Introduction
Glioblastoma (GB) is the most common and malignant
type of primary brain tumor in adults. The standard treatment of GB
includes surgery, radiation therapy, chemotherapy and also combined
treatment. However, no matter which treatment approach is used, the
therapeutic efficacy of GB is far from satisfactory.
There are many types of natural medicines that
possess potential antitumor efficacy in traditional Chinese
medicine (TCM). The antitumor activities of TCM mainly include: i)
inhibition of tumor proliferation and migration; ii) inhibition of
cell cycle progression; iii) promotion of cell apoptosis; and iv)
antiangiogenesis. Currently, more and more effective ingredients
are gradually being purified from natural medicines and have been
applied to treat various types of carcinomas, including glioma
(1–5). Zhang et al (6) reported that shikonin significantly
inhibited the cell proliferation, migration, invasion and the
expression of matrix metalloproteinase-2 (MMP-2) and MMP-9 in human
glioblastoma U87 and U251 cells. Cao et al (5) used a Chinese medicine formula named
‘Pingliu Keli’ (a mixture of Lycium chinense, Dendrobium
officinale and Arisaema heterophyllum) to treat SHG-44
glioma cells and they found that the folk remedy significantly
induced cell apoptosis in vitro.
Nuclear factor-κB (NF-κB) is a transcription factor
regulating a wide array of genes mediating numerous important
biological processes, such as cell proliferation, autophagy, DNA
repair, motility and protection against apoptosis (7). Proteins of the inhibitory κB family
(IκB) serve as inhibitors and regulators of NF-κB activity.
Phosphorylation of IκBs results in their proteasomal degradation
and the release of NF-κB for nuclear translocation and activation
of gene transcription (8). The
in vitro and in vivo studies have demonstrated that
some natural products such as isoflavone, curcumin, resveratrol and
lycopene exert inhibitory effects on human and animal cancers by
targeting NF-κB and its regulated gene products, including c-myc,
Bcl-2, Bcl-xL, MMPs and vascular endothelial growth factor (VEGF)
(9–13).
Aconitum coreanum is one of the most
important herbs, predominantly found in China, Korea and Japan.
A. coreanum has long been considered as a traditional folk
medicine with therapeutic effects against many disorders, such as
migraine headache, cardialgia, facial distortion, infantile
convulsion, epilepsy, tetanus, vertigo and rheumatic arthralgia
(14). In our previous study, we
succesfully extracted a polysaccharide from A. coreanum and
preliminarily investigated its bioefficacy in regards to inhibiting
the cell migration in breast cancer cells. However, whether it
induces cell apoptosis in cancer cells has not been elucidated.
In the present study, we prepared a polysaccharide
sulphated derivative from A. coreanum (ACP1-s) and examined
its inhibitory capacity on human malignant glioblastoma U87MG
cells. We also revealed the molecular mechanism underlying
ACP1-s-induced apoptosis. Our findings may contribute to the
further understanding of the biological efficacy of polysaccharide,
as well as highlight the possibility of ACP1-s as a novel
therapeutic agent for the treatment of glioma.
Materials and methods
Preparation of A. coreanum
polysaccharide
The A. coreanum polysaccharide (ACP) was
prepared by our research team, as previously reported (15). Briefly, the roots of A.
coreanum were grinded and defatted with ethanol. The residue
was extracted with hot water, and the extract supernatant was then
precipitated with ethanol. Crude polysaccharide precipitate was
collected and dried under reduced pressure. After removing the
proteins, the crude ACP was yielded by dialysis and lyophilisation.
The crude ACP was further applied to a DEAE-cellulose column and a
Sepharose CL-6B column to yield purified A. coreanum
polysaccharide named ACP1.
Physicochemical characterization and
sulphated modification of ACP1
We used high-performance gel permeation
chromatography (HPGPC) to determine the homogeneity and molecular
weight of ACP1. Monosaccharide compositions were identified and
quantified using gas chromatography (Gas Chromatograph, GC-2010
Plus; Shimadzu, Beijing, China). Fourier transform infrared
spectrum was measured with a Nicolet 5700 FT-IR spectrometer
(Thermo Fisher Scientific, Co., Ltd., Shanghai, China) in the
frequency range of 400-4,000 cm−1 (15).
The ACP1 with sulphated modification was designated
as ACP1-s. ACP1-s was prepared according to the chlorosulfonic
acid-pyridine method reported by Xu et al (16).
Cell culture
U87MG, a human brain glioblastoma cell line, was
purchased from the American Type Culture Collection (ATCC;
Manassas, VA, USA) and was routinely cultivated in MEM medium
(Gibco, Carlsbad, CA, USA), supplemented with 10% fetal bovine
serum (FBS; HyClone Laboratories, Beijing, China), 1X Non-essential
amino acid (NEAA; Invitrogen, Carlsbad, CA, USA), 1% glutamine and
100 U/ml penicillin-streptomycin (HyClone Laboratories). The NE-4C
neuroectodermal cells (ATCC) were cultivated in Dulbecco's modified
Eagle's medium (DMEM; Gibco) supplemented with 10% FBS (Gibco) and
penicillin-streptomycin. All cells were cultured in a humidified
incubator with 5% CO2 at 37°C.
Expression vector and cell
transfection
The NF-κB eukaryotic expression vector pcDNA3.1-P65
and its control vector pcDNA3.1 were maintained in our laboratory.
Before transfection, the U87MG cells were planted into a 6-well
plate. After 24 h, when the cells reached 70% confluence, U87MG
cells were transfected with an equal amount of pcDNA3.1-P65 or
pcDNA3.1 plasmid using Lipofectamine 3000 reagent (Invitrogen;
Thermo Fisher Scientific). Stable cells were selected using
neomycin.
Cell viability assay
Cell viability was determined by MTT assay. U87MG
and NE-4C cells were plated into a 96-well plate at a density of
1×104 cells/well and treated with different
concentrations of ACP1-s for 24 h. The medium was then replaced
with 100 µl fresh medium containing 0.5 mg/ml MTT (0.5 mg/ml;
Sigma-Aldrich, Shanghai, China). After 4 h of incubation, the
supernatants were discarded and 150 µl of dimethyl sulfoxide (DMSO;
Sigma-Aldrich) was added. Optical density (OD) at 570 nm was
measured using a microplate reader (BioTek Instruments, Beijing,
China). The cell growth inhibition rate (IR) was calculated as the
ratio between the OD of the ACP1-s treatment group and the OD of
the control group. All of the experiments were performed in
triplicate and repeated at least three times.
Apoptosis analysis
Cell apoptosis was determined using Annexin V-FITC
and PI double staining flow cytometric analysis. Briefly,
1×106 U87MG cells were treated with different
concentrations of ACP1-s for 24 h. Then, the cells were collected
and incubated with Annexin V-FITC/PI (BD Biosciences, Franklin
Lakes, NJ, USA) for 15 min in the dark and immediately analyzed
with flow cytometry (FACScan; BD Biosciences) with the FlowJo FACS
analysis software (FlowJo, LLC, Ashland, OR, USA). The cells in the
different portions represented the different cell states as
follows: late-apoptotic cells were presented in the upper right
quadrant, viable cells were presented in the lower left quadrant,
and early apoptotic cells were presented in the lower right
quadrant.
Western blotting
Cells were lysed in RIPA lysis buffer (Nanjing
KeyGen Biotech Co., Ltd., Nanjing, China) supplemented with
cocktail protease inhibitor (Roche Diagnostics, Shanghai, China).
Protein concentrations were determined by BCA protein assay kit
(Beyotime Institute of Biotechnology, Haimen, China). Equal amount
of proteins were separated by 5–12% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred to a nitrocellulose membrane (Millipore, Billerica, MA,
USA). The blots were blocked with 5% bovine serum albumin (BSA;
Sigma-Aldrich) in TBST at 37°C for 1 h. After that, the blots were
incubated with diluted solution of monoclonal antibodies against
NF-κB (1:1,000; #ab16502; Abcam, Shanghai, China), IκB (1:1,000;
#ab32518; Abcam), Bcl-2 (1:1,000; #ab32124; Abcam), Bax (1:1,000;
#ab32503; Abcam), cleaved caspase-3 (1:1,000; #9661; Cell Signaling
Technology, Shanghai, China) and β-actin (1:3,000; #sc47778; Santa
Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C overnight. After
being washed for 3 times in TBST, the blots were incubated with
horseradish peroxidase (HRP)-conjugated secondary antibodies (goat
anti-rabbit IgG-HRP second antibody, #sc2004; and goat anti-mouse
IgG-HRP second antibody, #sc2005; Santa Cruz Biotechnology) at room
temperature for 45 min. After being washed for another 3 times in
TBST, the blots were visualized by an enhanced chemiluminescence
system (ECL; Thermo Fisher Scientific). Protein expression was
determined semi-quantitatively by densitometric analysis with
Quantity One software (Bio-Rad Laboratories, Beijing, China).
Real-time PCR
Total cellular RNA was extracted using an
Eastep® Super Total RNA Isolation kit (Promega, Beijing,
China). RNA was converted to cDNA with SuperScript II Reverse
Transcriptase (Invitrogen) following the manufacturer's
instructions. Subsequently real-time PCR was performed using an ABI
SepOnePlus Real-Time PCR system (Applied Biosystems, Beijing,
China). The reaction system consisted of 20 µl containing an
aliquot of first-strand cDNA as a template, 10 µl 2X SYBR Premixed
buffer (Roche Diagnostics) and 2 µl forward and reverse primers.
The primers were as follows: Bcl-2 sense,
5′-AAAGGACCTGATCATTGGGG-3′ and antisense,
5′-CAACTCTTTTCCTCCCACCA-3′ (17);
β-actin sense, 5′-TCACCCACACTGTGCCCATCTACGA-3′ and antisense,
5′-CAGCGGAACCGCTCATTGCCAATGG-3′ (18). The PCR amplification process
consisted of one cycle at 95°C for 10 min, 30 cycles at 95°C for 10
sec and 55°C for 30 sec.
Statistical analysis
The SPSS 17.0 statistical software (SPSS, Inc.,
Chicago, IL, USA) was used for all data analysis. Data shown
represent mean ± SD. Student's t-test was used to compare
statistical differences for variables among the treatment groups.
Significance was defined as *P<0.05.
Results
Physicochemical and structural
characterization of ACP1 and ACP1-s
Physicochemical properties of ACP1 were reported in
our previous study (15). Briefly,
the carbohydrate content of ACP1 was 96.1%. HPGPC elution profile
revealed that ACP1 was a homogeneous polysaccharide with an average
molecular weight of 67.6 kDa. GC demonstrated that ACP1 was
composed of arabinose, mannose and glucose with a molar ratio of
0.24:1:3.23. The FT-IR spectrum demonstrated that α- and
β-configurations present simultaneously in ACP1. FT-IR spectrum
also showed some characteristic absorption of ACP1, such as O-H
bending, C-H stretching and C-O bending.
The sulphated derivative ACP1-s was prepared
according to the CSA-Pyr method. The sulfur content of ACP1-s was
7.57% (w/w). The FT-IR of ACP1-s showed characteristic absorption
bands of an asymmetrical S=O stretching vibration and a symmetrical
C-O-S vibration associated with a C-O-SO3 group, which
indicated that ACP1 was successfully sulphated (15).
ACP1-s inhibits the cell growth of
human brain glioblastoma U87MG cells
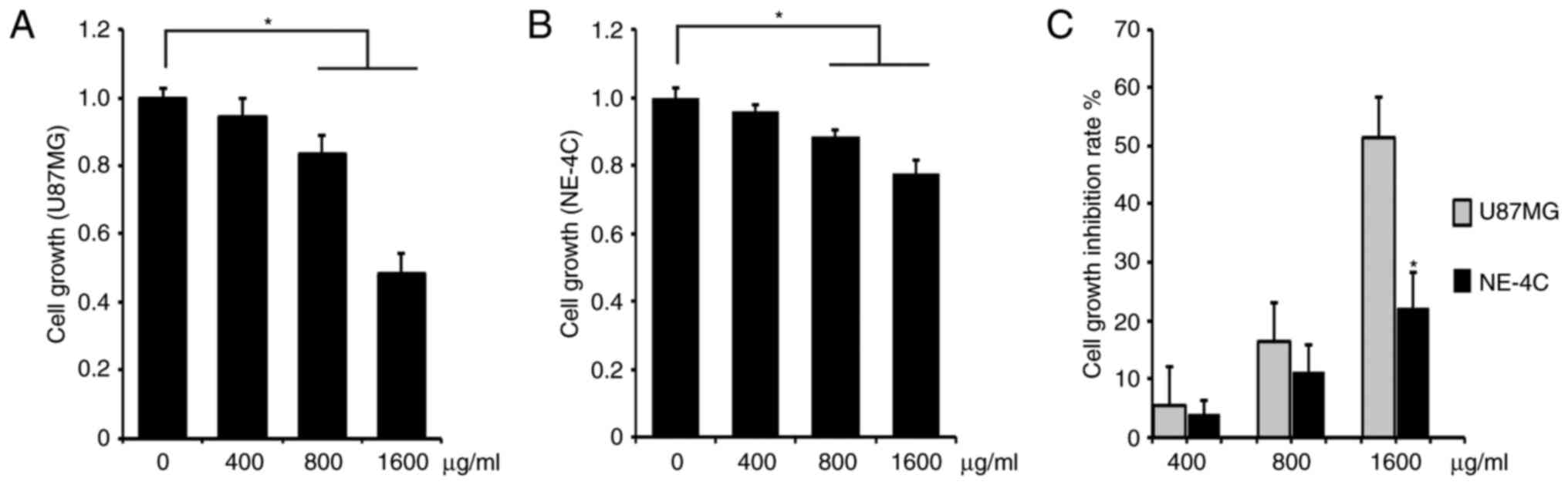
We investigated the cell growth inhibition capacity
of ACP1-s for U87MG glioblastoma cells. As a control group, mouse
neuroectodermal cell line NE-4C was investigated in parallel. We
added 400, 800 or 1,600 µg/ml ACP1-s in the cell culture media and
determined the cell proliferation by MTT assay. These three
concentrations of ACP1-s inhibited the cell growth in both the
U87MG and NE-4C cells, and the doses of 800 and 1,600 µg/ml ACP1-s
exhibited significantly higher activities than that of the 400
µg/ml ACP1-s treatment (Fig. 1A and
B; P<0.05). Then we compared the cell growth inhibition rate
(IR) of ACP1-s between the U87MG and the NE-4C cells. We found that
all three doses of ACP1-s generated higher IRs in the U87MG cells
(5.4 vs. 3.9%, 16.5 vs. 11.4% and 51.4 vs. 22.2%; Fig. 1C), and the IR of the 1,600 µg/ml
dose group was significantly higher in the U87MG cells
(P<0.05).
ACP1-s induces cell apoptosis in U87MG
cells
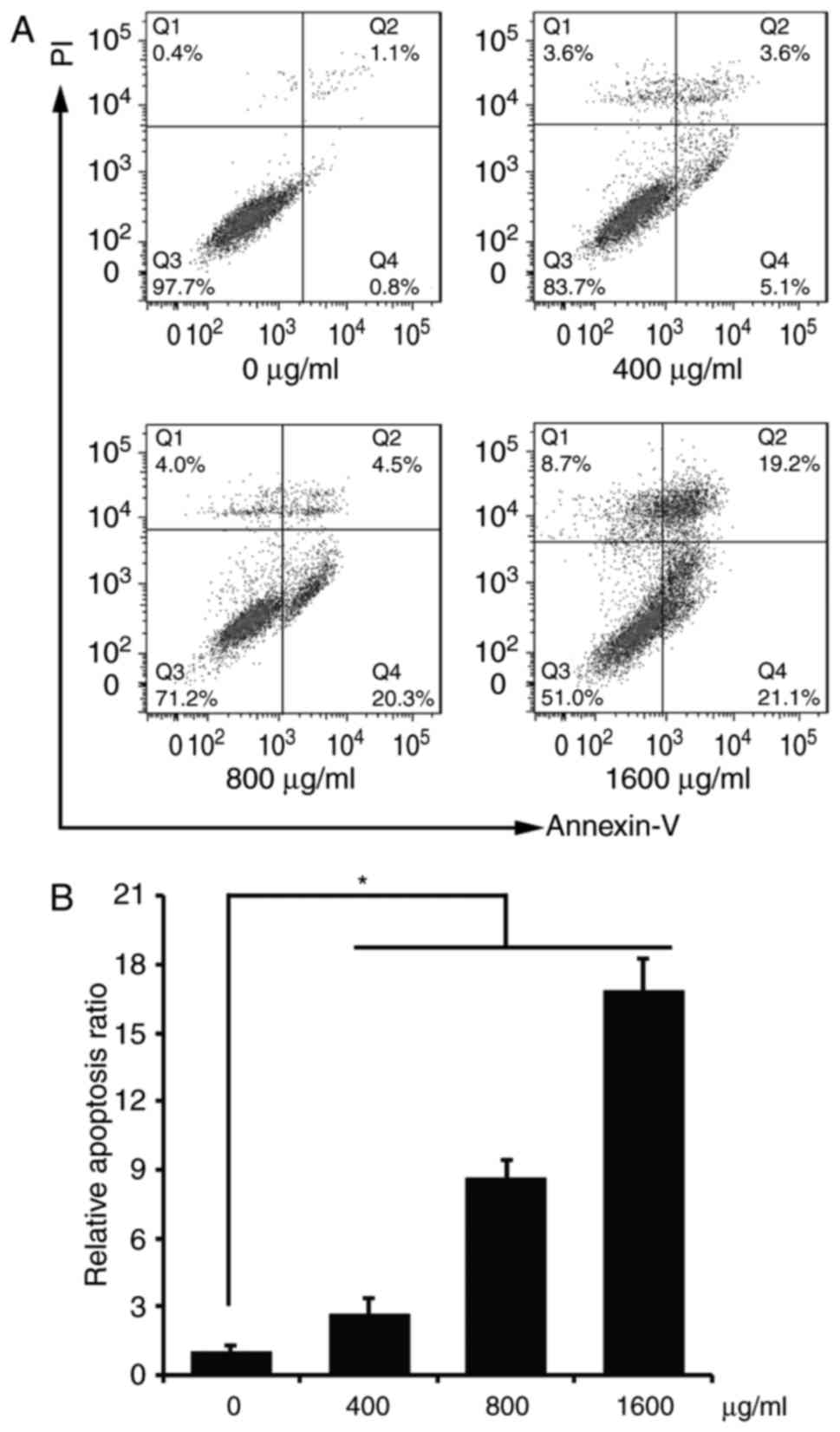
We examined the cell apoptosis of the ACP1-s-treated
U87MG cells with flow cytometry. As shown in Fig. 2, all three doses of 400, 800 and
1,600 µg/ml ACP1-s significantly induced cell apoptosis compared to
the control group (7 vs. 22.5 vs. 43.9%; Fig. 2B, P<0.05), and the cell apoptotic
percentage of the 1,600 µg/ml dose group was the highest (Fig. 2).
ACP1-s induces cell apoptosis through
the NF-κB/Bcl-2 signaling pathway
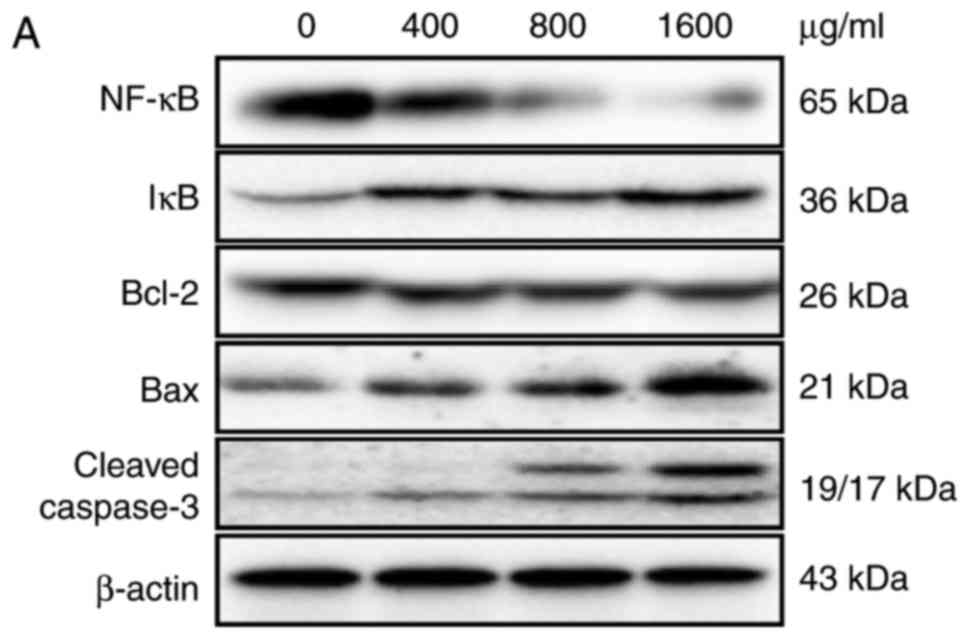
We analyzed the molecular mechanism underlying the
ACP1-s-induced cell apoptosis using western blot analysis and
real-time PCR methods. Western blot results indicated that after
treatment with ACP1-s, the level of IkB was increased 2.1-fold and
the level of NF-κB was accordingly reduced 5.2-fold (1,600 µg/ml
dose group; Fig. 3A and B,
P<0.05). The expression levels of NF-κB-regulated genes Bcl-2,
Bax and caspase-3 were also altered after treatment with ACP1-s.
The ratio of Bcl-2/Bax was reduced 3-fold and the level of cleaved
caspase-3 was increased 5.8-fold (1,600 µg/ml dose group; Fig. 3A and B, P<0.05).
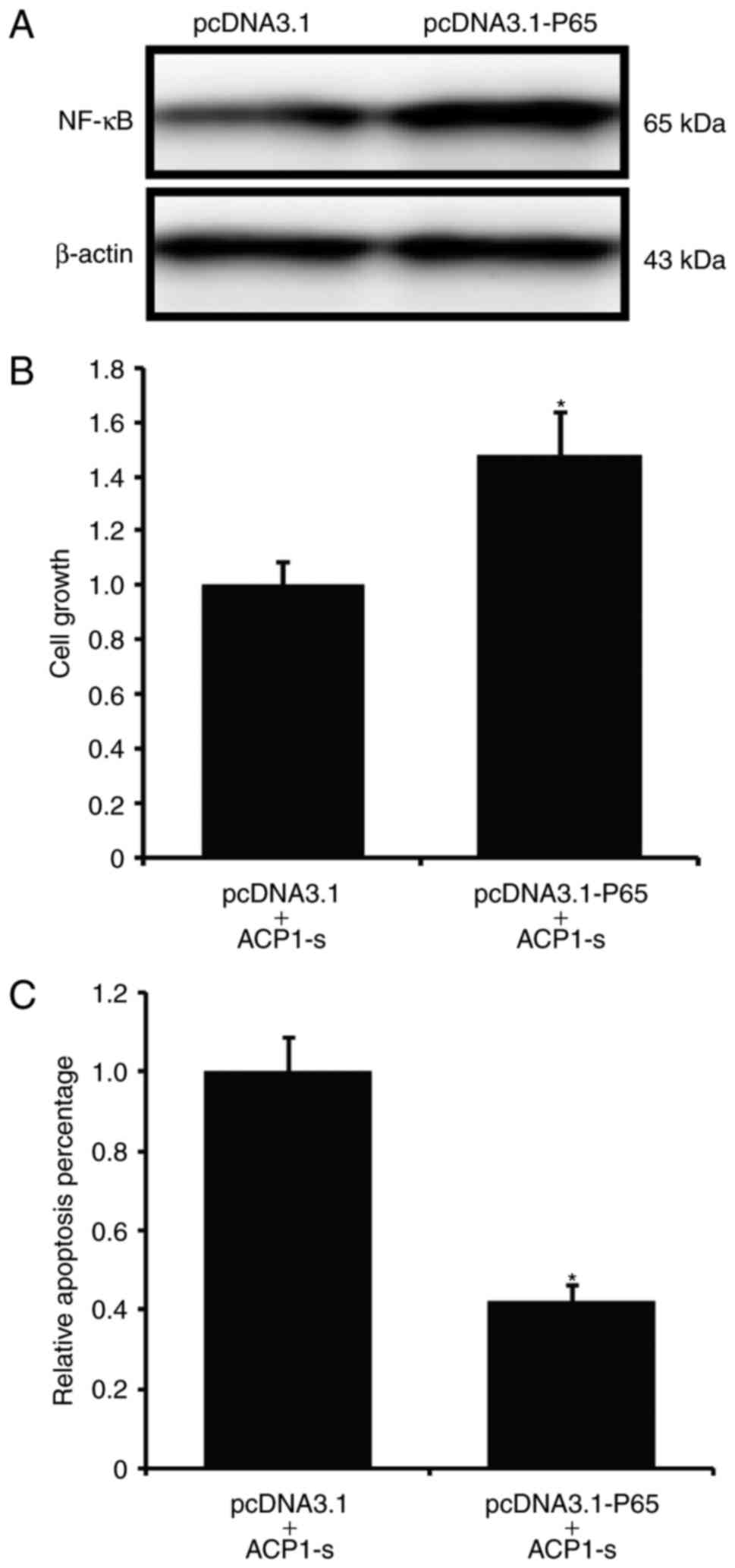
We then introduced exogenous p65 protein to
delineate the specificity of the ACP1-s-activated NF-κB/Bcl-2 cell
apoptotic signaling pathway. We first established a stable U87MG
cell line that overexpressed p65 (Fig.
4A), and then treated the stable cells with ACP1-s for 24 h to
investigate the cell growth and cell apoptosis. We found that after
introducing p65 to the U87MG cells, the ACP1-s-induced cell growth
inhibition and cell apoptosis were abolished (Fig. 4B and C; P<0.05).
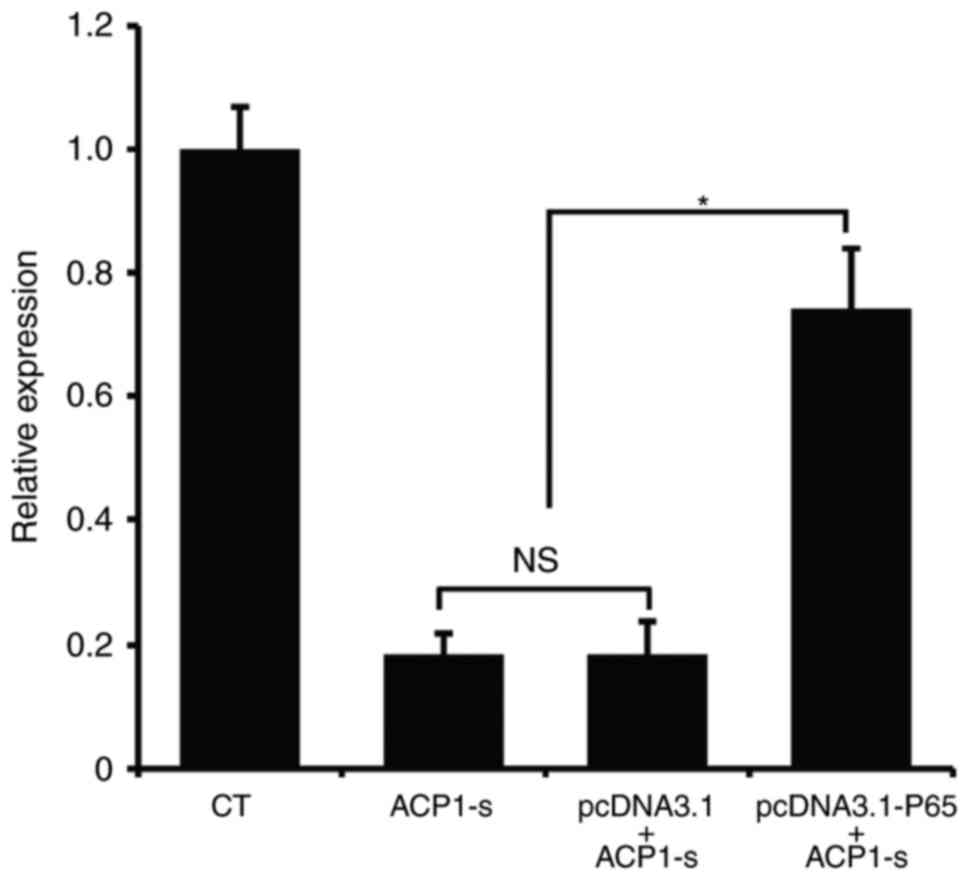
Then, we used real-time PCR to further examine the
Bcl-2 mRNA expression. Compared to the untransfected cells or the
cells transfected with the control plasmid, overexpression of p65
protein in U87MG cells partly neutralized the ACP1-s-induced Bcl-2
inhibition (Fig. 5; P<0.05).
Discussion
Medicinal plants have a long history of use in the
fight against diseases, and some medicinal plants now have been
developed into important drugs (19). There is growing interest in the use
of medicinal plant-derived drugs to combat human tumors in recent
years. It is estimated that currently more than 70% of anticancer
drugs have a natural origin (20).
Glioblastoma (GB) is the most damaging tumor of the
brain. The usual survival at identification of GB is only
approximately 1 year due to the therapeutic resistance and tumor
relapse after removal by surgery (21). Currently, the drugs of choice for
first-line therapy of GB include the methylating agent temozolomide
(TMZ) and chloroethyl-derivatives of nitrosourea: carmustine,
nimustine, lomustine and fotemustine (22). Although these drugs improve clinical
outcomes, chemoresistance remains one of the major problems
(23). Therefore, the development
of novel drugs is crucial to the treatment of GB.
Natural medicines have fewer side-effects compared
with conventional anticancer drugs. More recently, several
effective ingredients have been purified from medicinal plants and
have been used to kill glioma cells. For example, curcumin,
resveratrol and elemene can induce cell apoptosis, inhibit cell
proliferation, regulate the cell cycle and inhibit cell migration
and invation in glioma cells (24–29).
Natural polysaccharides possess many beneficial
health properties. Recent research has indicated that
polysaccharides and their sulphated derivative are able to activate
many cell signaling events that closely correlate with tumor
development (30–33). Therefore, polysaccharides from
medicinal plants may be a resource repository for the search for
novel therapeutic agents against cancer. In our previous study, we
succesfully extracted a polysaccharide from A. coreanum
(ACP1) and prepared its sulphated derivative (ACP1-s). We verified
that ACP1 can significantly inhibit the cell migration of human
breast cancer MDA-MB-435s cells in vitro and affect the
dynamic remodeling of the cell actin cytoskeleton. Moreover, we
demonstrated that ACP1-s possesses higher biological activity
compared with ACP1. In the present study, we investigated the
anti-glioma activity of ACP1-s in the human brain glioblastoma cell
line U87MG. Cell viability assay and flow cytometry results
demonstrated that 400, 800 and 1,600 µg/ml ACP1-s induced cell
growth inhibition and cell apoptosis through the NF-κB/Bcl-2 cell
apoptotic pathway. To explore the cytotoxicity of ACP1-s to normal
cells, a mouse neuroectodermal cell line NE-4C was employed as a
control in the cell viability assay. Our result demonstrated that
the cell growh inhibitory rate (IR) of the NE-4C cells was much
lower than that of the U87MG cells (22.2 vs. 51.4%; Fig. 1), which means that ACP1-s has
slighter cytotoxicity to normal neuronal cells.
NF-κB is highly activated in GB, and the NF-κB
pathway is one of the most related pathways in the natural medicine
induced cell apoptosis process (34). Cheng et al (35) found that a polysaccharide obtained
from highland barley inhibited the cell proliferation of human
colon cancer HT-29 cells through the activation of c-Jun N-terminal
kinase (JNK) and the inhibition of NF-κB. Zhang et al
(36) showed that a polysaccharide
from Lentinus edodes decreased the cell proliferation of
hepatocellular carcinoma cell lines HepG2 and H22 through the
inhibition of NF-κB, Stat3 and survivin signaling. Bcl-2 family
members regulate the mitochondrial pathway of apoptosis by complex
interactions, which dictate the integrity of the outer
mitochondrial membrane (37). The
ratio between Bcl-2 and Bax is important in regulating the release
of cytochrome c from mitochondria, which then activates
caspase-3 and induces apoptosis (38). Additionally, Bcl-2 is a target gene
for NF-κB, and there are multiple NF-κB binding sites on the Bcl-2
promoter (39). In the present
study, our data showed that IκB was activated after treatment with
ACP1-s and the level of NF-κB was accordingly reduced. Expression
levels of NF-κB-regulated genes Bcl-2, Bax and caspase-3 were also
altered following treatment with ACP1-s. These results indicated
that ACP1-s induced the cell apoptosis of U87MG glioma cells
through a NF-κB-mediated mitochondrial apoptosis. The p65
compensation experiment also confirmed our hypothesis.
Limited delivery of therapeutics across the
blood-brain barrier (BBB) makes GB one of the most dreaded cancers
in chemotherapy. Although the alkylating agent TMZ can cross BBB,
its efficacy is limited in GB patients (40). Some natural medicines exhibit
excellent brain penetration and efficacy against brain disorders,
such as ferulic acid and ligustilide (41). Therefore, natural medicine-based
therapies have a bright prospect for improving the efficacy of
current GB treatment. In the present study, although the in
vitro anti-glioma activity of ACP1-s was preliminarily
confirmed, whether ACP1-s can cross the BBB has not been
elucidated. In vitro model BBB transport and the animal
experiments are warranted.
In conclusion, we extracted a polysaccharide from
medicinal plant Aconitum coreanum and prepared its sulphated
derivative. We preliminarily investigated the anti-glioma
bioefficacy of ACP1-s using the U87MG cell line and revealed the
apoptotic molecular mechanism. Our findings suggest the potential
value of ACP1-s as a novel therapeutic agent for the treatment of
glioma.
Acknowledgements
We thank Dr Dehai Yu and Dr Haijun Li of the First
Hospital of Jilin University for kindly providing advice for the
cell experiments.
References
|
1
|
Cohen I, Tagliaferri M and Tripathy D:
Traditional Chinese medicine in the treatment of breast cancer.
Semin Oncol. 29:563–574. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xu ZY, Jin CJ, Zhou CC, Wang ZQ, Zhou WD,
Deng HB, Zhang M, Su W and Cai XY: Treatment of advanced
non-small-cell lung cancer with Chinese herbal medicine by stages
combined with chemotherapy. J Cancer Res Clin Oncol. 137:1117–1122.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mu J, Liu T, Jiang L, Wu X, Cao Y, Li M,
Dong Q, Liu Y and Xu H: The traditional Chinese medicine baicalein
potently inhibits gastric cancer cells. J Cancer. 7:453–461. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li W, Li C, Zheng H, Chen G and Hua B:
Therapeutic targets of Traditional Chinese medicine for colorectal
cancer. J Tradit Chin Med. 36:243–249. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao P, Cai X, Lu W, Zhou F and Huo J:
Growth inhibition and induction of apoptosis in SHG-44 glioma cells
by Chinese medicine formula ‘Pingliu Keli’. Evid Based Complement
Alternat Med. 2011:pii:9582432011. View Article : Google Scholar
|
|
6
|
Zhang FY, Hu Y, Que ZY, Wang P, Liu YH,
Wang ZH and Xue YX: Shikonin inhibits the migration and invasion of
human glioblastoma cells by targeting phosphorylated β-catenin and
phosphorylated PI3K/Akt: A potential mechanism for the anti-glioma
efficacy of a traditional Chinese herbal medicine. Int J Mol Sci.
16:23823–23848. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soubannier V and Stifani S: NF-kappaB
signalling in glioblastoma. Biomedicines. 5:292017. View Article : Google Scholar :
|
|
8
|
Napetschnig J and Wu H: Molecular basis of
NF-κB signaling. Annu Rev Biophys. 42:443–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Davis JN, Kucuk O and Sarkar FH: Genistein
inhibits NF-kappa B activation in prostate cancer cells. Nutr
Cancer. 35:167–174. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hussain AR, Ahmed M, Al-Jomah NA, Khan AS,
Manogaran P, Sultana M, Abubaker J, Platanias LC, Al-Kuraya KS and
Uddin S: Curcumin suppresses constitutive activation of nuclear
factor-kappaB and requires functional Bax to induce apoptosis in
Burkitt's lymphoma cell lines. Mol Cancer Ther. 7:3318–3329. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Estrov Z, Shishodia S, Faderl S, Harris D,
Van Q, Kantarjian HM, Talpaz M and Aggarwal BB: Resveratrol blocks
interleukin-1beta-induced activation of the nuclear transcription
factor NF-kappaB, inhibits proliferation, causes S-phase arrest,
and induces apoptosis of acute myeloid leukemia cells. Blood.
102:987–995. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kundu JK, Shin YK and Surh YJ: Resveratrol
modulates phorbol ester-induced pro-inflammatory signal
transduction pathways in mouse skin in vivo: NF-kappaB and AP-1 as
prime targets. Biochem Pharmacol. 72:1506–1515. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang CS, Fan YE, Lin CY and Hu ML:
Lycopene inhibits matrix metalloproteinase-9 expression and
down-regulates the binding activity of nuclear factor-kappa B and
stimulatory protein-1. J Nutr Biochem. 18:449–456. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tong H, Feng K, Zhang X, Tian D, Liu Y,
Chu X and Sun X: Purification and chemical compositions of a
proteoglycan isolated from Aconitum coreanum. Chemistry Natural
Compounds. 46:329–330. 2010. View Article : Google Scholar
|
|
15
|
Zhang Y, Wu W, Kang L, Yu D and Liu C:
Effect of Aconitum coreanum polysaccharide and its sulphated
derivative on the migration of human breast cancer MDA-MB-435s
cell. Int J Biol Macromol. 103:477–483. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu Y, Song S, Wei Y, Wang F, Zhao M, Guo J
and Zhang J: Sulfated modification of the polysaccharide from
Sphallerocarpus gracilis and its antioxidant activities. Int J Biol
Macromol. 87:180–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiao LJ, Chen YY, Lin P, Zou HF, Lin F,
Zhao LN, Li D, Guo L, Tang JB, Zheng XL and Yu XG: Hypoxia
increases CX3CR1 expression via HIF-1 and NF-κB in
androgen-independent prostate cancer cells. Int J Oncol.
41:1827–1836. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang G, Xu Y, Chen X and Hu G: IFITM1
plays an essential role in the antiproliferative action of
interferon-gamma. Oncogene. 26:594–603. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chao J, Dai Y, Verpoorte R, Lam W, Cheng
YC, Pao LH, Zhang W and Chen S: Major achievements of
evidence-based traditional Chinese medicine in treating major
diseases. Biochem Pharmacol. 139:94–104. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jacobo-Herrera NJ, Jacobo-Herrera FE,
Zentella-Dehesa A, Andrade-Cetto A, Heinrich M and Pérez-Plasencia
C: Medicinal plants used in Mexican traditional medicine for the
treatment of colorectal cancer. J Ethnopharmacol. 179:391–402.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Anjum K, Shagufta BI, Abbas SQ, Patel S,
Khan I, Shah SAA, Akhter N and Hassan SSU: Current status and
future therapeutic perspectives of glioblastoma multiforme (GBM)
therapy: A review. Biomed Pharmacother. 92:681–689. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Annovazzi L, Mellai M and Schiffer D:
Chemotherapeutic drugs: DNA damage and repair in glioblastoma.
Cancers (Basel). 9:pii: E572017. View Article : Google Scholar
|
|
23
|
Sarkaria JN, Kitange GJ, James CD, Plummer
R, Calvert H, Weller M and Wick W: Mechanisms of chemoresistance to
alkylating agents in malignant glioma. Clin Cancer Res.
14:2900–2908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perry MC, Demeule M, Régina A, Moumdjian R
and Béliveau R: Curcumin inhibits tumor growth and angiogenesis in
glioblastoma xenografts. Mol Nutr Food Res. 54:1192–1201.
2010.PubMed/NCBI
|
|
25
|
Dhandapani KM, Mahesh VB and Brann DW:
Curcumin suppresses growth and chemoresistance of human
glioblastoma cells via AP-1 and NFkappaB transcription factors. J
Neurochem. 102:522–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leone S, Basso E, Polticelli F and Cozzi
R: Resveratrol acts as a topoisomerase II poison in human glioma
cells. Int J Cancer. 131:E173–E178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ryu J, Ku BM, Lee YK, Jeong JY, Kang S,
Choi J, Yang Y, Lee DH, Roh GS, Kim HJ, et al: Resveratrol reduces
TNF-α-induced U373MG human glioma cell invasion through regulating
NF-κB activation and uPA/uPAR expression. Anticancer Res.
31:4223–4230. 2011.PubMed/NCBI
|
|
28
|
Zhu T, Zhao Y, Zhang J, Li L, Zou L, Yao Y
and Xu Y: β-Elemene inhibits proliferation of human glioblastoma
cells and causes cell-cycle G0/G1 arrest via mutually compensatory
activation of MKK3 and MKK6. Int J Oncol. 38:419–426.
2011.PubMed/NCBI
|
|
29
|
Zhao YS, Zhu TZ, Chen YW, Yao YQ, Wu CM,
Wei ZQ, Wang W and Xu YH: B-elemene inhibits Hsp90/Raf-1 molecular
complex inducing apoptosis of glioblastoma cells. J Neurooncol.
107:307–314. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun Q, Dong M, Wang Z, Wang C, Sheng D, Li
Z, Huang D and Yuan C: Selenium-enriched polysaccharides from
Pyracantha fortuneana (Se-PFPs) inhibit the growth and invasive
potential of ovarian cancer cells through inhibiting β-catenin
signaling. Oncotarget. 7:28369–28383. 2016.PubMed/NCBI
|
|
31
|
Chen Y, Liu ZJ, Liu J, Liu LK, Zhang ES
and Li WL: Inhibition of metastasis and invasion of ovarian cancer
cells by crude polysaccharides from rosa roxburghii tratt in vitro.
Asian Pac J Cancer Prev. 15:10351–10354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu J, Chen D, Liu C, Wu XZ, Dong CX and
Zhou J: Structural characterization and anti-tumor effects of an
inulin-type fructan from Atractylodes chinensis. Int J Biol
Macromol. 82:765–771. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han SB, Lee CW, Kang JS, Yoon YD, Lee KH,
Lee K, Park SK and Kim HM: Acidic polysaccharide from Phellinus
linteus inhibits melanoma cell metastasis by blocking cell adhesion
and invasion. Int Immunopharmacol. 6:697–702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cahill KE, Morshed RA and Yamini B:
Nuclear factor-κB in glioblastoma: Insights into regulators and
targeted therapy. Neuro Oncol. 18:329–339. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cheng D, Zhang X, Meng M, Han L, Li C, Hou
L, Qi W and Wang C: Inhibitory effect on HT-29 colon cancer cells
of a water-soluble polysaccharide obtained from highland barley.
Int J Biol Macromol. 92:88–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Li Q, Wang J, Cheng F, Huang X,
Cheng Y and Wang K: Polysaccharide from Lentinus edodes combined
with oxaliplatin possesses the synergy and attenuation effect in
hepatocellular carcinoma. Cancer Lett. 377:117–125. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang X: The expanding role of mitochondria
in apoptosis. Genes Dev. 15:2922–2933. 2001.PubMed/NCBI
|
|
39
|
Viatour P, Bentires-Alj M, Chariot A,
Deregowski V, de Leval L, Merville MP and Bours V: NF-kappa B2/p100
induces Bcl-2 expression. Leukemia. 17:1349–1356. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chamberlain MC: Temozolomide: Therapeutic
limitations in the treatment of adult high-grade gliomas. Expert
Rev Neurother. 10:1537–1544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu K, Wang ZZ, Liu D and Qi XR:
Pharmacokinetics, brain distribution, release and blood-brain
barrier transport of Shunaoxin pills. J Ethnopharmacol.
151:1133–1140. 2014. View Article : Google Scholar : PubMed/NCBI
|