Introduction
Esophageal cancer is one of the most aggressive
cancers worldwide. There are two main types of esophageal cancer:
esophageal squamous cell carcinoma (ESCC) and esophageal
adenocarcinoma (EAC) (1). In Asia,
ESCC is the predominant type and EAC remains rare (2). Treatment of ESCC has greatly relied
upon classical therapeutic means including surgery, radiotherapy or
chemotherapy. Although multimodality therapy can apparently improve
the prognosis of patients with ESCC, the overall 5-year survival
rate remains unsatisfactory. Therefore, seeking new potential
targets for the therapy of ESCC will help to improve the clinical
outcome of patients with ESCC (3),
which is reflecting our limited understanding on carcinogenesis and
metastasis of ESCC at the molecular and cellular levels. It is
urgent to find sensitive and specific early biomarkers for
diagnosis and prognosis of ESCC, as well as novel therapeutic
strategies.
The non-POU-domain-containing octamer (NONO) binding
protein, also called NONO, is involved in a variety of biological
processes, including regulating gene expression, DNA synthesis and
repair processes (4–6). Originally identified as an RNA-binding
protein (7), NONO interacts with
double-stranded DNA, single-stranded DNA and RNA and is involved in
multiple steps of gene transcription, RNA splicing and even
non-homologous DNA end joining (8).
Due to its roles in gene transcription, RNA processing and DNA
repairing, NONO may be implicated in cancer progression. It has
been demonstrated that NONO is associated with the initiation and
progression of human cancer. NONO protein expression is strongly
correlated with vascular invasions and poor patient survival of
bladder cancers (9). In fact,
increased NONO expression was reported in prostate cancer and
malignant melanoma (10,11). Knockdown NONO reduced melanoma cell
proliferation, promoted apoptosis and decreased mobility capacity.
Overexpression of NONO increased human umbilical vein endothelial
cells invasion (12). Chromosomal
translocation of NONO and TFE3 genes are frequently detected in
papillary rental cell carcinoma (13). Additionally, a previous study
indicates that NONO is an independent prognostic factor for human
neuroblastoma (14). One recent
study has identified that the nuclear protein NONO is highly
expressed in breast cancer tissues as compared with the adjacent
normal tissues in human patients. It is also demonstrated that
NONO-mediated regulation of breast cancer cell growth is dependent
on the presence of and interaction with SREBP-1 (15). Moreover, gene microarray studies
also reported that NONO mRNA levels are increased in ESCC (16,17).
However, validation of NONO protein expression in human ESCC
tissues has not been performed. The protein expression and
functional significance of NONO in ESCC are still unclear.
Materials and methods
Ethical approval
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. Collection of all tissues and data involving human
participants were approved by institutional ethics committee of
Beijing Friendship Hospital on human research.
Human tissue specimens and cell
lines
Human ESCC and adjacent normal esophageal tissues
were collected from 42 patients undergoing surgery of ESCC at the
Beijing Friendship Hospital (Beijing, China) between 2008 and 2012.
No patients had been treated with chemo- or radiation therapy
before surgery. Informed consent was obtained from each patient.
All tissues and data were collected in accordance with
institutional guidelines and approved by institutional ethics
committee of Beijing Friendship Hospital on human research. The
pathological tumor stage was assessed using the TNM system
according to the American Joint Committee on Cancer (AJCC) staging
manual (seventh edition) (18,19).
Human ESCC cell lines (TE-1, TE-2, TE-10, KYSE30, KYSE70, KYSE140,
KYSE450, EC109, EC9706) were generously provided by Cancer
Institute and Hospital, Chinese Academy of Medical Sciences and
cell authentication were reported (20,21).
Cells were maintained in RPMI-1640 medium containing 10% fetal
bovine serum, at 37°C in a 95% humidified incubator containing 5%
CO2.
Immunohistochemistry and pathological
scoring
Immunohistochemistry (IHC) followed the standard
protocol with NONO antibody (BD Biosciences, Bedford, MA, USA).
Slides were also counterstained with hematoxylin and mounted on
coverslips with glycerol-gelatin. Pathological scoring of NONO was
performed by the quickscore method (22). The percentage of stained cells (0–4,
5–19, 20–35, 40–59, 60–79 and 80–100%) was scored as 1–6, while the
staining intensity (no staining, low, moderate, and high) was
scored as 0–3. The quickscore of each tissue sample was calculated
by multiplying the score of percentage of positive cells with the
score of the staining intensity. Tumor samples with quickscore
>12 were recorded as strong, moderate (quickscore <12 and
>6), weak positive/negative (quickscore<6). Two doctors with
no prior knowledge of each patient's clinical information evaluated
all specimens independently.
siRNA and transfection
TE-1 and KYSE70 cells were plated at a density of
4×105 cells/well in 6-well plates. After 24 h of
culture, the cells were transfected with siRNA using
Lipofectamine® 2000 (Life Technologies, Grand Island,
NY, USA) according to the manufacturer's protocol as we previously
described. Small interfering RNA (siRNA) for NONO (siNONO) and
control siRNA (siCTRL) were purchased from GenePharma (Shanghai,
China). The siRNA sequences against NONO are
5′-CAGAGAAGCUGGUUAUAAAT-3′ and 5′-UUUAUAACCAGCUUCUCUGTT-3′. The
siRNA sequences against non-specific are
5′-UUCUCCGAACGUGUCACGUTT-3′ and 5′-ACGUGACACGUUCGGAGAATT-3′.
Real-time PCR
Total RNA was extracted from ESCC cell lines using
TRIzol (Life Technologies) as we previously described (23). Two micrograms of total RNA was
subjected to a random-primed reverse transcription using
SuperScript II reverse transcriptase (Life Technologies). Real-time
qPCR was conducted in triplicates using Applied Biosystems 7500
Fast with 5 ng of cDNA, 1 µM of each primer pair and SYBR Green PCR
master mix (Roche). The primers used were NONO forward,
5′-TTGTGGGAAATCTTCCTCCCGACA-3′ and reverse,
5′-GGGTTTCCAAGCGGATAAAGCCAA-3′. GAPDH forward,
5′-GGACCTGACCTGCCGTCTAGAA-3′ and reverse,
5′-GGTGTCGCTGTTGAAGTCAGAG-3′. Relative mRNA levels were normalized
to GAPDH.
Western blot analysis
Antibodies against Akt, phosphorylated Akt (p-Akt)
(Ser473), Erk, p-Erk1/2 (Thr202/Tyr204), caspase-3 and cleaved
PARP-1 were purchased from Cell Signaling Technology (Beverly, MA,
USA). The antibody against β-actin was purchased from
Sigma-Aldrich. Protein lysates were extracted by lysis buffer [50
mM Tris-HCl, pH 7.4; 10 mM EDTA; 5 mM EGTA; 0.5% NP40; 1% Triton
X-100 plus protease inhibitor (Roche)]. Fifty micrograms of total
protein were fractionated by SDS-polyacrylamide gel electrophoresis
and then transferred to PVDF membranes (Millipore, Billerica, MA,
USA). The membranes were probed with primary antibodies overnight
at 4°C and then incubated for 1 h with secondary
peroxidase-conjugated antibodies at room temperature, followed by
detection with an ECL plus system (Beyotime, Jiangsu, China).
β-actin was used as the loading control.
Cell proliferation assay
Cells were plated onto a 96-well plate at a density
of 1×103 cells per well. Viability of the cells was
measured using a CellTiter 96® Aqueous One Solution Cell
Proliferation assay (Promega, Madison, WI, USA) as described
previously with modifications (20). After 0, 24, 48 and 72 h, the optical
density at 492 nm was measured on a microplate reader (Smartspec
Model 450; Bio-Rad Laboratories, Inc., Hercules, CA, USA) at 490
nm. Three independent experiments were performed in triplicate.
Cell apoptosis analysis
Apoptosis rates of cells were measured using an
Annexin V-FITC/PI apoptosis kit (Becton Dickinson, Franklin Lakes,
NJ, USA) following the manufacturer's instructions. Briefly, cells
were harvested, washed with cold PBS, and resuspended in binding
buffer. Then, 5 µl Annexin V-FITC and 5 µl PI were added to each
sample containing 1×105 cells/100 µl. The samples were
incubated at 25°C in the dark for 15 min, followed by addition of
400 µl binding buffer. Within 1 h of preparation, the samples were
analyzed by a flow cytometer (Becton Dickinson). The experiments
were repeated three times independently.
Cell migration and invasion
assays
In cell migration assays, cells were grown on 6-well
plates until 100% confluent. A 20-µl pipette tip was used to
scratch and create a wound in the confluent monolayer at the center
of culture plates. After washes with PBS buffer, cells were
replenished with culture medium and cell migration was captured
under a microscope (Olympus Corp., Tokyo, Japan) at 0 and 24 h post
wound scratch. Migration rate was determined using the ImageJ
software as an average closed area of the wound relative to the
initial wound area at 24 h after wounding. Experiments were
performed in triplicate with three independent repeats. In cell
invasion assays, 10×104 of TE-1 cells, 12×104
of KYSE70 cells were suspended in serum-free RPMI-1640 medium and
seeded in BD Matrigel invasion chamber (Becton Dickinson). The
lower chamber contained 0.75 ml of RPMI-1640 medium plus 10% FBS.
After incubation for 30 h, non-invading cells in the upper chamber
were gently removed by cotton swabs. Cells that invaded through the
Matrigel and reached to the lower chamber were fixed, stained with
mounting medium containing DAPI (Vector Laboratories, Inc.,
Burlingame, CA, USA) and photographed by fluorescence microscope
(Olympus). Invaded cell numbers were counted by the ImageJ
software. Experiments were performed in triplicate with three
independent repeats.
Immunofluorescence assays
ESCC cell lines were grown on glass coverslips (Nest
Biotechnology Co., Ltd., Wuxi, China) to 50% confluence and then
fixed in 4% paraformaldehyde for 15 min at room temperature. The
cells were permeabilized with 0.3% Triton X-100 for 20 min, blocked
with 5% bovine serum albumin for 30 min, and then incubated with
the mouse monoclonal anti-NONO antibody (Becton Dickinson) at 4°C
overnight, followed by an Alexa Fluor 488-conjugated secondary
antibody (Jackson ImmunoResearch Laboratories, Inc., West Grove,
PA, USA) for 45 min at 37°C. The cells were rinsed in PBS between
incubation steps and mounted with medium containing
4′-6-diamidino-2-phenylindole (DAPI). Immunofluorescence images
were acquired using a fluorescence microscope (Olympus).
Statistical analysis
The Wilcoxon signed rank test was used for
comparison of quickscores between paired ESCC and adjacent benign
tissue (Fig. 1B). Comparison of
Quickscore distribution between benign and ESCC was performed by
χ2 test (Fig. 1C). The
associations of pathological features with NONO quickscores were
evaluated by Fisher's exact test in Table I. Data from Figs. 2–4
are presented as the mean ± SEM that was calculated from at least
three independent experiments. Statistical analysis was carried out
using GraphPad Prism (version 4) (GraphPad Software, Inc., La
Jolla, CA, USA) with the level of significance set at P<0.05,
P<0.01 and P<0.001. Paired Student's t-test was used to
compare two groups when the means follow the normal
distribution.
 | Table I.Pathological information of ESCC
patients and NONO expression. |
Table I.
Pathological information of ESCC
patients and NONO expression.
|
| NONO expression
level |
|
|
|---|
|
|
|
|
|
|---|
| Variables | Weak + moderate
(%) | Strong (%) | No. | P-value |
|---|
| Age |
|
|
|
|
|
>60 | 15 | 4
(28.6) | 11 (39.3) | 0.734 |
|
<60 | 27 | 10 (71.4) | 17 (60.7) |
|
| Sex |
|
|
|
|
|
Male | 32 | 11 (78.6) | 21 (75.0) | 1.000 |
|
Female | 10 | 3
(21.4) | 7
(25.0) |
|
| Tumor depth |
|
|
|
|
|
T1-2 | 6 | 5
(35.7) | 1
(3.6) |
0.011a |
|
T3-4 | 36 | 9
(64.3) | 27 (96.4) |
|
| LN metastasis |
|
|
|
|
| N0 | 36 | 11 (78.6) | 25 (89.3) | 0.652 |
|
N1/N2/N3 | 6 | 3
(21.4) | 3
(10.7) |
|
| Pathological
differentiation |
|
|
|
|
|
Well | 7 | 2
(14.2) | 5
(17.9) | 0.651 |
|
Moderate | 21 | 6
(42.9) | 15 (53.6) |
|
|
Poor | 14 | 6
(42.9) | 8 (28.5) |
|
| AJCC stage |
|
|
|
|
| I | 5 | 2
(14.3) | 3
(10.7) | 0.860 |
| II | 26 | 9
(64.3) | 17 (60.7) |
|
|
III–IV | 11 | 3
(21.4) | 8
(28.6) |
|
Results
NONO is upregulated in human ESCC
tissue samples
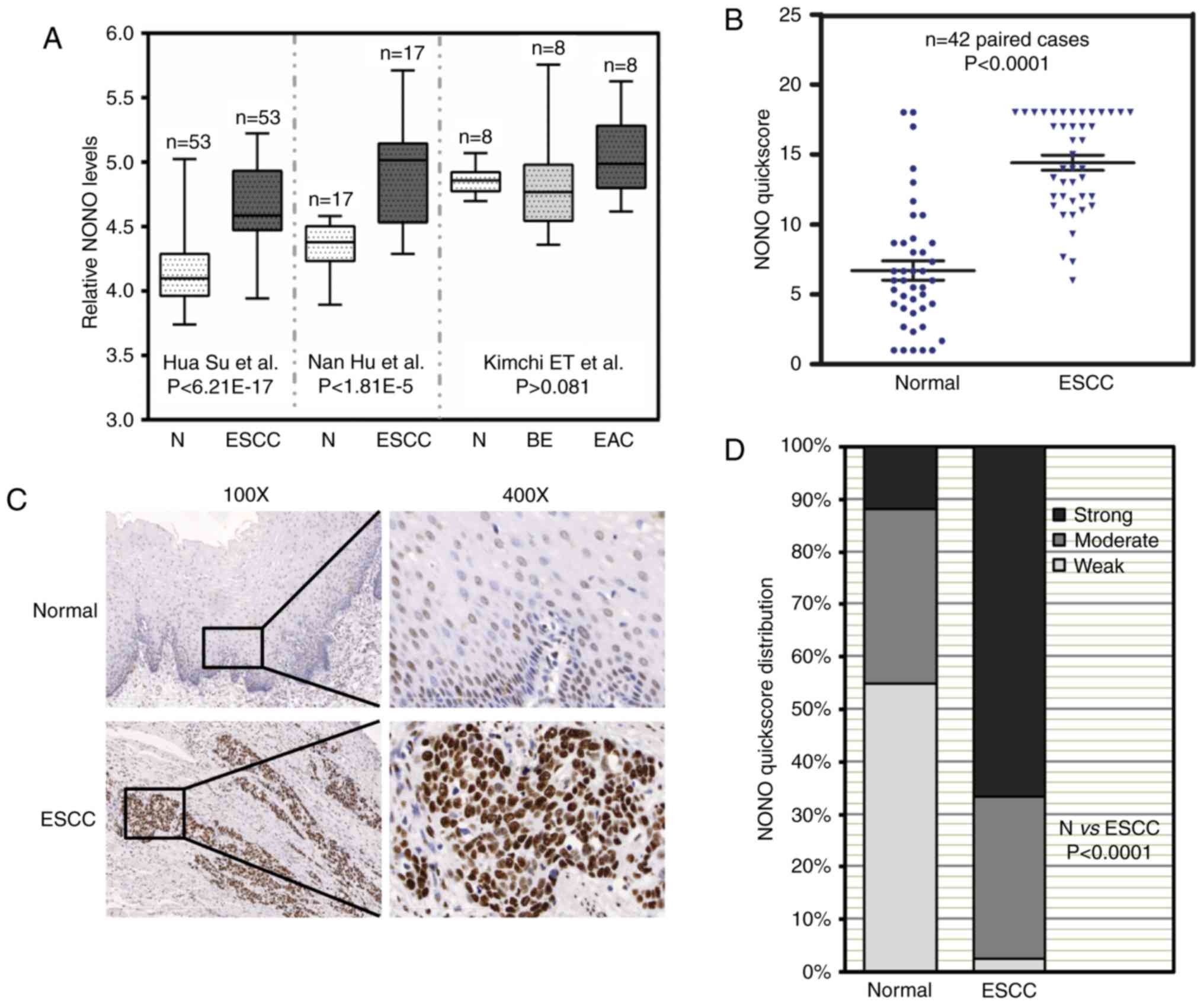
Three independent microarray studies reported that
higher mRNA levels of NONO were detected in ESCC tissues (Fig. 1A) (16,17,24).
By contrast, there were no significant differences of NONO mRNA
levels in Barrett's esophagus, EAC and adjacent normal esophagus.
In order to determine NONO protein expression in ESCC, we collected
42 paired patient samples containing ESCC and adjacent benign
tissue for IHC. IHC signal of NONO was localized in the nuclei of
esophageal epithelial cells as well as a sub-group of stromal and
muscle cells (Fig. 1B). NONO
expression was high in ESCC compared with that in adjacent benign
tissue samples (P<0.0001) (Fig.
1B). Representative IHC images are presented in Fig. 1C. Of the ESCC tissue samples 66.7%
showed strong NONO signal whereas only 11.9% of benign tissue
samples showed strong NONO staining (Fig. 1D). The relationship between
pathological background and NONO expression was further analyzed
(Table I). While there was no
correlation between NONO protein levels with patient age, gender,
lymph node metastasis, pathological differentiation and TNM stage,
strong NONO staining was detected in ESCC with greater tumor
invasion depth (P=0.011). These results suggested a potential role
of NONO in ESCC.
NONO expression in ESCC cell
lines
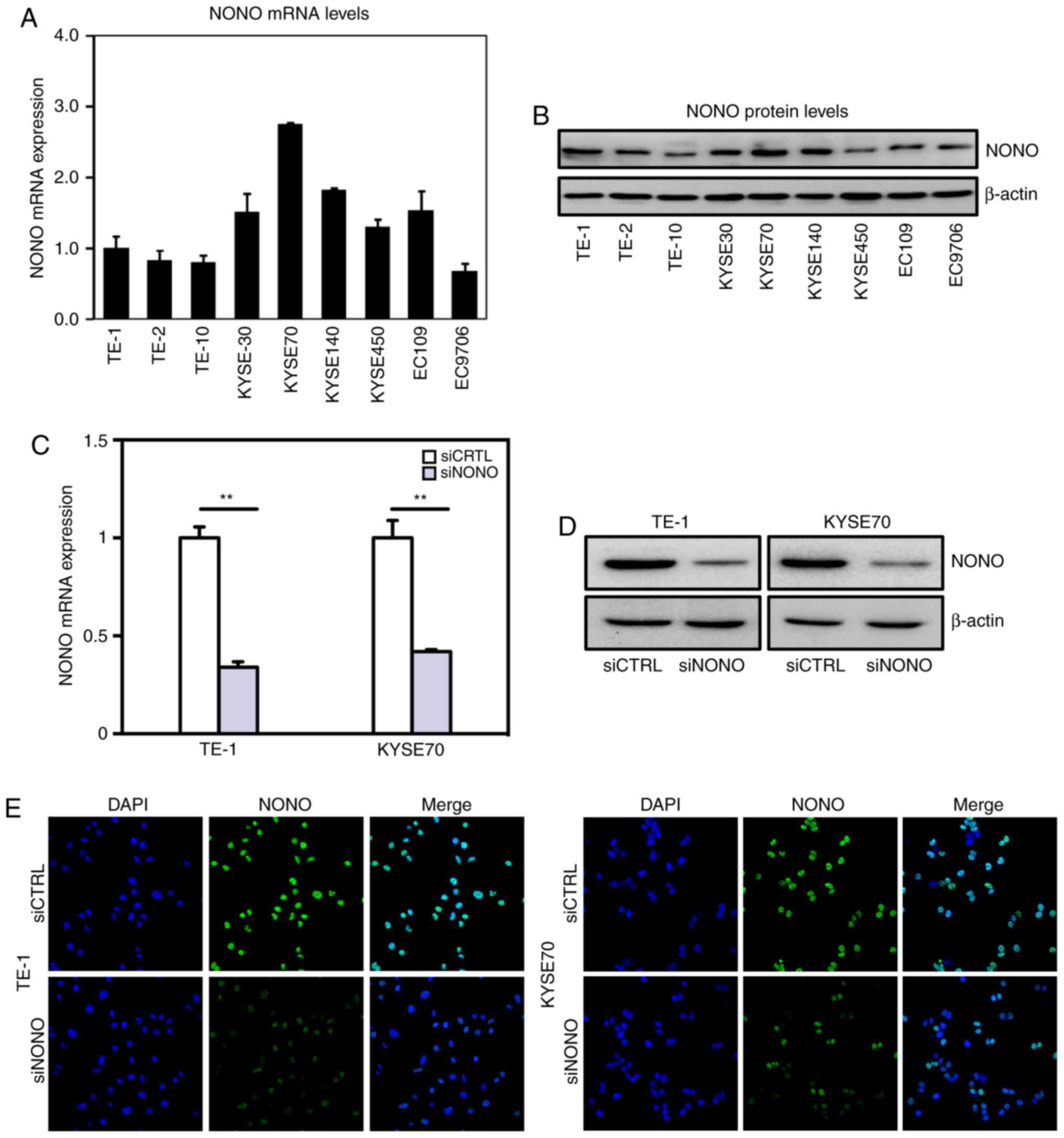
Real-time PCR and western blot assays indicated that
NONO is widely expressed in multiple ESCC cell lines (Fig. 2A and B). However, NONO mRNA levels
varies among these cell lines with relatively higher levels in
KYSE30 and KYSE70 cell lines and lower levels in TE-1, TE-2 and
TE-10 cell lines. TE-1 was derived from well differentiated ESCC,
whereas KYSE70 was derived from poorly differentiated tumors,
respectively (25). We chose TE-1
and KYSE70 cell lines for further functional analyses of NONO.
Knockdown NONO was achieved by transient transfection with
efficiencies shown in Fig.
2C-E.
Knockdown NONO inhibits ESCC cell
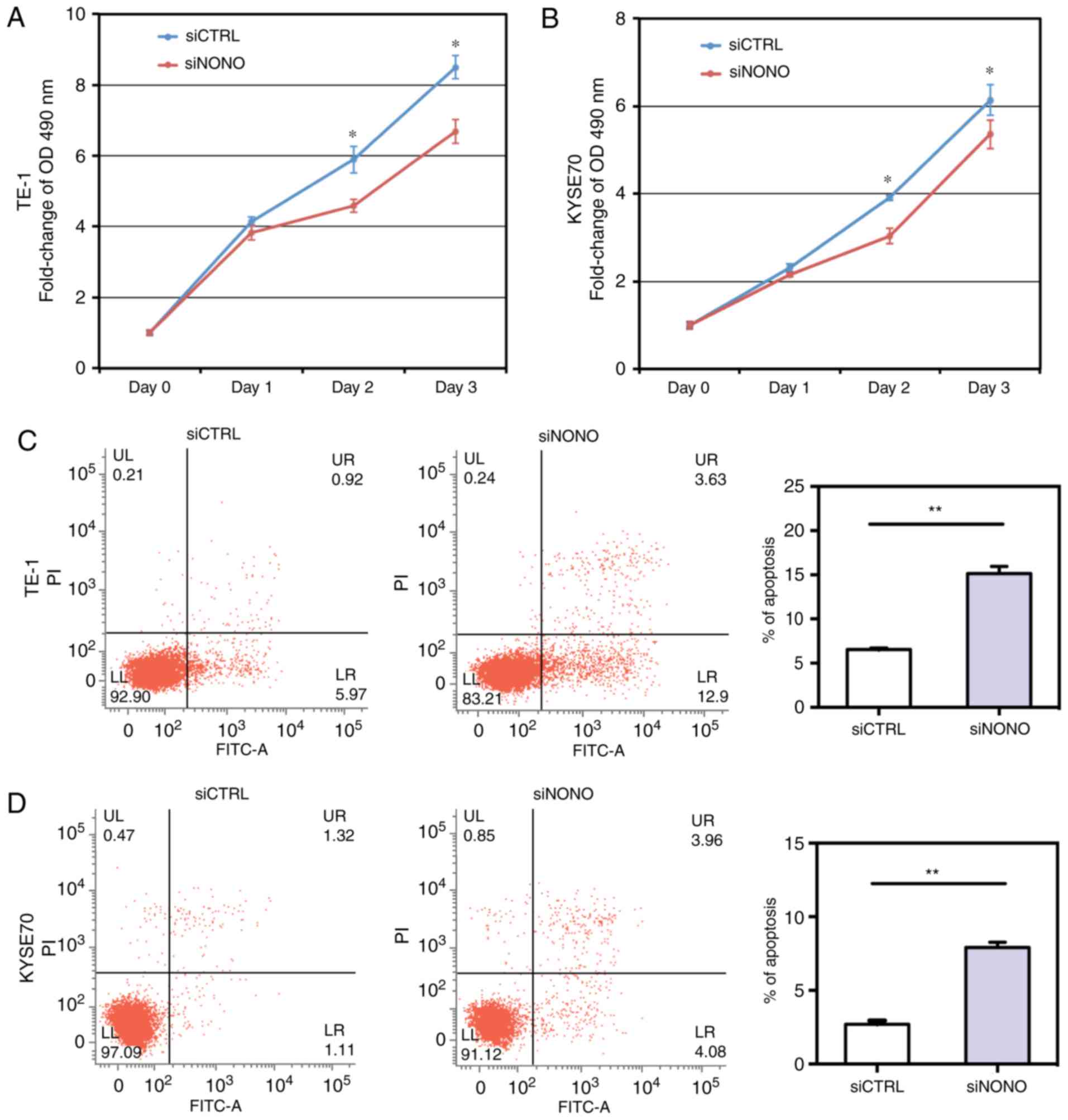
proliferation and induces cell apoptosis
Given the elevated expression of NONO in ESCC
tissues and cell lines, we examined whether NONO regulated ESCC
cell growth. As shown in Fig. 3A and
B, NONO knockdown by specific siRNA significantly inhibited
growth of TE-1 and KYSE70 cells.
To determine whether NONO could regulate apoptosis
of TE-1 and KYSE70 cells, we performed an Annexin V-FITC and PI
assay to detect apoptosis. Knockdown of NONO significantly induced
apoptosis of TE-1 and KYSE70 cells. After NONO knockdown, the
percentages of both early apoptotic (Annexin
V-positive/PI-negative) and late apoptotic (Annexin
V-positive/PI-positive) cells were significantly increased in
NONO-knockdown cells, compared with control cells (P<0.05,
Fig. 3C and D).
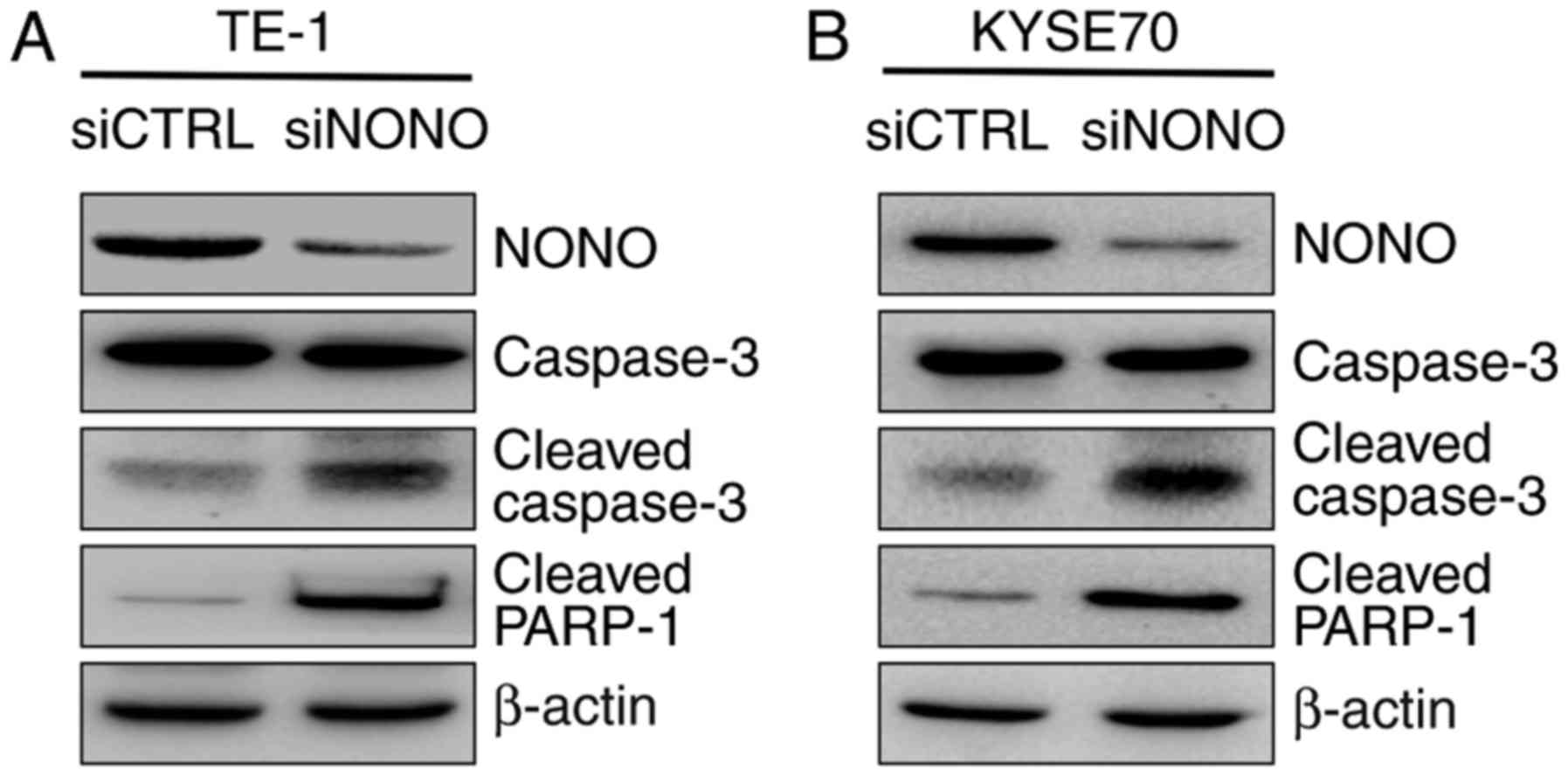
Because apoptosis is often mediated by the
activation of caspase-3 that leads to PARP binding to fragmented
DNA, western blot analysis was then used to detect caspase-3
activation. The result showed that cleavages of caspase-3 and
PARP-1 were dramatically increased in NONO-knockdown cells,
compared with control cells (Fig.
4).
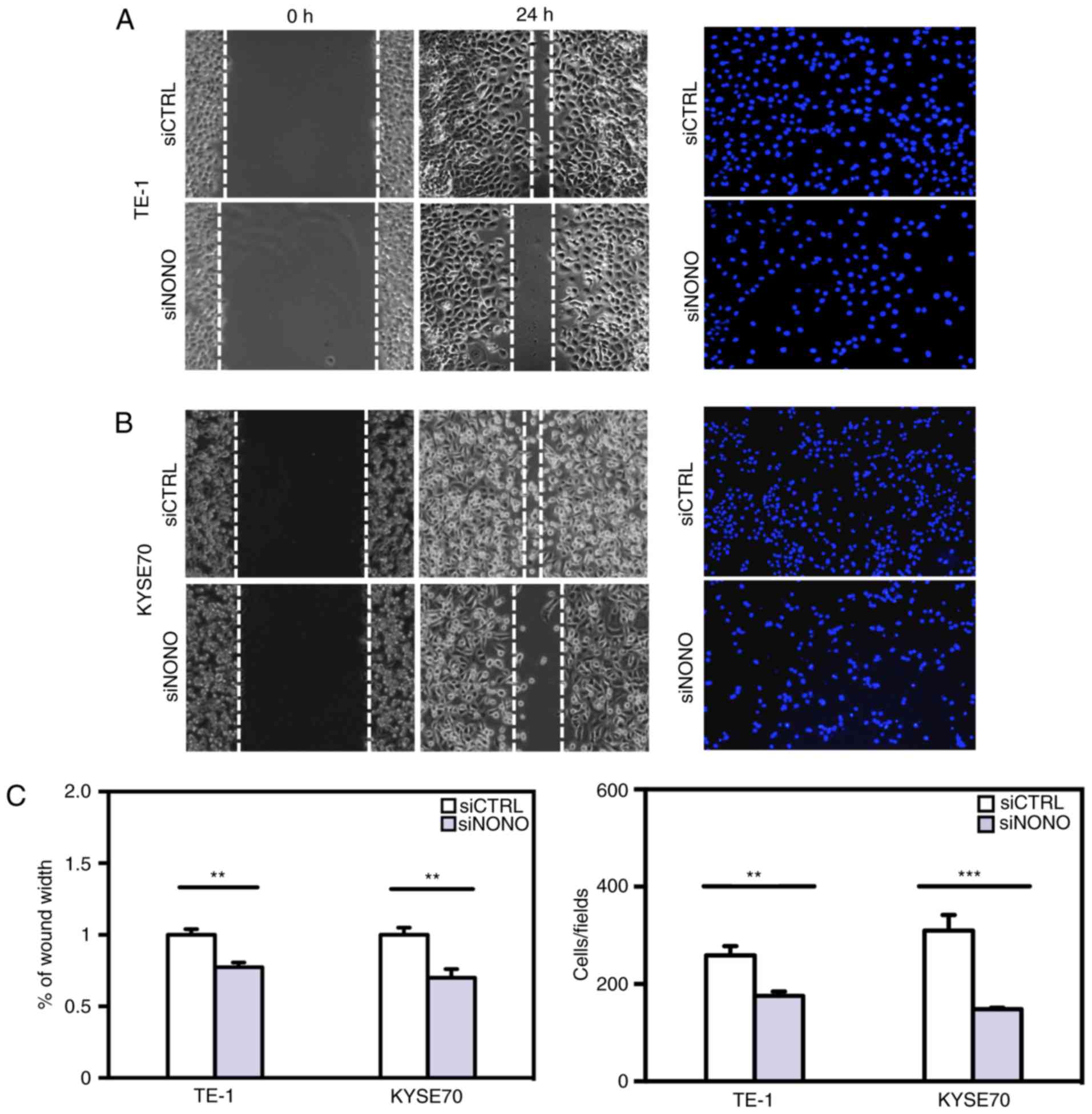
NONO regulates ESCC cell migration and
invasion in vitro
Since higher NONO protein expression was observed in
tumors with greater local invasion (Table I), we further studied whether NONO
is a regulator of ESCC cell migration and invasion in vitro.
Knockdown of endogenous NONO expression significantly reduced TE-1
and KYSE70 cell mobility in passing through the surface of
culturing plates or penetrating through Matrigel (Fig. 5). These results indicated that the
expression levels of NONO are important for ESCC cell migration and
invasion.
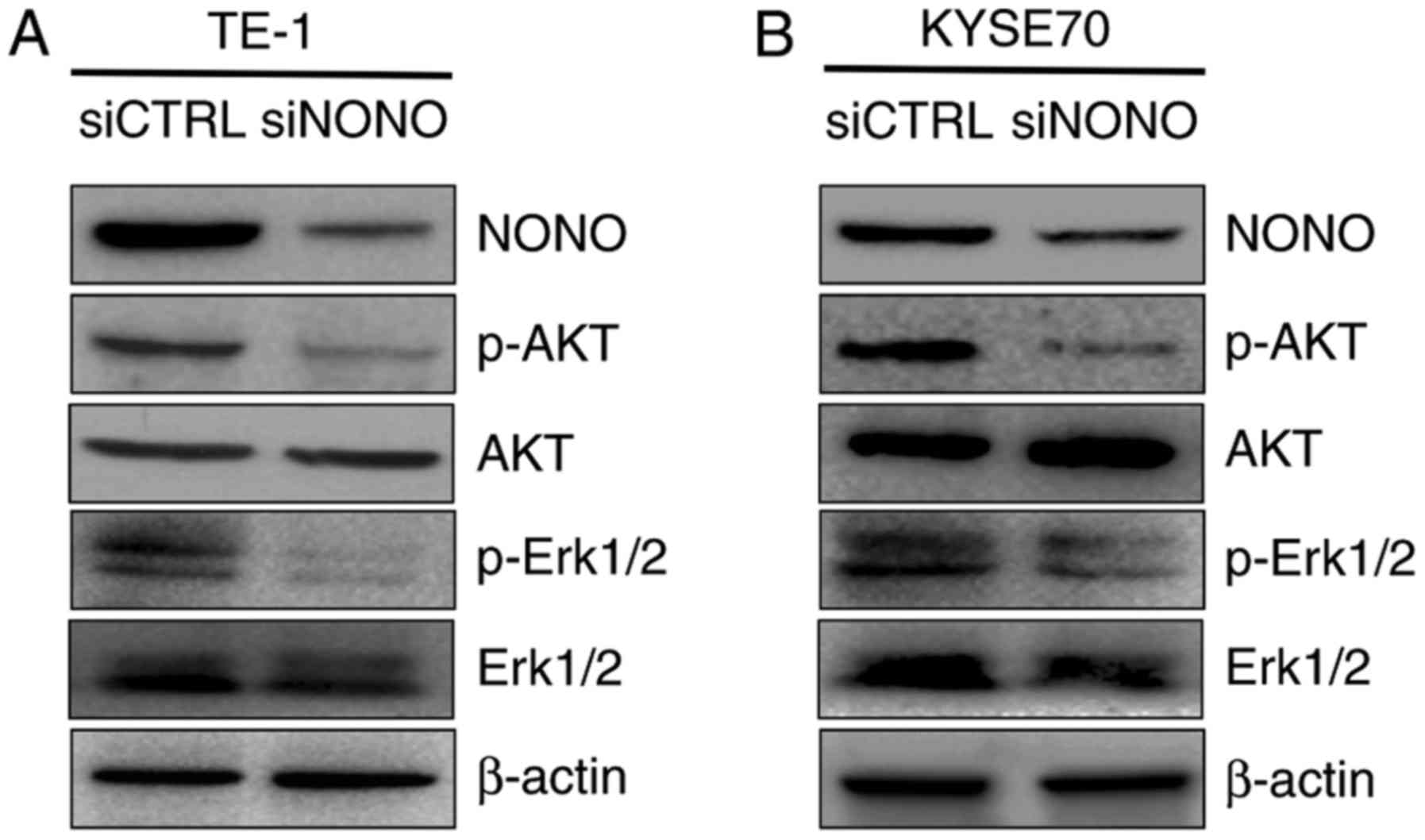
Knockdown NONO negatively regulates
p-Erk1/2 and p-AKT expression
As in vitro assays showed that NONO is
overexpressed in ESCC tissues and cells, and that knockdown NONO
inhibits proliferation, induces apoptosis and reduces mobility of
TE-1 and KYSE70 cells, we then explored the molecular mechanism
underlying the effects. Both Erk1/2/MAPK and PI3K/AKT activation
are frequent events in human cancer including ESCC (26,27).
Since Erk1/2 and AKT are activated through phosphorylation, we
detected the expression of the Thr202/Tyr204 phosphorylated form of
Erk1/2 and Ser473 phosphorylated form of AKT by western blotting.
TE-1 and KYSE70 cells were treated with siRNA targeting NONO or
control siRNA as a negative. We found that the expression levels of
phosphorylated Erk1/2 and AKT were dramatically decreased in both
TE-1 and KYSE70 NONO-knockdown cells (Fig. 6). These data suggested that Erk1/2
and PI3K/AKT pathway is required for the NONO-regulated growth,
apoptosis and invasion of ESCC cells.
Discussion
Being one of the most common causes of
cancer-related deaths in China, the average 5-year survival rate of
ESCC is still low. Several mechanisms promote ESCC progression and
provide with independence from normal regulation of cell control
(28). Treatment of ESCC especially
in advanced stages still remains a clinical challenge. Therefore,
new therapeutic targets remain to be elucidated. The nuclear NONO
protein is an RNA-binding molecule containing two RNA recognition
motifs. It is able to bind double-stranded DNA, single-stranded DNA
and RNA (29). Previous studies
showed that it retains hyper-edited messenger RNA in the nucleus
and therefore influences gene expression and differentiation of
stem cells (30). Recently, it has
been shown that NONO is strongly expressed in several types of
cancer. In addition, an increasing number of studies have reported
that NONO modulates various pivotal intermediate molecules and
ultimately regulates cell proliferation, apoptosis and mobility in
different types of human carcinomas. However, the expression
pattern and function of NONO in the tumorigenesis of ESCC remain
unknown. Our contribution in this study is for the first time to
demonstrate that NONO protein expression is upregulated in ESCC and
that higher NONO protein levels are associated with greater tumor
invasion depth. In addition, we demonstrated that NONO plays direct
regulatory roles in ESCC cell proliferation, apoptosis, migration
and invasion involving Erk1/2 and Akt signaling pathways.
Firstly, we observed a significantly elevated NONO
expression in most ESCC tissues compared with paired normal
esophageal epithelial tissues. Higher NONO expression was shown to
be associated with deeper local invasion, but no differences
between tumors with different degree of lymph node metastasis,
supporting a potential significance in early stage of ESCC
progression. However, the correlation of NONO expression and the
poor overall survival of ESCC patients need further study.
Secondly, we observed different oncogenic effects in
two ESCC cell lines following downregulation of NONO expression.
Targeted NONO silencing using siRNA inhibited cell growth in TE-1
and KYSE70 ESCC cell lines. Moreover, NONO silencing increased the
percentage of apoptotic cells in those cell lines, in which this
oncogene is endogenously overexpressed. In addition, we examined
the expression of cleaved caspase-3 and cleaved PARP-1 by western
blotting. We showed that knockdown of NONO could inhibit caspase-3
and PARP-1 cleavage, which was according to its role of apoptosis
inhibitor. Taken together, our data suggest that NONO may represent
a promising and effective target for antitumor therapy of ESCC.
Recent studies have also shown that the in
vitro proliferation, apoptosis and invasion of ESCC cell lines
were regulated by inhibition of PI3K or MAPK (26,31).
PI3K is a lipid kinase that generates second messengers involved in
regulation of a wide spectrum of cellular functions including
proliferation, survival and invasion (32). The effects of PI3K on tumor growth
and progression are thought to be mediated mainly by Akt. The MAPK
family includes the ERKs and the stress-activated protein kinases
(SAPKs), p38 and JNK. Erk/MAPK signaling is frequently activated
and promotes cancer cell proliferation, cell survival and
metastasis (33,34). Some studies observed multiple
biological aspects on NONO in tumor cells, such as NONO affecting
cell proliferation, apoptosis, and cell cycle of melanomas, which
is related to cx-43 (10). Besides,
NONO stimulates SREBP-1a-dependent cell proliferation and tumor
growth of breast cancer cells in vitro and in vivo
(15). This study is the first to
report that NONO silencing downregulated the expression level of
p-Akt and p-Erk1/2 protein without affecting the total Akt and
Erk1/2 expression level in ESCC cell lines, which suggested that
NONO might play an important role in growth, apoptosis and
metastasis of ESCC by activating both the PI3K/Akt and MAPK/Erk
signaling pathways. However, this study was performed only at the
cellular level. Further experiments to verify the influence of NONO
on ESCC will be done in animal models. More comprehensive
investigations are required to determine the associations between
NONO, Akt, Erk and tumorigenesis.
Taken together, our results demonstrated that
knockdown of NONO could inhibit proliferation and invasion, and
promote apoptosis through the PI3K/Akt and MAPK/Erk signaling
pathways in ESCC cancer cells. Our data may provide new evidence to
improve the understanding of carcinogenesis of ESCC and may help to
develop new therapeutic strategies for the treatment of ESCC.
Acknowledgements
This study was supported by Beijing Municipal
Administration of Hospitals Clinical Medicine Development of
Special Funding Support (no. ZY201308), and Beijing Natural Science
Foundation (no. 7152043).
References
|
1
|
Holmes RS and Vaughan TL: Epidemiology and
pathogenesis of esophageal cancer. Semin Radiat Oncol. 17:2–9.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang X, Fan JC, Wang AR, Leng Y, Li J, Bao
Y, Wang Y, Yang QF and Ren Y: Epidemiology of esophageal cancer in
Yanting - regional report of a national screening programme in
China. Asian Pac J Cancer Prev. 14:2429–2432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shav-Tal Y and Zipori D: PSF and p54
(nrb)/NonO - multi-functional nuclear proteins. FEBS Lett.
531:109–114. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patton JG, Porro EB, Galceran J, Tempst P
and Nadal-Ginard B: Cloning and characterization of PSF, a novel
pre-mRNA splicing factor. Genes Dev. 7:393–406. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li S, Kuhne WW, Kulharya A, Hudson FZ, Ha
K, Cao Z and Dynan WS: Involvement of p54 (nrb), a PSF partner
protein, in DNA double-strand break repair and radioresistance.
Nucleic Acids Res. 37:6746–6753. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong B, Horowitz DS, Kobayashi R and
Krainer AR: Purification and cDNA cloning of HeLa cell p54nrb, a
nuclear protein with two RNA recognition motifs and extensive
homology to human splicing factor PSF and Drosophila NONA/BJ6.
Nucleic Acids Res. 21:4085–4092. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ito T, Watanabe H, Yamamichi N, Kondo S,
Tando T, Haraguchi T, Mizutani T, Sakurai K, Fujita S, Izumi T, et
al: Brm transactivates the telomerase reverse transcriptase (TERT)
gene and modulates the splicing patterns of its transcripts in
concert with p54 (nrb). Biochem J. 411:201–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barboro P, Rubagotti A, Orecchia P, Spina
B, Truini M, Repaci E, Carmignani G, Romagnoli A, Introini C,
Boccardo F, et al: Differential proteomic analysis of nuclear
matrix in muscle-invasive bladder cancer: Potential to improve
diagnosis and prognosis. Cell Oncol. 30:13–26. 2008.PubMed/NCBI
|
|
10
|
Schiffner S, Zimara N, Schmid R and
Bosserhoff AK: p54nrb is a new regulator of progression of
malignant melanoma. Carcinogenesis. 32:1176–1182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ishiguro H, Uemura H, Fujinami K, Ikeda N,
Ohta S and Kubota Y: 55 kDa nuclear matrix protein (nmt55) mRNA is
expressed in human prostate cancer tissue and is associated with
the androgen receptor. Int J Cancer. 105:26–32. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim YM, Seo J, Kim YH, Jeong J, Joo HJ,
Lee DH, Koh GY and Lee KJ: Systemic analysis of tyrosine
phosphorylated proteins in angiopoietin-1 induced signaling pathway
of endothelial cells. J Proteome Res. 6:3278–3290. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clark J, Lu YJ, Sidhar SK, Parker C, Gill
S, Smedley D, Hamoudi R, Linehan WM, Shipley J and Cooper CS:
Fusion of splicing factor genes PSF and NonO (p54nrb) to the TFE3
gene in papillary renal cell carcinoma. Oncogene. 15:2233–2239.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu PY, Erriquez D, Marshall GM, Tee AE,
Polly P, Wong M, Liu B, Bell JL, Zhang XD, Milazzo G, et al:
Effects of a novel long noncoding RNA, lncUSMycN, on N-Myc
expression and neuroblastoma progression. J Natl Cancer Inst.
106:1062014. View Article : Google Scholar
|
|
15
|
Zhu Z, Zhao X, Zhao L, Yang H, Liu L, Li
J, Wu J, Yang F, Huang G and Liu J: p54 (nrb)/NONO regulates lipid
metabolism and breast cancer growth through SREBP-1A. Oncogene.
35:1399–1410. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu N, Clifford RJ, Yang HH, Wang C,
Goldstein AM, Ding T, Taylor PR and Lee MP: Genome wide analysis of
DNA copy number neutral loss of heterozygosity (CNNLOH) and its
relation to gene expression in esophageal squamous cell carcinoma.
BMC Genomics. 11:5762010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su H, Hu N, Yang HH, Wang C, Takikita M,
Wang QH, Giffen C, Clifford R, Hewitt SM, Shou JZ, et al: Global
gene expression profiling and validation in esophageal squamous
cell carcinoma and its association with clinical phenotypes. Clin
Cancer Res. 17:2955–2966. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Edge SBBD, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: American Joint Committee on Cancer Staging
Manual. 7th editon. Springer; New York, NY: 2009
|
|
19
|
Sobin LH and Compton CC: TNM seventh
edition: what's new, what's changed: communication from the
International Union Against Cancer and the American Joint Committee
on Cancer. Cancer. 116:5336–5339. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun X, Qiu JJ, Zhu S, Cao B, Sun L, Li S,
Li P, Zhang S and Dong S: Oncogenic features of PHF8 histone
demethylase in esophageal squamous cell carcinoma. PLoS One.
8:e773532013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meng XY, Zhu ST, Zhou QZ, Li P, Wang YJ
and Zhang ST: Promoter methylation regulates cigarette
smoke-stimulated cyclooxygenase-2 expression in esophageal squamous
cell carcinoma. J Dig Dis. 13:208–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Detre S, Saclani Jotti G and Dowsett M: A
‘quickscore’ method for immunohistochemical semiquantitation:
Validation for oestrogen receptor in breast carcinomas. J Clin
Pathol. 48:876–878. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang N, Sun X, Sun M, Zhu S, Wang L, Ma
D, Wang Y, Zhang S and Li P:
4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone promotes esophageal
squamous cell carcinoma growth via beta-adrenoceptors in vitro and
in vivo. PLoS One. 10:e01188452015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kimchi ET, Posner MC, Park JO, Darga TE,
Kocherginsky M, Karrison T, Hart J, Smith KD, Mezhir JJ,
Weichselbaum RR, et al: Progression of Barrett's metaplasia to
adenocarcinoma is associated with the suppression of the
transcriptional programs of epidermal differentiation. Cancer Res.
65:3146–3154. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishihira T, Hashimoto Y, Katayama M, Mori
S and Kuroki T: Molecular and cellular features of esophageal
cancer cells. J Cancer Res Clin Oncol. 119:441–449. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu F, Zheng S, Liu T, Liu Q, Liang M, Li
X, Sheyhidin I, Lu X and Liu W: MicroRNA-21 promotes the
proliferation and inhibits apoptosis in Eca109 via activating
ERK1/2/MAPK pathway. Mol Cell Biochem. 381:115–125. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Grille SJ, Bellacosa A, Upson J,
Klein-Szanto AJ, van Roy F, Lee-Kwon W, Donowitz M, Tsichlis PN and
Larue L: The protein kinase Akt induces epithelial mesenchymal
transition and promotes enhanced motility and invasiveness of
squamous cell carcinoma lines. Cancer Res. 63:2172–2178.
2003.PubMed/NCBI
|
|
28
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Basu A, Dong B, Krainer AR and Howe CC:
The intracisternal A-particle proximal enhancer-binding protein
activates transcription and is identical to the RNA- and
DNA-binding protein p54nrb/NonO. Mol Cell Biol. 17:677–686. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bond CS and Fox AH: Paraspeckles: Nuclear
bodies built on long noncoding RNA. J Cell Biol. 186:637–644. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li B, Tsao SW, Li YY, Wang X, Ling MT,
Wong YC, He QY and Cheung AL: Id-1 promotes tumorigenicity and
metastasis of human esophageal cancer cells through activation of
PI3K/AKT signaling pathway. Int J Cancer. 125:2576–2585. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vara Fresno JA, Casado E, de Castro J,
Cejas P, Belda-Iniesta C and González-Barón M: PI3K/Akt signalling
pathway and cancer. Cancer Treat Rev. 30:193–204. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wada T and Penninger JM: Mitogen-activated
protein kinases in apoptosis regulation. Oncogene. 23:2838–2849.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chan-Hui PY and Weaver R: Human
mitogen-activated protein kinase kinase kinase mediates the
stress-induced activation of mitogen-activated protein kinase
cascades. Biochem J. 336:599–609. 1998. View Article : Google Scholar : PubMed/NCBI
|