Introduction
Esophageal cancer is the eighth most common cancer
worldwide and the sixth leading cause of cancer-related mortality
(1). Esophageal cancer is generally
diagnosed at an advanced stage and has a dismal prognosis with a
17% 5-year overall survival rate (2). Currently, 5-fluorouracil (5-FU) and
cisplatin (Cis) are the mainstays of chemotherapy available for the
treatment of advanced esophageal cancer. However, the anticancer
effects provided by these chemotherapeutic agents are often
unsatisfactory. The response rates to Cis combined with 5-FU are
only 35–45% (2), and drug
resistance can be inherent or acquired during prolonged treatment.
Due to the toxic effects of traditional chemotherapy, an escalated
dosage often results in intensive adverse effect or even fatal
outcomes. Therefore, to date, efforts have focused on the cellular
and molecular mechanisms of the resistance phenotype to develop
more effective anticancer strategies.
Drug resistance in cancer cells is a multifactorial
phenomenon. Alterations of various molecules involved in DNA damage
repair, apoptosis induction and drug distribution have been
confirmed to contribute to the drug-resistance phenotype in cancer
cells (3). Among these molecules,
survivin has drawn interest from researchers due to its critical
roles in apoptosis inhibition and drug resistance induction
(4–7). As an inhibitor of apoptosis protein
(IAP) family member, survivin can inhibit apoptosis via intrinsic
and extrinsic pathways (5). At the
same time, survivin enhances the survival of cells through its
roles associated with various cellular signaling pathways (8,9).
Overexpression of survivin can be found in most cancer cell types
(including esophageal cancer cells) and confer drug-resistance to
cancer cells (10–12). Currently, survivin is considered as
an important therapeutic target in anticancer research.
The use of small-molecule anticancer compounds to
prevent or treat cancers is a novel idea (13–15).
α-solanine, one component of steroidal glycoalkaloids, is mainly
found in the potato tuber or the nightshade plant. Several studies
have shown that α-solanine demonstrates anti-metastatic activity in
different types of cancers (16–19)
and chemoprotective and chemotherapeutic effects in animal models
of breast cancer (20). In our
previous study, we found that α-solanine modulated the
radiosensitivity of esophageal cancer cells by inducing miR-138
expression (21). Therefore,
α-solanine may have effects on the chemosensitivity of esophageal
cancer cells. In the present study, we investigated the effect of
α-solanine on the chemosensitivity of EC9706 and KYSE30 cells to
5-FU and Cis and explored the potential molecular mechanisms.
Materials and methods
Cell culture and reagents
α-solanine and its solvent, dimethyl sulfoxide
(DMSO), were purchased from Sigma-Aldrich (St. Louis, MO, USA).
α-solanine was stored at −20°C after dissolution. Human esophageal
cancer cell lines (EC9706 and KYSE30) were purchased from the Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China). The cells were maintained in RPMI-1640 medium supplemented
with 10% fetal bovine serum (FBS; Gibco BRL, Gaithersburg, MD, USA)
and incubated in a 5% CO2 humidified incubator at
37°C.
CCK-8 assay
EC9706 and KYSE30 cells in logarithmic phase growth
were seeded into each well of a 96-well plate at a density of
1×104 cells/well. After 24 h, cells of each cell line
were treated with increasing concentrations (0, 2, 4 and 6 µmol/l)
of α-solanine combined with increasing concentrations (0, 10, 20,
30, 40 and 50 µg/ml) of 5-FU or increasing concentrations (0, 1, 2,
4, 6 and 8 µg/ml) of Cis, respectively, for 24 h. The blank group
was treated with PBS, and the control group was treated with 0
µmol/l α-solanine containing 1‰ DMSO. The viability of cells was
determined according to the protocol included in the Cell Counting
Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto, Japan). Cells were
cultured at 37°C in 5% CO2 for another 3 h with medium
inclusion with 10% CCK-8, and then the optical density was measured
at 450 nm. The experiments were performed in triplicate.
Flow cytometric assay
Apoptosis was analyzed by flow cytometric assay.
Flow cytometric assays were conducted using FITC Annexin V
Apoptosis Detection Kit I (BestBio, Shanghai, China), according to
the manufacturer's instructions. Briefly, EC9706 and KYSE30 cells
were cultured in a 96-well plate, in which the medium was added
with different concentrations of α-solanine (0, 2, 4 and 6 µmol/l)
with or without 40 µg/ml 5-FU or 6 µg/ml Cis for 48 h. The cells
were divided into a Blank group (treated with PBS containing 1‰
DMSO), additional treatment groups (treated with 40 µg/ml 5-FU or 6
µg/ml Cis and different concentrations of α-solanine), and the
control group treated with 40 µg/ml 5-FU or 6 µg/ml Cis with PBS
containing 1‰ DMSO. Cells from each group were harvested by
trypsinization and re-suspended at a density of 1×106
cells/ml in 1X binding buffer. After double staining with FITC
Annexin V and propidium iodide (PI), cells were analyzed using a
FACScan flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA)
equipped with CellQuest software (BD Biosciences).
Detection of caspase-3/7 activity
The enzymatic activity of caspase-3/7 was measured
using the Caspase-Glo 3/7 assay kit (Promega, Shanghai, China)
according to the manufacturer's instructions. In brief, EC9706 and
KYSE30 cells were seeded in 96-well plates and treated with or
without 40 µg/ml 5-FU or 6 µg/ml Cis and different concentrations
(0, 2, 4 and 6 µmol/l) of α-solanine for 48 h; the Blank group was
treated with PBS containing 1‰ DMSO. Then, the cells were lysed and
incubated with 100 µl of Apo-ONE Caspase-3/7 reagent (substrate and
buffer in the ratio of 1:100). After a one hour incubation in the
dark at room temperature (RT), the fluorescence of each well was
measured at 485–520 nm by reading in an Epoch microplate reader
(Biotek Instruments, Winooski, VT, USA).
In vivo tumor growth assay
EC9706 cells (5×106), which were labeled
with firefly luciferase, were subcutaneously inoculated into the
armpit of the right forelimb of 6-week-old female BALB/c nude mice
purchased from Vital River Laboratory Animal Technology Corp.
(Beijing, China). After one week, the mice were randomly divided
into six groups (5 mice per group): Blank group, α-solanine group,
5-FU group, Cis group, α-solanine and 5-FU group, α-solanine and
Cis group. Blank group was given normal saline weekly and 1‰ DMSO
every two days for 4 weeks. α-solanine was administered to the mice
at a dosage of 3.5 mg/kg every other day, which lasted 4 weeks.
5-FU was administered at a dosage of 20 mg/kg weekly for 4 weeks.
Cis was given to the mice at a dosage of 3 mg/kg weekly for 4
weeks. Before the mice were anesthetized with isoflurane, an
aqueous solution of luciferin (150 mg/kg) was intraperitoneally
injected 10 min prior to imaging. The mice were placed into the
light-tight chamber of a CCD camera system (Xenogen). The
luminescent area of the xenograft tumor was defined as the region
of interest (ROI) and the total signal in the ROI
(photon/sec/m2) was quantified using Living Image
software 3D (Xenogen). All procedures involving mice were performed
in compliance with the Guide for the Care and Use of Laboratory
Animals (National Institutes of Health).
Immunohistochemistry
Paraffin was washed away with xylene from tumor
tissue sections of 4 µm. The sections were then rehydrated in
graded ethanol, and pretreated in an electric waterbath at 100°C
with 0.01 M citric acid buffer (pH 6.0) for 20 min for antigen
retrieval. The sections were pre-incubated with 3% hydrogen
peroxide in deionized water for 10 min for inactivation of
endogenous peroxidase, after being washed 3 times with
phosphate-buffered saline (PBS) solution and then blocked with 5%
normal goat serum for 20 min. The specimens were then incubated
with rabbit anti-Ki67 nuclear antigen monoclonal antibody (Zhong
Shan Golden Bridge Biotechnology Co., Ltd., Beijing, China) at a
dilution of 1:100. Incubation was then carried out at 4°C in a
refrigerator overnight. After being washed with PBS three times,
the sections were incubated with biotin-conjugated secondary
antibody anti-rabbit IgG (Zhong Shan Golden Bridge Biotechnology
Co., Ltd.) for 30 min. The sections were washed thrice with PBS
followed by the application of biotin-streptavidin HRP for 30 min.
The bound complexes were visualized by the application of a 0.05%
diaminobenzidine (DAB) solution and counterstained with Harris
hematoxylin. The immune-histochemical expression of Ki67 in the
tumor tissues was semi-quantitatively scored on a five-point scale
(22). The Ki67 staining index was
scored by counting positive cells. Scoring was performed and
reviewed by two experienced investigators and average scores were
calculated.
RNA extraction and quantitative
real-time PCR
Relative levels of miR-138 and survivin mRNA in the
EC9706 and KYSE30 cells treated with different reagents or reagent
combination were determined by quantitative real-time PCR
(qRT-PCR). Total RNA was extracted using the Total RNA Kit I
(R6834-01; Omega Bio-Tek, Inc., Norcross, GA, USA) from the
aforementioned cells. Determination of relative levels of miRNA-138
was performed by using a High-Specificity miR-138 qRT-PCR Detection
Kit (Stratagene Corp. La Jolla, CA, USA) in conjunction with an ABI
7500 thermal cycler (Applied Bio-systems, USA). All protocols were
performed according to the manufacturer's instructions. We used U6
small nuclear RNA (U6 snRNA) for normalization. To determine the
relative levels of survivin mRNA, the qRT-PCR assays were performed
using ABI TaqMan PCR Master Mix (Applied Biosystems; Thermo Fisher
Scientific, Inc., Waltham, MA, USA). GAPDH was used as an internal
control. The corresponding CT values were recorded with ABI 7500
software, and then the relative expression levels were calculated
according to the formula 2−∆∆Ct.
Western blot assay
Total proteins from the transfected cells were
extracted using RIPA buffer containing phenylmethanesulfonyl
fluoride (PMSF). A BCA protein assay kit (Beyotime, Haimen, China)
was used to determine the protein concentrations. Proteins (40 µg)
were subjected to sodium dodecyl sulfate polyacrylamide gel
electrophoresis (SDS-PAGE) and transferred onto polyvinyl
difluoride (PVDF) membranes. After blocking, the membranes were
incubated overnight at 4°C with diluted (1:1,000) primary antibody
(polyclonal rabbit anti-survivin; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). Following extensive washes, the membranes were
incubated with diluted (1:5,000) horseradish peroxidase conjugated
goat anti-rabbit lgG (Santa Cruz Biotechnology, Inc.). Signals were
determined using DAB detection kit (Amersham Pharmacia Biotech,
Piscataway, NJ, USA). GAPDH (Santa Cruz Biotechnology, Inc.) served
as the endogenous reference.
Dual-luciferase assay
The human survivin 3′ untranslated region (3′UTR)
fragment containing putative binding sites for miR-138 were
amplified by PCR from human genomic DNA. The mutant survivin 3′UTRs
were obtained by overlap extension PCR. The fragments were cloned
into a pmirGLO-M-survivin. For the luciferase reporter assay, the
EC9706 cell line was transiently co-transfected with miRNA (miR-138
agomir or scrambled-miR-138 negative control) and reporter vectors
(wild-type reporter vectors or mutant-type reporter vectors), using
Lipofectamine® 2000. Luciferase activities were measured
using a Dual-Luciferase assay kit (Promega) according to the
manufacturer's instructions at 48 h post-transfection.
Statistical analysis
Statistical testing was conducted with the
assistance of SPSS 17.0 software (SPSS, Inc., Chicago IL, USA). All
data are expressed as means ± standard deviation (SD). One-way
ANOVA and LSD tests were used to analyze data. Results were
considered significantly significant at P-values <0.05.
Results
α-solanine enhances the
chemosensitivity of 5-FU and Cis in EC9706 and KYSE30 cells
We found that the treatment of different
concentrations of α-solanine (2, 4 and 6 µmol/l) for 24 h did not
alter the viability of both cell lines. The results revealed that
the concentrations of α-solanine used in the experiments in
vitro did not cause obvious cytotoxicity to the EC9706 and
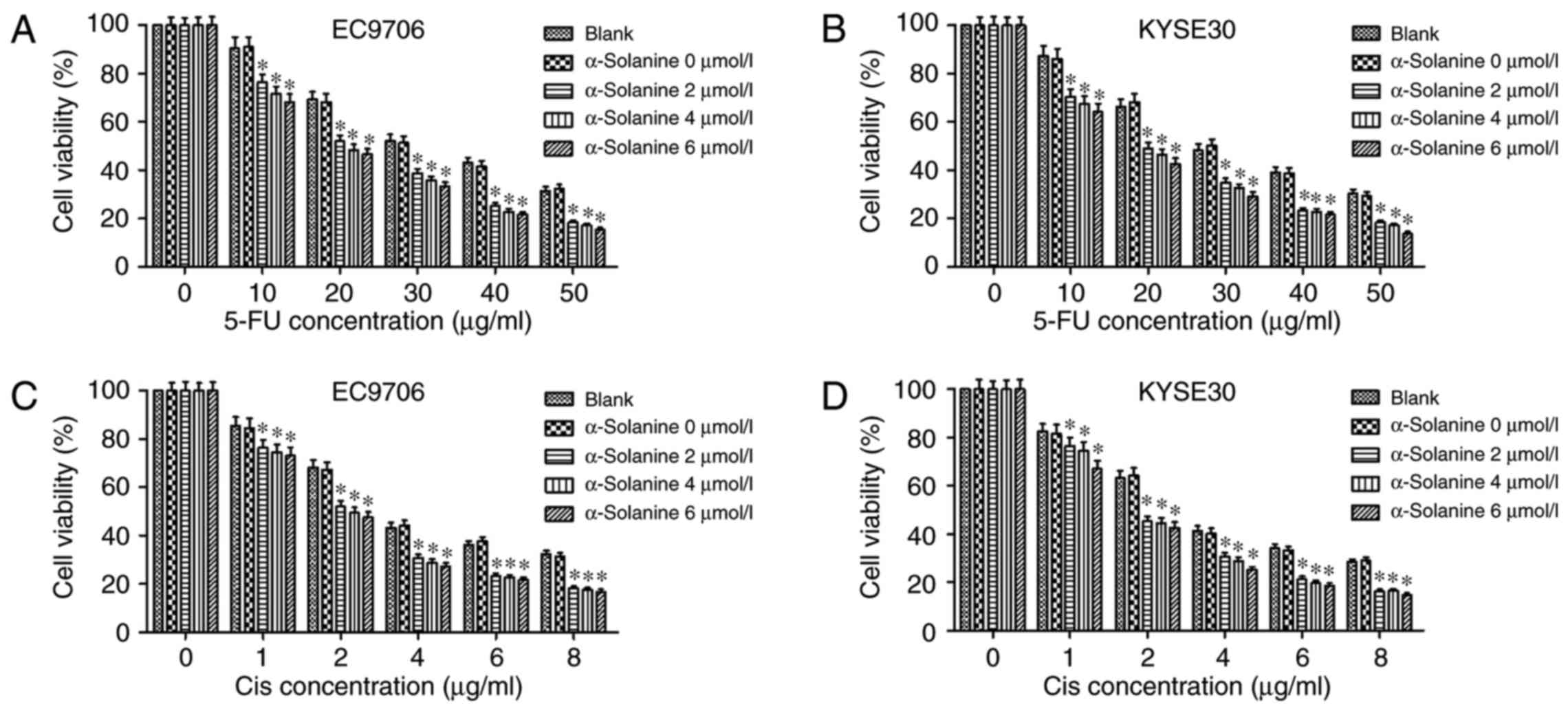
KYSE30 cells. The results of CCK-8 assay showed that α-solanine
enhanced the cytotoxic effect of 5-FU and Cis on EC9706 and KYSE30
cells. As depicted in Fig. 1, in
both cell lines, the semi-lethal concentration (LC50) of
5-FU and Cis were nearly 30 and 4 µg/ml, respectively. However,
when 4 µmol/l α-solanine was added, the LC50 of 5-FU and
Cis reduced to nearly 20 and 2 µg/ml, respectively, in the EC9706
and KYSE30 cells (P<0.05).
α-solanine increases the effect of
5-FU and Cis on the induction of apoptosis in EC9706 and KYSE30
cells
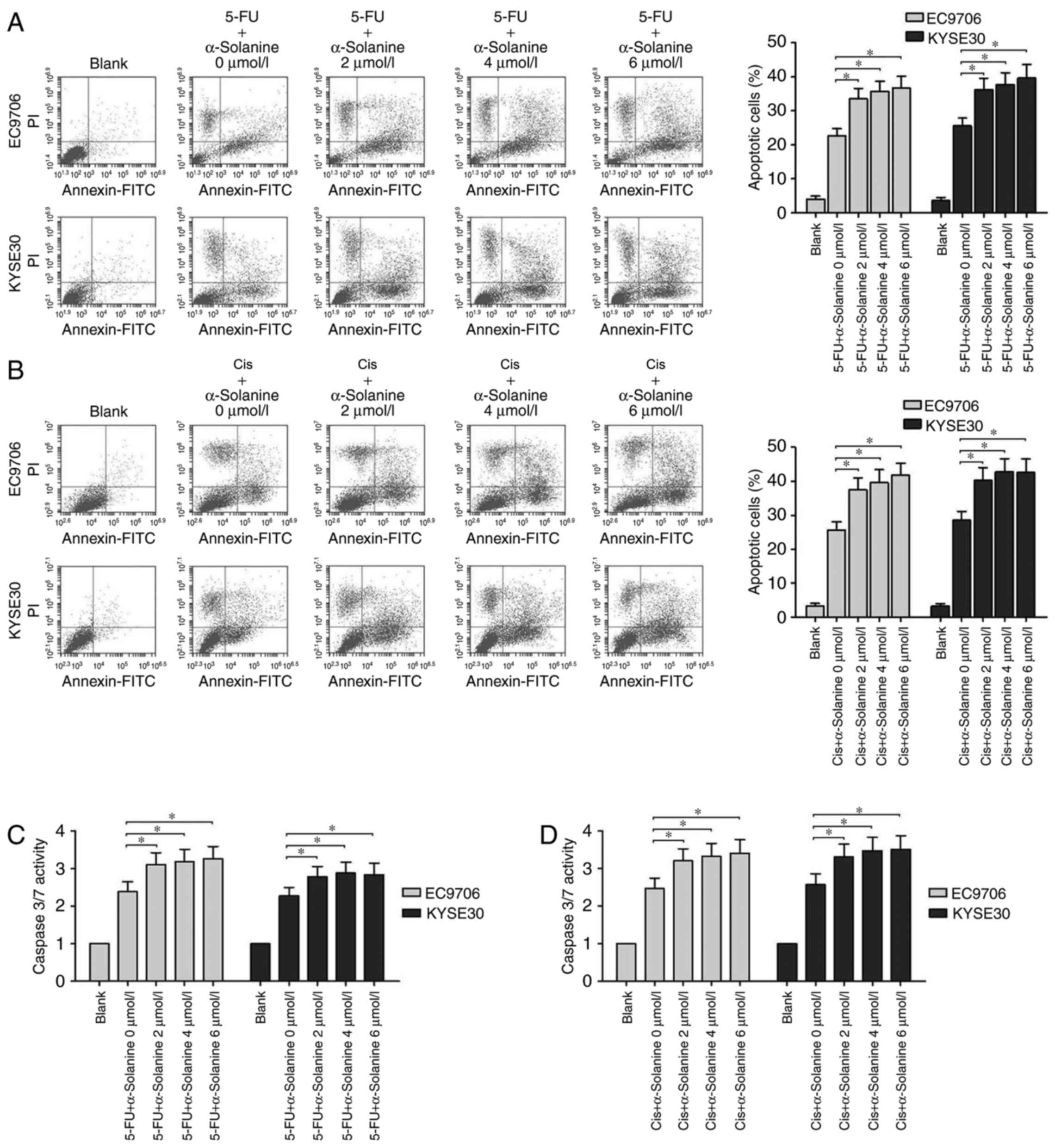
We studied the effects of α-solanine on the
apoptosis of EC9706 and KYSE30 cells treated with 5-FU or Cis. From
the flow cytometric assay results, we found that when the cells
were treated with 40 µg/ml 5-FU, the apoptosis rate of the EC9706
and KYSE30 cells ranged from 20 to 25%, and following treatment
with 4 µg/ml Cis, it was near 25%. However, when different
concentrations of α-solanine (2, 4 and 6 µmol/l) were added, the
apoptosis rates of both cell lines were significantly increased to
a range of 35 to 40% (Fig. 2A and
B, P<0.05). We also found that α-solanine and 5-FU or Cis
can activate caspase-3/7 more effectively than 5-FU or Cis
separately in EC9706 and KYSE30 cells (Fig. 2C and D, P<0.05). These results
indicated that α-solanine may exert its function as
chemosensitivity enhancer through apoptosis pathway.
α-solanine increases the inhibitory
effect of 5-FU and Cis on EC9706 cell-derived transplanted
tumors
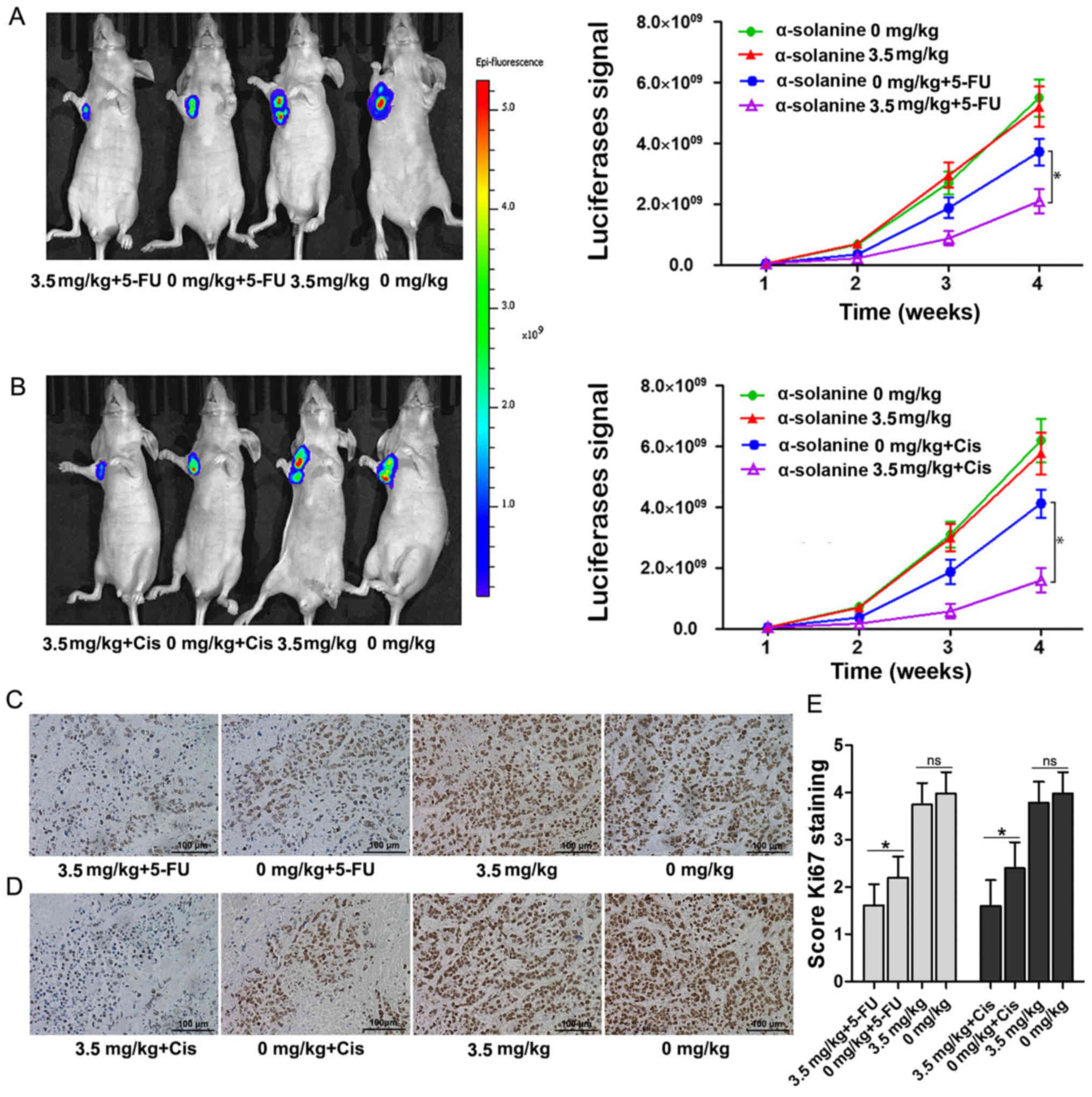
To further detect the chemosensitivity enhancing
effect of α-solanine on EC9706 cells, we performed a nude mouse
experiment and immunohistochemical test. The bioluminescence signal
was relatively weaker in the group treated with 5-FU or Cis and
α-solanine than groups that were treated with 5-FU or Cis
separately (Fig. 3A and B,
P<0.05). However, there were no significant differences between
the group that was treated with normal saline and 1‰ DMSO and that
with α-solanine in regards to the bioluminescence signal. The
immunohistochemistry and the semi-quantitative scoring results
showed that α-solanine enhanced the proliferation inhibition effect
of 5-FU or Cis in the EC9706 cells (Fig. 3C-E).
α-solanine increases the expression of
miR-138 and inhibits the expression of survivin protein in EC9706
and KYSE30 cells
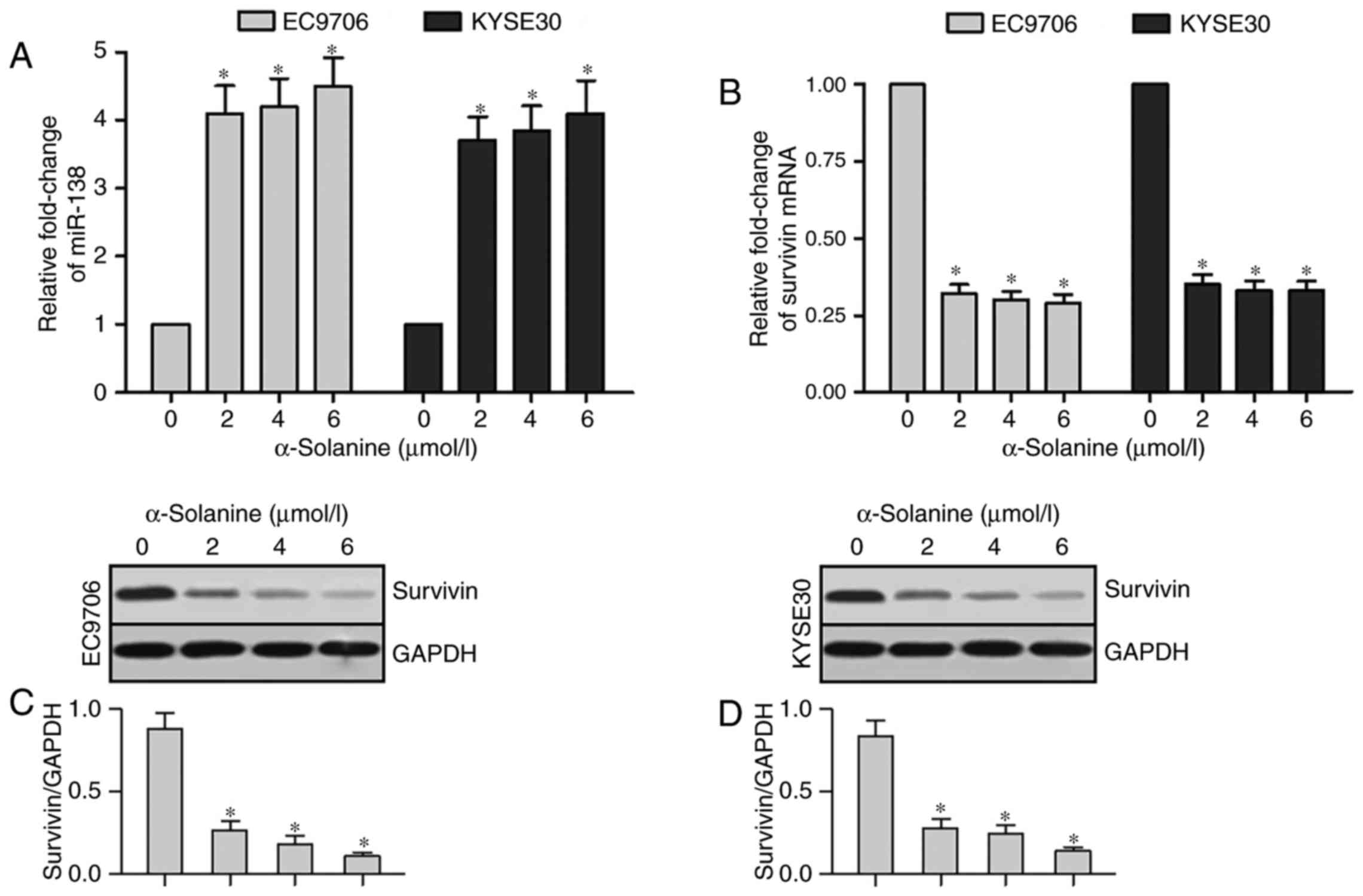
We performed qRT-PCR and western blot analysis tests
to detect the expression of miR-138 and survivin in EC9706 and
KYSE30 cells after treatment with different concentrations of
α-solanine (0, 2, 4 and 6 µmol/l). Expression of miR-138 had an
obvious upregulation in EC9706 and KYSE30 cells after treatment
with α-solanine (2, 4 and 6 µmol/l) compared with that of 0 µmol/l
α-solanine (Fig. 4A, P<0.05).
The results of qRT-PCR revealed that survivin mRNA expression was
significantly downregulated in the EC9706 and KYSE30 cells after
treatment with α-solanine (2, 4 and 6 µmol/l) compared with that of
0 µmol/l α-solanine (Fig. 4B,
P<0.05). Results of western blotting showed a significant
downregulation of survivin protein expression in the EC9706 and
KYSE30 cells treated with α-solanine (2, 4 and 6 µmol/l) when
compared with that of 0 µmol/l α-solanine (Fig. 4C and D, P<0.05). These results
demonstrated that α-solanine can induce the upregulation of miR-138
and downregulation of survivin protein expression in EC9706 and
KYSE30 cells.
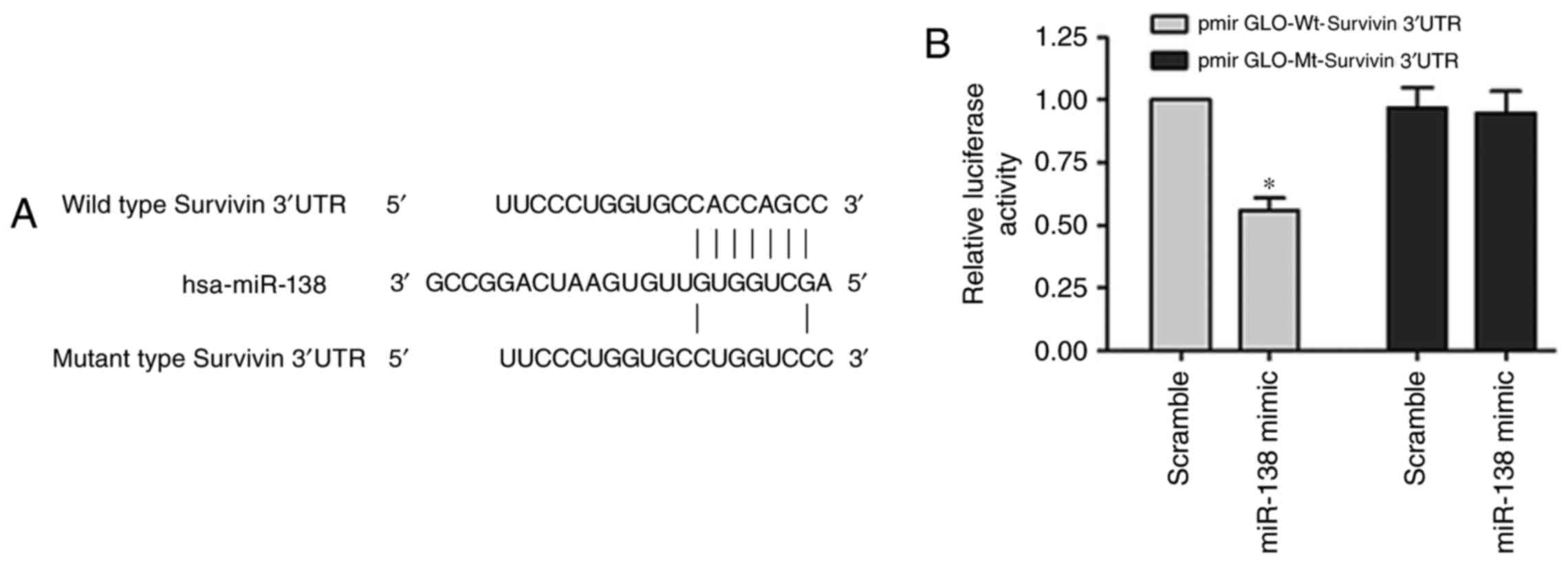
Survivin mRNA is a target of
miR-138
Bioinformatics analyses using TargetScan and miRanda
predicted that the 3′UTR of survivin contains binding sites for
miR-138 (Fig. 5A). The
Dual-Luciferase reporter assay was performed with systems
containing wild-type (pmirGLO-Wt-survivin) or mutant-type
(pmirGLO-Mt-survivin) 3′UTR of survivin, respectively.
Co-transfection with the miR-138 mimic significantly suppressed the
luciferase activity of the reporter containing the wild-type 3′UTR
of survivin (Fig. 5B, P<0.05).
These results indicated that miR-138 negatively regulates survivin
expression by directly binding on the sequence in the 3′UTR of its
mRNA.
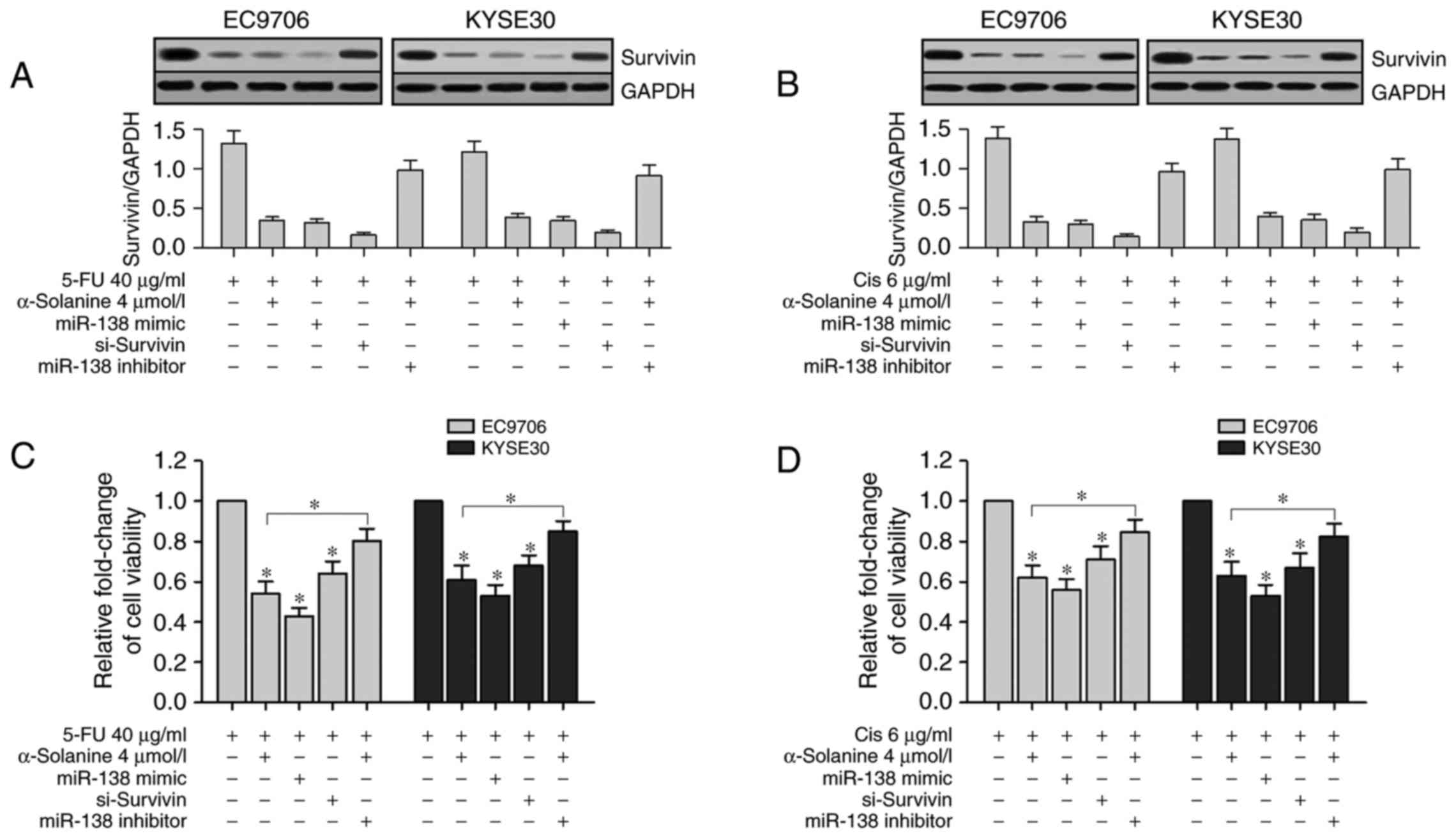
α-solanine enhances the
chemosensitivity of 5-FU and Cis in EC9706 and KYSE30 cells by
upregulation of miR-138 and downregulation of survivin
To validate the relationship between miR-138 and
survivin, we transfected miR-138 mimic, si-survivin and miR-138
inhibitor into EC9706 and KYSE30 cells separately and treated them
with 40 µg/ml 5-FU or 6 µg/ml Cis and/or 4 µmol/l α-solanine.
Western blotting results showed that survivin expression levels in
the α-solanine groups (cells treated with 4 µmol/l α-solanine),
miR-138 groups (cells transfected with miR-138 mimics) and
si-survivin groups (cells transfected with siRNA-survivin) were
significantly suppressed than the Blank group (cells treated with
1‰ DMSO). However, enhanced survivin expression was found in the
miR-138 inhibitor group (cells transfected with miR-138 inhibitor
and treated with 4 µmol/l α-solanine) when compared with the other
groups except the Blank group (Fig. 6A
and B, P<0.05). Then we conducted CCK-8 assay to study the
effects of α-solanine, miR-138, survivin and miR-138 inhibitor on
the chemosensitivity of EC9706 and KYSE30 cells to 5-FU and Cis.
CCK-8 assay revealed that the viability of EC9706 and KYSE30 cells
were significantly inhibited after treatment with 5-FU (40 µg/ml)
or Cis (6 µg/ml) for 24 h in the α-solanine, miR-138 and
si-survivin groups compared with the blank groups; in the miR-138
inhibitor group, the change was slight (Fig. 6C and D, P<0.05). These results
indicated that both the upregulation of miR-138 and the knockdown
of survivin enhanced the chemosensitivity of 5-FU and Cis in the
EC9706 and KYSE30 cells. These results also showed that inhibition
of miR-138 counteracted the function of α-solanine to enhance the
chemosensitivity of 5-FU and Cis in the EC9706 and KYSE30 cells. In
short, α-solanine enhanced the chemosensitivity of 5-FU and Cis in
EC9706 and KYSE30 cells by upregulation of miR-138 and
downregulation of survivin.
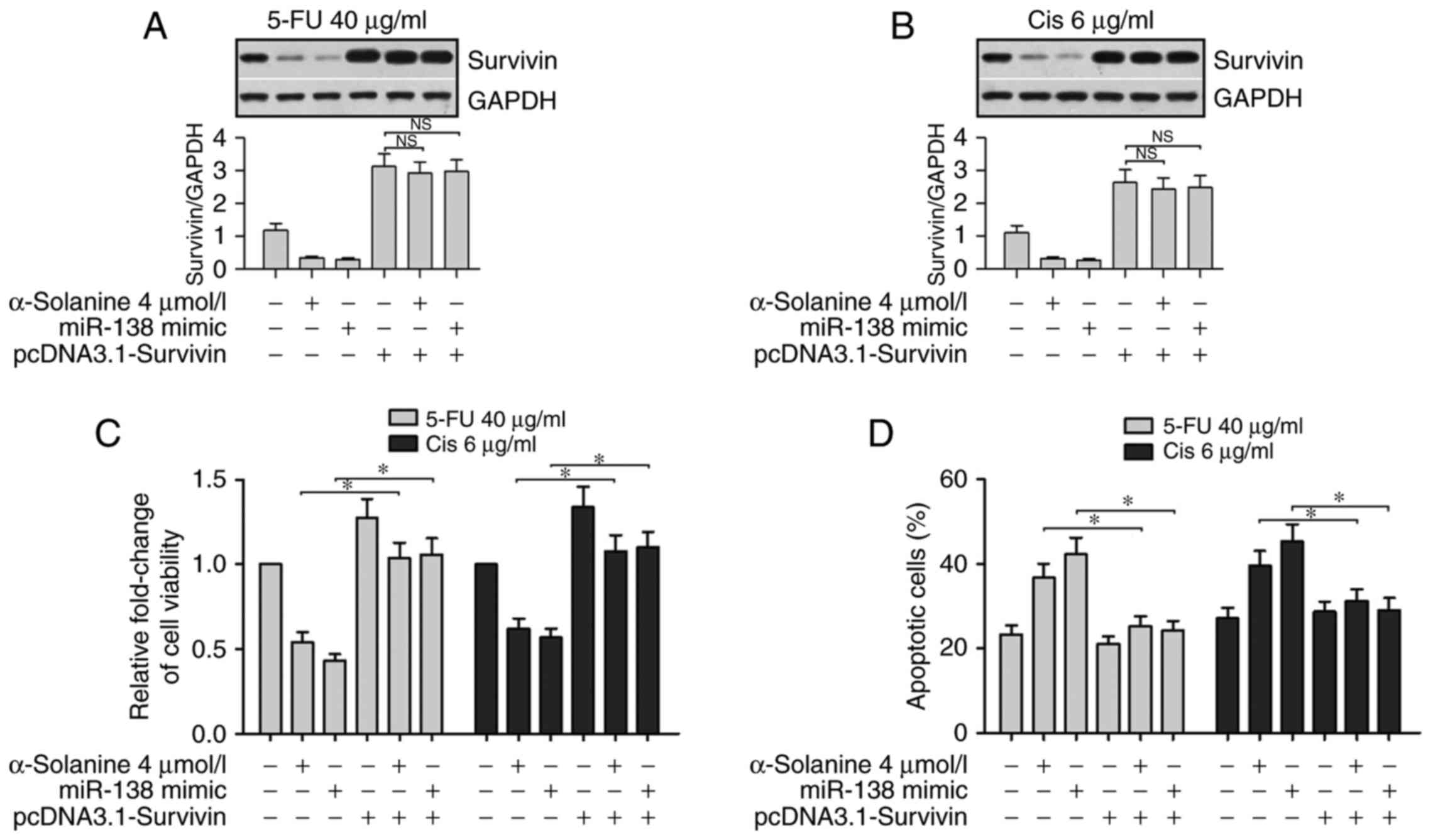
Overexpression of survivin counteracts
the effect of α-solanine and miR-138 on the chemosensitivity of
5-FU and Cis in EC9706 cells
To further validate the relationship between
α-solanine, miR-138 and survivin on the effect of 5-Fu and Cis in
EC9706 cells, we performed western blotting, CCK-8 and flow
cytometric assays. The western blot assay was performed to detect
survivin expression in cells after being treated with 5-FU or Cis
and being simultaneously or separately exposed to α-solanine (4
µmol/l), miR-138 mimics and the vector containing survivin but
lacking the 3′UTR sequence (pcDNA3.1-survivin). The results showed
that expression of survivin was significantly suppressed in the
cells transfected with miR-138 mimics or treated with α-solanine.
However, compared with the baseline level, the expression of
survivin, regardless of miR-138 and α-solanine, was significantly
enhanced in cells transfected with pcDNA3.1-survivin in the 5-FU
and Cis groups (Fig. 7A and B,
P<0.05). Results of the CCK-8 assay and flow cytometric assay
showed that the presence of the miR-138 mimic or α-solanine
significantly promoted the apoptosis of EC9706 cells when treated
with 5-FU (40 µg/ml) or Cis (6 µg/ml). Similarly, overexpression of
survivin offset the effects of miR-138 or α-solanine on the cells
(Fig. 7C and D, P<0.05). These
results further suggest that expression of survivin counteracted
the effect of α-solanine and miR-138 regarding the chemosensitivity
of EC9706 cells.
Discussion
Intrinsic and acquired drug resistance have become
the major challenge faced in anticancer chemotherapy. Recently,
many studies have focused on overcoming this issue by using
small-molecule anticancer compounds to re-sensitize cancer cells to
chemotherapy agents. Among these molecules, α-solanine is a
candidate, which has been proven to have impacts on proliferation,
invasion, metastasis and apoptosis of cancer cells (23–25).
In the present study, we conducted CCK-8 and flow cytometric assays
and found that α-solanine, under non-toxic concentrations, exerted
a sensitizing effect on esophageal cancer cell lines by enhancing
drug-induced apoptosis. In a mouse model, α-solanine also enhanced
the inhibitory effects of anticancer drugs on the xenograft tumor
growth. Therefore, the results suggest that α-solanine is a safe
and effective sensitivity enhancer when used with 5-FU or/and Cis
for esophageal cancer treatment, and it is worth clarifying the
underlying molecular mechanisms in the chemosensitizing function of
α-solanine.
It is estimated that microRNAs can regulate the
expression of more than 50% of human genes at the
post-transcriptional level (26).
Beyond the regulatory functions in normal cells, growing evidence
has highlighted the important roles of microRNAs in various
biological processes of cancers. Among the abundant microRNAs,
miR-138 is a member studied extensively and considered as a tumor
suppressor in many types of cancers (27,28).
In non-small cell lung cancer, overexpression of miR-138 was found
to inhibit the proliferation of cancer cells (29). By suppressing vimentin and zeste
homolog 2 expression, miR-138 restrained the metastasis of breast
and head and neck squamous cancer (30,31).
With regard to chemosensitivity, downregulation of miR-138
expression was observed in vincristine-induced multidrug resistant
leukemia cell line HL-60/VCR relative to HL-60 cells. However,
enforced expression of miR-138 reversed this resistant phenotype by
targeting P-glycoprotein and promoting drug-induced apoptosis
(32). Additionally, upregulation
of miR-138 can restore the sensitivity of lung cancer cell line,
A549/DPP, to cisplatin. Excision repair cross-complementation group
1 (ERCC1), a key enzyme in the DNA repair pathway, was identified
as a target of miR-138 in this case, and thus, downregulation of
ERCC1 impaired DNA damage repair and promoted apoptosis (33). In the present study, significant
upregulation of miR-138 was detected in esophageal cancer cells
treated with α-solanine in the qRT-PCR assay. Promotion of
anticancer drug-induced apoptosis was assessed by flow cytometric
assay. These results indicated that α-solanine exerted its
chemosensitizer function by elevating the expression of miR-138 in
esophageal cancer cells.
We aim to further investigate the molecular
mechanisms responsible for the chemosensitivity enhancing function
of miR-138, and the functions of microRNAs depend on their targets.
Thus, using bioinformatics tools, we predicted an IAP family
member, survivin, as the potential target of miR-138. Survivin is
the smallest IAP family member with only one baculovirus IAP repeat
(BIR) domain, which is essential for the anti-apoptosis function of
survivin. However, the anti-apoptotic mechanisms remain elusive.
Some researchers speculated that survivin, like other IAPs,
interferes with caspase-3 and −7 by directly binding them, and
blocks these terminal effectors to trigger apoptosis (5). Other investigators have reported that
survivin can interact with Smac/DIABLO to block activation of
caspase-9, initiator caspase of intrinsic apoptotic pathway
(34). As known, anticancer
chemotherapy can kill cancer cells by inducing caspase-dependent
apoptosis. However, upregulation of anti-apoptotic factors, such as
survivin is often noted in many types of cancers and renders cancer
cells drug resistant. Therefore, the therapeutic strategy to
suppress survivin expression can free these effector caspases from
inhibitory stress and re-sensitize cancer cells to apoptosis. In
this study, qRT-PCR and western blot assay showed that the
expression trend of survivin was inversely correlated with that of
miR-138 in esophageal cancer cells when treated with α-solanine.
Then, a series of validation assays demonstrated that α-solanine
enhanced the sensitivity of esophageal cancer cells to 5-FU or Cis
via the miR-138/survivin pathway. Survivin is highly expressed in
most human tumors but is completely absent in terminally
differentiated cells (35,36). Modulation of survivin is attractive
as it can focus on inducing cell death of cancer cells rather than
healthy cells. In addition, miR-138 regulates survivin expression
at the post-transcription level, thus it can bypass the upstream
signaling pathway that may cause dysregulation of survivin.
Therefore, we conclude that the α-solanine/miR-138/survivin cascade
may be an effective therapeutic target for esophageal cancer
treatment.
Acknowledgements
The present study was supported by the Education
Department of Henan Province Science and Technology Research
Projects (16A320028).
Glossary
Abbreviations
Abbreviations:
|
5-FU
|
5-fluorouracil
|
|
Cis
|
cisplatin
|
|
IAP
|
inhibitor of apoptosis protein
|
|
PBS
|
phosphate-buffered saline
|
|
LC50
|
semi-lethal concentration
|
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rustgi AK and El-Serag HB: Esophageal
carcinoma. N Engl J Med. 371:2499–2509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He SM, Li R, Kanwar JR and Zhou SF:
Structural and functional properties of human multidrug resistance
protein 1 (MRP1/ABCC1). Curr Med Chem. 18:439–481. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptosis gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tamm I, Wang Y, Sausville E, Scudiero DA,
Vigna N, Oltersdorf T and Reed JC: IAP-family protein survivin
inhibits caspase activity and apoptosis induced by Fas (CD95), Bax,
caspases, and anticancer drugs. Cancer Res. 58:5315–5320.
1998.PubMed/NCBI
|
|
6
|
Rathore R, McCallum JE, Varghese E, Florea
AM and Büsselberg D: Overcoming chemotherapy drug resistance by
targeting inhibitors of apoptosis proteins (IAPs). Apoptosis.
22:898–919. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pennati M, Folini M and Zaffaroni N:
Targeting survivin in cancer therapy. Expert Opin Ther Targets.
12:463–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ye Q, Cai W, Zheng Y, Evers BM and She QB:
ERK and AKT signaling cooperate to translationally regulate
survivin expression for metastatic progression of colorectal
cancer. Oncogene. 33:1828–1839. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Glienke W, Maute L, Wicht J and Bergmann
L: The dual PI3K/mTOR inhibitor NVP-BGT226 induces cell cycle
arrest and regulates Survivin gene expression in human pancreatic
cancer cell lines. Tumour Biol. 33:757–765. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ryan BM, Konecny GE, Kahlert S, Wang HJ,
Untch M, Meng G, Pegram MD, Podratz KC, Crown J, Slamon DJ, et al:
Survivin expression in breast cancer predicts clinical outcome and
is associated with HER2, VEGF, urokinase plasminogen activator and
PAI-1. Ann Oncol. 17:597–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rosato A, Pivetta M, Parenti A, Iaderosa
GA, Zoso A, Milan G, Mandruzzato S, Del Bianco P, Ruol A, Zaninotto
G, et al: Survivin in esophageal cancer: An accurate prognostic
marker for squamous cell carcinoma but not adenocarcinoma. Int J
Cancer. 119:1717–1722. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Singh N, Krishnakumar S, Kanwar RK, Cheung
CH and Kanwar JR: Clinical aspects for survivin: A crucial molecule
for targeting drug-resistant cancers. Drug Discov Today.
20:578–587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qian Y, Ma J, Guo X, Sun J, Yu Y, Cao B,
Zhang L, Ding X, Huang J and Shao JF: Curcumin enhances the
radiosensitivity of U87 cells by inducing DUSP-2 up-regulation.
Cell Physiol Biochem. 35:1381–1393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang SX, Qiu QH, Chen WB, Liang CH and
Huang B: Celecoxib enhances radiosensitivity via induction of
G2-M phase arrest and apoptosis in nasopharyngeal
carcinoma. Cell Physiol Biochem. 33:1484–1497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang BF, Wang XJ, Kang HF, Bai MH, Guan
HT, Wang ZW, Zan Y, Song LQ, Min WL, Lin S, et al: Saikosaponin-D
enhances radiosensitivity of hepatoma cells under hypoxic
conditions by inhibiting hypoxia-inducible factor-1α. Cell Physiol
Biochem. 33:37–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Punjabi S, Cook LJ, Kersey P, Marks R and
Cerio R: Solasodine glycoalkaloids: A novel topical therapy for
basal cell carcinoma. A double-blind, randomized,
placebo-controlled, parallel group, multicenter study. Int J
Dermatol. 47:78–82. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Friedman M, Lee KR, Kim HJ, Lee IS and
Kozukue N: Anticarcinogenic effects of glycoalkaloids from potatoes
against human cervical, liver, lymphoma, and stomach cancer cells.
J Agric Food Chem. 53:6162–6169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lee KR, Kozukue N, Han JS, Park JH, Chang
EY, Baek EJ, Chang JS and Friedman M: Glycoalkaloids and
metabolites inhibit the growth of human colon (HT29) and liver
(HepG2) cancer cells. J Agric Food Chem. 52:2832–2839. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lv C, Kong H, Dong G, Liu L, Tong K, Sun
H, Chen B, Zhang C and Zhou M: Antitumor efficacy of α-solanine
against pancreatic cancer in vitro and in vivo. PLoS One.
9:e878682014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mohsenikia M, Alizadeh AM, Khodayari S,
Khodayari H, Kouhpayeh SA, Karimi A, Zamani M, Azizian S and
Mohagheghi MA: The protective and therapeutic effects of
alpha-solanine on mice breast cancer. Eur J Pharmacol. 718:1–9.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Wu J, Guo W, Sun Q, Chen X, Zang
W, Dong Z and Zhao G: α-solanine modulates the radiosensitivity of
esophageal cancer cells by inducing microRNA 138 expression. Cell
Physiol Biochem. 39:996–1010. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Sleen Y, Wang Q, van der Geest KSM,
Westra J, Abdulahad WH, Heeringa P, Boots AMH and Brouwer E:
Involvement of Monocyte Subsets in the Immunopathology of Giant
Cell Arteritis. Sci Rep. 7:65532017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Meng XQ, Zhang W, Zhang F, Yin SY, Xie HY,
Zhou L and Zheng SS: Solanine-induced reactive oxygen species
inhibit the growth of human hepatocellular carcinoma HepG2 cells.
Oncol Lett. 11:2145–2151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wen Z, Huang C, Xu Y, Xiao Y, Tang L, Dai
J, Sun H, Chen B and Zhou M: α-solanine inhibits vascular
endothelial growth factor expression by down-regulating the
ERK1/2-HIF-1α and STAT3 signaling pathways. Eur J Pharmacol.
771:93–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang F, Yang R, Zhang G, Cheng R, Bai Y,
Zhao H, Lu X, Li H, Chen S, Li J, et al: Anticancer function of
α-solanine in lung adenocarcinoma cells by inducing microRNA-138
expression. Tumour Biol. 37:6437–6446. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Friedman RC, Farh KK, Burge CB and Bartel
DP: Most mammalian mRNAs are conserved targets of microRNAs. Genome
Res. 19:92–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Z, Tang J, Wang J, Duan G, Zhou L and
Zhou X: miR-138 acts as a tumor suppressor by targeting EZH2 and
enhances cisplatin-induced apoptosis in osteosarcoma cells. PLoS
One. 11:e01500262016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang B, Mu W, Wang J, Lu J, Jiang S, Li
L, Xu H and Tian H: MicroRNA-138 functions as a tumor suppressor in
osteosarcoma by targeting differentiated embryonic chondrocyte gene
2. J Exp Clin Cancer Res. 35:692016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye XW, Yu H, Jin YK, Jing XT, Xu M, Wan ZF
and Zhang XY: miR-138 inhibits proliferation by targeting
3-phosphoinositide-dependent protein kinase-1 in non-small cell
lung cancer cells. Clin Respir J. 9:27–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang J, Liu D, Feng Z, Mao J, Zhang C, Lu
Y, Li J, Zhang Q, Li Q and Li L: MicroRNA-138 modulates metastasis
and EMT in breast cancer cells by targeting vimentin. Biomed
Pharmacother. 77:135–141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu X, Wang C, Chen Z, Jin Y, Wang Y,
Kolokythas A, Dai Y and Zhou X: MicroRNA-138 suppresses
epithelial-mesenchymal transition in squamous cell carcinoma cell
lines. Biochem J. 440:23–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhao X, Yang L, Hu J and Ruan J: miR-138
might reverse multidrug resistance of leukemia cells. Leuk Res.
34:1078–1082. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Q, Zhong M, Liu W, Li J, Huang J and
Zheng L: Alterations of microRNAs in cisplatin-resistant human
non-small cell lung cancer cells (A549/DDP). Exp Lung Res.
37:427–434. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Du J, Kelly AE, Funabiki H and Patel DJ:
Structural basis for recognition of H3T3ph and Smac/DIABLO
N-terminal peptides by human Survivin. Structure. 20:185–195. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tanaka C, Uzawa K, Shibahara T, Yokoe H,
Noma H and Tanzawa H: Expression of an inhibitor of apoptosis,
survivin, in oral carcinogenesis. J Dent Res. 82:607–611. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Coumar MS, Tsai FY, Kanwar JR, Sarvagalla
S and Cheung CH: Treat cancers by targeting survivin: Just a dream
or future reality? Cancer Treat Rev. 39:802–811. 2013. View Article : Google Scholar : PubMed/NCBI
|