Introduction
Cachexia is a complex metabolic and behavioral
syndrome associated with underlying illness and characterized by
the loss of skeletal muscle (1).
Whereas malnutrition is reversible with conventional nutrition
intervention, cachexia is recognized as irreversible by
conventional nutrition support (2).
In particular, cancer cachexia occurs in approximately 80% of
patients with advanced cancer and is one of the most critical
aspects affecting their quality of life and mortality (3,4). There
is accumulating evidence that cancer cachexia is the direct cause
of cancer-related death in approximately 20% of all cancer patients
(5,6). Based on this background, it is
critical to evaluate the clinical significance of cancer cachexia
and clarify the factors associated with cachexia in cancer
patients.
MicroRNAs are an abundant class of small non-coding
RNA molecules 18–25 nucleotides in length that regulate gene
expression at the post-transcriptional level by promoting messenger
RNA degradation or blocking translation (7). Since a single miRNA molecule can
target hundreds of diverse mRNAs, miRNAs possess an enormous
regulatory potential concerning the protein-expression status and
represent critical regulators of various biological processes,
including cell differentiation, proliferation and apoptosis
(8). Besides the crucial biological
role of miRNAs in cancer development (9–13),
emerging evidence reveals that microvesicle-encapsulated miRNAs
stably exist in body fluids and are associated with the regulation
of cellular processes involved in cell communication, angiogenesis,
extracellular matrix remodeling and biological mechanisms in cancer
cachexia (14–16).
Colorectal cancer (CRC) is one of the most common
cancers worldwide and the second leading cause of cancer-related
death in the US (17,18). Despite advances in diagnostic and
therapeutic techniques in the last decade, the prognosis of CRC
patients with distant metastasis remains poor (19,20),
thus it is essential to identify new predictive biomarkers for
distant metastasis and poor outcome in order to improve both the
early detection of recurrence, as well as the prognosis of CRC
patients.
Previous studies by our research group revealed that
several serum markers reflecting systemic inflammatory response,
including interleukin (IL)-1b, IL-1ra, IL-6, IL-10, C-reactive
protein and albumin, are differentially expressed in serum from
patients with advanced CRC and can be used as predictive biomarkers
for postoperative nutritional status, morbidity and mortality in
CRC patients (21–24). Furthermore, we successfully revealed
that several circulating miRNAs could be used as biomarkers for
diagnosis, prognosis and metastasis prediction in CRC patients
(25–29). In the present study, we
systematically investigated the preoperative body composition
status of CRC patients and the expression profile of
sarcopenia-associated miRNAs in CRC tissues and matched serum to
clarify the clinical significance of preoperative body composition
and its correlation with cachexia-associated miRNA expression in
CRC patients.
Materials and methods
Patients and methods
Recently, our research group demonstrated that serum
miR-21 is a promising biomarker for the early detection and
prognosis in CRC patients (25).
From the validation cohort of this previous study, a total of 167
patients with CRC, who underwent primary resection at our Mie
University Hospital between 2005 and 2010 and had available
preoperative images of plain computed tomography (CT) were enrolled
in the present study. A written informed consent was obtained from
each patient and the study was approved by the review board of Mie
University Hospital. Patients who were treated with radiotherapy or
chemotherapy before surgery were excluded. No perioperative
mortalities were observed. The diagnosis of CRC was confirmed for
all patients based on clinicopathological findings. The
tumor-node-metastasis (TNM) staging system from the American Joint
Committee on Cancer was used for the pathological tumor staging of
CRC (30). All patients were
classified according to the UICC stage classification of resected
specimens: 33 patients were diagnosed with stage I disease, 54 with
stage II, 41 with stage III and 39 with stage IV. All CRC patients
diagnosed with stage III and IV disease received
5-fluorouracil-based chemotherapy, whereas no adjuvant chemotherapy
was given to patients with stage I and II disease. The locations of
tumors and distant metastases were determined by barium enema,
colonoscopy, CT and magnetic resonance imaging. Resection of the
primary tumor was performed in all patients and all patients were
followed up for tumor recurrence at regular intervals for up to 5
years. Patients were observed at 3-month intervals for 24 months
after the completion of surgery, followed by every 6 months for the
next 3 years and then, yearly. A history was taken and a physical
examination was performed in each visit and chest X-ray,
colonoscopy and CT were performed annually.
Image analysis
Using preoperative plain CT at the superior aspect
of the fourth lumbar vertebra (L4) as previously described
(31), we assessed the
cross-sectional area of the bilateral psoas muscles by manual
tracing and calculated the psoas muscle mass index (PMI) as
follows: PMI = cross-sectional area of bilateral psoas
muscle/height2 (cm2/m2). Low PMI
was considered as a proxy for low muscle volume as previously
described (32–34). Subfascial muscular tissue in the
multifidus muscle was estimated by manual tracing at the same level
on preoperative plain CT images and mean CT values [Hounsfield
units (HU)] for these areas were determined with the Aquarius NET
server (TeraRecon, Inc., San Mateo, CA, USA). We placed four
circles on areas of subcutaneous fat away from major vessels in CT
images, which were used as the regions of interest (ROIs) for
subcutaneous fat. The mean CT values (HU) for the ROI of
subcutaneous fat were assessed. IMAC was calculated by the ratio of
these CT values, as previously reported by Kitajima et al
(35,36), as follows: IMAC = mean CT value of
ROI of multifidus muscle (HU)/mean CT value of ROI of subcutaneous
fat (HU). High IMAC was considered as a proxy for low muscle
quality. For the assessment of postoperative PMI or IMAC, we
assessed these values on a postoperative plain CT image obtained 6
months after the resection of the primary tumor.
Sample collection, RNA isolation and
quantitative reverse-transcription polymerase chain reaction
The present study included the analysis of 287 serum
samples and matched surgical tissue specimens (serum, 153;
cancerous tissue, 134) obtained from the enrolled CRC patients. All
of these patients were previously described as the validation
cohort in a biomarker study of serum miR-21 (25). Blood samples were obtained by
venipuncture before surgery. Each sample was centrifuged at 3,000 ×
g for 5 min and stored at −80°C until analysis.
RNA isolation and qRT-PCR from
serum
RNA extraction and miRNA enrichment from all sera
were performed using the Qiagen miRNeasy kit (Qiagen, Valencia, CA,
USA). Briefly, 250 µl of serum or medium was thawed on ice and
centrifuged 1,700 × g for 15 min at room temperature to remove cell
debris and then, 200 µl of supernatant was lysed in 5 volumes of
Qiazol solution. To normalize any inadvertent sample-to-sample
variations during the RNA isolation procedure, reverse
transcription (RT) and PCR reaction, 25 fmol of synthetic C.
elegans miRNA (cel-miR-39) was added to each denatured sample.
Small RNAs were enriched and purified according to the
manufacturer's protocol, with the exception that the enriched small
RNAs were eluted in 40 µl of nuclease-free water. For the
miRNA-based RT-PCR assays, 1.67 µl of enriched small RNAs from
serum or cell-culture medium was reverse-transcribed using the
TaqMan MicroRNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, San Diego, CA, USA) in a total volume of
5.0 µl. reverse transcription (RT) products (1:15 dilution) were
used as template for the PCR. PCR reactions for the quantification
of miR-21 and cel-miR-39 were performed in duplicate with TaqMan 2X
Universal PCR Master Mix under previously described conditions
(25,37).
RNA isolation and qRT-PCR from
formalin-fixed, paraffin-embedded (FFPE) tissues
Total RNA was isolated from FFPE samples using the
RecoverAll Total Nucleic Acid Isolation kit (Ambion Inc.; Thermo
Fischer Scientific, Austin, TX, USA). Briefly, tissue sections were
microdissected to enrich for neoplastic cells, followed by
deparaffinization and RNA extraction according to the
manufacturer's protocol. Total RNA was eluted in the appropriate
buffer and quantified using a NanoDrop Spectrophotometer (NanoDrop
Technologies, Wilmington, DE, USA). Reverse transcription reactions
were carried out using the TaqMan MicroRNA Reverse Transcription
kit (Applied Biosystems, Foster City, CA, USA) in a total reaction
volume of 15 µl. miR-16 was chosen as an endogenous normalizer for
miRNAs based on the previous finding that miR-16 was one of the
most suitable reference genes for relative quantification of small
ncRNAs in colonic tissues including CRC and normal colonic mucosa
in microarray analysis of large cohorts. Furthermore, we previously
demonstrated that miR-16 is a reliable normalizer for tissue
samples (25–27,29,38–40).
miR-21 and miR-16 were quantified in duplicate by qRT-PCR using
MicroRNA Assay kits (Applied Biosystems). qRT-PCR was performed on
an Applied Biosystems 7000 Sequence Detection system with TaqMan
Universal PCR Master Mix 2X under the following cycling conditions:
95°C for 10 min, followed by 45 cycles at 95°C for 15 sec and 60°C
for 1 min. Cycle threshold (Ct) values were calculated using the
same threshold cut-off values for each assay to prevent
plate-to-plate variations and data were analyzed with Sequence
Detection Software v.1.4.1 (Applied Biosystems).
Calculation of the expression of
miRNA
The expression levels of serum or tissue miRNAs were
normalized using cel-miR-39 (for serum samples) and miR-16 (for
tissue samples) using the 2−ΔCt method as previously
described (25–27,29,38,39).
The differences between groups are presented as ΔCt, indicating
differences between the Ct values of the miRNAs of interest and the
Ct values of the normalizer miRNAs.
Statistical analysis
Statistical analysis was performed using MedCalc
software version 16.8.4 (Mariakerke, Belgium). The results are
expressed as the means ± standard deviation (SD). The differences
between groups were estimated using Chi-square and Mann-Whitney U
test. F-tests were conducted to assess the equality of variance for
comparable groups. Statistical differences in PMI between different
times (pre- and postoperative) from the same group of patients were
compared using the Wilcoxon test for paired samples. For
time-to-event analyses, survival estimates were calculated using
Kaplan-Meier analysis and groups were compared with the log-rank
test. Receiver operating characteristic (ROC) curves were
established to determine the cut-off values for analyzing prognosis
and various types of metastasis by Youden's index. Overall survival
(OS) was determined from the date the patient underwent surgery
until the date of death resulting from any cause (i.e.,
cancer-unrelated deaths were not censored) or the last known
follow-up for patients that were still alive. Disease-free survival
(DFS) was derermined from the date the patient underwent curative
surgery to the date of disease recurrence, death from any cause
(i.e., cancer-unrelated deaths were not censored), or last contact
with the patient. The Cox's proportional hazards models were used
to estimate hazard ratios (HR) for death and recurrence. Assumption
of proportionality was confirmed for the Cox proportional hazards
analyses by generating Kaplan-Meier survival curves (e.g., high vs.
low PMI groups) and ensuring that the two curves did not intersect
each other. Multivariate logistic regression models were used to
predict factors influencing hepatic, peritoneal, distant metastasis
and low PMI. Forced-entry regression was used to include these
variables in all multivariable equations to analyze whether each of
the predictors affected the outcome after adjusting for known
confounding factors. For the univariate and multivariate analyses
for patient prognosis, in addition to target PMI and IMAC status,
we included the following previously identified confounding
clinical factors that impact the prognosis of patients with rectal
cancer: sex, age at diagnosis, pathological differentiation
(well-moderate or poor), T stage (T1/2 or T3/4), venous invasion
(present or absent), lymphatic vessel invasion (present or absent),
lymph node metastasis (present or absent) and UICC stage
classification (stages I/II or stages III/IV). All P-values were
two-sided and P<0.05 was considered to indicate a statistically
significant difference.
Results
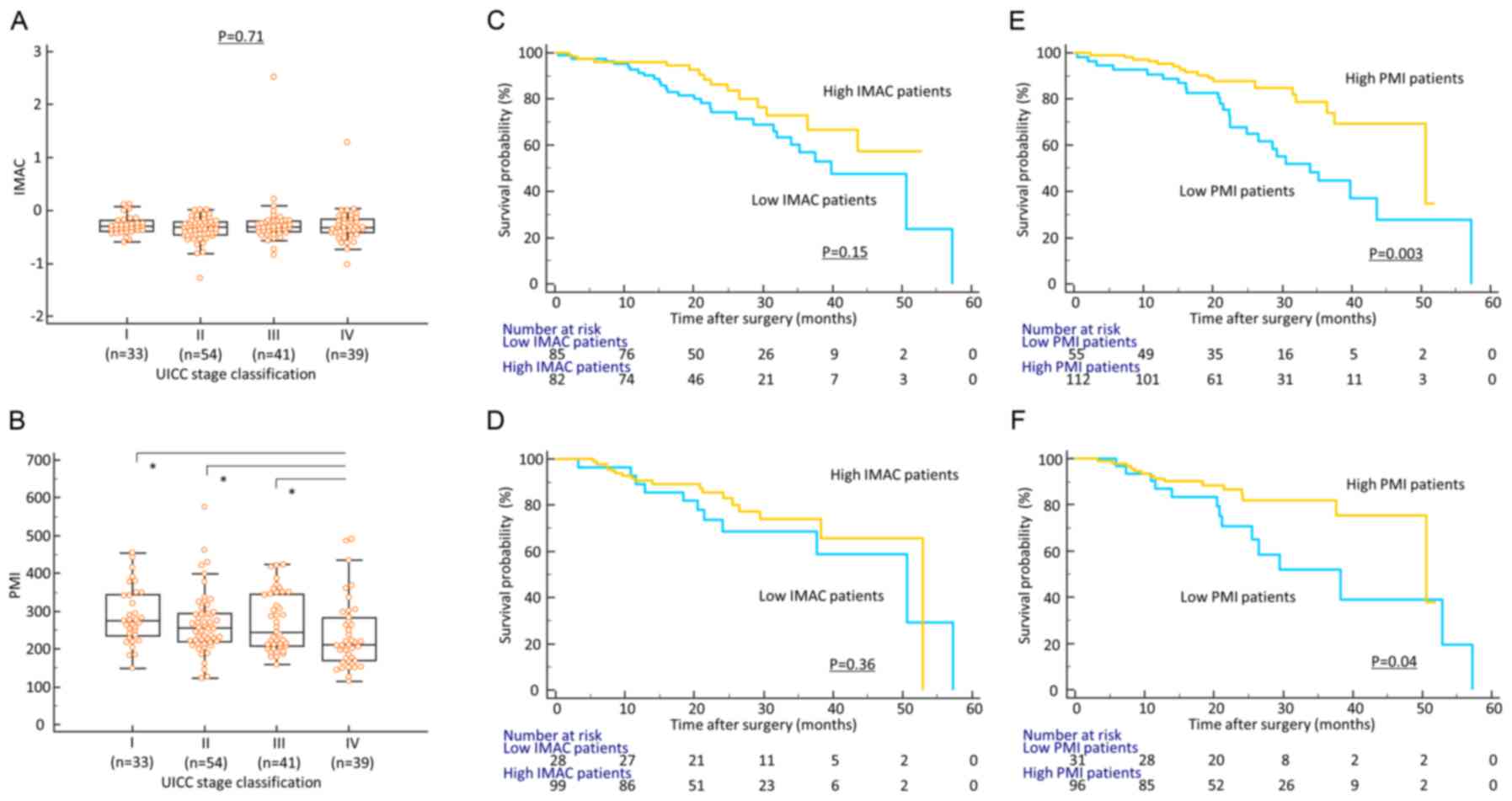
In contrast to the IMAC status,
preoperative PMI is significantly decreased with disease
progression in CRC patients
Firstly, to determine whether body composition
status had clinical significance in CRC patients, we analyzed the
association between preoperative PMI/IMAC and various
clinicopathological factors (Table
I). With the exception of a significant correlation with sex
(female) and older age, preoperative IMAC status was not
significantly correlated with other clinicopathological factors in
this cohort (Fig. 1A). In contrast,
decreased preoperative PMI was significantly associated with all
well-established clinicopathological factors for disease
progression, including advanced T stage (P=0.05), presence of
venous invasion (P=0.021), lymphatic vessel invasion (P=0.036),
lymph node metastasis (P=0.001), hepatic metastasis (P=0.028),
peritoneal metastasis (P=0.003), distant metastasis (P=0.0004) and
advanced TNM stage classification (P=0.0001) in CRC patients
(Table I; Fig. 1B).
 | Table I.Correlation between
clinicopathological variables and PMI/IMAC in colorectal cancer
patients. |
Table I.
Correlation between
clinicopathological variables and PMI/IMAC in colorectal cancer
patients.
|
|
| PMI |
| IMAC |
|
|---|
|
|
|
|
|
|
|
|---|
| Variable | n | High (n=112) | Low (n=55) | P-value | High (n=82) | Low (n=85) | P-value |
|---|
| Sex |
|
|
|
<0.0001b |
|
|
<0.0001b |
| sMale | 99 | 79 | 20 |
| 35 | 64 |
|
|
Female | 68 | 33 | 35 |
| 47 | 21 |
|
| Age (years) |
|
|
| 0.83 |
|
|
0.004b |
|
≥67a | 84 | 57 | 27 |
| 32 | 52 |
|
|
>67 | 83 | 55 | 28 |
| 50 | 33 |
|
| Histological
type |
|
|
| 0.13 |
|
| 0.33 |
|
Differentiated | 151 | 104 | 47 |
| 76 | 75 |
|
|
Undifferentiated | 16 | 8 | 8 |
| 6 | 10 |
|
| Pathological T
category |
|
|
| 0.05 |
|
| 0.39 |
|
pT1/2 | 50 | 39 | 11 |
| 22 | 28 |
|
|
pT3/4 | 117 | 73 | 44 |
| 60 | 57 |
|
| Vessel
invasion |
|
|
|
0.021b |
|
| 0.61 |
|
Present | 70 | 40 | 30 |
| 36 | 34 |
|
|
Absent | 97 | 72 | 25 |
| 46 | 51 |
|
| Lymphovascular
invasion |
|
|
|
0.036b |
|
| 0.68 |
|
Present | 126 | 79 | 47 |
| 63 | 63 |
|
|
Absent | 41 | 33 | 8 |
| 19 | 22 |
|
| Lymph node
metastasis |
|
|
|
0.001b |
|
| 0.91 |
| N0 | 97 | 75 | 22 |
| 48 | 49 |
|
| N1 | 70 | 37 | 32 |
| 34 | 36 |
|
| Hepatic
metastasis |
|
|
|
0.028b |
|
| 0.16 |
| H0 | 142 | 100 | 42 |
| 73 | 69 |
|
| H1 | 25 | 12 | 13 |
| 9 | 16 |
|
| Peritoneal
metastasis |
|
|
|
0.003b |
|
| 0.77 |
| P0 | 158 | 110 | 48 |
| 78 | 80 |
|
| P1 | 9 | 2 | 7 |
| 4 | 5 |
|
| Distant
metastasis |
|
|
|
0.0004b |
|
| 0.25 |
| M0 | 128 | 95 | 33 |
| 66 | 62 |
|
| M1 | 39 | 17 | 22 |
| 16 | 23 |
|
| UICC TNM
classification |
|
|
|
0.0001b |
|
| 0.36 |
| Stage
I | 33 | 29 | 5 |
| 18 | 15 |
|
| Stage
II | 54 | 41 | 13 |
| 26 | 28 |
|
| Stage
III | 41 | 26 | 15 |
| 22 | 19 |
|
| Stage
IV | 39 | 17 | 22 |
| 16 | 23 |
|
Decreased preoperative PMI is
associated with poor outcome in CRC patients
Subsequently, we performed time-to-event analysis to
evaluate the potential of preoperative PMI and IMAC levels as a
prognostic biomarker and generated Kaplan-Meier survival curves
subdivided by PMI and IMAC score. Although there was no significant
correlation between preoperative IMAC status and recurrence or
survival (log-rank test, P=0.15, P=0.36, respectively; Fig. 1C and D), patients with decreased
preoperative PMI had a significantly poor prognosis compared with
those with high PMI in terms of both OS and DFS (log-rank test,
P=0.003, P=0.04, respectively; Fig. 1E
and F). To determine the potential of preoperative PMI as a
predictive biomarker of recurrence and prognosis in CRC patients,
multivariate Cox's regression analysis was performed. In addition
to advanced T stage, presence of vascular invasion, lymphatic
vessel invasion and decreased IMAC, we revealed that decreased
preoperative PMI was an independent prognostic factor for OS in CRC
patients (HR: 2.99, 95%; CI: 1.37–6.55, P=0.006) (Table IIA). Furthermore, decreased
preoperative PMI was also an independent prognostic factor for poor
DFS in these patients (HR: 2.62, 95%; CI: 1.04–6.63, P=0.042;
Table IIB).
 | Table II.Multivariate analyses. |
Table II.
Multivariate analyses.
| A, predictors of
overall survival |
|---|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95%CI | P-value |
|---|
| Sex (male) | 1.29 | 0.67–2.49 | 0.45 | 1.5 | 0.67–3.39 | 0.33 |
| Age (>67
year-old)a | 1.07 | 0.58–1.97 | 0.84 | 1.3 | 0.63–2.67 | 0.48 |
| Histological type
(undifferentiated) | 2.2 | 0.97–5.0 | 0.06 | 1.19 | 0.49–2.03 | 0.69 |
| Location
(left) | 0.81 | 0.42–1.54 | 0.51 | 0.67 | 0.33–1.36 | 0.27 |
| T classification
(pT3/4) | 15.8 | 2.17–114.8 |
0.006b | 8.76 | 1.07–71.4 |
0.043b |
| Vessel involvement
(present) | 4.31 | 2.11–8.81 |
0.0001b | 2.21 | 1.02–4.78 |
0.045b |
| Lymphatic vessel
involvement (present) | 12.8 | 1.76–93.1 |
0.012b | 1.99 | 0.23–17.1 | 0.53 |
| Lymph node
metastasis (present) | 6.78 | 3.12–14.7 |
<0.0001b | 0.57 | 0.16–2.01 | 0.39 |
| Stage
classification (stage III/IV) | 10.3 | 4.03–26.4 |
<0.0001b | 9.67 | 2.13–44.0 |
0.003b |
| PMI (low) | 2.51 | 1.35–4.68 |
0.004b | 2.99 | 1.37–6.55 |
0.006b |
| IMAC (low) | 1.77 | 0.93–3.38 | 0.084 | 2.85 | 1.34–6.08 |
0.007b |
|
| B, predictors of
disease-free survival |
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
Variables | HR | 95% CI | P-value | HR | 95%CI | P-value |
|
| Sex (male) | 1.09 | 0.5–2.38 | 0.83 | 1.15 | 0.41–3.21 | 0.78 |
| Age (>67
year-old)a | 1.96 | 0.88–4.37 | 0.1 | 1.52 | 0.61–3.78 | 0.37 |
| Histological type
(undifferentiated) | 1.69 | 0.58–4.96 | 0.34 | 0.42 | 0.1–1.74 | 0.23 |
| Location
(left) | 0.64 | 0.29–1.38 | 0.25 | 0.4 | 0.15–1.07 | 0.07 |
| T classification
(pT3/4) | 4.29 | 1.29–14.2 |
0.017b | 2.06 | 0.51–8.25 | 0.31 |
| Vessel involvement
(present) | 4.4 | 1.98–9.81 |
0.0003b | 2.64 | 1.09–6.41 |
0.032b |
| Lymphatic vessel
involvement (present) | 11.5 | 1.56–84.7 | 0.017b | 2.22 | 0.24–20.3 | 0.48 |
| Lymph node
metastasis (present) | 3.68 | 1.72–7.88 |
0.0008b | 3.55 | 1.4–8.99 |
0.008b |
| PMI (low) | 2.18 | 1.02–4.68 |
0.045b | 2.62 | 1.04–6.63 |
0.042b |
| IMAC (low) | 1.43 | 0.65–3.12 | 0.37 | 2.1 | 0.81–5.44 | 0.13 |
Decreased PMI is an independent risk
factor for metastasis in CRC patients
Notably, low PMI status was significantly correlated
with metastasis-related clinicopathological factors such as
presence of hepatic, peritoneal and distant metastasis in CRC
patients. Based on these findings, we performed multivariate
logistic analysis to determine the clinical significance of low PMI
status as a predictive biomarker for metastasis (Table IIIA-C). Notably, low PMI status
was an independent predictive factor for hepatic metastasis (OR:
13.6, 95%; CI: 3.03–60.6, P=0.0006), peritoneal metastasis (OR:
5.73, 95% CI: 1.1–29.7, P=0.038) and distant metastasis (OR: 4.26,
95%; CI: 1.61–11.2, P=0.003), indicating that the presence of
distant metastasis was intimately correlated with sarcopenia in CRC
patients.
 | Table III.Multivariate analyses. |
Table III.
Multivariate analyses.
| A, predictors of
hepatic metastasis |
|---|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Sex (male) | 2.45 | 0.92–6.51 | 0.07 | 9.35 | 1.88–46.3 |
0.006b |
| Age (>67
years-old)a | 0.63 | 0.27–1.5 | 0.3 | 0.43 | 0.12–1.54 | 0.19 |
| Histological type
(differentiated) | 1.26 | 0.27–5.91 | 0.77 | 8.68 | 0.96–78.7 | 0.05 |
| Location
(left) | 0.46 | 0.19–1.08 | 0.07 | 0.21 | 0.06–0.77 |
0.018b |
| T classification
(pT3/4) | 3.63 | 1.03–12.7 |
0.044b | 0.68 | 0.13–3.61 | 0.65 |
| Vessel involvement
(present) | 3.57 | 1.44–8.83 |
0.006b | 2.77 | 0.77–10.0 | 0.12 |
| Lymphatic vessel
involvement (present) | 4.35 | 0.98–19.3 | 0.05 | 0.55 | 0.06–4.71 | 0.58 |
| Lymph node
metastasis (present) | 14.4 | 4.09–50.4 |
<0.0001b | 24.6 | 4.35–139 |
0.0003b |
| PMI (low) | 4.78 | 1.94–11.8 |
0.0007b | 13.6 | 3.03–60.6 |
0.0006b |
| IMAC (low) | 2.43 | 0.94–6.31 | 0.07 | 3.96 | 0.95–16.5 | 0.06 |
|
| B, predictors of
peritoneal metastasis |
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
|
| Sex (male) | 0.32 | 0.08–1.34 | 0.12 | 0.6 | 0.11–3.25 | 0.55 |
| Age (>67
year-old)a | 0.8 | 0.21–3.09 | 0.75 | 0.81 | 0.16–4.1 | 0.8 |
| Histological type
(differentiated) | 0.18 | 0.04–0.8 |
0.025b | 0.37 | 0.06–2.26 | 0.28 |
| Location
(Left) | 0.58 | 0.15–2.25 | 0.43 | 0.8 | 0.17–3.84 | 0.78 |
| T classification
(pT3/4) | – | – | 0.99 | – | – | 0.99 |
| Vessel involvement
(present) | 5.28 | 1.06–26.2 |
0.042b | 1.7 | 0.29–10.0 | 0.56 |
| Lymphatic vessel
involvement (present) | – | – | 0.99 | – | – | 0.99 |
| Lymph node
metastasis (present) | 5.28 | 1.06–26.2 |
0.042b | 2.03 | 0.35–11.9 | 0.43 |
| PMI (low) | 11.2 | 2.61–47.6 |
0.001b | 5.73 | 1.1–29.7 |
0.038b |
| IMAC (low) | 0.31 | 0.08–1.2 | 0.09 | 0.72 | 0.14–3.78 | 0.69 |
|
| C, predictors of
distant metastasis |
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
|
| Sex (male) | 1.3 | 0.62–2.74 | 0.48 | 2.1 | 0.77–5.96 | 0.15 |
| Age (>67
year-old)a | 0.73 | 0.35–1.49 | 0.38 | 0.59 | 0.24–1.47 | 0.26 |
| Histological type
(differentiated) | 0.47 | 0.16–1.38 | 0.17 | 0.97 | 0.25–3.71 | 0.96 |
| Location
(left) | 0.81 | 0.38–1.72 | 0.59 | 0.71 | 0.28–1.8 | 0.48 |
| T classification
(pT3/4) | 2.88 | 1.12–7.4 |
0.028b | 0.8 | 0.23–2.82 | 0.73 |
| Vessel involvement
(present) | 5.21 | 2.37–11.5 |
<0.0001b | 3.37 | 1.24–9.16 | 0.017
b |
| Lymphatic vessel
involvement (present) | 5.07 | 1.47–17.5 |
0.01b | 1.36 | 0.28–6.7 | 0.7 |
| Lymph node
metastasis (present) | 6.15 | 2.74–13.8 |
<0.0001b | 3.74 | 1.44–9.71 | 0.007
b |
| PMI (low) | 4.14 | 1.95–8.77 |
0.0002b | 4.26 | 1.61–11.2 |
0.003b |
| IMAC (low) | 1.53 | 0.74–3.16 | 0.25 | 1.79 | 0.68–4.71 | 0.24 |
|
| D, predictors of
decreased PMI |
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
Variables | OR | 95% CI | P-value | OR | 95% CI | P-value |
|
| Sex (female) | 4.19 | 2.12–8.3 |
<0.0001b | 5.02 | 2.12–11.9 |
0.0003b |
| Age (>67
year-old)a | 0.93 | 0.49–1.77 | 0.83 | 1.24 | 0.54–2.84 | 0.62 |
| Tissue miR-21
expression (high) | 0.8 | 0.39–1.62 | 0.53 | 0.81 | 0.38–2.02 | 0.76 |
| Serum miR-21
expression (high) | 2.18 | 1.02–4.66 |
0.044b | 2.68 | 1.07–6.74 |
0.036b |
| Serum albumin level
(low) | 3.17 | 1.62–6.19 |
0.0007b | 5.69 | 2.32–13.9 |
0.0001b |
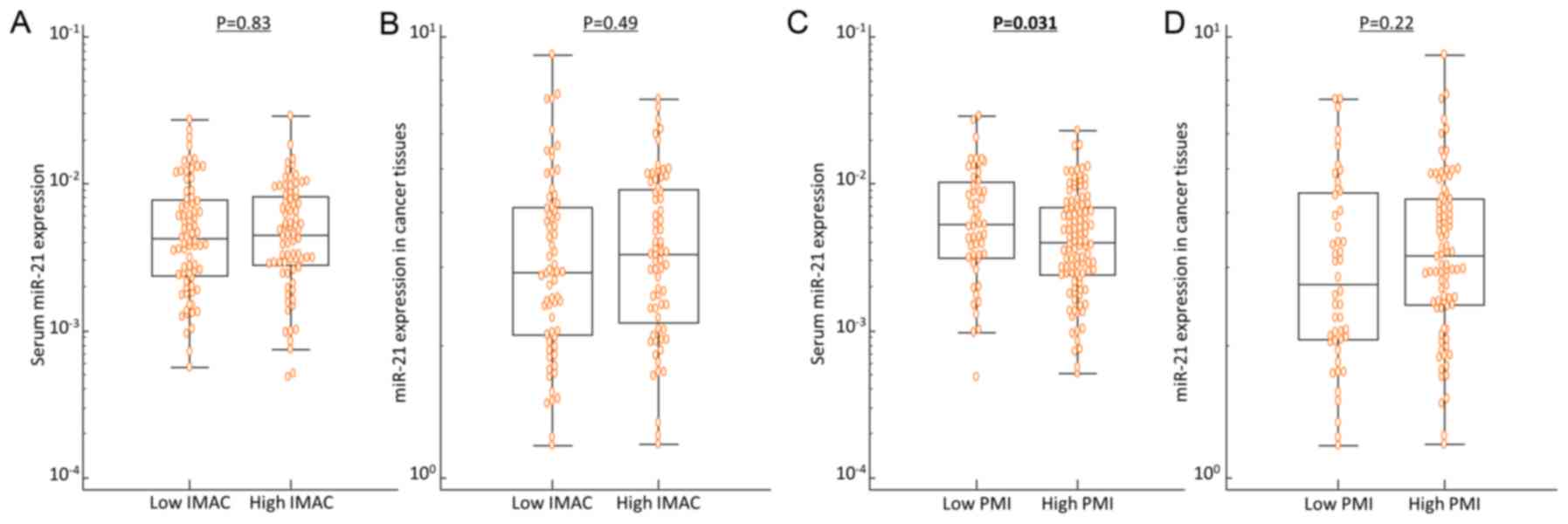
Serum miR-21 expression is
significantly increased in CRC patients with low PMI compared with
those with high PMI
miR-21 is one of the most representative oncogenic
secretory miRNAs (25,41) and a recent study revealed its
pivotal function in the pathogenesis of sarcopenia (42). Considering these findings, we
subsequently assessed serum and tissue miR-21 expression levels to
examine the correlation between miR-21 expression levels and
preoperative body composition status in CRC patients. Indeed,
despite the lack of significant correlation between the IMAC status
and miR-21 expression in cancer tissues or serum, serum miR-21
expression, but not tissue miR-21 expression, was significantly
increased in patients with low PMI compared with those with high
PMI (P=0.031; Fig. 2). Furthermore,
multivariate logistic regression analysis, adjusted by age, sex,
tissue miR-21 and serum albumin level, clearly demonstrated that
increased serum miR-21 level was an independent risk factor for
decreased PMI in CRC patients (Table
IIID).
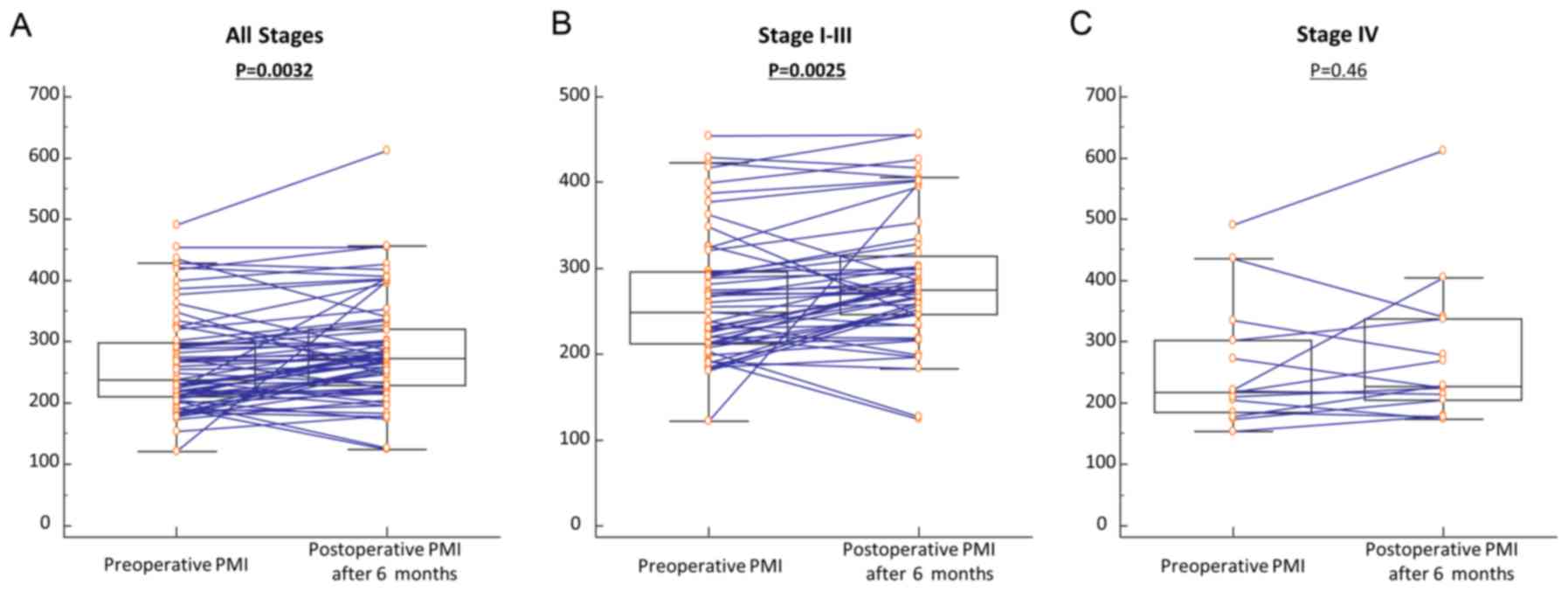
Changes in PMI in patients with CRC
before and after resection of primary tumors
In a previous study, we evaluated serum miR-21
expression levels in paired pre- and postoperative serum samples
and separately analyzed the data based on potentially curative
(Stages I–III) vs. non-curative surgeries (Stage IV) (25). Our study revealed postoperative
reductions in serum miR-21 levels among patients with potentially
curative surgeries (Stages I–III), but no significant differences
in patients with non-curative resections (Stage IV). These data
highlighted the feasibility of serum miR-21 as a disease-specific
biomarker for CRC. Based on these findings, we analyzed paired pre-
and postoperative PMI values in a subset of 65 CRC patients who
underwent surgical resection of their tumors. Among the 65 CRC
patients, 51 underwent potentially curative resection whereas 14
had multiple hepatic metastases and underwent primary resection to
prevent bleeding and bowel obstruction (non-curative resection).
Postoperative PMI was significantly increased compared with
preoperative PMI for total CRC patients (P=0.0032; Fig. 3A). Interestingly, postoperative PMI
was drastically improved compared with preoperative PMI in CRC
patients with potentially curative resection (P=0.0025; Fig. 3B), whereas PMI levels did not change
between pre- and postoperative status in CRC patients with
non-curative resection (P=0.46; Fig.
3C). These findings were consistent with previous findings of a
dysregulated pattern of serum miR-21 expression. Collectively,
these results revealed an intimate correlation between serum miR-21
and skeletal muscle mass and highlighted the expression of serum
miR-21 as a clinically feasible biomarker for monitoring cancer
cachexia in CRC patients.
Discussion
Cancer cachexia is recognized as severe malnutrition
accompanied by sarcopenia and is one of the major obstacles
affecting various clinical aspects in patients with malignancies.
Accumulating evidence has revealed that cancer cachexia is
intimately associated with the deterioration of functional status
and quality of life, low tolerance to chemotherapy and poor
prognosis in patients with gastrointestinal cancer (43,44).
However, its prognostic impact and clinical significance remain
unclear and the underlying pathological mechanism has not been
elucidated. We made several novel and interesting discoveries
during the course of our investigation. Firstly, despite no
significant correlation between IMAC and disease-correlated
factors, loss of PMI was significantly correlated with
well-established factors for disease progression, including
advanced T stage, presence of venous invasion, lymphatic vessel
invasion, lymph node, hepatic, peritoneal and distant metastasis as
well as advanced TNM stage classification in CRC patients.
Secondly, loss of PMI was significantly correlated with poor
prognosis and was an independent prognostic factor for both OS and
DFS in CRC patients by multivariate analysis. Furthermore, we
revealed that loss of PMI was an independent risk factor for
various types of metastasis in patients with CRC, including
hepatic, peritoneal and distant metastasis, indicating that
sarcopenia may be intimately associated with distant metastasis in
CRC. Thirdly, although tissue miR-21 expression was not correlated
with preoperative PMI status, serum miR-21 expression was
significantly increased in CRC patients with low PMI compared with
those with high PMI. Finally, we compared pre- and postoperative
PMI status. Although there was no significant change between pre-
and postoperative PMI in patients with non-curative resection,
postoperative PMI was drastically improved compared with
preoperative PMI in CRC patients with potentially curative
resection. These findings successfully validated previous results
revealing patterns of dysregulation between pre- and postoperative
serum miR-21 expression.
Currently, various components of body composition
are recognized as factors influencing surgical and oncological
outcome in patients with malignancies. Skeletal muscle mass is one
of the representative body composition components and loss of
skeletal muscle mass, called sarcopenia, is associated with aging,
inactivity and chronic benign and malignant disease (2,45).
Several studies revealed that sarcopenia frequently occurred in
patients with various malignancies and was a significant risk
factor for surgical complications in CRC patients (46–49).
Muscle steatosis, an increase in intramuscular adipose tissue, has
also attracted increasing attention as an important body
composition component in the field of oncological research
(32,33,50).
Okumura et al (32)
investigated 230 patients who underwent resection of pancreatic
cancer to assess the prognostic impact of muscle quantity and
quality in these patients. They demonstrated that low PMI and high
IMAC were independent prognostic factors of poor OS and
recurrence-free survival and suggested that low quality and
quantity of skeletal muscle may be closely related to mortality
after resection of pancreatic cancer. In the present study, we
performed matched analysis of preoperative PMI and IMAC to directly
compare the quality and quantity of skeletal muscle and to clarify
the clinical significance of these components in CRC patients. One
of the major findings in the present study is the clinical and
prognostic impact of low PMI in CRC patients. In contrast to IMAC,
low PMI status was an independent prognostic factor for both OS and
DFS in CRC patients. Furthermore, low PMI status was an independent
risk factor for various type σ of metastasis. These findings,
combined with previous evidence, indicated that assessment of PMI
status could be a promising prognostic marker for disease
progression in patients with CRC and that sarcopenia is closely
associated with disease progression, especially distant metastasis,
in CRC patients.
Another major finding of the present study is the
significant correlation between quantity of skeletal muscle mass
and circulating miR-21 expression in CRC patients. A recent study
(42) clearly demonstrated that
cancer-derived microvesicles containing miR-21 activated the TLR7
receptor on murine myoblasts and promoted muscle cell death through
c-Jun N-terminal kinase (JNK) activity in cancer cachexia.
Furthermore, our research group previously revealed several
specific features of serum miR-21 in CRC patients (25). Firstly, using supernatants from
cultured CRC cell lines, miR-21 was identified as a novel secretory
miRNA and serum expression of miR-21 in CRC patients correlated
positively with the expression status in tumor tissues. Secondly,
serum miR-21 expression was significantly increased in patients
with adenoma and CRC and quantification of serum miR-21 could
robustly discriminate the patients with adenoma and CRC from
healthy volunteers. Furthermore, we also demonstrated that serum
miR-21 expression decreased significantly in postoperative serum
samples from patients who underwent curative CRC surgery.
Consistent with these findings, the present study revealed that
serum miR-21 was significantly increased in patients with low PMI
compared with those with high PMI regardless of the lack of a
significant correlation between tissue miR-21 expression and PMI
status. Furthermore, postoperative PMI was significantly improved
in CRC patients who underwent curative surgery, supporting a
significant correlation between serum miR-21 and PMI status.
Collectively, our data revealed that circulating miR-21 was more
directly correlated with sarcopenia compared to tissue miR-21
expression in CRC patients and indicated that assessment of serum
miR-21 level may be used to evaluate the risk of sarcopenia and
cancer cachexia in CRC patients.
In conclusion we presented novel findings on the
clinical significance of sarcopenia and its intimate correlation
with circulating miR-21 expression in CRC. Skeletal muscle mass may
be used as a prognostic and predictive biomarker for various types
of distant metastasis in CRC patients and quantification of the
expression of serum miR-21 could be beneficial in planning a
nutrition intervention strategy for CRC patients.
Acknowledgements
We would like to thank Yuki Orito and Amphone Okada
for their excellent technical assistance. We also thank Mary Derry,
PhD ELS, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this
manuscript. The present study was supported by a research grant
from the Japanese Society for Palliative Medicine and the Mie
Medical Research Foundation.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
von Haehling S, Lainscak M, Springer J and
Anker SD: Cardiac cachexia: A systematic overview. Pharmacol Ther.
121:227–252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fearon K, Strasser F, Anker SD, Bosaeus I,
Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N,
Mantovani G, et al: Definition and classification of cancer
cachexia: An international consensus. Lancet Oncol. 12:489–495.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
von Haehling S and Anker SD: Cachexia as a
major underestimated and unmet medical need: Facts and numbers. J
Cachexia Sarcopenia Muscle. 1:1–5. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
von Haehling S, Anker MS and Anker SD:
Prevalence and clinical impact of cachexia in chronic illness in
Europe, USA, and Japan: Facts and numbers update 2016. J Cachexia
Sarcopenia Muscle. 7:507–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fearon KC: Cancer cachexia: Developing
multimodal therapy for a multidimensional problem. Eur J Cancer.
44:1124–1132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tisdale MJ: Cachexia in cancer patients.
Nat Rev Cancer. 2:862–871. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ksiazek-Winiarek DJ, Kacperska MJ and
Glabinski A: MicroRNAs as novel regulators of neuroinflammation.
Mediators Inflamm. 2013:1723512013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA, Dumitru CD, Shimizu M, Bichi R,
Zupo S, Noch E, Aldler H, Rattan S, Keating M, Rai K, et al:
Frequent deletions and down-regulation of micro-RNA genes miR15 and
miR16 at 13q14 in chronic lymphocytic leukemia. Proc Natl Acad Sci
USA. 99:pp. 15524–15529. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mirnezami AH, Pickard K, Zhang L, Primrose
JN and Packham G: MicroRNAs: Key players in carcinogenesis and
novel therapeutic targets. Eur J Surg Oncol. 35:339–347. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang L, Belaguli N and Berger DH: MicroRNA
and colorectal cancer. World J Surg. 33:638–646. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schetter AJ, Leung SY, Sohn JJ, Zanetti
KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, et
al: MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. Jama. 299:425–436.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cummins JM, He Y, Leary RJ, Pagliarini R,
Diaz LA Jr, Sjoblom T, Barad O, Bentwich Z, Szafranska AE,
Labourier E, et al: The colorectal microRNAome. Proc Natl Acad Sci
USA. 103:pp. 3687–3692. 2006; View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valadi H, Ekstrom K, Bossios A, Sjostrand
M, Lee JJ and Lotvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Montecalvo A, Larregina AT, Shufesky WJ,
Stolz DB, Sullivan ML, Karlsson JM, Baty CJ, Gibson GA, Erdos G,
Wang Z, et al: Mechanism of transfer of functional microRNAs
between mouse dendritic cells via exosomes. Blood. 119:756–766.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Castellana D, Zobairi F, Martinez MC,
Panaro MA, Mitolo V, Freyssinet JM and Kunzelmann C: Membrane
microvesicles as actors in the establishment of a favorable
prostatic tumoral niche: A role for activated fibroblasts and
CX3CL1-CX3CR1 axis. Cancer Res. 69:785–793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Andre N and Schmiegel W: Chemoradiotherapy
for colorectal cancer. Gut. 54:1194–1202. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lurje G, Zhang W and Lenz HJ: Molecular
prognostic markers in locally advanced colon cancer. Clin
Colorectal Cancer. 6:683–690. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Miki C, Tonouchi H, Wakuda R, Hatada T,
Inoue Y, Minato E, Kobayashi M and Kusunoki M: Intra-tumoral
interleukin-6 down-regulation system and genetic mutations of tumor
suppressor genes in colorectal carcinoma. Cancer. 94:1584–1592.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okugawa Y, Miki C, Toiyama Y, Yasuda H,
Yokoe T, Saigusa S, Hiro J, Tanaka K, Inoue Y and Kusunoki M: Loss
of tumoral expression of soluble IL-6 receptor is associated with
disease progression in colorectal cancer. Br J Cancer. 103:787–795.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koike Y, Miki C, Okugawa Y, Yokoe T,
Toiyama Y, Tanaka K, Inoue Y and Kusunoki M: Preoperative
C-reactive protein as a prognostic and therapeutic marker for
colorectal cancer. J Surg Oncol. 98:540–544. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shirai Y, Okugawa Y, Hishida A, Ogawa A,
Okamoto K, Shintani M, Morimoto Y, Nishikawa R, Yokoe T, Tanaka K,
et al: Fish oil-enriched nutrition combined with systemic
chemotherapy for gastrointestinal cancer patients with cancer
cachexia. Sci Rep. 7:48262017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Toiyama Y, Takahashi M, Hur K, Nagasaka T,
Tanaka K, Inoue Y, Kusunoki M, Boland CR and Goel A: Serum miR-21
as a diagnostic and prognostic biomarker in colorectal cancer. J
Natl Cancer Inst. 105:849–859. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hur K, Toiyama Y, Schetter AJ, Okugawa Y,
Harris CC, Boland CR and Goel A: Identification of a
metastasis-specific MicroRNA signature in human colorectal cancer.
J Natl Cancer Inst. 107:dju492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Okugawa Y, Toiyama Y, Toden S, Mitoma H,
Nagasaka T, Tanaka K, Inoue Y, Kusunoki M, Boland CR and Goel A:
Clinical significance of SNORA42 as an oncogene and a prognostic
biomarker in colorectal cancer. Gut. 66:107–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yamada A, Horimatsu T, Okugawa Y, Nishida
N, Honjo H, Ida H, Kou T, Kusaka T, Sasaki Y, Yagi M, et al: Serum
miR-21, miR-29a, and miR-125b are promising biomarkers for the
early detection of colorectal neoplasia. Clin Cancer Res.
21:4234–4242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hur K, Toiyama Y, Okugawa Y, Ide S, Imaoka
H, Boland CR and Goel A: Circulating microRNA-203 predicts
prognosis and metastasis in human colorectal cancer. Gut.
66:654–665. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual (7th). 2010.
|
|
31
|
Fujikawa H, Araki T, Okita Y, Kondo S,
Kawamura M, Hiro J, Toiyama Y, Kobayashi M, Tanaka K, Inoue Y, et
al: Impact of sarcopenia on surgical site infection after
restorative proctocolectomy for ulcerative colitis. Surg Today.
47:92–98. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Okumura S, Kaido T, Hamaguchi Y, Fujimoto
Y, Masui T, Mizumoto M, Hammad A, Mori A, Takaori K and Uemoto S:
Impact of preoperative quality as well as quantity of skeletal
muscle on survival after resection of pancreatic cancer. Surgery.
157:1088–1098. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kobayashi A, Kaido T, Hamaguchi Y, Okumura
S, Taura K, Hatano E, Okajima H and Uemoto S: Impact of
postoperative changes in sarcopenic factors on outcomes after
hepatectomy for hepatocellular carcinoma. J Hepatobiliary Pancreat
Sci. 23:57–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hamaguchi Y, Kaido T, Okumura S, Kobayashi
A, Hammad A, Tamai Y, Inagaki N and Uemoto S: Proposal for new
diagnostic criteria for low skeletal muscle mass based on computed
tomography imaging in Asian adults. Nutrition. 32:1200–1205. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kitajima Y, Eguchi Y, Ishibashi E,
Nakashita S, Aoki S, Toda S, Mizuta T, Ozaki I, Ono N, Eguchi T, et
al: Age-related fat deposition in multifidus muscle could be a
marker for nonalcoholic fatty liver disease. J Gastroenterol.
45:218–224. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kitajima Y, Hyogo H, Sumida Y, Eguchi Y,
Ono N, Kuwashiro T, Tanaka K, Takahashi H, Mizuta T, Ozaki I, et
al: Severity of non-alcoholic steatohepatitis is associated with
substitution of adipose tissue in skeletal muscle. J Gastroenterol
Hepatol. 28:1507–1514. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kroh EM, Parkin RK, Mitchell PS and Tewari
M: Analysis of circulating microRNA biomarkers in plasma and serum
using quantitative reverse transcription-PCR (qRT-PCR). Methods.
50:298–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hur K, Toiyama Y, Takahashi M, Balaguer F,
Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR and Goel A:
MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT)
in human colorectal cancer metastasis. Gut. 62:1315–1326. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Link A, Balaguer F, Shen Y, Nagasaka T,
Lozano JJ, Boland CR and Goel A: Fecal MicroRNAs as novel
biomarkers for colon cancer screening. Cancer Epidemiol Biomarkers
Prev. 19:1766–1774. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chang KH, Mestdagh P, Vandesompele J,
Kerin MJ and Miller N: MicroRNA expression profiling to identify
and validate reference genes for relative quantification in
colorectal cancer. BMC Cancer. 10:1732010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Okugawa Y, Grady WM and Goel A: Epigenetic
alterations in colorectal cancer: Emerging Biomarkers.
Gastroenterology. 149:1204–1225.e12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
He WA, Calore F, Londhe P, Canella A,
Guttridge DC and Croce CM: Microvesicles containing miRNAs promote
muscle cell death in cancer cachexia via TLR7. Proc Natl Acad Sci
USA. 111:pp. 4525–4529. 2014; View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Prado CM, Lieffers JR, McCargar LJ, Reiman
T, Sawyer MB, Martin L and Baracos VE: Prevalence and clinical
implications of sarcopenic obesity in patients with solid tumours
of the respiratory and gastrointestinal tracts: A population-based
study. Lancet Oncol. 9:629–635. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thoresen L, Frykholm G, Lydersen S,
Ulveland H, Baracos V, Prado CM, Birdsell L and Falkmer U:
Nutritional status, cachexia and survival in patients with advanced
colorectal carcinoma. Different assessment criteria for nutritional
status provide unequal results. Clin Nutr. 32:65–72. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fearon K, Arends J and Baracos V:
Understanding the mechanisms and treatment options in cancer
cachexia. Nat Rev Clin Oncol. 10:90–99. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
van Vledder MG, Levolger S, Ayez N,
Verhoef C, Tran TC and Ijzermans JN: Body composition and outcome
in patients undergoing resection of colorectal liver metastases. Br
J Surg. 99:550–557. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Voron T, Tselikas L, Pietrasz D, Pigneur
F, Laurent A, Compagnon P, Salloum C, Luciani A and Azoulay D:
Sarcopenia impacts on short- and long-term results of hepatectomy
for hepatocellular carcinoma. Ann Surg. 261:1173–1183. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tan BH, Birdsell LA, Martin L, Baracos VE
and Fearon KC: Sarcopenia in an overweight or obese patient is an
adverse prognostic factor in pancreatic cancer. Clin Cancer Res.
15:6973–6979. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lieffers JR, Bathe OF, Fassbender K,
Winget M and Baracos VE: Sarcopenia is associated with
postoperative infection and delayed recovery from colorectal cancer
resection surgery. Br J Cancer. 107:931–936. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hamaguchi Y, Kaido T, Okumura S, Ito T,
Fujimoto Y, Ogawa K, Mori A, Hammad A, Hatano E and Uemoto S:
Preoperative intramuscular adipose tissue content is a novel
prognostic predictor after hepatectomy for hepatocellular
carcinoma. J Hepatobiliary Pancreat Sci. 22:475–485. 2015.
View Article : Google Scholar : PubMed/NCBI
|