Introduction
Gastric cancer (GC) is the most lethal malignancy in
the digestive system (1,2). Due to the asymptomatic nature,
non-specific clinical manifestations, and the lack of efficient
screening programs, most GC patients were diagnosised with advanced
stages. The prognosis of patients with advanced GC (AGC) is still
poor even after multidisciplinary therapy combined with surgery,
chemotherapy, and radiation therapy (3). Human epidermal growth factor receptor
2 (HER2) is a member of a family of receptors acting as a
proto-oncogene. HER2 is overexpressed in ~20% of GC, and now it is
an important target for GC. Trastuzumab, a monoclonal antibody that
targets HER2, inhibits HER2-mediated signaling and thus induces
antibody-dependent cellular cytotoxicity against tumor cells
(4,5). The introduction of trastuzumab made a
new term in HER2-positive GC.
Trastuzumab combined with chemotherapy demonstrates
a significant survival benefit in patients with HER2-positive AGC
in the clinic. In ToGA study (trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-esophageal junction
cancer), an open-label, international, phase III, randomized
controlled trial, patients with HER2-postive GC were randomly
assigned to receive a standard chemotherapy regimen or chemotherapy
in combination with trastuzumab. Results showed that those who
received trastuzumab plus chemotherapy had prolonged overall
survival (OS) (6). Furthermore,
with the development of antibody-drug conjugate (ADC) technique,
strategies which take advantage of selective delivery of anticancer
drugs by monoclonal antibodies to antigen-expressing tumor cells
have proved efficacy. For example, ado-trastuzumab emtansine
(T-DM1) consists of trastuzumab linked to cytotoxic agent of
emtansine as a single agent, which shows statistically significant
antitumor efficacy and minor systemic toxicity in HER2-positive
tumors (7–9). In GC, T-DM1 showed a promising
antitumor efficacy in HER2-positive GC cell lines in vitro
and xenograft tumors in vivo (10,11).
Nowadays, several clinical trials are underway in patients with
HER2-positive AGC, including the efficacy and safety of T-DM1
compared with paclitaxel (PTX) (9).
However, T-DM1 does not show survival benefit in HER2-positive AGC
as the expected remarkable efficacy for HER2-positive breast cancer
(12). Therefore, HER2-directed
therapies for HER2-positive GC remains a challenge.
Fortunately, nanomedicine has a significant impact
on cancer therapy with the introduction of drug delivery system
based on nanobiotechnology, such as nanoparticle albumin-bound
paclitaxel (nab-paclitaxel). Nab-paclitaxel is a solvent-free
suspension of paclitaxel and human serum albumin (HSA) with an
average mini size, which allows better delivery of paclitaxel into
tumors via the unique mechanism (gp60-caveolin-1-SPARC) and passive
targeting of EPR (enhanced permeability and retention) effect. To
date, many studies have proved the superiority of nab-paclitaxel in
tumors, including GC (13–18). However, its clinical success is
stilled limited by unfavorable pharmacokinetics, suboptimal
biodistribution and toxicities (19). Antibody-nanoparticle conjugate (ANC)
offers opportunities for optimized targeted cancer treatment
(20,21). In view of these studies, here, the
conjugation of the two clinical drugs of nab-paclitaxel and
trastuzumab is presented as an ANC single-agent
(trastuzumab/nab-paclitaxel) for precisly targeted therapy of
HER2-positive GC.
Materials and methods
Main materials
Abraxane® [paclitaxel for injection
(albumin bound), lot no. 6109342] was from Celgene (Fresenius Kabi
USA, LLC Melrose Park, IL, USA). Taxol® (paclitaxel
injection) was from Bristol-Myers Sguibb (Corden Phama Latina
S.P.A, Sermoneta, Italy). Herceptin® (trastuzumab, lot
no. ab2428) was purchased from Abcam. FBS (fetal bovine serum, lot
no. 1698221) was purchased from Gibco Chemical Co. (Carlsbad, CA,
USA). Pen Strep (penicillin streptomycin, lot no. 1665735), 0.25%
trypsin-EDTA and PBS (phosphate-buffered saline, lot no. AAL211089)
were purchased from HyClone (GE Healthcare Life Sciences). Matrigel
basement membrane matrix (lot no. 356324) was from Corning Inc.
(Corning, New York, NY, USA). EDC (C8H17N3HCL,
N-(3-dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride, cat.
no. 25952-53-8) and NHS (C4H5NO2,
N-hydroxysuccinimide, cat. no. 6066-82-6) were from Sigma-Aldrich
(St. Louis, MO, USA). Cell Counting Kit-8 (CCK-8) was purchased
from Dojindo Laboratories, (Kumamoto, Japan). Annexin V-FITC/PI
Apoptosis Detection kit was from Nanjing KeyGen Biotech Co.
(Nanjing, China). Hematoxylin-eosin staining kit from Beyotime
Institute of Biotechnology (Shanghai, China). BALB/c nude mice
(weighing 20-22 g, 5 weeks old, half were male and half female)
were obtained from Comparative Medicine Centre, Yangzhou University
(Yangzhou, Jiangsu, China). Monoclonal antibodies of Bax (cat. no.
ssc-493), Bcl-2 (cat. no. sc-509), caspase-3 (cat. no. ssc-271759),
caspase-8 (cat. no. sc-56071), caspase-9 (cat. no. sc-17784) and
survivin (cat. no. sc-101433) was from Santa Cruz Biotechnology
Inc. (Santa Cruz, CA, USA). NIR797-isothiocyanate (MW 880.14 Da)
was purchased from Sigma-Aldrich Co. The transmission electron
microscopic (TEM) images were obtained by a JEM-2100, JEOL Ltd.
(Tokyo, Japan). Flow cytometry analysis was performed by BD
FACSCalibur (BD Biosciences, San Jose, CA, USA). Cells were imaged
using a confocal microscope (Zeiss LSM 710; Carl Zeiss, Oberkochen,
Germany), and the tumor-bearing nude mice were imaged with a
Caliper IVIS (in vivo imaging system; Perkin-Elmer, Waltham,
MA, USA). All the experiments were conducted according to the
manufacturer's protocols. The reagents were of analytical
grade.
Preparation of ANC of
trastuzumab/nab-paclitaxel
ANC of trastuzumab/nab-paclitaxel was synthesized
using EDC/NHS by surface activation method. Briefly, 500 µl of
nab-paclitaxel was dissolved in 1 ml of PBS followed by the
addition of 100 µl NHS (5.75×10−7 g/ml) and 100 µl EDC
(2.3×10−7 g/ml). After spining at 10 rpm for 120 min, 20
µl of trastuzumab (0.2 mg/ml) was added in the suspension. After
further rotation at 10 rpm for 120 min at 4°C, it was
ultracentrifuged at 1×104 rpm, 4°C for 15 min to remove
excess EDC/NHS and unconjugated trastuzumab. The process was
repeated 3 times after sonication. Further, the ANC of
trastuzumab/nab-paclitaxel were resuspended in 1 ml PBS and stored
at −20°C. Morphological characteristics of the ANC of
trastuzumab/nab-paclitaxel were examined using a TEM. Dynamic light
scattering (DLS) was performed to determine the hydrodynamic radius
(Rh) of trastuzumab/nab-paclitaxel at 25°C using a Dynapro™ plate
reader (Wyatt Technology, Santa Barbara, CA, USA).
Cell culture
The human HER2-postive GC cell line, NCI-N87,
purchased from Cell Bank of Chinese Academy of Sciences (Shanghai,
China) and were cultured in RPMI-1640 supplemented with 10% heat
inactivated fetal bovine serum (FBS), 1% Pen Strep in a humidified
incubator in 5% CO2 at 37°C.
Cell viability assay
NCI-N87 cells were seeded in 96-well plates at a
density of ~5,000 cells per well and treated with different
treatments, paclitaxel, nab-paclitaxel and
trastuzumab/nab-paclitaxel, respectively. Cell without treatment
were used as a control. After further incubation for 48 h at 37°C,
the relative cell viability was assessed using CCK-8 assays.
Briefly, after the different treatments, CCK-8 (10 µl) was added to
each well and incubated for a further 2 h. Optical density (OD) at
450 nm was read on an ELx800 microplate reader (BioTek, Vermont,
WI, USA), and then the cell viability was calculated as
follows:
OD(450 nm in test cells)/OD(450 nm
in control cells) × 100%
Cell cycle analysis
NCI-N87 cells were seeded in 6-well plates. After
different treatments, cells were collected, fixed in 75% cold
ethanol at 4°C for at least 2 h, washed by cold phosphate-buffered
saline, stained with PI/RNase staining buffer for 15 min, and then
measured by flow cytometry.
Apoptosis assay
Quantification of apoptotic cells was determined
using an Annexin V-fluorescein isothiocyanate (FITC)/propidum
iodide (PI) detection kit (BD Pharmingen, San Diego, CA, USA)
according to the manufacturer's instructions. Cells were collected
after different treatments, washed twice with cold PBS and
resuspended in 100 µl binding buffer, followed by staining with 5
µl of Annexin V-FITC and 10 µl of PI solution at room temperature
in the dark for 15 min. Analyses were then performed using a
FACSCalibur™ flow cytometer.
Morphological characteristics of the
nucleus by DAPI stain
The cells were first cultured on slides in 24-well
plates at a density of 1×104 cells/well. After treatment
with different agents, the cells on the slides were fixed by
incubation in 4% paraformaldehyde (PFA) for 30 min. After washing
with PBS three times, the cells were incubated in 1 mg/ml DAPI in
methanol for 30 min in the dark. The cells were then observed with
a fluorescence microscope.
Western blot analysis
After different treatments, cells were lysed, and
the isolated protein was quantified using the Bradford method,
subjected to sodium dodecyl sulfate polyacrylamide gel
electrophoresis, and then transferred on to a polyvinylidene
fluoride membrane. After being blocked, the membrane was incubated
with primary polyclonal antibodies of anti-Bax, Bcl-2, caspase-3,
caspase-8, caspase-9, survivin and GAPDH overnight at 4°C, and
subsequently incubated with horseradish peroxidase-conjugated IgG
antibody as the secondary antibody for 1 h at room temperature. The
protein bands were detected using an enhanced
electrochemiluminescence detection system (ECL system; Amersham
Pharmacia Biotech, Amersham, UK). After normalization using the
corresponding GAPDH expression, the expression levels of Bax,
Bcl-2, caspase-3, caspase-8, caspase-9 and survivin were determined
using densitometry scans.
Gastric cancer xenograft model in nude
mice
In vivo antitumor efficacy of paclitaxel,
nab-paclitaxel and trastuzumab/nab-paclitaxel were evaluated
through tumor-bearing mice. BALB/c nude mice were kept in
filter-topped cages with standard rodent chow, water available
ad libitum, and a 12-h light/dark cycle. The experiment
protocol was approved by the Committee on Ethical Animal
Experiments at Southeast University. The mice were fed with sterile
food in a specific pathogen-free facility. All mice were injected
subcutaneously with 5×106 NCI-N87 cells. The length (a)
and width (b) of the tumor were measured every other day. Tumor
volumes (V/mm3) were calculated using the formula: V = ½
× a × b2. When tumor volumes reached ~60 mm3
(22), the mice were randomly
divided into four groups: PBS as control group, paclitaxel,
nab-paclitaxel and trastuzumab/nab-paclitaxel (all the groups were
treated with equivalent paclitaxel concentration at 20 mg/kg). The
intravenous treatment was done twice a week for four times. The RTV
(relative tumor volume) = VX/V1, where
VX and V1 represent the volumes on day X and
the first day of tumor treatment. The antitumor efficacy of tumor
inhibition rate is defined as both of the tumor volume and weight
inhibitory rate, which is calculated using the formula: volume
inhibitory rate (%) = (1-RTVaverage experimental
group/RTVaverage control group) × 100%.
In vivo imaging of NIR-797-labeled
trastuzumab/nab-paclitaxel
Firstly, NIR-797-labeld trastuzumab/nab-paclitaxel
were synthesised by physical adsorption. Briefly, 1 mg of NIR-797
was added to 1 ml of paclitaxel, nab-paclitaxel and
trastuzumab/nab-paclitaxel (5 mg/ml paclitaxel equivalent)
respectively. After spining at 10 rpm overnight, the suspensions of
trastuzumab/nab-paclitaxel were ultracentrifuged at 10,000 rpm, 4°C
for 15 min to remove unadsorpted dye, finally the precipitations
were resuspended in 1 ml of PBS for injection. For in vivo
imaging, the tumor-bearing mice were injected via tail vein at a
single dose of NIR-797-labled paclitaxel, NIR-797-labled
nab-paclitaxel and NIR-797-labled trastuzumab/nab-paclitaxel at 20
mg/kg paclitaxel equivalent concentration when the tumors reached
~60 mm3. The mice were imaged in a small animal imaging
system by X-ray and fluorescence at 0, 2, 4, 8, 24, 48 and 72 h
after injection.
Histopathological examination
After different treatments, the mice were sacrificed
by cervical dislocation. The organs, including lung, heart, liver,
spleen, kidney and tumor tissues isolated from each group were
immersed in 4% PFA solution, embedded in paraffin, and stained with
hematoxylin-eosin. Thereafter, the tissues were examined using an
Olympus IX51 microscope (×200; Olympus Corp., Tokyo, Japan).
Statistical analysis
GraphPad Prism 5.0 (GraphPad software; San Diego,
CA, USA) was used for all statistical analysis. The mean ± SD was
determined for each group in the individual experiments. The
Student's t-test was used to determine the significance of
differences between different groups. P-values <0.05 were
considered to indicate statistically significant difference.
Results
Characterization of
trastuzumab/nab-paclitaxel
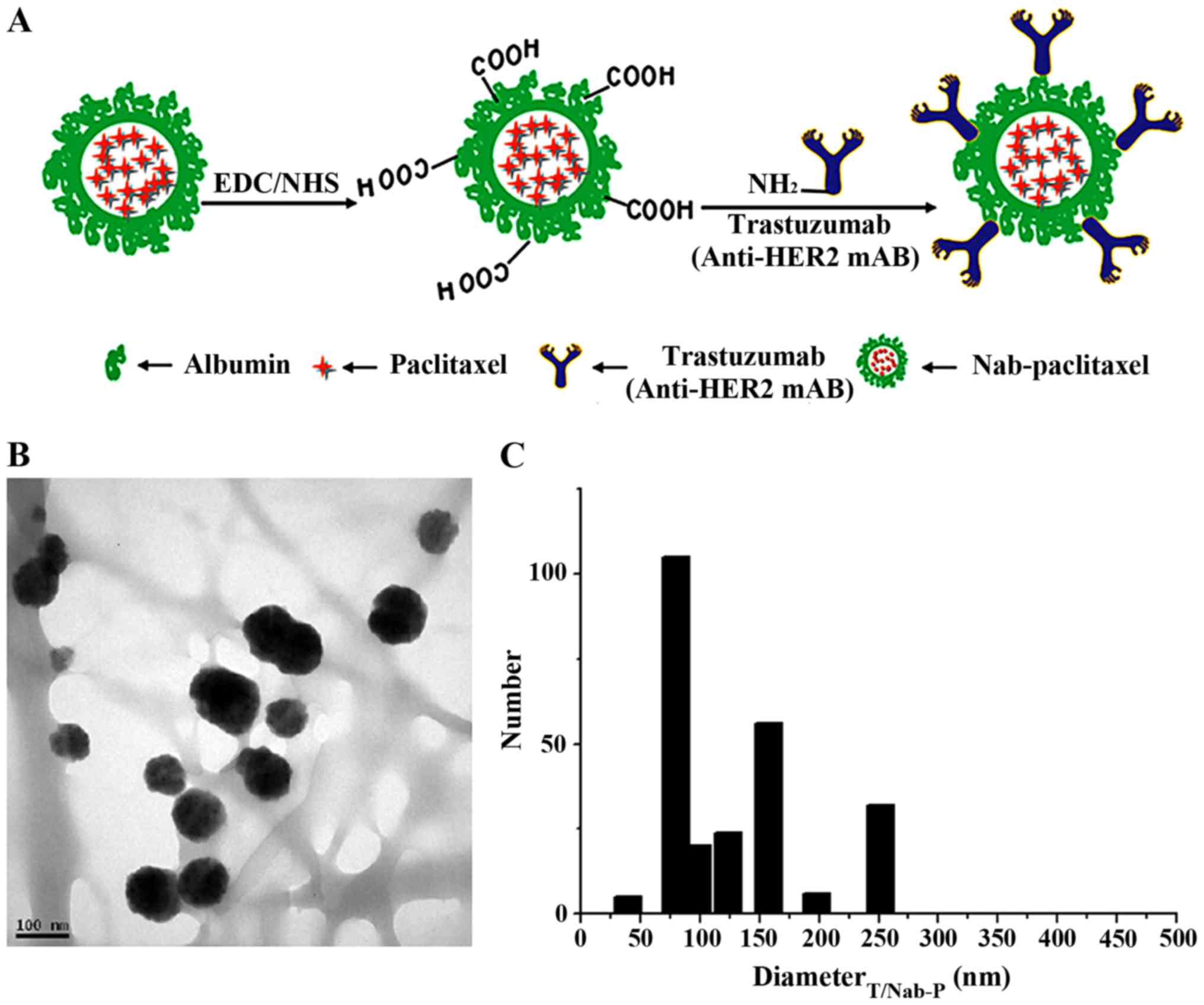
The scheme for the preparation of
trastuzumab/nab-paclitaxel is presented in Fig. 1A. The TEM images in Fig. 1B show the trastuzumab/nab-paclitaxel
is spherical in shape and in a suitable size (139.18±32.06 nm)
measured by DLS (Fig. 1C) for drug
delivery. The concentration of paclitaxel of
trastuzumab/nab-paclitaxel by UV spectrophotometry indicates that
the concentration of paclitaxel was one-tenth of
nab-paclitaxel.
In vitro antitumor efficacy
To determine the antitumor efficacy in vitro,
we measured the cytotoxicity following treatment of HER2-postive GC
NCI-N87 cells with paclitaxel, nab-paclitaxel and
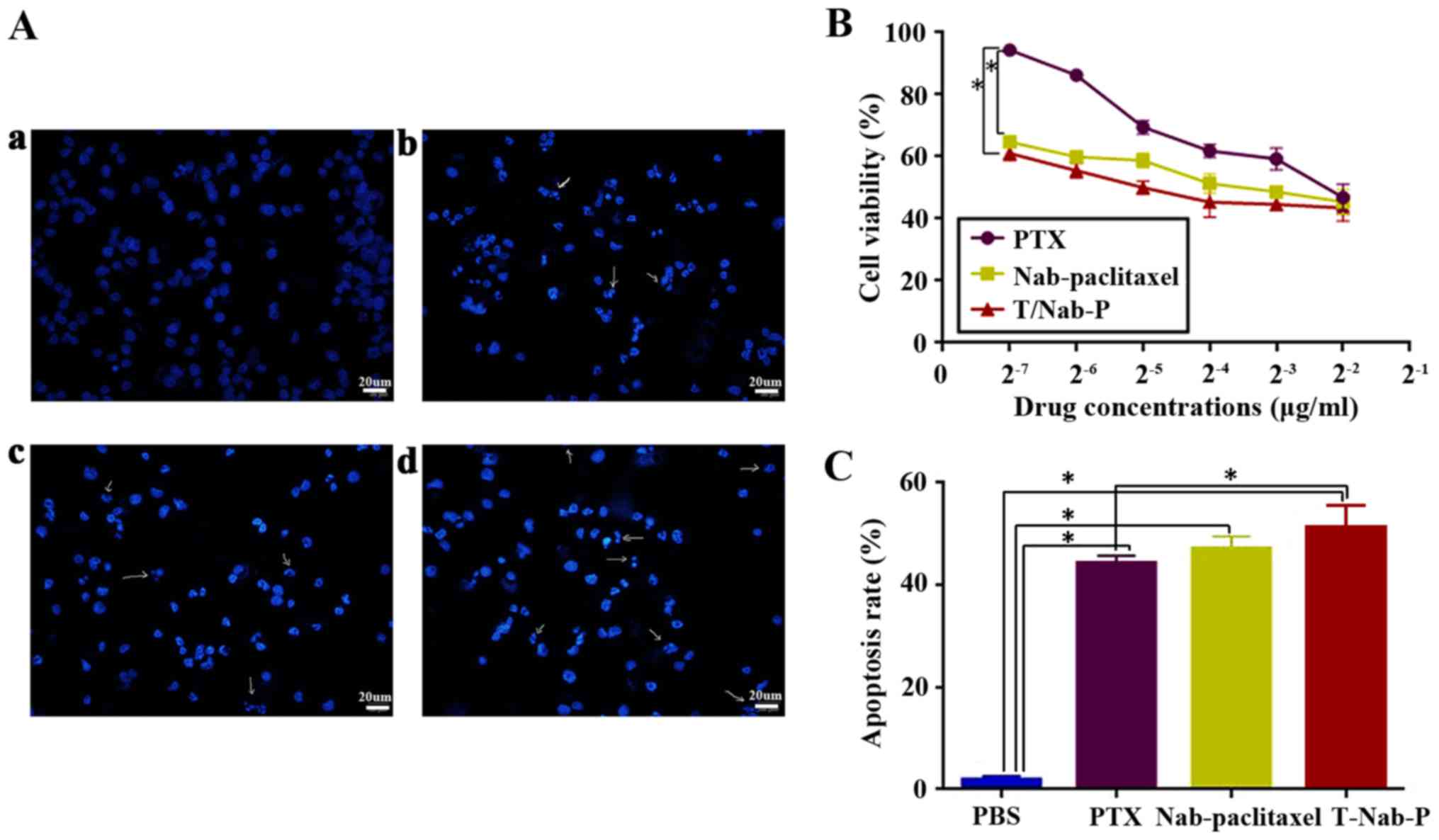
trastuzumab/nab-paclitaxel for 48 h. As shown in Fig. 2A, typical characteristic apoptotic
changes, such as chromatin condensation, convoluted nuclei with
cavitations, fragmentation of the nucleus, and apoptotic bodies,
could be found after different treatments. That is to say,
paclitaxel, nab-paclitaxel, and trastuzumab/nab-paclitaxel could
inhibit the growth of NCI-N87 cells, whereas
trastuzumab/nab-paclitaxel was found to be more cytotoxic than
paclitaxel and nab-paclitaxel (Fig.
2B). The half-maximal inhibitory concentration
(IC50), defined as the concentration of paclitaxel to
kill 50% of cells, was found to be 0.24±0.08, 0.13±0.03 and
0.048±0.01 µg/ml for paclitaxel, nab-paclitaxel and
trastuzumab/nab-paclitaxel, respectively, with an excellent
dose-effect relationship, suggesting that the killing effects of
these drugs were dose-dependent. Similarly, the apoptosis rate was
higher in trastuzumab/nab-paclitaxel group (51.30±2.28%) than
paclitaxel group (43.32±1.08%) and nab-paclitaxel group
(46.64±1.47%) in NCI-N87 cells (Fig.
2C). These data clearly suggest that trastuzumab/nab-paclitaxel
could enhance cytotoxic effect against NCI-N87 cells better than
paclitaxel and nab-paclitaxel in vitro.
Cell cycle distributions of NCI-N87
cells
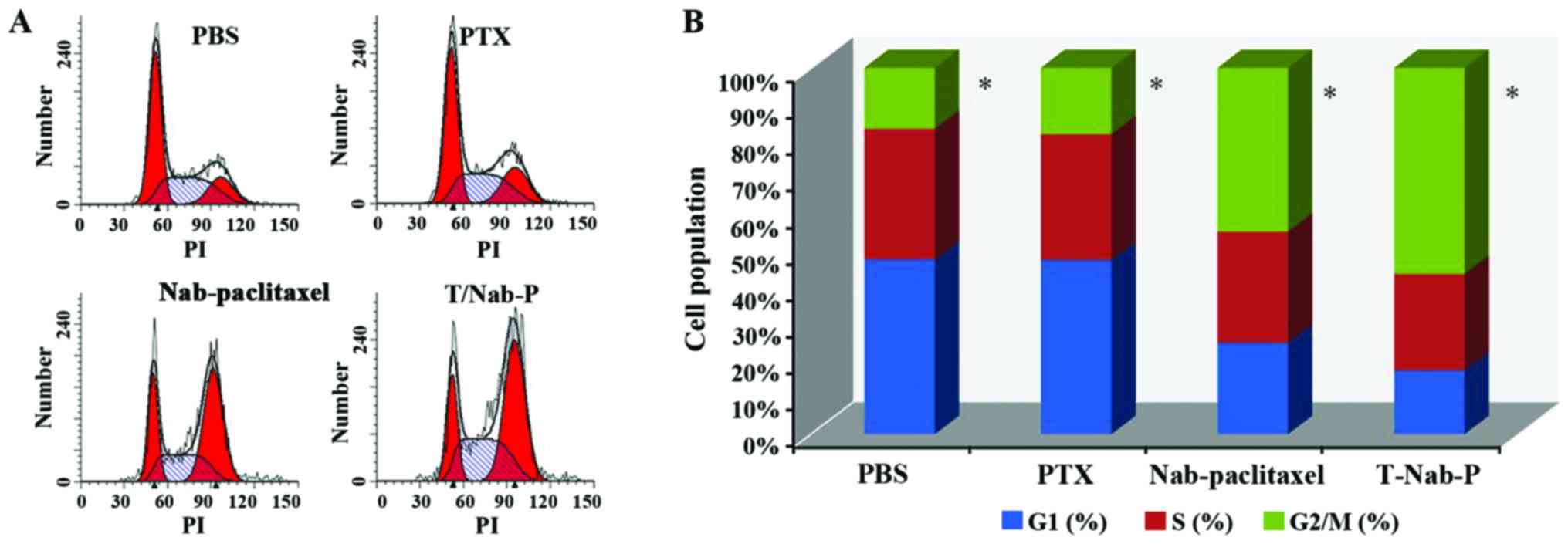
We quantified the population of NCI-N87 cells in
different stages of the cell cycle upon treatments with paclitaxel,
nab-paclitaxel and trastuzumab/nab-paclitaxel, respectively, at a
concentration of 0.24 µg/ml paclitaxel equivalent for 48 h. In our
study, after different treatments, G2/M arrest was frequently
observed in NCI-N87 cells. Moreover, trastuzumab/nab-paclitaxel
showed more significant G2/M arrest (56.35±2.14%) than that of
paclitaxel (18.17±1.34%) and nab-paclitaxel (44.83±2.58%), which is
shown in Fig. 3.
Apoptosis induction in NCI-N87
cells
Similar to the two clinical drugs paclitaxel and
nab-paclitaxel, the novel ANC of trastuzumab/nab-paclitaxel could
also induce apoptosis in NCI-N87 cells (Fig. 4A). The early apoptotic rates of
NCI-N87 cells treated by trastuzumab/nab-paclitaxel (20.8±0.28%) is
higher than that of nab-paclitaxel (14.9±0.17%), paclitaxel
(12.7±0.65%), and PBS as control (2.8±0.12%) (Fig. 4B). To explore the possible signaling
pathways involved in apoptosis, we examined the changes of the
apoptosis-related proteins, including caspase-3, caspase-8,
caspase-9, Bax, Bcl-2, and survivin by western blotting. As shown
in Fig. 4C and D, when treated with
paclitaxel, nab-paclitaxel and trastuzumab/nab-paclitaxel for 48 h,
the levels of both Bcl-2 and survivin in NCI-N87 cells were
significantly downregulated, while those of Bax, caspase-3,
caspase-8, caspase-9 protein were upregulated as well as the ratio
of Bax/Bcl-2.
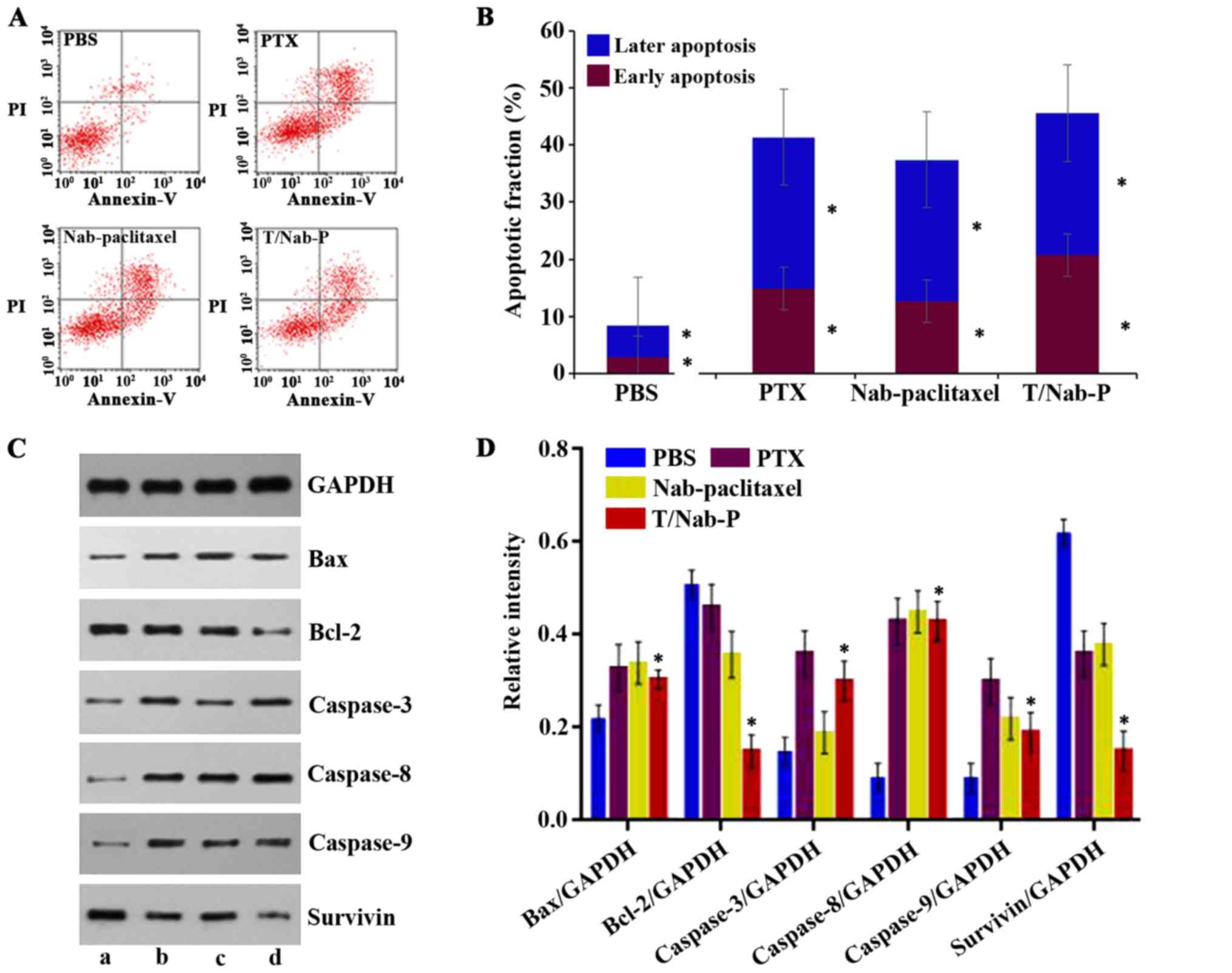 | Figure 4.Induction of apoptosis in NCI-N87
cells by different treatments. (A) NCI-N87 cells were treated with
paclitaxel, nab-paclitaxel and trastuzumab/nab-paclitaxel at
concentration of 0.24 µg/ml for 48 h, while PBS group was the
control. (B) The apoptosis rates are calculated by adding up the
percentage of early apoptosis rate and later apoptosis rate. (C)
Western blot analysis of endogenous and different treatments (a)
PBS, (b) paclitaxel, (c) nab-paclitaxel, (d)
trastuzumab/nab-paclitaxel)-induced Bax, Bcl-2, caspase-3,
caspase-8, caspase-9 and survivin protein levels in NCI-N87 cells.
(D) The relative intensity of Bax, Bcl-2, caspase-3, caspase-8,
caspase-9 and survivin protein after 48-h treatment. |
In vivo antitumor efficacy
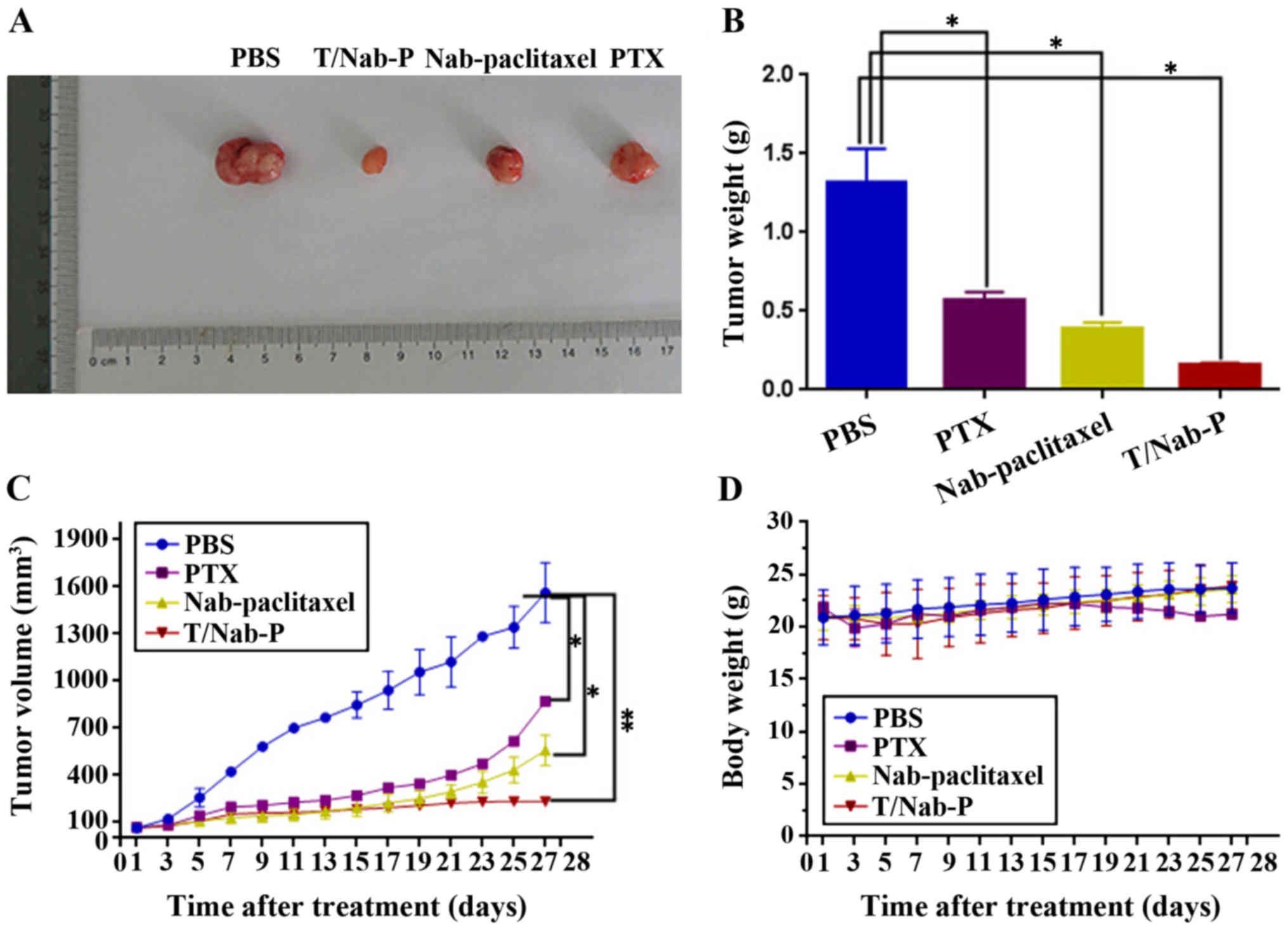
Antitumor efficacy of trastuzumab/nab-paclitaxel
compared with paclitaxel and nab-paclitaxel were further evaluated
in NCI-N87 xenograft models at a 20 mg/kg paclitaxel equivalent
dose in vivo. During the experiment period, all the mice
were weighed and tumor volumes were measured every other day. At 4
weeks after treatment, mice were sacrificed. Then the mouse tumors
were imaged (Fig. 5A) and tumor
weight was recorded (Fig. 5B). Mean
tumor volume treated with trastuzumab/nab-paclitaxel was 233±24
mm3, nab-paclitaxel of 559±97 mm3, paclitaxel
of 871±94 mm3 and PBS as control of 1,576±190
mm3 (Fig. 5C). Obvious
significant tumor regression was obtained in mice treated with
trastuzumab/nab-paclitaxel compared with paclitaxel and
nab-paclitaxel. In addition, trastuzumab/nab-paclitaxel compared
with nab-paclitaxel and paclitaxel did not have significanly
increased body weight (Fig.
5D).
NIRF imaging and biodistribution of
NIR-797-labeled drugs in tumor-bearing mice
To visualize the biodistribution of
trastuzumab/nab-paclitaxel in NCI-N87 tumor-bearing mice, a
near-infrared fluorescent (NIRF) dye, NIR-797, was labeled to
paclitaxel, nab-paclitaxel and trastuzumab/nab-paclitaxel. NIR
fluorescence signals were clearly and dynamically observed in the
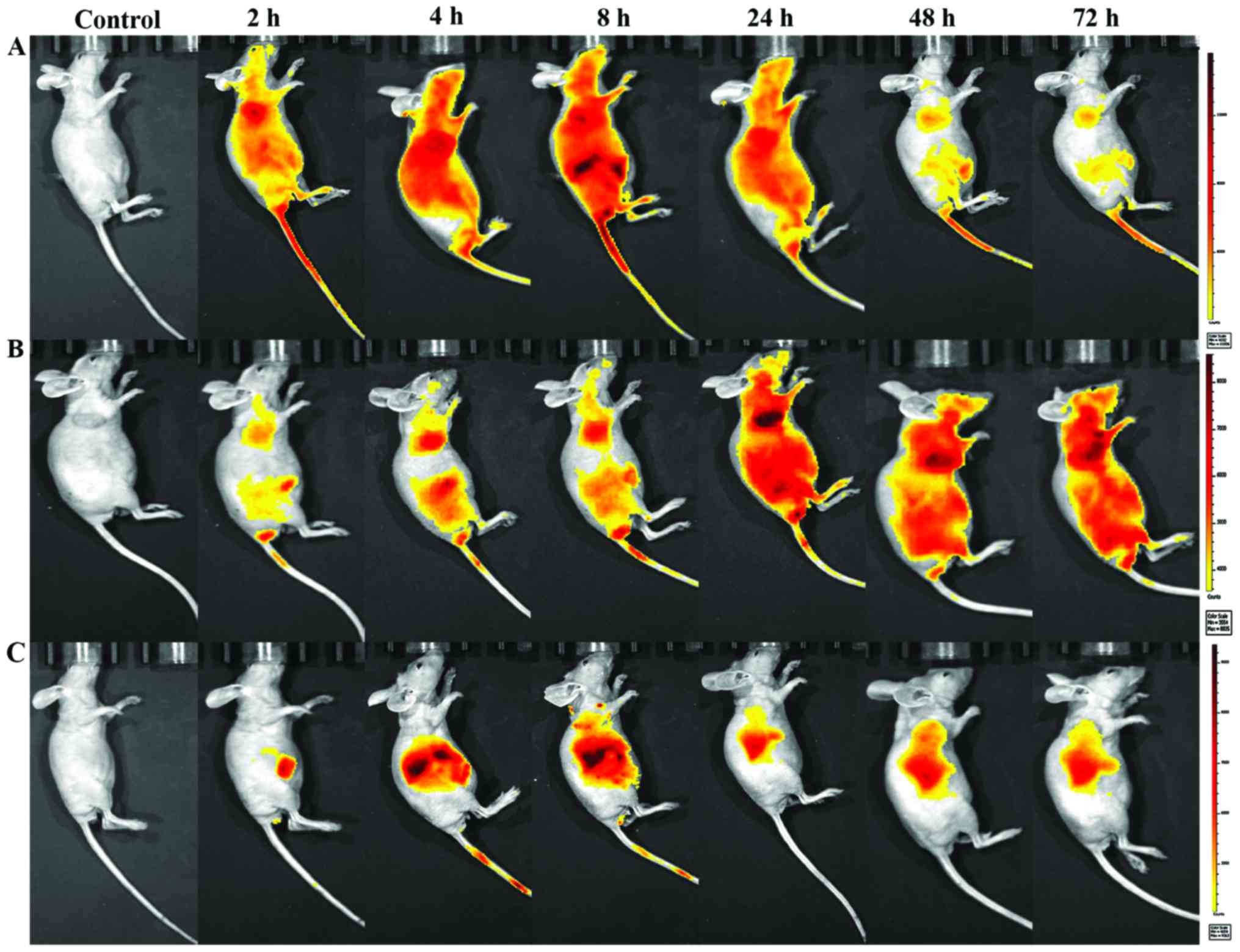
mice. As shown in Fig. 6, 2 h after
injection, these fluorescence signals of NIR-797-labeled paclitaxel
were strong and mainly localized in the body area, but it began to
decrease after 8 h and decreased gradually especially in the tumor
site with no distribution in the brain. Obvious increased
accumulation of NIR fluorescence signals was observed in the tumor
site of the mice injected nab-paclitaxel and
trastuzumab/nab-paclitaxel. What is inspiring, as time increased,
the fluorescence signals of tumors became stronger and the
fluorescence signals of liver began to weaken. Fluorescence signals
of NIR-797-labeled trastuzumab/nab-paclitaxel remains strong until
48 h after injection, at which point the fluorescence signals of
NIR-797-labeled nab-paclitaxel has already decreased. In addition,
72 h after injection, fluorescence signals could only be seen in
the liver and tumor site which indicated a better targeting and
sustained release of trastuzumab/nab-paclitaxel. Furthermore, these
fluorescence signals of trastuzumab/nab-paclitaxel group were only
observed in liver and tumor areas.
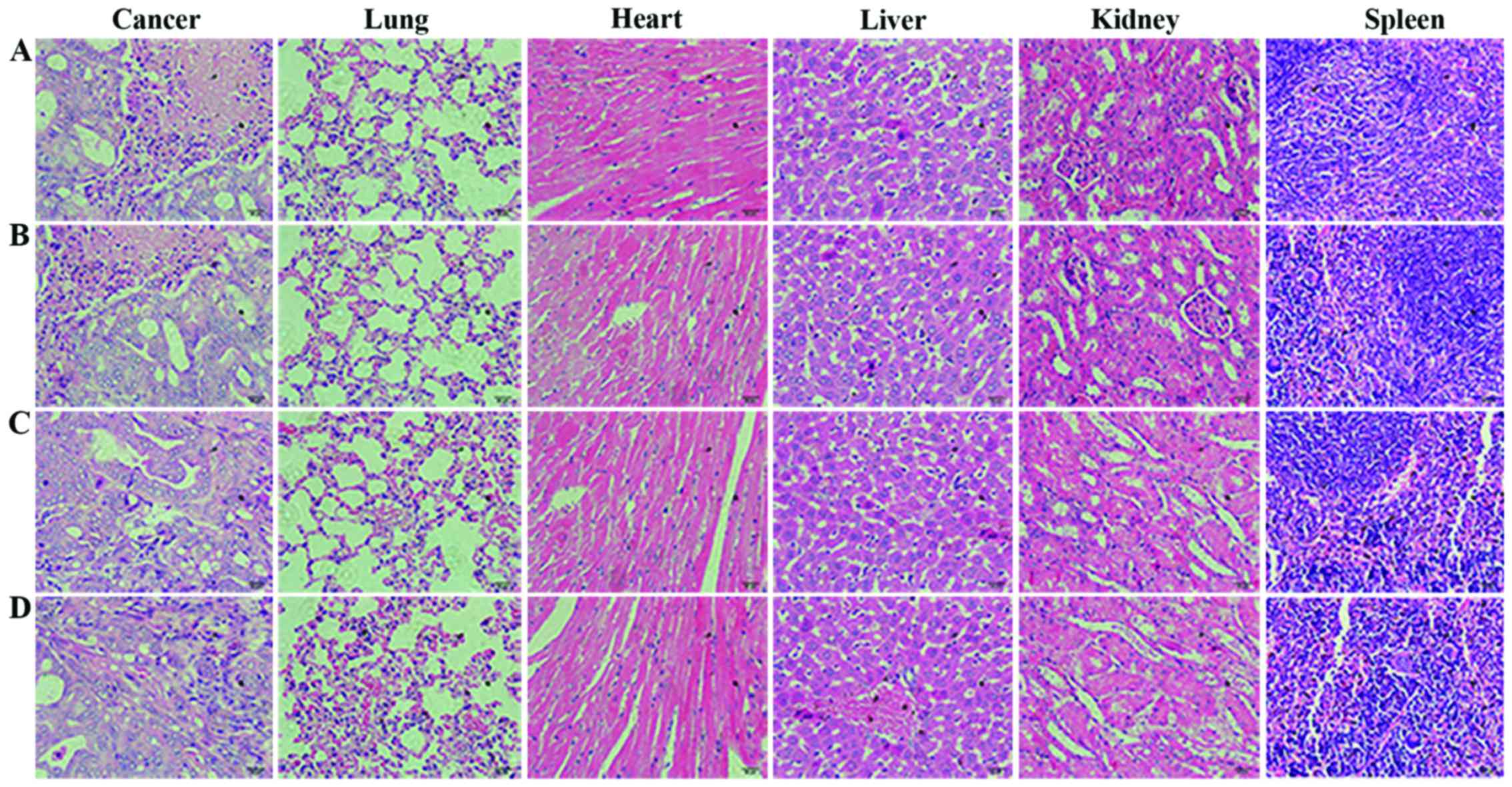
Histopathological examination
We carried out histological bioanalysis of organs to
evaluate the potential side effects of trastuzumab/nab-paclitaxel
on the main organs of mice in vivo. As shown in Fig. 7, there were no apparent
histopathologic changes in the tissues, including heart, liver,
spleen, lung and kidney.
Discussion
AGC remains one of the most lethal malignancies due
to its intrinsic resistance and its aggressiveness to standard
chemotherapy and targeted therapy. Nab-paclitaxel as carriers of
chemotherapeutic drugs to reverse the toxicity of paclitaxel have
displayed that nab-paclitaxel can significantly increase the
antitumor effection and still maintain a certain concentration. In
clinic, trastuzumab combined with chemotherapy as ADC demonstrates
a significant survival benefit in patients with HER2-positive AGC.
Thus, in this study, we further constructed the ANC of
trastuzumab/nab-paclitaxel for AGC.
Apoptosis is the preferred anticancer manner
regulated by genes (23–25). Many oncogenes and tumor suppressor
genes are involved in the regulation of apoptosis; the
proto-oncogene Bcl-2 is the most important (26). Bcl-2 and Bax are a pair of positive
and negative regulators of genes; Bax induced apoptosis, while
Bcl-2 inhibited apoptosis (27).
Caspase-3 may promote apoptosis, while survivin is the strongest
inhibitor of apoptosis in AGC, also promoting cell proliferation,
which can directly inhibit the activity of caspase-3 and caspase-8
and caspase-9, thereby blocking the apoptotic process (28,29).
These results of apoptosis phenomenon in NCI-N87 cells treated with
PBS, paclitaxel, nab-paclitaxel and trastuzumab/nab-paclitaxel by
western blotting observation showed that ANC of
trastuzumab/nab-paclitaxel could decrease Bcl-2 and survivin
protein expression, and increase Bax and caspase-3 protein
expression; the difference was more significant than the control
group. Caspase activation is generally considered to be the
hallmark of apoptosis, and caspase-3 is the main effector caspase
that is involved in apoptosis. These results indicate that
trastuzumab/nab-paclitaxel could induce anticancer activity in a
caspase-dependent manner, and effectiveness of the survivin
pathway.
Paclitaxel's mode of antitumor action is the
disruption of microtubule dynamics, and paclitaxel is believed to
mediate G2/M cell cycle arrest in various cancer cells, including
GC (30–32). In our study, after different
treatments, G2/M arrest was frequently observed in NCI-N87 cells.
Furthermore, trastuzumab/nab-paclitaxel showed more significant
G2/M arrest (56.35±2.14%) than that of paclitaxel (18.17±1.34%) and
nab-paclitaxel (44.83±2.58%). Conjugation of paclitaxel and
trastuzumab as an ADC was thought to be promising and worthy
(33,34). However, clinical results from ADC
failed to demonstrate therapeutic benefit with shortcomings of poor
in vitro potency, modest in vivo activity and
localization in human tumors (35).
Albumin-bound formulation for paclitaxel based on nano-drug
delivery system, nab-paclitaxel, has the advantage of passive
target for tumor due to EPR effect. Now, deliberate modifications
of ligand or antibody to the surface of nanoparticles were
conducted to achieve specific targeting to the corresponding
receptor on tumor cells (22,36).
The unique targeting mechanism of HSA (22,37)
and passive targeting property of nab-paclitaxel along with the
active targeting property of anti-HER2 antibody may introduce
sequentially dual-targeting and more precise paclitaxel
delivery.
Currently, the NIRF imaging technique is widely used
for cancer molecular imaging research; it has a clearer and longer
visualization for tracking in vivo. In our study,
fluorescence signals of NIR-797-labeled trastuzumab/nab-paclitaxel
remains strong until 48 h after injection. Furthermore, the
fluorescence signals of trastuzumab/nab-paclitaxel group were only
observed in liver and tumor area, illustrating that the ANC of
trastuzumab/nab-paclitaxel could target the tumor tissue more
precisely than nanoparticles. In vivo antitumor efficacy
demonstrated that the ANC of the trastuzumab/nab-paclitaxel had
more significant tumor regression than other treatment groups. In
addition, trastuzumab/nab-paclitaxel compared with nab-paclitaxel
and paclitaxel had no obviously effect on the quality of life.
In conclusion, this study successfully synthesized
antibody-nanoparticle conjugate of trastuzumab/nab-paclitaxel with
properties of passive and active target. In vitro and in
vivo findings illustrated that trastuzumab/nab-paclitaxel could
enhance antitumor efficacy, which could represent a novel precisely
targeting therapeutic agent for HER2-positive GC.
Acknowledgements
This study was supported by the National Nature
Science Foundation of People's Republic of China (81371678).
Glossary
Abbreviations
Abbreviations:
|
AGC
|
advanced gastric cancer
|
|
ANC
|
antibody-nanoparticle conjugate
|
|
HER2
|
human epidermal growth factor receptor
2
|
|
T-DM1
|
ado-trastuzumab emtansine
|
|
HSA
|
human serum albumin
|
|
OS
|
overall survival
|
|
ADC
|
antibody-drug conjugates
|
|
GAPDH
|
glyceraldehyde-3-phosphate
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu B and Xie J: Identifying therapeutic
targets in gastric cancer: The current status and future direction.
Acta Biochim Biophys Sin (Shanghai). 48:90–96. 2016.PubMed/NCBI
|
|
4
|
Sakai D, Satoh T, Kurokawa Y, Kudo T,
Nishikawa K, Oka Y, Tsujinaka T, Shimokawa T, Doki Y and Furukawa
H: A phase II trial of trastuzumab combined with irinotecan in
patients with advanced HER2-positive chemo-refractory gastric
cancer: Osaka Gastrointestinal Cancer Chemotherapy Study Group
OGSG1203 (HERBIS-5). Jpn J Clin Oncol. 43:838–840. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gumusay O, Benekli M, Ekinci O, Baykara M,
Ozet A, Coskun U, Demirci U, Uner A, Dursun A, Atak EY, et al:
Discordances in HER2 status between primary gastric cancer and
corresponding metastatic sites. Jpn J Clin Oncol. 45:416–421. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Corrigan PA, Cicci TA, Auten JJ and Lowe
DK: Ado-trastuzumab emtansine: A HER2-positive targeted
antibody-drug conjugate. Ann Pharmacother. 48:1484–1493. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahn ER, Wang E and Glück S: Is the
improved efficacy of trastuzumab and lapatinib combination worth
the added toxicity? A discussion of current evidence,
recommendations, and ethical issues regarding dual HER2-targeted
therapy. Breast Cancer (Auckl). 6:191–207. 2012.PubMed/NCBI
|
|
9
|
Moghaddas A and Borhani A: Whether
HER2-positive non-breast cancers are candidates for treatment with
Ado-trastuzumab emtansine? J Res Pharm Pract. 5:227–233. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barok M, Tanner M, Köninki K and Isola J:
Trastuzumab-DM1 is highly effective in preclinical models of
HER2-positive gastric cancer. Cancer Lett. 306:171–179. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamashita-Kashima Y, Shu S, Harada N and
Fujimoto-Ouchi K: Enhanced antitumor activity of trastuzumab
emtansine (T-DM1) in combination with pertuzumab in a HER2-positive
gastric cancer model. Oncol Rep. 30:1087–1093. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shitara K and Ohtsu A: Advances in
systemic therapy for metastatic or advanced gastric cancer. J Natl
Compr Canc Netw. 14:1313–1320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gupta N, Hatoum H and Dy GK: First line
treatment of advanced non-small-cell lung cancer - specific focus
on albumin bound paclitaxel. Int J Nanomed. 9:209–221. 2014.
|
|
14
|
Yamamoto T, Miyazaki T, Kurashima Y, Ohata
K, Okawa M, Tanaka S, Uenishi T and Ohno K: A case report of
successful chemotherapy with tegafur/gimeracil/oteracil and
nab-paclitaxel for gastric cancer with chronic renal failure. Gan
To Kagaku Ryoho. 42:735–738. 2015.(In Japanese). PubMed/NCBI
|
|
15
|
Vogel A, Kullmann F, Kunzmann V, Al-Batran
SE, Oettle H, Plentz R, Siveke J, Springfeld C and Riess H:
Patients with advanced pancreatic cancer and hyperbilirubinaemia:
Review and German expert opinion on treatment with nab-paclitaxel
plus Gemcitabine. Oncol Res Treat. 38:596–603. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirsh V: nab-paclitaxel for the management
of patients with advanced non-small-cell lung cancer. Expert Rev
Anticancer Ther. 14:129–141. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Montero AJ, Adams B, Diaz-Montero CM and
Glück S: nab-paclitaxel in the treatment of metastatic breast
cancer: A comprehensive review. Expert Rev Clin Pharmacol.
4:329–334. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ruttala HB, Ramasamy T, Shin BS, Choi HG,
Yong CS and Kim JO: Layer-by-layer assembly of hierarchical
nanoarchitectures to enhance the systemic performance of
nanoparticle albumin-bound paclitaxel. Int J Pharm. 519:11–21.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
van Lith SA, van Duijnhoven SM, Navis AC,
Leenders WP, Dolk E, Wennink JW, van Nostrum CF and van Hest JC:
Legomedicine - A versatile chemo-enzymatic approach for the
preparation of targeted dual-labeled llama antibody-nanoparticle
conjugates. Bioconjug Chem. 28:539–548. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Obaid G, Chambrier I, Cook MJ and Russell
DA: Cancer targeting with biomolecules: A comparative study of
photodynamic therapy efficacy using antibody or lectin conjugated
phthalocyanine-PEG gold nanoparticles. Photochem Photobiol Sci.
14:737–747. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen L, Chen F, Zhao M, Zhu X, Ke C, Yu J,
Yan Z, Zhang F, Sun Y, Chen D, et al: A redox-sensitive
micelle-like nanoparticle self-assembled from amphiphilic
adriamycin-human serum albumin conjugates for tumor targeted
therapy. Biomed Res Int. 2015:9874042015.PubMed/NCBI
|
|
23
|
Su LY, Shi YX, Yan MR, Xi Y and Su XL:
Anticancer bioactive peptides suppress human colorectal tumor cell
growth and induce apoptosis via modulating the PARP-p53-Mcl-1
signaling pathway. Acta Pharmacol Sin. 36:1514–1519. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moon JY, Cho M, Ahn KS and Cho SK:
Nobiletin induces apoptosis and potentiates the effects of the
anticancer drug 5-fluorouracil in p53-mutated SNU-16 human gastric
cancer cells. Nutr Cancer. 65:286–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Korbakis D and Scorilas A: Quantitative
expression analysis of the apoptosis-related genes BCL2, BAX and
BCL2L12 in gastric adenocarcinoma cells following treatment with
the anticancer drugs cisplatin, etoposide and taxol. Tumour Biol.
33:865–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lindsay J, Esposti MD and Gilmore AP:
Bcl-2 proteins and mitochondria - specificity in membrane targeting
for death. Biochim Biophys Acta. 1813:532–539. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gonzalez-Campora R, Davalos-Casanova G,
Beato-Moreno A, Garcia-Escudero A, Pareja Megia MJ, Montironi R and
Lopez-Beltran A: BCL-2, TP53 and BAX protein expression in
superficial urothelial bladder carcinoma. Cancer Lett. 250:292–299.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sriramoju B, Kanwar RK and Kanwar JR:
Nanoformulated cell-penetrating survivin mutant and its dual
actions. Int J Nanomed. 9:3279–3298. 2014.
|
|
29
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 8:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Toiyama Y, Tanaka K, Konishi N, Mohri Y,
Tonouchi H, Miki C and Kusunoki M: Administration
sequence-dependent antitumor effects of paclitaxel and
5-fluorouracil in the human gastric cancer cell line MKN45. Cancer
Chemother Pharmacol. 57:368–375. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsuhashi N, Saio M, Matsuo A, Sugiyama Y
and Saji S: Apoptosis induced by 5-fluorouracil, cisplatin and
paclitaxel are associated with p53 gene status in gastric cancer
cell lines. Int J Oncol. 26:1563–1567. 2005.PubMed/NCBI
|
|
32
|
Arranja A, Gouveia LF, Gener P, Rafael DF,
Pereira C, Schwartz S Jr and Videira MA: Self-assembly PEGylation
assists SLN-paclitaxel delivery inducing cancer cell apoptosis upon
internalization. Int J Pharm. 501:180–189. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bender B, Leipold DD, Xu K, Shen BQ,
Tibbitts J and Friberg LE: A mechanistic pharmacokinetic model
elucidating the disposition of trastuzumab emtansine (T-DM1), an
antibody-drug conjugate (ADC) for treatment of metastatic breast
cancer. AAPS J. 16:994–1008. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Krop IE, Lin NU, Blackwell K, Guardino E,
Huober J, Lu M, Miles D, Samant M, Welslau M and Diéras V:
Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in
patients with HER2-positive metastatic breast cancer and central
nervous system metastases: A retrospective, exploratory analysis in
EMILIA. Ann Oncol. 26:113–119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Elzoghby AO, Samy WM and Elgindy NA:
Protein-based nanocarriers as promising drug and gene delivery
systems. J Control Release. 161:38–49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ruan J, Ji J, Song H, Qian Q, Wang K, Wang
C and Cui D: Fluorescent magnetic nanoparticle-labeled mesenchymal
stem cells for targeted imaging and hyperthermia therapy of in vivo
gastric cancer. Nanoscale Res Lett. 7:3092012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Regino CAS, Ogawa M, Alford R, Wong KJ,
Kosaka N, Williams M, Feild BJ, Takahashi M, Choyke PL and
Kobayashi H: Two-step synthesis of galactosylated human serum
albumin as a targeted optical imaging agent for peritoneal
carcinomatosis. J Med Chem. 53:1579–1586. 2010. View Article : Google Scholar : PubMed/NCBI
|