Introduction
Oral cancer is the 9th most common cancer among
males and the 14th among females in the US according to a recent
study (1). Approximately 90% of
oral cancer cases are oral squamous cell carcinoma (OSCC) (2). As early stage OSCC is asymptomatic,
patients with OSCC are often diagnosed at the middle and advanced
stages resulting in a poor prognosis. Although multidisciplinary
treatment strategy has been made in OSCC, the 5-year overall
survival of OSCC is still less than 50%. Little improvement has
been made in the last 35 years (3).
Thus, it is an urgent need to achieve a better understanding of the
mechanisms of OSCC and discover effective therapeutic targets.
Ten hallmarks of cancer have been published in cells
(4). One of these is angiogenesis.
The newly-formed endothelial vessels of which are thought to arise
by sprouting of pre-existing capillaries, supply nutrients and
oxygen for tumor growth and metastasis (4). Therefore, an astonishing outpouring of
anti-angiogenic inhibitors has been developed as a promising method
to ‘starve’ tumors in the past decade. However, anti-angiogenesis
targeted to endothelium have shown limited efficacy. This indicates
that there may be other supplementary blood supply patterns to
nourish tumors. In 1999, Maniotis and his colleagues found a new
manner of blood supply named vasculogenic mimicry (VM), which is
independent of traditional angiogenesis (5). VM is challengeable and complementary
to traditional angiogenesis. The structure of VM lacks endothelial
lining, instead of aggressive cancer cells forming vessel-like
structure in patients' aggressive tumors (5). The features of positive periodic
acid-schiff (PAS) and negative CD34 are regarded as the golden
standard for tumor cell-lined VM. Myriad of studies have
contributed to the understanding of VM since its introduction.
Collectively, VM is closely associated with metastasis and poor
prognosis in many cancers including melanoma, lung cancer,
hepatocellular carcinoma and also oral cancer (6). However, certain studies showed that
traditional angiogenesis inhibitors could not inhibit the formation
of VM, and even caused extracellular matrix-rich tubular network
formation in vitro (7).
Therefore, therapeutic strategies that target VM hold a great
promise in the treatment of cancer; but up to this point, there is
no effective inhibitor reported to VM.
Niclosamide is a FDA-proven oral anti-helminthic
drug used worldwide against human tapeworms for approximately 50
years (8). Recently, more
beneficial effects of niclosamide have been found in several
diseases that are irrelevant to parasites. These diseases include
cancer, type II diabetes, bacterial and viral infection,
neuropathic pain, rheumatoid arthritis, and bone loss diseases
(9). Detailed mechanism studies
show that niclosamide exerts anticancer effect in many types of
cancers including acute myelogenous leukemia, colon cancer,
prostate cancer, lung cancer, breast cancer and ovarian cancer
through inhibition of WNT, Notch, mTOR and STAT3 pathways (10). However, the precise mechanism
underlying this anticancer activity has yet to be elucidated.
In addition, a large fraction of the genome
sequences are active but only 2% of it encodes a protein according
to the analysis of human genome sequence, thus the majority of
transcripts are known as non-coding RNAs (ncRNAs) (11,12).
Currently, small non-coding RNAs such as microRNAs (miRNAs) have
been studied extensively and their potential to regulate the gene
expression and cell function have emerged as a tool for diagnosis
of numerous cancers (12,13). Previous studies show that
microRNA-124 (miR-124) acts as a tumor suppressor in head and neck
squamous cell carcinoma (14) and
overexpression of miR-124 correlates with better breast cancer
prognosis (15). Moreover, miR-124
has been reported to be associated with VM in cervical cancer cells
(16). However, whether miR-124 is
involved in the niclosamide's anticancer effect in oral cancer
remains unclear.
In this study, we found that niclosamide inhibited
proliferation and promoted apoptosis of oral cancer cells by using
two OSCC cell lines WSU-HN6 and Tca83. Furthermore, niclosamide not
only inhibited VM formation of OSCC cancer cells in vitro,
but also decreased the tumor size and the number of VM in
vivo. Molecular assays demonstrated that, niclosamide markedly
downregulated VM-associated genes VEGFA, MMP2, ROCK1 and Cdc42,
whereas, it upregulated miR-124 and downregulated p-STAT3
significantly. Intriguingly, like niclosamide, stably expressing
miR-124 OSCC cancer cell line inhibited the p-STAT3 expression.
Moreover, the stable cell line HN6-miR-124 could decrease the
ability of mobility, invasiveness and VM formation. Together, our
study suggests that niclosamide shows potential to be a new
inhibitor of VM in oral cancer through upregulation of miR-124 and
downregulation of STAT3.
Materials and methods
Cell culture
WSU-HN6 cells were cultured in Dulbecco's modified
Eagle's medium (DMEM, Gibco, Carlsbad, CA, USA) and Tca83 cells in
Roswell Park Memorial Institute (RPMI)-1640 medium (Gibco),
supplemented with 10% fetal bovine serum (FBS; Hyclone). They were
maintained at 37°C in a humidified incubator with 5%
CO2.
Cell proliferation assay
The cell proliferation was determined in
vitro using Cell Counting Kit-8 (CCK-8) assay. Briefly, cells
were seeded at a density of 1×104 cells per well with
100 µl growth medium in 96-well plates. Following overnight
incubation, cells were cultured in complete culture medium
containing 5 µM niclosamide [Sigma-Aldrich, St. Louis, MO, USA,
dissolved in dimethyl sulfoxide (DMSO)]. After 24 h of treatment,
CCK-8 reagent (10 µl) was added to each well for color development.
Cell viability was determined photometrically at 450 nm using an
ELx808 absorbance microplate reader (BioTek Instruments, Winooski,
VT, USA).
Apoptotic assay
Apoptosis caused by niclosamide was assessed by Cell
Death Detection Kit II (Roche Diagnostics, Indianapolis, IN, USA).
Briefly, WSU-HN6 and Tca83 cells were treated with DMSO and 5 µM
niclosamide in 100-mm dishes for 24 h. The cells were harvested and
washed with ice-cold PBS twice. Apoptosis was detected by flow
cytometry after the cells were stained with FITC-conjugated Annexin
V and propidium iodide (PI) according to manufacturer's
instructions.
Generation of stably expressing
miR-124 oral cancer cell line
WSU-HN6 cells were transfected with
pCDNA3.2/V5-hsa-mir-124 plasmids carrying miR-124 overexpression
cassette. pcDNA3.2/V5 hsa-mir-124 was a gift from David Bartel
[Addgene plasmid #26306 (17)].
Cells were screened by G418 for 2 weeks. The cells transfected with
empty vector served as control. The single colonies were picked up
for detection of miR-124 expression.
Capillary-like tube formation
assay
Pre-chilled 96-well plates were coated with 50 µl
Matrigel (BD Biosciences, Franklin Lakes, NJ, USA) for 1 h at 37°C.
Tumor cells (2×104 per well) were seeded in Matrigel
coated plates. Then culture plates were exposed to 5 µM niclosamide
for 8 h. Tubular structures were photographed under a microscope
with recording digital camera (DP72; Olympus, Tokyo, Japan), and
five representative fields per well were used to evaluate the
ability of tube formation.
Real-time PCR
Total RNA was isolated using the TRIzol reagent
(Invitrogen, Carlsbad, CA, USA). RNA (2 µg) was reversely
transcribed into cDNA with a moloney murine leukaemia virus reverse
transcriptase (M-MLV RTase; Promega, Madison, WI, USA). VEGFA,
MMP2, Cdc42 and ROCK1 were detected with the following respective
primers: VEGFA (5′-GCAGAATCATCACGAAGTGG-3′ and
5′-GCAACGCGAGTCTGTGTTTTTG-3′); MMP2 (5′-GCCCCAGACAGGTGATCTTG-3′ and
5′-GCTTGCGAGGGAAGAAGTTGT-3′); Cdc42 (5′-CCATCGGAATATGTACCGACTG-3′
and 5′-CTCAGCGGTCGTAATCTGTCA-3′); ROCK1
(5′-AACATGCTGCTGGATAAATCTGG-3′ and 5′-TGTATCACATCGTACCATGCCT-3′);
GAPDH (5′-ATGGGGAAGGTGAAGGTCG-3′ and 5′-GGGGTCATTGATGGCAACAATA-3′),
respectively. miRNA quantifiation was performed using miDETECT A
Track™ miRNA qRT-PCR kit (RiboBio, Guangzhou, China) following the
manufacturer's instruction. Real-time PCR was performed using the
SYBR Green master mix (Roche Diagnostics) on an ABI 7500 instrument
(Applied Biosystems, Foster, CA, USA). miR-124 and U6 primers were
from Ribobio. GAPDH and U6 served as mRNA and miRNA endogenous
controls, respectively. The fold-change was determined as
2−∆∆Ct. All real-time PCR reactions were performed in
triplicate and repeated three times.
Western blot assay
Cells were harvested and lysed in RIPA buffer
(Applygen Technologies, Beijing, China) with protease inhibitors
(Roche Diagnostics). Protein (30 µg) was loaded. Antibodies against
phosphorylated (p)-STAT3 (1:1,000), total (t)-STAT3 (1:1,000),
VEGFA (1:500), MMP2 (1:500), p-Cdc42 (1:1,000), and GAPDH (1:1,000)
were incubated, and then membranes were placed overnight at 4°C.
Then, relevant secondary antibodies were incubated on the
membranes. Immunoreactive bands were visualized with a
chemiluminescence detection system (Applygen Technologies).
Wound healing assay
Cells (5×105) were cultured as confluent
monolayers, and then serum-starved for 30 h followed by wound
scratching across the well with a 200-µl pipette tip. The detached
cells were removed by D-Hanks. Then the cells were treated with 5
µM niclosamide for indicated times. The monolayer was photographed
at a magnification of ×10 using an inverted microscope (Nikon
Corp., Tokyo, Japan) and the speed of the cell movement was
calculated.
Transwell invasion assay
A Transwell chamber plate (8 µm pore size,
Millipore, Bedford, MA, USA) with a polycarbonate membrane coated
with 100 µl of diluted Matrigel (BD Biosciences, Minneapolis, MN,
USA) was used for the cell invasion assays. Briefly,
1×105 of 24-h serum-starved cells in 100 µl serum-free
medium were seeded in the matrigel coated upper chamber of the
Transwell, while 500 µl of culture medium supplemented with 20% FBS
was added to the bottom chamber. Niclosamide (5 µM) was added in
each upper chamber. The cells were allowed to migrate for 20 h, and
then fixed with 95% ethanol, stained with 1% crystal violet
(Sigma-Aldrich). After wiping off the non-migrated cells in the
upper chamber of the Transwell, migrated cells were counted and
photographed using a light microscopy at a magnification of ×20
(Olympus). Ten high-power fields of each treatment were used to
calculate the relative migration speed, and the experiment was
repeated three times.
Oral cancer xenografts and
treatment
Medical ethics committee of the Peking University
Health Center approved this study. WSU-HN6 cells
(3×106/mouse) were s.c. injected into 6-week-old female
BALB/c nude mice, 5 mice in each group. Mice were treated with
vehicle control (Kolliphor® EL) and niclosamide (20
mg/kg/day i.p.). Tumor volume was calculated with the formula: V=
(L × W2)/2 (L, length; W, width). Mice were sacrificed
and tumors were harvested on the 15th day of treatment.
Periodic acid-Schiff (PAS)-CD34 dual
staining
Immunostaining was applied to perform CD34 staining.
PAS staining was performed using PAS staining kit (Solarbio Co.,
Ltd., Beijing, China). Briefly, after DAB reaction for CD34
immunostaining, the sections were treated with 0.5% periodic acid
solution for 10 min, rinsed with distilled water for 5 min,
followed by staining in periodic acid-Schiff solution for 15–30
min. After rinsing with distilled water, sections were
counterstained with hematoxylin, dehydrated, cleared and
mounted.
Statistical analysis
Statistical analysis was performed with GraphPad
Prism v5.0 software. All data are presented means ± standard
deviation of the mean (SD), P<0.05 was considered to indicate a
statistically significant difference.
Results
Niclosamide inhibits cell
proliferation of oral cancer cells
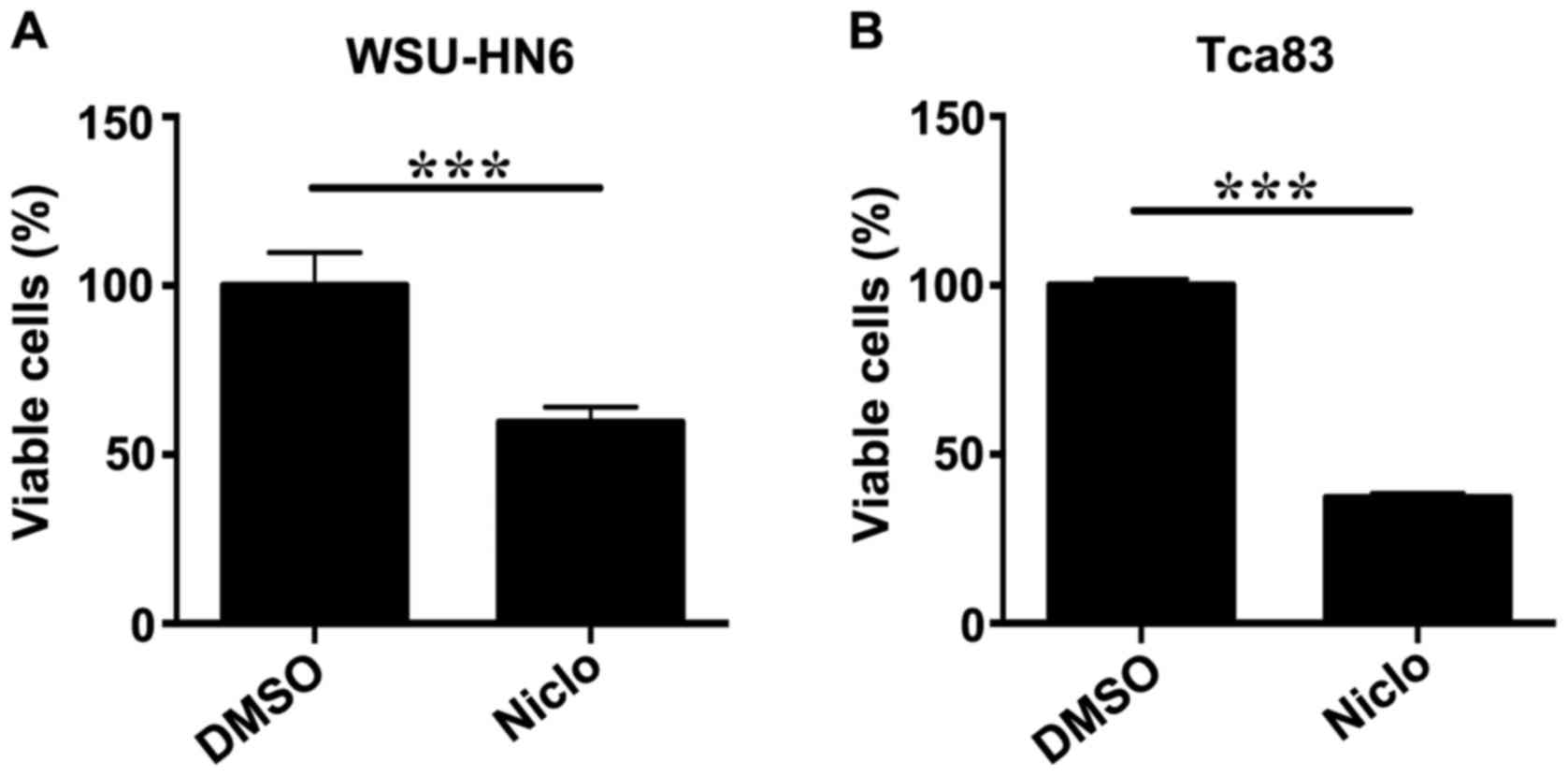
To determine whether niclosamide inhibits the growth
of WSU-HN6 and Tca83 cells, cell viability was determined by CCK-8
assay, respectively. After the cells were treated with vehicle DMSO
or 5 µM niclosamide for 24 h, the results showed that niclosamide
significantly inhibited cell proliferation of WSU-HN6 (Fig. 1A) and Tca83 (Fig. 1B) in both oral cancer cell
lines.
Niclosamide induces apoptosis of oral
cancer cells
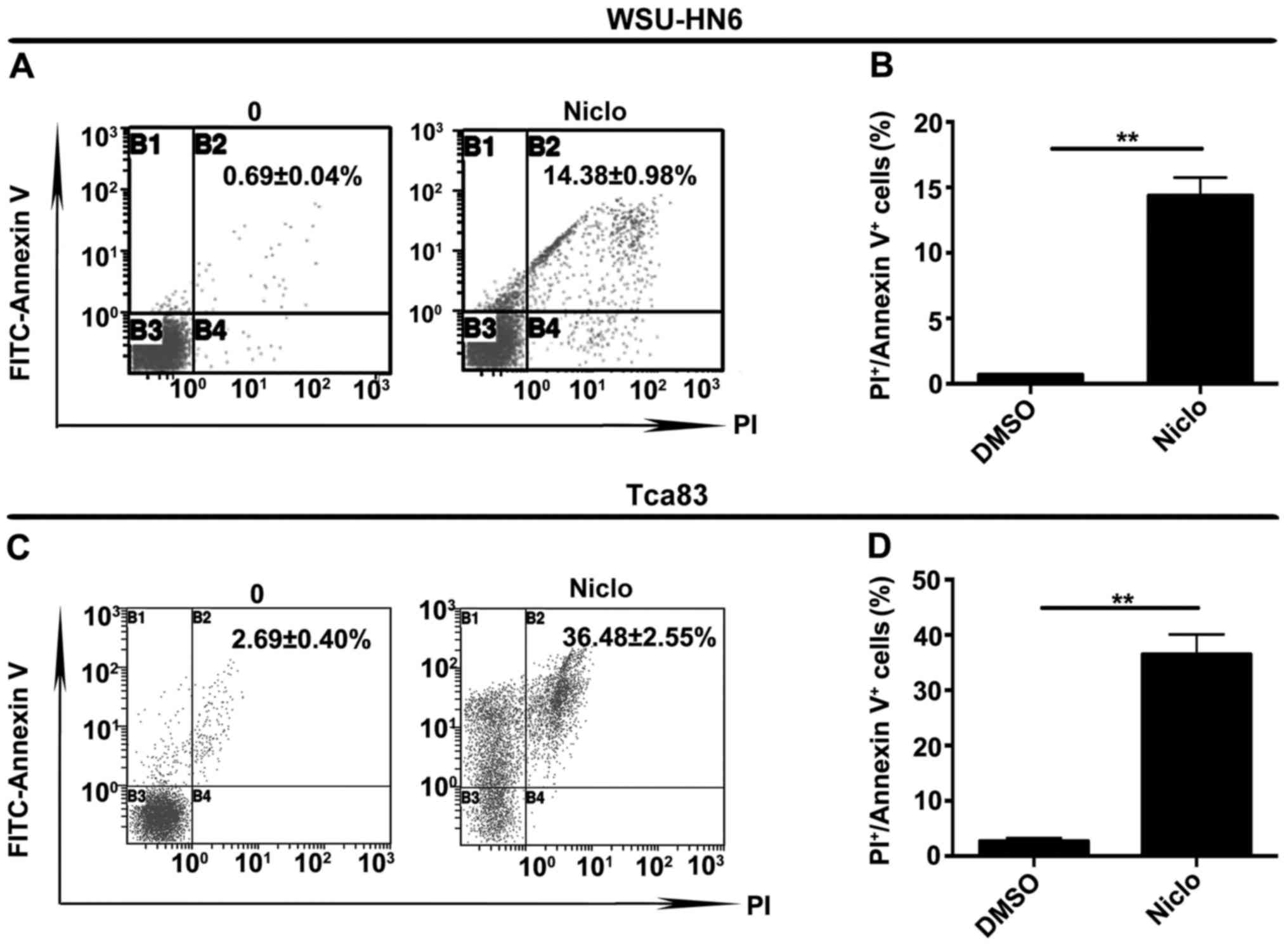
To investigate whether niclosamide inhibits oral
cancer cell growth through induction of apoptosis, we performed
apoptosis assay as shown in Fig. 2.
Cells were treated with vehicle dimethyl sulfoxide (DMSO) or 5 µM
niclosamide for 24 h, respectively. The apoptosis rates of the
treated cells were 0.69±0.04% and 14.38±0.98% in WSU-HN6
(P<0.05, Fig. 2A and B), and
2.69±0.40%, 36.48±2.55% in Tca83 (P<0.05, Fig. 2C and D).
Niclosamide decreases capillary-like
tube formation in vitro, and inhibits tumor growth and VM in
vivo
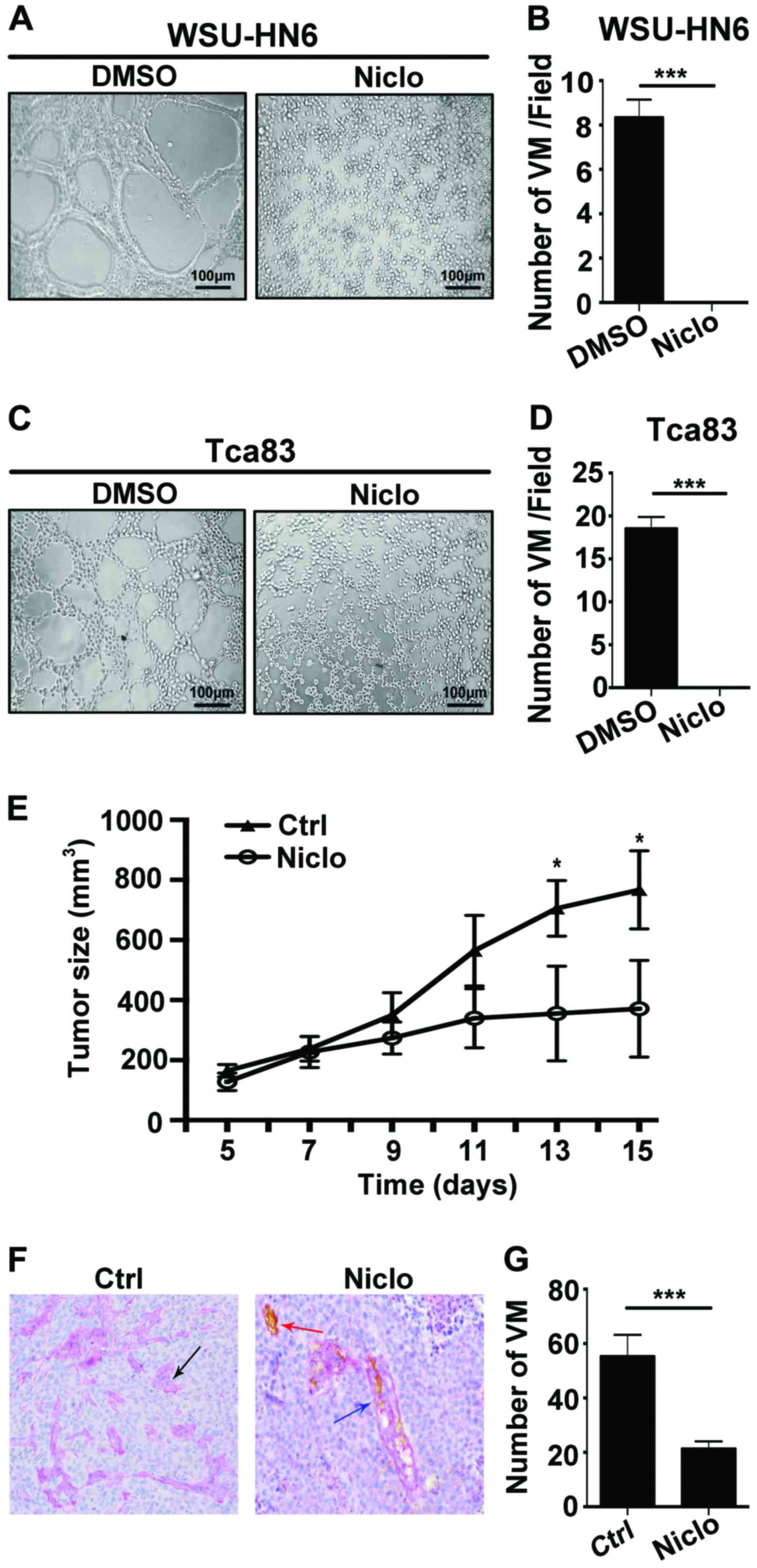
To investigate whether niclosamide plays an
anticancer role through VM, we carried out capillary-like tube
formation assay in vitro and VM detection by PAS-CD34 dual
staining in niclosamide treated WSU-HN6 xenografts in vivo.
The results showed that 5 µM niclosamide completely inhibited the
capacity of tube formation in the OSCC cell lines WSU-HN6 (Fig. 3A and B) and Tca83 (Fig. 3C and D) in vitro. Next, we
generated oral cancer xenograft mice by subcutaneously implanting
WSU-HN6 cells into nude mice. We found that compared with control
group, 20 mg/kg/day niclosamide significantly decreased the tumor
size (Fig. 3E) and the number of VM
in vivo (Fig. 3F and G). We
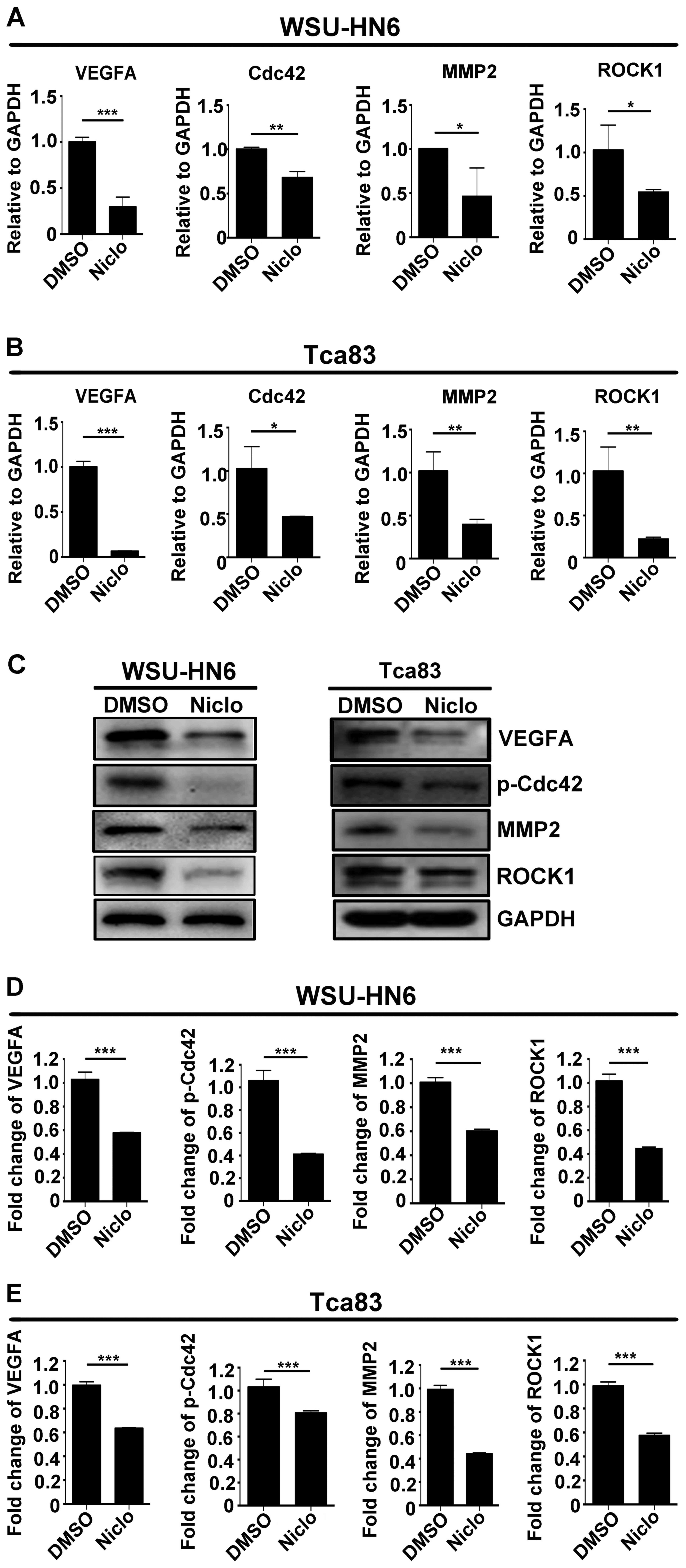
performed real-time PCR and western blotting to detect VM-related
gene expression including VEGFA, MMP2, ROCK1 and Cdc42 in 5 µM
niclosamide-treated WSU-HN6 and Tca83 cell lines. The real-time PCR
result showed that niclosamide markedly inhibited VEGFA, MMP2,
ROCK1 and Cdc42 expression at mRNA level in WSU-HN6 (Fig. 4A) and Tca83 (Fig. 4B) cell lines. The western blot assay
showed that niclosamide decreased the expression of VEGFA, MMP2,
ROCK1 and p-Cdc42 at protein level in the two oral cancer cell
lines (Fig. 4C-E).
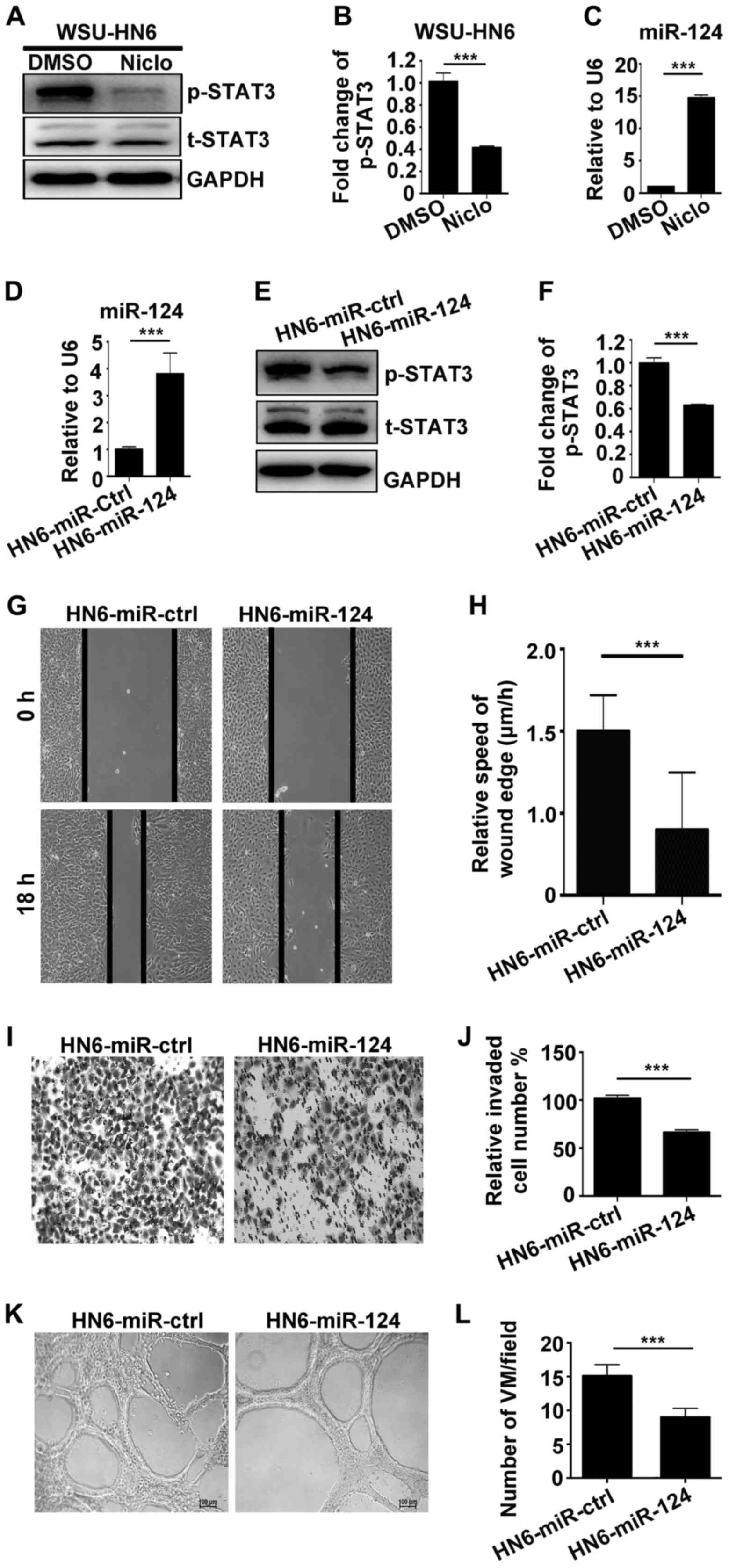
miR-124 is involved in the anticancer
effects of niclosamide through downregulation of p-STAT3
expression
To explore whether p-STAT3 and non-coding RNA is
involved in the anticancer effects of niclosamide, we performed
western blotting and real-time PCR assays. The results showed that
p-STAT3 was significantly downregulated at protein level (Fig. 5A and B); miR-124 was upregulated at
mRNA level (Fig. 5C) by 5 µM
niclosamide in WSU-HN6 cells. To further confirm the results, we
generated stably overexpressing miR-124 cell line in WSU-HN6. The
stable cell line HN6-miR-124 and empty vector control cell line
were used to detect the expression of miR-124. Real-time PCR
clearly showed that miR-124 was highly expressed in HN6-miR-124
(Fig. 5D), which suggested that
HN6-miR-124 cell line was successfully established. In addition,
p-STAT3 was downregulated in HN6-miR-124 compared with HN6-miR-ctrl
cells (Fig. 5E and F). Furthermore,
to investigate whether HN6-miR-124 affects the mobility,
invasiveness and VM formation of oral cancer cells, we carried out
wound healing, Transwell and capillary-like tube formation assays.
The results show that HN6-miR-124 could significant reduce the
migration (Fig. 5G and H), invasion
(Fig. 5I and J) and the number of
VM formation (Fig. 5K and L)
compared with the HN6-miR-ctrl group.
Discussion
Bringing a new drug to market would take an average
of 15 years and US $800 million according to an analysis (18). In 2007, an article, entitled ‘New
uses for old drugs’ by Chong and Sullivan (19) was published on 17 existing drugs
that are in various stages of clinical and animal testing for new
uses. Because of the known pharmacokinetics and safety profiles, it
can bypass almost 40% of the overall cost of bringing a drug to
market. Finding new uses for existing drugs is a proven low
manufacturing costs and high stability, it is very attractive to be
researched. In this study, we found a new anticancer mechanism of
niclosamide through inhibition of vasculogenic mimicry (VM)
formation.
VM is a tumor microcirculation pattern that usually
exists in highly aggressive cancers. For example, the ability of
highly invasive instead of poorly invasive, melanoma cells to
generate patterned vascular channels in vitro (5). This helps to understand the strong
association between the presence of vascular channel and poor
cancer patient prognosis. Multiple molecular mechanisms, especially
MMPs and VEGFA, have been reported to participate in VM formation
which are regarded as significant factors in tumor migration and
invasion. VEGFA belongs to an angiogenic growth factor family, and
can be secreted by almost all tumor cells, and be associated with
tumor angiogenesis. Expression of MMPs could be upregulated by
VEGFA, contributing to matrix plasticity and VM formation (20). Furthermore, hypoxia is the most
common phenomena. Hypoxia stimulates tumor cells secreting HIF-1α,
which in turn activates VEGFA and contributes to VM formation
(21). Traditional anti-angiogenic
drugs targeted to endothelium reduce the density of blood vessel,
as well as energy and oxygen supply, which can aggravate hypoxia of
tumor cells. As tumors grow, the increasing nutrient and oxygen
deficiency as a compensatory stimulus will contribute to VM
formation and indirectly promote aggressiveness and therapeutic
resistances of cancers. This can well explain why the common
anti-angiogenic drugs have limited effect on VM.
To investigate the underlying mechanism that
niclosamide could inhibit VM in vitro and in vivo, we
explored the VM-related gene expression at mRNA and protein levels.
We verified that while niclosamide inhibits VM, the expression of
VEGFA, MMP2, ROCK1 and Cdc42 genes are downregulated (Fig. 4A). VEGFA and MMP2 play important
roles in VM formation. ROCK and Cdc42 are cytoskeleton genes, which
regulate the cell morphology, as well as epithelial-mesenchymal
transition (EMT), migration and invasion of cancer cells (22,23).
Research shows that ROCK is a regulator in VM in hepatocellular
carcinoma cells (24) and
osteosarcoma cells (25). Cdc42 is
involved in VM, which can be downregulated by BNIP3 in melanoma
cells (26). Thus, we can conclude
that niclosamide could downregulate VM-related genes to inhibit VM
formation. More importantly, VEGFA (27), MMP2 (28) and Cdc42 (29) can be regulated by STAT3.
STAT3 activation is frequently observed in advanced
tumors and significantly involved in the process of proliferation,
differentiation, apoptosis, migration and invasion of cancer cells
(30), accumulating research shows
that STAT3 can play an important role in angiogenesis both in
physiological and pathological situations (31,32).
Furthermore, STAT3 activation plays a positive role in VM formation
of gastric adenocarcinoma (33).
Curcumin could suppress VM of laryngeal squamous cell carcinoma
(34) and hepatocellular carcinoma
(35) in vitro through the
inhibition of STAT3 signaling pathway. Moreover, our results show
that niclosamide could downregulate the expression of p-STAT3 at
protein level. Based on the above data and evidence, we deduce that
STAT3 may play a central role in the anticancer effects of
niclosamide.
miRNAs are small noncoding RNAs that play important
roles in many cellular processes (36). Up to this point, more than 30,424
human miRNAs have been identified and miRNAs usually function as
potential oncogenes or oncosuppressor genes through imperfectly
complementary to the 3′ UTR of target mRNA (37,38).
In this study, we focused on miR-124 because it has been reported
to be downregulated and affect metastasis in several types of
cancer including oral cancer (39),
suggesting that miR-124 has the potential as therapeutic target for
oral cancer. However, there is no report on the effect of
niclosamide on miR-124. In a recent study, our results show that
niclosamide increases the expression of miR-124. Furthermore,
reports suggested that there is interplay between miR-124 and STAT3
signaling pathway in multiple human cancer cells including
endometrial carcinoma (40),
hepatocellular carcinoma (41) and
colorectal cancer (42). Therefore,
we deduce that STAT3 may play an important role in the anticancer
effects of niclosamide. To clarify the function of STAT3 and
miR-124 in oral cancer, we detected the expression of p-STAT3 and
the ability of VM in stable cell line HN6-miR-124. As our results
show, such as niclosamide, HN6-miR-124 can decrease the expression
of p-STAT3. Also, HN6-miR-124 has lower mobility, invasiveness and
capillary-like tube formation ability compared with control. Based
on the above data, we deduce that both miR-124 and STAT3 play key
roles in the anticancer effects of niclosamide. Niclosamide exerts
its anticancer effects partly through miR-124/STAT3/VM axis.
In conclusion, our study, for the first time shows
that niclosamide exerts its anticancer effects partly through
modulating VM via upregulation of miR-124 and downregulation of
STAT3. These findings support the possibility of using niclosamide
as a target for anti-VM therapy in OSCC.
Acknowledgements
This work was supported by the Research Fund for
Capital Medical Development (2011-0425-02), the Research Grants
from Nature Foundation of Heilongjiang Province (no. QC2014C107),
Tianjin Natural Science Foundation (14JCQNJC12500), the National
Nature Science Foundation of China (grant no. 81470707, 81300901
and 81772873) and Beijing Natural Science Foundation (7172240).
References
|
1
|
Jemal A, Ward EM, Johnson CJ, Cronin KA,
Ma J, Ryerson B, Mariotto A, Lake AJ, Wilson R, Sherman RL, et al:
Annual Report to the Nation on the Status of Cancer, 1975–2014,
Featuring Survival. J Natl Cancer Inst. 109:djx0302017. View Article : Google Scholar :
|
|
2
|
Zini A, Czerninski R and Sgan-Cohen HD:
Oral cancer over four decades: Epidemiology, trends, histology, and
survival by anatomical sites. J Oral Pathol Med. 39:299–305. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yadav P: Recent advances in head and neck
cancer reconstruction. Indian J Plast Surg. 47:185–190. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maniotis AJ, Folberg R, Hess A, Seftor EA,
Gardner LM, Pe'er J, Trent JM, Meltzer PS and Hendrix MJ: Vascular
channel formation by human melanoma cells in vivo and in vitro:
Vasculogenic mimicry. Am J Pathol. 155:739–752. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang S, Zhang D and Sun B: Vasculogenic
mimicry: Current status and future prospects. Cancer Lett.
254:157–164. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van der Schaft DW, Seftor RE, Seftor EA,
Hess AR, Gruman LM, Kirschmann DA, Yokoyama Y, Griffioen AW and
Hendrix MJ: Effects of angiogenesis inhibitors on vascular network
formation by human endothelial and melanoma cells. J Natl Cancer
Inst. 96:1473–1477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xiang D, Yuan Y, Chen L, Liu X, Belani C
and Cheng H: Niclosamide, an anti-helminthic molecule,
downregulates the retroviral oncoprotein Tax and pro-survival Bcl-2
proteins in HTLV-1-transformed T lymphocytes. Biochem Biophys Res
Commun. 464:221–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen W, Mook RA Jr, Premont RT and Wang J:
Niclosamide: Beyond an antihelminthic drug. Cell Signal Apr.
4:2017(Epub ahead of print).
|
|
10
|
Li Y, Li PK, Roberts MJ, Arend RC, Samant
RS and Buchsbaum DJ: Multi-targeted therapy of cancer by
niclosamide: A new application for an old drug. Cancer Lett.
349:8–14. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Djebali S, Davis CA, Merkel A, Dobin A,
Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F,
et al: Landscape of transcription in human cells. Nature.
489:101–108. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Martens-Uzunova ES, Böttcher R, Croce CM,
Jenster G, Visakorpi T and Calin GA: Long noncoding RNA in
prostate, bladder, and kidney cancer. Eur Urol. 65:1140–1151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li M, Marin-Muller C, Bharadwaj U, Chow
KH, Yao Q and Chen C: MicroRNAs: Control and loss of control in
human physiology and disease. World J Surg. 33:667–684. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Ling Z, Hao Y, Pang X, Han X,
Califano JA, Shan L and Gu X: MiR-124 acts as a tumor suppressor by
inhibiting the expression of sphingosine kinase 1 and its
downstream signaling in head and neck squamous cell carcinoma.
Oncotarget. 8:25005–25020. 2017.PubMed/NCBI
|
|
15
|
Chen SM, Chou WC, Hu LY, Hsiung CN, Chu
HW, Huang YL, Hsu HM, Yu JC and Shen CY: The effect of MicroRNA-124
overexpression on anti-tumor drug sensitivity. PLoS One.
10:e01284722015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wan HY, Li QQ, Zhang Y, Tian W, Li YN, Liu
M, Li X and Tang H: MiR-124 represses vasculogenic mimicry and cell
motility by targeting amotL1 in cervical cancer cells. Cancer Lett.
355:148–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chiang HR, Schoenfeld LW, Ruby JG, Auyeung
VC, Spies N, Baek D, Johnston WK, Russ C, Luo S, Babiarz JE, et al:
Mammalian microRNAs: Experimental evaluation of novel and
previously annotated genes. Genes Dev. 24:992–1009. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
DiMasi JA, Hansen RW and Grabowski HG: The
price of innovation: New estimates of drug development costs. J
Health Econ. 22:151–185. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chong CR and Sullivan DJ Jr: New uses for
old drugs. Nature. 448:645–646. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang JY, Sun T, Zhao XL, Zhang SW, Zhang
DF, Gu Q, Wang XH, Zhao N, Qie S and Sun BC: Functional
significance of VEGF-a in human ovarian carcinoma: Role in
vasculogenic mimicry. Cancer Biol Ther. 7:758–766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jones MK, Szabó IL, Kawanaka H, Husain SS
and Tarnawski AS: von Hippel Lindau tumor suppressor and
HIF-1alpha: New targets of NSAIDs inhibition of hypoxia-induced
angiogenesis. FASEB J. 16:264–266. 2002.PubMed/NCBI
|
|
22
|
Feltrin D and Pertz O: Assessment of Rho
GTPase signaling during neurite outgrowth. Methods Mol Biol.
827:181–194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kwon J, Kim NH and Choi I: ROCK activity
regulates functional tight junction assembly during blastocyst
formation in porcine parthenogenetic embryos. PeerJ. 4:e19142016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang JG, Li XY, Wang YZ, Zhang QD, Gu SY,
Wu X, Zhu GH, Li Q and Liu GL: ROCK is involved in vasculogenic
mimicry formation in hepatocellular carcinoma cell line. PLoS One.
9:e1076612014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xia Y, Cai X, Fan J, Zhang L, Li Z, Ren J,
Wu G and Zhu F: RhoA/ROCK pathway inhibition by fasudil suppresses
the vasculogenic mimicry of U2OS osteosarcoma cells in vitro.
Anticancer Drugs. 28:514–521. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Maes H, Van Eygen S, Krysko DV,
Vandenabeele P, Nys K, Rillaerts K, Garg AD, Verfaillie T and
Agostinis P: BNIP3 supports melanoma cell migration and
vasculogenic mimicry by orchestrating the actin cytoskeleton. Cell
Death Dis. 5:e11272014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Z and Han ZC: STAT3: A critical
transcription activator in angiogenesis. Med Res Rev. 28:185–200.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu W, Sun W, Zhang JT, Liu ZY, Li XP and
Fan YZ: Norcantharidin enhances TIMP-2 anti-vasculogenic mimicry
activity for human gallbladder cancers through downregulating MMP-2
and MT1-MMP. Int J Oncol. 46:627–640. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu F, Chen Y, Li Y, Ju J, Wang Z and Yan
D: RNA-interference-mediated Cdc42 silencing down-regulates
phosphorylation of STAT3 and suppresses growth in human
bladder-cancer cells. Biotechnol Appl Biochem. 49:121–128. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu H and Jove R: The STATs of cancer - new
molecular targets come of age. Nat Rev Cancer. 4:97–105. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Valdembri D, Serini G, Vacca A, Ribatti D
and Bussolino F: In vivo activation of JAK2/STAT-3 pathway during
angiogenesis induced by GM-CSF. FASEB J. 16:225–227.
2002.PubMed/NCBI
|
|
32
|
Osugi T, Oshima Y, Fujio Y, Funamoto M,
Yamashita A, Negoro S, Kunisada K, Izumi M, Nakaoka Y, Hirota H, et
al: Cardiac-specific activation of signal transducer and activator
of transcription 3 promotes vascular formation in the heart. J Biol
Chem. 277:6676–6681. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song YY, Sun LD, Liu ML, Liu ZL, Chen F,
Zhang YZ, Zheng Y and Zhang JP: STAT3, p-STAT3 and HIF-1α are
associated with vasculogenic mimicry and impact on survival in
gastric adenocarcinoma. Oncol Lett. 8:431–437. 2014.PubMed/NCBI
|
|
34
|
Hu A, Huang JJ, Jin XJ, Li JP, Tang YJ,
Huang XF, Cui HJ, Xu WH and Sun GB: Curcumin suppresses
invasiveness and vasculogenic mimicry of squamous cell carcinoma of
the larynx through the inhibition of JAK-2/STAT-3 signaling
pathway. Am J Cancer Res. 5:278–288. 2014.PubMed/NCBI
|
|
35
|
Chiablaem K, Lirdprapamongkol K,
Keeratichamroen S, Surarit R and Svasti J: Curcumin suppresses
vasculogenic mimicry capacity of hepatocellular carcinoma cells
through STAT3 and PI3K/AKT inhibition. Anticancer Res.
34:1857–1864. 2014.PubMed/NCBI
|
|
36
|
Qureshi IA and Mehler MF: Regulation of
non-coding RNA networks in the nervous system - what's the REST of
the story? Neurosci Lett. 466:73–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Carroll AP, Tooney PA and Cairns MJ:
Context-specific microRNA function in developmental complexity. J
Mol Cell Biol. 5:73–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kozomara A and Griffiths-Jones S: miRBase:
Annotating high confidence microRNAs using deep sequencing data.
Nucleic Acids Res. 42(Database issue): D68–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jin L, Miao J, Liu Y, Li X, Jie Y, Niu Q
and Han X: Icaritin induces mitochondrial apoptosis by
up-regulating miR-124 in human oral squamous cell carcinoma cells.
Biomed Pharmacother. 85:287–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Zhang Z, Liu X, Huang T, He W, Shen
Y, Liu X, Hong K and Cao Q: miR-124 functions as a tumor suppressor
in the endometrial carcinoma cell line HEC-1B partly by suppressing
STAT3. Mol Cell Biochem. 388:219–231. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu Y, Yue X, Cui Y, Zhang J and Wang K:
MicroRNA-124 suppresses growth of human hepatocellular carcinoma by
targeting STAT3. Biochem Biophys Res Commun. 441:873–879. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang J, Lu Y, Yue X, Li H, Luo X, Wang Y,
Wang K and Wan J: MiR-124 suppresses growth of human colorectal
cancer by inhibiting STAT3. PLoS One. 8:e703002013. View Article : Google Scholar : PubMed/NCBI
|