Introduction
Programmed death-ligand 1 (PD-L1) and PD-1
expression is variably regulated in immune cells and tumor cells to
maintain immunological tolerance, which controls the occurrence of
an autoimmune reaction against self-antigens (1,2).
PD-L1-expressing antigen-presenting cells, such as monocytes,
macrophages, dendritic and tumor cells regulate excess immune
reactions and inhibit activated T-cell function (3,4).
Meanwhile, PD-1, the receptor for PD-L1, is expressed on activated
T, B and NK cells in the tumor microenvironment. Anti-PD-1 blockade
therapy promotes exhaustive marker-positive T-cell expansion and
survival (5), resulting in an
antitumor response in vivo.
Since the recent success of immune checkpoint
antibodies, such as ipilimumab and nivolumab, as reported for
metastatic melanoma patients, many ongoing clinical trials have
been underway to evaluate their efficacy in various solid cancers
other than melanomas (6–8). Despite these promising results, the
response rate associated with the single antibody treatment is
~20–40% while 60–70% of cancer patients belong to the
non-responding group. Furthermore, it is still difficult to
accurately predict the responders to antibody therapy based on the
current preclinical studies (9,10).
In the present study, we used a previously reported
immune response-associated gene panel consisting of 164 genes (56
antigen-presenting cells and T-cell-associated genes, 34 cytokine-
and metabolism-associated genes, 47 TNF and TNF receptor
superfamily genes and 27 regulatory T-cell-associated genes)
(11). The present study
investigated the association of the gene panel expression with
immunological and clinical parameters, such as i) PD-L1 expression;
ii) a high mutation load [single nucleotide variant (SNV) number];
iii) a driver gene mutation; iv) CD8 expression; and v) survival
time, using the genomic data from 13 melanoma patients in the
Project High-tech Omics-based Patient Evaluation (HOPE). Since
2014, 1,685 cancer patients have been enrolled in Project HOPE in
which the simultaneous analyses of whole-exome sequencing (WES) and
gene expression profiling (GEP) have been performed (12,13).
We aimed to evaluate the immunological status in the tumor tissues
using next-generation sequencing and to better obtain a prediction
of the responders to immune checkpoint antibody treatment through
suitable biomarker detection.
Materials and methods
Patient registration
Project HOPE uses comprehensive whole-exome
sequencing and gene expression profiling of various tumor tissues
and is conducted in accordance with the ‘Ethical Guidelines for
Human Genome and Genetic Analysis Research’ in Japan. Informed
consent was obtained from all the patients participating in Project
HOPE, and the study was approved by the Institutional Review Board
of Shizuoka Cancer Center (SCC), Japan. Tumor tissues, along with
the surrounding normal tissues, were dissected from surgical
specimens by trained pathologists. A total of 1,685 cancer patients
were registered in Project HOPE from 2014 to 2015. Characteristics
of the 13 melanoma patients listed are shown in Table I.
 | Table I.Melanoma patient list registered in
Project HOPE. |
Table I.
Melanoma patient list registered in
Project HOPE.
| Case | Age | Sex | Status | Relapse-free
survival (M) | Overall survival
(M) | PD-L1b | SNV no. (exon) | Vogelstein mutation
no. | CD8b |
|---|
|
MEL-001a | 69 | F | Alive | 19 | 28 | 1 | 2712 | 12 | 2 |
| MEL-002 | 79 | F | Dead | – | – | 2 | 84 | 0 | 3 |
| MEL-003 | 41 | F | Alive | 24 | 24 | 0 | 15 | 0 | 2 |
| MEL-004 | 50 | M | Alive | 22 | 22 | 1 | 35 | 1 | 4 |
| MEL-005 | 60 | M | Alive | 22 | 22 | 0 | 31 | 0 | 1 |
| MEL-006 | 31 | F | Alive | 19 | 19 | 0 | 45 | 3 | 2 |
| MEL-007 | 88 | F | Dead | 4 | 8 | 1 | 737 | 8 | 3 |
| MEL-008 | 78 | M | Alive | 19 | 19 | 0 | 88 | 1 | 4 |
| MEL-009 | 81 | M | Alive | 17 | 17 | 0 | 30 | 0 | 1 |
| MEL-010 | 85 | M | Dead | 5 | 16 | 1 | 297 | 3 | 1 |
| MEL-011 | 58 | F | Alive | 12 | 14 | 0 | 114 | 1 | 0 |
| MEL-012 | 82 | F | Dead | 3 | 4 | 0 | 69 | 2 | 3 |
| MEL-013 | 58 | M | Alive | 10 | 10 | 0 | 39 | 0 | 2 |
Comprehensive gene expression analysis
using DNA microarray
Total RNA was extracted from ~10 mg of tissue
samples using the miRNeasy Mini kit (Qiagen, Hilden, Germany)
according to the manufacturer's instructions. The method of
performing the DNA microarray analysis was previously described
(13,14). Briefly, the ratio of the expression
intensity between the tumor tissue (T) and the surrounding normal
tissue (N) was calculated from the normalized values. The
expression values for all probes were log (base 2) transformed
before performing the statistical analysis.
Whole-exome sequencing (WES) analysis
of the melanoma tissues using next-generation sequencing
WES analysis including mapping, variant calling and
identification of somatic mutation were performed using the Ion
Proton system with the Ion AmpliSeq™ Exome kit, Torrent Suite
Software and Ion Reporter™ Server system (Thermo Fisher Scientific,
Waltham, MA USA) as previously reported (12). Briefly, all the variants called by
the variant caller were available. However, the data presented in
SCC represent those variants considered to be of good quality,
based on the filtering in which the sequences were discarded with a
quality <30, variant allele frequency <10% or depth of
coverage <20. Those mutations that were identified in tumor
samples and not observed in matched normal samples were extracted
as somatic mutations. Single-nucleotide variants (SNVs) of the
total exonic mutations for each sequenced tumor included
non-synonymous, synonymous, and indels/frameshift mutations. In the
present study we focused on somatic SNVs. Additionally, Vogelstein
driver gene mutation (15)
profiling was investigated.
Immunohistochemistry
For the immune checkpoint protein staining, the
anti-PD-L1 antibody (rabbit monoclonal, cat. 13684; 1:200 dilution)
was purchased (Cell Signaling, Danvers, MA, USA). For the
tumor-infiltrating lymphocyte (TIL) staining, anti-CD4 (mouse
monoclonal, cat. MS-1528-S; 1:20 dilution) and anti-CD8 (mouse
monoclonal, cat. MS-457-S; 1:50 dilution) antibodies (Thermo Fisher
Scientific) were purchased and were used for the
immunohistochemistry analysis. In each section stained with the
various antibodies, 10 high-magnification (×200) fields were
analyzed using WinROOF image-analyzing software (Mitani
Corporation, Tokyo, Japan). The PD-L1 staining was evaluated as the
percentage of tumor cells exhibiting positive membranous staining
as follows: score 0, <1%; score 1, 1–5%; score 2, >5-50%; and
score 3, >50% (16). The TIL
level was assessed by a semi-quantitative estimation of the density
of the CD8+ T cells inside the tumor site as follows:
score 0, no or sporadic CD8+ T cells; score 1, moderate
number of CD8+ T cells; score 2, abundant number of
CD8+ T cells; and score 3, highly abundant number of
CD8+ T cells (17). The
score that was most frequent in entire sections was assigned.
Statistical analysis
The differentially expressed genes derived from the
164 immune response-associated gene panels between the
immunological parameter-positive and parameter-negative groups were
identified using the volcano plot method. Each microarray probe was
considered significantly differentially expressed between two
groups of samples if they satisfied the following criteria: i)
corrected t-test P-value <0.05; ii) a Benjamini-Hochberg false
discovery rate (FDR) <0.1; and iii) a fold change >2.0 or
below 1/2. Correlations between the immune response-associated gene
expression and the clinicopathological features, including the
survival data, were analyzed using an unpaired two-tailed t-test or
a Spearman coefficiency test. Values of P<0.05 were considered
significant. The relapse-free survival (RFS) was calculated from
the date of the diagnosis until the date of distant relapse. The
overall survival (OS) was calculated from the date of the diagnosis
to the date of death from cancer. Follow-up was assessed from the
date of the diagnosis to the last contact date with the event-free
patients.
Results
PD-L1 and CD8 expression, Vogelstein
driver genes mutations, and SNV number in melanoma tumors
PD-L1 expression was evaluated according to the
criteria of the staining score, such that the scores of 1 and 2
were positive and a score of 0 was negative. Five cases were
positive and 8 were negative for PD-L1 expression. According to the
Vogelstein driver mutation number, the WES analysis revealed that 5
cases had ≥2 mutations, and 8 had <2 mutations. For the SNV
number, 4 cases had ≥100 SNVs and 9 had <100. The CD8 expression
level was high in 5 (scores 3 and 4) and low in 8 cases (scores
0–2) based on the IHC scoring denotations (Table I).
Association of the immune
response-associated gene expression with immunological parameters
using a volcano plot
We previously established an immune
response-associated gene panel, consisting of 164 genes (11). The association of the immune
response-associated gene expression obtained by the GEP data from
Project HOPE with PD-L1 expression, SNV number, Vogelstein driver
gene mutation number and CD8 expression was investigated using a
volcano plot analysis.
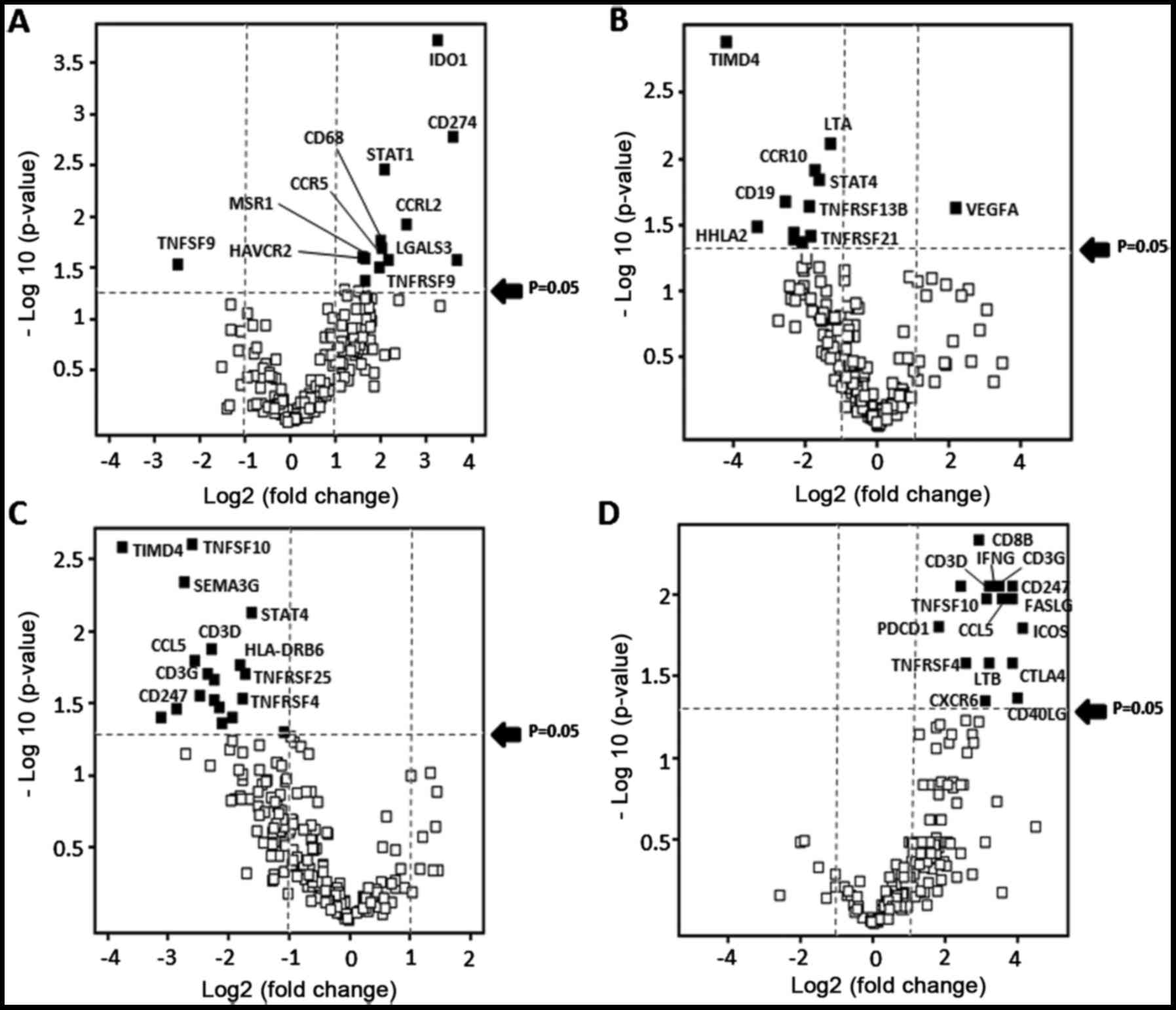
With regard to the PD-L1 expression, 12 immune
response-associated genes were identified as upregulated genes in
the PD-L1-positive melanomas, in which 6 genes were involved in
T-cell suppression and 6 were related to T-cell activation
(Fig. 1A and Table II). In addition, the VEGF gene
alone was identified as an upregulated gene in high SNV number with
>100 melanomas (Fig. 1B).
Regarding the Vogelstein driver mutation number, in contrast, 18
immune response-associated genes were downregulated in the
Vogelstein mutation high-number group. Notably, 9 genes involved in
T-cell activation, such as CD3 (D, G and Z), CD40LG, STAT4, CCL5,
TNFRSF4, TNFSF8 and TNFSF14, were identified (Fig. 1C and Table III). Notably, 14 immune
response-associated genes were identified as upregulated genes in
the TIL marker CD8-high melanomas, which were mostly correlated to
T-cell activation favoring a Th1 response leading to tumor killing
by CTLs (Fig. 1D).
 | Table II.Upregulated gene list in
PD-L1-positive melanomas. |
Table II.
Upregulated gene list in
PD-L1-positive melanomas.
| Probe name | Gene symbol | FC | Log FC | Regulation | P-value |
|---|
| A_23_P412321 | CCR5 | 3.948 | 1.981 | Up |
1.99×10−2 |
| A_23_P69310 | CCRL2 | 5.753 | 2.524 | Up |
1.16×10−2 |
| A_23_P338479 | CD274 | 11.825 | 3.564 | Up |
1.60×10−3 |
| A_23_P15394 | CD68 | 3.930 | 1.975 | Up |
1.68×10−2 |
| A_24_P411561 | HAVCR2 | 2.953 | 1.562 | Up |
2.43×10−2 |
| A_32_P351968 | HLA-DMB | 3.057 | 1.612 | Up |
4.15×10−2 |
| A_23_P112026 | IDO1 | 9.284 | 3.215 | Up |
1.80×10−4 |
| A_23_P128919 | LGALS3 | 4.402 | 2.138 | Up |
2.58×10−2 |
| A_24_P372223 | MSR1 | 3.088 | 1.627 | Up |
2.53×10−2 |
| A_24_P274270 | STAT1 | 4.126 | 2.045 | Up |
3.31×10−3 |
| A_23_P49338 | TNFRSF12A | 12.512 | 3.645 | Up |
2.56×10−2 |
| A_23_P51936 | TNFRSF9 | 3.808 | 1.929 | Up |
3.06×10−2 |
| A_33_P3397763 | TNFSF9 | −5.673 | −2.504 | Down |
2.84×10−2 |
 | Table III.Downregulated gene list in driver
mutation high melanomas. |
Table III.
Downregulated gene list in driver
mutation high melanomas.
| Probe name | Gene symbol | FC | Regulation | P-value |
|---|
| A_24_P63380 | BMPR1B | −4.37088 | Down |
4.24×10−2 |
| A_33_P3358923 | BTLA | −4.76685 | Down |
2.12×10−2 |
| A_23_P152838 | CCL5 | −5.93301 | Down |
1.55×10−2 |
| A_23_P34676 | CD247
(CD3ζ) | −5.64369 | Down |
2.71×10−2 |
| A_33_P3375541 | CD3D | −4.90473 | Down |
1.29×10−2 |
| A_23_P98410 | CD3G | −5.11053 | Down |
1.91×10−2 |
| A_33_P3250680 | CD40LG | −7.29745 | Down |
3.38×10−2 |
| A_33_P3218980 | ENTPD1 | −3.87063 | Down |
3.87×10−2 |
| A_24_P169013 | HLA-DRB6 | −3.53737 | Down |
1.66×10−2 |
| A_33_P3248265 | LTB | −4.80739 | Down |
2.93×10−2 |
| A_23_P6818 | SEMA3G | −6.73986 | Down |
4.43×10−3 |
| A_23_P68031 | STAT4 | −3.11526 | Down |
7.24×10−3 |
| A_23_P7503 | TIMD4 | −13.6631 | Down |
2.52×10−3 |
| A_33_P3234530 | TNFRSF25 | −3.34808 | Down |
1.91×10−2 |
| A_33_P3286157 | TNFRSF4 | −3.47391 | Down |
2.84×10−2 |
| A_23_P121253 | TNFSF10 | −4.52915 | Down |
3.28×10−2 |
| A_21_P0000113 | TNFSF10 | −6.11132 | Down |
2.40×10−3 |
| A_24_P237036 | TNFSF14 | −8.744 | Down |
3.86×10−2 |
| A_23_P169257 | TNFSF8 | −2.1605 | Down |
4.88×10−2 |
Correlation of the immune
response-associated gene expression with the survival time of the
melanoma patients
The correlation of the expression of 164 immune
response-associated genes with the overall and relapse-free
survival time was investigated using a Spearman's rank-order
correlation. Fourteen genes and 17 genes were significantly
correlated with the overall and relapse-free survival time,
respectively (Table IV). Eight
genes, including CD27, CXCR6, IL17RB, PDCD1, TNFRSF11A, ADAM12,
EDA2R and TREM1, were commonly identified in both the overall and
relapse-free survival time groups, and 5 were positively correlated
and 3 were negatively correlated.
 | Table IV.Correlation of immune
response-associated genes with survival time. |
Table IV.
Correlation of immune
response-associated genes with survival time.
| Overall
survival |
|---|
|
|---|
| Gene name | r-value | P-value |
|---|
| CCR6 | 0.6829 | 0.0295 |
| CD27 | 0.7561 | 0.0114 |
| CDH3 | 0.7744 | 0.0085 |
| CXCR6 | 0.7561 | 0.0114 |
| IL17RB | 0.6646 | 0.0036 |
| PDCD1 | 0.6525 | 0.0409 |
|
TNFRSF11A | 0.9147 | 0.0002 |
| ADAM12 | −0.8721 | 0.0011 |
| EDA2R | −0.8598 | 0.0014 |
| GREB1 | −0.7073 | 0.0221 |
| IL6 | −0.6829 | 0.0295 |
| STAT5A | −0.7622 | 0.0014 |
| TDO2 | −0.6342 | 0.0489 |
| TREM1 | −0.7012 | 0.0239 |
|
| Relapse-free
survival |
|
| Gene
name | r-value | P-value |
|
| BTLA | 0.7078 | 0.0221 |
| B7H5 | 0.6585 | 0.0384 |
| B7H7 | 0.7632 | 0.0102 |
| CD27 | 0.8493 | 0.0019 |
| CD3E | 0.6893 | 0.0274 |
| CD8B | 0.7816 | 0.0076 |
| CXCR6 | 0.8555 | 0.0016 |
| FASLG | 0.6401 | 0.0462 |
| IL17RB | 0.7571 | 0.0112 |
| LAG3 | 0.7447 | 0.0135 |
| PDCD1 | 0.7936 | 0.0061 |
| TIMD4 | 0.7509 | 0.0123 |
|
TNFRSF11A | 0.7755 | 0.0084 |
| TNFRSF21 | 0.6647 | 0.0362 |
| ADAM12 | −0.7385 | 0.0147 |
| EDA2R | −0.7016 | 0.0237 |
| TREM1 | −0.6524 | 0.0409 |
Discussion
In the present study, we used a previously reported
immune response-associated gene panel that consisted of 164 genes
(11), and investigated the
association of the expression of the gene panel with immunological
and clinical parameters, such as: i) PD-L1 expression; ii) a high
mutation load [single nucleotide variant (SNV) number]; iii) driver
gene mutation; iv) CD8 expression; and v) survival time, using the
genomic data from 13 melanoma patients registered in Project
HOPE.
With advances in cancer genomic sequencing, specific
gene signatures involved in the therapeutic response and prognosis
have been reported, and their accuracy and efficiency have been
investigated in various types of cancer, such as breast, stomach,
non-small cell lung cancers and melanomas (18,19).
However, few studies focusing on immune-related gene panels or
signature identifications have been reported since the development
of cancer genomic technologies such as next-generation sequencing.
The identification of cancer-specific T-cell receptor (TCR)
sequences has been attempted in immunological routine analyses
(20), but not much success has
been obtained. Small scale genetic studies focusing on renal cell
cancer or polypoid precancerous colorectal lesions revealed that
tumor-associated macrophage markers or TIL markers were involved in
the prognosis or the progression of precancerous to cancerous
lesions (21,22). However, Lee et al (23) obtained biopsy tissues from 55
triple-negative breast cancer patients treated with combined
chemotherapy, and evaluated immune responses using the NanoString
nCounter GX human immunology panel (579 immune-related genes),
which demonstrated that a higher expression of cytotoxic molecules,
TCR signaling pathway molecules, Th1 cytokines and B cell markers
were associated with a pathological complete response (CR).
First, the association of the immune
response-associated gene panel expression with the expression level
of PD-L1 was investigated in the present study. Twelve immune
response-associated genes were identified as upregulated in the
PD-L1-positive melanomas; 6 of these genes were involved in T-cell
suppression and 6 were related to T-cell activation. In particular,
the 6 T-cell stimulation-related genes were: CCRL2 (attraction of
TILs) (24); CD68 (M1 macrophage
activation); CCR5 (T-cell migration); HLA-DMB (increase of
CD8+ TIL and IFN-γ level, and improvement of survival)
(25); STAT1 (IFN-γ signal
activation in T cells) (26) and
TNFRSF9 (T-cell activation) (27).
However, the others were T-cell inhibition-related genes,
including; CD274 (PD-L1), IDO-1, HAVCR2 (TIM-3), LGALS3
(galectin-3), MSR1 and TNFRSF12A. Taube et al (28) reported similar results using a
volcano plot of 11 melanoma patients, which demonstrated 12
upregulated genes in PD-L1-positive melanomas including 4
immuno-regulatory genes, such as CD274, PDCD1 (PD-1), LAG3 and
IL-10. The upregulated gene profile in the PD-L1-positive melanomas
in our study was similar to their analysis.
Second, the association of the immune
response-associated gene panel expression with Vogelstein driver
mutation number was investigated. Eighteen immune
response-associated genes were downregulated in the Vogelstein
mutation high-number (>2) group. Notably, 9 genes involved in
T-cell activation, including CD3 (D, G and Z), CD40LG, STAT4, CCL5,
TNFRSF4, TNFSF8 and TNFSF14, were identified. Among these, TNFRSF4
(OX40), TNFSF8 (CD30-L) and TNFSE14 (HVEM-L) are TNF ligand
superfamily members and trigger T-cell stimulating signals by
binding to their specific receptors. A constitutive signal
activation, such as MAPK, STAT3, NF-κB, and β-catenin, in cancer
cells induces an immunosuppressive effect that is mediated by the
TGF-β, IL-6, IL-10 and VEGF produced by the cancer cells, resulting
in regulatory T-cell and myeloid derived suppressor cell (MDSC)
induction (29). Specifically, an
STK11 mutation and RAS/MAPK activation were linked to CD3 gene
downregulation or a TIL reduction in the tumor (30,31)
Additionally, Frederick et al (32) demonstrated that a BRAF inhibition
was associated with an upregulation of melanoma antigen expression
and a favorable tumor microenvironment through the reduction of
immunosuppressive cytokines, such as IL-6 and IL-8. In the present
study, an extensive immunosuppressive effect on the T-cell
activation signal was ascertained, but the upregulation of melanoma
antigens was not significant because of the small number of cases
in the evaluation.
Third, the correlation of the expression of 164
immune response-associated genes with the overall and relapse-free
survival time was investigated using a Spearman's rank-order
correlation. Eventually, 8 genes, such as CD27, CXCR6, IL17RB,
PDCD1, TNFRSF11A, ADAM12, EDA2R and TREM1, were commonly identified
in both the overall and relapse-free survival time groups, of which
5 were positively correlated and 3 were negatively correlated.
Briefly, the survival-correlated gene profiling was as follows:
CD27 (expressed on CD8+ TIL was associated with a good
prognosis) (33); CXCR6 (the
CXCR6/CXCL16 axis in the tumor was associated with a TIL increase
and a good prognosis) (34); IL17RB
(a higher HOXB13-to-IL17RB ratio was linked to a worse outcome)
(35); TNFRSF11A (RANK upregulation
may be linked to mammary tumorigenesis in BRCA1-mutant carriers)
(36); ADAM12 [an aggressive
ovarian cancer marker was associated with a TGF-β-induced
epithelial to mesenchymal transition (EMT)] (37); EDA2R (highly expressed in ovarian
cancer and was associated with a poor prognosis); and TREM1
(induced a proinflammatory and protumor microenvironment and was
associated with a poor prognosis) (38). Based on these observations, the
protein expression of the 8 markers, using previously resected
melanoma tissues, warrants future investigation, and the specific
association of the protein expression with the survival data based
on a Kaplan-Meier analysis should be precisely performed.
Finally, in the present study, we investigated the
association of the expression of the immune response-associated
gene panel with various parameters, mainly PD-L1 and CD8
expression, driver gene mutation and survival time, and several
gene signatures involved in patient prognosis were identified.
These results revealed that cancer genomic data may be associated
with specific immunological gene signatures closely linked to the
immunological status in the tumor microenvironment, which could
contribute to the development of specific cancer immunotherapies
for tailored medicine called precision immunotherapy (39).
Acknowledgements
The authors thank the staff at the Shizuoka Cancer
Center Hospital for the clinical support and sample
preparation.
Glossary
Abbreviations
Abbreviations:
|
WES
|
whole-exome sequencing
|
|
GEP
|
gene expression profiling
|
|
PD-1
|
programmed death-1
|
|
PD-L1
|
programmed death-ligand 1
|
|
NGS
|
next-generation sequencing
|
|
SNV
|
single nucleotide variant
|
|
TIL
|
tumor-infiltrating lymphocyte
|
|
OS
|
overall survival
|
|
RFS
|
relapse-free survival
|
References
|
1
|
Zhang B, Chikuma S, Hori S, Fagarasan S
and Honjo T: Nonoverlapping roles of PD-1 and FoxP3 in maintaining
immune tolerance in a novel autoimmune pancreatitis mouse model.
Proc Natl Acad Sci USA. 113:pp. 8490–8495. 2016; View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang J, Okazaki IM, Yoshida T, Chikuma S,
Kato Y, Nakaki F, Hiai H, Honjo T and Okazaki T: PD-1 deficiency
results in the development of fatal myocarditis in MRL mice. Int
Immunol. 22:443–452. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heeren AM, Koster BD, Samuels S, Ferns DM,
Chondronasiou D, Kenter GG, Jordanova ES and de Gruijl TD: High and
interrelated rates of PD-L1+CD14+
antigen-presenting cells and regulatory T cells mark the
microenvironment of metastatic lymph nodes from patients with
cervical cancer. Cancer Immunol Res. 3:48–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yaguchi T and Kawakami Y: Cancer-induced
heterogeneous immunosuppressive tumor microenvironments and their
personalized modulation. Int Immunol. 28:393–399. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fourcade J, Sun Z, Pagliano O, Chauvin JM,
Sander C, Janjic B, Tarhini AA, Tawbi HA, Kirkwood JM, Moschos S,
et al: PD-1 and Tim-3 regulate the expansion of tumor
antigen-specific CD8+ T cells induced by melanoma
vaccines. Cancer Res. 74:1045–1055. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Topalian SL, Hodi FS, Brahmer JR,
Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD,
Sosman JA, Atkins MB, et al: Safety, activity, and immune
correlates of anti-PD-1 antibody in cancer. N Engl J Med.
366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weber JS, O'Day S, Urba W, Powderly J,
Nichol G, Yellin M, Snively J and Hersh E: Phase I/II study of
ipilimumab for patients with metastatic melanoma. J Clin Oncol.
26:5950–5956. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wolchok JD, Kluger H, Callahan MK, Postow
MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K,
et al: Nivolumab plus ipilimumab in advanced melanoma. N Engl J
Med. 369:122–133. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Okazaki T, Chikuma S, Iwai Y, Fagarasan S
and Honjo T: A rheostat for immune responses: The unique properties
of PD-1 and their advantages for clinical application. Nat Immunol.
14:1212–1218. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Weber JS, D'Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Akiyama Y, Kondou R, Iizuka A, Ohshima K,
Urakami K, Nagashima T, Shimoda Y, Tanabe T, Ohnami S, Ohnami S, et
al: Immune response-associated gene analysis of 1,000 cancer
patients using whole-exome sequencing and gene expression
profiling-Project HOPE. Biomed Res. 37:233–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urakami K, Shimoda Y, Ohshima K, Nagashima
T, Serizawa M, Tanabe T, Saito J, Usui T, Watanabe Y, Naruoka A, et
al: Next generation sequencing approach for detecting 491 fusion
genes from human cancer. Biomed Res. 37:51–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamaguchi K, Urakami K, Ohshima K,
Mochizuki T, Akiyama Y, Uesaka K, Nakajima T, Takahashi M, Tamai S
and Kusuhara M: Implementation of individualized medicine for
cancer patients by multiomics-based analyses-the Project HOPE-.
Biomed Res. 35:407–412. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ohshima K, Hatakeyama K, Nagashima T,
Watanabe Y, Kanto K, Doi Y, Ide T, Shimoda Y, Tanabe T, Ohnami S,
et al: Integrated analysis of gene expression and copy number
identified potential cancer driver genes with
amplification-dependent overexpression in 1,454 solid tumors. Sci
Rep. 7:6412017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vogelstein B, Papadopoulos N, Velculescu
VE, Zhou S, Diaz LA Jr and Kinzler KW: Cancer genome landscapes.
Science. 339:1546–1558. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: KEYNOTE-001 Investigators: Pembrolizumab for the treatment
of non-small-cell lung cancer. N Engl J Med. 372:2018–2028. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dahlin AM, Henriksson ML, Van Guelpen B,
Stenling R, Oberg A, Rutegård J and Palmqvist R: Colorectal cancer
prognosis depends on T-cell infiltration and molecular
characteristics of the tumor. Mod Pathol. 24:671–682. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hallett RM, Dvorkin-Gheva A, Bane A and
Hassell JA: A gene signature for predicting outcome in patients
with basal-like breast cancer. Sci Rep. 2:2272012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cristescu R, Lee J, Nebozhyn M, Kim KM,
Ting JC, Wong SS, Liu J, Yue YG, Wang J, Yu K, et al: Molecular
analysis of gastric cancer identifies subtypes associated with
distinct clinical outcomes. Nat Med. 21:449–456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Munson DJ, Egelston CA, Chiotti KE, Parra
ZE, Bruno TC, Moore BL, Nakano TA, Simons DL, Jimenez G, Yim JH, et
al: Identification of shared TCR sequences from T cells in human
breast cancer using emulsion RT-PCR. Proc Natl Acad Sci USA.
113:pp. 8272–8277. 2016; View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mickley A, Kovaleva O, Kzhyshkowska J and
Gratchev A: Molecular and immunologic markers of kidney
cancer-potential applications in predictive, preventive and
personalized medicine. EPMA J. 6:202015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maglietta A, Maglietta R, Staiano T,
Bertoni R, Ancona N, Marra G and Resta L: The immune landscapes of
polypoid and nonpolypoid precancerous colorectal lesions. PLoS One.
11:e01593732016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee HJ, Lee JJ, Song IH, Park IA, Kang J,
Yu JH, Ahn JH and Gong G: Prognostic and predictive value of
NanoString-based immune-related gene signatures in a neoadjuvant
setting of triple-negative breast cancer: Relationship to
tumor-infiltrating lymphocytes. Breast Cancer Res Treat.
151:619–627. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang LP, Cao J, Zhang J, Wang BY, Hu XC,
Shao ZM, Wang ZH and Ou ZL: The human chemokine receptor CCRL2
suppresses chemotaxis and invasion by blocking CCL2-induced
phosphorylation of p38 MAPK in human breast cancer cells. Med
Oncol. 32:2542015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Callahan MJ, Nagymanyoki Z, Bonome T,
Johnson ME, Litkouhi B, Sullivan EH, Hirsch MS, Matulonis UA, Liu
J, Birrer MJ, et al: Increased HLA-DMB expression in the tumor
epithelium is associated with increased CTL infiltration and
improved prognosis in advanced-stage serous ovarian cancer. Clin
Cancer Res. 14:7667–7673. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Avalle L, Pensa S, Regis G, Novelli F and
Poli V: STAT1 and STAT3 in tumorigenesis: A matter of balance.
JAK-STAT. 1:65–72. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nam KO, Kang WJ, Kwon BS, Kim SJ and Lee
HW: The therapeutic potential of 4–1BB (CD137) in cancer. Curr
Cancer Drug Targets. 5:357–363. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Taube JM, Young GD, McMiller TL, Chen S,
Salas JT, Pritchard TS, Xu H, Meeker AK, Fan J, Cheadle C, et al:
Differential expression of immune-regulatory genes associated with
PD-L1 display in melanoma: Implications for PD-1 pathway blockade.
Clin Cancer Res. 21:3969–3976. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawakami Y, Yaguchi T, Sumimoto H,
Kudo-Saito C, Tsukamoto N, Iwata-Kajihara T, Nakamura S, Nishio H,
Satomi R, Kobayashi A, et al: Roles of signaling pathways in cancer
cells and immune cells in generation of immunosuppressive
tumor-associated microenvironmentsThe Tumor Immunoenvironment.
Shurin MR, Umansky V and Malyguine A: Springer Science+Buisiness
Media B.V.; Dordrecht, The Netherlands: pp. 307–323. 2013,
View Article : Google Scholar
|
|
30
|
Schabath MB, Welsh EA, Fulp WJ, Chen L,
Teer JK, Thompson ZJ, Engel BE, Xie M, Berglund AE, Creelan BC, et
al: Differential association of STK11 and TP53 with KRAS
mutation-associated gene expression, proliferation and immune
surveillance in lung adenocarcinoma. Oncogene. 35:3209–3216. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Loi S, Dushyanthen S, Beavis PA, Salgado
R, Denkert C, Savas P, Combs S, Rimm DL, Giltnane JM, Estrada MV,
et al: RAS/MAPK activation is associated with reduced
tumor-infiltrating lymphocytes in triple-negative breast cancer:
Therapeutic cooperation between MEK and PD-1/PD-L1 immune
checkpoint inhibitors. Clin Cancer Res. 22:1499–1509. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frederick DT, Piris A, Cogdill AP, Cooper
ZA, Lezcano C, Ferrone CR, Mitra D, Boni A, Newton LP, Liu C, et
al: BRAF inhibition is associated with enhanced melanoma antigen
expression and a move favorable tumor microenvironment in patients
with metastatic melanoma. Clin Cancer Res. 19:1225–1231. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wouters MC, Komdeur FL, Workel HH, Klip
HG, Plat A, Kooi NM, Wisman GB, Mourits MJ, Arts HJ, Oonk MH, et
al: Treatment regimen, surgical outcome, and T-cell differentiation
influence prognostic benefit of tumor-infiltrating lymphocytes in
high-grade serous ovarian cancer. Clin Cancer Res. 22:714–724.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hojo S, Koizumi K, Tsuneyama K, Arita Y,
Cui Z, Shinohara K, Minami T, Hashimoto I, Nakayama T, Sakurai H,
et al: High-level expression of chemokine CXCL16 by tumor cells
correlates with a good prognosis and increased tumor-infiltrating
lymphocytes in colorectal cancer. Cancer Res. 67:4725–4731. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhao L, Zhu S, Gao Y and Wang Y: Two-gene
expression ratio as predictor for breast cancer treated with
tamoxifen: Evidence from meta-analysis. Tumour Biol. 35:3113–3117.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nolan E, Vaillant F, Branstetter D, Pal B,
Giner G, Whitehead L, Lok SW, Mann GB, Rohrbach K, Huang LY, et al
Kathleen Cuningham Foundation Consortium for Research into Familial
Breast Cancer (kConFab), : RANK ligand as a potential target for
breast cancer prevention in BRCA1-mutation carriers. Nat Med.
22:933–939. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cheon DJ, Li AJ, Beach JA, Walts AE, Tran
H, Lester J, Karlan BY and Orsulic S: ADAM12 is a prognostic factor
associated with an aggressive molecular subtype of high-grade
serous ovarian carcinoma. Carcinogenesis. 36:739–747. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Duan M, Wang ZC, Wang XY, Shi JY, Yang LX,
Ding ZB, Gao Q, Zhou J and Fan J: TREM-1, an inflammatory
modulator, is expressed in hepatocellular carcinoma cells and
significantly promotes tumor progression. Ann Surg Oncol.
22:3121–3129. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mandal R and Chan TA: Personalized
oncology meets immunology: The path toward precision immunotherapy.
Cancer Discov. 6:703–713. 2016. View Article : Google Scholar : PubMed/NCBI
|