Introduction
Head and neck squamous cell carcinoma (HNSCC)
accounts for 90% of all head and neck cancers, and is the sixth
most common malignancy worldwide (1). Despite improved locoregional control
and more effective therapeutic strategies for HNSCC, the 5-year
survival rate for HNSCC patients remains 50–60%. Lymph node
metastasis is one of the major reasons associated with the poor
prognosis of patients with HNSCC (2). It is reported that the lymph node
metastasis rate of oral tongue squamous cell carcinoma (OTSCC) is
nearly 50%, and lymph node metastasis is the primary factor
influencing prognosis (3–5). Thus, understanding the mechanisms that
underlie the metastasis of OTSCC is urgently needed to improve
therapeutic strategies and ultimately patient prognosis.
In the present study, we investigated the mechanisms
underlying the lymph node metastasis of OTSCC. FNDC3B (fibronectin
type III domain containing 3B), also named FAD104, was found to be
upregulated in tongue cancer through a web-based bioinformatic
meta-analysis (Oncomine). FNDC3B is known to be a member of the
FNDC3 family (including FNDC3A, FNDC3B and FNDC3C). FNDC3B has a
proline-rich region, nine fibronectin type III domains and a
transmembrane region (6,7). The fibronectin type III (FNIII)
domains act as a scoffold and can integrate with different
proteins, which plays an important role in cell adhesion and growth
signaling (8). Initially, FNDC3B
was identified as a known regulator of adipocyte and osteoblast
differentiation, and FNDC3B-deficient mice died at birth due to
lung abnormalities (9). Moreover,
analyses using mouse embryonic fibroblasts (MEFs) revealed that
loss of FNDC3B suppressed cell adhesion, migration and
proliferation (10). These results
suggest that FNDC3B has important roles in cell adhesion, migration
and proliferation, which raised the question as to whether FNDC3B
regulates the invasion and metastasis of cancer cells and promoted
us to investigate its role in the metastasis of cancer cells.
Previous studies have demonstrated that expression of FNDC3B
protein was upregulated in a series of human tumors, including
breast carcinoma (11), esophageal
carcinoma (12), glioblastoma
(13,14) and hepatocellular carcinoma (11,15).
Moreover, FNDC3B has also been confirmed to serve as an oncogene
and play a significant role in the regulation of the motility and
invasion of glioma and hepatocellular carcinoma cells. However,
there is little research concerning its biological functions,
particularly in the metastasis of tongue squamous cell
carcinoma.
A hypoxic enviroment plays an important role in the
metastasis of cancer cells (16).
The induction of hypoxia-inducible factor (HIF)-1 is the
best-characterized transcription factor that is responsive to
hypoxia (17). HIF-1 is composed of
αβ heterodimers; expression of the α-subunit is tightly regulated
by oxygen, whereas the β-subunit is constitutively expressed
(18). Numerous studies have shown
that HIF-1α overexpression is frequently observed in human cancers
and is associated with metastasis of several types of solid types
of carcinoma, including breast carcinoma, chondrosarcoma,
colorectal adenocarcinoma and head and neck cancer (19–21).
In the present study, we demonstrated that FNDC3B
expression is positively correlated with lymph node metastasis and
clinical tumor-node-metastasis (cTNM) stage in OTSCC patients.
Furthermore, we demonstrated that cobalt chloride
(CoCl2, a hypoxia mimetic agent) promoted FNDC3B
expression, and then induced EMT in OTSCC cells, ultimately
promoting their migratory and invasive abilities. This study
elucidated the important role played by FNDC3B in OTSCC metastasis
and the identification of possible drug targets.
Materials and methods
Patients and clinical tissue
specimens
The present study was approved by the Ethics
Committee of the Kunming Medical University. All patients provided
written informed consent in order to participate in the study. A
total of 116 paraffin-embedded OTSCC samples, which were
consecutively pathologically diagnosed between January 2004 and
January 2014 at the First Affiliated Hospital of Kunming Medical
University, were collected for immunohistochemical (IHC) analysis.
These samples were obtained from 56 men and 60 women, with a mean
age of 55 years (ranging from 35 to 86 years). All patients were
followed up until July 2016. cTNM stages were assessed according to
the TNM classification of the American Joint Committee on Cancer
(AJCC) (22). Correlations between
FNDC3B expression levels and clinical features in the patient
cohort are shown in Table I.
 | Table I.Correlation between FNDC3B expression
and clinicopathological features of the patients with OTSCC. |
Table I.
Correlation between FNDC3B expression
and clinicopathological features of the patients with OTSCC.
|
| FNDC3B |
|
|
|---|
|
|
|
|
|
|---|
| Characteristics | Low | High | χ2 | P-value |
|---|
| Sex |
|
| 0.534 | 0.577 |
|
Male | 29 | 27 |
|
|
|
Female | 27 | 33 |
|
|
| Age (years) |
|
| 0.011 | 1.000 |
|
<50 | 20 | 22 |
|
|
|
≥50 | 36 | 38 |
|
|
| Clinical T
phase |
|
| 4.388 | 0.045 |
|
T1-2 | 51 | 46 |
|
|
|
T3-4 | 5 | 14 |
|
|
| Clinical N
phase |
|
| 5.712 | 0.022 |
| N0 | 41 | 31 |
|
|
|
N1+2 | 15 | 29 |
|
|
| Histological
differentiation |
|
| 0.267 | 0.709 |
| I | 30 | 35 |
|
|
|
II–III | 26 | 25 |
|
|
| cTNM stage |
|
| 4.342 | 0.042 |
|
I–II | 36 | 27 |
|
|
|
III–IV | 20 | 33 |
|
|
Silico gene expression studies
We used the Oncomine database (Compendia Bioscience;
http://www.oncomine.org) to identify upregulated
genes in tongue carcinoma on April 23, 2017 and performed a
microarray meta-analysis to compare all genes across 5 different
datasets (23–27) that were identified by the following
parameters: ‘mRNA’, ‘tongue carcinoma’ and ‘cancer vs. normal
analysis’. To compare FNDC3B expression between cancer and normal
tissues, FNDC3B expression fold changes were limited to
P<0.05.
Immunohistochemical assay (IHC)
Paraffin-embedded OTSCC tissue specimens were cut
into 3-µm-thick sections and incubated at 60°C for 2 h. All
sections were deparaffinized with xylene and rehydrated with a
gradient of ethanol to distilled water. After soaking with 3%
H2O2 for 15 min to block endogenous
peroxidase, the sections were microwaved in sodium citrate buffer
(pH 8.0) for antigen retrieval. Then, incubation was carried out
with a rabbit anti-FNDC3B antibody (Proteintech Group, Rosemont,
IL, USA) overnight at 4°C (dilution of 1:200). The slides were
washed with phosphate-buffered saline (PBS) three times and
incubated with anti-rabbit secondary antibody for 50 min at 37°C.
Diaminobenzidine (DAB; Zhongshan Biological and Technical Co.,
Beijing, China) was used as a colorimetric reagent for protein
detection. Normal goat serum was used as a negative control.
Semi-quantitative expression levels were measured by assessing the
intensities and percentages of the stained cancer cells. The
percentage of positive cancer cells was divided into four levels: 0
(0–25%), 1 (6–50%), 2 (51–75%) and 3 (76–100%). Staining intensity
was divided into four grades: 0 (no staining), 1 (yellow), 2 (deep
yellow) and 3 (brown). Scoring of both parameters was calculated
after counting at least 4 fields. The overall score was the
addition of the positive cell percentage and the staining
intensity. Each specimen was classified into 1 of 2 groups
according to overall scores: scores of 0–3 points were classified
as negative or low expression, and scores >3 points were
classified as high expression for FNDC3B. All specimens were
independently assessed by two pathologists who were blindly to the
patient identity and clinical outcome.
Cell lines and cell culture
The tongue squamous cell carcinoma (TCA8113, TSCCA
and CAL27) cell lines were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA). The human normal epithelial
carcinoma cell line NTEC was a gift from Musheng Zeng (Sun Yat-sen
University Cancer Center, Guangzhou, China). All cells were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
streptomycin (100 U/ml) and penicillin (100 U/ml) in a humidified
5% CO2 incubator at 37°C.
Antibodies and reagents
Mouse anti-E-cadherin and anti-vimentin antibodies
were obtained from BD Technologies (La Jolla, CA, USA). DMOG was
obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Antibody against β-catenin was purchased from Cell Signaling
Technology (Beverly, MA, USA). Rabbit anti-HIF-1α antibody was
purchased from Abcam (Cambridge, MA, USA), and mouse anti-β-actin
antibody and anti-GAPDH were obtained from Proteintech Group
(Chicago, IL, USA). Goat anti-rabbit and anti-mouse goat
peroxidase-conjugated secondary antibodies were purchased from
Pierce Biotechnology (Rockford, IL, USA). All other reagents were
obtained from Sigma-Aldrich (St. Louis, MO, USA).
Generation of target-specific silenced
cells
Retrovirus particles were produced in 293FT cells by
transfection of pSuper-Retro-Puro plasmid harboring specific shRNA.
The shRNAs were determined by Invitrogens siRNA design tool
(Invitrogen, Carlsbad, CA, USA). The target sequences were as
follows: FNDC3B shRNA#1: 5-GCAGGTTATTCTCGTTCAA-3; FNDC3B shRNA#2:
5-GCTTACTACCCACCTGTTA-3; FNDC3B shRNA#3: 5-GCAGCTGCACAACAGTATA-3. A
standard calcium phosphate co-transfection was performed with a PIK
packaging plasmid in the 293FT cells. The supernatant was collected
and filtered using 0.45-µm filters. The target cells were incubated
with 2 µg/ml Polybrene (Sigma-Aldrich). Twenty-four hours after
infection, the cells were incubated with 1 µg/ml puromycin for 3
days for selection of positive cells.
Western blotting
Cells were washed twice with PBS and then lysed in
1X sodium dodecyl sulfate (SDS) sample buffer. A total of 25 µg
protein was loaded on a 9% SDS-polyacrylamide gel by
electrophoresis, transferred to a polyvinylidene fluoride (PVDF)
membrane at 100 mA for 2 h, blocked with 5% skim milk for 1 h in
room temperature, and then incubated with the primary antibodies
overnight at 4°C. On the folllowing day, incubation with the
secondary antibodies was carried out at room temperature for 45
min. After washing, bound antibodies were visualized via
electrochemiluminescence, which was captured by XAR film. Scanning
and analysis of the western bloting bands were performed using the
Quantity One program (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Quantitative real-time polymerase
chain reaction (real-time PCR)
TRIzol reagent (Invitrogen) was used to extract
total RNA from the cultured cells, and 2 µg of each sample was
reverse-transcribed using M-MLV reverse transcriptase (Promega,
Madison, WI, USA) following the kit instructions. Real-time PCR was
performed with the Fast SYBR-Green Master Mix (Bio-Rad
Laboratories, Inc.). The housekeeping gene GAPDH was used as an
internal control. The PCR amplifications were performed in a
PTC-200 PCR system (Bio-Rad Laboratories, Inc.) using the following
cycle parameters: 10 min at 95°C, followed by 45 cycles of 10 sec
at 95°C, 10 sec at 55°C, and 20 sec at 72°C, with a final extension
at 72°C for 10 min. The FNDC3B sense primer was
5-CCACCTGTTACCGGACCTG-3 and the antisense primer was
5-GGGTGATGTAGGTTGACATTCC-3. The mRNA relative expression levels
were quantified using the 2−∆∆Ct method.
Cell invasion and migration
assays
After being serum-starvation for 24 h,
4×104 cells were plated into a 24-well Transwell plate
(Corning Costar, Cambridge, MA, USA) with or without a Matrigel
coating (BD Biosciences, Bedford, MA, USA). A total of 500 µl of
RPMI-1640 supplemented with 10% FBS was placed in the lower
chamber. After incubation at 37°C for 12 (migration) or 24 h
(invasion), the cells that migrated to the reverse sides of the
inserts were fixed in methanol and stained with 0.1% crystal violet
(Sigma-Aldrich) for 10 min, photographed and counted (6 random 100×
fields per well) under an inverted microscope (Olympus Corp.,
Tokyo, Japan). Each experiment was repeated at least three
times.
Statistical analysis
The SPSS statistical package 19.0 (SPSS, Inc.,
Chicago, IL, USA) was used to analyze the data. Data are presented
as the means ± SEM of values from three independent experiments.
Correlations between FNDC3B expression and the clinical features of
the OTSCC patient cohort were assessed by the χ2 test.
The Students t-test was used for multiple comparisons. P<0.05
was considered statistically significant. Error bars represent SEM.
*P<0.05, **P<0.01, ***P<0.001 were indicative of
statistically significant results as shown in the figures. Each
experiment was repeated at least three times.
Results
Overexpression of FNDC3B in OTSCC
samples is related to the clinicopathological features of the OTSCC
patients
To identify the molecules involved in lymph node
metastasis of human OTSCC, a microarray meta-analysis was performed
to compare all genes across 5 different datasets (including 107
cancer tissues and 87 adjacent normal tissues from OTSCC patients).
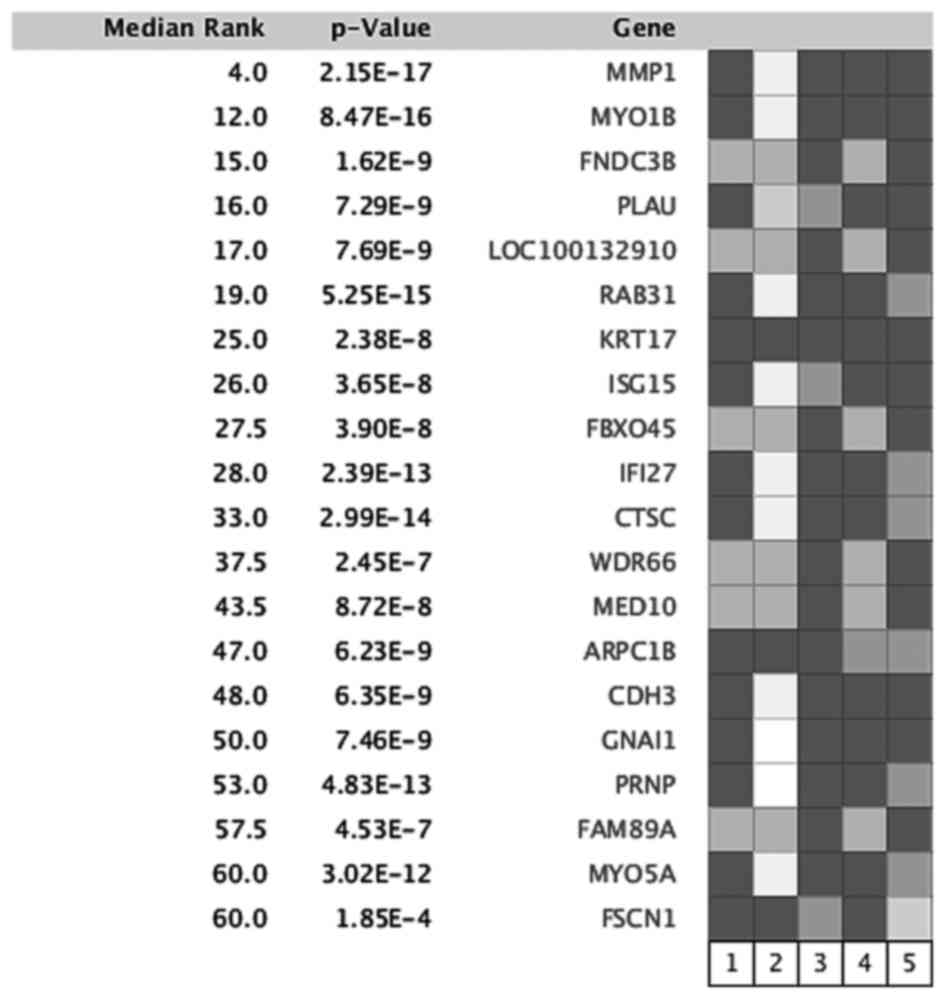
The overexpressed genes were ranked by median ranked analyses, and
FNDC3B was identified as one of the most frequently upregulated
genes in OTSCC tissues compared to adjacent tissues (Fig. 1). Therefore, we further evaluated
the roles of FNDC3B in OTSCC progression in the present study.
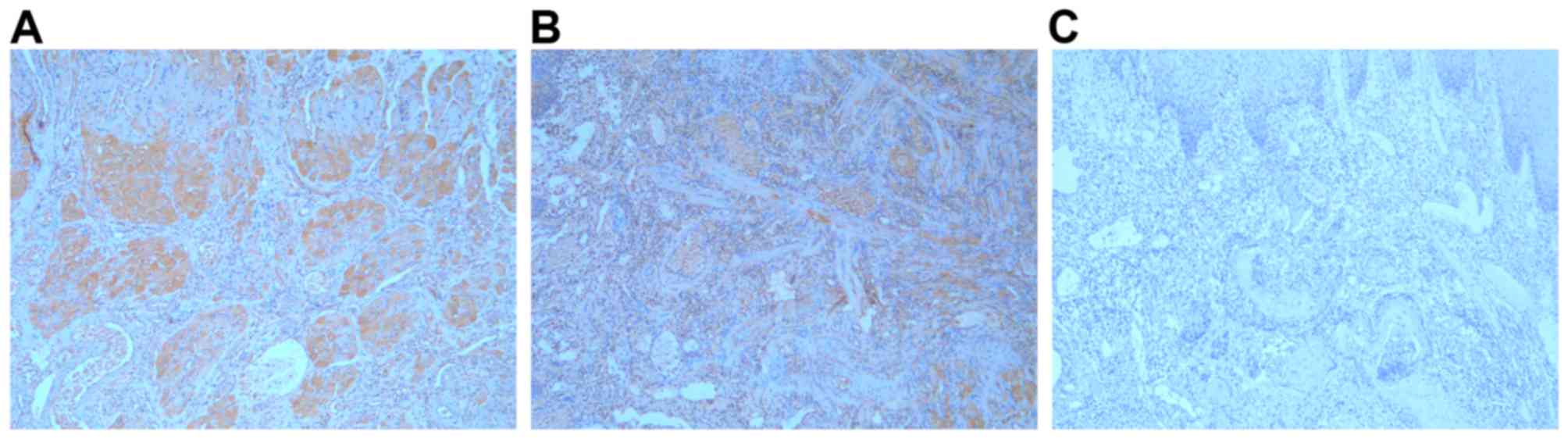
To assess the clinical significance of FNDC3B in
OTSCC samples, we examined FNDC3B expression in 116
paraffin-embedded OTSCC samples by immunohistochemistry. As a
result, 56 cases (48.3%) showed negative/low FNDC3B expression, and
60 (51.7%) cases showed high FNDC3B expression (Fig. 2). In the correlation analysis,
positive FNDC3B expression was associated with lymph node
metastasis and advanced cTNM stage (Table I).
OTSCC cell lines exhibit increased
FNDC3B expression
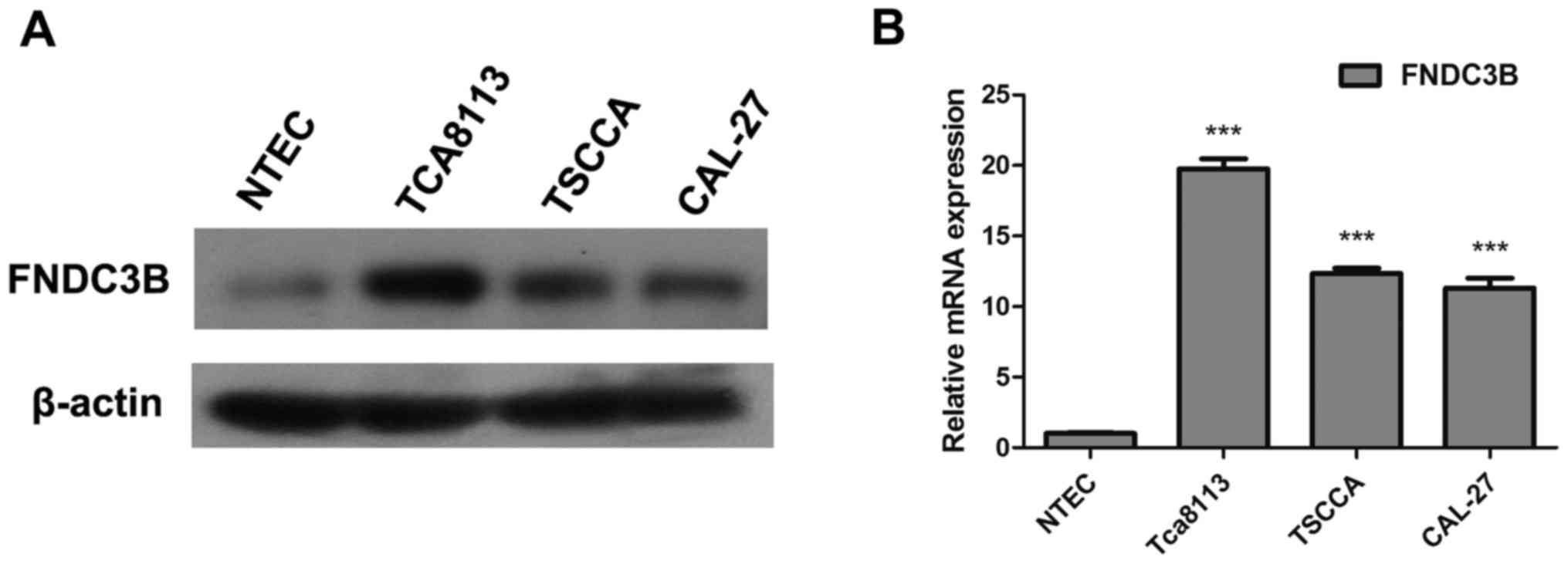
Real-time PCR and western blotting were conducted to
compare FNDC3B expression in OTSCC cell lines (TCA8113, TSCCA and
CAL27) and a normal tongue epithelial cell line (NTEC). Western
blotting revealed higher FNDC3B protein expression in the OTSCC
cell lines compared with the NTEC cells (Fig. 3A). Similar result was found for
FNDC3B mRNA expression based on real-time PCR (Fig. 3B). Thus, our data indicated that
FNDC3B mRNA and protein expression were significantly increased in
the OTSCC cell lines.
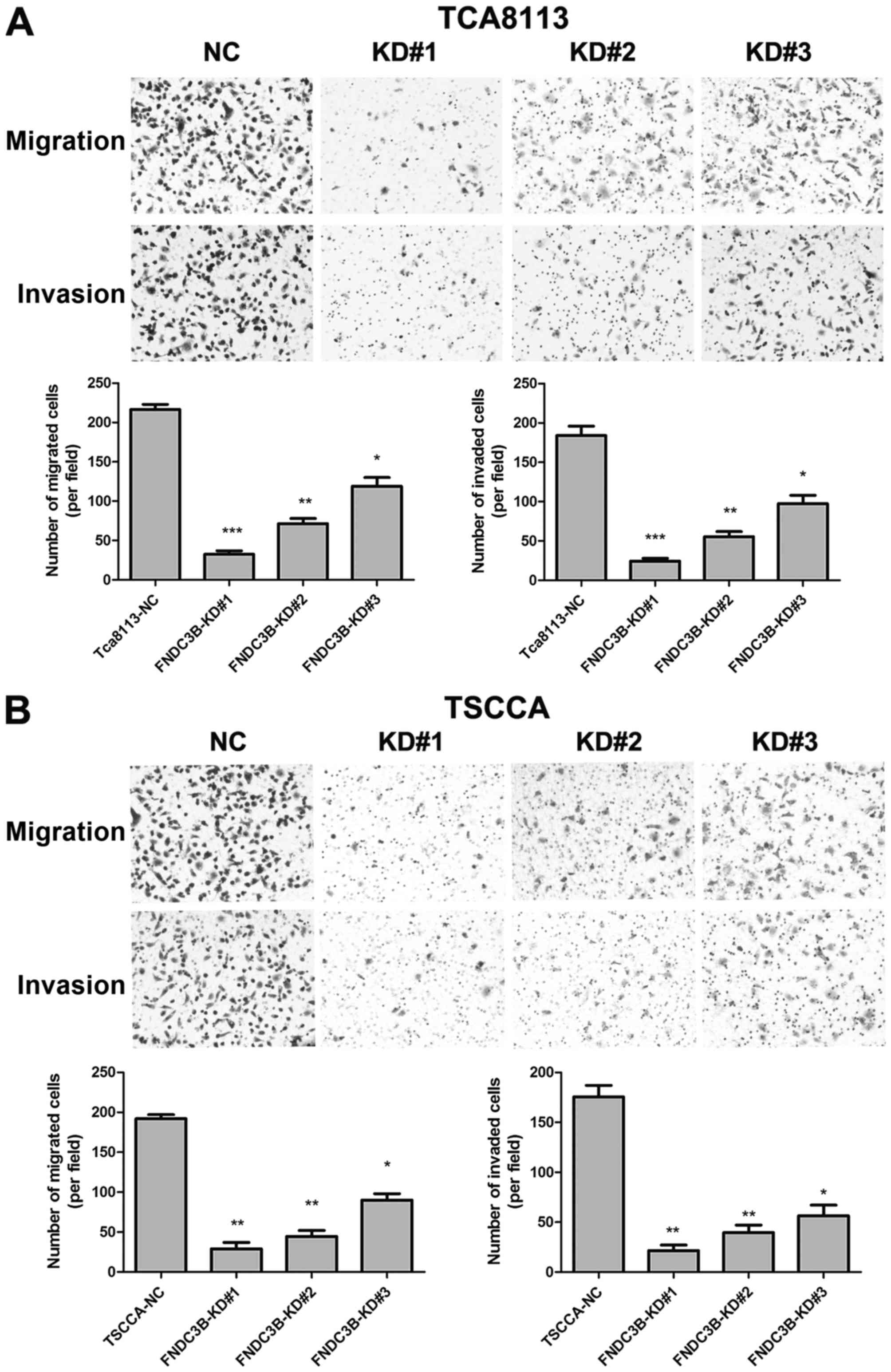
FNDC3B is closely associated with the
invasive and migratory abilities of OTSCC cells
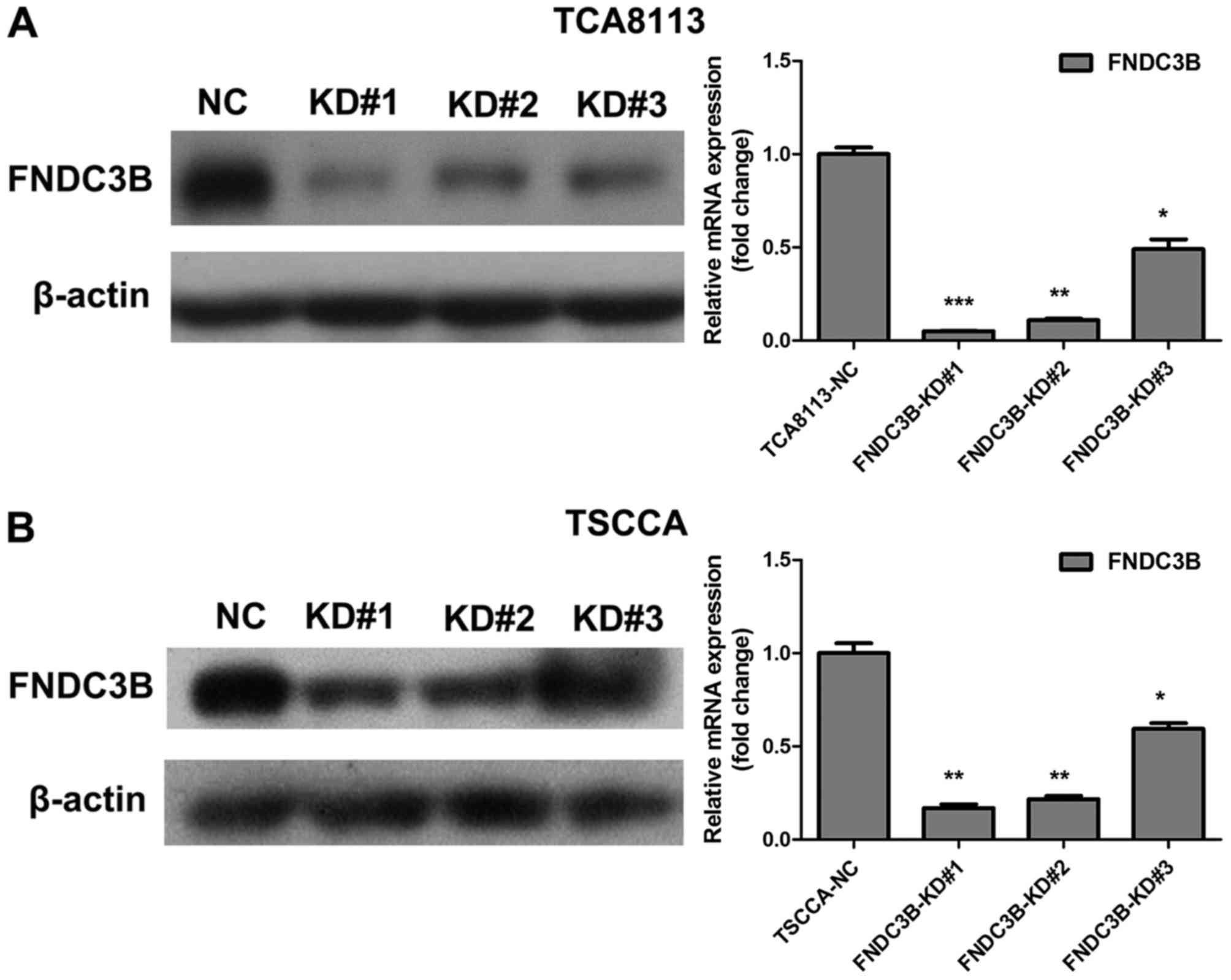
To clarify the role of FNDC3B in OTSCC cell invasion
and migration, we generated TCA8113 cells with stably knocked down
FNDC3B expression using retroviral vectors (FNDC3B-KD#1, KD#2 and
KD#3), and scrambled shRNA was used as a negative control (NC).
Real-time PCR and western blotting were performed to assess the
FNDC3B knockdown efficiency. As shown in Fig. 4, FNDC3B mRNA and protein levels were
significantly lower in FNDC3B-KD cells than NC cells, confirming
the knockdown efficiency of FNDC3B in these cells. Invasion and
migration assay results revealed that knockdown of FNDC3B markedly
reduced the invasive and migratory abilities of TCA8113 and TSCCA
cells (Fig. 5). Taken together,
these results suggest that FNDC3B promotes OTSCC cell invasion and
migration.
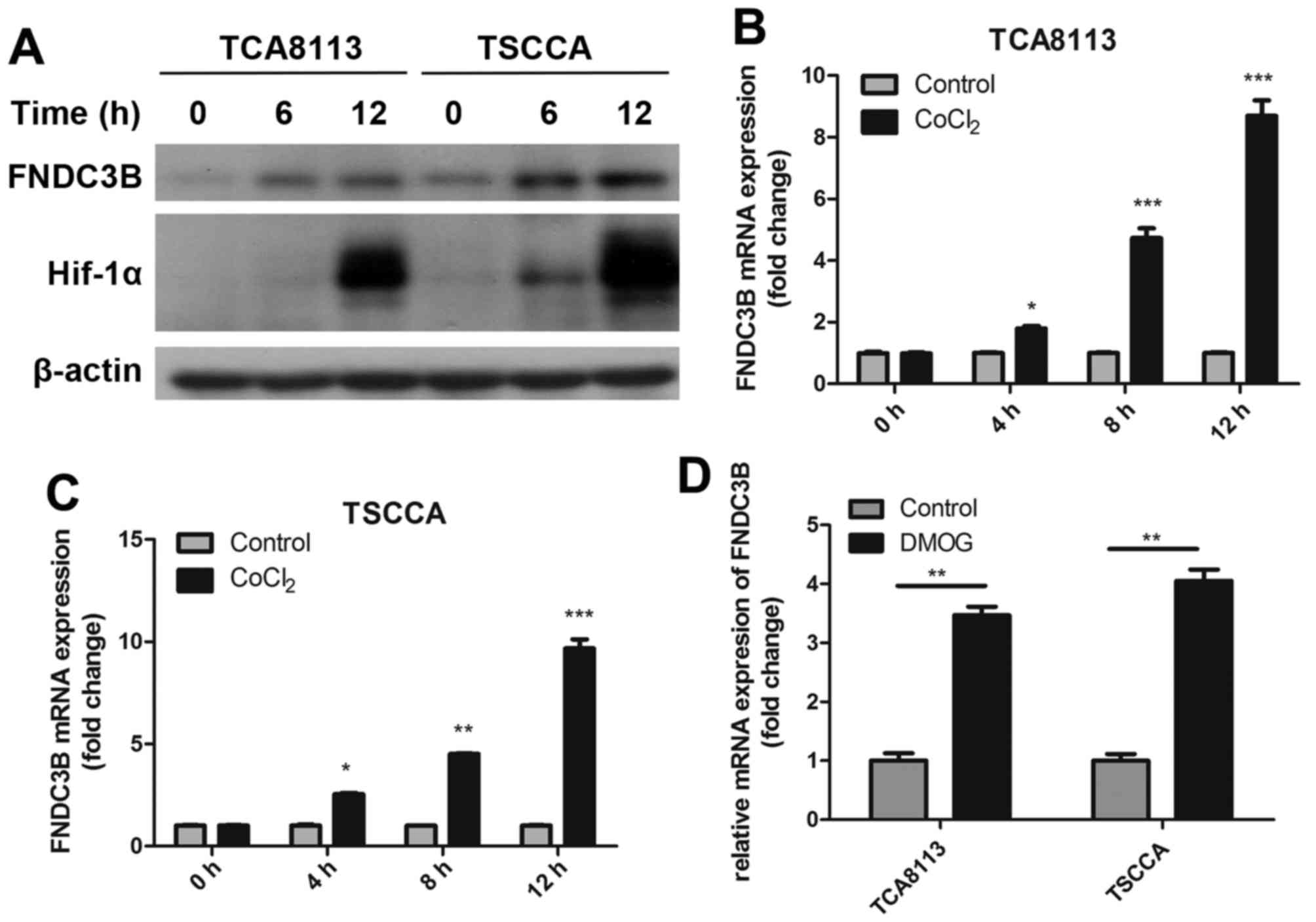
Hypoxia promotes FNDC3B expression in
OTSCC cells via HIF-1α
Hypoxia microenvironment plays an important role
during the metastasis of malignancies. To identify the relationship
between hypoxia and FNDC3B, we treated OTSCC cells with 0.1 mM
CoCl2 (a hypoxia mimetic) to simulate chemical hypoxia.
Treatment with CoCl2 significantly increased FNDC3B mRNA
and protein production in a time-dependent manner (Fig. 6A-C). Further evidence in support of
the involvement of HIF-1α in the hypoxic induction of FNDC3B mRNA
was provided by experiments conducted in the presence of
dimethyloxallyl glycine (DMOG, 1 mM), which blocks degradation of
HIF-1α and promotes normoxic accumulation of HIF-1α (Fig. 6D). Levels of FNDC3B mRNA increased
by 3- to 4-fold in cells treated with DMOG in normal oxygen
condition, relative to the untreated cells. These findings suggest
that hypoxia promotes FNDC3B expression in OTSCC cells via HIF-1α
induction.
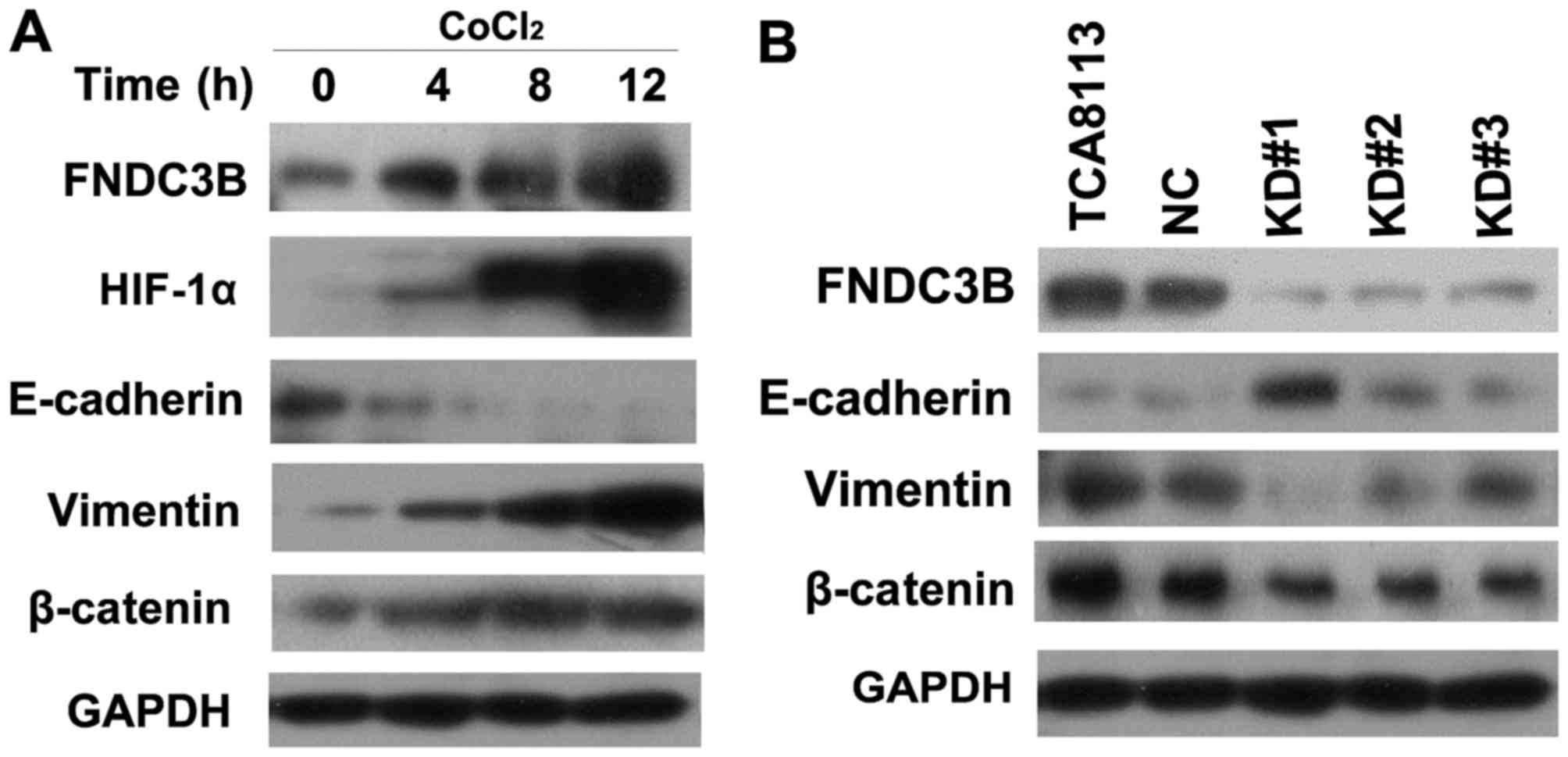
FNDC3B promotes EMT in a hypoxic
environment
Hypoxia can promote EMT in many cancer cell types
via HIF-1α induction. Thus, we investigated the role of FNDC3B in
HIF-1α-induced EMT. We treated TCA8113 cells with CoCl2
(0.1 mM). We found that CoCl2 upregulated HIF-1α
expression in OTSCC cells, meanwhile it significantly decreased
E-cadherin expression and increased vimentin and β-catenin
expression compared with the NC cells (Fig. 7A). On the other hand, we found that
cells with FNDC3B knockdown exhibited significantly increased
E-cadherin expression and decreased vimentin expression (Fig. 7B). These results demonstrated that
FNDC3B plays an important role in the process of hypoxia-induced
EMT in TCA8113 cells.
Discussion
Metastasis and replase are the main reasons of
treatment failure for the patients with oral tongue squamous cell
carcinoma (OTSCC). Lymph node metastasis is the primary factor
influencing the prognosis (1,5).
Despite its clinical importance, little is known about the genetic
and biochemical determinants of OTSCC metastasis. Thus, clarifying
the mechanism of OTSCC metastasis is of significance in improving
prognosis of OTSCC patients. The present study found that FNDC3B is
overexpression in OTSCC tissues and positive FNDC3B expression is
associated with lymph node metastasis and clinical cTNM stage.
The process of metastasis is highly complex and
occurs through a series of sequential steps, including tumor cell
detachment from the primary tumor; invasion through the
extracellular matrix, basement membrane and endothelial wall; entry
into the vascular system; secondary site plantation and growth in a
target organ (18,28). However, this process is also highly
inefficient as few cells that migrate from the primary tumor
successfully colonize at distant sites. Nearly 50% of OTSCC
patients have lymph node metastases, indicating that OTSCC cells
have strong invasive and migratory capacities. For cellular
progression, epithelial cancer cells must undergo increased
invasive and migratory abilities during EMT, which is an essential
step for metastatic spreading (19,20).
EMT is a multi-step process where epithelial cells acquire a
mesenchymal phenotype which is characterized by enhanced motility
ability, downregulation of epithelial proteins including
E-cadherin, claudins and α-catenin, and overexpression of
mesenchymal phenotype proteins, such as vimentin, N-cadherin and
fibronectin. These changes are activated by transcription factors,
including Twist1, Snail, Slug and β-catenin (21,29).
Tissue hypoxia is a common microenvironment for
solid tumors with rapid growth (16). The present study showed that OTSCC
cells upregulate FNDC3B expression in a time-dependent manner under
conditions that mimic hypoxia (treatment with CoCl2).
During this process, mimicking hypoxia (via treatment with
CoCl2) promotes EMT and enhances invasion and migration
of OTSCC cells. These results indicate that hypoxic conditions
increase FNDC3B expression, subsequently induce EMT in OTSCC cells,
ultimately improving their invasive and migratory potentials.
Our data suggest the important role of FNDC3B in
promoting the migratory and invasive abilities of OTSCC cells and
are consistent with the findings of numerous previous studies
(12,13,30).
FNDC3B is usually amplified and highly expressed in esophageal,
lung, glioblastoma, hepatocellular and breast cancers (11–15).
Lin et al (12) found that
overexpression of FNDC3B facilitated cell migration and tumor
metastasis in hepatocellular carcinoma. Cai et al (30) found that the 3q amplified oncogene
FNDC3B promoted proferiration of hepatocellular carcinoma cells,
and activated several cancer pathways, including PI3-kinase/Akt,
Rb1 and TGFβ signaling. MicroRNA-129-5p inhibits cell processes
including viability, proliferation, migration and invasiveness of
glioblastoma cells U87 through targeting FNDC3B (13). In addition, direct evidence from our
in vitro experiments indicates that FNDC3B promotes cell
migration and tumor metastasis via activation of EMT in OTSCC.
In conclusion, to the best of our knowledge, this is
the first study to show that FNDC3B expression is positively
correlated with the rate of lymph node metastasis and cTNM stage in
patients with OTSCC. The mechanism underlying the FNDC3B-mediated
promotion of metastasis may be related to hypoxia-induced
stimulation of FNDC3B production, which promotes OTSCC cell
migration, invasion and EMT. This study demonstrated that FNDC3B is
a potential therapeutic target for cancer cell metastasis. However,
many related issues, such as the exact mechanism of the
hypoxia-induced overexpression of FNDC3B and other signaling
pathways that may be activated in the process of FNCD3B-induced
EMT, warrant further research.
Acknowledgements
The present study was supported by National Natural
Science Foundation of China (81560470 and 81260402).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Otsuka Y, Sato H, Oikawa T, Onodera Y, Nam
JM, Hashimoto A, Fukunaga K, Hatanaka KC, Hatanaka Y, Matsuno Y, et
al: High expression of EPB41L5, an integral component of the
Arf6-driven mesenchymal program, correlates with poor prognosis of
squamous cell carcinoma of the tongue. Cell Commun Signal.
14:282016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burusapat C, Jarungroongruangchai W and
Charoenpitakchai M: Prognostic factors of cervical node status in
head and neck squamous cell carcinoma. World J Surg Oncol.
13:512015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haksever M, Inançlı HM, Tunçel U,
Kürkçüoğlu SS, Uyar M, Genç O and Irkkan C: The effects of tumor
size, degree of differentiation, and depth of invasion on the risk
of neck node metastasis in squamous cell carcinoma of the oral
cavity. Ear Nose Throat J. 91:130–135. 2012.PubMed/NCBI
|
|
5
|
Feng HJ, Bao YL, Liang ZP, Zhao FP, Xu SE,
Xu W, Zhao C and Qin G: Silencing of FANCD2 enhances the
radiosensitivity of metastatic cervical lymph node-derived head and
neck squamous cell carcinoma HSC-4 cells. Int J Oncol.
50:1241–1250. 2017. View Article : Google Scholar
|
|
6
|
Katoh D, Nishizuka M, Osada S and Imagawa
M and Imagawa M: Fad104, a positive regulator of adipocyte
differentiation, suppresses invasion and metastasis of melanoma
cells by inhibition of STAT3 activity. PLoS One. 10:e01171972015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang HY, McMahon C, Ali SM, Young LE,
Yekezare S, Ross JS and Ball ED: Novel FNDC3B and MECOM fusion and
WT1 L378fs* 7 frameshift mutation in an acute myeloid leukaemia
patient with cytomorphological and immunophenotypic features
reminiscent of acute promyelocytic leukaemia. Br J Haematol.
172:987–990. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nishizuka M, Kishimoto K, Kato A, Ikawa M,
Okabe M, Sato R, Niida H, Nakanishi M, Osada S and Imagawa M:
Disruption of the novel gene fad104 causes rapid postnatal death
and attenuation of cell proliferation, adhesion, spreading and
migration. Exp Cell Res. 315:809–819. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kishimoto K, Nishizuka M, Ueda T, Kajita
K, Ugawa S, Shimada S, Osada S and Imagawa M: Indispensable role of
factor for adipocyte differentiation 104 (fad104) in lung
maturation. Exp Cell Res. 317:2110–2123. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Urtreger AJWS, Werbajh SE, Verrecchia F,
Mauviel A, Puricelli LI, Kornblihtt AR and Bal de Kier Joffé ED:
Fibronectin is distinctly downregulated in murine mammary
adenocarcinoma cells with high metastatic potential. Oncol Rep.
16:1403–1410. 2006.PubMed/NCBI
|
|
11
|
Lin CH, Lin YW, Chen YC, Liao CC, Jou YS,
Hsu MT and Chen CF: FNDC3B promotes cell migration and tumor
metastasis in hepatocellular carcinoma. Oncotarget. 7:49498–49508.
2016.PubMed/NCBI
|
|
12
|
Yang Y, Li D, Yang Y and Jiang G: An
integrated analysis of the effects of microRNA and mRNA on
esophageal squamous cell carcinoma. Mol Med Rep. 12:945–952. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu H, Hu Y and Qiu W: Potential mechanisms
of microRNA-129-5p in inhibiting cell processes including
viability, proliferation, migration and invasiveness of
glioblastoma cells U87 through targeting FNDC3B. Biomed
Pharmacother. 87:405–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stangeland B, Mughal AA, Grieg Z, Sandberg
CJ, Joel M, Nygård S, Meling T, Murrell W, Vik Mo EO and Langmoen
IA: Combined expressional analysis, bioinformatics and targeted
proteomics identify new potential therapeutic targets in
glioblastoma stem cells. Oncotarget. 6:26192–26215. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sawey ET, Chanrion M, Cai C, Wu G, Zhang
J, Zender L, Zhao A, Busuttil RW, Yee H, Stein L, et al:
Identification of a therapeutic strategy targeting amplified FGF19
in liver cancer by Oncogenomic screening. Cancer Cell. 19:347–358.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ibrahim AA, Schmithals C, Kowarz E,
Köberle V, Kakoschky B, Pleli T, Kollmar O, Nitsch S, Waidmann O,
Finkelmeier F, et al: Hypoxia causes down-regulation of Dicer in
hepatocellular carcinoma, which is required for up-regulation of
hypoxia inducible factor 1alpha and epithelial-mesenchymal
transition. Clin Cancer Res. 23:3896–3905. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, Xia
XF, Sun X, Li GG, Hu QD, Fu QH, et al: Hypoxia-induced
epithelial-to-mesenchymal transition in hepatocellular carcinoma
induces an immunosuppressive tumor microenvironment to promote
metastasis. Cancer Res. 76:818–830. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhong Z, Hu Z, Jiang Y, Sun R, Chen X, Chu
H, Zeng M and Sun C: Interleukin-11 promotes epithelial-mesenchymal
transition in anaplastic thyroid carcinoma cells through
PI3K/Akt/GSK3β signaling pathway activation. Oncotarget.
7:59652–59663. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu Y, Wang Y, Lin Y, Liu Y, Wang Y, Jia J,
Singh P, Chi YI, Wang C, Dong C, et al: Dub3 inhibition suppresses
breast cancer invasion and metastasis by promoting Snail1
degradation. Nat Commun. 8:142282017.doi: 10.1038/ncomms14228.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang T, Liang L, Liu X, Wu JN, Chen J, Su
K, Zheng Q, Huang H and Liao GQ: TGFβ1-Smad3-Jagged1-Notch1-Slug
signaling pathway takes part in tumorigenesis and progress of
tongue squamous cell carcinoma. J Oral Pathol Med. 45:486–493.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi ZM, Wang L, Shen H, Jiang CF, Ge X, Li
DM, Wen YY, Sun HR, Pan MH, Li W, et al: Downregulation of miR-218
contributes to epithelial-mesenchymal transition and tumor
metastasis in lung cancer by targeting Slug/ZEB2 signaling.
Oncogene. 36:2577–2588. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th. Springer;
New York: 2010
|
|
23
|
Estilo CL, O-charoenrat P, Talbot S, Socci
ND, Carlson DL, Ghossein R, Williams T, Yonekawa Y, Ramanathan Y,
Boyle JO, et al: Oral tongue cancer gene expression profiling:
Identification of novel potential prognosticators by
oligonucleotide microarray analysis. BMC Cancer. 9:112009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kuriakose MA, Chen WT, He ZM, Sikora AG,
Zhang P, Zhang ZY, Qiu WL, Hsu DF, McMunn-Coffran C, Brown SM, et
al: Selection and validation of differentially expressed genes in
head and neck cancer. Cell Mol Life Sci. 61:1372–1383. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pyeon D, Newton MA, Lambert PF, den Boon
JA, Sengupta S, Marsit CJ, Woodworth CD, Connor JP, Haugen TH,
Smith EM, et al: Fundamental differences in cell cycle deregulation
in human papillomavirus-positive and human papillomavirus-negative
head/neck and cervical cancers. Cancer Res. 67:4605–4619. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Talbot SG, Estilo C, Maghami E, Sarkaria
IS, Pham DK, O-charoenrat P, Socci ND, Ngai I, Carlson D, Ghossein
R, et al: Gene expression profiling allows distinction between
primary and metastatic squamous cell carcinomas in the lung. Cancer
Res. 65:3063–3071. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye H, Yu T, Temam S, Ziober BL, Wang J,
Schwartz JL, Mao L, Wong DT and Zhou X: Transcriptomic dissection
of tongue squamous cell carcinoma. BMC Genomics. 9:692008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Książkiewicz M, Markiewicz A and Zaczek
AJ: Epithelial-mesenchymal transition: A hallmark in metastasis
formation linking circulating tumor cells and cancer stem cells.
Pathobiology. 79:195–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nieto MA: Context-specific roles of EMT
programmes in cancer cell dissemination. Nat Cell Biol. 19:416–418.
2017. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cai C, Rajaram M, Zhou X, Liu Q, Marchica
J, Li J and Powers RS: Activation of multiple cancer pathways and
tumor maintenance function of the 3q amplified oncogene FNDC3B.
Cell Cycle. 11:1773–1781. 2012. View
Article : Google Scholar : PubMed/NCBI
|