Introduction
Pancreatic cancer, which is dominated by pancreatic
ductal adenocarcinoma (PDAC), is currently the fourth leading cause
of cancer-related mortality in developed countries, with a 5-year
survival rate <8% (1). The
initiation and progression of pancreatic cancer are always
associated with non-specific symptoms. At diagnosis, most patients
have developed to an advanced stage with local invasion and distant
metastasis that preclude surgical resection of pancreatic cancer
due to the lack of early diagnostic procedures (2). More than 80% of patients miss the
opportunity for complete surgical eradication, and even for those
who undergo surgery, the 5-year survival rate is still ~25%
(3). Gemcitabine has been regarded
as the mainstay of chemotherapy for pancreatic cancer. In recent
years, gemcitabine combined with FOLFIRINOX (folinic acid,
5-fluorouracil, irinotecan and oxaliplatin) or albumin-bound
paclitaxel has been introduced and has shown improved therapeutic
efficacy compared to gemcitabine monotherapy at the cost of serious
adverse effects (4,5).
Epithelial-to-mesenchymal transition (EMT) has been
considered as a major driver of the initiation and progression of
pancreatic cancer (6). The process
of EMT is associated with elevated expression of N-cadherin and
vimentin as well as decreased expression of E-cadherin (7). The EMT process could be triggered by a
number of transcriptional factors, including Snail and Slug, ZEB1,
ZEB2 and Twist (6,8). A previous study showed that deletion
of Zeb1, one of the EMT transcriptional factors, reduced the
stemness, tumorigenic and colonization capacities of cancer cells;
the deletion of Zeb1 suppressed the grading, invasion and distant
metastasis of pancreatic cancer in vivo (9). Further research revealed that the
transcriptional factor Snail- or Twist-induced EMT program is of
great importance in mediating the chemoresistance of pancreatic
cancer to gemcitabine (10), which
is in accordance with previous evidence that EMT is implicated in
stemness and treatment resistance (6).
Members of the transforming growth factor-β (TGF-β)
family play important roles in the growth, differentiation and
apoptosis of cells, and they exert their functions during the
process of embryonic development (11). Previous studies have revealed that
pancreatic cancer expresses high levels of TGF-β, and it correlates
with poor prognosis (12,13). Smad2 and Smad3, two members of the
Smad family, mediate the signal transduction of TGF-β. Binding of
TGF-β with transmembrane serine-threonine kinase receptors leads to
the phosphorylation and activation of downstream Smad2 and Smad3,
which then translocate to the nucleus to control the transcription
of target genes (11).
Itraconazole (ITZ) is a type of triazole anti-fungal
agent that is widely applied clinically in the treatment and
prevention of fungal infections. Recently, ITZ has been found to
have great potential in the treatment of several types of cancers
(14–17). A non-comparative, randomized, phase
II study showed that high-dose ITZ exhibited anticancer properties
in patients with metastatic castration-resistant prostate cancer
(18). Another phase II study that
enrolled 23 patients with progressive non-squamous non-small cell
lung cancer found that ITZ combined with pemetrexed achieved longer
progression-free survival and significantly prolonged overall
survival compared to treatment with pemetrexed alone (19). In addition, ITZ also showed
therapeutic efficacy in basal cell carcinoma by reducing cell
proliferation and tumor size (20).
In addition, numerous preclinical studies have been conducted to
investigate the therapeutic effect of ITZ in other types of
cancers, including ovarian cancer (21), endometrial cancer (22), gastrointestinal cancer (23) and bladder cancer (17).
However, whether ITZ has antitumor effects on
pancreatic cancer cells and the underlying mechanisms remain to be
elucidated. In the present study, we investigated the effects of
ITZ on the biological behavior of pancreatic cancer cells and the
potential underlying molecular mechanisms.
Materials and methods
Cell culture and reagents
We purchased human MiaPaCa-2, Panc-1, and BxPC-3
tumor cells from the Chinese Academy of Sciences Cell Bank of Type
Culture Collection (CBTCCCAS, Shanghai, China). The MiaPaCa-2 and
Panc-1 cell lines were cultured in Dulbecco's modified Eagle's
medium (DMEM) with 10% fetal bovine serum (FBS) (Hyclone, Logan,
UT, USA), and BxPC-3 was cultured in RPMI-1640 supplemented with
10% FBS and 1% penicillin-streptomycin. The pancreatic cancer cell
lines were cultured under standard conditions at 37°C with a 5%
CO2 atmosphere. The antibodies against E-cadherin,
N-cadherin, and vimentin were purchased from Cell Signaling
Technology (Danvers, MA, USA); the antibody against β-actin was
obtained from Sigma (St. Louis, MO, USA); and the antibodies
against TGF-β and p-SMAD2/3 were obtained from Abcam (Cambridge,
MA, USA). ITZ was initially dissolved in DMSO (dimethyl sulfoxide)
at a stock concentration of 2 mM. Working dilutions of ITZ were
made in culture medium immediately before use.
Cell viability assays
The viability of pancreatic cancer cells was
measured using
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
(MTT) (Sigma) assays. Panc-1, BxPC-3 and MiaPaCa-2 cells were
seeded onto 96-well plates at a density of 5,000 cells per well and
incubated overnight in 10% FBS medium. After treatment with ITZ for
24, 48 and 72 h at 37°C and 5% CO2, the inhibition rate
of the pancreatic cancer cells was assessed. Briefly, 20 µl of MTT
solution (5 mg/ml in distilled water) was added to each well, and
the cells were incubated for 4 h at 37°C for the absorption of MTT;
then, the medium was removed and replaced by 200 µl of DMSO, and
the optical density (OD) was measured at 490 nm on a
multifunctional microplate reader (POLARstar OPTIMA; BMG,
Offenburg, Germany).
Colony formation assays
Panc-1 and BxPC-3 cells were seeded onto 35-mm petri
dishes (1,000 cells per dish) and allowed to adhere overnight.
Then, cells were treated with different concentrations of ITZ and
further cultured for 2 weeks to allow colonies to form. At the
indicated time point, colonies were fixed with 4% paraformaldehyde
for 2 h, stained with 0.1% crystal violet solution and rinsed. The
colonies >0.5 mm in diameter were counted using a microscope
(Nikon Eclipse Ti-S; Nikon Instruments, Inc., Tokyo, Japan) at a
magnification, ×400.
Apoptosis assays
We assessed apoptosis of Panc-1 and BxPC-3 cells
using an Annexin V-FITC/PI apoptosis detection kit (Beyotime
Institute of Biotechnology, Shanghai, China) according to the
manufacturer's instructions. Pancreatic cancer cells
(105 cells/well) were seeded onto 6-well plates and
pretreated with FBS-free DMEM or RPMI-1640 for 8 h; then, the
medium was replaced with fresh medium containing varying
concentrations of ITZ. A total of 48 h later, the cancer cells were
trypsinized, washed with PBS and then stained with Annexin V and
propidium iodide in the dark. The percentage of apoptotic cells was
quantified by flow cytometry.
Western blot assays
Whole-cell lysates of Panc-1 and BxPC-3 cells were
prepared using RIPA buffer (Beyotime, Guangzhou, China) according
to the manufacturer's instructions. Protein concentration was
determined using a BCA protein assay kit (Pierce, Rockford, IL,
USA). The protein lysates were resolved on a 10% polyacrylamide gel
with a 5% stacking gel. The proteins were subsequently blotted onto
polyvinylidene difluoride (PVDF) membranes. The membranes were
blocked for 2 h in TBS containing 0.1% (vol/vol) Tween-20 and 10%
(wt/vol) non-fat dry milk powder and then incubated with primary
antibodies overnight at 4°C. Following incubation with secondary
HRP-coupled antibodies for 2 h at room temperature, the membranes
were washed with 0.1% TBS-Tween-20, and the immunocomplexes were
detected using the enhanced chemiluminescence (ECL) kit and a
Molecular Imager ChemiDoc XRS system (Bio-Rad Laboratories,
Hercules, CA, USA). β-actin was used as an internal loading
control.
Immunofluorescence analysis
The cells were fixed in 4% formaldehyde diluted in
phosphate-buffered saline (PBS) for 20 min. Following
permeabilization with 0.3% Triton X-100, the cells were treated
with blocking buffer (5% BSA in PBS) for 1 h and then incubated
with a primary antibody at 4°C overnight. Green conjugated
secondary antibodies from Jackson ImmunoResearch Laboratories (West
Grove, PA, USA) were applied at room temperature, and the nuclei
were stained with 4′-6-diamidino-2-phenylindole (DAPI). Images were
pseudocolored using a Zeiss Instruments confocal microscope.
Cell invasion assays
Matrigel invasion assays were performed as
previously described to assess the invasive viability of pancreatic
cancer cells (24). In brief, the
upper chambers of the wells were coated with Matrigel (BD
Biosciences, Franklin Lakes, NJ, USA). The tumor cells
(105) were suspended in serum-free DMEM and seeded onto
the upper chamber. The cells were allowed to invade toward the
lower chamber, which contained DMEM with 10% FBS, for 48 h and were
then fixed with 4% formaldehyde, followed by the removal of
non-invasive cells in the upper chamber with a cotton swab. The
invading cells were stained with 0.1% crystal violet. The number of
invaded cells was quantified by counting the stained cells under a
microscope.
Wound healing assays
The migration ability of the cancer cells was
assessed by wound-healing assays. The cancer cells were pretreated
as indicated, and then, the cells were scratched using a 10-µl
pipette tip. Next, the cancer cells were washed 3 times with PBS
and cultured in serum-starved medium. Images were obtained under a
microscope (Nikon Instruments, Inc.).
In vivo study
LSL-KrasG12D/+; p53fl/+;
Pdx1-Cre (KPC) mice were used to study the therapeutic effect of
ITZ in vivo. The breeding of KPC mice was achieved by
crossing the Pdx1-Cre mice with LSL-KrasG12D mice and
p53fl/fl mice (purchased from the Nanjing Biomedical
Research Institute of Nanjing University, Nanjing, China). All mice
were housed under pathogen-free conditions and had free access to
water and food. All experimental protocols were approved by the
Ethics Committee of the First Affiliated Hospital of Medical
College, Xi'an Jiaotong University (Xi'an, China).
Statistical analysis
Each experiment was performed at least three times.
The data are presented as the mean ± standard deviation.
Comparisons between groups were analyzed by Student's t-test using
SPSS (version 15.0; SPSS, Inc., Chicago, IL, USA). P-values
<0.05 were considered to be statistically significant.
Results
ITZ inhibits the viability of
pancreatic cancer cells in a concentration-dependent manner
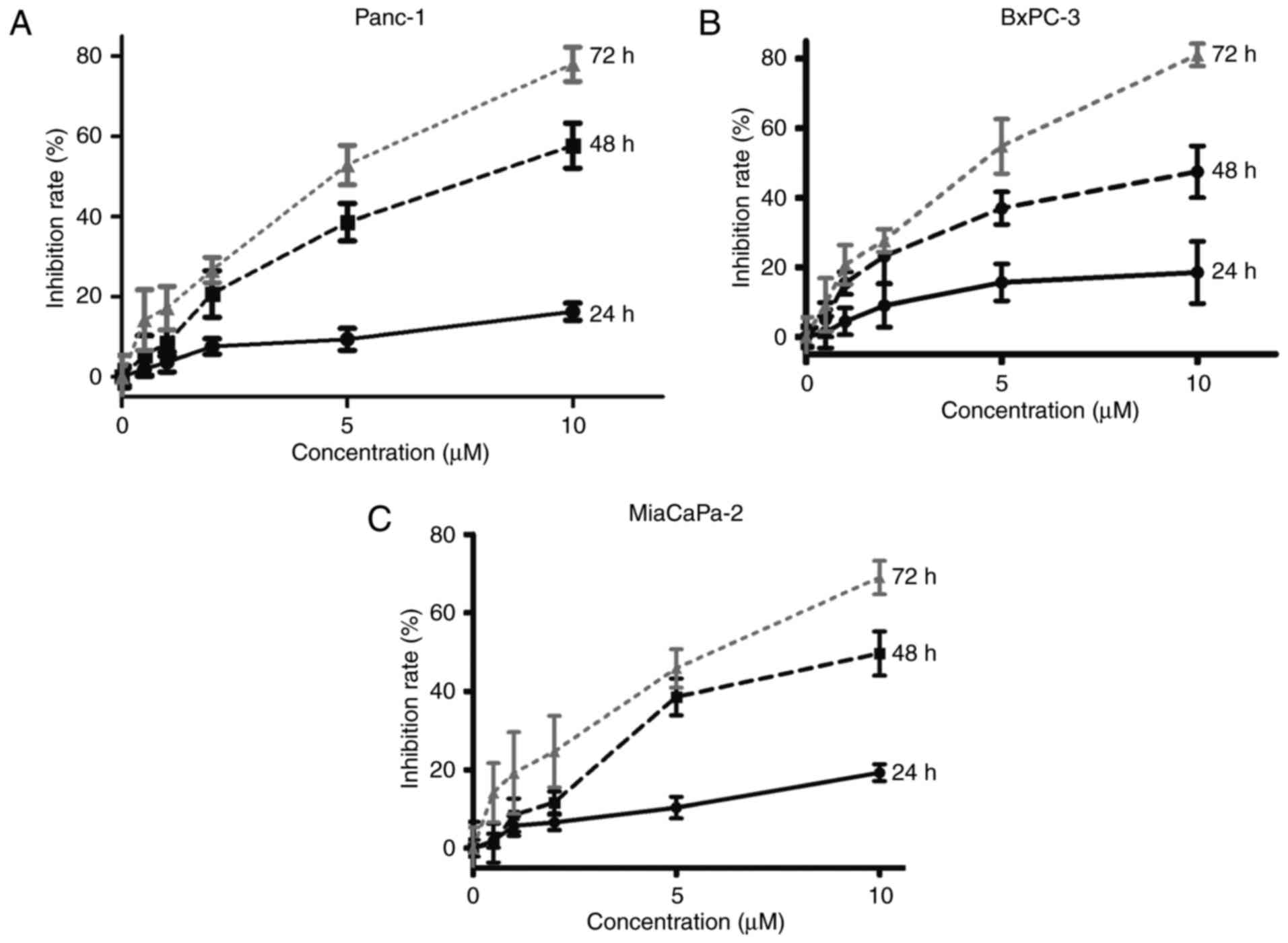
We first investigated the effect of ITZ on the
viability of pancreatic cancer cells. Pancreatic cancer cell lines
Panc-1, BxPC-3 and MiaPaCa-2 were treated with increasing
concentrations of ITZ (0, 0.5, 1, 2, 5 and 10 µM). The viability of
pancreatic cancer cell lines was assessed by MTT assays at the
indicated time points (24, 48 and 72 h). Our results showed that
ITZ inhibited the viability of pancreatic cancer cell lines in a
dose- and time-dependent manner (Fig.
1). We observed that ITZ at a concentration of 2 µM began to
suppress the viability of pancreatic cancer, and 5 µM ITZ showed a
sufficient effect. A previous study that involved thirty healthy
men revealed that successive intake of ITZ every 12 h for 14 days
produced a steady plasma concentration of 2.67 µM (25). According to the results of the
clinical study and the MTT assay, the concentrations of 2 and 5 µM
were chosen for further experiments.
ITZ inhibits colony formation and
induces apoptosis in pancreatic cancer cells
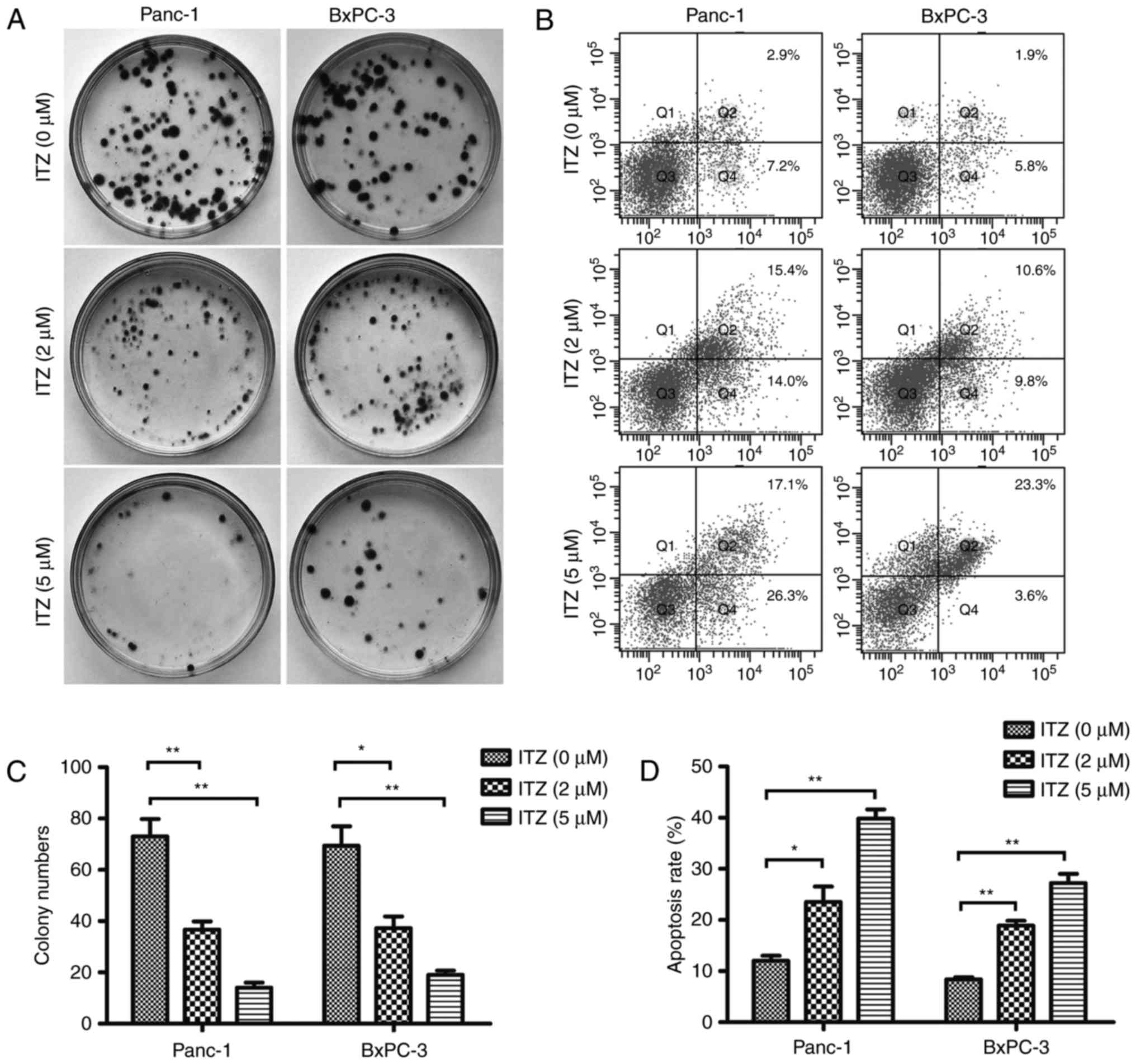
To further investigate the therapeutic effect of ITZ
in pancreatic cancer, we set out to detect the effect of ITZ on the
clone formation capability of Panc-1 and BxPC-3 cell lines. Panc-1
and BxPC-3 cells were seeded onto 35-mm Petri dishes and allowed to
adhere overnight; then, the cells were treated with 2 or 5 µM ITZ
for 48 h. Cancer cells were cultured for two weeks, and the number
of colonies were calculated. As shown in Fig. 2A, we found that ITZ at a
concentration of 2 µM significantly suppressed colony formation in
the Panc-1 and BxPC-3 cell lines. In the dishes that were treated
with 5 µM of ITZ, few colonies were observed (Fig. 2C).
Next, flow cytometric analyses were performed to
evaluate whether ITZ could induce apoptosis in pancreatic cancer
cells. Panc-1 and BxPC-3 cells were pretreated with FBS-free DMEM
or FBS-free RPMI-1640 for 12 h and then treated with 2 or 5 µM ITZ
for 48 h. We found that compared to the vehicle-treated cells,
cells treated with ITZ showed an increased percentage of apoptotic
cells (Fig. 2B and D). These
results revealed the role of ITZ in inhibiting colony formation and
inducing pancreatic cancer cell apoptosis.
ITZ inhibits the invasion and
migration of pancreatic cancer cells
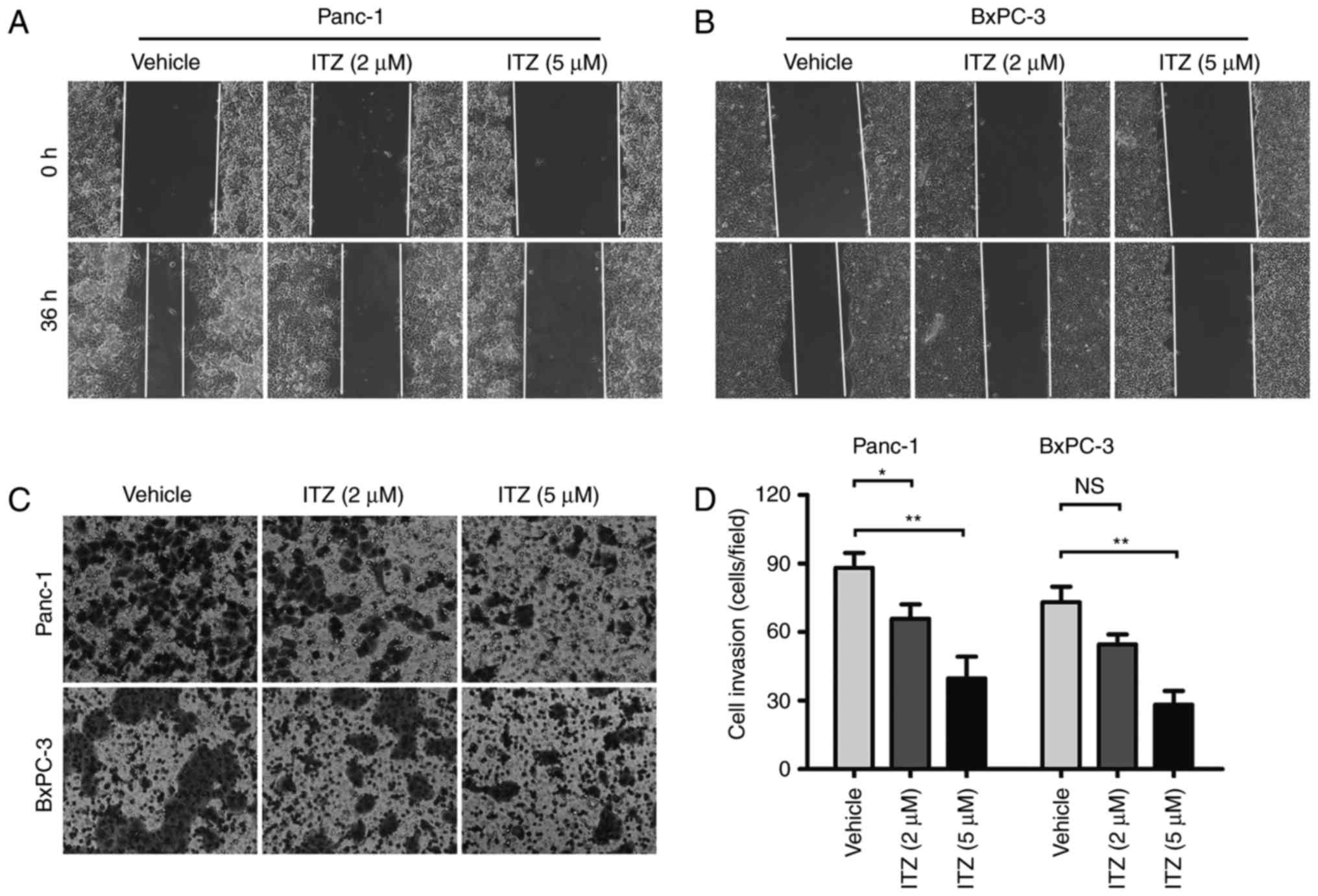
Invasion and metastasis are important
characteristics of pancreatic cancer, and most patients with
pancreatic cancer have already developed to advanced stages with
complications involving distant metastasis at the time of diagnosis
(5). Thus, we explored the effect
of ITZ on the invasion and migration of pancreatic cancer cells.
After starvation in FBS-free DMEM or FBS-free RPMI-1640 for 12 h,
Panc-1 and BxPC-3 cells were treated with 2 µM or 5 µM ITZ for 48
h. The wound healing assays were performed under serum-free
conditions to evaluate the migration of cancer cells. We found that
the migration abilities of Panc-1 and BxPC-3 cells were impaired by
ITZ intervention compared to vehicle-treated cells (Fig. 3A and B).
To investigate the invasive ability of cancer cells
after ITZ intervention, Matrigel invasion assays were conducted. We
found that ITZ at the concentration of 2 µM significantly inhibited
the invasion of Panc-1 cells; at the concentration of 5 µM, the
invasive abilities of Panc-1 and BxPC-3 were both suppressed
(Fig. 3C and D). These findings
suggest that ITZ inhibits the invasion and migration capacities of
pancreatic cancer cells in vitro.
ITZ inhibits EMT and TGF-β/SMAD2/3
signaling in pancreatic cancer cells
EMT is a complicated program that plays an important
role in the invasion and metastasis of cancer (26,27).
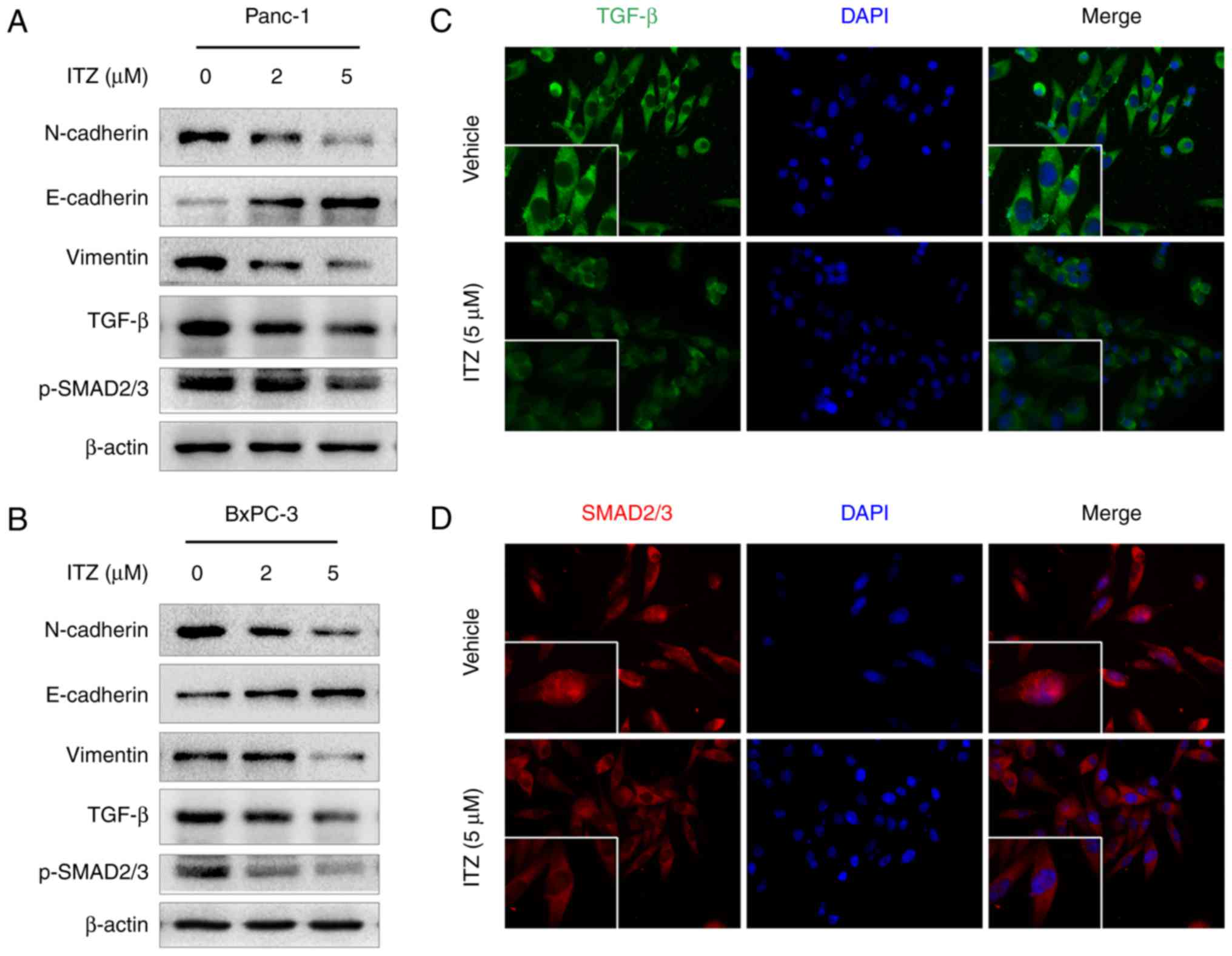
We then investigated whether ITZ could affect the EMT process. We
found that in Panc-1 cells, ITZ treatment (2 or 5 µM) decreased the
expression of the mesenchymal markers, N-cadherin and vimentin.
However, expression of the epithelial marker, E-cadherin, was
elevated (Fig. 4A). A similar
effect was also observed regarding EMT in BxPC-3 cells (Fig. 4B).
Given previous evidence that TGF-β is involved in
EMT as well as the invasion and migration of cancer cells (28,29),
we set out to investigate the effect of ITZ on TGF-β/SMAD2/3
signaling. Western blotting was performed to detect the expression
of TGF-β and p-SMAD2/3. As expected, we observed high expression of
TGF-β and p-SMAD2/3 in Panc-1 and BxPC-1 cells. However, compared
to vehicle-treated cells, the expression of TGF-β was downregulated
after ITZ treatment. Additionally, the level of p-SMAD2/3 was also
decreased (Fig. 4A and B). Binding
of TGF-β with its membrane-bound receptor leads to phosphorylation
of the SMAD family. The latter, in turn, translocates into the
nucleus and acts as transcriptional factors to exert their
functions (30). Then, we conducted
immunofluorescence assays to study the effect of ITZ on
TGF-β/SMAD2/3 signaling. We observed that ITZ treatment decreased
the level of TGF-β and suppressed the nuclear accumulation of
SMAD2/3 (Fig. 4C and D). These data
suggest that ITZ treatment inhibits the EMT process and
TGF-β/SMAD2/3 signaling in pancreatic cancer.
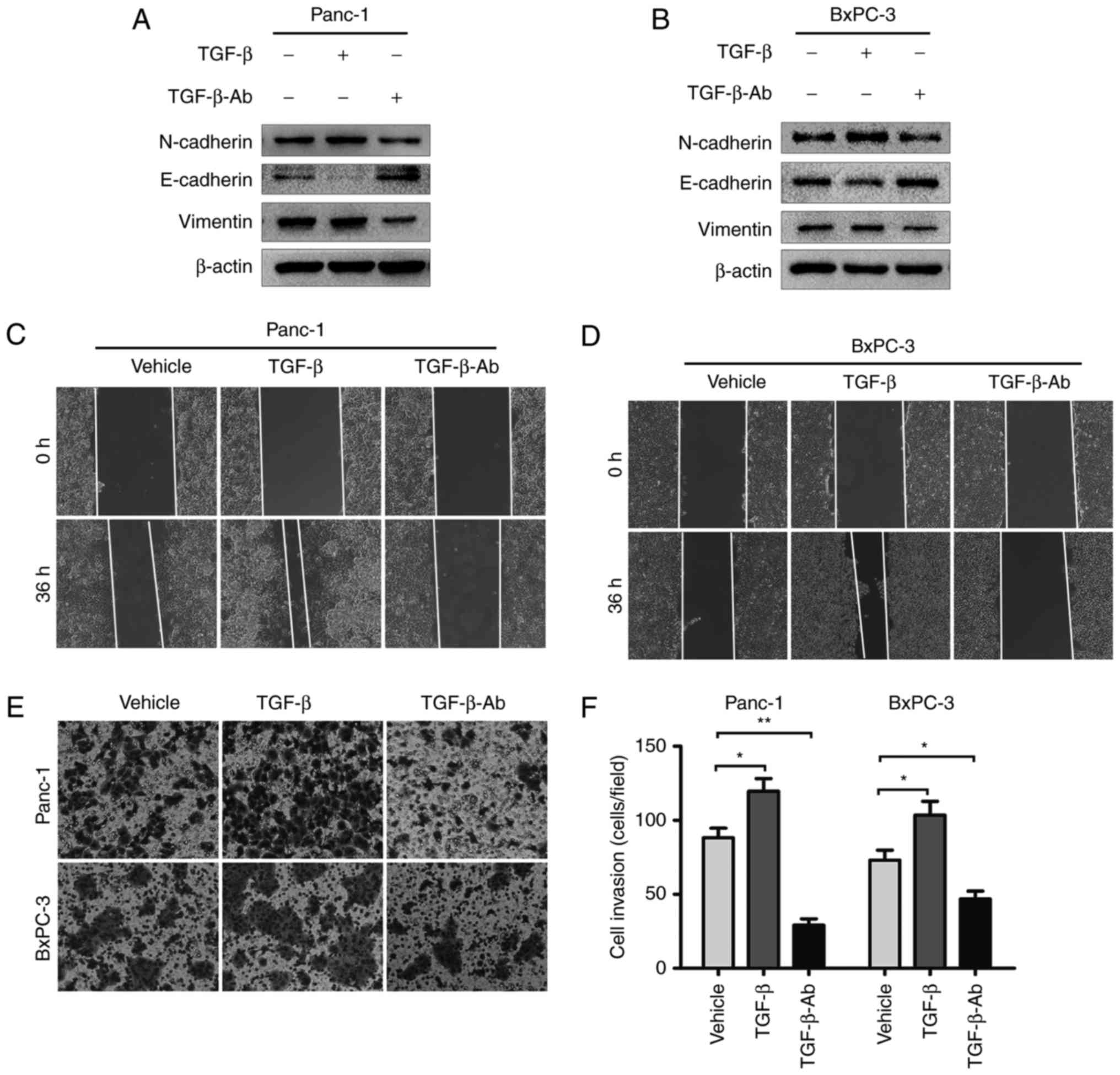
TGF-β is a potent factor mediating EMT
in pancreatic cancer cells
To investigate whether the effect of ITZ on EMT is
mediated by impaired TGF-β/SMAD2/3 signaling, we reassessed the
effect of TGF-β/SMAD2/3 signaling on EMT in pancreatic cancer
cells. After starvation in FBS-free medium for 8 h, Panc-1 and
BxPC-3 cells were treated with recombinant TGF-β1 (5 ng/ml) for 24
h, and then, the EMT markers were detected by western blot assays.
Recombinant TGF-β1 significantly suppressed the expression of
E-cadherin and elevated the expression of N-cadherin and vimentin
in Panc-1 and BxPC-3 cells (Fig. 5A and
B). The wound healing assays showed that treatment with
recombinant TGF-β1 promoted the migration of cancer cells (Fig. 5C and D). Accordingly, the Matrigel
invasion assays showed that the invasive capability of cancer cells
was also increased by recombinant TGF-β1 treatment (Fig. 5E and F).
Next, we set out to observe the effect of TGF-β1
deprivation on the invasion and migration of pancreatic cancer.
TGF-β1 neutralizing antibody was used to block the function of
TGF-β1. As expected, treatment with TGF-β1 neutralizing antibody
elevated the expression of E-cadherin and decreased the levels of
N-cadherin and vimentin compared to vehicle-treated cancer cells
(Fig. 5A and B). The wound healing
and Matrigel invasion assays also showed that TGF-β1 neutralizing
antibody inhibited the invasion and migration of Panc-1 and BxPC-3
cells (Fig. 5C-F). We revealed that
TGF-β is a potent factor that mediates EMT, invasion and migration
in pancreatic cancer cells.
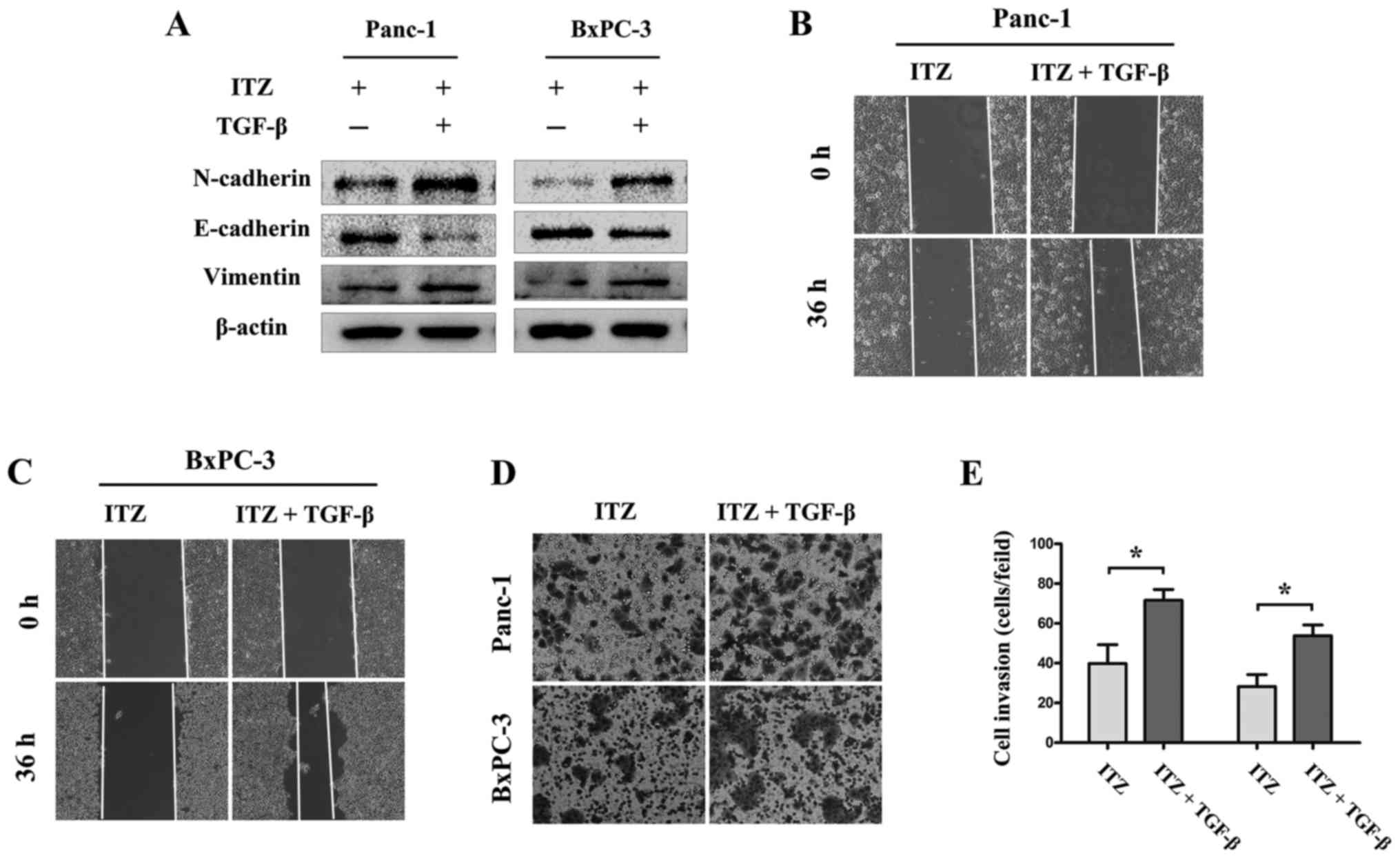
TGF-β/SMAD2/3 signaling is required
for the effects of ITZ on pancreatic cancer
Given the results that ITZ inhibited TGF-β/SMAD2/3
signaling as well as the invasion and migration of pancreatic
cancer cells, we wondered whether TGF-β/SMAD2/3 signaling is
required for ITZ to exert its anticancer effects on pancreatic
cancer. To verify our hypothesis, Panc-1 and BxPC-3 cells were
pretreated with ITZ (5 µM) for 24 h, and then, the cells were
treated with recombinant TGF-β1 (5 ng/ml). Western blot assays
showed that recombinant TGF-β1 recovered the expression of
N-cadherin and vimentin, both of which were suppressed by ITZ
treatment. Accordingly, recombinant TGF-β1 treatment inhibited the
ITZ-induced expression of E-cadherin (Fig. 6A and B). In addition, the invasion
and migration capabilities of Panc-1 and BxPC-3 cells were also
recovered (Fig. B-E). These results showed that recombinant TGF-β1
reversed the effect of ITZ on pancreatic cancer. We revealed that
TGF-β/SMAD2/3 signaling mediates the effects of ITZ on pancreatic
cancer.
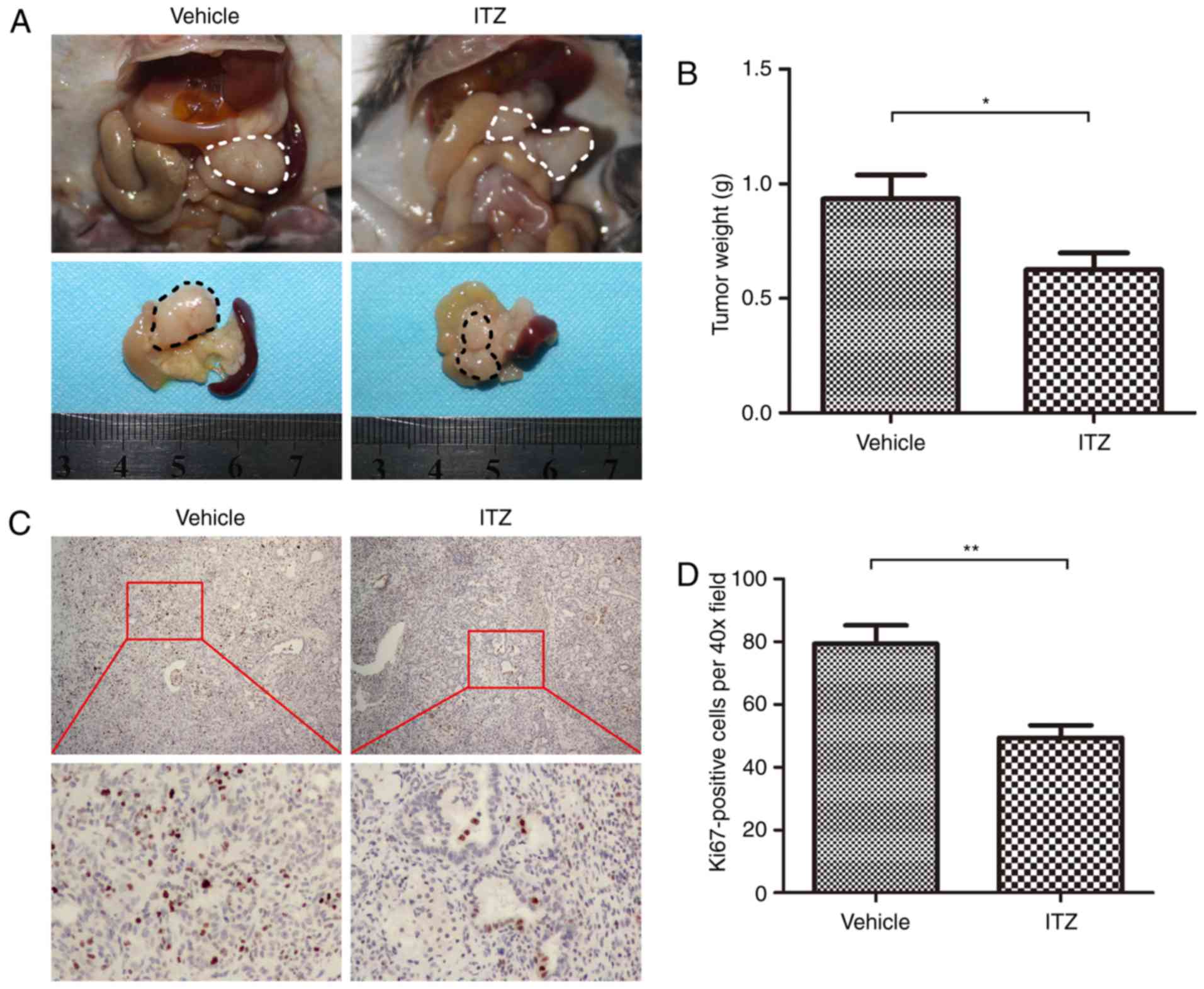
ITZ suppresses growth of pancreatic
cancer in vivo
To further investigate the therapeutic effect of ITZ
in vivo, we used KPC transgenic mice harboring spontaneous
pancreatic cancer. Starting at 8 weeks of age, when KPC mice have
developed widespread advanced pancreatic neoplasia, we treated the
KPC mice with vehicle or ITZ. KPC mice were sacrificed when
end-staged disease developed. We found that ITZ treatment
significantly decreased the tumor weight (Fig. 7A and B). Next, immunohistochemistry
was performed to detect the proliferation index, Ki67. We found
that treatment with ITZ decreased the number of Ki67-positive cells
(Fig. 7C and D). These results
suggest the therapeutic effect of ITZ in vivo.
Discussion
Pancreatic cancer is the major cause of
cancer-associated mortality, with dismal prognosis, invasion and
distant metastasis as some of its dominant features. The treatment
of pancreatic cancer remains a challenge because of the lack of
techniques for early diagnosis and the resistance to traditional
chemotherapeutic drugs (2).
Therefore, new drug candidates are needed for pancreatic cancer.
ITZ is a broad-spectrum anti-fungal agent that exerts its effect
through the decrease of ergosterol synthesis, which is required for
fungal cell membrane integrity (14). Accumulating evidence suggests that
ITZ exhibits anticancer activity against various types of cancers,
and therefore, we hypothesized that ITZ may be a promising
potential drug in the treatment of pancreatic cancer. Here, we
found that ITZ inhibits the viability of pancreatic cancer cell
lines. Treatment with ITZ significantly reduced colony formation
and induced apoptosis in Panc-1 and BxPC-3 cells. We noted that ITZ
treatment impaired the invasion and migration abilities of
pancreatic cancer cells. In addition, ITZ suppressed EMT in
pancreatic cancer cells, partly through the inhibition of
TGF-β/SMAD2/3 signaling. Furthermore, ITZ suppressed the growth of
pancreatic cancer in vivo.
TGF-β/SMAD2/3 signaling plays vital roles in the
progression of pancreatic cancer. A previous study reported that in
genetically engineered mice harboring spontaneous pancreatic
cancer, haploinsufficiency for the Tgfbr2 gene, which encodes the
receptor for TGF-β, achieved a higher frequency of
well-differentiated pancreatic cancer. In addition,
haploinsufficiency for Tgfbr2 also reduced hematogenous metastasis
and suppressed the colonization of cancer cells into the liver
(31), indicating the role of
TGF-β/SMAD2/3 signaling in the distant metastasis of pancreatic
cancer. TGF-β is also involved in the establishment of the
immunosuppressive microenvironment of cancer, in part through
inducing the synthesis and secretion of immunosuppressive
cytokines, such as IL-2, IL-6 and IL-17; on the other hand, it
promotes the induction of CD8(+) regulatory T cells, which
contribute to cancer progression and immune evasion (32,33).
TGF-β silencing combined with immune activation induced profound
tumor cell apoptosis, which is mediated by the recruitment of
activated CD8-positive T cells and the reduced infiltration of
CD11b(+) Gr-1(−) myeloid cells into the tumor (34). Here, we report that TGF-β is
sufficient to induce the invasion and migration of pancreatic
cancer cells, and blocking TGF-β significantly inhibited the
invasion and migration abilities of cancer cells. We also revealed
that TGF-β is an important mediator of EMT in pancreatic cancer,
and we reconfirmed the cancer-promoting role of the TGF-β/SMAD2/3
signaling pathway in pancreatic cancer.
The anticancer activity of ITZ is mediated by
various mechanisms. Aftab, et al reported that in non-small
cell lung cancer, ITZ inhibited the proliferation, migration and
tube formation of endothelial cells in response to the induction of
the pro-angiogenic factors, including vascular endothelial growth
factor (VEGF) and basic fibroblast growth factor (bFGF);
additionally, oral ITZ significantly reduced tumor vascularity
in vivo (35). In addition,
inhibition of the Hedgehog pathway, which has been shown to
contribute to the growth and progression of many types of cancers,
is another important mechanism mediating the anticancer effect of
ITZ (36). A recent report also
revealed that ITZ-mediated Hedgehog pathway inhibition inhibited
proliferation as well as induced apoptosis and autophagic cell
death in breast cancer, thus inhibiting tumor growth in vivo
(37). Besides the Hedgehog
pathway, a recent study reported that ITZ exerts its anticancer
effect by impairing a regulatory network involving the Hedgehog,
Wnt, and PI3K/mTOR signaling pathways, thus inhibiting the growth
of melanoma and extending the survival of mice harboring melanoma
xenografts (38). Here, we found
that treatment with ITZ may interfere with TGF-β/SMAD2/3 signaling.
We revealed a novel mechanism by which ITZ suppresses the
progression of pancreatic cancer.
EMT enables cancer cells to invade the basement
membranes and spread to the vasculature or lymphatic ducts to form
distant metastases. Previous studies have reported that in
pancreatic cancer, EMT plays context-specific roles depending on
the different EMT-associated transcriptional factors; they reported
that genetic ablation of Snail or Twist contributes to enhanced
sensitivity to gemcitabine treatment without altering the incidence
of systemic dissemination and metastasis, but the transcriptional
factor Zeb1-induced EMT is associated with invasiveness and distant
metastasis (9,10). We found that ITZ is sufficient to
impair EMT. Accordingly, ITZ suppressed the invasion and migration
of pancreatic cancer.
For the treatment of pancreatic cancer, gemcitabine
combined with FOLFIRINOX (folinic acid, 5-fluorouracil, irinotecan
and oxaliplatin) or albumin-bound paclitaxel could act as the
first-line chemotherapy, and the two regimens achieved an overall
survival of 11.1 and 8.5 months, respectively, compared to the 6.8
months achieved by gemcitabine treatment alone (4,5).
However, for the patients who are resistant to the first-line
regimens, a more efficient second-line regimen is required. A
previous study reported that ITZ combined with other cytotoxic
chemotherapeutic drugs showed a promising effect in patients with
pancreatic cancer who had first received chemotherapy and had a
history of progression during or after prior treatment (39). ITZ in combination with a cytotoxic
regimen consisting of docetaxel, gemcitabine, and carboplatin (DGC)
achieved a median overall survival of 11.4 months. Among 38
patients, the authors observed one complete and 13 partial
responses (39). Here, our study
provides further evidence for the therapeutic efficacy of ITZ in
pancreatic cancer.
In conlusion, our results suggest that ITZ has
potent anticancer properties. ITZ was able to induce apoptosis,
inhibit cellular proliferation, suppress EMT and suppress the
invasion and migration of pancreatic cancer cells. As a drug that
has been tested for toxicity in humans and has been approved for
human use, ITZ shows great potential in the treatment of pancreatic
cancer.
Acknowledgements
This study was supported by grants from the National
Natural Science Foundation of China (nos. 81672434, 81472248 and
81402971).
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kleeff J, Korc M, Apte M, La Vecchia C,
Johnson CD, Biankin AV, Neale RE, Tempero M, Tuveson DA, Hruban RH,
et al: Pancreatic cancer. Nat Rev Dis Primers. 2:160222016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kamisawa T, Wood LD, Itoi T and Takaori K:
Pancreatic cancer. Lancet. 388:73–85. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Von Hoff DD, Ervin T, Arena FP, Chiorean
EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et
al: Increased survival in pancreatic cancer with nab-paclitaxel
plus gemcitabine. N Engl J Med. 369:1691–1703. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Conroy T, Desseigne F, Ychou M, Bouché O,
Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de
la Fouchardière C, et al Groupe Tumeurs Digestives of Unicancer, ;
PRODIGE Intergroup, : FOLFIRINOX versus gemcitabine for metastatic
pancreatic cancer. N Engl J Med. 364:1817–1825. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou P, Li B, Liu F, Zhang M, Wang Q, Liu
Y, Yao Y and Li D: The epithelial to mesenchymal transition (EMT)
and cancer stem cells: Implication for treatment resistance in
pancreatic cancer. Mol Cancer. 16:522017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sánchez-Tilló E, Liu Y, de Barrios O,
Siles L, Fanlo L, Cuatrecasas M, Darling DS, Dean DC, Castells A
and Postigo A: EMT-activating transcription factors in cancer:
Beyond EMT and tumor invasiveness. Cell Mol Life Sci. 69:3429–3456.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krebs AM, Mitschke J, Lasierra Losada M,
Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D,
Reichardt W, Bronsert P, et al: The EMT-activator Zeb1 is a key
factor for cell plasticity and promotes metastasis in pancreatic
cancer. Nat Cell Biol. 19:518–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh P, Wig JD and Srinivasan R: The Smad
family and its role in pancreatic cancer. Indian J Cancer.
48:351–360. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Friess H, Yamanaka Y, Büchler M, Ebert M,
Beger HG, Gold LI and Korc M: Enhanced expression of transforming
growth factor beta isoforms in pancreatic cancer correlates with
decreased survival. Gastroenterology. 105:1846–1856. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Javle M, Li Y, Tan D, Dong X, Chang P, Kar
S and Li D: Biomarkers of TGF-β signaling pathway and prognosis of
pancreatic cancer. PLoS One. 9:e859422014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pantziarka P, Sukhatme V, Bouche G, Meheus
L and Sukhatme VP: Repurposing Drugs in Oncology
(ReDO)-itraconazole as an anti-cancer agent. E Cancer Med Sci.
9:5212015.
|
|
15
|
Hu Q, Hou YC, Huang J, Fang JY and Xiong
H: Itraconazole induces apoptosis and cell cycle arrest via
inhibiting Hedgehog signaling in gastric cancer cells. J Exp Clin
Cancer Res. 36:502017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsubamoto H, Inoue K, Sakata K, Ueda T,
Takeyama R, Shibahara H and Sonoda T: Itraconazole inhibits
AKT/mTOR signaling and proliferation in endometrial cancer cells.
Anticancer Res. 37:515–519. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mamtani R, Yang YX, Scott FI, Lewis JD and
Boursi B: Association of itraconazole, a Hedgehog inhibitor, and
bladder Cancer. J Urol. 196:343–348. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antonarakis ES, Heath EI, Smith DC,
Rathkopf D, Blackford AL, Danila DC, King S, Frost A, Ajiboye AS,
Zhao M, et al: Repurposing itraconazole as a treatment for advanced
prostate cancer: A noncomparative randomized phase II trial in men
with metastatic castration-resistant prostate cancer. Oncologist.
18:163–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rudin CM, Brahmer JR, Juergens RA, Hann
CL, Ettinger DS, Sebree R, Smith R, Aftab BT, Huang P and Liu JO:
Phase 2 study of pemetrexed and itraconazole as second-line therapy
for metastatic nonsquamous non-small-cell lung cancer. J Thorac
Oncol. 8:619–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim DJ, Kim J, Spaunhurst K, Montoya J,
Khodosh R, Chandra K, Fu T, Gilliam A, Molgo M, Beachy PA, et al:
Open-label, exploratory phase II trial of oral itraconazole for the
treatment of basal cell carcinoma. J Clin Oncol. 32:745–751. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsubamoto H, Sonoda T, Yamasaki M and
Inoue K: Impact of combination chemotherapy with itraconazole on
survival of patients with refractory ovarian cancer. Anticancer
Res. 34:2481–2487. 2014.PubMed/NCBI
|
|
22
|
Inoue K, Tsubamoto H, Sakata K, Sakane R,
Hao H, Hirota S, Sonoda T and Shibahara H: Expression of Hedgehog
signals and growth inhibition by itraconazole in endometrial
cancer. Anticancer Res. 36:149–153. 2016.PubMed/NCBI
|
|
23
|
Hara M, Nagasaki T, Shiga K and Takeyama
H: Suppression of cancer-associated fibroblasts and endothelial
cells by itraconazole in Bevacizumab-resistant gastrointestinal
cancer. Anticancer Res. 36:169–177. 2016.PubMed/NCBI
|
|
24
|
Zhang D, Lei J, Ma J, Chen X, Sheng L,
Jiang Z, Nan L, Xu Q, Duan W, Wang Z, et al: β2-adrenogenic
signaling regulates NNK-induced pancreatic cancer progression via
upregulation of HIF-1α. Oncotarget. 7:17760–17772. 2016.PubMed/NCBI
|
|
25
|
Barone JA, Moskovitz BL, Guarnieri J,
Hassell AE, Colaizzi JL, Bierman RH and Jessen L: Food interaction
and steady-state pharmacokinetics of itraconazole oral solution in
healthy volunteers. Pharmacotherapy. 18:295–301. 1998.PubMed/NCBI
|
|
26
|
Guo J, Wang B, Fu Z, Wei J and Lu W:
Hypoxic microenvironment induces EMT and upgrades stem-like
properties of gastric cancer cells. Technol Cancer Res Treat.
15:60–68. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Carstens JL, Lovisa S and Kalluri R:
Microenvironment-dependent cues trigger miRNA-regulated feedback
loop to facilitate the EMT/MET switch. J Clin Invest.
124:1458–1460. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yin T, Wang C, Liu T, Zhao G and Zhou F:
Implication of EMT induced by TGF-beta1 in pancreatic cancer. J
Huazhong Univ Sci Technolog Med Sci. 26:700–702. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kong B, Michalski CW, Hong X, Valkovskaya
N, Rieder S, Abiatari I, Streit S, Erkan M, Esposito I, Friess H,
et al: AZGP1 is a tumor suppressor in pancreatic cancer inducing
mesenchymal-to-epithelial transdifferentiation by inhibiting
TGF-β-mediated ERK signaling. Oncogene. 29:5146–5158. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Javelaud D, Alexaki VI, Dennler S,
Mohammad KS, Guise TA and Mauviel A: TGF-β/SMAD/GLI2 signaling axis
in cancer progression and metastasis. Cancer Res. 71:5606–5610.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhong Y, Macgregor-Das A, Saunders T,
Whittle MC, Makohon-Moore A, Kohutek ZA, Poling J, Herbst BT,
Javier BM, Cope L, et al: Mutant p53 together with TGFβ signaling
influence organ-specific hematogenous colonization patterns of
pancreatic cancer. Clin Cancer Res. 23:1607–1620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou X, Mao Y, Zhu J, Meng F, Chen Q, Tao
L, Li R, Fu F, Liu C, Hu Y, et al: TGF-β1 promotes colorectal
cancer immune escape by elevating B7-H3 and B7-H4 via the
miR-155/miR-143 axis. Oncotarget. 7:67196–67211. 2016.PubMed/NCBI
|
|
33
|
Wu M, Chen X, Lou J, Zhang S, Zhang X,
Huang L, Sun R, Huang P, Wang F and Pan S: TGF-β1 contributes to
CD8+ Treg induction through p38 MAPK signaling in
ovarian cancer microenvironment. Oncotarget. 7:44534–44544.
2016.PubMed/NCBI
|
|
34
|
Ellermeier J, Wei J, Duewell P, Hoves S,
Stieg MR, Adunka T, Noerenberg D, Anders HJ, Mayr D, Poeck H, et
al: Therapeutic efficacy of bifunctional siRNA combining TGF-β1
silencing with RIG-I activation in pancreatic cancer. Cancer Res.
73:1709–1720. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Aftab BT, Dobromilskaya I, Liu JO and
Rudin CM: Itraconazole inhibits angiogenesis and tumor growth in
non-small cell lung cancer. Cancer Res. 71:6764–6772. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim J, Tang JY, Gong R, Kim J, Lee JJ,
Clemons KV, Chong CR, Chang KS, Fereshteh M, Gardner D, et al:
Itraconazole, a commonly used antifungal that inhibits Hedgehog
pathway activity and cancer growth. Cancer Cell. 17:388–399. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Wei S, Zhao Y, Shi C, Liu P, Zhang
C, Lei Y, Zhang B, Bai B, Huang Y, et al: Anti-proliferation of
breast cancer cells with itraconazole: Hedgehog pathway inhibition
induces apoptosis and autophagic cell death. Cancer Lett.
385:128–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liang G, Liu M, Wang Q, Shen Y, Mei H, Li
D and Liu W: Itraconazole exerts its anti-melanoma effect by
suppressing Hedgehog, Wnt, and PI3K/mTOR signaling pathways.
Oncotarget. 8:28510–28525. 2017.PubMed/NCBI
|
|
39
|
Tsubamoto H, Sonoda T, Ikuta S, Tani S,
Inoue K and Yamanaka N: Combination chemotherapy with itraconazole
for treating metastatic pancreatic cancer in the second-line or
additional setting. Anticancer Res. 35:4191–4196. 2015.PubMed/NCBI
|