Introduction
Lung cancer is a malignancy with high morbidity and
mortality; approximately 80–85% of all lung cancers are non-small
cell lung cancer (NSCLC) (1).
Significant progress has been observed in NSCLC treatment, yet the
5-year survival rate of NSCLC patients is <15% (2) and represents more than one-quarter
(27%) of all cancer deaths due to lung cancer in the US (3). Currently, low- and middle-income
countries account for >50% of lung cancer-related deaths
annually (4). Many NSCLC patients
are at an advanced stage at initial diagnosis due to its aggressive
and early metastatic potential, thereby leading to a poor long-term
prognosis. Thus, identification of effective strategies to predict
and control NSCLC is essential.
Raf-1 proto-oncogene, serine/threonine kinase (Raf1)
is an important part of the human RAS/RAF/MEK/ERK signaling pathway
and is an important signaling molecule. Raf1 is closely associated
with the regulation of cell proliferation and differentiation
(5). Abnormal activation is found
in many types of tumors and is mainly related to Raf1 expression.
An association between Raf1 and tumor invasion is found in prostate
(6), colorectal (7,8) and
thyroid cancer (9).
We found that the prognosis of NSCLC after
radiotherapy varies, even with the same stage and treatment. Raf1
plays a critical role in many types of tumors, but its expression
and clinical significance remain to be reported in NSCLC primary
tissues. Considering these findings, the present study aimed to
determine the clinical value and prognostic significance of Raf1 in
NSCLC patients following radiotherapy.
Materials and methods
Sample collection
In the present study, 110 samples of formalin-fixed
paraffin-embedded NSCLC tissue were obtained between December 2011
and April 2014 from the Affiliated Jiangsu Cancer Hospital at the
Nanjing Medical University in China. The tissue samples were
collected from diagnosed patients by puncture or bronchoscopy
biopsy. All cases were classified into the clinical types of NSCLC
according to the American Joint Committee on Cancer (AJCC, 2010).
All patients only received radiotherapy due to the financial
concerns regarding targeted therapy and rejected other
treatments.
Ethics statement
This study was approved by the Ethics Committee of
Jiangsu Cancer Hospital. Written informed consent was obtained from
all patients before treatment initiation.
Immunohistochemistry
Immunohistochemistry was performed using the
standard streptavidin-peroxidase technique to determine Raf1
expression. Paraffin-embedded tissues were cut into 4-µm sections,
dewaxed, and rehydrated using routine methods. All slides were
subjected to heat-induced antigen retrieval using Tris buffer (0.01
mmol/l, pH, 6.0) in a pressure cooker. Then, the slides were placed
in 3% hydrogen peroxide for 10 min to quench endogenous peroxidase.
The sections were further incubated with anti-Raf1 (ab32025,
GR176309-2; 1:1,000 dilution; Abcam, Hong Kong) overnight at 4°C
after thoroughly rinsing thrice with phosphate-buffered saline
(PBS) for 1 min each time. After washing in PBS, the sections were
incubated with biotinylated secondary antibodies (Dako Denmark A/S,
Glostrup, Denmark) for 30 min at room temperature and stained with
freshly prepared 3,3′-diaminobenzidine and light hematoxylin as
counterstain. Known positive controls were included in each
staining procedure. PBS was used to replace primary antibodies in
the negative control.
Immunohistochemical evaluation
Raf1 expression was quantified simultaneously by two
independent observers who were blinded to the patient data. If the
results of the two independent observers were different, then we
would request for an additional pathology expert. In each case,
four representative areas were selected, and ≥400 tumor cells were
observed at ×400 magnification. The percentage of positive cells
was evaluated according to the number of positive cells divided by
all cancer cells under a microscope at four selected foci. The
following scale was adopted: 0, no positive tumor cells; 1, 1–10%
positive tumor cells; 2, 11–50%; and 3, 51–100%. The staining
intensity was evaluated by the presence of yellow- or brown-colored
end product at the target antigen site. Furthermore, the staining
intensity (no staining, mild, moderate, and intense) was graded as
0, 1, 2, or 3 points, respectively (0, no detectable staining; 1,
mild staining-light yellow; 2, moderate-yellow; and 3,
intense-brown). Final scores were obtained by multiplying the
positive tumor grade by the staining intensity score (0, 1, 2, 3,
4, 6 and 9). The final scores ≤4 and ≥6 were considered tumors with
low and high expression levels, respectively.
Cell culture
Human pulmonary carcinoma cell lines H1299 and A549
and transfected cell lines A549-7/Pb and A549-7/Raf1 were obtained
from the Research Center of Clinical Oncology of the Affiliated
Jiangsu Cancer Hospital, Nanjing Medical University, Nanjing, China
and maintained in Dulbecco's modified Eagle's medium (DMEM)
(Corning, Manassas, VA, USA) containing 10% fetal bovine serum
(FBS) (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA
USA). Cultures were grown at 37°C in an atmosphere with 5%
CO2. Cell lines A549-7/Pb and A549-7/Raf1 were
established with transfected Pb-puro empty vector and Pb-puro-Raf1
plasmid using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), respectively.
Cell transfection
Raf1-siRNA and NC-siRNA were purchased from RiboBio
(Guangzhou, China). The Lipofectamine 2000 transfection reagent was
used to transfect the H1299 cell lines according to the
manufacturer's instructions.
Western blot assay
Cells transfected after 48 h were extracted and
prepared in RIPA buffer (Beyotime, Shanghai, China). BCA protein
assay kit (Beyotime) was used to detect the protein concentration.
The primary antibodies were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA USA). The anti-Raf1 was obtained
from Abcam. β-actin was used as the loading control. Immunoreactive
bands were measured with ECL detection reagent (Millipore,
Billerica, MA, USA).
RNA isolation using quantitative RT-PC
reaction (qRT-PCR)
Fold changes for Raf1/β-actin expression levels were
calculated using the 2−ΔΔCt method. The total RNA from
cells was extracted with TRIzol (Invitrogen; Thermo Fisher
Scientific, Inc.). The PrimeScript First Strand cDNA Synthesis kit
(Takara, Dalian, China) was used to synthesize first-strand cDNA.
The SYBR-Green qRT-PCR was performed on an ABI7300 real-time PCR
system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
primer pairs were 5′-GGGAGCTTGGAAGACGATCAG-3′ and
5′-ACACGGATAGTGTTGCTTGTC-3′ for Raf1 and
5′-TTCTACAATGAGCTGCGTCTG-3′ and 5′-CAGCCTGGATAGCAACGTATC-3′ for
β-actin. Each experiment was performed in triplicate.
Wound healing assay
Approximately 1.5×105 cells were plated
in 6-well dishes and subsequently transfected with siRNA. An
incision was created at the center of the cell monolayer as an
artificial wound. Images of the wound area were captured using an
optical microscope (Olympus Corp, Tokyo, Japan) with a
magnification of ×100 36 h after injury.
Invasion assay
Cell invasion was measured using 24-well BD Matrigel
Invasion Chambers (BD Biosciences, Franklin Lakes, NJ, USA)
according to the manufacturer's instructions. The A549 and H1299
cells were seeded into the upper well of the invasion chamber
resuspended (1.5×104 cells per well) in 200 µl
serum-free medium after transfection. The lower chamber well
containing 500 µl DMEM and 20% FBS was used to stimulate invasion.
After 36 h of incubation, the invading cells were fixed with 4%
paraformaldehyde and subsequently stained with crystal violet,
whereas non-invading cells were removed. Cells were counted on
optical microscope in ×40 magnification fields.
Statistical analysis
All statistical analyses were conducted using SPSS
17.0 software (SPSS, Inc., Chicago, IL, USA). Overall survival (OS)
was defined as the interval between the date of definite diagnosis
and the date of death or last follow-up. Progression-free survival
was defined as the time between the date of first recurrence and
the last follow-up. The relationships between Raf1 and
clinicopathological parameters were analyzed using the Chi-square
test. For survival data, Kaplan-Meier curves were generated, and
analysis was performed using the log-rank test. A prognostic
analysis was performed using the univariate and multivariate Cox
regression models. P<0.05 was considered statistically
significant.
Results
Protein expression of Raf1 in
NSCLC
Immunohistochemical staining of Raf1 was performed
in 110 samples of primary NSCLC. Raf1 immunoreactivity was
predominantly detected in the membrane, although weak cytoplasmic
staining was observed. A total of 21 (19.1%) and 28 (25.5%) of the
110 samples exhibited high and low Raf1 expression levels,
correspondingly. In addition, 61 (54.6%) of the 110 specimens were
assumed to have no staining (intensity score 0) (Table I and Fig. 1).
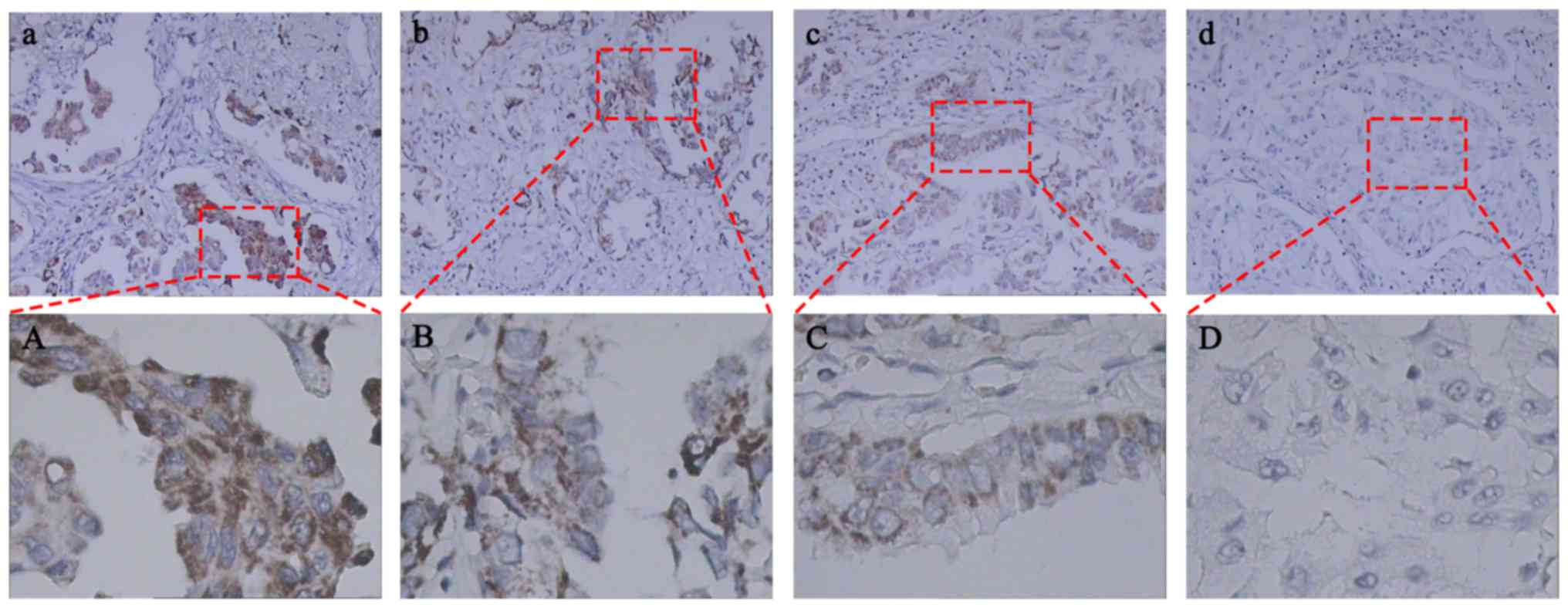 | Figure 1.Immunohistochemical expression of Raf1
in human primary non-small cell lung cancer tissues. Raf1 protein
was mainly expressed in the membrane with high (a), moderate (b),
mild (c), and negative (d) expression levels with brownish-yellow
staining. Images (a, b, c, and d) show high-power fields of the
boxed areas in (A, B, C and D), respectively. Significantly
increased Raf1 expression was detected in lung adenocarcinoma
cells. (a-d), magnification ×100; (A-D), magnification ×400. |
 | Table I.Clinicopathological characteristics of
110 non-small cell lung cancer patients. |
Table I.
Clinicopathological characteristics of
110 non-small cell lung cancer patients.
|
|
| Raf1 |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameter | No. of cases | Negative | Low | High | P-value |
|---|
| Sex |
|
|
|
| 0.995 |
| Male | 67 | 37 | 17 | 13 |
|
|
Female | 43 | 24 | 11 | 8 |
|
| Age (years) |
|
|
|
| 0.740 |
| ≤70 | 31 | 19 | 7 | 5 |
|
|
>70 | 79 | 42 | 21 | 16 |
|
| Smoking status |
|
|
|
| 0.955 |
|
Non-smoker | 69 | 39 | 17 | 13 |
|
|
Smoker | 41 | 22 | 11 | 8 |
|
| Lesion |
|
|
|
| 0.837 |
|
Peripheral | 78 | 42 | 21 | 15 |
|
|
Central | 32 | 19 | 7 | 6 |
|
| Histology |
|
|
|
| 0.437 |
|
SQCC | 78 | 43 | 16 | 13 |
|
|
ADC | 32 | 18 | 12 | 8 |
|
|
Differentiation |
|
|
|
| 0.029 |
|
Moderate | 71 | 46 | 14 | 11 |
|
|
Poor | 39 | 15 | 14 | 10 |
|
| N stage |
|
|
|
| 0.014 |
|
cN0 | 68 | 45 | 14 | 9 |
|
|
cN1/N2 | 42 | 16 | 14 | 12 |
|
| T stage |
|
|
|
| 0.038 |
| T1 | 22 | 16 | 4 | 2 |
|
| T2 | 53 | 33 | 11 | 9 |
|
|
T3-4 | 35 | 12 | 13 | 10 |
|
| Radiotherapy |
|
|
|
| 0.074 |
| CR | 45 | 31 | 9 | 4 |
|
| PR | 40 | 20 | 10 | 10 |
|
| SD | 25 | 10 | 9 | 7 |
|
Correlations between Raf1 expression
and clinicopathological parameters
The clinicopathological characteristics of the
patients are summarized in Table I.
The immunohistochemical analyses of NSCLC tissues showed that Raf1
protein expression was significantly correlated with lymph node
metastasis and T stage and was associated with poor histological
differentiation (P=0.014, 0.038 and 0.029, respectively). However,
no significant correlations were observed between Raf1 expression
and age, gender, smoking status, tumor location, and lesion
(P>0.05).
Univariate and multivariate analyses
of prognostic factors
We analyzed the effect of Raf1 expression on
clinical outcomes in these patients. According to the univariate
analysis (Table II), histological
differentiation (P<0.05 and P<0.05), T stage (P<0.05 and
P<0.05), lymph node metastasis (P<0.05 and P<0.05), and
Raf1 expression (P<0.05 and P<0.05) were significantly
associated with time to progression (TTP) and OS. The prognoses
obtained were better in patients with Raf1-negative expression than
in patients with positive expression. Abnormal Raf1 expression was
associated with disease progression in patients with NSCLC. In the
multivariate analysis (Table
III), Raf1 expression was an independent risk factor for TTP
(HR, 1.94, 95% CI 1.16–3.25, P=0.01) and OS (HR, 1.64, 95% CI
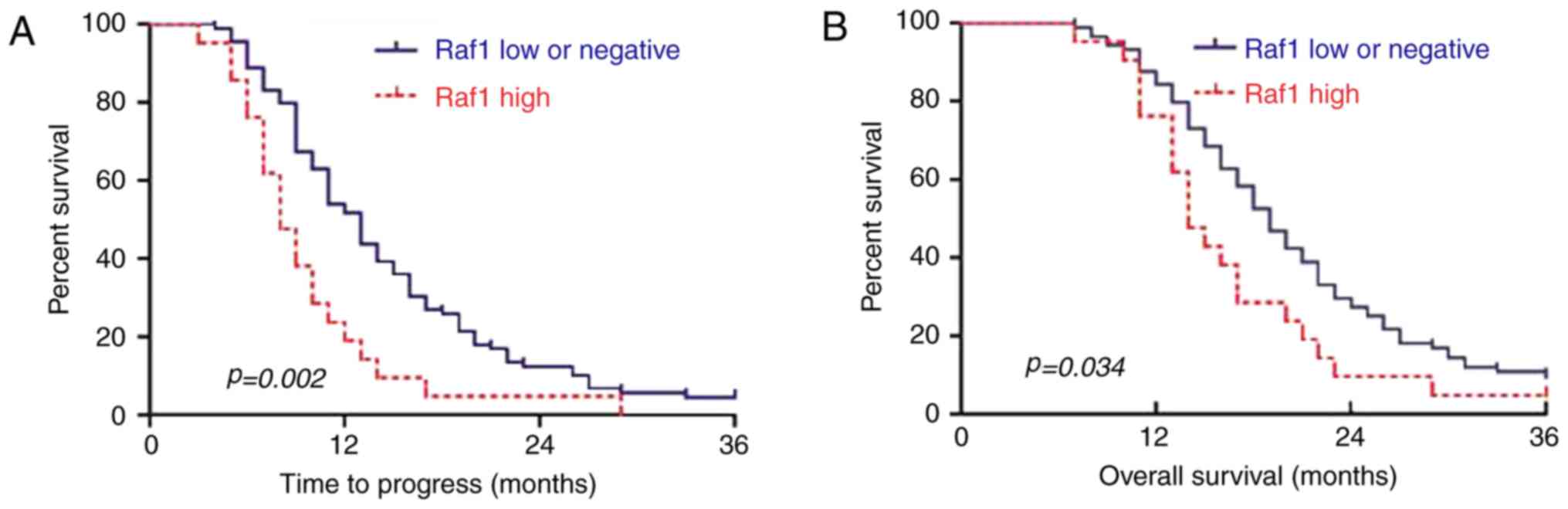
0.98–2.75, P=0.06). Survival curves showed that the TTP was earlier
in the Raf1 low and negative group than in the high group
(P=0.002). Moreover, the cumulative 3-year survival rate was higher
in the Raf1 low and negative group than in the high group (P=0.034)
(Fig. 2). These data showed that
Raf1 overexpression may lead to early TTP in patients with NSCLC
after radiotherapy.
 | Table II.Univariate prognostic factor analyses
for various clinical endpoints. |
Table II.
Univariate prognostic factor analyses
for various clinical endpoints.
|
|
| TTP | OS |
|---|
|
|
|
|
|
|---|
| Factor | N | (Median, M) | P-value | % (3-year) | P-value |
|---|
| Sex |
|
| 0.734 |
| 0.689 |
|
Male | 67 | 11 |
| 9.8 |
|
|
Female | 43 | 12 |
| 27.9 |
|
| Age (years) |
|
| 0.642 |
| 0.536 |
|
≤70 | 31 | 14 |
| 10.1 |
|
|
>70 | 79 | 11 |
| 15.0 |
|
| Smoking status |
|
| 0.600 |
| 0.537 |
|
Non-smoker | 69 | 12 |
| 15.0 |
|
|
Smoker | 41 | 10 |
| 10.9 |
|
| Lesion |
|
| 0.194 |
| 0.655 |
|
Peripheral | 78 | 11 |
| 14.1 |
|
|
Central | 32 | 13 |
| 12.5 |
|
| Histology |
|
| 0.651 |
| 0.680 |
|
SQCC | 72 | 10 |
| 15.2 |
|
|
ADC | 38 | 11 |
| 13.7 |
|
| Differentation |
|
| 0.000 |
| 0.000 |
|
Mederate | 71 | 14 |
| 52.1 |
|
|
Poor | 39 | 7 |
| 32.4 |
|
| N stage |
|
| 0.000 |
| 0.000 |
|
cN0 | 68 | 14 |
| 47.9 |
|
|
cN1/N2 | 42 | 9 |
| 22.7 |
|
| T stage |
|
| 0.000 |
| 0.000 |
| T1 | 22 | 27 |
| 44.4 |
|
| T2 | 53 | 13 |
| 35.1 |
|
|
T3-4 | 35 | 8 |
| 27.0 |
|
| Radiotherapy |
|
| 0.000 |
| 0.000 |
| CR | 45 | 16 |
| 22.0 |
|
| PR | 40 | 11 |
| 10.0 |
|
| SD | 25 | 7 |
| 0.3 |
|
| Raf1 |
|
| 0.002 |
| 0.009 |
|
Negative or low | 89 | 13 |
| 10.9 |
|
|
High | 21 | 8 |
| 0.1 |
|
 | Table III.Multivariable prognostic factor
analyses for various clinical endpoints. |
Table III.
Multivariable prognostic factor
analyses for various clinical endpoints.
|
| TTP | OS |
|---|
|
|
|
|
|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Differentation | 4.02 | 2.35–6.85 | 0.00 | 2.83 | 1.70–4.72 | 0.00 |
| N stage | 1.35 | 0.85–2.16 | 0.20 | 1.24 | 0.77–1.99 | 0.38 |
| T stage | 2.63 | 1.78–3.87 | 0.00 | 2.19 | 1.52–3.16 | 0.00 |
| Radiotherapy | 1.80 | 1.32–2.47 | 0.00 | 1.40 | 1.04–1.88 | 0.03 |
| Raf1
expression | 1.94 | 1.16–3.25 | 0.01 | 1.64 | 0.98–2.75 | 0.06 |
We selected the poor and strong metastatic
capabilities of lung cancer cell lines A549 and H1299 to further
investigate the prognostic value of Raf1 in NSCLC after
radiotherapy. The relationship between Raf1 expression and the
proliferation and invasion of lung cancer cells was also studied by
the following methods.
Raf1 expression in lung cancer A549
and H1299 cells
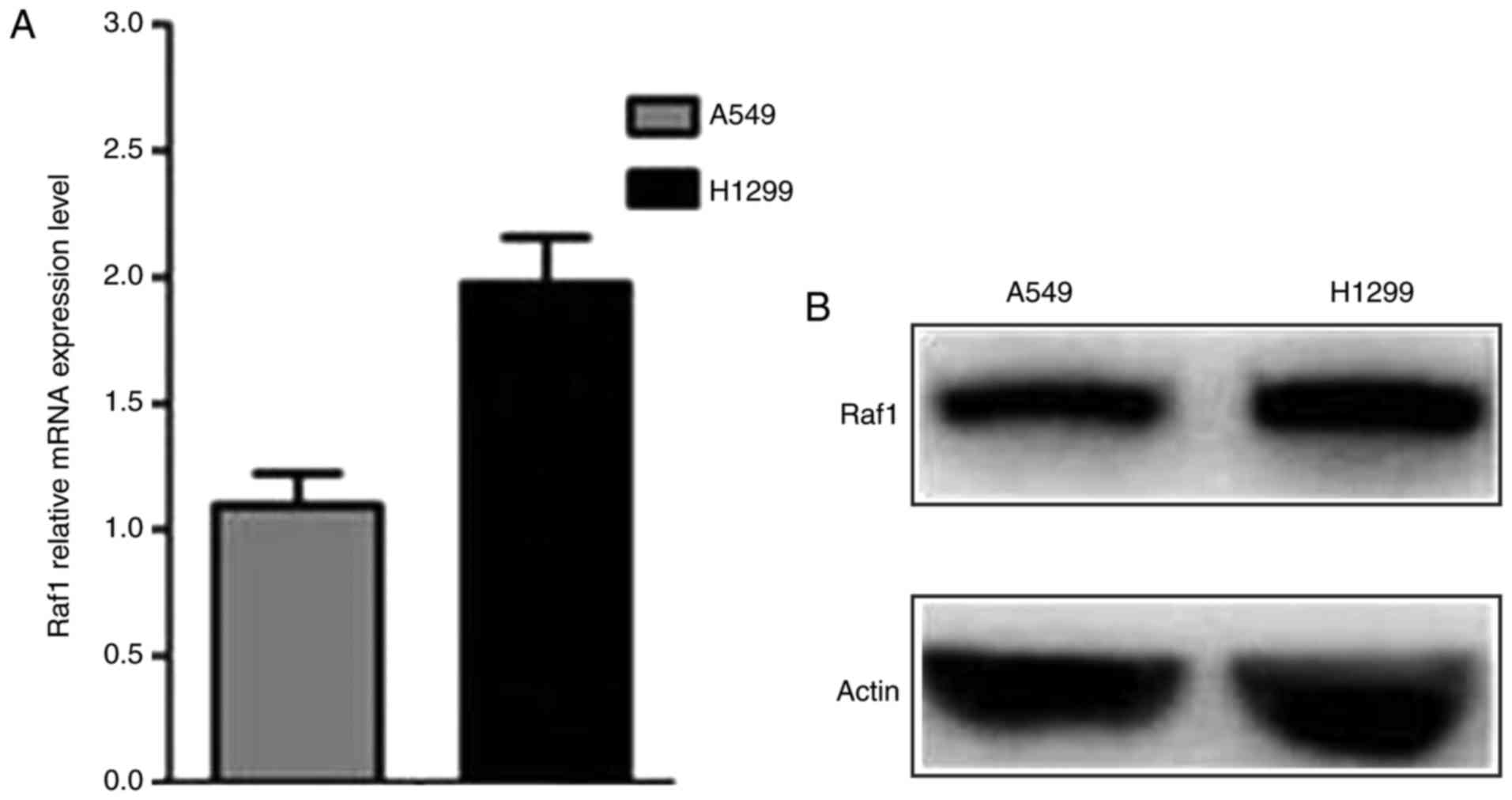
The qRT-PCR and western blot assay were conducted to
determine the Raf1 expression in pulmonary carcinoma cell lines.
The results showed that Raf1 was expressed in both cell lines and
was higher in H1299 cells than in A549 cells (Fig. 3). The Raf1 expression in A549 and
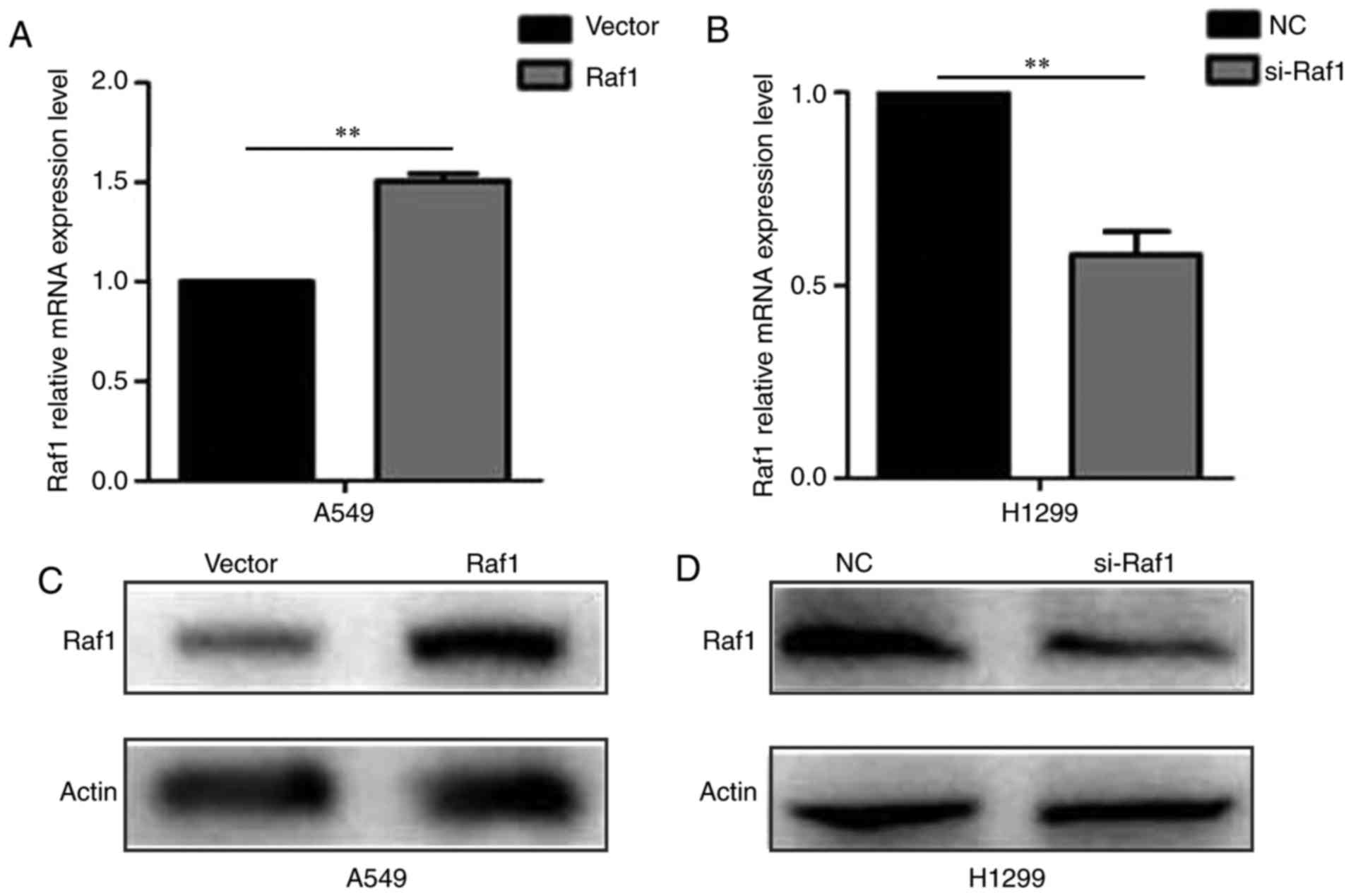
H1299 cells were upregulated and downregulated, respectively, after
being transfected (Fig. 4).
Raf1 promotes pulmonary carcinoma cell
invasion and migration
We performed a gain-of-function analysis in
vitro to examine the effect of Raf1 on the invasion and
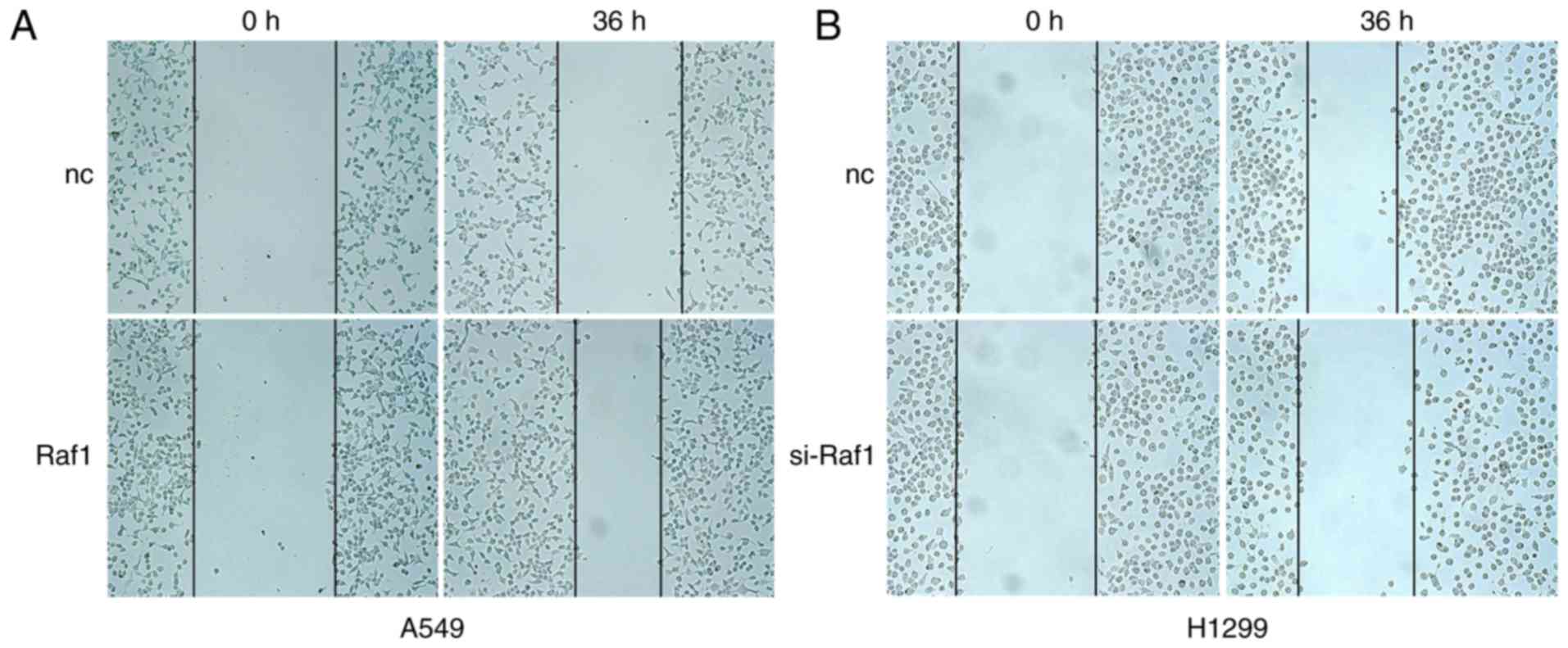
migration of pulmonary carcinoma cells. The wound healing assay
showed that the lateral migration capability was increased in the
Raf1-overexpressing A549 cells and decreased in the Raf1-silenced
H1299 cells (Fig. 5). Vertical
migration capability with the same result was observed in the
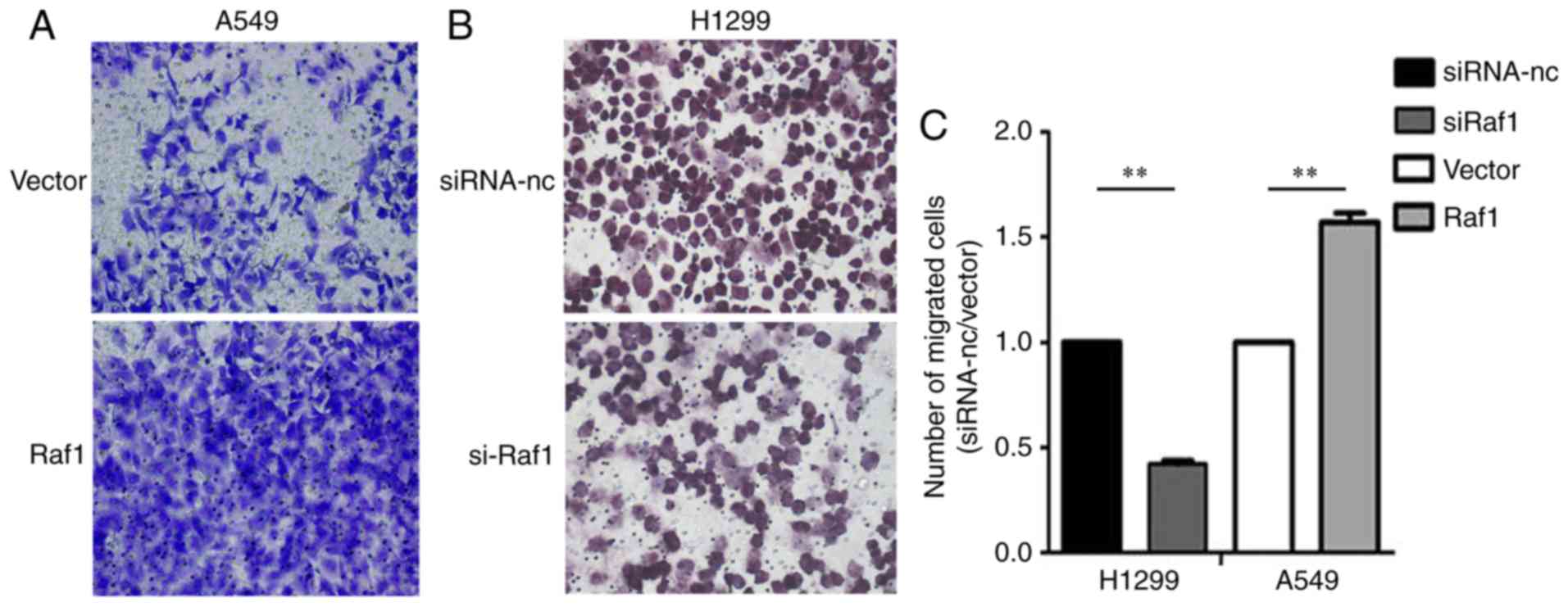
Transwell migration assay (Fig. 6).
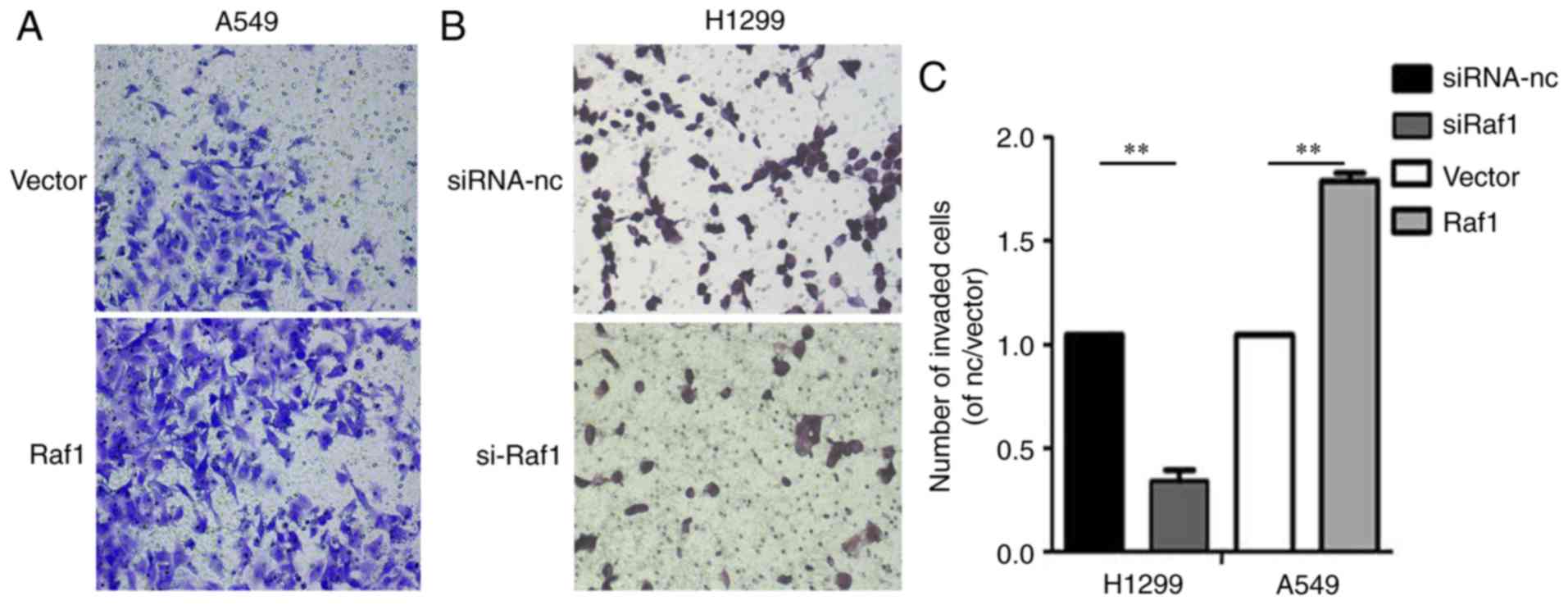
In the Transwell invasion assay, the invasion capability was
enhanced in the Raf1-overexpressing A549 cells and decreased in the
Raf1-silenced H1299 cells (Fig. 7).
Collectively, these results suggest that Raf1 accelerated the
migration and invasion of pulmonary carcinoma cells.
Discussion
In the present study, we examined the Raf1
expression in NSCLC patients, analyzed the correlations between
Raf1 expression and clinicopathological parameters and assessed the
value of the Raf1 expression and predicted the prognosis in NSCLC
patients with radiotherapy. The effect of Raf1 on the metastasis
and invasion of lung cancer A549 and H1299 cells was also
confirmed.
Raf1 is the main transmitter of cell growth and
reproduction signal conversion (10). It guides the receptor signals from
the cell membrane to the nucleus (11). Once activated, Raf1 phosphorylates
and activates the RAF/MEK/ERK signaling pathway, which then
regulates the cell cycle, proliferation, apoptosis, and migration
(12–15). Extensive studies have shown that
Raf1 is overexpressed in various types of cancers, and Raf1 plays
important roles in conferring resistance to erlotinib in NSCLC cell
lines (16), disease risk in oral
squamous cell carcinoma (17) and
osteosarcoma progression (18).
Truncated Raf1 expression was found to confer resistance to
mesenchymal-epithelial transition factor inhibition (19), and the Raf1/ERK tyrosine kinase
pathway was found to be involved in regulating the gene expression
of multidrug resistance protein in pancreatic cancer cells
(20). The Raf1 gene is expressed
in prostate cancer samples (6) and
potential clinically actionable fusions in prostate cancer cases
(21). Raf1 fusions have also been
reported to stimulate a mitogen-activated kinase-like protein in
pilocytic astrocytoma (22).
Activated Raf1 can phosphorylate activated protein kinases MEK1 and
MEK2, which consequently phosphorylate to activate the
serine/threonine-specific protein kinases ERK1 and ERK2 (23); these protein kinases play an
important role in controlling the gene expression involved in cell
division, apoptosis, differentiation, and migration (24). In a transgenic mouse model,
overexpression of Raf1 kinase was found to induce genetic events
associated with dysplasia in a genetic model of lung cancer
(25). These findings suggest that
Raf1 may play a role in human malignancies.
Recent studies have shown that Raf1 is positively
expressed in thyroid cancer compared with adjacent normal tissues
(9). Raf1 signals were detected in
28 (15%) of 186 Chinese prostate cancer samples. High Raf1 genomic
copy (>2 copies) was found to be correlated significantly with
old age (>65 years) and high baseline PSA (>50 ng/ml)
(6). In the present study, we
determined the Raf1 expression in 110 NSCLC specimens. The
percentage of Raf1-positive expression was 44.5% (49/110), and the
rates of high and low expression were 19.1% (21/110) and 25.5%
(28/110), respectively. The univariate analyses showed that Raf1
expression was significantly correlated with histological
differentiation, lymph node metastasis, and T stage. The patients
with Raf1 expression displayed early TTP and poor OS. The
multivariate analyses showed that Raf1 was an independent risk
factor for TTP in these patients, and Raf1 overexpression also
played an important role in a poor 3-year OS compared with the
patients with low and negative Raf1 expression. Yet, the difference
was statistically insignificant (P=0.06). Significant results may
have been obtained if the follow-up period was extended or the
number of patients was increased. Furthermore, pcDNA3.1-Raf1 and
Raf1-siRNA were used to transfect the A549 and H1299 cell lines,
respectively, to further confirm the evidence linking Raf1
expression with metastasis or NSCLC recurrence. The wound healing
and Transwell assays showed that Raf1 high-expressing cells showed
strong invasion and migration capabilites when compared with the
low expressing A549 and H1299 cells. These results agree partially
with the findings that Raf1 is associated with cancer cell
proliferation and invasion in pancreatic and colorectal cancer
types (20,26). Studies have also shown that histone
deacetylase inhibitors through transcriptional downregulation of
Raf1 suppressed c-Jun/Fra-1-mediated proliferation in neuroblastoma
cells (27). Blocking Raf1
expression with microRNAs was found to inhibit the proliferation
and invasion of thyroid and colorectal cancer types (9,28). The
molecular mechanisms related to the participation of Raf1 in the
invasive process in NSCLC remain elusive. Therefore, further
studies are required to clarify these mechanisms.
In conclusion, our results demonstrated that the
high level of Raf1 expression in NSCLC tissue samples was
associated with an early TTP and an adverse prognosis in patients
with NSCLC. Raf1 expression was positively correlated with the
invasion and migration capabilities of lung cancer A549 and H1299
cells.
Acknowledgements
The authors would like to thank the members of the
Department of Pathology and the Research Center of Clinical
Oncology, The Affiliated Jiangsu Cancer Hospital of Nanjing Medical
University for providing the paraffin-embedded biopsies and
technical assistance, respectively.
Funding
No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
XH and HT conceived and designed the experiments. HT
performed the experiments. JZW and LY coordinated the research and
analyzed the data. HT and LY wrote the manuscript. KD, XHW and YYX
supported the experiments and helped to draft the manuscript. JZW
provided the financial support and supervised laboratorial
processes. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Jiangsu Cancer Hospital. Written informed consent was obtained from
all patients before treatment initiation.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ettinger DS, Akerley W, Borghaei H, Chang
AC, Cheney RT, Chirieac LR, D'Amico TA, Demmy TL, Ganti AK,
Govindan R, et al: Non-small cell lung cancer. J Natl Compr Canc
Netw. 10:1236–1271. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torre LA, Siegel RL and Jemal A: Lung
Cancer statistics. Adv Exp Med Biol. 893:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cobb MH, Hepler JE, Cheng M and Robbins D:
The mitogen-activated protein kinases, ERK1 and ERK2. Semin Cancer
Biol. 5:261–268. 1994.PubMed/NCBI
|
|
6
|
Ren G, Liu X, Mao X, Zhang Y, Stankiewicz
E, Hylands L, Song R, Berney DM, Clark J, Cooper C and Lu YJ:
Identification of frequent BRAF copy number gain and alterations of
RAF genes in Chinese prostate cancer. Genes Chromosomes Cancer.
51:1014–1023. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Slattery ML, Lundgreen A and Wolff RK: MAP
kinase genes and colon and rectal cancer. Carcinogenesis.
33:2398–2408. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Borovski T, Vellinga TT, Laoukili J, Santo
EE, Fatrai S, van Schelven S, Verheem A, Marvin DL, Ubink I, Borel
Rinkes IHM and Kranenburg O: Inhibition of RAF1 kinase activity
restores apicobasal polarity and impairs tumour growth in human
colorectal cancer. Gut. 66:1106–1115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang F, Jiang C, Sun Q, Yan F, Wang L, Fu
Z, Liu T and Hu F: miR-195 is a key regulator of raf1 in thyroid
cancer. Onco Targets Ther. 8:3021–3028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wellbrock C, Karasarides M and Marais R:
The RAF proteins take centre stage. Nat Rev Mol Cell Biol.
5:875–885. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
McKay MM and Morrison DK: Integrating
signals from RTKs to ERK/MAPK. Oncogene. 26:3113–3121. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
McCubrey JA, Steelman LS, Chappell WH,
Abrams SL, Wong EW, Chang F, Lehmann B, Terrian DM, Milella M,
Tafuri A, et al: Roles of the Raf/MEK/ERK pathway in cell growth,
malignant transformation and drug resistance. Biochim Biophys Acta.
1773:1263–1284. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Steelman LS, Chappell WH, Abrams SL, Kempf
RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F,
Mazzarino MC, et al: Roles of the Raf/MEK/ERK and
PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity
to therapy-implications for cancer and aging. Aging (Albany NY).
3:192–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hwang YH, Choi JY, Kim S, Chung ES, Kim T,
Koh SS, Lee B, Bae SH, Kim J and Park YM: Over-expression of
c-raf-1 proto-oncogene in liver cirrhosis and hepatocellular
carcinoma. Hepatol Res. 29:113–121. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kyriakis JM, App H, Zhang XF, Banerjee P,
Brautigan DL, Rapp UR and Avruch J: Raf-1 activates MAP
kinase-kinase. Nature. 358:417–421. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu ZH, Hang JB, Hu JA and Gao BL:
RAF1-MEK1-ERK/AKT axis may confer NSCLC cell lines resistance to
erlotinib. Int J Clin Exp Pathol. 6:1493–504. 2013.PubMed/NCBI
|
|
17
|
Kordi-Tamandani DM, Saberi E, Jamali S and
Ladiz MA: ERK and RAF1 gene: Analysis of methylation and expression
profiles in patients with oral squamous cell carcinoma. Br J Biomed
Sci. 71:100–103. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen L, Wang Q, Wang GD, Wang HS, Huang Y,
Liu XM and Cai XH: Mir-16 inhibits cell proliferation by targeting
IGF1R and the Raf1-MEK1/2-ERK1/2 pathway in osteosarcoma. FEBS
Lett. 587:1366–1372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Petti C, Picco G, Martelli ML, Trisolini
E, Bucci E, Perera T, Isella C and Medico E: Truncated RAF kinases
drive resistance to MET inhibition in MET-addicted cancer cells.
Oncotarget. 6:221–233. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao Z, Ding N, Xiao G, Wang S, Wu Y and
Tang L: Reversal of multidrug resistance by gefitinib via RAF1/ERK
pathway in pancreatic cancer cell line. Anat Rec (Hoboken).
295:2122–2128. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Robinson D, Van Allen EM, Wu YM, Schultz
N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC
and Attard G: Integrative clinical genomics of advanced prostate
cancer. Cell. 161:1215–1228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lawson AR, Tatevossian RG, Phipps KP,
Picker SR, Michalski A, Sheer D, Jacques TS and Forshew T: RAF gene
fusions are specific to pilocytic astrocytoma in a broad paediatric
brain tumour cohort. Acta Neuropathol. 120:271–273. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brennan DF, Dar AC, Hertz NT, Chao WC,
Burlingame AL, Shokat KM and Barford D: A Raf-induced allosteric
transition of KSR stimulates phosphorylation of MEK. Nature.
472:366–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li P, Wood K, Mamon H, Haser W and Roberts
T: Raf-1: A kinase currently without a cause but not lacking in
effects. Cell. 64:479–482. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rohrbeck A, Müller VS and Borlak J:
Molecular characterization of lung dysplasia induced by c-Raf-1.
PLoS One. 4:e56372009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li X, Stevens PD, Liu J, Yang H, Wang W,
Wang C, Zeng Z, Schmidt MD, Yang M, Lee EY and Gao T: PHLPP is a
negative regulator of RAF1, which reduces colorectal cancer cell
motility and prevents tumor progression in mice. Gastroenterology.
146:1301–1312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He W, Wu Y, Tang X, Xia Y, He G, Min Z, Li
C, Xiong S, Shi Z, Lu Y and Yuan Z: HDAC inhibitors suppress
c-Jun/Fra-1-mediated proliferation through transcriptionally
downregulating MKK7 and Raf1 in neuroblastoma cells. Oncotarget.
7:6727–6747. 2016.PubMed/NCBI
|
|
28
|
Chai J, Wang S, Han D, Dong W, Xie C and
Guo H: MicroRNA-455 inhibits proliferation and invasion of
colorectal cancer by targeting RAF proto-oncogene
serine/threonine-protein kinase. Tumour Biol. 36:1313–1321. 2015.
View Article : Google Scholar : PubMed/NCBI
|