Introduction
Hepatocellular carcinoma is known to be highly
malignant and has a poor prognosis, constituting the most common
cause of cancer-related deaths worldwide after lung cancer
(1). Standard therapies for liver
cancer treatment include the administration of gemcitabine and
sorafenib (2). However, these have
high rates of unfavorable treatment outcomes (3).
Almost all tumors, including hepatocellular
carcinomas, contain an heterogeneous vascular network due to
angiogenic imbalance and the aberrant proliferation of cancer cells
(4–7). Since oxygen is supplied through the
bloodstream, anoxic (<0.5% O2), hypoxic (0.5–1.5%
O2) and normoxic (>1.5% O2) regions are
supposed to intermingle in tumor tissues (8–10).
Many studies have reported that hypoxic environments dramatically
reduce the effectiveness of existing anticancer agents (11–14).
Therefore, there is an urgent need for the discovery of novel
targets that would lead to the development of pharmaceutical agents
able to exert their anticancer activity in a hypoxic
environment.
Induction of the stabilization of hypoxia-inducible
factor 1 (HIF-1) is crucial for the adaptation of cancer cells to
hypoxic conditions (15), as the
stabilized protein is a transcription factor that regulates the
expression of various genes in order to shift energy production
from oxidative phosphorylation in mitochondria to glycolysis and/or
to facilitate angiogenesis (16).
It was recently reported that HIF-1-dependent activation of
autophagy occurs under hypoxic conditions (17). Autophagy is an intracellular
cleaning mechanism in which cellular components are digested by
lysosomal enzymes after being isolated from the cytoplasm by a
bilayer membrane. Autophagy is induced not only by hypoxia, but
also by other types of intense cellular stress including nutrient
deprivation, growth factor withdrawal and treatment with anticancer
agents (18–20). Hypoxia constitutes a severe
metabolic stress and the induction of autophagy is likely to
contribute to adaptation by allowing damaged organelles and
proteins to be properly processed, which is a crucial part of the
intracellular quality control system.
However, the significance and roles of autophagy
under hypoxic conditions are subject of extreme controversy, as
they appear to vary among cancers and/or cell types. Some studies
reveal that autophagy contributes to cell survival, whereas others
indicate that it negatively affects this outcome (21–23).
For that reason, it is necessary to thoroughly analyze the
significance of autophagy under hypoxic conditions in individual
primary tumors. The present study aimed to clarify the influence of
autophagy induced by cobalt chloride, a hypoxia-mimicking agent, on
the survival of hepatocellular carcinoma cells, as well as the
potential usefulness of this process as a therapeutic target. The
results of the present study revealed that autophagy under such
conditions was dependent on the AMPK pathway and suppressed
apoptosis, whereas blockage of this pathway may constitute an
attractive therapeutic target as it reduces the ability of cancer
cells to adapt to hypoxia.
Materials and methods
Reagents
The materials for the present study were obtained
from the following sources: LY294002 was obtained from Calbiochem
(Merck KGaA, Darmstadt, Germany). Dulbecco's modified Eagle's
medium (DMEM), dimethyl sulfoxide, cobalt chloride, bafilomycin A1,
MitoTEMPO and a proteinase inhibitor cocktail were purchased from
Sigma-Aldrich (St. Louis, MO, USA). MitoTracker Green FM and
MitoSOX Red were obtained from Thermo Fisher Scientific (Waltham,
MA, USA). Anti-LC3 antibody (1:1,000; rabbit polyclonal; cat no.
PM036) was purchased from MBL (Medical and Biological Laboratories
Co., Ltd., Nagoya, Japan). The antibodies against phospho-p70 S6
kinase (Thr389) (1:1,000; rabbit monoclonal; cat. no. 9234), p70 S6
kinase (1:1,000; rabbit polyclonal; cat. no. 9202), phospho-Akt
(Ser473) (1:1,000; rabbit monoclonal; cat. no. 4058), AKT (1:1,000;
rabbit polyclonal; cat. no. 9272), phospho-AMPKα (Thr172) (1:1,000;
rabbit monoclonal; cat. no. 2535) and AMPKα (1:1,000; rabbit
polyclonal; cat. no. 2532), as well as Alexa Flour 555-(1:1,000;
cat. no. 4409) or Alexa Flour 488-conjugated secondary antibodies
(1:1,000; cat. no. 4412) were obtained from Cell Signaling
Technology (Danvers, MA, USA). Anti-HIF-1α antibody (1:1,000;
rabbit polyclonal; cat. no. GTX127309) was purchased from GeneTex
(Irvine, CA, USA), anti-actin antibody (1:10,000; mouse monoclonal;
cat. no. 013-24553) was obtained from Wako Pure Chemical Industries
(Osaka, Japan). The anti-LAMP1 (1:500; mouse monoclonal; cat. no.
sc-20011) and anti-Tom20 (1:500; mouse monoclonal; cat. no.
sc-17764) antibodies were obtained from Santa Cruz Biotechnology
(Dallas, TX, USA).
Cell lines and culture conditions
The human hepatocellular carcinoma cell lines Huh7
and HepG2 were purchased from the Japanese Collection of Research
Bioresources Cell Bank (JCRB Cell Bank, Osaka, Japan). Both cell
lines were cultured in DMEM supplemented with 10% fetal bovine
serum (FBS), 50 U/ml penicillin, 50 µg/ml streptomycin and
non-essential amino acids (Gibco BRL; Thermo Fisher Scientific,
Paisley, UK). The cells were cultured at 37°C, under 5%
CO2-95% air.
Cytotoxicity assay
Cytotoxicity assays were performed using the Cell
Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Kumamoto,
Japan). In brief, HepG2 or Huh7 cells were seeded in 96-well plates
(1×104 cells/well) and incubated for 24 h. The medium
was then changed to DMEM with or without 300 µM cobalt chloride,
followed by addition of serial dilutions of autophagy inhibitors.
After another 24 h, the cells were washed with phosphate-buffered
saline (PBS) and 100 µl of DMEM containing 10% WST-8 solution was
added to each well and each plate was incubated for another 3 h.
Subsequently, the absorbance at 460 nm was assessed and cell
viability values were expressed as percentages, with the cell
numbers in the corresponding control cultures (absence of autophagy
inhibitors under each culture condition) set as 100%.
Western blot analysis
Protein extraction and western blot analysis were
performed as previously described (24). The antibody dilutions were used
according to the manufacturer's instructions.
Immunostaining analysis
Cells grown on 4-well glass chamber slides
(Sigma-Aldrich) were treated with 300 µM cobalt chloride
(CoCl2) for 24 h. After incubation, the slides were
washed with PBS, fixed with PBS containing 4% formaldehyde and 0.1%
Triton X-100 for 30 min at 25°C, blocked with 3% bovine serum
albumin in PBS for 1 h and incubated first with the indicated
primary antibody for 1 h at 25°C and then, with the corresponding
secondary antibody at 25°C for another 1 h. DNA was counterstained
with SlowFade mounting medium containing DAPI
(4′,6-diamidino-2-phenylindole dihydrochloride) that was obtained
from Cell Signaling Technology. Images of optical sections with 0.7
µm thickness were captured using a Zeiss LSM 700 confocal laser
scanning microscope (Carl Zeiss Microscopy, GmbH, Oberkochen,
Germany) under a 63× lens objective (numerical aperture, 1.2 W).
The Alexa Fluor 488 dye was excited by a 488-nm laser and the
resulting fluorescence emission was detected through a filter that
transmitted wavelengths ranging from 420 to 550 nm. The Alexa Fluor
555 dye was excited by a 555-nm laser and the resulting
fluorescence emission was detected through a filter that
transmitted wavelengths over 560 nm. The number of puncta was
counted using ImageJ v1.51n software (NIH, Bethesda, MD, USA). DAPI
was used for nuclear counterstaining and its 405-nm-excited
fluorescence emission was detected through a filter that
transmitted wavelengths over 420 nm.
Plasmids and stable transfection
The HepG2 cells were transfected with
MISSION® short hairpin targeting human AMPKa·1
(sh-AMPKCCGGCCATCCTGAAAGAGTACCATTCTCGAGAATGGTACTCTTTCAGGATGGTTTTT)-containing
plasmids (Sigma-Aldrich) using Lipofectamine LTX Reagent with PLUS
Reagent (Thermo Fisher Scientific) for 48 h. The cells were then
transferred into medium containing 1.5 µg/ml puromycin
(Sigma-Aldrich) for 3 weeks for single-cell clone selection to
obtain a stable-expression cell line.
Statistical analysis
Bars or symbols in the graphs represent the means ±
standard deviation (SD) generated from at least three independent
experiments. Significant differences were determined by one-way
analysis of variance (ANOVA), two-way ANOVA or t-tests. A P-value
of <0.05 was considered to indicate a statistically significant
difference.
Results
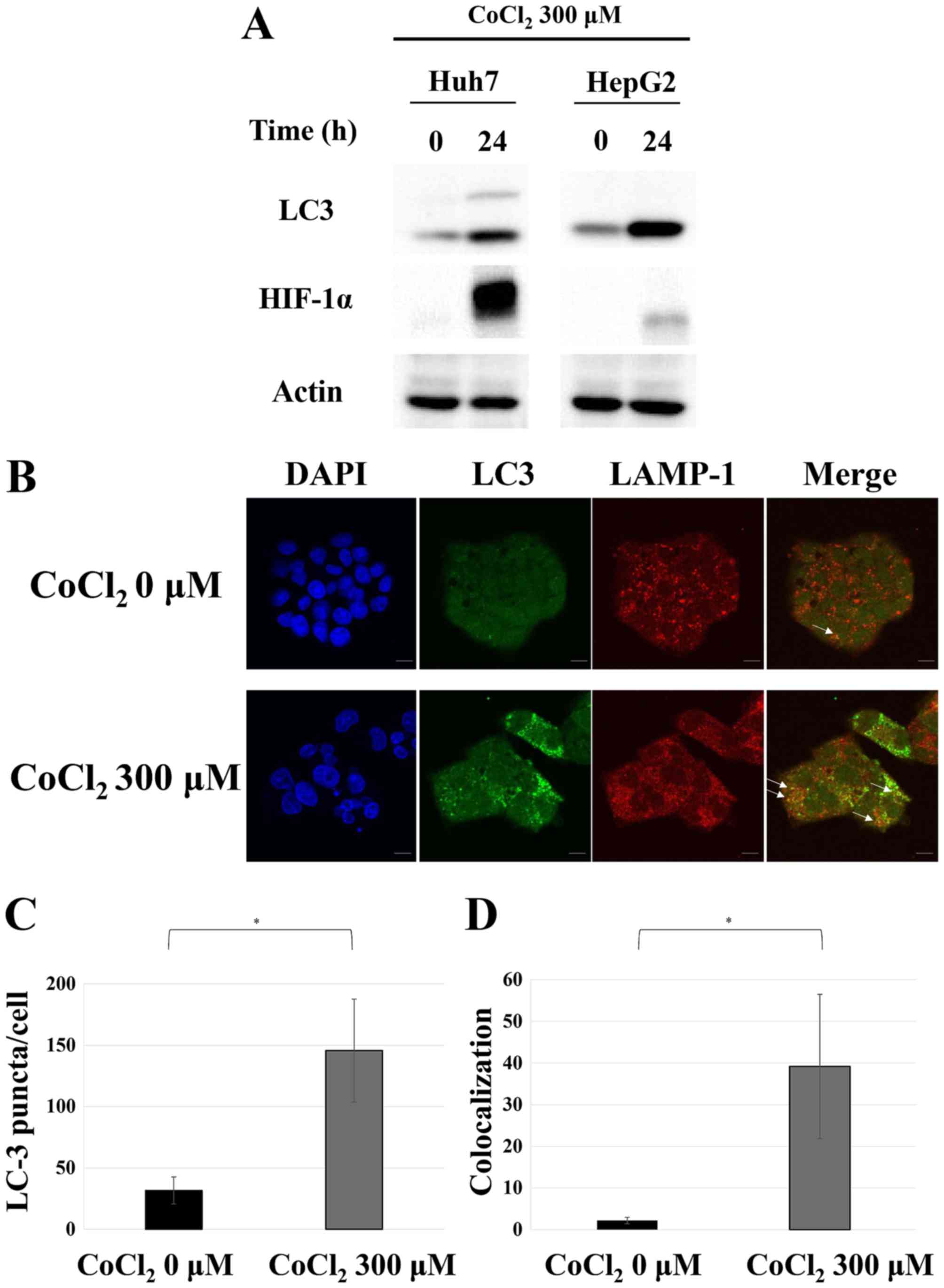
Autophagy is induced by cobalt
chloride treatment
In order to confirm the induction of autophagy in a
hypoxic environment, we treated the Huh7 and HepG2 cells with a
hypoxia-mimicking agent, cobalt chloride (25,26).
This resulted in a significant accumulation of HIF-1α, which is a
hypoxia marker, as well as an increase in the band of LC3-II, which
is a modified form of LC3 that can be found in autophagosomes and
autolysosomes (Fig. 1A). Both
changes indicated the induction of autophagy under
hypoxia-mimicking conditions. HepG2 cells were also subjected to
double immunostaining for LC3 and LAMP1, a lysosomal marker. As
displayed in Fig. 1B-D, cobalt
chloride treatment resulted in an increase in LC3 puncta,
confirming autophagy induction. Furthermore, the observed
colocalization of LC3 and LAMP1 indicated the fusion of
autophagosomes and lysosomes. These results strongly indicated that
autophagy was induced in hepatocellular carcinoma cells under
hypoxic conditions.
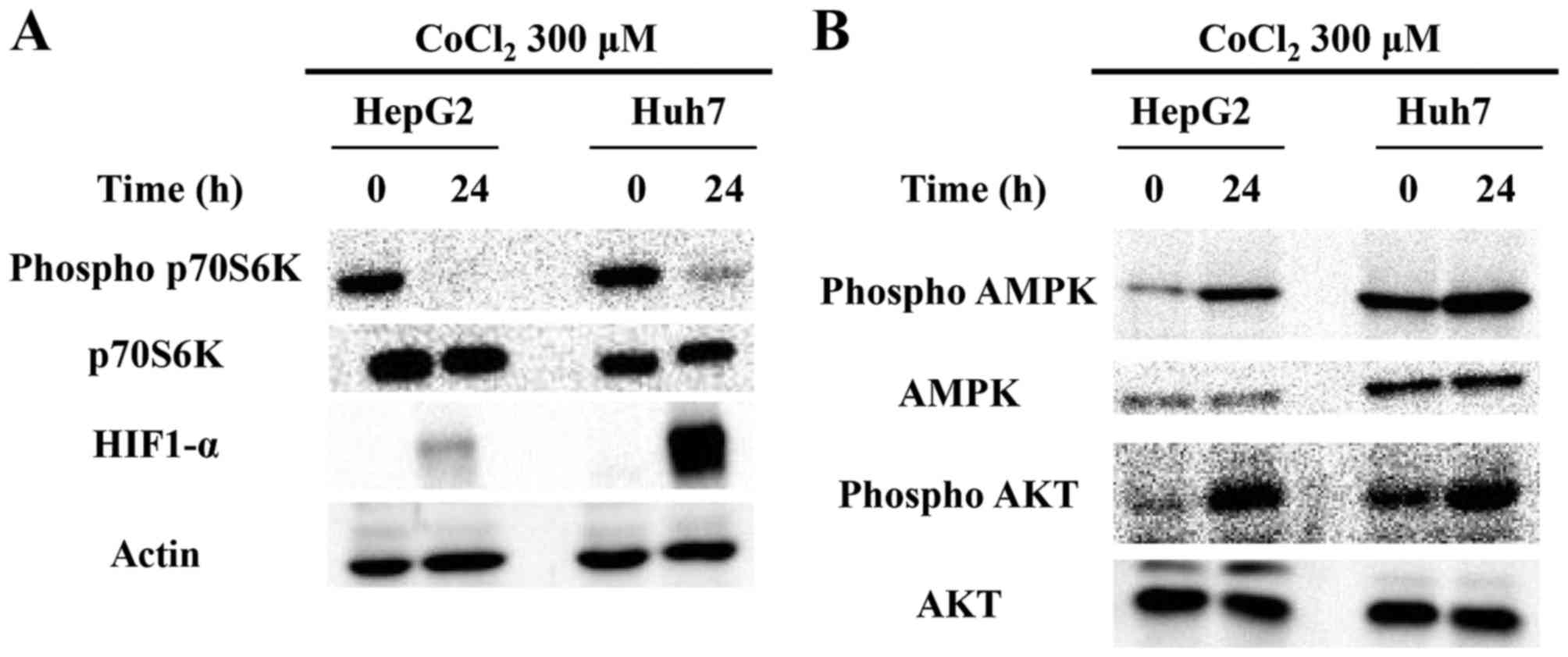
Autophagy induced by cobalt chloride
is dependent on the AMPK/mTOR pathway
Subsequently, we investigated in detail the
signaling pathway mediating the induction of autophagy by cobalt
chloride treatment. The activity of mTOR, a regulator of autophagy,
was examined using the phosphorylation status of p70S6K, which is a
direct target of mTOR (27), as an
indicator. Cobalt chloride treatment led to a significant
attenuation of p70S6K phosphorylation, strongly indicative of lower
mTOR activity in both cell lines (Fig.
2A). Previous studies have revealed that the phosphorylation of
mTOR is positively regulated by AKT (28) and negatively regulated by AMPK
(29). Thus, the lower mTOR
activity that we observed, may be attributed to a reduction of the
AKT activity (e.g., through decreased phosphorylation) and/or an
increase in the AMPK activity (e.g., through increased
phosphorylation), based on which pathways are involved. However, as
demonstrated in Fig. 2B, the
phosphorylation of both AKT and AMPK was increased (Fig. 2B). Thus, we concluded that the
induction of autophagy by cobalt chloride treatment is likely to be
independent of the AKT pathway and dependent on the AMPK/mTOR
pathway. This result was consistent with a previous study that
examined the mechanism of hypoxia-induced autophagy in human dental
pulp cells (30).
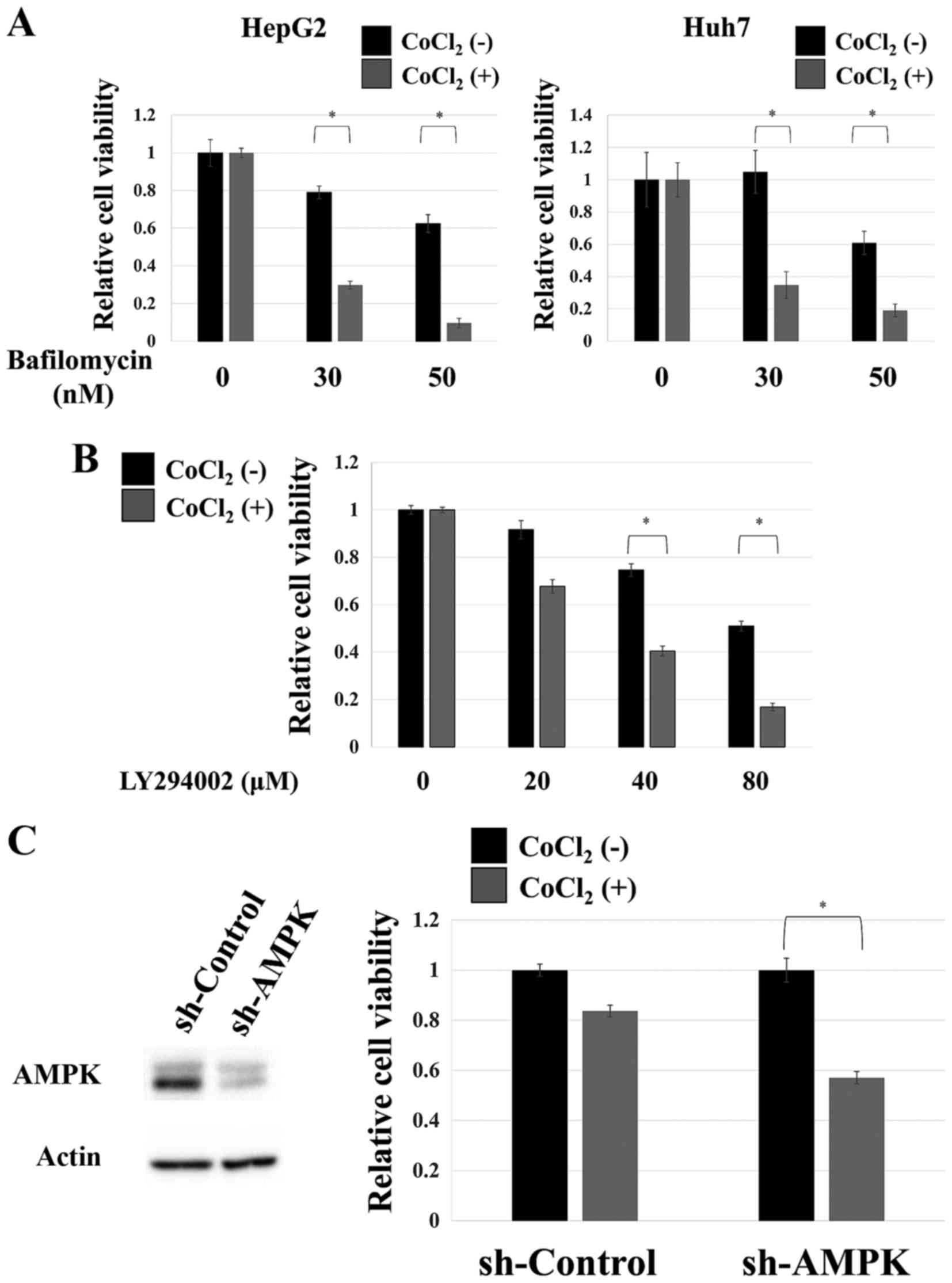
Autophagy inhibition results in
selective cytotoxicity under hypoxia-mimicking conditions
Both hepatocellular carcinoma cell lines were
treated with bafilomycin A1, an autophagy inhibitor (31,32).
In both cell lines, the inhibitor displayed modest toxicity under
normal culture conditions, whereas it dramatically reduced cell
survival when added to cells in hypoxia-mimicking conditions
(Fig. 3A). High toxicity was also
observed when the Huh7 cells were treated with LY294002, a
different autophagy inhibitor (33), under hypoxia-mimicking conditions
(Fig. 3B). As aforementioned, AMPK
probably mediates the induction of autophagy by cobalt chloride
treatment. Thus, we attempted to examine the role of this kinase in
the adaptation of cells to this condition. As displayed in Fig. 3C, the knockdown of AMPK was
extremely toxic under hypoxia-mimicking conditions in HepG2 cells.
These results strongly indicated that the proper functioning of
autophagy is essential for the adaptation to hypoxia-mimicking
conditions and that the AMPK pathway plays a critical role in the
process of adaptation as it mediates cobalt chloride-induced
autophagy.
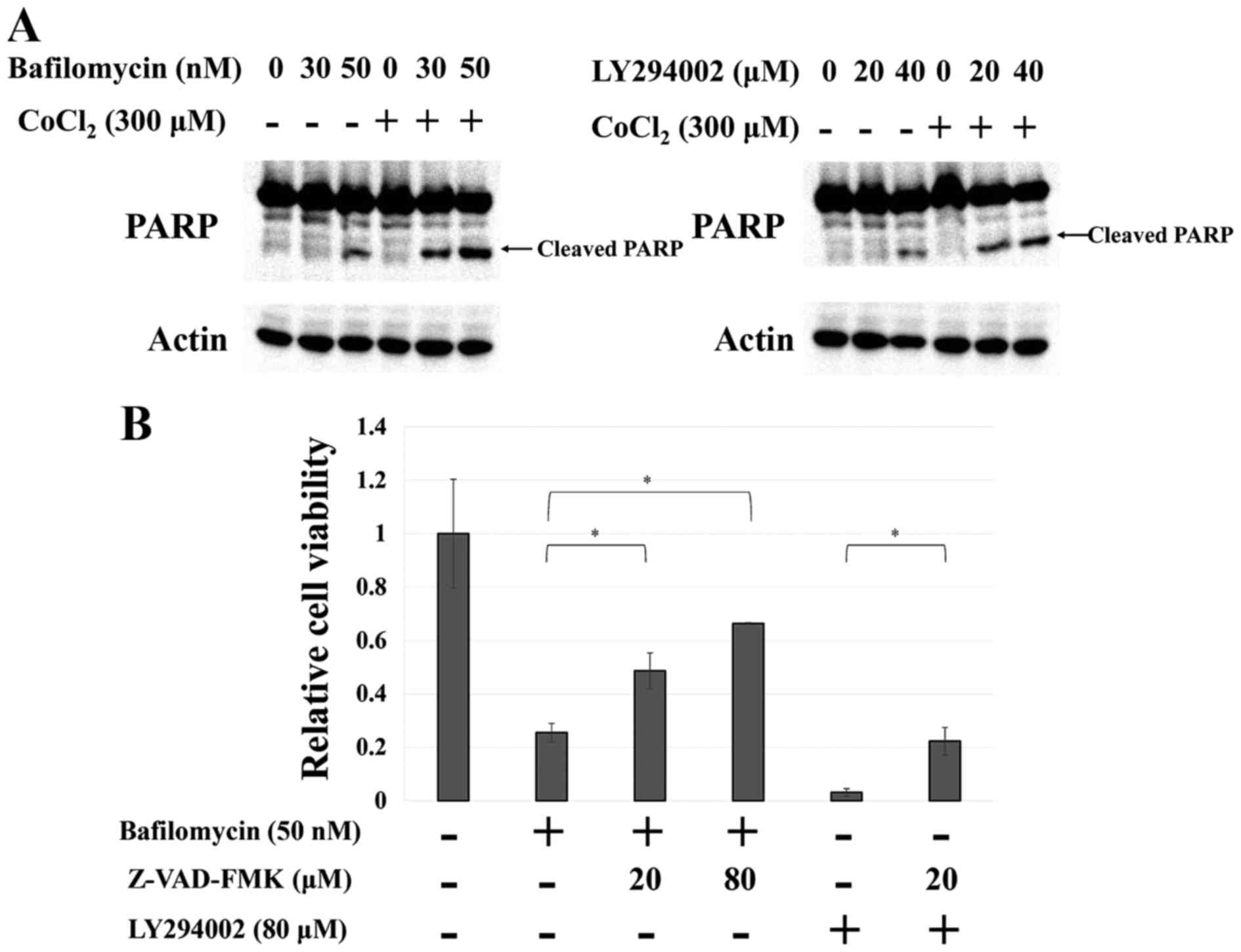
Cobalt chloride-induced autophagy
suppresses apoptosis
It has been reported that autophagy supports cell
survival through the suppression of apoptosis (34). Thus, we examined whether apoptosis
would be induced in the Huh7 cells when adaptation to
hypoxia-mimicking conditions was blocked by autophagy inhibitors.
Notably, inhibiting autophagy under hypoxia-mimicking conditions
resulted in a large increase in PARP cleavage (Fig. 4A), which is an apoptosis indicator,
whereas co-treatment with z-VAD-FMK, an apoptosis inhibitor,
strongly attenuated this effect (Fig.
4B). These results indicated that hepatocellular carcinoma
cells adapted to hypoxia using autophagy to suppress apoptosis.
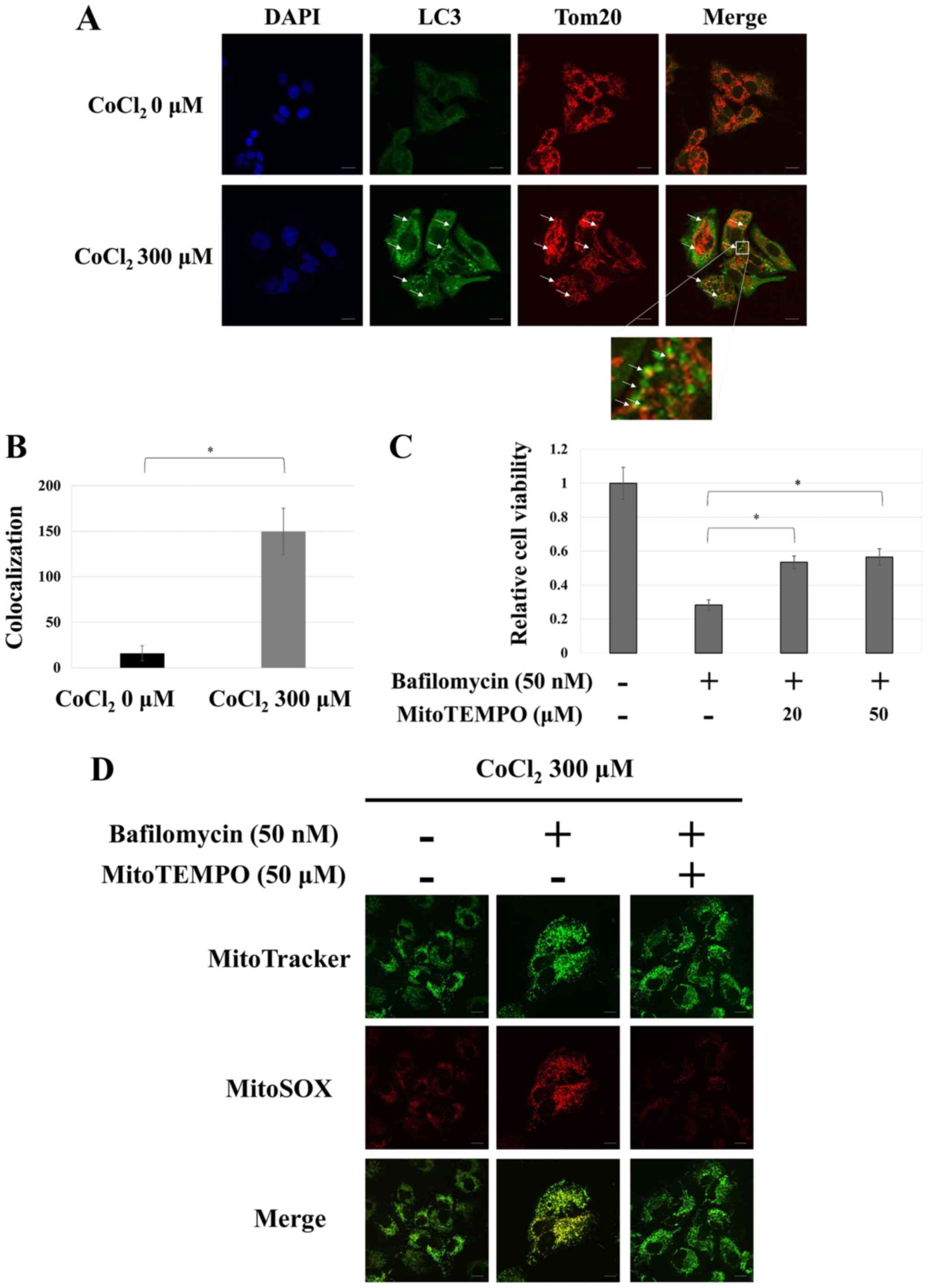
Mitophagy is a critical adaptive
response to hypoxia-mimicking conditions
It has been reported that cells use autophagy to
destroy mitochondria in order to maintain the intracellular redox
status, which allows them to evade apoptosis (17). This ‘mitochondria-selective’ type of
autophagy is termed mitophagy. We examined whether mitophagy is
induced during adaptation to hypoxia-mimicking conditions by
immunostaining HepG2 cells for LC3 and Tom20, a known mitochondrial
marker. The distribution of most of the LC3 puncta corresponded to
one of the Tom20 puncta under hypoxia-mimicking conditions,
strongly indicating that mitophagy had been induced (Fig. 5A and B). In addition, treatment with
MitoTEMPO, a mitochondria-selective antioxidant reagent (35), significantly reduced cytotoxicity
and mitochondrial reactive oxygen species (ROS) resulting from the
inhibition of autophagy during hypoxia-mimicking conditions
(Fig. 5C and D). These results
indicated mitophagy as the type of autophagy mainly used by
hepatocellular carcinoma cells to adapt to hypoxia conditions and
avoid apoptosis.
Discussion
In the present study, we used hepatocellular
carcinoma cells to examine the importance of autophagy on their
ability to adapt to hypoxia-mimicking conditions, which were
achieved using cobalt chloride treatment. We found that cobalt
chloride induces autophagy through the AMPK pathway and that this
process allows cells to evade apoptosis and adapt to the
hypoxia-mimicking environment. Furthermore, autophagy inhibitors
exhibited significant cytotoxicity under hypoxia-mimicking
conditions. Since cobalt chloride induces stabilization of HIF-1
and is known as a hypoxia-mimicking agent, further investigation of
the effects of exposure to other hypoxia-mimicking agents and to a
hypoxic environment is necessary to gain a more precise
understanding of the significance of autophagy in the process of
adaptation to hypoxic environments.
It is known that AMPK can be activated by two
distinct mechanisms (36). The
first, which is considered the classical activation mechanism, is
sensing the intracellular ATP/AMP ratio. Notably, the intracellular
ATP level has been reported to decrease under hypoxic conditions
(37). Thus, the activation of AMPK
in the present hypoxia-mimicking cell culture system may be
attributed, at least partly, to this mechanism. The second AMPK
activation mechanism is stimulation by ROS (36). It has recently been revealed that,
beyond their ability to damage DNA and proteins, ROS also play an
important role as mediator molecules controlling cellular signaling
when their levels are low (38,39).
More important with respect to our findings, is that hypoxia is
known to cause an intracellular accumulation of ROS, which are
produced both in mitochondria and by the activity of NOX4 and
contribute to cell proliferation, survival and motility (40,41).
These data indicate that the phagocytosis-inducing activation of
the AMPK pathway in our hypoxia-mimicking cell culture system may
be attributed to either of the two known AMPK activation
mechanisms. Further studies are required to determine the extent of
the contribution of each mechanism. In addition, it is important to
note that the AKT phosphorylation was enhanced by the treatment
with cobalt chloride in the present study. The activation of AKT is
involved in cell survival and proliferation. In turn, LY294002, an
autophagy inhibitor used in this study, is known to be the pan-PI3K
inhibitor. Therefore, it was difficult to clearly distinguish
whether the high level of cytotoxicity induced by LY294002, during
cobalt-chloride treatment, was due to the inhibition of autophagy
or the blockade of the survival signal of AKT. It is necessary to
elucidate the detailed role of AKT in hypoxic adaptation in the
future.
It has been reported that autophagy induced in a
hypoxic environment negatively affects survival in glioma and
breast cancer cell lines, as autophagic cell death is triggered
under these conditions (21). To
our knowledge, the present study is the first to report an
anti-apoptotic, pro-survival effect for cobalt chloride-induced
autophagy in hepatocellular carcinoma cell lines, indicating that
autophagy may constitute a potential therapeutic target for this
type of cancer. Furthermore, our results indicated that mitophagy
is a critical system that allows cells to adapt to hypoxia through
the regulation of intracellular ROS levels. This is both rational,
since mitochondria serve as major loci of ROS production and
consistent with previous data that support a mitophagy-mediated
apoptosis avoidance mechanism (17). Thus, it is likely that cells avert
excessive ROS accumulation by properly processing mitochondria
damaged by hypoxic stress via mitophagy.
Notably, the present study indicated that the
cytotoxicity of autophagy inhibitors was selective to
hypoxia-mimicking conditions, as their effect on cell survival
under normoxic culture conditions was limited. Since the reduced
form of glutathione, which determines the cellular antioxidant
capacity, decreases in a hypoxic environment (42), the selective character of the
cytotoxicity displayed by autophagy inhibitors may be attributed to
excess ROS being produced due to mitophagy failure to overwhelm the
antioxidant capacity of the cell, mostly when this capacity is
already compromised, such as under hypoxia-mimicking conditions.
Under normoxic conditions, conversely, the small number of damaged
mitochondria and the intact glutathione-based anti-oxidant defense
maintains cytotoxicity at relatively low levels despite autophagy
failure. Future studies focusing on the intracellular redox status
may be required in order to elucidate the underlying molecular
mechanism of these processes.
Considering the microenvironments that can be found
inside a tumor, the dependence of cancer tissues on the bloodstream
to obtain oxygen and nutrients causes parts of the tumor to be
exposed not only to hypoxia, but also to a lack of nutrients.
Identifying valid therapeutic targets in these microenvironments
requires determining in what way cancer cells adapt to both types
of stress and, importantly, discovering mechanisms that may be
shared among the two adaptation processes. Since autophagy in
hepatocellular carcinoma cells is induced even in a nutrient-poor
environment, supporting cell survival (Endo et al
unpublished data), it may represent such a common mechanism.
Consistent with the importance of autophagy in tumor
microenvironment, an analysis using human surgical specimens
indicated that cancerous tissues had a higher autophagic activity
than non-cancerous tissues (43).
Therefore, autophagy may be viewed as a potential therapeutic
target not only in the context of the hypoxic stress response, but
also in the whole cancer microenvironment.
In the present study, we revealed that autophagy
induced by cobalt chloride treatment did not trigger autophagic
cell death in hepatocellular carcinoma cells but rather contributed
significantly to cell survival. Additionally, we demonstrated that
autophagy may constitute a very attractive target for developing
novel therapeutic strategies for the treatment of hepatocellular
carcinoma.
Acknowledgements
We wish to thank Makiko Sato and Akiko Sakuyama from
the Department of Preventive Medicine, Tokai University School of
Medicine, for their excellent secretarial support and Kazuhiro
Yoshida from the Support Center for Medical Research and Education,
Tokai University, for providing excellent technical support. We
would also like to thank Editage (www.editage.jp) for English language editing. The
present study was supported in part by the 2016 Research and Study
Project of Tokai University Educational System General Research
Organization (SO), the 2016 Tokai University School of Medicine
Research Aid (SO), the 2017 Research and Study Program of Tokai
University Educational System General Research Organization (SO),
the 2017 Tokai University School of Medicine Research Aid (HE) and
a Grant-in-Aid for Scientific Research (nos. 23701113 and 26870600
to HE) from the Ministry of Education, Culture, Sports, Science and
Technology of Japan.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wörns M and Galle PR: HCC
therapies-lessons learned. Nat Rev Gastroenterol Hepatol.
11:447–452. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brown JM and Giaccia AJ: The unique
physiology of solid tumors: Opportunities (and problems) for cancer
therapy. Cancer Res. 58:1408–1416. 1998.PubMed/NCBI
|
|
5
|
Jain RK: Molecular regulation of vessel
maturation. Nat Med. 9:685–693. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Less JR, Skalak TC, Sevick EM and Jain RK:
Microvascular architecture in a mammary carcinoma: Branching
patterns and vessel dimensions. Cancer Res. 51:265–273.
1991.PubMed/NCBI
|
|
7
|
Thomlinson RH and Gray LH: The
histological structure of some human lung cancers and the possible
implications for radiotherapy. Br J Cancer. 9:539–549. 1955.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Helmlinger G, Yuan F, Dellian M and Jain
RK: Interstitial pH and pO2 gradients in solid tumors in
vivo: High-resolution measurements reveal a lack of correlation.
Nat Med. 3:177–182. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vaupel P, Höckel M and Mayer A: Detection
and characterization of tumor hypoxia using pO2
histography. Antioxid Redox Signal. 9:1221–1235. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heindryckx F, Mertens K, Charette N,
Vandeghinste B, Casteleyn C, Van Steenkiste C, Slaets D, Libbrecht
L, Staelens S, Starkel P, et al: Kinetics of angiogenic changes in
a new mouse model for hepatocellular carcinoma. Mol Cancer.
9:2192010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Ning X, Sun L, Zhang H, Shi Y, Guo
C, Han S, Liu J, Sun S, Han Z, et al: Hypoxia-inducible factor-1
alpha contributes to hypoxia-induced chemoresistance in gastric
cancer. Cancer Sci. 99:121–128. 2008.PubMed/NCBI
|
|
12
|
Song J, Qu Z, Guo X, Zhao Q, Zhao X, Gao
L, Sun K, Shen F, Wu M and Wei L: Hypoxia-induced autophagy
contributes to the chemoresistance of hepatocellular carcinoma
cells. Autophagy. 5:1131–1144. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sullivan R, Paré GC, Frederiksen LJ,
Semenza GL and Graham CH: Hypoxia-induced resistance to anticancer
drugs is associated with decreased senescence and requires
hypoxia-inducible factor-1 activity. Mol Cancer Ther. 7:1961–1973.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokoi K and Fidler IJ: Hypoxia increases
resistance of human pancreatic cancer cells to apoptosis induced by
gemcitabine. Clin Cancer Res. 10:2299–2306. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Semenza GL: Hypoxia-inducible factor 1:
Master regulator of O2 homeostasis. Curr Opin Genet Dev.
8:588–594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Semenza GL: HIF-1 and tumor progression:
Pathophysiology and therapeutics. Trends Mol Med. 8:S62–S67. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang H, Bosch-Marce M, Shimoda LA, Tan
YS, Baek JH, Wesley JB, Gonzalez FJ and Semenza GL: Mitochondrial
autophagy is an HIF-1-dependent adaptive metabolic response to
hypoxia. J Biol Chem. 283:10892–10903. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizushima N, Yamamoto A, Matsui M,
Yoshimori T and Ohsumi Y: In vivo analysis of autophagy in response
to nutrient starvation using transgenic mice expressing a
fluorescent autophagosome marker. Mol Biol Cell. 15:1101–1111.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lum JJ, Bauer DE, Kong M, Harris MH, Li C,
Lindsten T and Thompson CB: Growth factor regulation of autophagy
and cell survival in the absence of apoptosis. Cell. 120:237–248.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bursch W, Ellinger A, Kienzl H, Török L,
Pandey S, Sikorska M, Walker R and Hermann RS: Active cell death
induced by the anti-estrogens tamoxifen and ICI 164 384 in human
mammary carcinoma cells (MCF-7) in culture: The role of autophagy.
Carcinogenesis. 17:1595–1607. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Azad MB, Chen Y, Henson ES, Cizeau J,
McMillan-Ward E, Israels SJ and Gibson SB: Hypoxia induces
autophagic cell death in apoptosis-competent cells through a
mechanism involving BNIP3. Autophagy. 4:195–204. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tasdemir E, Maiuri MC, Galluzzi L, Vitale
I, Djavaheri-Mergny M, D'Amelio M, Criollo A, Morselli E, Zhu C,
Harper F, et al: Regulation of autophagy by cytoplasmic p53. Nat
Cell Biol. 10:676–687. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang R, Zhu F, Ren J, Huang L, Liu P and
Wu G: Beclin1/PI3K-mediated autophagy prevents hypoxia-induced
apoptosis in EAhy926 cell line. Cancer Biother Radiopharm.
26:335–343. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Endo H, Niioka M, Kobayashi N, Tanaka M
and Watanabe T: Butyrate-producing probiotics reduce nonalcoholic
fatty liver disease progression in rats: New insight into the
probiotics for the gut-liver axis. PLoS One. 8:e633882013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan RH, Chen PS, Zhao D and Zhang WD:
Hypoxia induced by CoCl2 influencing the expression and the
activity of matrix metalloproteinase-2 in rat hepatic stellate
cells. Zhonghua Gan Zang Bing Za Zhi. 15:654–657. 2007.(In
Chinese). PubMed/NCBI
|
|
26
|
Karovic O, Tonazzini I, Rebola N, Edström
E, Lövdahl C, Fredholm BB and Daré E: Toxic effects of cobalt in
primary cultures of mouse astrocytes. Similarities with hypoxia and
role of HIF-1alpha. Biochem Pharmacol. 73:694–708. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Blommaart EF, Luiken JJ, Blommaart PJ, van
Woerkom GM and Meijer AJ: Phosphorylation of ribosomal protein S6
is inhibitory for autophagy in isolated rat hepatocytes. J Biol
Chem. 270:2320–2326. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hahn-Windgassen A, Nogueira V, Chen CC,
Skeen JE, Sonenberg N and Hay N: Akt activates the mammalian target
of rapamycin by regulating cellular ATP level and AMPK activity. J
Biol Chem. 280:32081–32089. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inoki K, Zhu T and Guan K: TSC2 mediates
cellular energy response to control cell growth and survival. Cell.
115:577–590. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Q, Liu H, Sun Q, Zhang L, Lin H, Yuan
G, Zhang L and Chen Z: Adenosine monophosphate-activated protein
kinase/mammalian target of rapamycin-dependent autophagy protects
human dental pulp cells against hypoxia. J Endod. 39:768–773. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bowman EJ, Siebers A and Altendorf K:
Bafilomycins: A class of inhibitors of membrane ATPases from
microorganisms, animal cells, and plant cells. Proc Natl Acad Sci
USA. 85:pp. 7972–7976. 1988; View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoshimori T, Yamamoto A, Moriyama Y, Futai
M and Tashiro Y: Bafilomycin A1, a specific inhibitor of
vacuolar-type H(+)-ATPase, inhibits acidification and protein
degradation in lysosomes of cultured cells. J Biol Chem.
266:17707–17712. 1991.PubMed/NCBI
|
|
33
|
Blommaart EF, Krause U, Schellens JP,
Vreeling-Sindelárová H and Meijer AJ: The phosphatidylinositol
3-kinase inhibitors wortmannin and LY294002 inhibit autophagy in
isolated rat hepatocytes. Eur J Biochem. 243:240–246. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim SE, Park HJ, Jeong HK, Kim MJ, Kim M,
Bae ON and Baek SH: Autophagy sustains the survival of human
pancreatic cancer PANC-1 cells under extreme nutrient deprivation
conditions. Biochem Biophys Res Commun. 463:205–210. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Murphy MP and Smith RA: Targeting
antioxidants to mitochondria by conjugation to lipophilic cations.
Annu Rev Pharmacol Toxicol. 47:629–656. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hardie DG, Ross FA and Hawley SA: AMPK: A
nutrient and energy sensor that maintains energy homeostasis. Nat
Rev Mol Cell Biology. 13:251–262. 2012. View Article : Google Scholar
|
|
37
|
Heerlein K, Schulze A, Hotz L, Bärtsch P
and Mairbäurl H: Hypoxia decreases cellular ATP demand and inhibits
mitochondrial respiration of a549 cells. Am J Respir Cell Mol Biol.
32:44–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Owada S, Shimoda Y, Tsuchihara K and Esumi
H: Critical role of H2O2 generated by NOX4
during cellular response under glucose deprivation. PLoS One.
8:e566282013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nogueira V and Hay N: Molecular pathways:
Reactive oxygen species homeostasis in cancer cells and
implications for cancer therapy. Clin Cancer Res. 19:4309–4314.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chandel NS, McClintock DS, Feliciano CE,
Wood TM, Melendez JA, Rodriguez AM and Schumacker PT: Reactive
oxygen species generated at mitochondrial complex III stabilize
hypoxia-inducible factor-1alpha during hypoxia: A mechanism of
O2 sensing. J Biol Chem. 275:25130–25138. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Diebold I, Petry A, Hess J and Görlach A:
The NADPH oxidase subunit NOX4 is a new target gene of the
hypoxia-inducible factor-1. Mol Biol Cell. 21:2087–2096. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mansfield KD, Simon MC and Keith B:
Hypoxic reduction in cellular glutathione levels requires
mitochondrial reactive oxygen species. J Appl Physiol (1985).
97:1358–1366. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hirayama A, Kami K, Sugimoto M, Sugawara
M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, et
al: Quantitative metabolome profiling of colon and stomach cancer
microenvironment by capillary electrophoresis time-of-flight mass
spectrometry. Cancer Res. 69:4918–4925. 2009. View Article : Google Scholar : PubMed/NCBI
|