Introduction
Osteosarcoma is the most common primary bone
malignancy in children and young adults, and accounts for
approximately 60% of all malignant bone tumors diagnosed in the
first two decades of life (1).
Currently, pulmonary metastasis is the most common cause of
osteosarcoma-related death (2).
Despite advanced strategies such as surgery, adjuvant chemotherapy,
and radiotherapy, the prognosis of osteosarcoma still remains poor,
and the survival of osteosarcoma patients has reached a plateau
(3–5). Genetic changes as well as dysfunction
of oncogenes or tumor suppressors have been demonstrated to be
tightly associated with the development and progression of
osteosarcoma (6,7). Hence, identification of new molecules
involved in tumor progression is of crucial importance to reduce
the morbidity and mortality of this devastating disease.
Hypoxia-inducible factor-1α (HIF-1α) is a
transcription factor normally regulated by the oxygen concentration
but is often overexpressed in solid tumors such as cancers of the
colon, breast, pancreas, kidney, prostate and bladder (8,9). Many
tumor promoter genes are transactivated by HIF-1α, however, its
interaction with other clusters of genes are not well known, such
as long non-coding RNAs (lncRNAs). lncRNAs are most commonly
defined as RNA transcripts of more than 200 nucleotides (nt) and
located in nuclear or cytosolic fractions with no protein-coding
capacity (10). Recent research
suggests that lncRNAs can regulate gene expression at the
transcriptional or post-transcriptional level, and may facilitate
the diagnosis and prognosis of human cancers (11–13). A
previous study indicated that many lncRNAs participate in
osteosarcoma progression, including MALAT1, H19, TUG1, HIF3PUT and
LOC285194 (14). However, only a
few lncRNAs have been functionally identified and validated to be
potential regulators of osteosarcoma, and more research is needed
to clarify the role of other lncRNAs.
Urothelial cancer associated 1 (UCA1) gene is a
lncRNA located at 19p13.12, which was initially discovered and
investigated in bladder cancer, which has oncogenic roles in tumor
proliferation and metastasis (15,16).
Subsequently, it was found to be upregulated in other cancers, such
as prostate and breast cancer, and has a potential oncogenic role
(17,18). It is reported that some
transcription factors such as Ets-2, TGF-β1 and C/EBPα-p30 protein
bind the core promoter of UCA1 to enhance its expression (19). Previously, Li et al
demonstrated that UCA1 promotes osteosarcoma progression and
correlates with poor prognosis (20). However, the specific role and
underlying mechanism of UCA1 in regards to proliferation and
apoptosis in osteosarcoma remain unknown.
In the present study, we aimed to determine the
expression level of UCA1 in osteosarcoma samples and cell lines. In
addition, we further investigated the effect of UCA1 on
osteosarcoma cell proliferation and apoptosis, and the underlying
regulatory mechanism. The aim of the present study was to clarify
i) the expression and role of UCA1 in osteosarcoma; ii) the
mechanism underlying the UCA1 overexpression in osteosarcoma cells;
and iii) the potential downstream target and pathway of UCA1
involved in proliferation and apoptosis in osteosarcoma.
Materials and methods
Cell culture
Human osteosarcoma cell lines MG-63, SAOS-2, U-2OS,
HOS, SW1353 and one osteoblastic cell line (hFOB1.19) were obtained
from the Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). All osteosarcoma cell lines were maintained in
Dulbecco's modified Eagle's medium (DMEM) (Invitrogen, Carlsbad,
CA, USA) containing 10% fetal bovine serum (FBS) (Sigma-Aldrich,
St. Louis, MO, USA), 100 U/ml penicillin and 100 g/ml streptomycin
(Life Technologies, Grand Island, NY, USA) at 37°C in 5%
CO2 and 95% air. Osteoblastic hFOB cells were grown in
DMEM/F12 1:1 medium with 10% FBS, 2.5 mM L-glutamine and 0.3 mg/ml
G418 at 37°C in 5% CO2 and 95% air. The cell lines
passed the DNA profiling test [short tandem repeat (STR)].
RNA oligoribonucleotides and cell
transfection
RNA interference was conducted using synthetic small
interfering RNA (siRNA) oligo (RiboBio Co., Guangzhou, China). Two
synthetic siRNA oligos against UCA1 and a negative control sequence
are as follows: si-UCA1-1: (sense)
5′-TGGTAATGTATCATCGGCTTAGTTCAAGAGACTAAGCCGATGATACATTACCTTTTTTC-3′,
(antisense)
5′-TCGAGAAAAAAGGTAATGTATCATCGGCTTAGTCTCTTGAACTAAGCCGATGATACATTACCA-3′;
si-UCA1-2: (sense)
5′-GATCCGGCTAATATGCCTGATTACTTTCAAGAGAAGTAATCAGGCATATTAGCTTTTTTGGAAA-3′,
(antisense)
5′-AGCTTTTCCAAAAAAGCTAATATGCCTGATTACTTCTCTTGAAAGTAATCAGGCATATTAGCCG-3′;
siRNA-NC: (sense)
5′-TTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTTC-3′,
(antisense)
5′-TCGAGAAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAAA-3′.
UCA1 complementary DNA (p-UCA1) fragment, HIF-1α expressing vector
(p-HIF-1α), PTEN expressing vector (p-PTEN) and control vector were
purchased from RiboBio. Osteosarcoma cells were plated in 24-well
plates at 1×105/well. Forty-eight hours after plating,
100 nM of RNA oligoribonucleotides were transfected into the cells
with Lipofectamine 2000 (Invitrogen) according to the
manufacturer's instructions.
RNA extraction, reverse transcription
and RT-qPCR
Total RNA was isolated from primary osteosarcoma
cell lines using TRIzol reagent (Invitrogen). Τhen, the cDNA was
synthesized from 200 ng extracted total RNA using the PrimeScript
RT reagent kit (Takara Bio Company, Shiga, Japan) and amplified by
RT-qPCR with an SYBR-Green kit (Takara Bio Co., Ltd., Dalian,
China) on an ABI PRISM 7500 Sequence Detection System (Applied
Biosystems, Foster City, CA, USA) with the housekeeping gene GAPDH
as an internal control. The 2−ΔΔCt method was used to
determine the relative quantification of gene expression levels.
All the premier sequences were synthesized by RiboBio, and the
premier sequences were as follows: UCA1 (forward)
5′-CTCTCCTATCTCCCTTCACTGA-3′, (reverse) 5′-CTTTGGGTTGAGGTTCCTGT-3′;
HIF-1α (forward) 5′-TCTAGACTCGAGTACAAGGCAGCAGAAAC-3′, (reverse)
5′-TCTAGAGTTTGTGCAGTATTGTAGCC-3′; GAPDH (forward)
5′-AGTGGCAAAGTGGAGATT-3′, (reverse) 5′-GTGGAGTCATACTGGAACA-3′. Each
experiment was performed in triplicate.
Cell proliferation assay
Cell growth was quantified using the Cell Counting
Kit-8 (CCK-8; Beyotime Corporation, Shanghai, China). Briefly, 100
µl of cells from the different transfection groups were seeded onto
a 96-well plate at a concentration of 2,000 cells/well and were
incubated at 37°C. At different time points, the optical density
was measured at 450 nm using a microtiter plate reader, and the
rate of cell survival was expressed as the absorbance. The results
represent the mean of three replicates under the same
conditions.
Cell cycle assay
Cells were washed in PBS and fixed in 70% ethanol at
4°C for 2 h. DNA staining was carried out with 10 mg propidium
iodide/ml PBS and 2.5 µg DNase-free RNase/ml PBS for at least 30
min before flow cytometry in a Coulter EPICS XL flow cytometer
(Beckman Coulter, Inc., Fullerton, CA). Cell cycle profiles were
generated from flow cytometry analysis with Modifit software (BD
Biosciences, San Jose, CA, USA).
Dual-luciferase reporter assay
Using MG-63 genomic DNA, the identified UCA1
promoter DNA region was amplified, and the PCR products were cloned
into the pGEM-T Easy vector system (Promega, Madison, WI, USA).
Then, the UCA1 promoter DNA region was incorporated into the pGL4
luciferase expression vector (Promega). Luciferase activity was
assessed using the Dual-Luciferase Reporter Assay System (Promega)
48 h after transfection, and the ratio of Firefly/Renilla
luciferase activity was determined.
Chromatin immunoprecipitation
(ChIP)
ChIP was performed using the EZ ChIP™
Chromatin Immunoprecipitation kit (Millipore, Bedford, MA, USA),
according to the manufacturer's protocol. Briefly, cross-linked
chromatin was sonicated into 200–1,000 bp fragments. The chromatin
located on the promoter of lncRNA UCA1 was immunoprecipitated using
anti-HIF-1α antibodies (#3434T, 1:1,000; Cell Signaling Technology,
Beverly, MA, USA). An isotype-matched IgG was used as a negative
control, and the total RNA that immunoprecipitated by the HIF-1α
antibody served as a positive control. RT-qPCR was conducted to
detect the relative enrichment of the lncRNA UCA1 promoter.
Western blotting and antibodies
The primary antibodies were: rabbit anti-human
HIF-1α antibody (#3434T, 1:1,000), PTEN antibody (#9559T, 1:1,000),
rabbit anti-human phospho-AKT antibody (#9271T, 1:1,000), and
rabbit anti-human β-actin antibody (#4970T, 1:1,000) (all from Cell
Signaling Technology). Horseradish peroxidase-conjugated (HRP)
anti-rabbit antibodies (1:5,000; Santa Cruz Biotechnology, Santa
Cruz, CA, USA) were used as the secondary antibodies. The AKT
inhibitor wortmannin was purchased from Sigma-Aldrich. The
concentration used was 50 µM and the cells were treated for 12 h
before further experiments. Cell lysates in 1X SDS loading buffer
(60 mM Tris-HCl, pH 6.8; 2% SDS; 20% glycerol; 0.25% bromophenol
blue; and 1.25% 2-mercaptoethanol) were incubated at 100°C for 10
min to facilitate sample loading for conventional western blot
analysis. The relative protein levels were quantified using
densitometry with a Gel-Pro Analyzer (Media Cybernetics, Rockville,
MD, USA).
Signal transduction reporter
array
Cignal Signal Transduction Reporter Array (Qiagen,
Valencia, CA, USA) was used to simultaneously investigate
alterations in the activities of 50 canonical signalling pathways
in response to UCA1 knockdown. Cells were transfected with
antisense oligonucleotides-targeting UCA1 for 24 h and were
subsequently transfected with a mixture of a transcription
factor-responsive firefly luciferase reporter and a constitutively
expressing Renilla construct. The relative activity of each
pathway was determined by luciferase/Renilla and normalized
to the untreated controls. Experiments were performed in
triplicates.
Statistical analysis
Kolmogorov-Smirnov test was used to determine the
normality of the distribution of data in each group. Data are
presented as median (interquartile range). Differences in cell
growth curves and cell cytotoxicity curves were determined by
repeated measures analysis of variance. The differences in lncRNA
or mRNA expression level between different groups were analyzed by
the Mann-Whitney U-test or Kruskal-Wallis test. Count data were
described as frequency and examined using Fisher's exact test. All
differences were regarded as statistically significant at
P<0.05. Statistical analyses were performed with GraphPad Prism
5.01 (GraphPad Software, La Jolla, CA, USA).
Results
lncRNA UCA1 is upregulated in
osteosarcoma cells and promotes cell growth
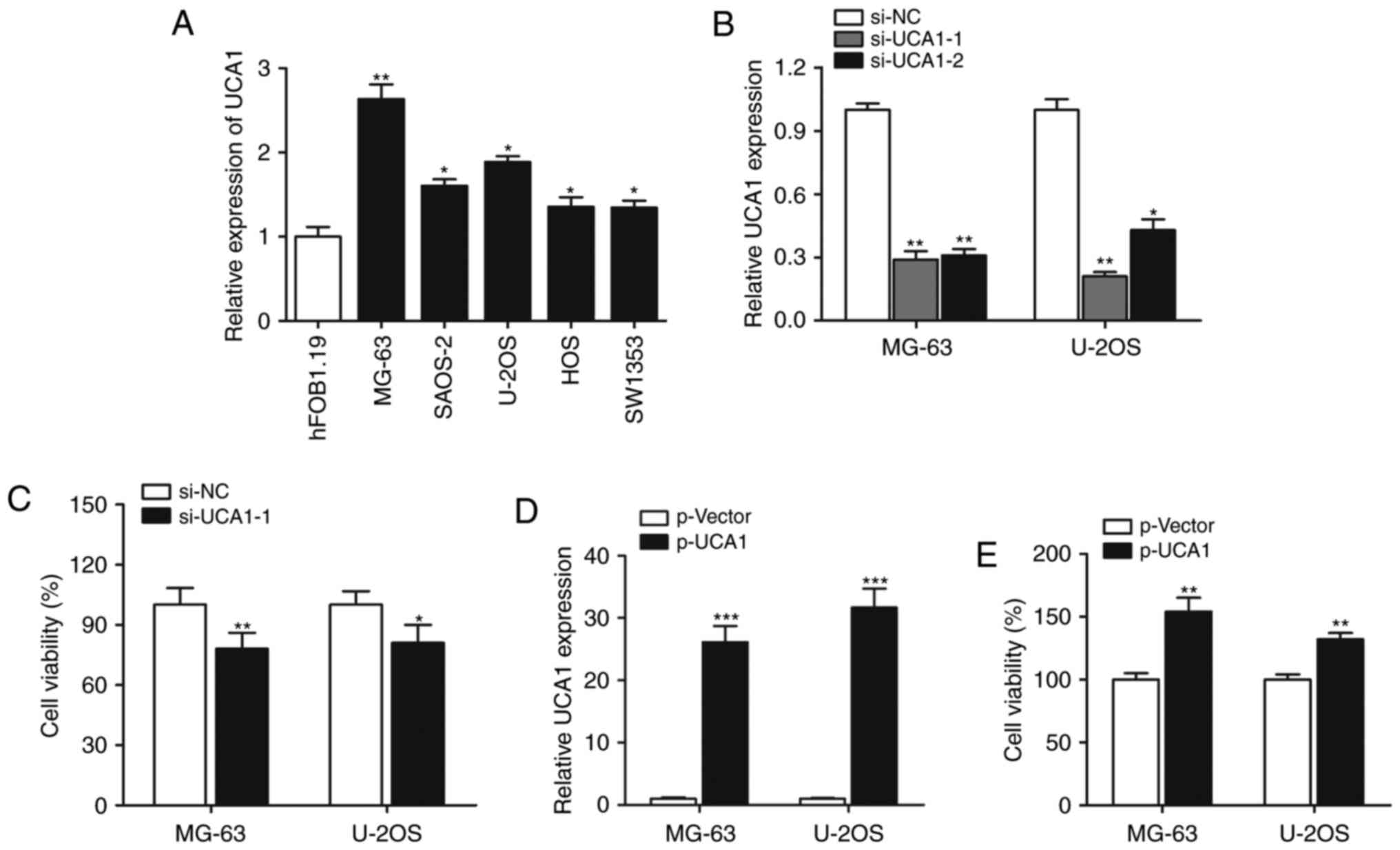
We firstly performed RT-qPCR to determine the
expression of lncRNA UCA1, and the results showed that expression
of lncRNA UCA1 was significantly increased in all the five
osteosarcoma cell lines when compared with the expression level in
the normal hFOB1.19 cells (Fig.
1A). The MG-63 and U-2OS cell lines were selected for
subsequent experiments. We investigated the functional role of UCA1
in cell growth. As shown in Fig.
1B, UCA1 was silenced by si-UCA1-1 or si-UCA1-2, and si-UCA1-1
was chosen for further gain- and loss-of-function assays. CCK-8
assay showed that knockdown of UCA1 significantly suppressed the
cell proliferation rate (Fig. 1C).
When UCA1 was overexpressed by transfection with p-UCA1 (Fig. 1D), cell growth was significantly
promoted (Fig. 1E), suggesting that
lncRNA UCA1 positively regulates osteosarcoma cell growth.
lncRNA UCA1 is induced by HIF-1α and
HIF-1α interacts with the HIF-1α response element in the promoter
region of UCA1
In order to determine the mechanism underlying the
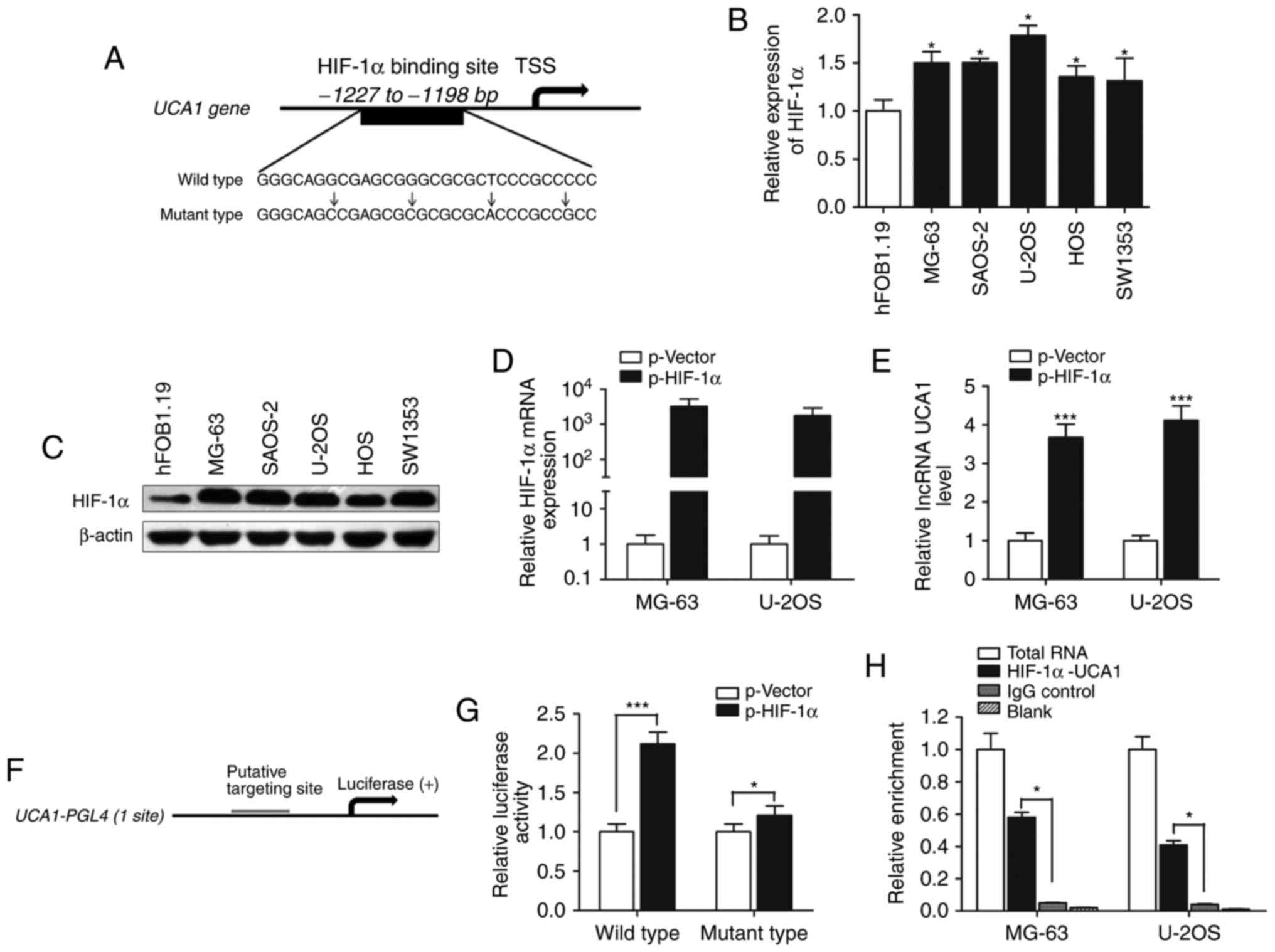
upregulation of lncRNA UCA1 in osteosarcoma cells, we focused on
transcription factors that potentially bind to the UCA1 promoter.
It has been reported that HIF-1α is a positive regulator of
osteosarcoma progression under a hypoxic condition. Thus, we aimed
to ascertain whether HIF-1α regulates UCA1 expression. Based on
computer algorithms PROMO (http://alggen.lsi.upc.es/cgi-bin/promo_v3/promo/promoinit.cgi?dirDB=TF_8.3),
and GeneCards (http://www.genecards.org/cgi-bin/carddisp.pl?), we
analyzed the promoter region of UCA1 and detected the presence of
HIF-1α-binding sites (Fig. 2A). We
then determined the expression of HIF-1α and found that both HIF-1α
mRNA and protein expression levels were upregulated in osteosarcoma
cells compared with the level noted in the osteoblastic cell line
hFOB (Fig. 2B and C). lncRNA UCA1
expression was significantly increased after HIF-1α was
overexpressed in MG-63 and U2OS cells (Fig. 2D and E).
To investigate the direct binding of HIF-1α to the
UCA1 promoter, we cloned the promoter region (~1.5 kb) of UCA1 into
luciferase reporter plasmid (pGL4 basic, Fig. 2F). As shown in Fig. 2G, luciferase activity was
significantly increased in wild-type HIF-1α-transfected cells
compared with the control vector in the MG-63 cells (P<0.001),
while mutant HIF-1α had less impact on the promoter activity of
UCA1 (P<0.05). In addition, ChIP experiments showed that HIF-1α
immunoprecipitation was observed at the promoter of UCA1 in
osteosarcoma cell lines (Fig. 2H).
Taken together, these results demonstrate that HIF-1α interacts
with the HIF-1α response element in the UCA1 promoter, thus
inducing its transcription.
HIF-1α promotes cell growth of
osteosarcoma by inducing lncRNA UCA1 expression
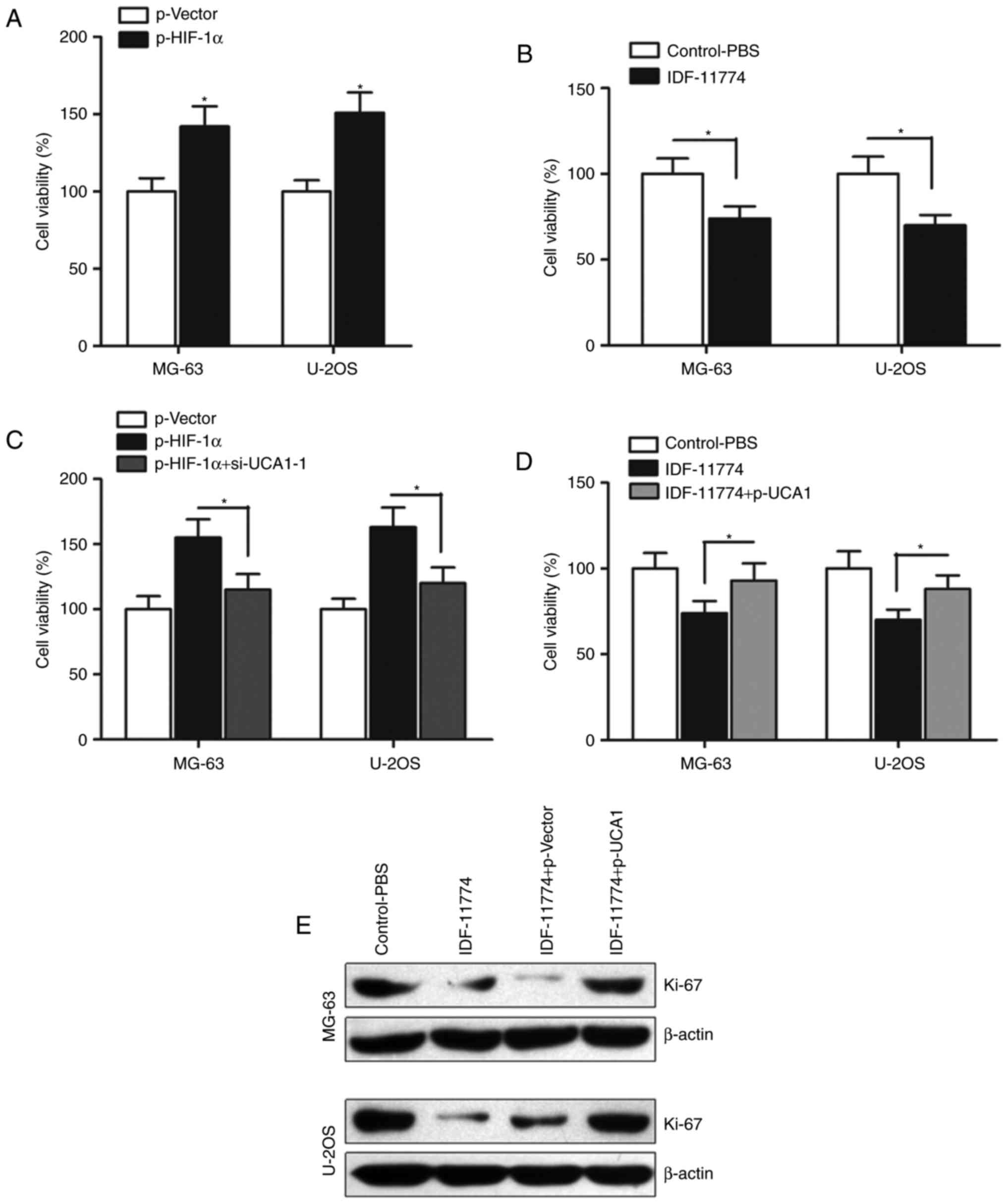
We then determined the effect of HIF-1α on
osteosarcoma cell growth. CCK-8 assay indicated that enhanced
expression of HIF-1α promoted cell growth (Fig. 3A), however, when HIF-1α was
inhibited by its specific inhibitor IDF-11774, the cell growth was
significantly suppressed (Fig. 3B).
To further determine whether HIF-1α regulates cell growth by
promoting lncRNA UCA1 expression, we transfected si-UCA1-1 into
HIF-1α-overexpressing osteosarcoma cells. Our results indicated
that si-UCA1-1 significantly abrogated the HIF-1α-induced promotion
of cell growth (Fig. 3C).
Similarly, overexpression of UCA1 by p-UCA1 partially reversed the
HIF-1α inhibitor IDF-11774-induced suppression of cell growth in
the MG-63 and U-2OS cells (Fig.
3D). We also detected the expression of proliferation marker
Ki-67, and found that p-UCA1 significantly reversed the suppression
of Ki-67 expression induced by IDF-11774 (Fig. 3E). Collectively, HIF-1α regulates
cell growth by influencing the function of lncRNA UCA1 in
osteosarcoma.
lncRNA UCA1 regulates cell growth
through inactivation of the PTEN/AKT signaling pathway
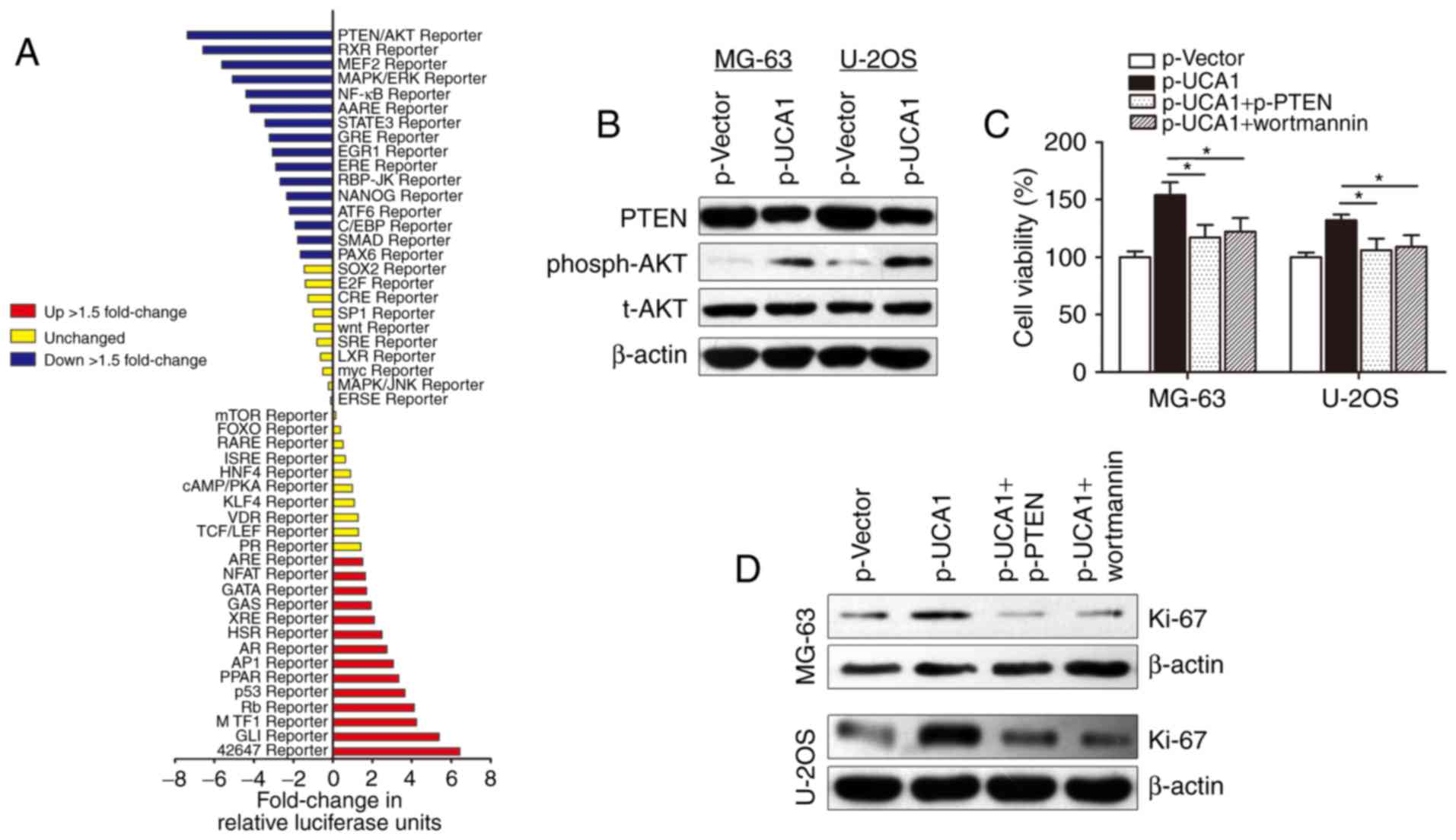
To investigate the molecular mechanisms underlying
how lncRNA UCA1 contributes to osteosarcoma cell growth, we used
Cignal Signal Transduction Reporter Array to simultaneously
investigate the activities of 50 canonical signaling pathways upon
UCA1 overexpression in MG-63 cells. This assay involved a mixture
of a pathway-specific transcription factor-responsive firefly
luciferase reporter, which contains a specific transcription
factor-responsive element in the promoter (TRE), and a
constitutively expressed Renilla luciferase reporter, which
were co-transfected to monitor alterations in the activity of that
signaling pathway. Notably, we identified PTEN/AKT signaling as one
of the most significantly repressed pathways upon UCA1
overexpression (Fig. 4A). PTEN/AKT
signaling pathway participates in the regulation of proliferation
and cell cycle in tumors, and it is well accepted that there are
functional interactions between HIF-1α and the PTEN/AKT signaling
pathway (21). Herein, we sought to
determine whether the PTEN/AKT pathway is responsible for the
lncRNA UCA1-induced promotion of cell growth. The PTEN and
phosph-AKT (phosphorylation site is Thr308) blot was then
immunostained using the total extract and the western blot
experiments showed that lncRNA UCA1 suppressed PTEN expression and
promoted phosph-AKT expression. However, no change in total AKT
(t-AKT) protein level was found (Fig.
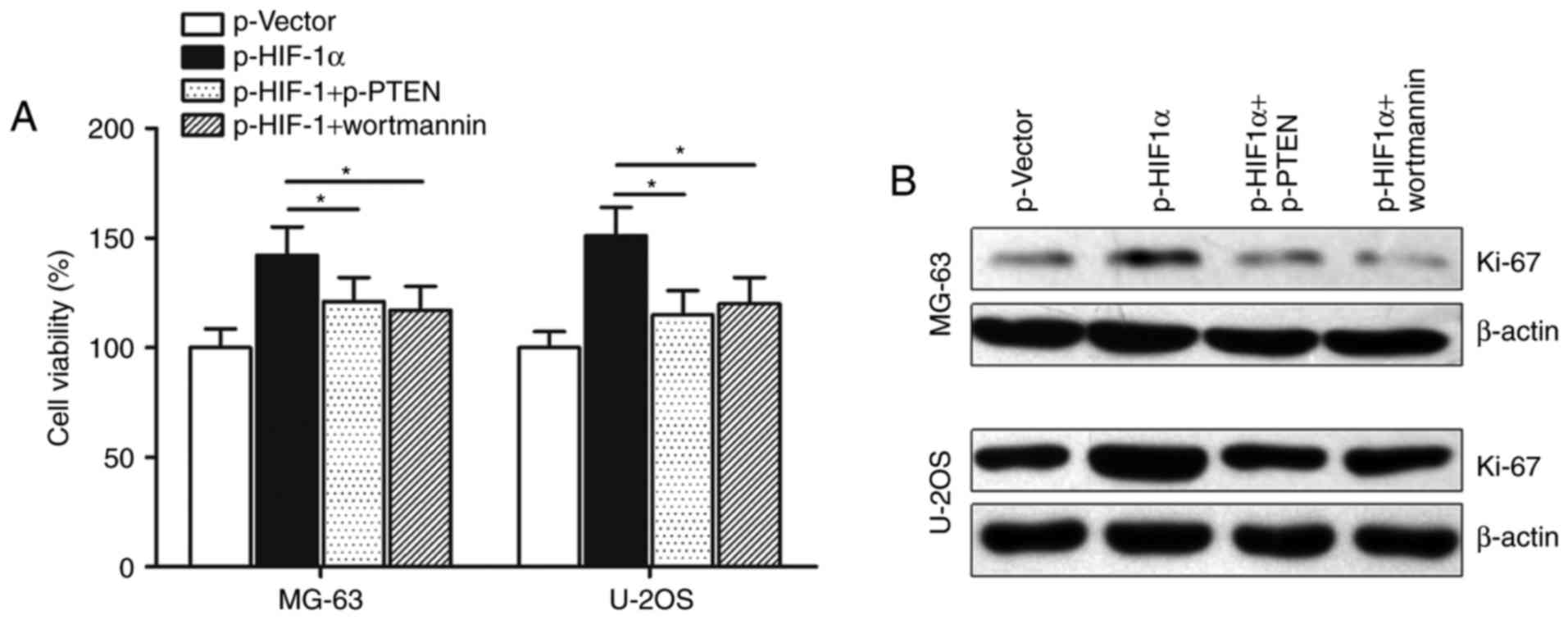
4B). In addition, CCK-8 assay showed that transfection with
p-PTEN or treatment with AKT inhibitor wortmannin potently
abolished p-UCA1-induced promotion of cell growth in osteosarcoma
cells (Fig. 4C). Cell proliferation
marker Ki-67 was also reversed by p-PTEN or wortmannin, suggesting
that lncRNA UCA1 may regulate osteosarcoma cell growth via the
PTEN/AKT pathway (Fig. 4D).
HIF-1α induces cell growth with cell
cycle arrest via the UCA1/PTEN/AKT signaling pathway
The biologic consequences of UCA1 and PTEN/AKT
pathway in HIF-1α regulation of cell growth were then examined. We
validated that HIF-1α can promote cell growth by targeting UCA1,
and then we determined the interaction between HIF-1α and the
PTEN/AKT pathway. As shown in Fig.
5A, HIF-1α-induced promotion of cell growth was abrogated by
pPTEN or AKT inhibitor wortmannin in the MG-63 and U-2OS cells.
Similarly, western blot assays showed that HIF-1α-induced
upregulation of Ki-67 was almost abolished by p-PTEN and wortmannin
in the MG-63 and U-2OS cells (Fig.
5B).
We then determined whether the HIF-1α/UCA1 pathway
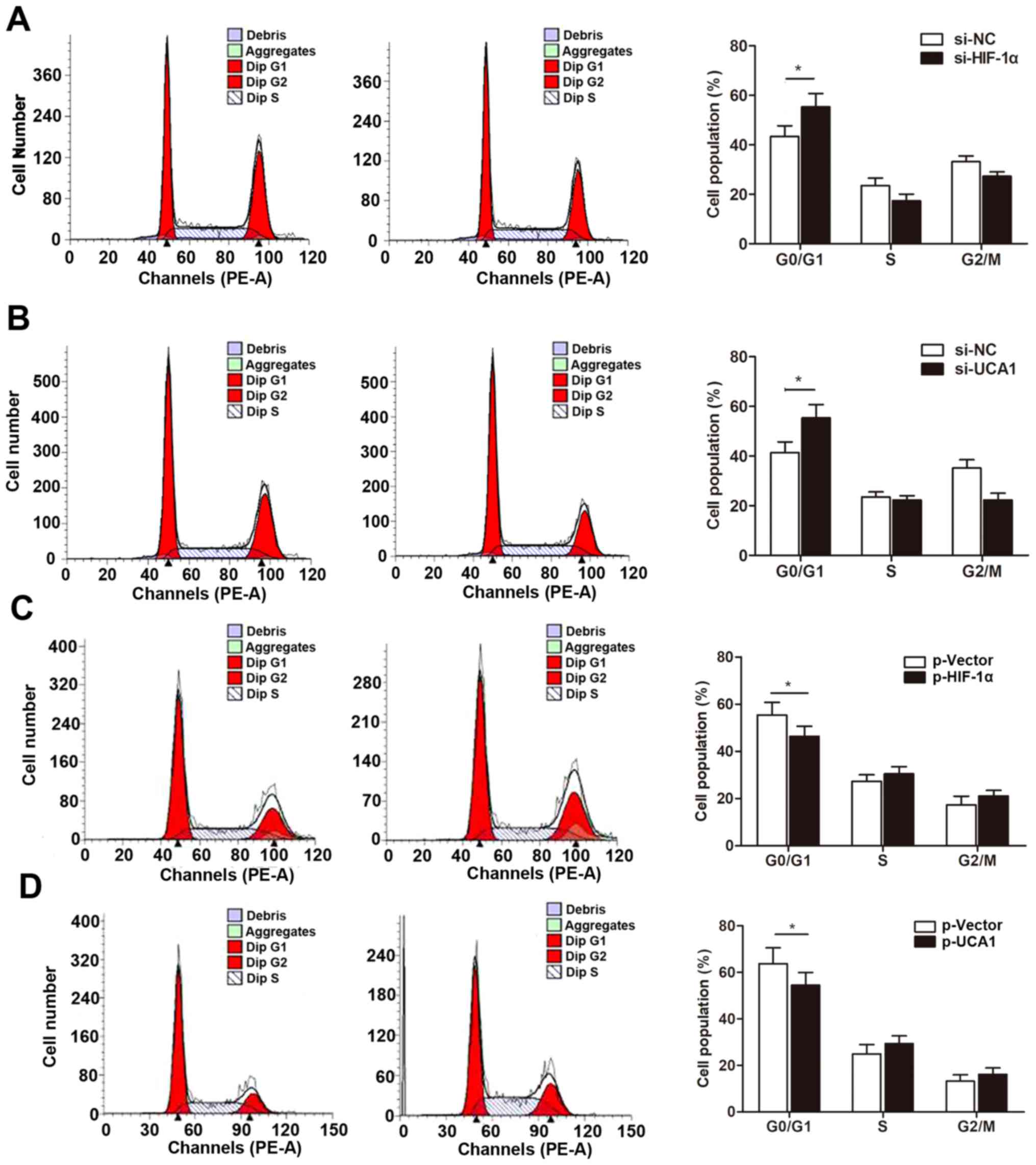
regulates cell growth by inducing cell cycle arrest. Our cell cycle
assay indicated that knockdown of HIF-1α significantly increased
the percentage of cells in the G0/G1 phase (Fig. 6A). More importantly, si-UCA1 also
caused cell cycle arrest in a the G0/G1 phase (Fig. 6B), suggesting that HIF-1α-induced
UCA1 regulates cell growth through the PTEN/AKT pathway with G0/G1
cell cycle arrest. In contast, overexpression of HIF-1α or UCA1
downregulated the proportion of cells arrested at the G0/G1 phase
(Fig. 6C and D).
Discussion
Recent advances in the analysis of the non-protein
coding region of the human genome has allowed the discovery of
extensive transcription of large RNA transcripts that lack
protein-coding function, termed non-coding RNAs (22). It has become evident that lncRNAs
may be an important class of genes involved in carcinogenesis
(23). Currently, rapid tumor
growth and pulmonary metastasis are the major reasons for the death
of patients with osteosarcoma, revealing that effective prognostic
factors and therapeutic targets could help improve treatment
strategies to overcome metastatic osteosarcoma. Therefore, it
stands to reason that defining the molecular mechanisms whereby
lncRNAs have an impact on cancer progression may provide novel
opportunities to treat osteosarcoma. Here in the present study, we
found that lncRNA UCA1 was significantly upregulated in
osteosarcoma cells compared with normal osteoblastic cells by Hiseq
screening and RT-qPCR validation. Enhanced UCA1 expression was
activated by the transcription factor HIF-1α which promoted the
viability of osteosarcoma cells. We also identified that the
HIF-1α-induced UCA1 promotion of cell growth was inactivated by the
PTEN/AKT signaling pathway.
lncRNA UCA1 was first reported to be overexpressed
and valuable as a prognostic marker for bladder cancer but has also
been linked to several other human tumor entities (16). Numerous studies indicate that UCA1
plays critical roles in the development and progression of cancers,
such as breast (24) and colorectal
cancer (25), and esophageal
squamous cell carcinoma (26).
Currently, there are two studies that have focused on the role of
UCA1 in osteosarcoma. Wen et al found that UCA1 was
significantly increased in osteosarcoma specimens including primary
tissues and serum samples when compared with controls, and it could
be a specific and non-invasive candidate biomarker for the
diagnosis and prognosis of osteosarcoma (27). Li et al demonstrated that
enhanced expression of UCA1 was correlated with the poor prognosis
of osteosarcoma patients and promoted proliferation and metastasis
of osteosarcoma cells (20). Our
data suggest that UCA1 was upregulated in osteosarcoma cells and
promoted cell growth, which is consistent with the two previous
reports. However, the underlying mechanism of why UCA1 is
upregulated and how UCA1 regulates osteosarcoma progression remains
unknown.
We firstly investigated the reason for high UCA1
expression in osetosarcoma. Bioinformatic databases including PROMO
and GeneCards were used to screen for potential proto-oncogenic
transcription factors. Finally, we focused on the HIF-1α
transcription factor due to a previous study involving HIF-1α
regulation in osteosarcoma (28).
As expected, we identified the presence of the HIF-1α-binding sites
on UCA1 promoter region, and HIF-1α presented a relatively higher
score than other regulators according to analysis of bioinformatic
databases. Increased HIF-1α levels have been found in many tumor
types, accompanied by increased expression of HIF-1 target genes,
including but not limited to VEGFA, PGK1, ANGPTL4 and HK2 (29). HIF-1α overexpression has been
correlated with a high risk of metastasis and high mortality in
many human cancers, including osteosarcoma (30). We also found that HIF-1α was
overexpressed in osteosarcoma cells and UCA1 was markedly
upregulated after transfection of the HIF-1α-expressing vector.
Following luciferase reporter assay and ChIP assay, both suggest
that HIF-1α could interact with the promoter region of lncRNA UCA1.
In addition, functional biological assays indicated that HIF-1α can
enhance cell growth by targeting UCA1. It must be said that the
positive regulation of HIF-1α on lncRNA UCA1 expression is under
normoxic conditions. Further research is required to verify whether
this interaction also applies under a hypoxic condition.
Collectively, the integrated approach suggests that HIF-1α
activates UCA1 translational expression in osteosarcoma under
normoxic conditions.
In addition, we sought to determine the underlying
regulatory mechanisms by which UCA1 exerts its function in
osteosarcoma. To reveal whether lncRNA UCA1 participates in the
regulation of cell proliferation via targeting the downstream
pathway, we performed Cignal Signal Transduction Reporter Array.
This array involved a mixture of a pathway-specific transcription
factor-responsive firefly luciferase reporter, which contains a
specific transcription factor-responsive element in the promoter,
and a constitutively expressed Renilla luciferase reporter,
which were co-transfected to monitor alterations in the activity of
this signaling pathway. This high-throughput dual-luciferase assay
led us to identify the PTEN/AKT pathway as one putatively affected
by lncRNA UCA1. The western blot experiments showed that lncRNA
UCA1 suppressed PTEN expression. Additionally, the phosphorylation
of AKT, which was under the regulation of PTEN, was altered
accordingly after the overexpression or inhibition of UCA1.
PTEN, a well-known tumor suppressor, has been found
to play an important role in the development and progression of
various human cancers (31,32). It is a major negative regulator of
the PI3K/Akt signaling pathway (33). PI3K/PTEN balance is involved in the
expression of long-term potentiation (LTP) and the regulation of
postsynaptic α-amino-3-hydroxy- 5-methyl-4-isoxazolepropionic acid
(AMPA) receptor densities. Herein, the phosphatase and tensin
homologue on PTEN/Akt signaling pathway participates in the
regulatory process of synaptic plasticity associated with glutamate
receptors, and PTEN/AKT signaling is frequently activated in
various cancers (34). lncRNAs were
reported to be involved in the regulation of the PTEN/AKT pathway
in various cancers. Guo et al demonstrated that lncRNA
AFAP1-AS1 promotes the cell proliferation of gastric cancer cells
via the PTEN/p-AKT pathway (35);
Liao et al found that lncRNA CASC2 interacts with miR-181a
to modulate glioma growth and resistance to TMZ through the
PTEN/AKT pathway (36); Yang et
al suggested that MEG3 regulates the growth of testicular germ
cell tumors through the PTEN/PI3K/AKT pathway (37). However, the interaction of lncRNA
and this signaling pathway in osteosarcoma is not well known. The
present study demonstrated that lncRNA UCA1 regulates osteosarcoma
cell growth by suppressing PTEN and activating the p-AKT protein
level, indicating that the interaction between UCA1 and the
PTEN/AKT pathway may exert important regulatory function.
After having established the interaction between
HIF-α and lncRNA UCA1 and the subsequent downstream PTEN/AKT
pathway, we then sought to identify whether HIF-α promoted cell
proliferation by inducing UCA1 and suppressing the PTEN/AKT
pathway. Gain- and loss-of-function assays showed that
HIF-1α-induced promotion of cell growth was abrogated by pPTEN or
AKT inhibitor wortmannin in the MG-63 and U-2OS cells. Notably, the
degree of overexpression of UAC1 and/or HIF-α were not related to
that of cell growth. It is reasonable that the cell growth was
regulated by different pathways and different factors, and the
interval of cell growth changes may be limited due to the
characteristics of the cell lines. Moreover, knockdown of HIF-1α
and UCA1 induced cell cycle arrest in the G0/G1 phase, which
further validated the co-regulation of HIF-1α and UCA1 on the
PTEN/AKT signaling pathway. One of the limitations of the present
study is that no in-vivo experiments were performed to
support our in-vitro findings. We will extend our study in
the future to validate the data in vivo.
In conclusion, our integrated approach reveals that
UCA1 is upregulated in osteosarcoma cells. Moreover, it promotes
cell growth and caused cell cycle arrest through inactivation of
the PTEN/AKT signaling pathway. Hence, UCA1 may be a potential
prognostic marker and therapeutic target for osteosarcoma
patients.
References
|
1
|
Ma O, Cai WW, Zender L, Dayaram T, Shen J,
Herron AJ, Lowe SW, Man TK, Lau CC and Donehower LA: MMP13, Birc2
(cIAP1), and Birc3 (cIAP2), amplified on chromosome 9, collaborate
with p53 deficiency in mouse osteosarcoma progression. Cancer Res.
69:2559–2567. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li PL, Zhang X, Wang H, Wang L, Liu T, Du
L, Yang Y and Wang C: MALAT1 is associated with poor response to
oxaliplatin-based chemotherapy in colorectal cancer patients and
promotes chemoresistance through EZH2. Mol Cancer Ther. 16:739–751.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen L, Wang Q, Wang GD, Wang HS, Huang Y,
Liu XM and Cai XH: Mir-16 inhibits cell proliferation by targeting
IGF1R and the RAF1-MEK1/2-ERK1/2 pathway in osteosarcoma. FEBS
Lett. 587:1366–1372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Han K, Chen X, Bian N, Ma B, Yang T, Cai
C, Fan Q, Zhou Y and Zhao TB: MicroRNA profiling identifies MiR-195
suppresses osteosarcoma cell metastasis by targeting CCND1.
Oncotarget. 6:8875–8889. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu K, Huang J, Ni J, Song D, Ding M, Wang
J, Huang X and Li W: MALAT1 promotes osteosarcoma development by
regulation of HMGB1 via miR-142-3p and miR-129-5p. Cell Cycle.
16:578–587. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li PL, Zhang X, Wang LL, Du LT, Yang YM,
Li J and Wang CX: MicroRNA-218 is a prognostic indicator in
colorectal cancer and enhances 5-fluorouracil-induced apoptosis by
targeting BIRC5. Carcinogenesis. 36:1484–1493. 2015.PubMed/NCBI
|
|
7
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Talks KL, Turley H, Gatter KC, Maxwell PH,
Pugh CW, Ratcliffe PJ and Harris AL: The expression and
distribution of the hypoxia-inducible factors HIF-1alpha and
HIF-2alpha in normal human tissues, cancers, and tumor-associated
macrophages. Am J Pathol. 157:411–421. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
10
|
Kapranov P, Cheng J, Dike S, Nix DA,
Duttagupta R, Willingham AT, Stadler PF, Hertel J, Hackermüller J,
Hofacker IL, et al: RNA maps reveal new RNA classes and a possible
function for pervasive transcription. Science. 316:1484–1488. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ponting CP, Oliverand PL and Reik W:
Evolution and functions of long non-coding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi X, Sun M, Liu H, Yao Y and Song Y:
Long non-coding RNAs: A new frontier in the study of human
diseases. Cancer Lett. 339:159–166. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long non-coding RNAs: Novel insights into
hepatocelluar carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li Z, Yu X and Shen J: Long non-coding
RNAs: Emerging players in osteosarcoma. Tumor Biol. 37:2811–2816.
2016. View Article : Google Scholar
|
|
15
|
Boon RA, Jaé N, Holdt L and Dimmeler S:
Long non-coding RNAs: From clinical genetics to therapeutic
targets? J Am Coll Cardiol. 67:1214–1226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XS, Zhang Z, Wang HC, Cai JL, Xu QW,
Li MQ, Chen YC, Qian XP, Lu TJ, Yu LZ, et al: Rapid identification
of UCA1 as a very sensitive and specific unique marker for human
bladder carcinoma. Clin Cancer Res. 12:4851–4858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Na XY, Liu ZY, Ren PP, Yu R and Shang XS:
Long non-coding RNA UCA1 contributes to the progression of prostate
cancer and regulates proliferation through KLF4-KRT6/13 signaling
pathway. Int J Clin Exp Med. 8:12609–12616. 2015.PubMed/NCBI
|
|
18
|
Tuo YL, Li XM and Luo J: Long non-coding
RNA UCA1 modulates breast cancer cell growth and apoptosis through
decreasing tumor suppressive miR-143. Eur Rev Med Pharmacol Sci.
19:3403–3411. 2015.PubMed/NCBI
|
|
19
|
Wu W, Zhang S, Li X, Xue M, Cao S and Chen
W: Ets-2 regulates cell apoptosis via the Akt pathway, through the
regulation of urothelial cancer associated 1, a long non-coding
RNA, in bladder cancer cells. PLoS One. 8:e739202013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li W, Xie P and Ruan WH: Overexpression of
lncRNA UCA1 promotes osteosarcoma progression and correlates with
poor prognosis. J Bone Oncol. 5:80–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Park JH, Lee JY, Shin DH, Jang KS, Kim HJ
and Kong G: Loss of Mel-18 induces tumor angiogenesis through
enhancing the activity and expression of HIF-1α mediated by the
PTEN/PI3K/Akt pathway. Oncogene. 30:4578–4589. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gutschner T and Diederichs S: The hall
marks of cancer: A long non-coding RNA point of view. RNA Biol.
9:703–719. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mattick JS: The genetic signatures of
non-coding RNAs. PLoS Genet. 5:e10004592009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang J, Zhou N, Watabe K, Lu Z, Wu F, Xu
M and Mo YY: Long non-coding RNA UCA1 promotes breast tumor growth
by suppression of p27 (Kip1). Cell Death Dis. 5:e10082014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han Y, Yang YN, Yuan HH, Zhang TT, Sui H,
Wei XL, Liu L, Huang P, Zhang WJ and Bai YX: UCA1, a long
non-coding RNA up-regulated in colorectal cancer influences cell
proliferation, apoptosis and cell cycle distribution. Pathology.
46:396–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li JY, Ma X and Zhang CB: Overexpression
of long non-coding RNA UCA1 predicts a poor prognosis in patients
with esophageal squamous cell carcinoma. Int J Clin Exp Pathol.
7:7938–7944. 2014.PubMed/NCBI
|
|
27
|
Wen JJ, Ma YD, Yang GS and Wang GM:
Analysis of circulating long non-coding RNA UCA1 as potential
biomarkers for diagnosis and prognosis of osteosarcoma. Eur Rev Med
Pharmacol Sci. 21:498–503. 2017.PubMed/NCBI
|
|
28
|
Guo S, Bai R, Liu W, Zhao A, Zhao Z, Wang
Y, Wang Y, Zhao W and Wang W: MicroRNA-210 is upregulated by
hypoxia-inducible factor-1α in the stromal cells of giant cell
tumors of bone. Mol Med Rep. 12:6185–6192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amelio I and Melino G: The ‘Sharp’ blade
against HIF-mediated metastasis. Cell Cycle. 11:4530–4535. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hu T, He N, Yang Y, Yin C, Sang N and Yang
Q: DEC2 expression is positively correlated with HIF-1 activation
and the invasiveness of human osteosarcomas. J Exp Clin Cancer Res.
34:222015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Ann Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar
|
|
32
|
Di Cristofano A and Pandolf PP: The
multiple roles of PTEN in tumor suppression. Cell. 100:387–390.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kwon CH, Luikart BW, Powell CM, Zhou J,
Matheny SA, Zhang W, Li Y, Baker SJ and Parada LF: Pten regulates
neuronal arborization and social interaction in mice. Neuron.
50:377–388. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xiao ZD, Jiao CY, Huang HT, He LJ, Zhao
JJ, Lu ZY and Liu LX: miR-218 modulate hepatocellular carcinoma
cell proliferation through PTEN/AKT/PI3K pathway and HoxA10. Int J
Clin Exp Pathol. 7:4039–4044. 2014.PubMed/NCBI
|
|
35
|
Guo JQ, Li SJ and Guo GX: Long non-coding
RNA AFAP1-AS1 promotes cell proliferation and apoptosis of gastric
cancer cells via PTEN/p-AKT pathway. Dig Dis Sci. 62:2004–2010.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liao Y, Shen L, Zhao H, Liu Q, Fu J, Guo
Y, Peng R and Cheng L: lncRNA CASC2 Interacts With miR-181a to
modulate glioma growth and resistance to TMZ through PTEN pathway.
J Cell Biochem. 118:1889–1899. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang NQ, Luo XJ, Zhang J, Wang GM and Guo
JM: Crosstalk between Meg3 and miR-1297 regulates growth of
testicular germ cell tumor through PTEN/PI3K/AKT pathway. Am J
Transl Res. 8:1091–1099. 2016.PubMed/NCBI
|