cancer and
pancreatic cancer as an oncogene (10). LRH1 can promote proliferation of
breast cancer cells through upregulating Erα transcription that
involves GREB1. In addition, it plays an important role in
promoting the invasion and migration of breast cancer cells through
activating remodeling of protein cytoskeleton and decomposition of
E-cadherin (11). Furthermore, LRH1
gene mutation is related to susceptibility of cancer, which
accelerates OS cell proliferation and tumor growth in nude mice
through upregulation of cyclin D1/E1 (12).
As reported, activation of Wnt/β-catenin pathway is
an important inducing factor for the metastasis of OS cells
(13). Expression of β-catenin gene
is upregulated in highly metastatic OS cell lines. Apoptotic
resistance, which is a key molecular mechanism guaranteeing
survival of the metastatic tumor cells, plays an important role
during the entire metastasis process. Metastasis can only take
place in case the tumor cells survive during metastasis.
Wnt/β-catenin pathway also plays an important resisting role during
apoptotic resistance, which is considered to be a key signaling
pathway for the invasion and metastasis of tumor cells. In the
present study, we aimed to systematically detect the function of
microRNA-381 on osteosarcoma cell growth and anticancer mechanism
for clinic treatment.
Materials and methods
Collection of human samples
Osteosarcoma tissues (n=7) and adjacent normal
tissues (n=7) were collected from Department of Orthopedics, The
Second Hospital of Jilin University, Changchun, Jilin, China. Our
study protocol was approved by Research Ethics Committee of The
Second Hospital of Jilin University.
Quantitative real-time polymerase
chain reaction (qRTPCR)
Total RNA was harvested using the RNA Isolation kit
(Ambion, USA) from tissue samples and cells. Then cDNA synthesis
kit (Qiagen, Germany) was carried out to conduct reverse to cDNA.
qRT-PCR was performed in the Applied Biosystems 7900 Fast Real-Time
PCR system (Applied Biosystems, USA) and a TaqMan Universal PCR
Master Mix (Applied Biosystems). MicroRNA-381 expression was
calculated using the 2−∆∆Ct method.
Cell culture
The human osteosarcoma cancer cell line 143B was
purchased from the American Type Culture Collection (ATCC) and
cultured in Dulbecco's modified Eagle's medium (DMEM, Gibco,
Invitrogen, Carlsbad, CA, USA), supplemented with 10% fetal bovine
serum (FBS, Gibco, Invitrogen) in a humidified incubator containing
5% CO2 at 37°C.
MicroRNA-381 mimics, antisense and
transfection
MicroRNA-381 mimics, antisense microRNA-381 mimics,
Si-LRH-1 or negative control mimics were obtained from Genepharm
Co. (Shanghai). Cells were transfected with microRNA-381 mimics,
antisense microRNA-381 mimics or negative control mimics by
Lipofectamine™ 2000 reagent (Invitrogen). Cells were transfected
with microRNA-381 mimics and Si-LRH-1 or negative control mimics by
Lipofectamine 2000 reagent (Invitrogen).
MTT assays and LDH activity
Cells were incubated with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT;
5 mg/ml; Sigma, St. Louis, MO, USA) for 4 h at 37°C. After
incubation for 4 h, medium was removed and dimethyl sulfoxide
(DMSO) was added to dissolve the formazan product for 20 min at
37°C. Absorbance was determined by an ELISA reader (Bio-Rad,
Hercules, CA, USA) at a wavelength of 490 nm. LDH of the cells were
incubated with LDH activity kit (Beyotime, Haimen, China) for 1 h
at 37°C. Absorbance was determined by an ELISA reader (Bio-Rad) at
a wavelength of 450 nm.
Cell apoptosis assay and caspase-3/9
activity
Cells were stained with FITC-labeled Annexin V
(Sigma-Aldrich) and propidium iodide (Sigma-Aldrich) at dark for 30
min. Apoptosis rate was analyzed via flow cytometer (FACSCalibur,
BD Biosciences, San Jose, CA, USA). Cells were harvested, washed
with PBS and lysed in a cell lysis buffer (Beyotime). Protein
concentration was quantified using a BCA kit Beyotime). Proteins
(10 µg) were incubated with caspase-3/9 activity kits (Beyotime) at
37°C for 2 h. Absorbance was determined by an ELISA reader
(Bio-Rad) at a wavelength of 405 nm.
DAPI measuring
Cells were washed with PBS, and stained with DAPI
assay for 30 min at 37°C. Then, cells were washed with PBS and
observed using a microscope (Olympus, Tokyo, Japan).
Western blotting
Cells were harvested, washed with PBS and lysed in a
cell lysis buffer (Beyotime). Protein concentration was quantified
using a BCA kit (Beyotime). Proteins (50 µg) were separated by
8–10% SDS-agarose gel, transferred to nitrocellulose membranes
(Bio-Rad). After blocking with 5% non-fat milk in PBS, membranes
were immunoblotted with the antibodies Bax, Bcl-2, p53, LRH-1, Wn,
β-catenin and GAPDH (Santa Cruz, CA, USA) at 4°C, followed by HRP
secondary antibodies (Cell Signaling) for 1 h at 37°C. The protein
bands were visualized by an ECL Western blotting kit (Millipore,
Boston, MA, USA) and analyzed by Image-Pro Plus 6.0 software (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
Data are expressed as the mean ± SEM.
Independent-samples t-test or ANOVA (one-way analysis of variance)
was used to determine data. P-value <0.05 was considered
statistically significant.
Results
MicroRNA-381 expression of
osteosarcoma patients
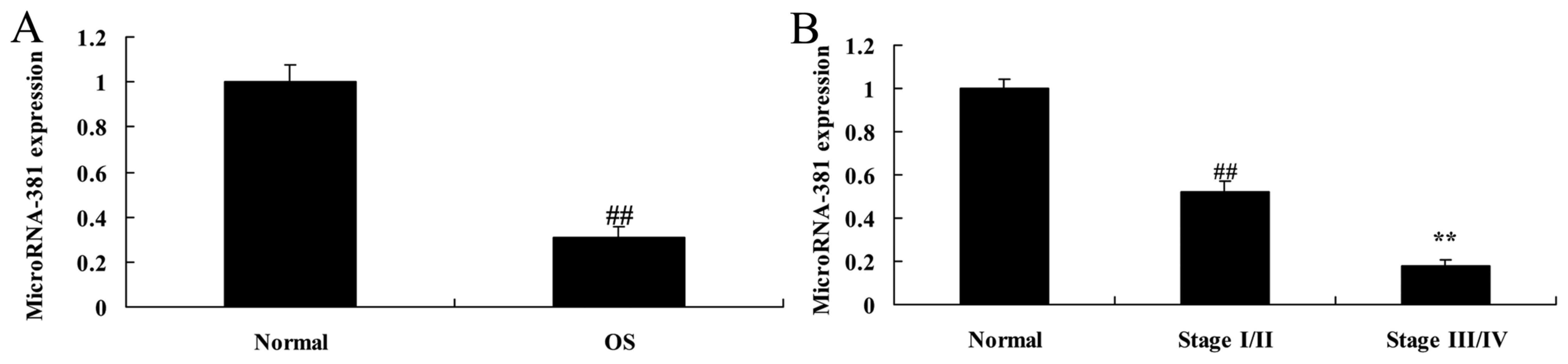
To determine the function of microRNA-381 in
osteosarcoma, its expression levels were analyzed in osteosarcoma
tissues and pair-matched adjacent normal. As showed in Fig. 1, microRNA-381 expression of
osteosarcoma patients was decreased, microRNA-381 levels were more
downregulated at stages III/IV than those at stage I/II in
osteosarcoma patients.
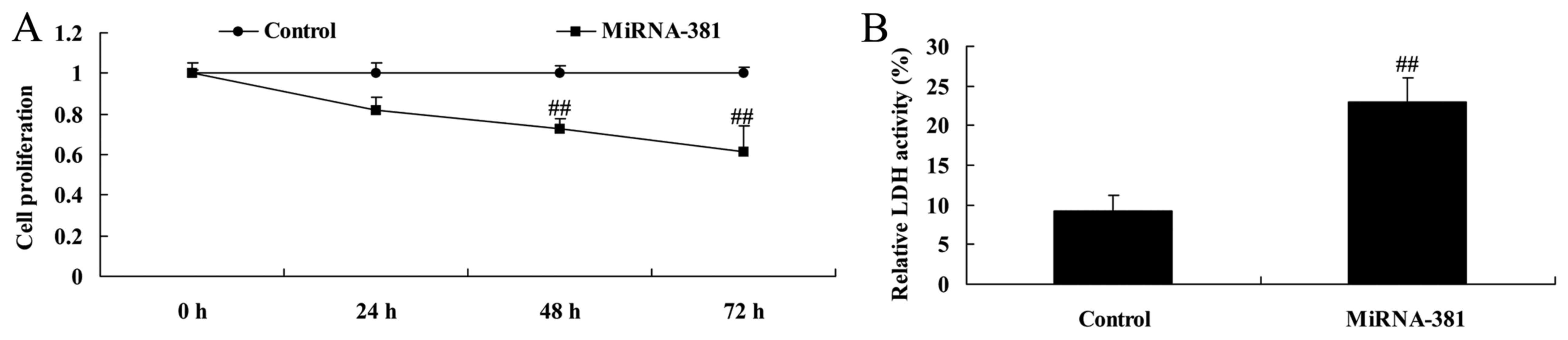
Upregulation of microRNA-381 on cell
proliferation and LDH activity of osteosarcoma
To determine the function of microRNA-381 in
osteosarcoma, we used microRNA-381 mimics to inhibit microRNA-381
expression. As shown in Fig. 2,
results from ELISA reader showed that microRNA-381 upregulation
significantly inhibited cell proliferation and increased LDH
activity of osteosarcoma.
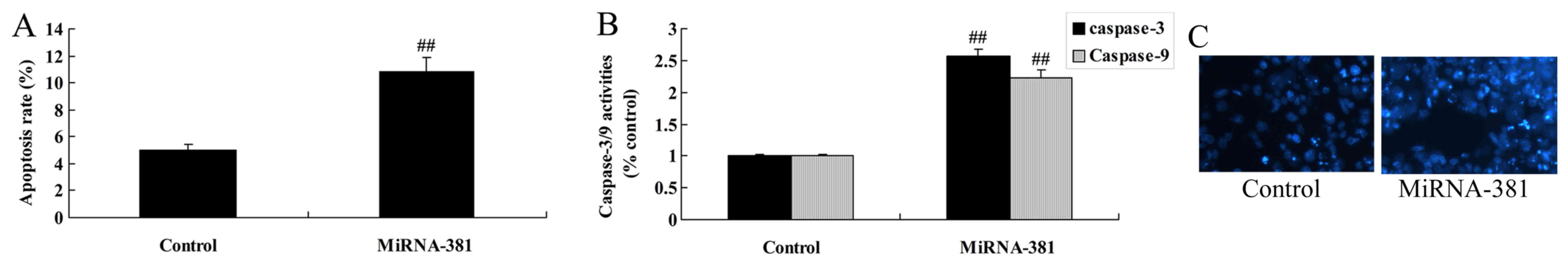
Upregulation of microRNA-381 on
apoptosis of osteosarcoma
Then, we determined the function of microRNA-381 on
apoptosis rate, caspase-3/9 activities and DAPI assay of
osteosarcoma. As showed in Fig. 3A and
B, significant increases of apoptosis rate, caspase-3/9
activities, and nucleus apoptosis were remarkably observed in
osteosarcoma by microRNA-381 upregulation.
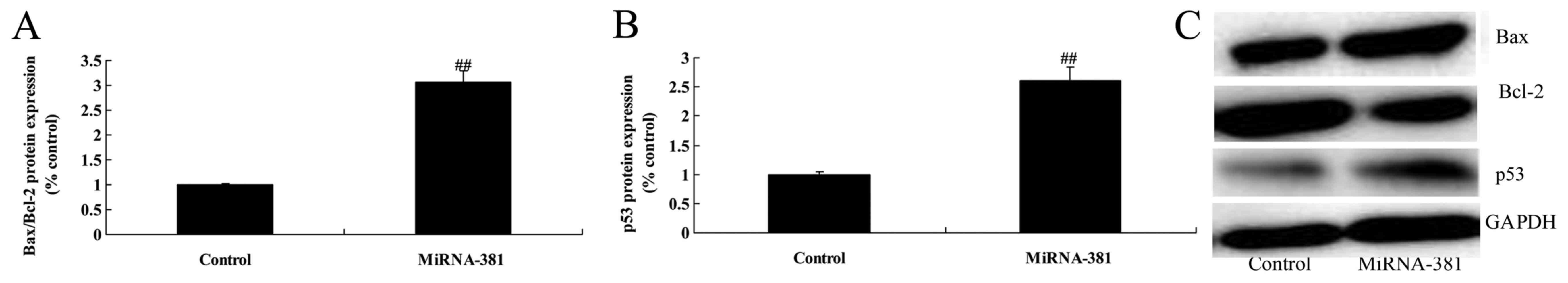
Upregulation of microRNA-381 on
Bax/Bcl-2 and p53 protein expression of osteosarcoma
Therefore, we aimed to identify its regulated target
and reveal the underlying molecular mechanisms of microRNA-381 on
Bax/Bcl-2 and p53 protein expression of osteosarcoma. As Fig. 4 shows, upregulation of microRNA-381
significantly induced Bax/Bcl-2 and p53 protein expression of
osteosarcoma.
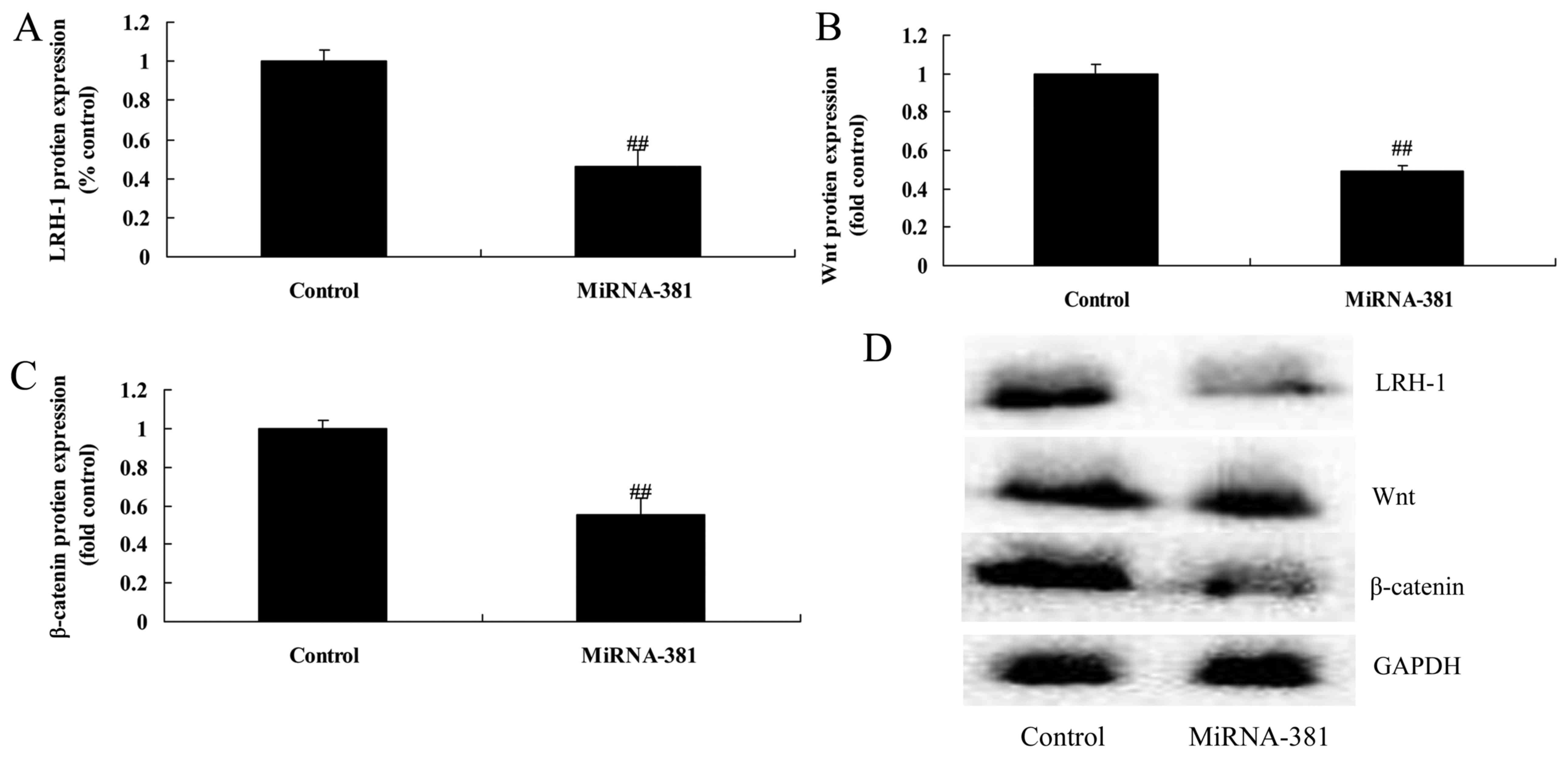
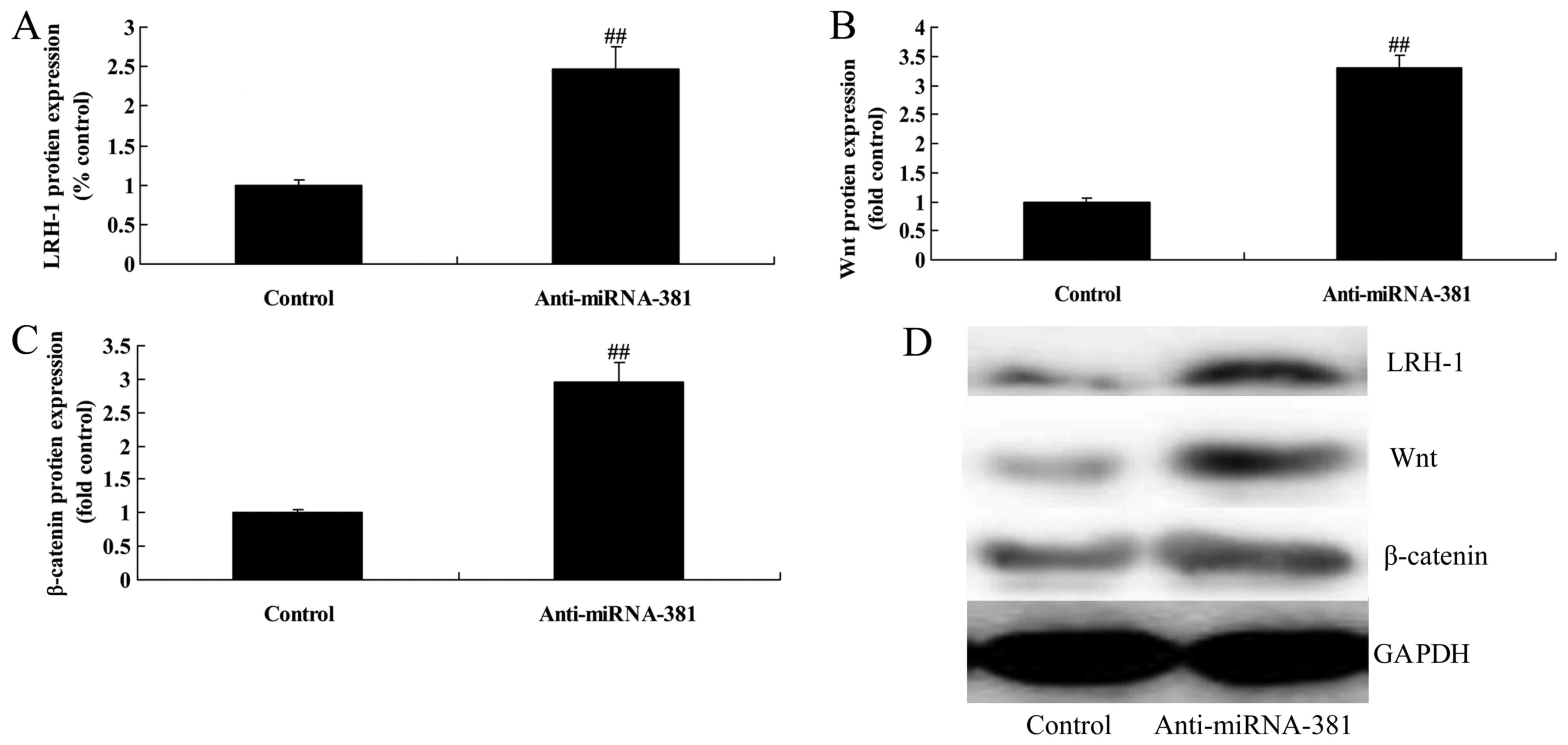
Upregulation of microRNA-381 on
LRH-1/Wnt/β-catenin signaling pathway of osteosarcoma
To determine if LRH-1 is targeted by microRNA-381 in
osteosarcoma, LRH-1, Wnt and β-catenin protein expression were
measured in this study. As shown in Fig. 5, upregulation microRNA-381
significantly suppressed LRH-1/Wnt/β-catenin signaling pathway of
osteosarcoma.
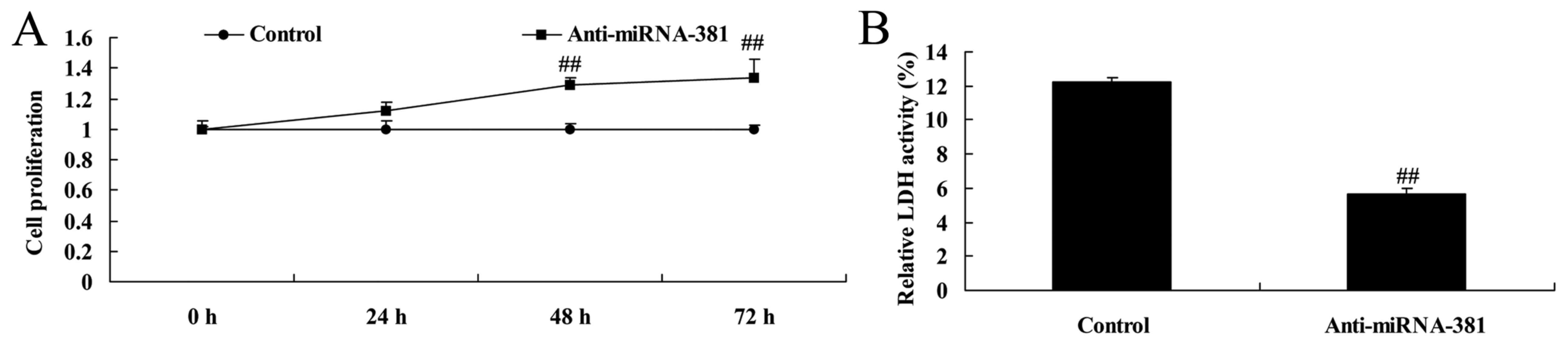
Downregulation of microRNA-381 on cell
proliferation and LDH activity of osteosarcoma
Then, we also upregulated microRNA-381 using
anti-microRNA-381 mimics, cell proliferation and LDH activity of
osteosarcoma were measured. Besides, downregulation of microRNA-381
significantly increased cell proliferation and inhibited LDH
activity of osteosarcoma (Fig.
6).
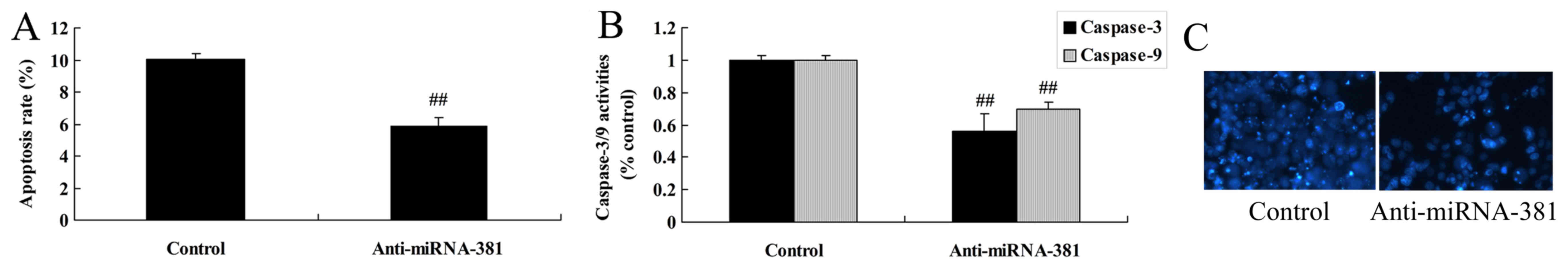
Downregulation of microRNA-381 on
apoptosis of osteosarcoma
There was significant inhibition of the apoptosis
rate and caspse-3/9 activities in osteosarcoma by microRNA-381
upregulation (Fig. 7A and B),
whereas, microRNA-381 downregulation reduced nucleus apoptosis of
osteosarcoma (Fig. 7C).
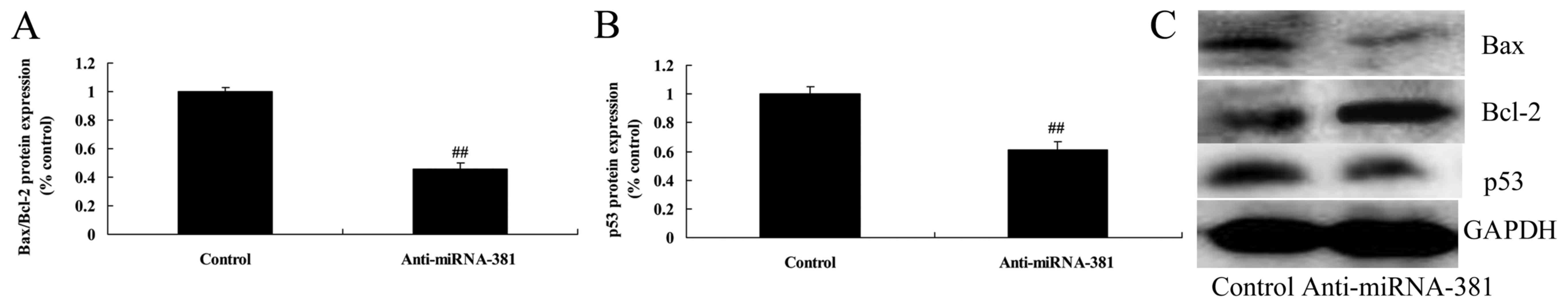
Downregulation of microRNA-381 on
Bax/Bcl-2 and p53 protein expression of osteosarcoma
We determined the potential tumor suppressing
effects of microRNA-381 on Bax/Bcl-2 and p53 protein expression of
osteosarcoma. As shown in Fig. 8,
expression of Bax/Bcl-2 and p53 protein of osteosarcoma was also
reduced by microRNA-381 downregulation.
Downregulation of microRNA-381 on
LRH-1/Wnt/β-catenin signaling pathway of osteosarcoma
Next, to clarify the roles of deregulated
microRNA-381 in osteosarcoma, LRH-1/Wnt/β-catenin signaling pathway
of osteosarcoma by microRNA-381 upregulation was measured. As a
result, microRNA-381 downregulation significantly induced
LRH-1/Wnt/β-catenin signaling pathway of osteosarcoma (Fig. 9).
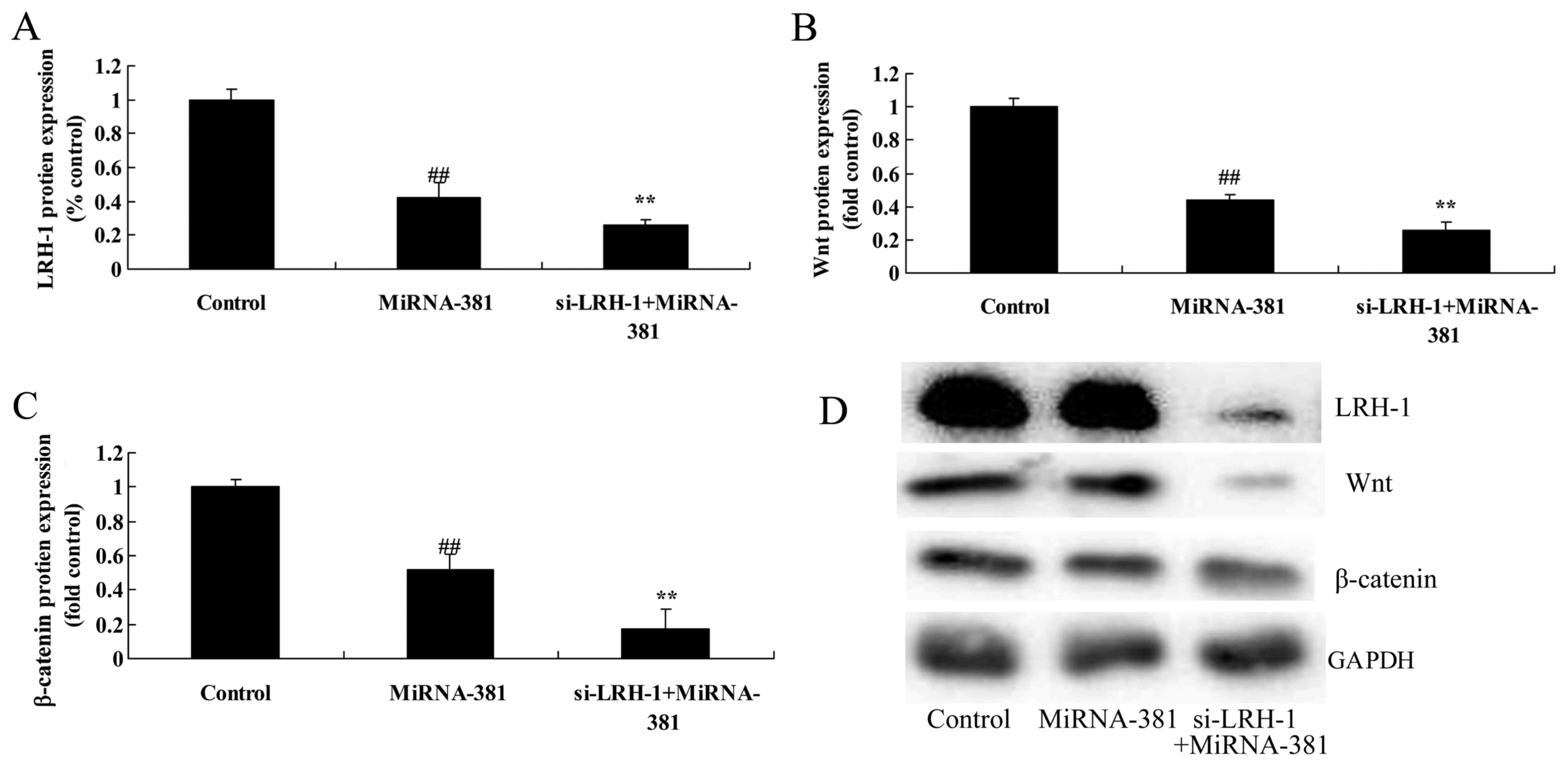
The inhibition of LRH-1 on
LRH-1/Wnt/β-catenin signaling pathway of osteosarcoma by
microRNA-381
To further confirm the above findings, we used
Si-LRH-1 to inhibit LRH-1 expression in osteosarcoma by
microRNA-381. However, a significant reduction of LRH-1, Wnt and
β-catenin protein expression was observed when transfected with
microRNA-381 and Si-LRH-1 (Fig.
10).
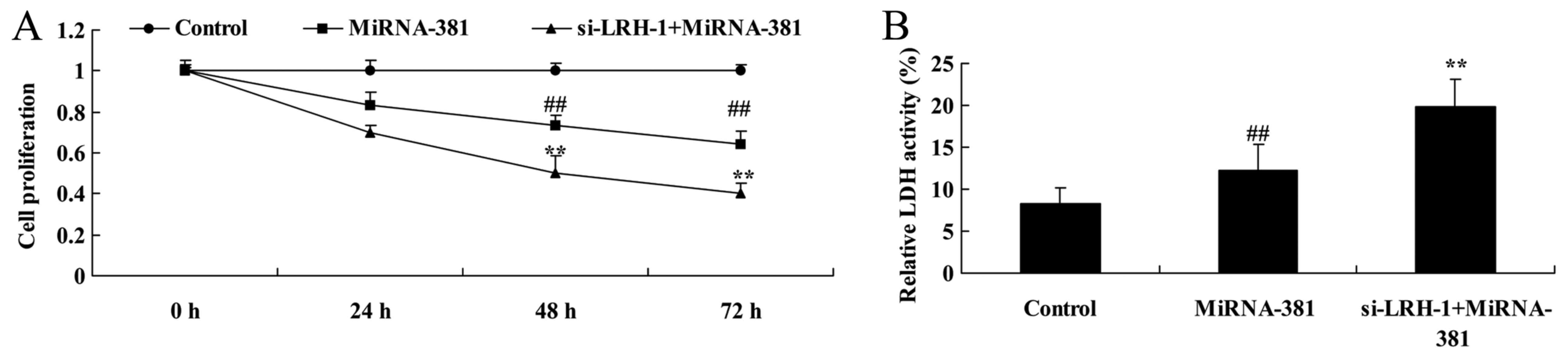
The inhibition of LRH-1 on cell
proliferation and LDH activity of osteosarcoma by microRNA-381
Therefore, LRH-1 regulation of the function of
microRNA-381 on osteosarcoma was investigated to clarify the
underlying molecular mechanisms. As showed in Fig. 11, the inhibition of LRH-1
significantly inhibited cell proliferation and increased LDH
activity of osteosarcoma by microRNA-381 (Fig. 11).
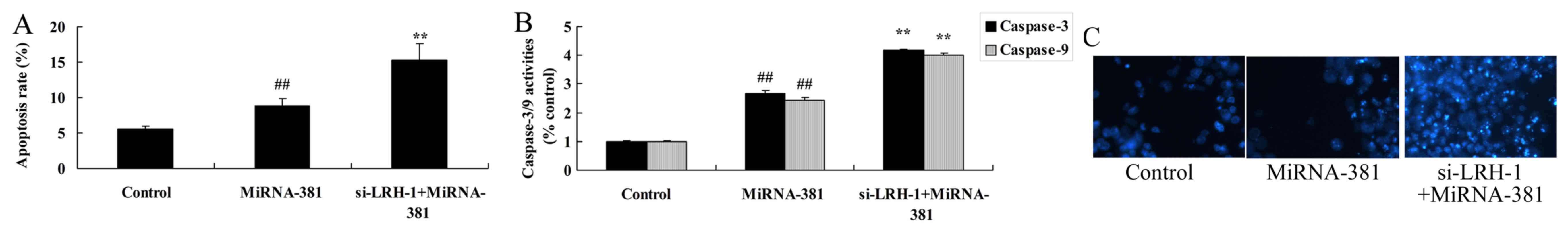
The inhibition of LRH-1 on apoptosis
of osteosarcoma by microRNA-381
On the other hand, the inhibition of LRH-1
significantly induced apoptosis and caspase-3/9 activities of
osteosarcoma by microRNA-381 (Fig.
12).
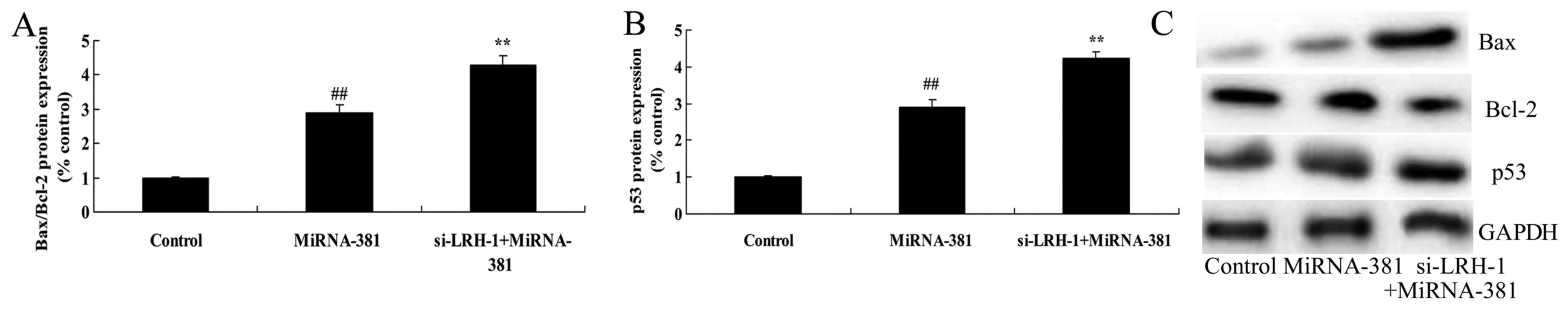
The inhibition of LRH-1 on Bax/Bcl-2
and p53 protein expression of osteosarcoma by microRNA-381
To further verify the functional connection between
LRH-1 and Bax/Bcl-2 and p53 protein expression of osteosarcoma by
microRNA-381, osteosarcoma cells were transfected with Si-LRH-1 and
microRNA-381. As shown in Fig. 13,
the inhibition of LRH-1 significantly increased the function of
microRNA-381 on Bax/Bcl-2 and p53 protein expression of
osteosarcoma (Fig. 13).
Discussion
OS is the most common primary malignant bone tumor,
75% of which occur at the age of 10–30 years (14). Morbidity of OS is relatively low
compared with other malignant tumors; however, it frequently occurs
in adolescent and is associated with the clinical manifestations of
rapid growth and short course of disease, which severely affects
the physical and mental health of adolescent (15). The comprehensive application of
neoadjuvant chemotherapy, limb salvage surgery and pulmonary
metastasis dissection in recent years has greatly improved the
prognosis for OS; however, its major pathogenesis remains unclear
(16). Therefore, in-depth
investigation of the molecular and biological characteristics of OS
is of great significance to the diagnosis and treatment of OS
(17). The roles of microRNAs in
genesis and development of tumors are increasingly prominent,
mainly exerting their functions through specifically regulating
expression of tumor-related genes (6). In our study, we for the first time
determined that microRNA-381 of osteosarcoma patients was
decreased, microRNA-381 levels were more downregulated at stages
III/IV than those at stage I/II in osteosarcoma patients. Zhang
et al showed that microRNA-381 suppresses cell growth and
invasion of liver cancer through LRH-1 (18).
miRNAs are involved in early embryonic development,
cell proliferation, apoptosis, differentiation and metabolism in
animals. Furthermore, they can also regulate the differentiation
and maturity of stem cells (19).
miRNAs play extremely important roles in regulating gene expression
of cells, and their abnormal expression is closely related to the
genesis of numerous tumors. Recent research has discovered that
abnormal expression of miRNAs can be seen in a number of human
tumors (20). It is also found
through hybridization techniques in some research that expression
quantities of miRNAs in tumor tissue are mostly lower than those in
normal tissue. In addition, the expression is associated with
distinct tissue specificity, which can even be used to judge the
histological origin of the undifferentiated tumor (7). It indicates that miRNAs play important
roles in tumor differentiation, proliferation and metastasis, but
their molecular mechanisms remain to be further investigated
(20). Therefore, these results
suggest that microRNA-381 downregulation increased cell
proliferation, decreased LDH activity and apoptosis, and inhibited
caspase-3/9 activities, Bax/Bcl-2 and p53 protein expressions in
osteosarcoma cells in vitro.
Many signaling pathway changes are involved in the
molecular mechanism of tumor metastasis. Wnt/β-catenin signaling
pathway is a pathway in evolution that plays an important role in
tumor metastasis-related signaling pathways (13). Wnt signaling pathway is at present
the hot research pathway regarding signaling pathways. Abnormal
changes of Wnt signaling pathway is found to exist in numerous
tumors such as nasopharyngeal carcinoma, breast cancer, gastric
cancer, liver cancer and prostate cancer (21). Wnt signaling pathway is closely
related to tumor invasion- and metastasis-related events; for
instance, cytoskeleton formation, migration and adhesion of tumor
cells, as well as tumor angiogenesis, plays an important role in
tumor invasion and metastasis, as found in research in recent years
(22). In this study, we found that
downregulation of microRNA-381 upregulated LRH-1/Wnt/β-catenin
signaling pathway.
Wnt competitively binds onto a receptor of
β-catenin, inhibits the degradation of β-catenin in cytoplasm, and
induces increase in β-catenin concentration (23). High concentration of β-catenin can
regulate transcription of relevant target genes and cell cycle
through nuclear translocation, promotes tumor cell survival and
proliferation, thus showing anti-apoptotic effects (23). One of the major characteristics of
the metastatic tumor cells is the activity of apoptotic resistance,
which is also referred to as apoptosis tolerance (24). It facilitates the survival of
metastatic OS cells in the circulatory system; and manifests
insensitivity to chemotherapeutics (24). As a result, regulating Wnt/β-catenin
signaling pathway, and constructing the balance between
pro-apoptotic and anti-apoptotic signals, so as to reduce the
activity of apoptotic resistance in tumor cells, contributes to the
recovery of sensitivity of tumor cells to chemotherapeutics
(25). Our study showed that
overexpression of microRNA-381 downregulated LRH-1/Wnt/β-catenin
signaling pathway. Zhang et al showed that microRNA-381
suppresses cell growth and invasion of liver cancer through
LRH-1/Wnt/β-catenin signaling pathway (18).
Positive expression of LRH1 in immunohistochemical
sections of OS tissue is not related to patient sex, lesion site
and pathological type; instead, it is associated with surgical
stage and distant metastasis (12).
It further reveals that LRH1 can promote genesis, development and
metastasis of tumors (12). OS
patients with high LRH1 protein expression have remarkably shorter
average survival time and average metastasis time compared with
those with low LRH1 protein expression, and the difference is of
statistical significance (26). It
is verified through Cox multifactor survival analysis that LRH1
expression is one of the independent factors influencing prognosis
for OS patients (27).
Overexpression of LRH1 is an important factor affecting the
biological behavior of OS in clinic, which promotes OS progression,
and it is closely associated with the role of LRH1 in promoting
tumor cell proliferation and metastasis (26). Thus, we provided evidence that
downregulation of microRNA-381 upregulated LRH-1/Wnt/β-catenin
signaling pathway. Si-LRH-1 promoted the anticancer effects of
microRNA-381 on osteosarcoma cell growth through Wnt/β-catenin
signaling pathway. Liang et al suggested that downregulation
of microRNA-381 promotes cell proliferation and invasion through
upregulation of LRH-1 in colon cancer (28).
In conclusion, microRNA-381 suppressed the
proliferation and induced apoptosis of osteosarcoma cells through
LRH-1/Wnt/β-catenin signaling pathway. Besides, microRNA-381 may be
an independent diagnostic and prognostic marker for
osteosarcoma.
References
|
1
|
Morris CD, Teot LA, Bernstein ML, Marina
N, Krailo MD, Villaluna D, Janeway KA, DuBois SG, Gorlick RG and
Randall RL: Assessment of extent of surgical resection of primary
high-grade osteosarcoma by treating institutions: A report from the
Children's Oncology Group. J Surg Oncol. 113:351–354. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grignani G, Palmerini E, Dileo P, Asaftei
SD, D'Ambrosio L, Pignochino Y, Mercuri M, Picci P, Fagioli F,
Casali PG, et al: A phase II trial of sorafenib in relapsed and
unresectable high-grade osteosarcoma after failure of standard
multimodal therapy: An Italian Sarcoma Group study. Ann Oncol.
23:508–516. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hugate RR, Wilkins RM, Kelly CM, Madsen W,
Hinshaw I and Camozzi AB: Intraarterial chemotherapy for extremity
osteosarcoma and MFH in adults. Clin Orthop Relat Res.
466:1292–1301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nataraj V, Batra A, Rastogi S, Khan SA,
Sharma MC, Vishnubhatla S and Bakhshi S: Developing a prognostic
model for patients with localized osteosarcoma treated with uniform
chemotherapy protocol without high dose methotrexate: A
single-center experience of 237 patients. J Surg Oncol.
112:662–668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim YH, Goh TS, Lee CS, Oh SO, Kim JI,
Jeung SH and Pak K: Prognostic value of microRNAs in osteosarcoma:
A meta-analysis. Oncotarget. 8:8726–8737. 2017.PubMed/NCBI
|
|
6
|
Li H, Zhang K, Liu LH, Ouyang Y, Guo HB,
Zhang H, Bu J and Xiao T: MicroRNA screening identifies circulating
microRNAs as potential biomarkers for osteosarcoma. Oncol Lett.
10:1662–1668. 2015.PubMed/NCBI
|
|
7
|
Torreggiani E, Roncuzzi L, Perut F, Zini N
and Baldini N: Multimodal transfer of MDR by exosomes in human
osteosarcoma. Int J Oncol. 49:189–196. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi Y, Huang J, Zhou J, Liu Y, Fu X, Li Y,
Yin G and Wen J: MicroRNA-204 inhibits proliferation, migration,
invasion and epithelial-mesenchymal transition in osteosarcoma
cells via targeting Sirtuin 1. Oncol Rep. 34:399–406. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li C, Dong J, Han Z and Zhang K:
MicroRNA-219-5p represses the proliferation, migration, and
invasion of gastric cancer cells by targeting the
LRH-1/Wnt/β-catenin signaling pathway. Oncol Res. 25:617–627. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang W, Tian Y, Jiang S, Liu S, Zhao X
and Tian D: MicroRNA-376c suppresses non-small-cell lung cancer
cell growth and invasion by targeting LRH-1-mediated Wnt signaling
pathway. Biochem Biophys Res Commun. 473:980–986. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bianco S, Brunelle M, Jangal M, Magnani L
and Gévry N: LRH-1 governs vital transcriptional programs in
endocrine-sensitive and -resistant breast cancer cells. Cancer Res.
74:2015–2025. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Wu S, Lv S, Wang H, Wang Y and Guo
Q: Suppression of liver receptor homolog-1 by microRNA-451
represses the proliferation of osteosarcoma cells. Biochem Biophys
Res Commun. 461:450–455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xue YL, Meng XQ, Ma LJ and Yuan Z:
Plumbagin exhibits an anti-proliferative effect in human
osteosarcoma cells by downregulating FHL2 and interfering with
Wnt/β-catenin signalling. Oncol Lett. 12:1095–1100. 2016.PubMed/NCBI
|
|
14
|
London CA, Gardner HL, Mathie T, Stingle
N, Portela R, Pennell ML, Clifford CA, Rosenberg MP, Vail DM,
Williams LE, et al: Impact of toceranib/piroxicam/cyclophosphamide
maintenance therapy on outcome of dogs with appendicular
osteosarcoma following amputation and carboplatin chemotherapy: A
multi-institutional study. PLoS One. 10:e01248892015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pappo AS, Vassal G, Crowley JJ, Bolejack
V, Hogendoorn PC, Chugh R, Ladanyi M, Grippo JF, Dall G, Staddon
AP, et al: A phase 2 trial of R1507, a monoclonal antibody to the
insulin-like growth factor-1 receptor (IGF-1R), in patients with
recurrent or refractory rhabdomyosarcoma, osteosarcoma, synovial
sarcoma, and other soft tissue sarcomas: Results of a Sarcoma
Alliance for Research Through Collaboration study. Cancer.
120:2448–2456. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Song BS, Seo J, Kim DH, Lim JS, Yoo JY and
Lee JA: Gemcitabine and docetaxel for the treatment of children and
adolescents with recurrent or refractory osteosarcoma: Korea Cancer
Center Hospital experience. Pediatr Blood Cancer. 61:1376–1381.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lewis IJ, Nooij MA, Whelan J, Sydes MR,
Grimer R, Hogendoorn PC, Memon MA, Weeden S, Uscinska BM, van
Glabbeke M, et al MRC BO06 and EORTC 80931 collaborators, ;
European Osteosarcoma Intergroup, : Improvement in histologic
response but not survival in osteosarcoma patients treated with
intensified chemotherapy: A randomized phase III trial of the
European Osteosarcoma Intergroup. J Natl Cancer Inst. 99:112–128.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang Q, Zhao S, Pang X and Chi B:
MicroRNA-381 suppresses cell growth and invasion by targeting the
liver receptor homolog-1 in hepatocellular carcinoma. Oncol Rep.
35:1831–1840. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang T, Xu Z, Wang K and Wang N: Network
analysis of microRNAs and genes in human osteosarcoma. Exp Ther
Med. 10:1507–1514. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ram Kumar RM, Boro A and Fuchs B:
Involvement and clinical aspects of microRNA in osteosarcoma. Int J
Mol Sci. 17:8772016. View Article : Google Scholar :
|
|
21
|
Li Z, Zhao L and Wang Q: Overexpression of
long non-coding RNA HOTTIP increases chemoresistance of
osteosarcoma cell by activating the Wnt/β-catenin pathway. Am J
Transl Res. 8:2385–2393. 2016.PubMed/NCBI
|
|
22
|
Zhou L, An N, Jiang W, Haydon R, Cheng H,
Zhou Q, Breyer B, Feng T and He TC: Fluorescence-based functional
assay for Wnt/beta-catenin signaling activity. Biotechniques.
33:1126–1128, 1130, 1132 passim. 2002.PubMed/NCBI
|
|
23
|
Tian J, He H and Lei G: Wnt/β-catenin
pathway in bone cancers. Tumour Biol. 35:9439–9445. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Reimann E, Kõks S, Ho XD, Maasalu K and
Märtson A: Whole exome sequencing of a single osteosarcoma case -
integrative analysis with whole transcriptome RNA-seq data. Hum
Genomics. 8:202014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martins-Neves SR, Corver WE,
Paiva-Oliveira DI, Van den Akker BE, Briaire-de-Bruijn IH, Bovée
JV, Gomes CM and Cleton-Jansen AM: Osteosarcoma stem cells have
active Wnt/β-catenin and overexpress SOX2 and KLF4. J Cell Physiol.
231:876–886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lonati C, Sordi A, Giuliani D, Spaccapelo
L, Leonardi P, Carlin A, Ottani A, Galantucci M, Grieco P, Catania
A, et al: Molecular changes induced in rat liver by hemorrhage and
effects of melanocortin treatment. Anesthesiology. 116:692–700.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kovacevic A, Hammer A, Stadelmeyer E,
Windischhofer W, Sundl M, Ray A, Schweighofer N, Friedl G,
Windhager R, Sattler W, et al: Expression of serum amyloid A
transcripts in human bone tissues, differentiated osteoblast-like
stem cells and human osteosarcoma cell lines. J Cell Biochem.
103:994–1004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang Y, Zhao Q, Fan L, Zhang Z, Tan B,
Liu Y and Li Y: Down-regulation of microRNA-381 promotes cell
proliferation and invasion in colon cancer through up-regulation of
LRH-1. Biomed Pharmacother. 75:137–141. 2015. View Article : Google Scholar : PubMed/NCBI
|