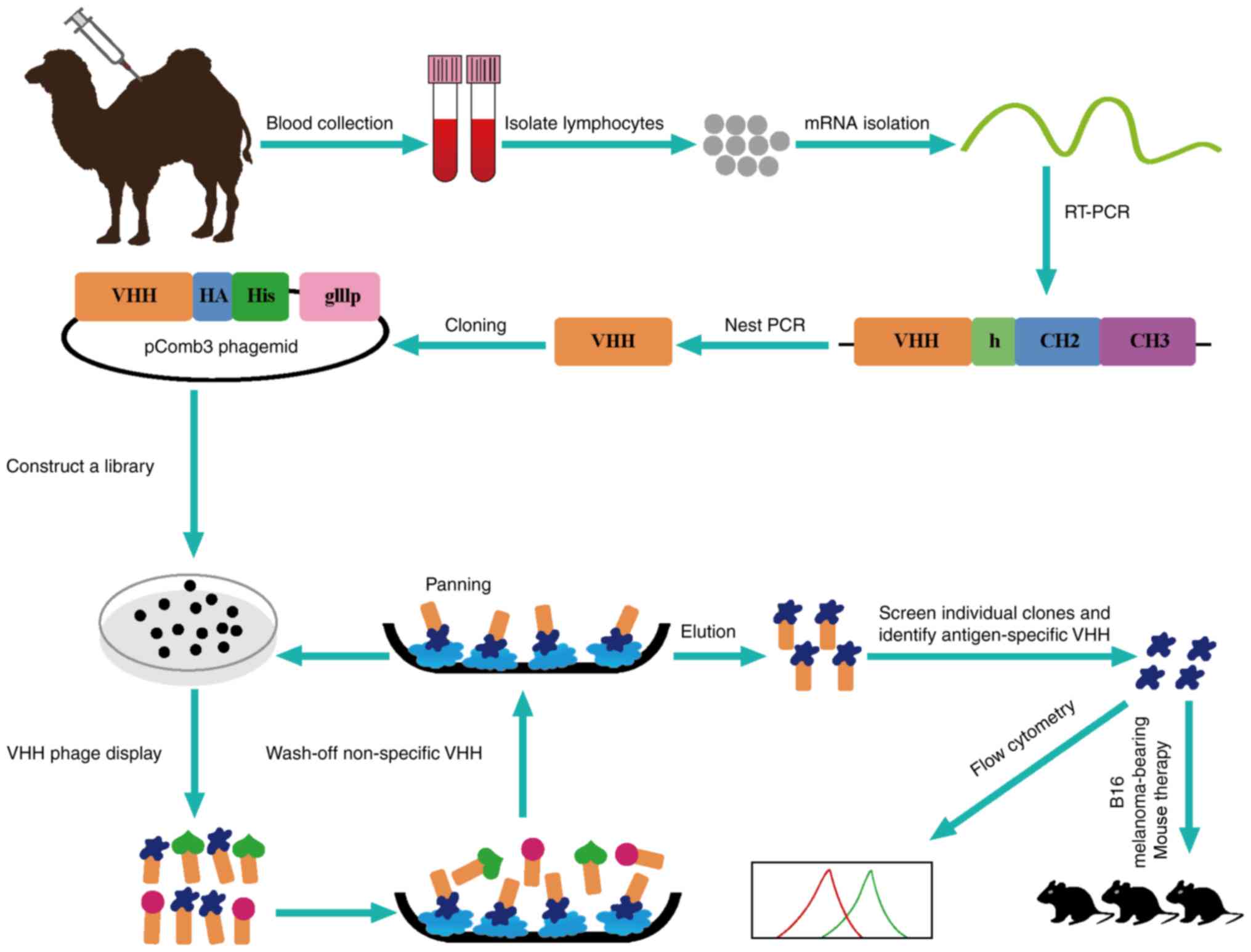
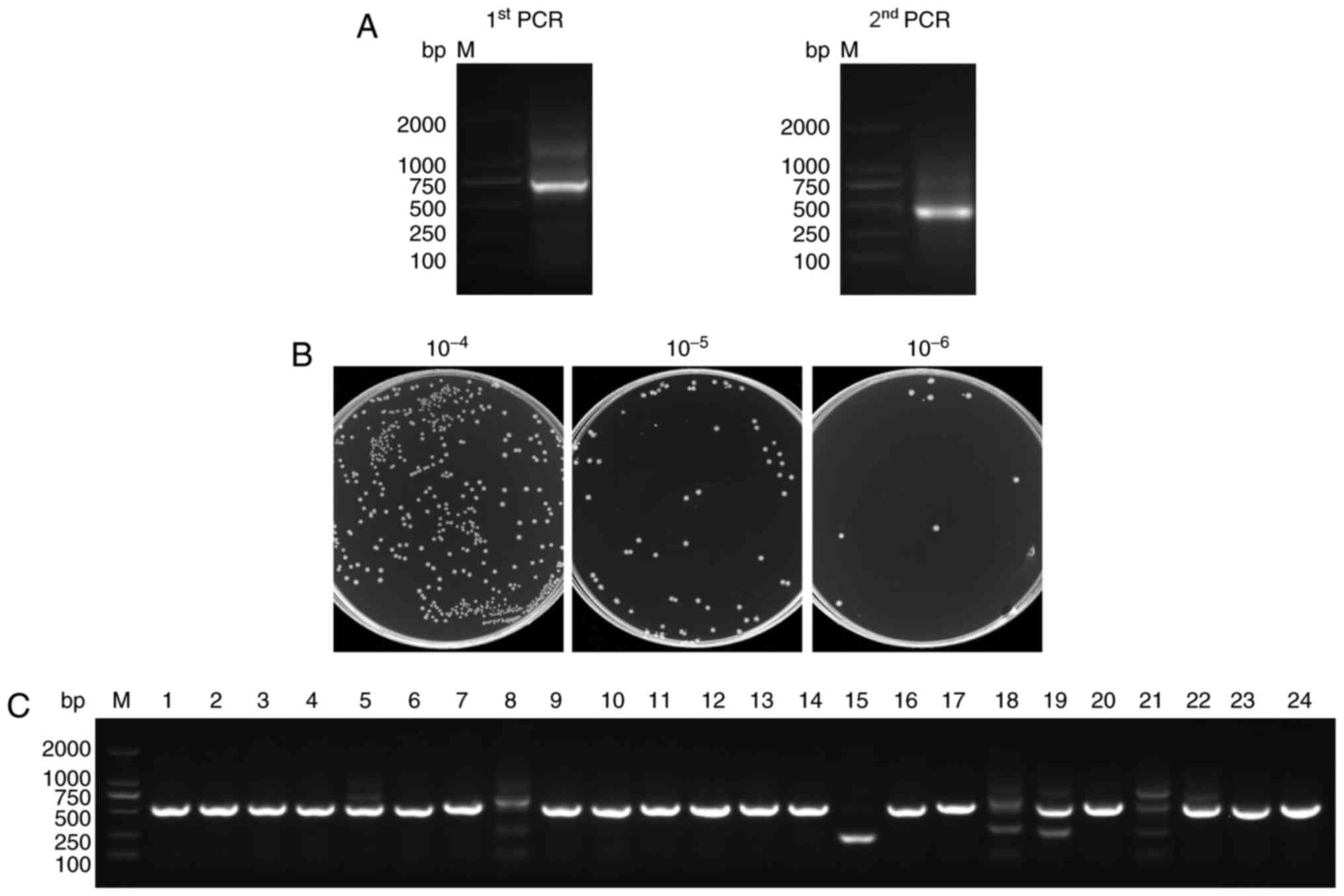
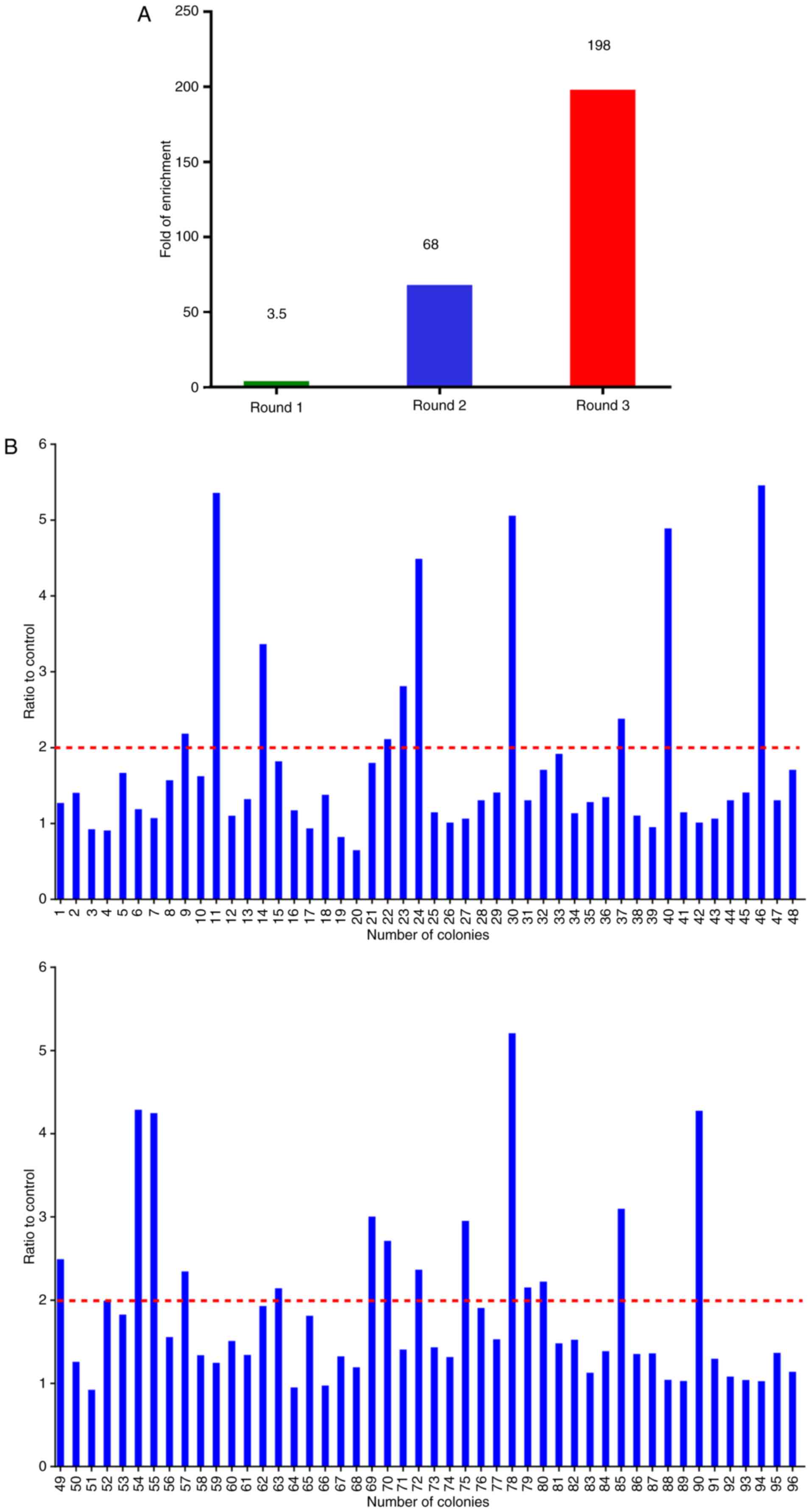
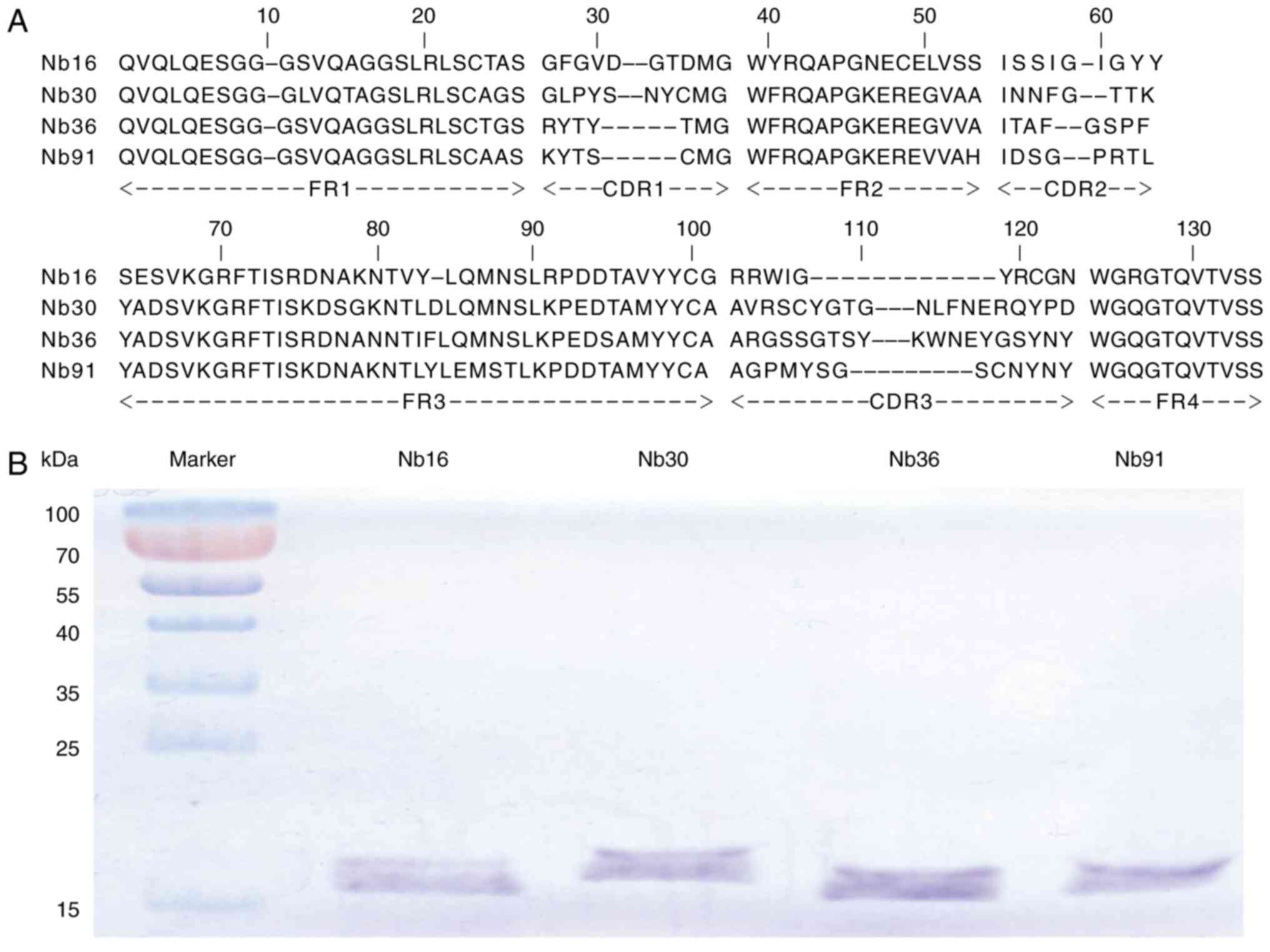
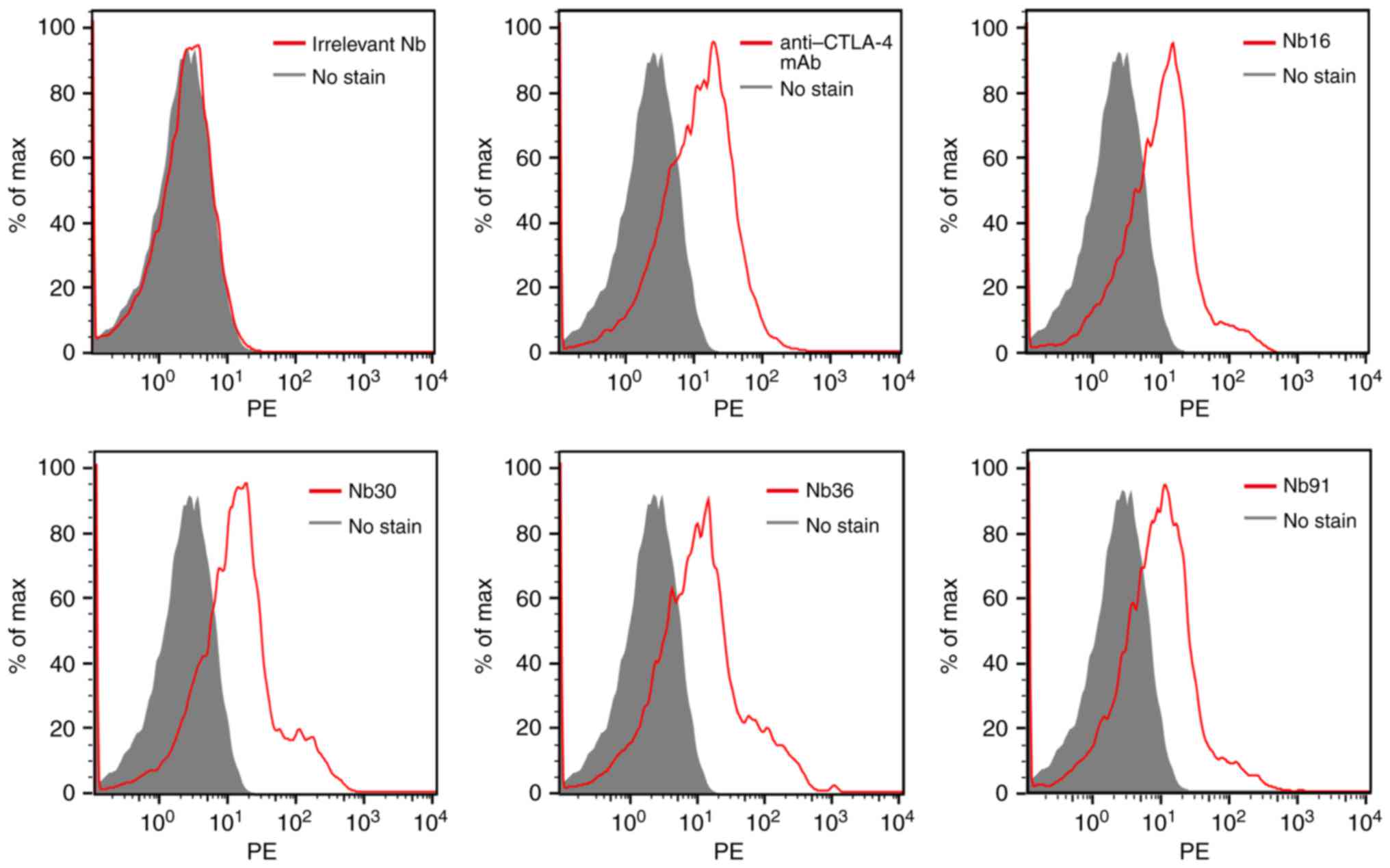
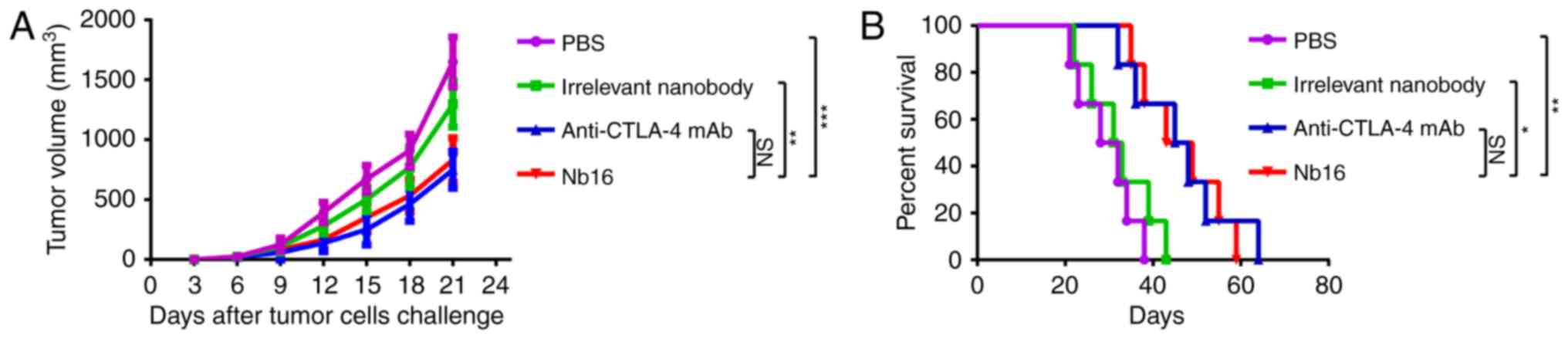
|
1
|
Peggs KS, Quezada SA, Chambers CA, Korman
AJ and Allison JP: Blockade of CTLA-4 on both effector and
regulatory T cell compartments contributes to the antitumor
activity of anti-CTLA-4 antibodies. J Exp Med. 206:1717–1725. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zamarin D, Holmgaard RB, Subudhi SK, Park
JS, Mansour M, Palese P, Merghoub T, Wolchok JD and Allison JP:
Localized oncolytic virotherapy overcomes systemic tumor resistance
to immune checkpoint blockade immunotherapy. Sci Transl Med.
6:226ra322014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Butte MJ, Keir ME, Phamduy TB, Sharpe AH
and Freeman GJ: Programmed death-1 ligand 1 interacts specifically
with the B7-1 costimulatory molecule to inhibit T cell responses.
Immunity. 27:111–122. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Motoshima T, Komohara Y, Horlad H,
Takeuchi A, Maeda Y, Tanoue K, Kawano Y, Harada M, Takeya M and Eto
M: Sorafenib enhances the antitumor effects of anti-CTLA-4 antibody
in a murine cancer model by inhibiting myeloid-derived suppressor
cells. Oncol Rep. 33:2947–2953. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hurwitz AA, Foster BA, Kwon ED, Truong T,
Choi EM, Greenberg NM, Brug MB and Allison JP: Combination
immunotherapy of primary prostate cancer in a transgenic mouse
model using CTLA-4 blockade. Cancer Res. 60:2444–2448.
2000.PubMed/NCBI
|
|
6
|
Van Elsas A, Hurwitz AA and Allison JP:
Combination immunotherapy of B16 melanoma using anti-cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage
colony-stimulating factor (GM-CSF)-producing vaccines induces
rejection of subcutaneous and metastatic tumors accompanied by
autoimmune depigmentation. J Exp Med. 190:355–366. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leach DR, Krummel MF and Allison JP:
Enhancement of antitumor immunity by CTLA-4 blockade. Science.
271:1734–1736. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ribas A, Camacho LH, Lopez-Berestein G,
Pavlov D, Bulanhagui CA, Millham R, Comin-Anduix B, Reuben JM, Seja
E, Parker CA, et al: Antitumor activity in melanoma and anti-self
responses in a phase I trial with the anti-cytotoxic T
lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J
Clin Oncol. 23:8968–8977. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fernández LÁ and Muyldermans S: Recent
developments in engineering and delivery of protein and antibody
therapeutics. Curr Opin Biotech. 22:839–842. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saerens D, Frederix F, Reekmans G, Conrath
K, Jans K, Brys L, Huang L, Bosmans E, Maes G, Borghs G and
Muyldermans S: Engineering camel single-domain antibodies and
immobilization chemistry for human prostate-specific antigen
sensing. Anal Chem. 77:7547–7555. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hassanzadeh-Ghassabeh G, Devoogdt N, De
Pauw P, Vincke C and Muyldermans S: Nanobodies and their potential
applications. Nanomedicine. 8:1013–1026. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Market E and Papavasiliou FN: V(D)J
recombination and the evolution of the adaptive immune system. PLoS
Biol. 1:E162003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holt LJ, Herring C, Jespers LS, Woolven BP
and Tomlinson IM: Domain antibodies: proteins for therapy. Trends
Biotechnol. 21:484–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Muyldermans S: Nanobodies: Natural
single-domain antibodies. Annu Rev Biochem. 82:775–797. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gueorguieva D, Li S, Walsh N, Mukerji A,
Tanha J and Pandey S: Identification of single-domain, Bax-specific
intra-bodies that confer resistance to mammalian cells against
oxidative-stress-induced apoptosis. FASEB J. 20:2636–2638. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pérez JM, Renisio JG, Prompers JJ, van
Platerink CJ, Cambillau C, Darbon H and Frenken LG: Thermal
unfolding of a llama antibody fragment: A two-state reversible
process. Biochemistry. 40:74–83. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muyldermans S: Single domain camel
antibodies: Current status. J Biotechnol. 74:277–302.
2001.PubMed/NCBI
|
|
19
|
De Genst E, Silence K, Decanniere K,
Conrath K, Loris R, Kinne J, Muyldermans S and Wyns L: Molecular
basis for the preferential cleft recognition by dromedary
heavy-chain antibodies. Proc Natl Acad Sci USA. 103:pp. 4586–4591.
2006; View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kijanka M, Warnders FJ, El Khattabi M,
Lub-de Hooge M, van Dam GM, Ntziachristos V, de Vries L, Oliveira S
and van Bergen En Henegouwen PM: Rapid optical imaging of human
breast tumour xenografts using anti-HER2 VHHs site-directly
conjugated to IRDye 800CW for image-guided surgery. Eur J Nucl Med
Mol Imaging. 40:1718–1729. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vosjan MJ, Vercammen J, Kolkman JA,
Stigter-van Walsum M, Revets H and van Dongen GA: Nanobodies
targeting the hepatocyte growth factor: Potential new drugs for
molecular cancer therapy. Mol Cancer Ther. 11:1017–1025. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
De Meyer T, Eeckhout D, De Rycke R, De
Buck S, Muyldermans S and Depicker A: Generation of VHH antibodies
against the Arabidopsis thaliana seed storage proteins. Plant Mol
Biol. 84:83–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Conrath KE, Lauwereys M, Galleni M,
Matagne A, Frère JM, Kinne J, Wyns L and Muyldermans S:
Beta-lactamase inhibitors derived from single-domain antibody
fragments elicited in the camelidae. Antimicrob Agents Chemother.
45:2807–2812. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vincke C, Gutiérrez C, Wernery U, Devoogdt
N, Hassanzadeh-Ghassabeh G and Muyldermans S: Generation of single
domain antibody fragments derived from camelids and generation of
manifold constructs. Methods Mol Biol. 907:145–176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu M, Gong X, Hu Y, Ou W and Wan Y:
Streptavidin-biotin-based directional double Nanobody sandwich
ELISA for clinical rapid and sensitive detection of influenza H5N1.
J Transl Med. 12:3522014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nevoltris D, Lombard B, Dupuis E, Mathis
G, Chames P and Baty D: Conformational nanobodies reveal tethered
epidermal growth factor receptor involved in EGFR/ErbB2 predimers.
ACS Nano. 9:1388–1399. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hernot S, Unnikrishnan S, Du Z, Shevchenko
T, Cosyns B, Broisat A, Toczek J, Caveliers V, Muyldermans S,
Lahoutte T, et al: Nanobody-coupled microbubbles as novel molecular
tracer. J Control Release. 158:346–353. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Muyldermans S, Baral T, Retamozzo VC, De
Baetselier P, De Genst E, Kinne J, Leonhardt H, Magez S, Nguyen V,
Revets H, et al: Camelid immunoglobulins and nanobody technology.
Vet Immunol Immunopathol. 128:178–183. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Curran MA, Montalvo W, Yagita H and
Allison JP: PD-1 and CTLA-4 combination blockade expands
infiltrating T cells and reduces regulatory T and myeloid cells
within B16 melanoma tumors. Proc Natl Acad Sci USA. 107:pp.
4275–4280. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dumoulin M, Conrath K, Van Meirhaeghe A,
Meersman F, Heremans K, Frenken LG, Muyldermans S, Wyns L and
Matagne A: Single-domain antibody fragments with high
conformational stability. Protein Sci. 11:500–515. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vu KB, Ghahroudi MA, Wyns L and
Muyldermans S: Comparison of llama VH sequences from conventional
and heavy chain antibodies. Mol Immunol. 34:1121–1131. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vaneycken I, Govaert J, Vincke C,
Caveliers V, Lahoutte T, De Baetselier P, Raes G, Bossuyt A,
Muyldermans S and Devoogdt N: In vitro analysis and in vivo tumor
targeting of a humanized, grafted nanobody in mice using pinhole
SPECT/micro-CT. J Nucl Med. 51:1099–1106. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vincke C, Loris R, Saerens D,
Martinez-Rodriguez S, Muyldermans S and Conrath K: General strategy
to humanize a camelid single-domain antibody and identification of
a universal humanized nanobody scaffold. J Biol Chem.
284:3273–3284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao Y, Gao W, Chen X, Cha N, Wang X, Jia
X, Wang B, Ren M and Ren J: Enhancing the treatment effect on
melanoma by heat shock protein 70-peptide complexes purified from
human melanoma cell lines. Oncol Rep. 36:1243–1250. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vosjan MJ, Vercammen J, Kolkman JA,
Stigter-van Walsum M, Revets H and van Dongen GA: Nanobodies
targeting the hepatocyte growth factor: Potential new drugs for
molecular cancer therapy. Mol Cancer Ther. 11:1017–1025. 2012.
View Article : Google Scholar : PubMed/NCBI
|