Introduction
Epithelial ovarian cancer (EOC) is regarded as the
most common type of ovarian cancers, which is usually diagnosed at
an advanced stage due to the ineffective screening strategies, and
causes immense morbidity and mortality worldwide (1). Although the surgical resection
combined with cisplatin-based chemotherapy has greatly benefited
cancer patients, the total survival of patients with advanced
disease is <30% due to chemoresistance (2,3).
Therefore, it is meaningful to find the sensitizer of EOC to
cisplatin and clarify its mechanisms.
Numerous studies indicate that the mechanism of
tumor occurrence, development, invasion, metastasis and resistance
is complex (4–9). As one of the most well-known natural
products, triptolide (TPL) had been used as an anti-inflammatory
agent for rheumatoid arthritis for a long time in China, and was
also recognized as a potential medicine for various types of
cancers (10–16) although some researchers showed its
potential toxicity on animal liver, kidney, testes, ovary and heart
(17). Recent studies indicated
that TPL induced cell apoptosis by inhibiting nuclear factor
κ-light-chain-enhancer of activated B cells (NF-κB) in a
p53-independent pathway, producing reactive oxygen species (ROS)
and inactivating the PI3K/Akt signal pathway (9,16,18–21).
In previous studies, our group proved that the TPL effectively
inhibited cell growth, proliferation, metabolism, survival and
cancer genesis by regulating the PI3K/Akt pathway (22). However, scarce study is carried out
to evaluate the antitumor effect of TPL on cellular immunity and
angiogenesis.
In the present study, we investigated the TPL
anticancer effects using SKOV3/DDP cell line and a mouse model, and
studied the TPL sensitization effects via inhibiting protein
expression of angiogenesis and immunology.
Materials and methods
Cell experiments
Platinum-resistant SKOV3/DDP cell line (purchased
from China Center for Type Culture Collection, Wuhan, China), which
was derived from human ovarian carcinoma, was cultured in RPMI-1640
medium containing 10% fetal bovine serum (FBS) and 100 U/ml
penicillin/streptomycin. Cells were cultured at 37°C in a 5%
humidified CO2 atmosphere, and 0.3 µg/ml cisplatin was
added to the culture media to maintain the acquired resistance to
DDP.
Cellular migration and invasion
assays
To evaluate the effect of TPL on cell migration, a
scratch assay was applied. SKOV3/DDP cells were seeded onto 6-well
plates to make a confluent monolayer, and then a p200 pipette tip
was used to create a straight line to make a ‘scratch’. Suspended
cells were washed using PBS, then 200 µl RPMI-1640 medium
containing 2% FBS was added. SKOV3/DDP cells were co-cultured with
10 µg/ml DDP, 8 ng/ml TPL and 10 µg/ml DDP + 8 ng/ml TPL for 24 h.
The wound area was calculated using Image-Pro Plus software (IPP;
Media Cybernetics, Rockville, MD, USA).
For cell invasion assay, SKOV3/DDP cells
(5×104 cells/well) were seeded to the upper chamber of
the Transwell plates (Corning Life Sciences, Lowell, MA, USA) and
co-cultured with RPMI-1640 media containing 2% FBS, and 500 µl
RPMI-1640 media supplemented with 10% FBS were added into the
bottom wells. Then, 10 µg/ml DDP, 8 ng/ml TPL and 10 µg/ml DDP + 8
ng/ml TPL were added to the chambers, respectively. Incubated for
24 h, the SKOV3/DDP cells that invaded through the Matrigel matrix
membrane were stained with crystal violet for 30–40 min, and their
number was counted using an inverted microscope.
Apoptosis analysis
The treated SKOV3/DDP cells were digested using
trypsin and washed twice using cold Hanks' solution. Then,
SKOV3/DDP cells were suspended in a binding buffer containing
Annexin V-FITC and PI. The cell mixture was incubated at room
temperature (RT) (in dark) for 15 min, and were then sorted by cell
flow cytometry (Becton-Dickinson, San Jose, CA, USA).
Western blotting
Cell lysis buffer supplemented with protease
inhibitor cocktail and 1 mM phenylmethanesulfonyl fluoride (PMSF)
were used to prepare whole-cell lysates, and protein concentrations
were measured (10). Then, samples
were resolved by polyacrylamide electrophoresis, the polyvinylidene
difluoride membranes were blocked with 5% non-fat milk in TBST for
1 h at RT, and were incubated with primary antibodies for 3 h at
RT, and then incubated with the appropriate HRP-conjugated
secondary antibody for another 1 h.
Mouse model of ovarian cancer
To establish primary tumor xenografts, five million
SKOV3/DDP cells were injected in the BALB/c-nu nude mice in a
volume of 20 µl of PBS. When tumors reached 100 mm3, PBS
(50 ml/kg/day, every day), DDP (4 mg/kg/day on the 1st and 8th
days), TPL (0.15 mg/kg/day every day), DDP + TPL (4 mg/kg/day of
DDP on the 1st and 8th days, 0.15 mg/kg/day of TPL every day) was
injected i.p. into the mice. In the end, all mice were cervically
sacrificed and their orbital blood were collected.
The present study was approved by the Ethics
Committee of the Second Affiliated Hospital of Nanchang University,
and all the research was carried out based on the approved
guidelines.
ELISA
The yields of IL-2 and TNF-α in mouse sera were
evaluated using the IL-2 ELISA kit) and TNF-α ELISA kit (both from
eBioscience, San Diego, CA, USA).
Immunohistochemical staining
Resected tumors were fixed in the 10% buffered
formalin, and then embedded in paraffin and mounted on slides.
Tumor sections were paraffinized and suppressed in endogenous
peroxidase activity incubation in 3% hydrogen peroxide, and were
microwaved in 10-mM sodium citrate (pH 6.0) to achieve the antigen
retrieval. Then, 2.5% horse serum were used to block sections, and
corresponding antibodies were used and incubated for 16 h at 4°C.
3,3′-Diaminobenzidine and hematoxylin were used to stain slides,
and detection was achieved using Avidin-Biotin Complex System
(Vector Laboratories, Burlingame, CA, USA), which was analyzed
using the IPP based on their density mean, area sum and integrated
optical density.
Statistical analysis
The data are presented as mean ± SD, P<0.05 was
considered statistically significant (23–25).
Results
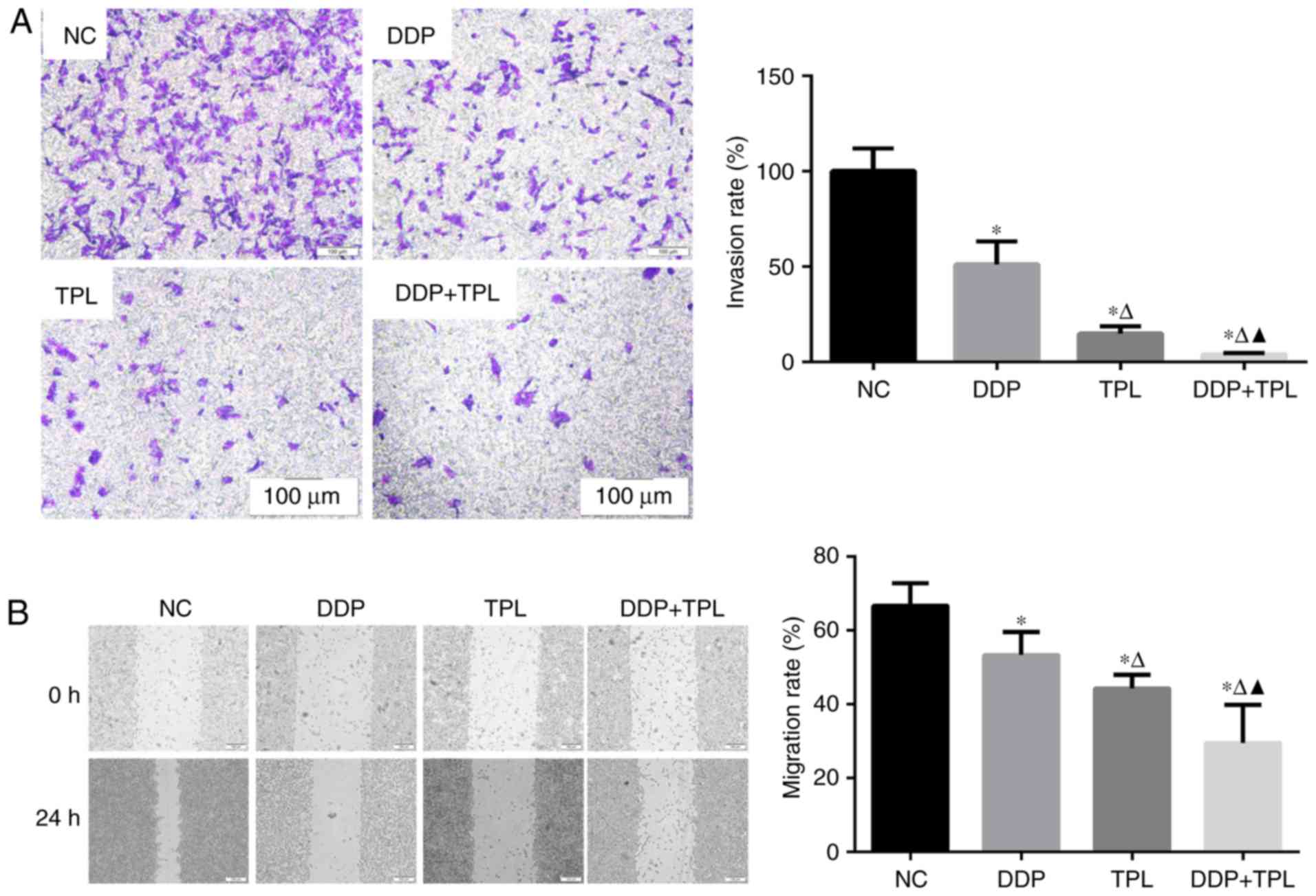
Effects of TPL and DDP + TPL on
migration and invasion of SKOV3/DDP cells
To the best of our knowledge, cellular invasion is
an important part of cellular migration. Cells with high migration
usually possess high invasion, while cells with high invasion may
not possess high migration. So we simultaneously tested the
migration and invasion of SKOV3/DDP cells. As shown in Fig. 1, the addition of DDP, TPL and DDP +
TPL significantly reduced the cellular invasion and migration of
SKOV3/DDP compared with the control group (NC) at 24 h (P<0.05),
and the synergistic effect of TPL and DDP had significantly
enhanced the inhibition effect on cellular invasion and migration
compared with DDP and TPL group (P<0.05).
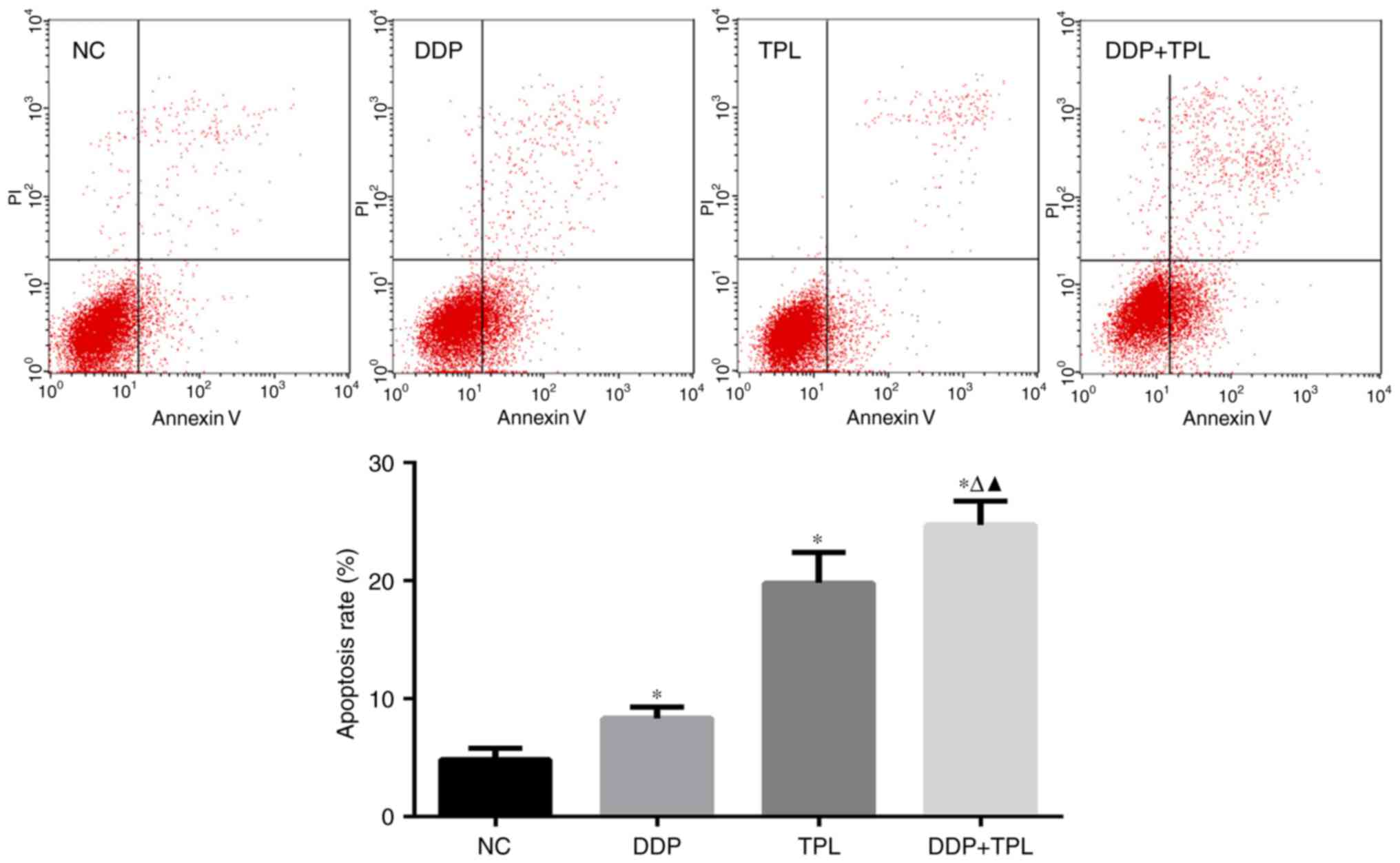
TPL + DDP increases the apoptosis rate
of SKOV3/DDP cells
When treated with different concentrations of
reagents, the apoptosis rates in the control, DDP, TPL and TPL +
DDP group were (4.863±0.930), (8.333±0.965), (19.823±2.558) and
(24.733±2.009)%, respectively. For the single drug group, DDP and
TPL greatly enhanced the apoptosis rate of SKOV3/DDP cells
(P<0.05), and the TPL + DDP had the best promoting effect on
apoptosis of SKOV3/DDP cells compared with DDP and TPL group
(P<0.05) (Fig. 2).
Effects of TPL and DDP + TPL on
protein expression of SKOV3/DDP cells
As tumor development is a complex process, so
cancer-related proteins were tested. As shown in Fig. 3, the treatment of DDP, TPL and DDP +
TPL significantly reduced the expression of adhesion-related
protein integrin β1 (ITGβ1) and apoptosis-inhibiting proteins
survivin, matrix metalloproteinase 2 (MMP-2) and MMP-9 (DDP + TPL
group >TPL >DDP; P<0.05), and significantly increased the
yields of apoptosis-promoting proteins cleaved caspase-3 and Smac
(DDP + TPL group > TPL > DDP; P<0.05).
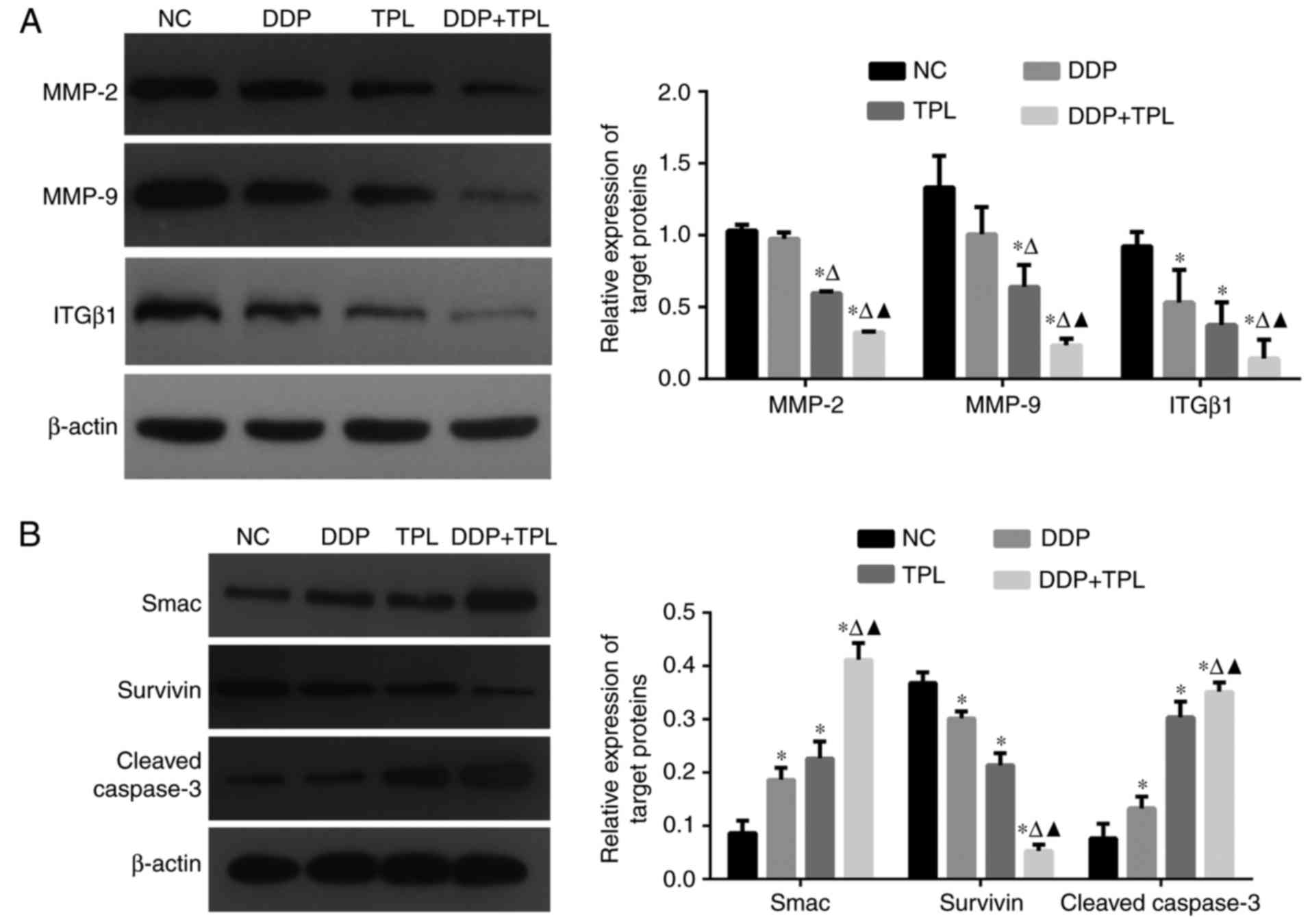 | Figure 3.(A) Effects of DDP, TPL and DDP + TPL
on protein expression profiles of MMP-2, MMP-9, ITGβ1 and β-actin;
(B) Effects of DDP, TPL and DDP + TPL on protein expression
profiles of Smac, survivin, cleaved caspase-3 and β-actin. The DDP
+ TPL significantly reduced the production of ITGβ1, MMP-2 and
MMP-9, and significantly enhanced the yield of cleaved caspase-3
and Smac. Compared with blank group, *P<0.05; compared with TPL
group, ∆P<0.05; compared with DDP group,
▲P<0.05. |
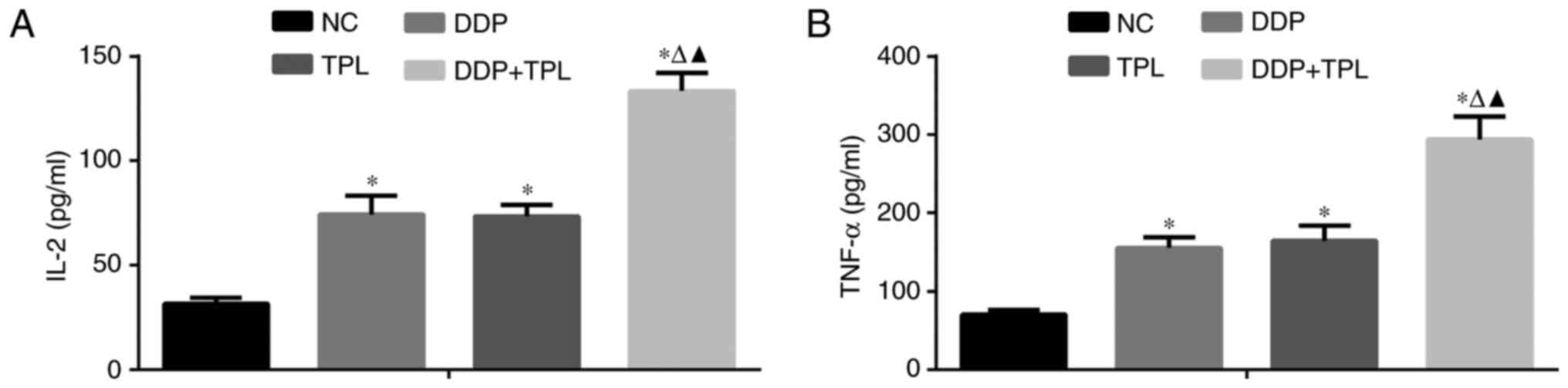
Effects of TPL and DDP + TPL on IL-2
and TNF-α expression
To further study the anticancer effects of TPL and
DDP + TPL on tumors, the DDP, TPL and DDP + TPL were
intraperitoneally injected or orally administered to mice, and
their effects on related cytokines were evaluated. As shown in
Fig. 4, the 4 mg/kg/day DDP, 0.15
mg/kg/day TPL, 4 mg/kg/day TPL and 0.15 mg/kg/day TPL greatly
enhanced the inflammatory factors IL-2 and TNF-α in serum, and the
DDP + TPL possessed the best enhancement effects compared to the
other two groups.
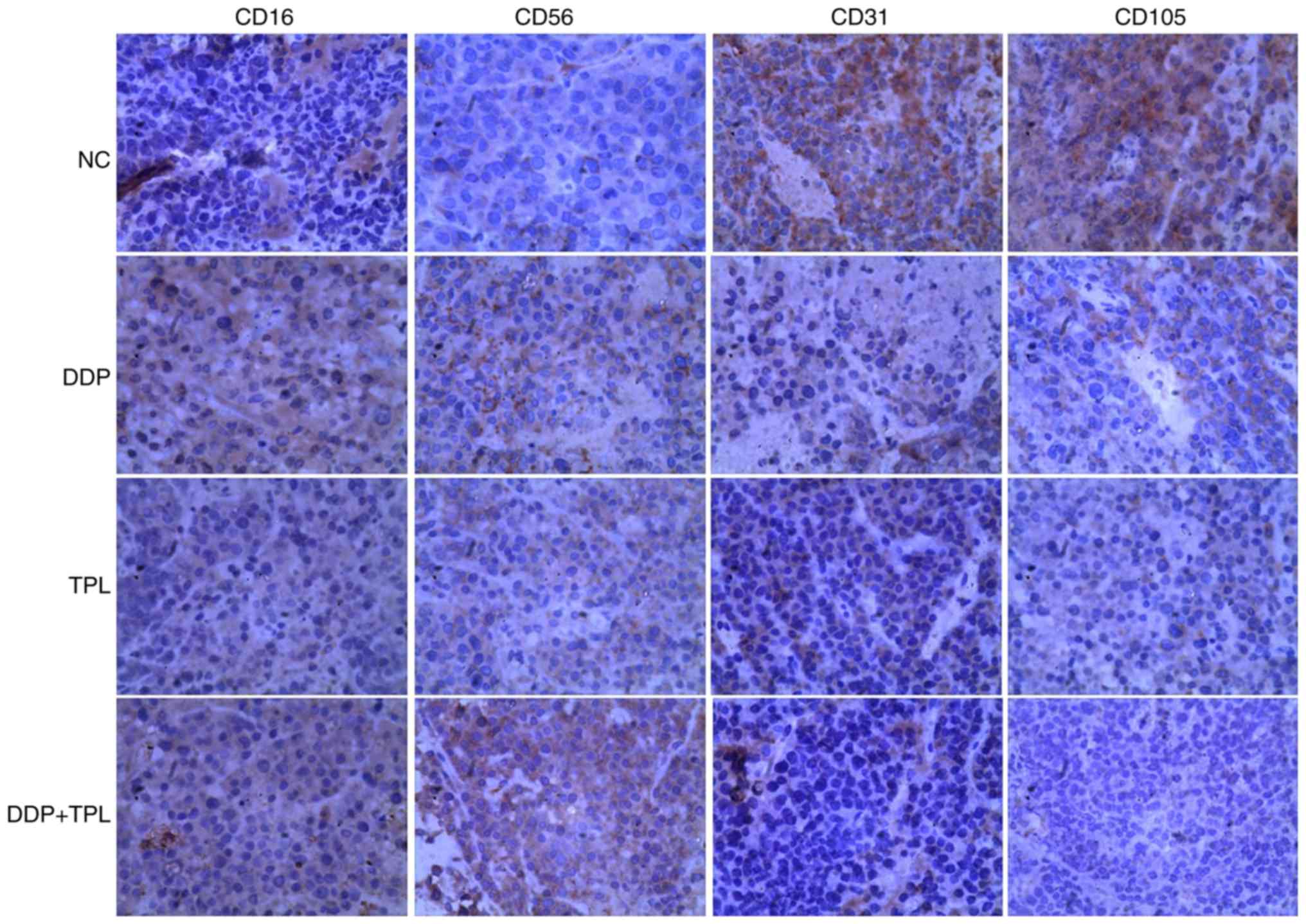
Effects of TPL and DDP + TPL on
protein expression of cellular immunity and angiogenesis
To find the synergistic mechanisms of TPL and DDP,
proteins related to tumor immunity and angiogenesis were studied in
control, DDP, TPL, and TPL + DDP groups. As shown in Fig. 5, the NK cell-related protein levels
of CD16 and CD56 obviously increased in treatment groups, and the
TPL + DDP had the best effect. Moreover, the addition of DDP, TPL
and DDP + TPL greatly inhibited the production of vascular
endothelial growth factor (VEGF) related proteins CD31 and CD105
(DDP + TPL group >TPL group >DDP group).
Discussion
The platinum-based chemotherapy combined with
curative resection was widely used in various cancer treatments,
while drug resistance to DDP has emerged as the major hinderence to
this method (26), thus
naturally-occurring, plant-derived compounds have become a research
hotspot in cancer therapies, which have been proven to influence
multiple signaling pathways and can enhance the activity of
conventional chemotherapy and radiation therapy (27).
As one of the well-known phytochemicals, TPL has
been investigated for its pleiotropic anticancer activities by
inhibiting cancer cell proliferation and inducing apoptosis of
various cancers (11,12,16,28–35),
while few studies were carried out on the effect of TPL on the
cellular immunity and angiogenesis of human epithelial ovarian
cancer (EOC).
In the present study, we first evaluated the
combination of DDP and TPL on cell invasion, migration and
apoptosis of cisplatin-resistance cell SKOV3/DDP in vitro,
and determined the combined effects of DDP and TPL. The expected
value of combination effect between DDP and TPL was calculated as:
[(observed DDP value)/(control value)] × [(observed TPL
value)/(control value)] × (control value); and the combination
index is calculated as the ratio of (expected value)/(observed DDP
+ TPL value), and the ratio of >1 indicated a synergistic effect
(36). As the ratio of the
combination of DDP + TPL was >1, thus, they presented a
synergistic effect on the inhibition of human EOC. Our results
indicated that the DDP + TPL group significantly inhibited the
invasion and migration of SKOV3/DDP cells compared with DDP and TPL
group, and the apoptosis rate in DDP + TPL group was as high as
(24.733±2.009)% (Figs. 1 and
2). Moreover, the western blot
results confirmed that addition of TPL to DDP group greatly
enhanced the yields of apoptosis-promoting proteins of cleaved
caspase-3 and Smac, and obviously reduced the production of ITGβ1,
survivin, MMP-2 and MMP-9 (P<0.05). As is known, caspase-3
belongs to the cysteine protease family, playing a key role in
apoptotic pathways via cleaving a series of key cellular proteins,
while survivin is a member of the inhibitor of apoptosis (IAP)
family to inhibit caspase activation (37), and Smac is a member of promoter for
caspase activation via binding to inhibitors of apoptosis-related
proteins (38), therefore the
negative regulation of survivin and positive regulation of Smac to
caspase-3 lead to apoptosis (programmed cell death) of SKOV3/DDP
cells.
In addition, the significant reduction of the
membrane protein ITGβ1 (39) (a key
protein in tumor invasion and metastasis, via mediating the
adhesion of cells to the matrix and regulating the adhesion growth,
migration, invasion and angiogenesis and chemotherapy resistance of
many tumor cells) and MMP-2/MMP-9 (40) (whose altered expression and activity
levels had been strongly implicated in the progression and
metastasis of many forms of cancers) contributed to tumor apoptosis
and tumor suppression. Fig. 4 shows
the DDP + TPL greatly enhanced expression of IL-2 (which promotes
the differentiation of T cells into effector T cells and into
memory T cells to enhance the immunity of host) and TNF-α (whose
primary role is to regulate immune cells, and is able to inhibit
tumorigenesis), creating a inflammatory environment to promote the
death of cancer cells (41,42).
In conclusion, our results indicated that both the
TPL and DDP + TPL greatly enhanced cell apoptosis and tumor
suppression via adjusting cellular immunity and angiogenesis of
human EOC. Therefore, we proposed that TPL can lower the resistance
of EOC to cisplatin, and can serve as a promising reagent for the
treatment of human ovarian cancer.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81760729,
81503364 and 31560264), and the Jiangxi Government (20161BBG70218,
2016A078, 20171BCB23028 and 20175526).
References
|
1
|
Cannistra SA: Cancer of the ovary. New
Engl J Med. 351:2519–2529. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shepherd JE: Current strategies for
prevention, detection, and treatment of ovarian cancer. J Am Pharm
Assoc. 40:392–401. 2000.
|
|
3
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haynes-Gimore N, Banach M, Brown E, Dawes
R, Edholm ES, Kim M and Robert J: Semi-solid tumor model in Xenopus
laevis/gilli cloned tadpoles for intravital study of
neovascularization, immune cells and melanophore infiltration. Dev
Biol. 408:205–212. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Facciabene A, Motz GT and Coukos G:
T-regulatory cells: Key players in tumor immune escape and
angiogenesis. Cancer Res. 72:2162–2171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou R, He PL, Ren YX, Wang WH, Zhou RY,
Wan H, Ono S, Fujiwara H and Zuo JP: Myeloid suppressor
cell-associated immune dysfunction in CSA1M fibrosarcoma
tumor-bearing mice. Cancer Sci. 98:882–889. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kiessling R, Wasserman K, Horiguchi S,
Kono K, Sjöberg J, Pisa P and Petersson M: Tumor-induced immune
dysfunction. Cancer Immunol Immunother. 48:353–362. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Z, Yun R, Yu X, Hu H, Huang G, Tan B
and Chen T: Overexpression of Notch3 and pS6 is associated with
poor prognosis in human ovarian epithelial cancer. Mediators
Inflamm. 2016:59534982016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hu H, Luo L, Liu F, Zou D, Zhu S, Tan B
and Chen T: Anti-cancer and sensibilisation effect of triptolide on
human epithelial ovarian cancer. J Cancer. 7:2093–2099. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson SM, Wang X and Evers BM:
Triptolide inhibits proliferation and migration of colon cancer
cells by inhibition of cell cycle regulators and cytokine
receptors. J Surg Res. 168:197–205. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shu B, Duan W, Yao J, Huang J, Jiang Z and
Zhang L: Caspase 3 is involved in the apoptosis induced by
triptolide in HK-2 cells. Toxicol In Vitro. 23:598–602. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Manzo SG, Zhou ZL, Wang YQ, Marinello J,
He JX, Li YC, Ding J, Capranico G and Miao ZH: Natural product
triptolide mediates cancer cell death by triggering CDK7-dependent
degradation of RNA polymerase II. Cancer Res. 72:5363–5373. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shao H, Ma J, Guo T and Hu R: Triptolide
induces apoptosis of breast cancer cells via a mechanism associated
with the Wnt/β catenin signaling pathway. Exp Ther Med. 8:505–508.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H, Pan GF, Jiang ZZ, Yang J, Sun LX and
Zhang LY: Triptolide inhibits human breast cancer MCF-7 cell growth
via downregulation of the ERα-mediated signaling pathway. Acta
Pharmacol Sin. 36:606–613. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ziaei S and Halaby R: Immunosuppressive,
anti-inflammatory and anti-cancer properties of triptolide: A mini
review. Avicenna J Phytomed. 149–164. 2016.PubMed/NCBI
|
|
16
|
Zhong YY, Chen HP, Tan BZ, Yu HH and Huang
XS: Triptolide avoids cisplatin resistance and induces apoptosis
via the reactive oxygen species/nuclear factor-κB pathway in
SKOV3PT platinum-resistant human ovarian cancer cells.
Oncol Lett. 6:1084–1092. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xi C, Peng S, Wu Z, Zhou Q and Zhou J:
Toxicity of triptolide and the molecular mechanisms involved.
Biomed Pharmacother. 90:531–541. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim MJ, Lee TH, Kim SH, Choi YJ, Heo J and
Kim YH: Triptolide inactivates Akt and induces caspase-dependent
death in cervical cancer cells via the mitochondrial pathway. Int J
Oncol. 37:1177–1185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lee KY, Park JS, Jee YK and Rosen GD:
Triptolide sensitizes lung cancer cells to TNF-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis by inhibition
of NF-kappaB activation. Exp Mol Med. 34:462–468. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhu W, Hu H, Qiu P and Yan G: Triptolide
induces apoptosis in human anaplastic thyroid carcinoma cells by a
p53-independent but NF-κB-related mechanism. Oncol Rep.
22:1397–1401. 2009.PubMed/NCBI
|
|
21
|
Morgan MJ and Liu ZG: Crosstalk of
reactive oxygen species and NF-κB signaling. Cell Res. 21:103–115.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zeng C, Chen T, Zhang Y and Chen Q:
Hedgehog signaling pathway regulates ovarian cancer invasion and
migration via adhesion molecule CD24. J Cancer. 8:786–792. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ye YZ, Zhang ZH, Fan XY, Xu XL, Chen ML,
Chang BW and Zhang YB: Notch3 overexpression associates with poor
prognosis in human non-small-cell lung cancer. Med Oncol.
30:5952013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mirone G, Perna S, Shukla A and Marfe G:
Involvement of Notch-1 in resistance to regorafenib in colon cancer
cells. J Cell Physiol. 231:1097–1105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan X, Wu H, Xu H, Xiong H, Chu Q, Yu S,
Wu GS and Wu K: Notch signaling: An emerging therapeutic target for
cancer treatment. Cancer Lett. 369:20–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Berkenblit A and Cannistra SA: Advances in
the management of epithelial ovarian cancer. J Reprod Med.
50:426–438. 2005.PubMed/NCBI
|
|
27
|
Aggarwal BB, Sethi G, Baladandayuthapani
V, Krishnan S and Shishodia S: Targeting cell signaling pathways
for drug discovery: An old lock needs a new key. J Cell Biochem.
102:580–592. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang M, Huang J, Pan HZ and Jin J:
Triptolide overcomes dexamethasone resistance and enhanced
PS-341-induced apoptosis via PI3k/Akt/NF-κB pathways in human
multiple myeloma cells. Int J Mol Med. 22:489–496. 2008.PubMed/NCBI
|
|
29
|
Chen YW, Lin GJ, Chuang YP, Chia WT, Hueng
DY, Lin CK, Nieh S and Sytwu HK: Triptolide circumvents
drug-resistant effect and enhances 5-fluorouracil antitumor effect
on KB cells. Anticancer Drug. 21:502–513. 2010. View Article : Google Scholar
|
|
30
|
Carter BZ, Mak DH, Schober WD, McQueen T,
Harris D, Estrov Z, Evans RL and Andreeff M: Triptolide induces
caspase-dependent cell death mediated via the mitochondrial pathway
in leukemic cells. Blood. 108:630–637. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Jin H, Xu R, Mei Q and Fan D:
Triptolide downregulates Rac1 and the JAK/STAT3 pathway and
inhibits colitis-related colon cancer progression. Exp Mol Med.
41:717–727. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang S, Chen J, Guo Z, Xu XM, Wang L, Pei
XF, Yang J, Underhill CB and Zhang L: Triptolide inhibits the
growth and metastasis of solid tumors. Mol Cancer Ther. 2:65–72.
2003.PubMed/NCBI
|
|
33
|
Antonoff MB, Chugh R, Borja-Cacho D,
Dudeja V, Clawson KA, Skube SJ, Sorenson BS, Saltzman DA, Vickers
SM and Saluja AK: Triptolide therapy for neuroblastoma decreases
cell viability in vitro and inhibits tumor growth in vivo. Surgery.
146:282–290. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li CJ, Chu CY, Huang LH, Wang MH, Sheu LF,
Yeh JI and Hsu HY: Synergistic anticancer activity of triptolide
combined with cisplatin enhances apoptosis in gastric cancer in
vitro and in vivo. Cancer Lett. 319:203–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miyata Y, Sato T and Ito A: Triptolide, a
diterpenoid triepoxide, induces antitumor proliferation via
activation of c-Jun NH 2-terminal kinase 1 by decreasing
phosphatidylinositol 3-kinase activity in human tumor cells.
Biochem Biophys Res Commun. 336:1081–1086. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhou JR, Yu L, Mai Z and Blackburn GL:
Combined inhibition of estrogen-dependent human breast carcinoma by
soy and tea bioactive components in mice. Int J Cancer. 108:8–14.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Altieri DC: Molecular cloning of effector
cell protease receptor-1, a novel cell surface receptor for the
protease factor Xa. J Biol Chem. 269:3139–3142. 1994.PubMed/NCBI
|
|
38
|
Petersen SL, Wang L, Yalcin-Chin A, Li L,
Peyton M, Minna J, Harran P and Wang X: Autocrine TNFalpha
signaling renders human cancer cells susceptible to
Smac-mimetic-induced apoptosis. Cancer Cell. 12:445–456. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu Y, Jin X, Huang Y, Dong J, Wang H, Wang
X and Cao X: Inhibition of peritoneal metastasis of human gastric
cancer cells by dextran sulphate through the reduction in HIF-1α
and ITGβ1 expression. Oncol Rep. 35:2624–2634. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Overall CM and López-Otín C: Strategies
for MMP inhibition in cancer: Innovations for the post-trial era.
Nat Rev Cancer. 2:657–672. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
41
|
Swardfager W, Lanctôt K, Rothenburg L,
Wong A, Cappell J and Herrmann N: A meta-analysis of cytokines in
Alzheimer's disease. Biol Psychiat. 68:930–941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liao W, Lin JX and Leonard WJ: IL-2 family
cytokines: New insights into the complex roles of IL-2 as a broad
regulator of T helper cell differentiation. Curr Opin Immunol.
23:598–604. 2011. View Article : Google Scholar : PubMed/NCBI
|