Introduction
In the central nervous system, gliomas are the most
common and malignant types of tumor. The WHO tumor grades are
significantly associated with patient prognosis (1). Due to the tumor invasiveness and
difficulty in identifying tumor boundaries, the prognosis remains
poor even with use of the current standard therapies. Scientists
have previously indicated that tumor microenvironmental changes and
gene mutations [including microRNAs (miRNAs) and long non-coding
RNAs (lncRNAs)] each control glioma progression and prognosis
(2–5). Although modern healthcare has
developed rapidly, especially in the application of individualized
treatments (6,7), effective and accessible novel
therapeutic strategies are urgently required to combat this
malignancy.
miRNAs are a class of small non-coding RNAs that
negatively regulate their targets by binding to the 3′-untranslated
regions (3′-UTRs) of the mRNA, and then degrade their target mRNA
or hinder the expression of their target protein (8). Studies have shown that miRNAs regulate
various physiological and pathological processes, such as cell
division, differentiation and death, as well as cancer progression,
stem-cell differentiation and self-renewal (4,9,10). In
recent years, miRNAs have been widely studied and noted as
biomarkers (11). miRNA genes are
generally located at fragile chromosomal sites, and increasing
evidence suggests that miRNAs serve an important role in cancer
progression (12). Various human
cancer types are accompanied by aberrant expression of miRNAs,
which can function as tumor suppressors or oncogenes. Recently,
miRNA-320a has been reported to regulate cell proliferation,
differentiation and invasion in human colon cancer (13), and erythroid differentiation
(14) as well as bladder carcinoma
(15). However, there are few
studies demonstrating an association between miRNA-320a and
glioma.
Aquaporin 4 (AQP4) is a protein of the aquaporin
family, which is distributed in different areas of the central
nervous system (16). AQP4 is the
AQP subtype abundantly expressed on astrocytes, and serves an
important role in fluid exchange between the cerebrospinal fluid
compartments and the brain (17,18).
Recently, AQP4 regulation has been reported to have a role in
various brain diseases, including cerebral edema (19), Alzheimer's disease (20) and gliomas (21,22).
It not only controls water exchange, but can also regulate the
expression of amyloid-β peptides (Aβ), and influence K+
and Ca2+ transport (20). Numerous studies have suggested the
importance of AQP4 in the physiological and pathological processes
of the brain. In cancer research, AQP4 has also been associated
with cell apoptosis and adhesion (23,24);
thus, AQP4 may be a target for certain cancer therapies.
AQP4 is highly expressed at perivascular astrocyte
end-feet, influencing cell membrane dissociation and recombination.
In addition, it is highly expressed in gliomas and contributes to
tumor progression (17,25,26).
Therefore, we hypothesized that AQP4 could regulate cell invasion
and migration in glioma.
In this study, we obtained further knowledge
regarding the associations between AQP4 and miRNA-320a. We found
that miRNA-320a was downregulated in glioma tissues compared to
normal tissues, and we identified AQP4 to be a direct target of
miRNA-320a that mediates glioma cell invasion and migration.
Materials and methods
CGGA data analyses and miRNA target
prediction
miRNA-320a expression values and associated
prognostic information from 198 glioma cases were obtained from the
Chinese Glioma Genome Atlas (CGGA; http://www.cgga.org.cn). These 198 samples were
comprised of 135 WHO III and IV tumors, and 63 WHO II tumors. The
Kaplan-Meier estimation method was used for overall survival
analysis of patients based on miRNA expression. Candidate targets
of miRNA-320a were predicted by miRBase (http://mirtarbase.mbc.nctu.edu.tw/index.php),
TargetScan (http://www.targetscan.org/vert_71) and miRanda
(http://www.microrna.org/microrna/home.do).
Cell culture and human tissue
samples
The U87 and U251 human glioma cell lines were
cultured in DMEM (HyClone; GE Healthcare Life Sciences, Logan, UT,
USA) supplemented with 10% fetal bovine serum (FBS; Gemini Bio
Products, West Sacramento, CA, USA), 100 U/ml penicillin and 100
ng/ml streptomycin (both from Beyotime Institute of Biotechnology,
Haimen, China). All cells were incubated at 37°C in an atmosphere
containing 5% CO2. Human glioma and non-cancerous human
brain paraffin-embedded tissues were obtained from the Institute of
Neuroscience Ran Jianhua research group. These tissue samples were
subsequently used for immunohistochemistry (IHC) and for
hematoxylin and eosin (H&E) staining.
miRNA mimic and siRNA
transfection
Hsa-miRNA-320a, miRNA-negative control (NC) mimics
and AQP4 siRNA were chemically synthesized by RiboBio Co., Ltd.
(Guangzhou, China). Transfection of hsa-miRNA-320a, miRNA-NC or
siRNA was performed using the riboFECT™ CP reagent (RiboBio Co.,
Ltd.), according to the manufacturer's instructions. miRNA-320a or
siRNA (Table I) was transfected
into the U87 and U251 glioma cells for 48 h, prior to further
experiments being performed.
 | Table I.The interference sequences of
AQP4. |
Table I.
The interference sequences of
AQP4.
| siRNAs | Sequences |
|---|
| AQP4 siRNA1 |
CCAAGTCTGTCTTCTACAT |
| AQP4 siRNA2 |
GTTGAATTCAAACGTCGTT |
| AQP4 siRNA3 |
TTTACCGGTCGACATGGTT |
Dual-luciferase reporter assay
The 3′-UTR of AQP4 contains three predicted matching
regions for miRNA-320a. For the luciferase reporter assay,
Luci-AQP4 and the NC were designed and synthesized by Gene Create
Biological Engineering Co., Ltd. (Wuhan, China). First, 293T cells
were transfected with an AQP4-3′-UTR-luciferase plasmid, followed
by transfection with the miRNA-320a mimic or miRNA-NC in 48-well
plates. The cells were then collected and lysed for a luciferase
assay 48 h after transfection. Renilla luciferase was used
for normalization.
RNA isolation and reverse
transcription-quantitative PCR (RT-qPCR) analysis
The total RNAs were extracted from U87 and U251
cells using a HiPure Universal miRNA kit (Magen, Guangzhou, China).
The RT-qPCR analysis was performed using an All-in-One miRNA
RT-qPCR Detection kit (GeneCopoeia, Inc., Rockville, MD, USA), as
previously described (27), with a
T100™ Thermal Cycler and a CFX Connect™ Real-Time System (Bio-Rad
Laboratories, Inc., Hercules, CA, USA), according to the
manufacturer's instructions. The associated expression values of
miRNA-320a were calculated using a comparative method following
normalization to U6 rRNA. The specific primers were all designed
and synthesized by GeneCopoeia, Inc. (mature has-miRNA-320a:
5′-AAAAGCUGGGUUGAGAGGGCGA-3′). The mRNA was extracted using RNAiso
plus, and converted to cDNA using a PrimeScript™ RT Reagent kit
(both from Takara Bio, Inc., Otsu, Japan). RT-qPCR was performed
using SYBR Premix Ex Taq II (Takara Bio, Inc.). SYBR-Green primer
sequences were as follows: AQP4 forward, 5′-TCAGCATCGCCAAGTCTGTC-3′
and reverse, 5′-CTGGGAGGTGTGACCAGATAG-3′; MMP9 forward,
5′-CCCGGACCAAGGATACAGT-3′ and reverse, 5′-GCCATTCACGTCGTCCTTA-3′;
β-actin forward, 5′-ACTGGGACGACATGGAAAAG-3′ and reverse,
5′-TACATGGCTGGGACATTGAA-3′.
Invasion and migration assays
A Transwell chamber assay was used to assess cell
invasion and migration, according to the manufacturer's
instructions. For the invasion assay, 2×104 transfected
cells were seeded into the upper chambers, which were coated with
extracellular matrix (ECM) (BD Biosciences, San Jose, CA, USA). The
migration assay did not use ECM-coated chambers (Costar; Corning
Incorporated, Corning, NY, USA). After incubation at 37°C for 24 h,
the cells that were adherent to the upper surface of the filter
were removed, and the migrated cells on the lower surfaces were
fixed and then stained with crystal violet (Beyotime Institute of
Biotechnology).
IHC and western blotting
IHC and western blot analyses were performed as
previously described (28). Primary
antibodies included the following: Mouse mAb AQP4 (1:300; cat. no.
sc-32739) for IHC (Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA), rabbit mAb AQP4 for western blotting (1:1,000; cat. no.
BS3436) and rabbit mAb β-actin (1:10,000; cat. no. AP0060) (both
from Bioworld Technology, Inc., St. Louis Park, MN, USA), and a
rabbit mAb MMP9 (1:800; cat. no. wl01580) (Wanleibio Co., Ltd.,
Shenyang, China). A secondary anti-rabbit antibody (1:10,000; cat.
no. BS1043) (Bioworld Technology, Inc.) was used, as well as a
biotin-streptavidin HRP detection system (ZSGB-Bio, Beijing,
China). The western blot analyses were visualized via
chemiluminescence reagents (EMD Millipore, Billerica, MA, USA) and
a Bio-Rad ChemiDoc™ Touch Imaging System (Bio-Rad Laboratories,
Inc.). IHC staining was visualized using a light microscope
(Olympus Corporation, Tokyo, Japan) and MicroPublisher v.5.0 RTV
software (QImaging, Surrey, BC, Canada).
H&E staining
The paraffin-embedded tissue sections were sliced
into 4-µm-thick sections, deparaffinized in dimethylbenzene, and
then rehydrated in 100% and 80% alcohol and distilled water.
Subsequently, H&E was used to stain the tissue sections, which
were then imaged under a light microscope (Olympus Corporation) and
analyzed with MicroPublisher v.5.0 RTV software.
Immunofluorescence staining
In brief, the U87 and U251 glioma cells were
transfected with miRNA-320a or miRNA-NC and AQP4-siRNA or NC at
37°C with 5% CO2 for 48 h. The cells were then washed
three times with ice-cold PBS and fixed in 4% paraformaldehyde.
Normal goat serum (HyClone; GE Healthcare Life Sciences) was used
to block the cell membranes for 30 min at room temperature, then
the primary mouse mAb AQP4 (1:1,000; cat. no. sc-32739) was added
and incubated overnight at 4°C. The anti-mouse secondary
fluorescent antibody (1:64; cat. no. BA1101) (Boster Biological
Technology, Ltd., Wuhan, China) was subsequently added and
incubated for 1 h at 37°C, followed by staining with PI (Beyotime
Institute of Biotechnology). Following this, 50% glycerol was used
for mounting, and images were obtained via fluorescence microscopy
(Olympus Corporation).
Statistical analysis
The data were analyzed using SPSS v.20.0 (IBM SPSS,
Chicago, IL, USA) and the results from each transfected group were
compared against the NC group using a t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
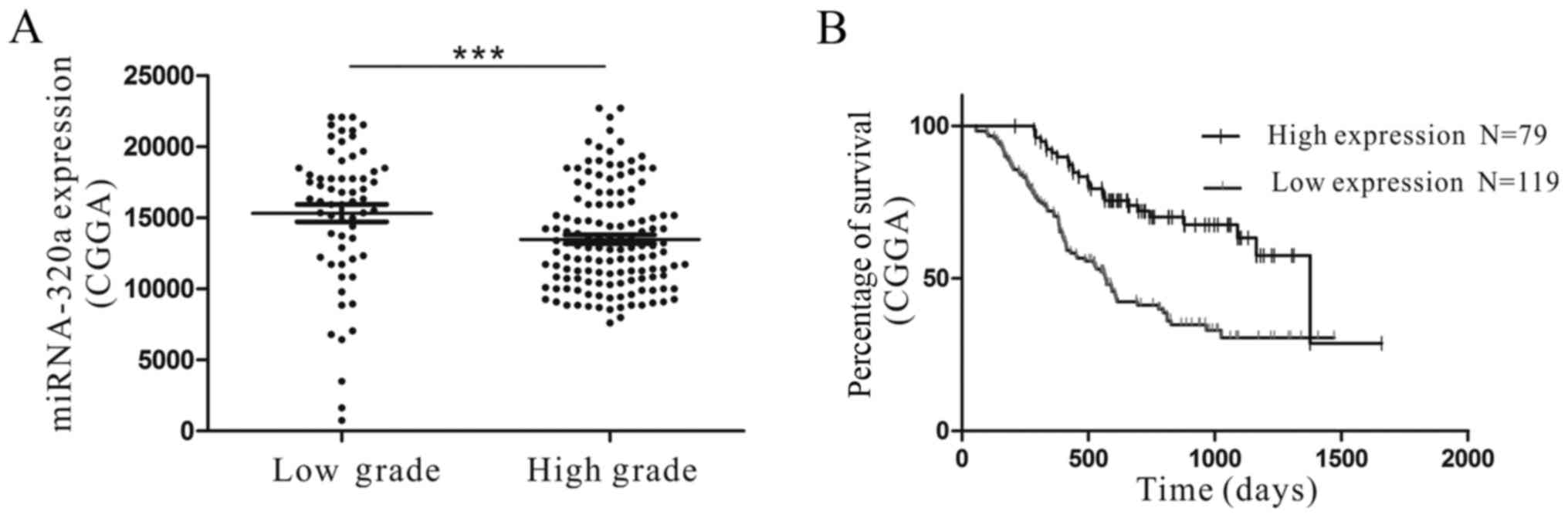
miRNA-320a is downregulated in glioma
tissues and is associated with patient prognosis
miRNA-320a has been reported to be downregulated in
chronic myeloid leukemia and non-small cell lung cancer (29,30).
To investigate the expression of miRNA-320a in glioma, microarray
data from the CGGA were analyzed. The microarray data and clinical
information from the CGGA included a total of 198 high-grade and
low-grade glioma samples. The results indicated that the miRNA-320a
expression was significantly lower in high-grade (grade III–IV)
glioma samples compared with in low-grade (grade II) glioma samples
(P<0.01; Fig. 1A). In addition,
the miRNA-320a expression in grade IV glioma tissues was
significantly different compared with that in grade II glioma and
normal brain tissues. Furthermore, in human glioma cell lines,
miRNA-320a was significantly decreased compared with that in human
astrocytes (31).
We identified that the expression of miRNA-320a was
associated with overall survival in each of the 198 glioma samples
from the CGGA. We determined that high miRNA-320a expression was
significantly associated with a better prognosis (P<0.01;
Fig. 1B). The results demonstrated
that miRNA-320a expression was downregulated in glioma tissues and
cell lines, and that high miRNA-320a expression was associated with
an improved patient prognosis, consistent with prior studies
(32). Therefore, the data revealed
that miRNA-320a may function as a tumor suppressor in glioma.
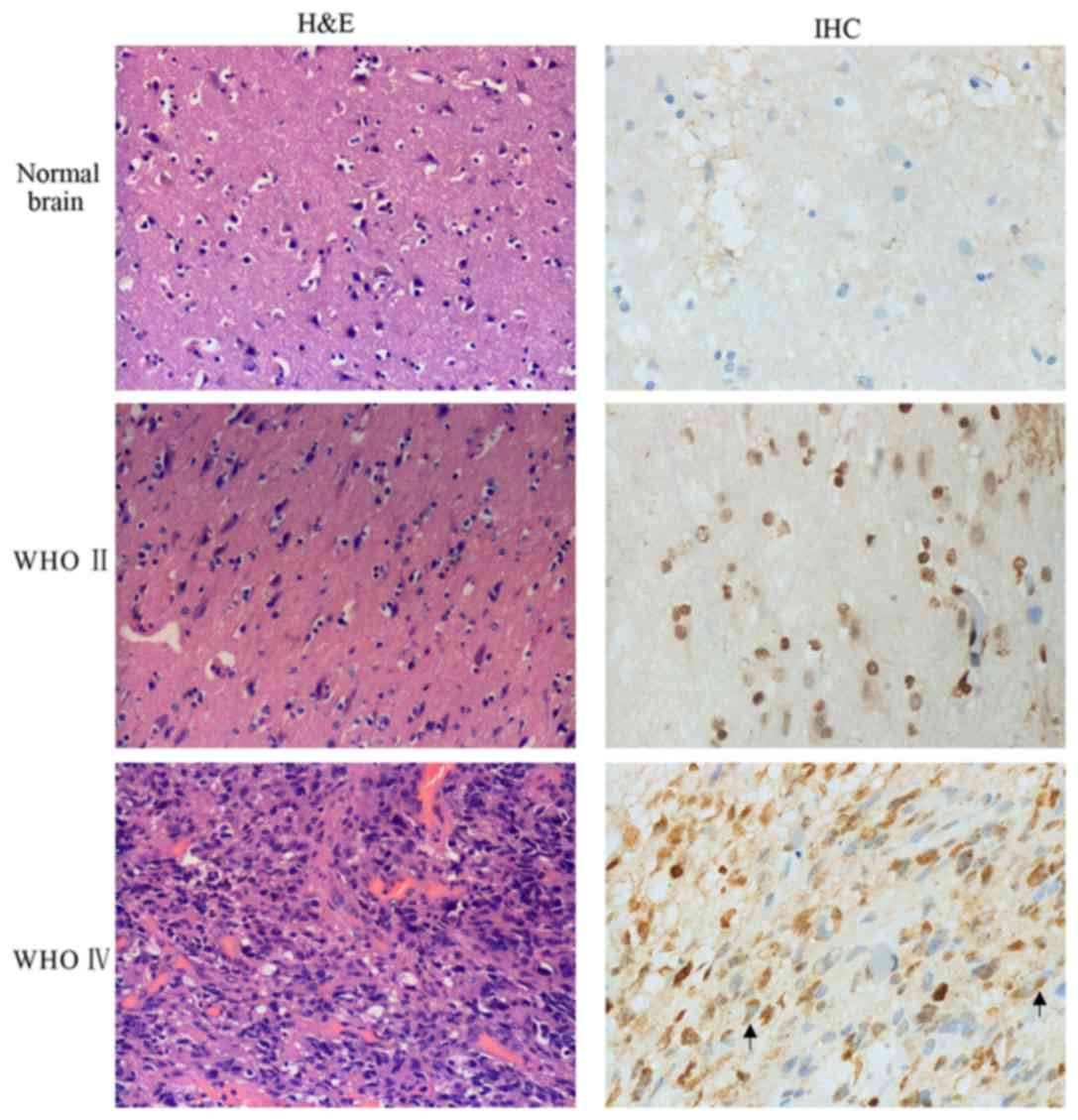
AQP4 is upregulated in human glioma
tissue samples
IHC was used to investigate the expression of AQP4
in human glioma and normal brain tissues (Fig. 2). Compared with the WHO grade II and
normal brain tissues, H&E staining clearly revealed cellular
atypia and an abundance of tumor cells in the WHO grade IV glioma
samples. The nuclei were non-uniform in size, and exhibited
hyperchromatism and nuclear division. Regarding AQP4 expression,
the high-grade glioma tissues almost express AQP4 in whole cells,
and showed a polarized distribution. In grade II glioma tissues,
few cells expressed AQP4 in the nucleus. By comparison, in normal
brain tissues, AQP4 was expressed only in perivascular cells. Prior
studies indicated that downregulated AQP4 could suppress cell
proliferation and motility (33).
Those results demonstrated that the expression of AQP4 was
significantly correlated with the glioma WHO grade, and had a key
role in the cancer progression.
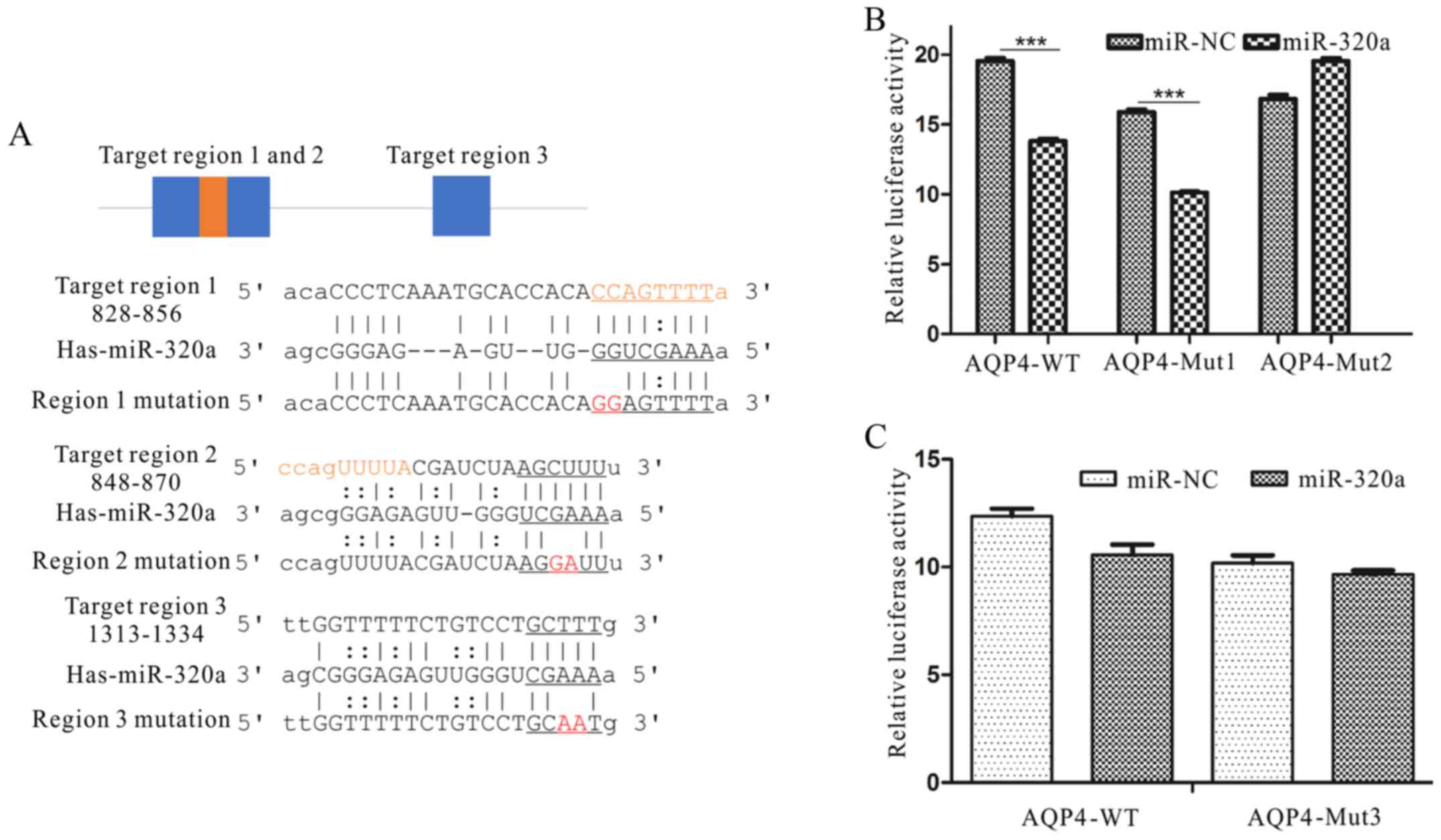
AQP4 is a direct target of
miRNA-320a
To demonstrate the mechanism of miRNA-320a in
glioma, we searched for miRNA-320a targets using the algorithms
TargetScan, miRanda and miRbase. AQP4 was selected from several
putative miRNA-320a targets since it plays key roles in brain
diseases, cell adhesion and motility (16,19,24,33).
Luciferase assays were conducted to examine whether AQP4 was a
direct target of miRNA-320a. As shown in Fig. 3, three 3′-UTR regions of AQP4 mRNA
were complementary to miRNA-320a. The results indicated that
miRNA-320a could directly target a region within the 2,000 bp of
the AQP4 3′-UTR (Fig. 3A).
Therefore, using the luciferase reporter system, we ascertained
that the overexpression of miRNA-320a in U87 and U251 cells
suppressed the activity of AQP4 (P<0.01; Fig. 3B and C).
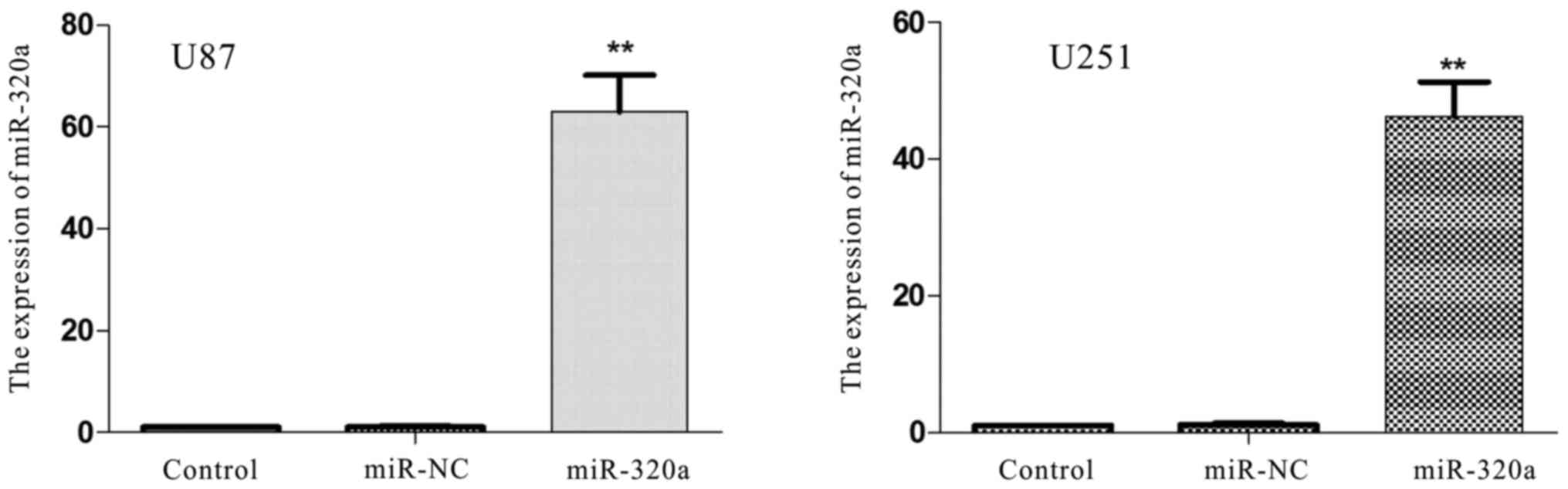
Upregulation miRNA-320a inhibits cell
invasion and migration
To further explore the biological functions of
miRNA-320a in glioma cell lines, U87 and U251 cells were
transfected with miRNA-320a or miRNA-NC mimics. RT-qPCR revealed
that miRNA-320a expression was significantly increased in the
transfected U87 and U251 cells, compared with that in the control
and miR-NC groups (P<0.01; Fig.
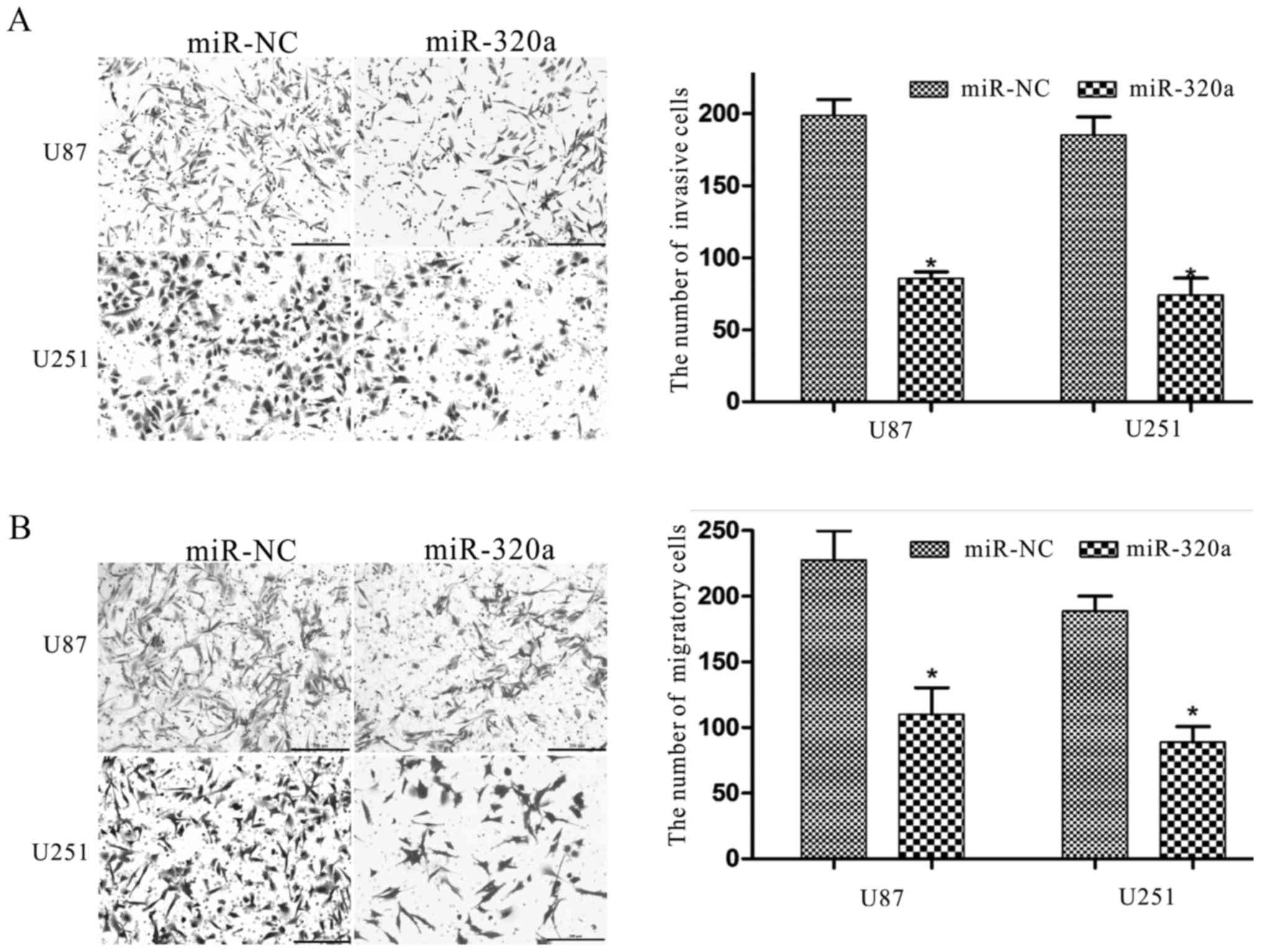
4). A Transwell assay was conducted to determine the migratory
and invasive behavior of U87 and U251 cells, and was used to detect
the function of miRNA-320a in the progression of glioma. We found
that miRNA-320a overexpression significantly decreased the
migratory and invasive capacities of U87 and U251 cells (Fig. 5A and B). In addition, miRNA-320a has
been demonstrated to suppress the proliferation of K-562 chronic
myelogenous leukemia, LN-229 glioblastoma and U2OS osteosarcoma
cells (29,31,34).
The results of these prior studies have each demonstrated that
miRNA-320a could function as a tumor suppressor, and regulate
progression and motility in glioma cells, as well as in other types
of cancer.
Upregulation of miRNA-320a suppresses
the expression of AQP4
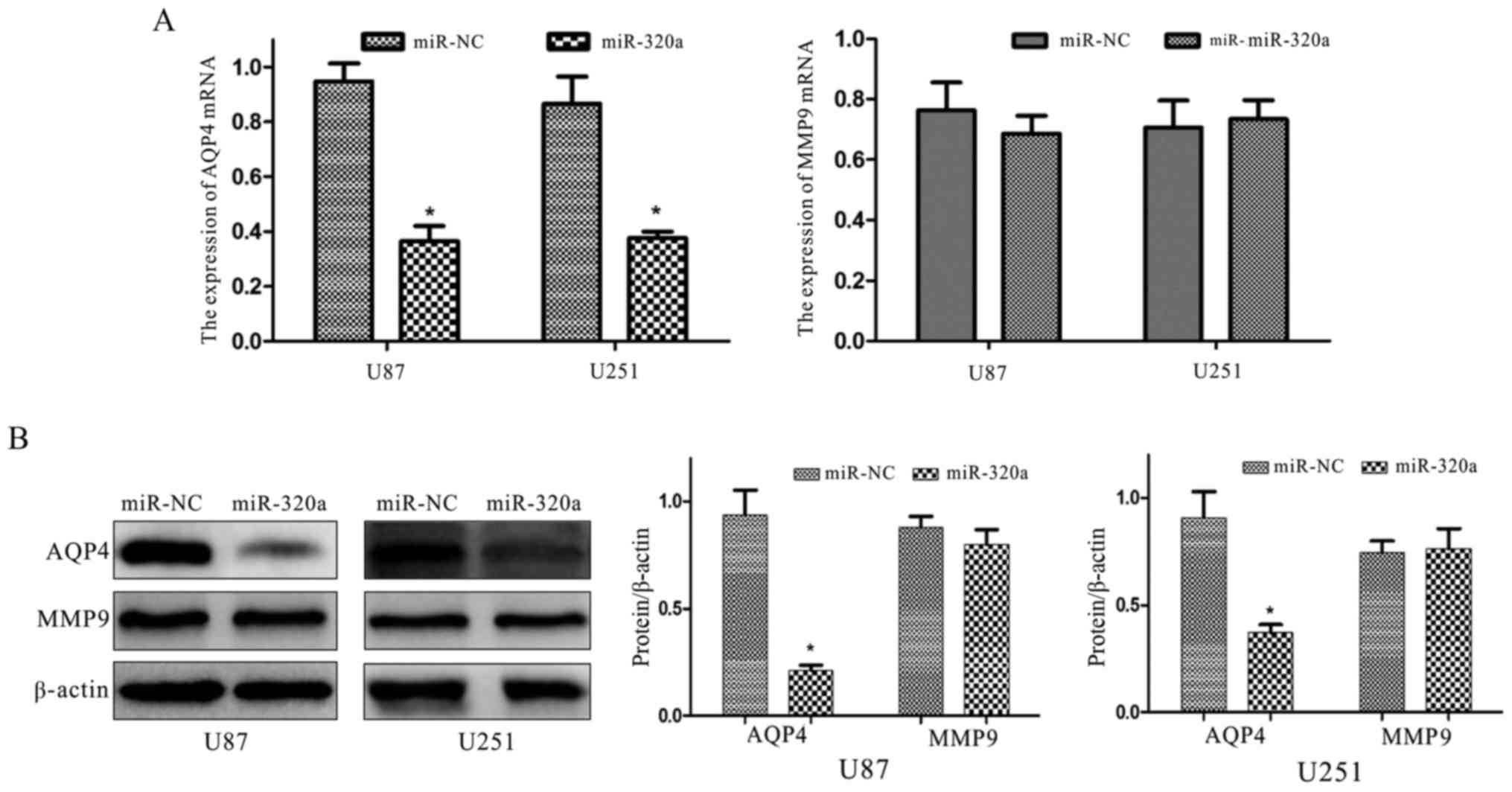
Luciferase assays were conducted using U87 and U251
cells in order to identify changes in miRNA-320a expression, and
whether these changes could regulate the expression of the target
protein AQP4, and MMP9. The results revealed that the mRNA and
protein levels of AQP4 were significantly decreased however, MMP9
expression was not markedly altered (Fig. 6). Furthermore, the
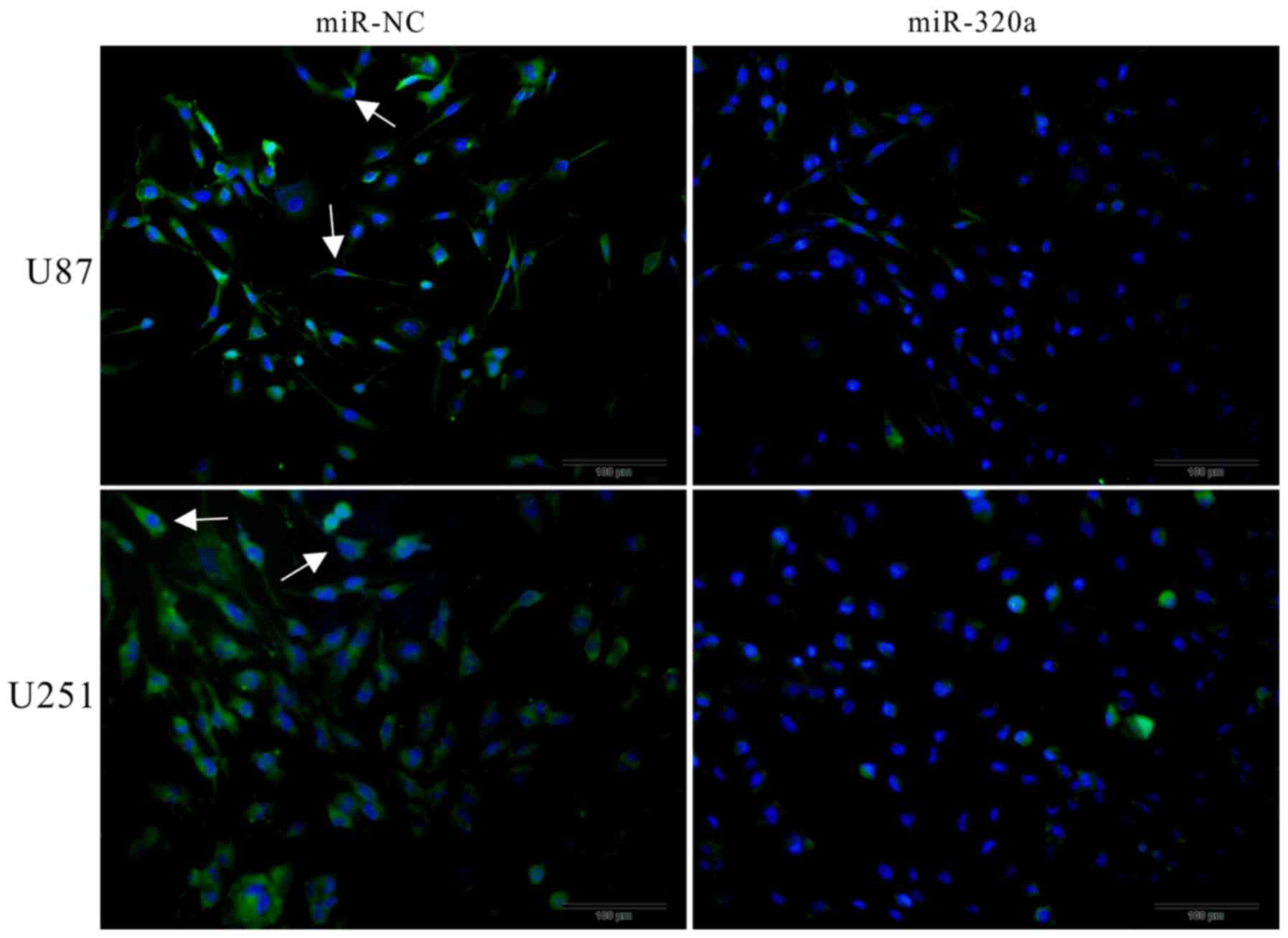
immunofluorescence assay revealed that AQP4 was mainly expressed on
the cell membrane, that it had a polarized distribution, and that
it was inhibited by the overexpression of miRNA-320a (Fig. 7). These results indicated that
miRNA-320a suppressed invasion and migration through direct
interference with AQP4, and not with MMP9, in the U87 and U251 cell
lines.
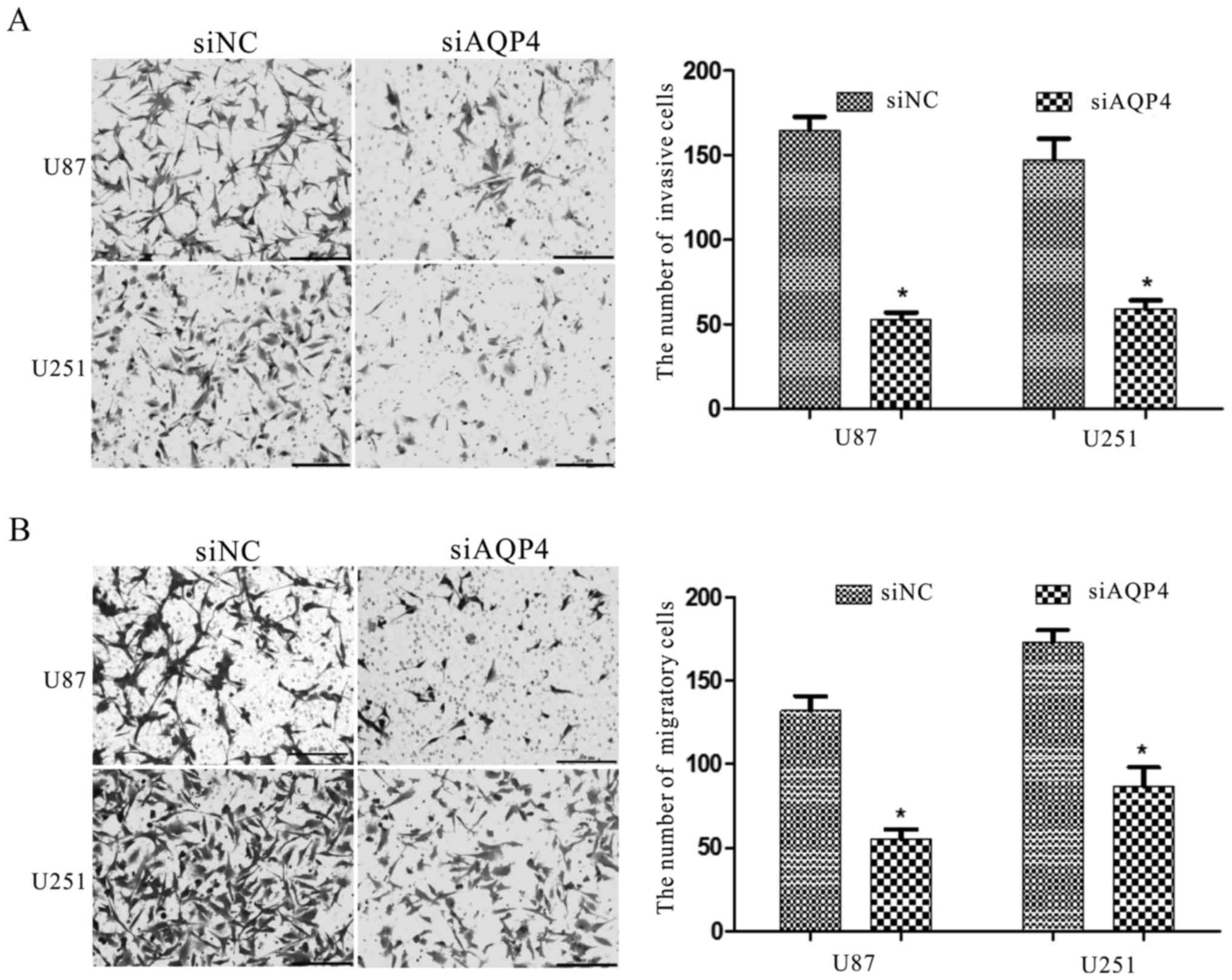
Interference with AQP4 expression
inhibits cell invasion and migration
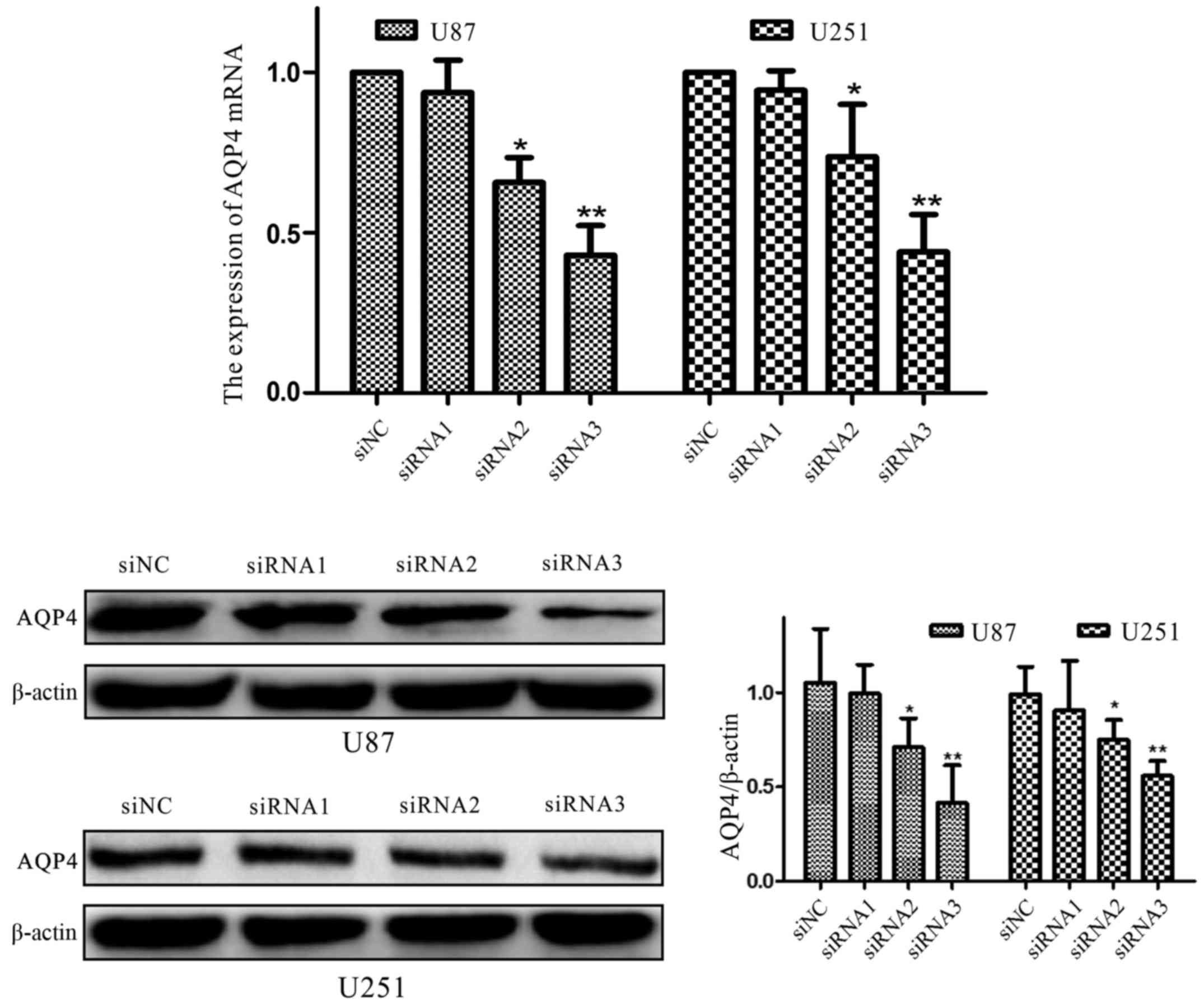
To further demonstrate the functions of AQP4 in U87
and U251 cells, we used RNA interference to downregulate the AQP4
protein (P<0.01; Fig. 8).
Immunofluorescence also was used to co-verify (Fig. 9) that the expression of AQP4 protein
was significantly downregulated. The Transwell assay revealed that
siAQP4 could markedly inhibit the invasion and migration of U87 and
U251 cells (Fig. 9). These results
demonstrated that AQP4 has a key function in glioma cell
motility.
Discussion
An increasing number of studies have been focusing
on the roles of various miRNAs in cancer. Many studies have
revealed that miRNAs can serve key roles in regulating the
expression of tumor suppressor genes or oncogenes, and that the
aberrant expression of miRNAs could promote or inhibit oncogenesis
(4,10,12).
Certain studies have stated that miRNAs regulate the self-renewal,
differentiation and division of cells via post-transcriptional gene
silencing (10). Aberrant miRNA
levels, particularly an overall downregulation are present in
various cancer types compared with their normal tissue
counterparts. Thus, miRNAs are potential biomarkers for monitoring
the progress and prognosis of patients with cancer.
Our results showed that miRNA-320a was significantly
decreased in the glioma tissue samples analyzed with the CGGA
microarray data. Combining the data with those of prior studies
confirms that miRNA-320a is markedly lower in glioma tissues than
in normal brain tissues, and lower in glioma cell lines than in
normal human astrocytes (31).
Thus, we hypothesized that the expression of miRNA-320a was
aberrant in gliomas. As aforementioned, miRNA-320a has many
targets. It can suppress the gene expression of IGF-1R, β-catenin,
BCL/ABL and ARPP-19, among others (13,29,31,35),
acting as a tumor suppressor in these cancer types. Thus,
miRNA-320a is significantly downregulated in gliomas and in other
malignant tumor types, and miRNA-320a overexpression could suppress
tumor development in vivo and in vitro, potentially
improving the prognosis.
The AQP family has thirteen members, of which AQP4
is the most studied; it has been described in the brain cells of
both rodents and primates (19).
The results of this particular study indicated that AQP4 is
primarily permeable to water. In brain tissues, a high
concentration of AQP4 has been documented at the astrocyte end-feet
where they are in contact with blood vessels (19). However, AQP4 distribution differs
significantly between various brain structures, including between
astrocytes, the hippocampus, the cerebellum and the corpus callosum
(36–40). The different AQP4 distribution
patterns suggest that AQP4 has multiple functions in the brain.
AQP4 may also be involved in cell adhesion (24). It has been reported in human breast
cancer that the downregulation of AQP4 could inhibit cell
proliferation, migration and invasion (33). In glioblastoma, AQP4 knockdown was
revealed to induce cell apoptosis (23). In the present study, we determined
that downregulation of AQP4 inhibited the invasion and migration of
U87 and U251 glioma cells (Fig. 9).
In autoimmune and neurodegenerative diseases, AQP4 has been
associated with neuroinflammation (41,42).
Integration of this information revealed that AQP4 not only has a
key role in cerebral fluid transportation, but also serves
significant functions in pathological processes of the central
nervous system. However, the potential functions of AQP4 in the
central nervous system still need to be fully elucidated.
Matrix metalloproteinases (MMPs), which are secreted
by cells, are able to degrade all components of the ECM, a process
conducive to cell invasion and migration (43,44).
In particular, MMP9 has been demonstrated to be associated with
numerous aggressive tumor types in humans, including glioma
(45–47). In the present study MMP9 expression
was not altered with the overexpression of miRNA-320a, thus
confirming that miRNA-320a inhibits glioma cell invasion and
migration by a mechanism other than targeting MMP9. However, we
hypothesized that AQP4 and MMP9 may act synergistically in glioma
cell invasion and migration to inhibit these processes.
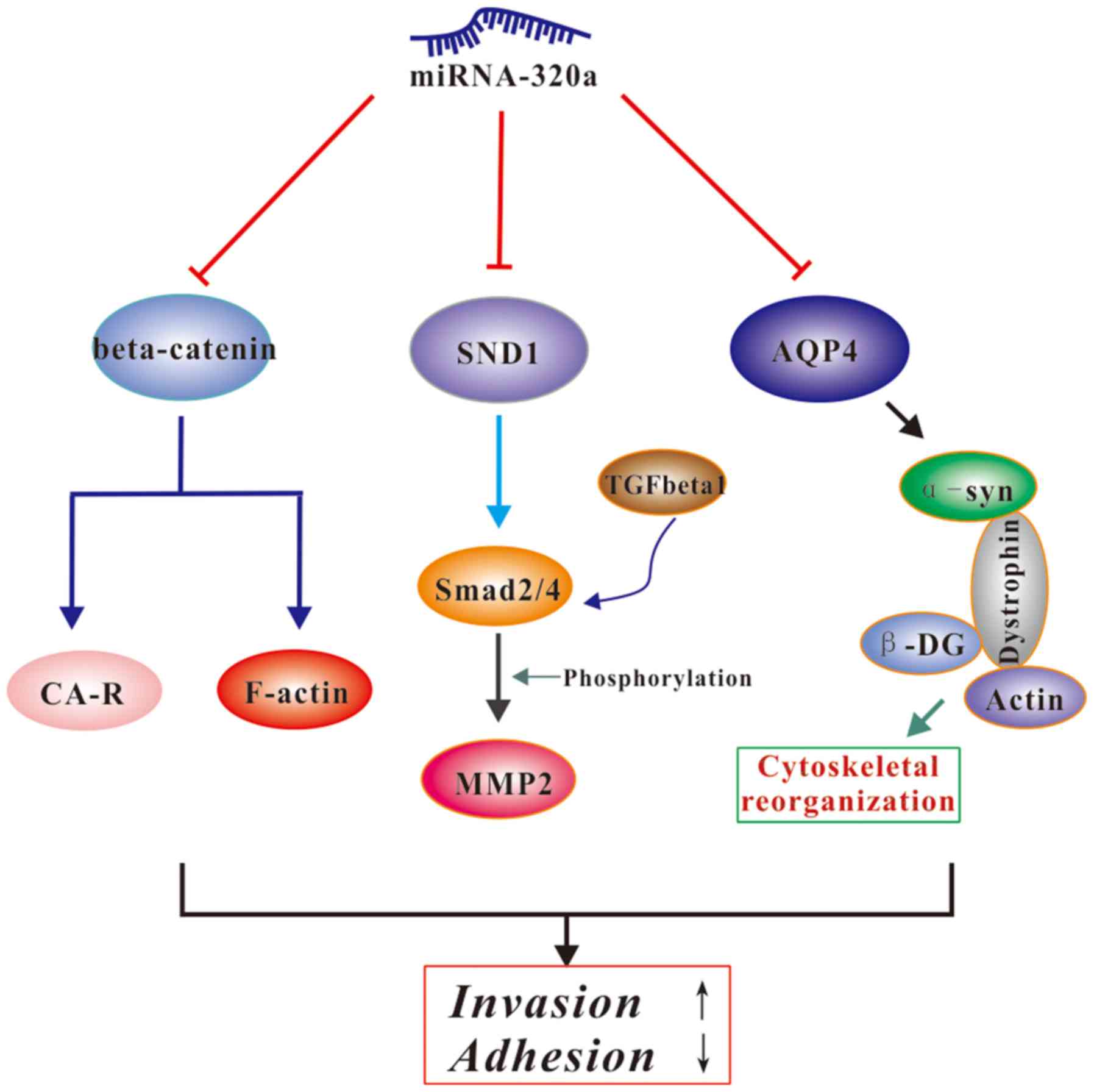
A recent study found that miRNA-320a can directly
target SND1 and β-catenin to inhibit glioma cell invasion and
proliferation (32). Another study
indicated that β-catenin could bind to cadherin adhesion receptors
and F-actin in order to bridge the extracellular adhesive activity
of cadherins with the actin cytoskeleton of tumor cells (48). Lan et al revealed that AQP4
was involved in cytoskeletal organization. Briefly, AQP4 interacts
with α-syntrophin; this is comprised of dystrophin and utrophin,
linker molecules between the actin cytoskeleton and β-dystroglycan
(22). SND1, also known as P100
co-activator or Tudor domain-containing protein 11, promotes tumor
angiogenesis, cell invasion and cell migration via the NF-κB and
TGF-β signaling pathways (49,50).
Li et al reported that SND1 promotes Smad2/4 expression, a
pivotal downstream signaling protein of SND1 in the TGF-β1 pathway,
and then enhances MMP2 expression (32). Collectively, the results indicated
that AQP4, β-catenin and SND1 may all be regulated by miRNA-320a,
that β-catenin and AQP4 could interact with adhesion-associated
proteins and cytoskeletal proteins, and regulate the invasive
ability of cells, and that SND1 promotes cell aggressiveness by
degrading the ECM (Fig. 10).
In the present study, we identified AQP4 to be a
direct and functional target of miRNA-320a. Overexpression of
miRNA-320a in glioma cells suppressed cell invasion and migration.
In addition, evaluation of the microarray data and survival
analysis from the CGGA samples revealed that miRNA-320a was
significantly downregulated in glioma tissues, which was correlated
with prognosis. The present study demonstrated that miRNA-320a
functions as a tumor suppressor in glioma, and that AQP4 serves an
important function in cell invasion and migration. Although
miRNA-based therapeutics are still under development, our results
are encouraging, and suggest that miRNA-320a and AQP4 could serve
as clinical targets for the treatment of glioma or other tumor
types in the future.
Acknowledgements
The present study was supported by the National
Science Foundation of China (grant no. 31271368). We thank Wei
Qiang of The University of Chicago Molecular Oncology Laboratory
for support with the theory.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vredenburgh JJ, Desjardins A, Reardon DA
and Friedman HS: Experience with irinotecan for the treatment of
malignant glioma. Neuro Oncol. 11:80–91. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Katakowski M, Charteris N, Chopp M and
Khain E: Density-dependent regulation of glioma cell proliferation
and invasion mediated by miR-9. Cancer Microenviron. 9:149–159.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim Y, Jeon H and Othmer H: The role of
the tumor microenvironment in glioblastoma: A mathematical model.
IEEE Trans Biomed Eng. 64:519–527. 2017.PubMed/NCBI
|
|
4
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang T, Wang YR, Zeng F, Cao HY, Zhou HD
and Wang YJ: LncRNA H19 is overexpressed in glioma tissue, is
negatively associated with patient survival, and promotes tumor
growth through its derivative miR-675. Eur Rev Med Pharmacol Sci.
20:4891–4897. 2016.PubMed/NCBI
|
|
6
|
Oberheim Bush NA and Chang S: Treatment
strategies for low-grade glioma in adults. J Oncol Pract.
12:1235–1241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Weathers SS and Gilbert MR: Toward
personalized targeted therapeutics: An overview. Neurotherapeutics.
14:256–264. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miska EA: How microRNAs control cell
division, differentiation and death. Curr Opin Genet Dev.
15:563–568. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zimmerman AL and Wu S: MicroRNAs, cancer
and cancer stem cells. Cancer Lett. 300:10–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Reid G: MicroRNAs in mesothelioma: From
tumour suppressors and biomarkers to therapeutic targets. J Thorac
Dis. 7:1031–1040. 2015.PubMed/NCBI
|
|
12
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun JY, Huang Y, Li JP, Zhang X, Wang L,
Meng YL, Yan B, Bian YQ, Zhao J, Wang WZ, et al: MicroRNA-320a
suppresses human colon cancer cell proliferation by directly
targeting β-catenin. Biochem Biophys Res Commun. 420:787–792. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mittal SP, Mathai J, Kulkarni AP, Pal JK
and Chattopadhyay S: miR-320a regulates erythroid differentiation
through MAR binding protein SMAR1. Int J Biochem Cell Biol.
45:2519–2529. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shang C, Zhang H, Guo Y, Hong Y, Liu Y and
Xue Y: miR-320a down-regulation mediates bladder carcinoma invasion
by targeting ITGB3. Mol Biol Rep. 41:2521–2527. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nagelhus EA and Ottersen OP: Physiological
roles of aquaporin-4 in brain. Physiol Rev. 93:1543–1562. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camassa LM, Lunde LK, Hoddevik EH,
Stensland M, Boldt HB, De Souza GA, Ottersen OP and Amiry-Moghaddam
M: Mechanisms underlying AQP4 accumulation in astrocyte endfeet.
Glia. 63:2073–2091. 2015. View Article : Google Scholar
|
|
18
|
Hubbard JA, Hsu MS, Seldin MM and Binder
DK: Expression of the astrocyte water channel aquaporin-4 in the
mouse brain. ASN Neuro. 7:17590914156054862015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Badaut J, Fukuda AM, Jullienne A and Petry
KG: Aquaporin and brain diseases. Biochim Biophys Acta.
1840:1554–1565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lan YL, Zhao J, Ma T and Li S: The
potential roles of aquaporin 4 in Alzheimer's disease. Mol
Neurobiol. 53:5300–5309. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang WC, Zhou LJ, Zhang R, Yue ZY, Dong H,
Song CY, Qian H, Lu SJ and Chang FF: Effects of propofol and
sevoflurane on aquaporin-4 and aquaporin-9 expression in patients
performed gliomas resection. Brain Res. 1622:1–6. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lan YL, Wang X, Lou JC, Ma XC and Zhang B:
The potential roles of aquaporin 4 in malignant gliomas.
Oncotarget. 8:32345–32355. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ding T, Zhou Y, Sun K, Jiang W, Li W, Liu
X, Tian C, Li Z, Ying G, Fu L, et al: Knockdown a water channel
protein, aquaporin-4, induced glioblastoma cell apoptosis. PLoS
One. 8:e667512013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hiroaki Y, Tani K, Kamegawa A, Gyobu N,
Nishikawa K, Suzuki H, Walz T, Sasaki S, Mitsuoka K, Kimura K, et
al: Implications of the aquaporin-4 structure on array formation
and cell adhesion. J Mol Biol. 355:628–639. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang L, Wang X, Zhen S, Zhang S, Kang D
and Lin Z: Aquaporin-4 upregulated expression in glioma tissue is a
reaction to glioma-associated edema induced by vascular endothelial
growth factor. Oncol Rep. 28:1633–1638. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao WJ, Zhang W, Li GL, Cui Y, Shi ZF and
Yuan F: Differential expression of MMP-9 and AQP4 in human glioma
samples. Folia Neuropathol. 50:176–186. 2012.PubMed/NCBI
|
|
27
|
Peng J, Omran A, Ashhab MU, Kong H, Gan N,
He F and Yin F: Expression patterns of miR-124, miR-134, miR-132,
and miR-21 in an immature rat model and children with mesial
temporal lobe epilepsy. J Mol Neurosci. 50:291–297. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu ZH, Li J, Xia J, Jiang R, Zuo GW, Li
XP, Chen Y, Xiong W and Chen DL: Ginsenoside 20(s)-Rh2 as potent
natural histone deacetylase inhibitors suppressing the growth of
human leukemia cells. Chem Biol Interact. 242:227–234. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xishan Z, Ziying L, Jing D and Gang L:
MicroRNA-320a acts as a tumor suppressor by targeting BCR/ABL
oncogene in chronic myeloid leukemia. Sci Rep. 5:124602015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang G, Jiang G, Wang C, Zhong K, Zhang
J, Xue Q, Li X, Jin H and Li B: Decreased expression of
microRNA-320a promotes proliferation and invasion of non-small cell
lung cancer cells by increasing VDAC1 expression. Oncotarget.
7:49470–49480. 2016.PubMed/NCBI
|
|
31
|
Guo T, Feng Y, Liu Q, Yang X, Jiang T,
Chen Y and Zhang Q: MicroRNA-320a suppresses in GBM patients and
modulates glioma cell functions by targeting IGF-1R. Tumour Biol.
35:11269–11275. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li H, Yu L, Liu J, Bian X, Shi C, Sun C,
Zhou X, Wen Y, Hua D, Zhao S, et al: miR-320a functions as a
suppressor for gliomas by targeting SND1 and β-catenin, and
predicts the prognosis of patients. Oncotarget. 8:19723–19737.
2017.PubMed/NCBI
|
|
33
|
Li YB, Sun SR and Han XH: Down-regulation
of AQP4 inhibits proliferation, migration and invasion of human
breast cancer cells. Folia Biol (Praha). 62:131–137.
2016.PubMed/NCBI
|
|
34
|
Cheng C, Chen ZQ and Shi XT: MicroRNA-320
inhibits osteosarcoma cells proliferation by directly targeting
fatty acid synthase. Tumour Biol. 35:4177–4183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lü M, Ding K, Zhang G, Yin M, Yao G, Tian
H, Lian J, Liu L, Liang M, Zhu T, et al: MicroRNA-320a sensitizes
tamoxifen-resistant breast cancer cells to tamoxifen by targeting
ARPP-19 and ERRγ. Sci Rep. 5:87352015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Badaut J, Nehlig A, Verbavatz J, Stoeckel
M, Freund-Mercier MJ and Lasbennes F: Hypervascularization in the
magnocellular nuclei of the rat hypothalamus: Relationship with the
distribution of aquaporin-4 and markers of energy metabolism. J
Neuroendocrinol. 12:960–969. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Badaut J, Verbavatz JM, Freund-Mercier MJ
and Lasbennes F: Presence of aquaporin-4 and muscarinic receptors
in astrocytes and ependymal cells in rat brain: A clue to a common
function? Neurosci Lett. 292:75–78. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hsu MS, Seldin M, Lee DJ, Seifert G,
Steinhäuser C and Binder DK: Laminar-specific and developmental
expression of aquaporin-4 in the mouse hippocampus. Neuroscience.
178:21–32. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nielsen S, Nagelhus EA, Amiry-Moghaddam M,
Bourque C, Agre P and Ottersen OP: Specialized membrane domains for
water transport in glial cells: High-resolution immunogold
cytochemistry of aquaporin-4 in rat brain. J Neurosci. 17:171–180.
1997.PubMed/NCBI
|
|
40
|
Wen H, Nagelhus EA, Amiry-Moghaddam M,
Agre P, Ottersen OP and Nielsen S: Ontogeny of water transport in
rat brain: Postnatal expression of the aquaporin-4 water channel.
Eur J Neurosci. 11:935–945. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Juryńczyk M, Tackley G, Kong Y, Geraldes
R, Matthews L, Woodhall M, Waters P, Kuker W, Craner M, Weir A, et
al: Brain lesion distribution criteria distinguish MS from
AQP4-antibody NMOSD and MOG-antibody disease. J Neurol Neurosurg
Psychiatry. 88:132–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Vaknin-Dembinsky A, Brill L, Kassis I,
Petrou P, Ovadia H, Ben-Hur T, Abramsky O and Karussis D: T-cell
responses to distinct AQP4 peptides in patients with neuromyelitis
optica (NMO). Mult Scler Relat Disord. 6:28–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Inhibition of invasion and MMPs by a nutrient
mixture in human cancer cell lines: A correlation study. Exp Oncol.
32:243–248. 2010.PubMed/NCBI
|
|
44
|
Singh RD, Haridas N, Patel JB, Shah FD,
Shukla SN, Shah PM and Patel PS: Matrix metalloproteinases and
their inhibitors: Correlation with invasion and metastasis in oral
cancer. Indian J Clin Biochem. 25:250–259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Das G, Shiras A, Shanmuganandam K and
Shastry P: Rictor regulates MMP-9 activity and invasion through
Raf-1-MEK-ERK signaling pathway in glioma cells. Mol Carcinog.
50:412–423. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ruiz-Morales JM, Dorantes-Heredia R,
Arrieta O, Chávez-Tapia NC and Motola-Kuba D: Neutrophil
gelatinase-associated lipocalin (NGAL) and matrix
metalloproteinase-9 (MMP-9) prognostic value in lung
adenocarcinoma. Tumour Biol. 36:3601–3610. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Shi W, Xiao H, Xue F and Wu J: Dynamic
changes of matrix metalloproteinase 9 in heterotopic ossification
of rat model. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi.
28:1133–1138. 2014.(In Chinese). PubMed/NCBI
|
|
48
|
Rimm DL, Koslov ER, Kebriaei P, Cianci CD
and Morrow JS: Alpha 1(E)-catenin is an actin-binding and -bundling
protein mediating the attachment of F-actin to the membrane
adhesion complex. Proc Natl Acad Sci U S A. 92:pp. 8813–8817. 1995;
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Santhekadur PK, Akiel M, Emdad L, Gredler
R, Srivastava J, Rajasekaran D, Robertson CL, Mukhopadhyay ND,
Fisher PB and Sarkar D: Staphylococcal nuclease domain containing-1
(SND1) promotes migration and invasion via angiotensin II type 1
receptor (AT1R) and TGFβ signaling. FEBS Open Bio. 4:353–361. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Santhekadur PK, Das SK, Gredler R, Chen D,
Srivastava J, Robertson C, Baldwin AS Jr, Fisher PB and Sarkar D:
Multifunction protein staphylococcal nuclease domain containing 1
(SND1) promotes tumor angiogenesis in human hepatocellular
carcinoma through novel pathway that involves nuclear factor κB and
miR-221. J Biol Chem. 287:13952–13958. 2012. View Article : Google Scholar : PubMed/NCBI
|