Introduction
Colorectal cancer (CRC) is the third most common
malignancy and one of the leading causes of cancer-related deaths
worldwide (1–3). The global burden of CRC is increasing
and is likely to persist until the year 2035 and beyond (4). With the introduction of CRC screening,
more and more patients benefit from the early detection of
precancerous lesions. However, despite the developments in
colonoscopy as well as in treatment, the therapeutic effect of CRC
remains unsatisfactory. The main obstacles in CRC therapy are
metastasis and drug resistance. Therefore, understanding the
molecular mechanism of CRC is important for the development of an
effective therapy.
β-arrestin1 and β-arrestin2 belong to the nonvisual
β-arrestins and are ubiquitous proteins. β-arrestins are
multifunctional proteins and are well-known for their classical
role in the G protein-coupled receptor (GPCR) desensitization,
sequestration and internalization (5,6).
Furthermore, β-arrestins are scaffold proteins that can interact
with many other signaling molecules and regulate cellular
responses, such as proliferation, migration and invasion as well as
apoptosis (7,8). β-arrestins play an important role in
physiological and pathological conditions.
Numerous studies have demonstrated that β-arrestin2
is abnormally expressed in many types of cancer, including breast,
lung, castration-resistant prostate and hepatocellular cancer
(9,10). β-arrestin2 is essential for the
tumorigenesis of chronic myelogenous leukemia and colon cancer
(11,12). It contributes to the proliferation
of castration-resistant prostate cancer but inhibits lung cancer
growth (13,14). It also decreases metastasis of
hepatocellular cancer, but promotes breast cancer migration and
invasion (15,16). Above all, β-arrestin2 is involved in
the tumorigenesis and progression of cancer in multifunctional
ways.
Bonnans et al (12) demonstrated that β-arrestin2 is
required for the initiation of colon cancer through the elevated
Wnt pathway in vivo and in vitro (12). Nonetheless, the expression and
clinicopathological significance of β-arrestin2 in CRC have not
been reported. Liu et al (17) demonstrated that β-arrestin2
deficiency protracts the activation of the NF-κB pathway and
suppresses radiation-induced intestinal crypt progenitor cell
apoptosis (17). However, the role
of β-arrestin2 in chemo-induced colon epithelial cell apoptosis
remains to be explored. The aim of this study was to investigate
the role of β-arrestin2 in CRC and CRC cell apoptosis. To assess
the expression and clinical significance of β-arrestin2 in CRC, CRC
tissues were analyzed. Immunohistochemistry assay demonstrated that
β-arrestin2 was overexpressed in CRC tissues compared with normal
tissues, although its high expression was not related with the
clinicopathological features. Furthermore, β-arrestin2
downregulation did not alter the cell proliferation rate, migration
and invasion capacity in vitro, although, the data indicated
that β-arrestin2 downregulation inhibited the 5-FU-induced CRC cell
apoptosis, reduced the expression of cleaved-caspase-3 and Bax and
increased the expression of Bcl-2. In addition, β-arrestin2
overexpression increased the apoptosis rate of CRC cells stimulated
by 5-FU. The p-p65 expression increased following β-arrestin2
downregulation and decreased following β-arrestin2 overexpression.
Collectively, these data indicated that β-arrestin2 played a
critical role in CRC and contributed to CRC cell apoptosis via the
NF-κB signaling pathway.
Materials and methods
Patient tissue
From April 2009 to February 2016, 59 primary CRC
samples (41 cases) and normal colon tissues (18 cases) were
collected at the Peking University People's Hospital (Beijing,
China). The CRC group consisted of 20 women and 21 men aged between
48–88 years. The control group consisted of adjacent non-cancerous
mucosa tissue from 12 CRC patients and adjacent non-inflammation
mucosa tissue from 6 inflammation bowel disease patients. CRC
diagnosis was confirmed by 2 pathologists. Patients who received
chemotherapy and radiotherapy prior to surgery were not enrolled in
this study. The present study was approved by the Human Ethics
Review Board of the Peking University People's Hospital (Beijing,
China). All patients obtained informed consent to donate their
tissue samples and clinical information for research, and written
consent was given from all the patients.
Cell lines
The human colon cancer cell lines LoVo and HCT116
were obtained from the American Type Culture Collection (Manassas,
VA, USA). The cells were cultured in DMEM medium (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10%
fetal bovine serum (FBS) and 1% penicillin/streptomycin (Thermo
Fisher Scientific, Inc.) in a humidified atmosphere containing 5%
CO2 and 95% air at 37°C.
Immunohistochemistry assay
Paraffin sections were deparaffinized in xylene and
hydrated in alcohol gradient. The slides were incubated with 3%
H2O2 for 10 min. Antigen retrieval was
performed at 95°C for 20 min in sodium citrate solution (Solarbio
Science and Technology, Beijing, China). The slides were blocked
with 5% bovine serum albumin (BSA) for 1 h and incubated with
rabbit anti-β-arrestin2 monoclonal antibody (1:200; cat. no. 3857;
Cell Signaling Technology, Inc., Danvers, MA, USA) at 4°C
overnight. After being washed with PBS, the slides were incubated
with anti-rabbit horseradish peroxidase-conjugated secondary
antibody (1:1,000; cat. no. ZB2301; ZSGB-BIO, Beijing, China) for 1
h at room temperature. After washing with PBS, the DAB solution was
used to visualize β-arrestin2 expression and then the nuclei were
stained with hematoxylin. PBS were used as a negative control. Data
were analyzed on an Olympus microscope (Olympus Inc., Tokyo, Japan)
by two independent single-blinded pathologists.
Five random fields were selected for scoring under
×200 magnification. The scoring was performed based on staining
scope: 1 (0–25%); 2 (25–50%); 3 (50–75%); and 4 (75–100%). The
staining intensity was also divided into 4 levels: 0, negative; 1,
weakly positive (light yellow); 2, moderately positive (yellow
brown); 3, strongly positive (dark brown). The expression score was
calculated as follows: Staining scope × intensity. Scores ≥4
reflected positive expression, while those below 4 represented
negative expression (18).
Cell transfection
Small interfering RNA (siRNA) was synthesized by
GenePharm Co. (Suzhou, Jiangsu, China). The target sequences for
β-arrestin2 were as follows: 5′-CGUAGACUUUGAGAUUCGATT-3′,
β-arrestin2-siRNA-1; 5′-CUCAACUCGAACAAGAUGATT-3′,
β-arrestin2-siRNA-2; 5′-CCAACCUCAUUGAAUUUGATT-3′,
β-arrestin2-siRNA-3. The sequence of unrelated siRNA was
5′-UUCUCCGAACGUGUCACGUTT-3′ (NC-siRNA). The full length of
β-arrestin2 were cloned into pCMV vector in frame with GFP (Sino
Biological, Beijing, China). The cells were seeded in proper dishes
and transfected at 70% confluency. In 6-cell culture cluster, the
transfection was conducted with siRNAs (100 nmol/well) or plasmids
(2,500 ng/well) using Lipofectamine 3000 (5 µl/well) according to
the manufacturer's instructions (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Quantitative real-time PCR
Real-time PCR was used to detect gene silencing
expression of β-arrestin2. Total RNA was isolated using the RNA
Isolation kit (Omega Bio-Tek, Inc., Norcross, GA, USA) and
complementary DNA (cDNA) was synthesized using a ReverTra Ace qPCR
RT kit (Toyobo Life Science, Osaka, Japan), according to the
manufacturer's instructions. Real-time PCR was performed using
SYBR-Green Mix kit (cat. no. 4385612; Applied Biosystems; Thermo
Fisher Scientific, Inc.) and Applied Biosystems ABI 7500 system
(Applied Biosystems; Thermo Fisher Scientific, Inc.). The thermal
cycling conditions for RT-PCR were as follows: Denaturation at 95°C
for 2 min, followed by 40 cycles of denaturation 95°C for 30 sec,
annealing at 60°C for 30 sec and extension at 72°C for 45 sec. The
prime sequences for each gene were as follows: β-arrestin2 sense,
TCCATGCTCCGTCACACTG and antisense, ACAGAAGGCTCGAATCTCAAAG
(length=82 bp); GAPDH sense, GTCTCCTCTGACTTCAACAGCG and antisense,
ACCACCCTGTTGCTGTAGCCAA (length=131 bp). The data were calculated
using the 2−ΔΔCt method and GAPDH was the reference
gene.
Western blot analysis
Total proteins were prepared from the cell lines
after 48 h transfection and stimulation with 5-FU. Briefly, cells
were washed twice with ice-cold PBS and lysed in RIPA lysis buffer
(containing 25 mM Tris-HCl pH 7.6, 150 mM NaCl, 1% NP-40, 1% sodium
deoxycholate, 0.1% SDS, 1 mM phenylmethylsulfonyl fluoride and 1%
protease inhibitor cocktails) for 20 min on ice. Samples were then
centrifuged at 13,400 × g for 20 min. The supernatant was collected
and the protein concentration was determined by BCA protein assay
(Pierce Chemical, Rockford, IL, USA). The supernatants were added
appropriate volume 5X SDS-PAGE loading buffer (Applygen
Technologies Inc., Beijing, China) and incubated at 100°C for 5
min. Equal amounts of protein were separated by 10% SDS-PAGE and
then transferred to polyvinylidene fluoride (PVDF) membranes
(Millipore; Merck KGaA, Darmstadt, Germany). The membranes were
blocked with 5% non-fat dry milk in TBS for 2 h and then incubated
with mouse anti-β-arrestin2 monoclonal antibody (1:500; cat. no.
ab54790; Abcam, Cambridge, MA, USA), rabbit anti-Bax polyclonal
antibody (1:500; cat. no. sc-526; Santa Cruz Biotechnology, Inc.,
Dallas, Texas, USA) and mouse anti-Bcl-2 monoclonal antibody
(1:500; cat. no. sc-7382; Santa Cruz Biotechnology), rabbit
anti-cleaved-caspase-3 monoclonal antibody (cat. no. 5A1E; Cell
Signaling Technology, Inc.) and rabbit anti-p-p65 monoclonal
antibody (cat. no. 3033P; Cell Signaling Technology, Inc.) and
rabbit anti-GAPDH polyclonal antibody (1:1,000; cat. no. AF0911;
Abmart, Shanghai, China) at 4°C overnight. The membranes were
washed with TBST and incubated with the appropriate horseradish
peroxidase-conjugated second antibodies (cat. nos. ZB2305 and
ZB2301; ZSGB-BIO) for 1 h at room temperature. Proteins were
visualized by enhanced ECL detection kit (Pierce Chemical). Band
intensities were analyzed using the ImageJ analysis software
(National Institutes of Health, Bethesda, MD, USA).
Cell proliferation assay
Cell proliferation was conducted using the Cell
Counting Kit-8 (CCK-8; Dojindo, Tokyo, Japan). Forty-eight hours
after transfection with siRNA, equal amounts of cells
(3×103 cells/well) were seeded in a 96-well plate and
cultured in the medium supplemented with 10% FBS at indicated
time-points. Every 24 h, 10 µl of CCK-8 were added and the cells
were incubated for 3 h in the humidified incubator that contained
5% CO2 at 37°C. Relative proliferation was obtained by
scanning with an ELISA reader with a 450-nm filter.
Migration and invasion assay
Cell migration and invasion were analyzed using a
Boyden chamber (Corning Costar, Rochester, NY, USA) with a
gelatin-coated polycarbonate membrane filter (6.5 mm diameter, 8 µm
pore size). For the invasion assay the upper surface of the filter
was coated with 20 µl Matrigel (BD Biosciences, Bedford, MA, USA)
at 37°C for 1 h. After being transfected with siRNA for 48 h, the
cells were trypsinized and resuspended with 1% FBS culture medium
at a final density of 5×105 cells/ml. Cell suspension
(200 µl) was added to the upper chamber, and 10% FBS culture medium
was added to the lower chamber as a chemoattractant. The cells were
incubated in the humidified incubator that contained 5%
CO2 at 37°C. After 24 h, the upper surface of the filter
was scrubbed with a cotton swab and then the non-migrated or
non-invaded cells were removed. The cells at the lower surface of
the chamber were fixed with 4% paraformaldehyde for 30 min. After
being washed twice with PBS, migration or invasion cells were
stained with 0.5% (w/v) crystal violet for 15 min. Cells were then
counted using a light microscope (Olympus Inc.). Five random fields
were selected for cell counting under ×100 magnification.
Caspase-3 activity assay
Caspase-3 activity kit (Beyotime Institute of
Biotechnology, Haimen, Jiangsu, China) was used to estimate the
caspase-3 activity according to the manufacturer's instructions.
After being transfected with siRNA, LoVo cells were stimulated with
5-FU for 48 h and then the cells were collected for the caspase-3
activity assay. Briefly, the cells were trypsinized and washed with
cold PBS. Subsequently, the cells were resuspended in lysis buffer
(2×106 cells/100 µl) and were shaken on ice for 15 min.
Cell lysis was then centrifuged at 13,400 × g and 4°C for 20 min.
The supernatants were collected and the protein concentrations were
determined by Bradford protein assay (Beyotime Institute of
Biotechnology). Protein supernatants (50 µl), 40 µl reaction buffer
and 10 µl caspase-3 substrate (Ac-DEVD-pNA, 2 mM) were added to the
96-well microtiter plates and then incubated at 37°C for 4 h.
Caspase-3 activity was quantified using a microplate reader at an
absorbance of 405 nm and then was demonstrated as a percentage of
enzyme activity compared to the negative control group.
Terminal deoxynucleotidyl
transferase-mediated deoxyuridine triphosphate nick end labeling
(TUNEL) assay
Nucleosomal DNA fragmentation was determined by
TUNEL assay using an in situ Apoptosis Detection kit (KeyGen
Biotech. Co., Ltd., Nanjing, Jiangsu, China) according to the
manufacturer's instructions. After being transfected with
β-arrestin2 overexpression plasmid, the HCT116 cells were
stimulated with 5-FU for 48 h and then cells were collected for the
TUNEL assay. The cells were fixed with 4% paraformaldehyde for 30
min at 4°C and washed three times with PBS. The fixed cells were
then incubated in PBS containing 1% Triton X-100 for 15 min at room
temperature. Subsequently, cells were incubated with 3%
H2O2-methanol for 15 min at room temperature.
TdT enzyme solution (10 µl) was added into the samples and
incubated for 1 h at 37°Cin the dark, and then with 10 µl
streptavidin-HRP for 30 min, at 37°C in the dark. DAB solution was
used to visualize DNA fragmentation. The nucleus was stained with
hematoxylin. Data were analyzed on an Olympus microscope. Five
random fields were selected for counting the apoptosis rate under
×100 magnification.
Statistical analysis
Statistical analyses were performed using the SPSS
software version 19.0 (IBM Corp., Armonk, NY, USA). Chi-square test
was used to analyze the correlation between β-arrestin2 expression
and clinicopathological characteristics. Data were presented as the
mean ± SE. Group differences were determined by one-way ANOVA and
Student's t-test. P-values <0.05 were considered to indicate
statistically significant differences.
Results
Increased expression of β-arrestin2 is
observed in CRC tissues compared to healthy colon tissues
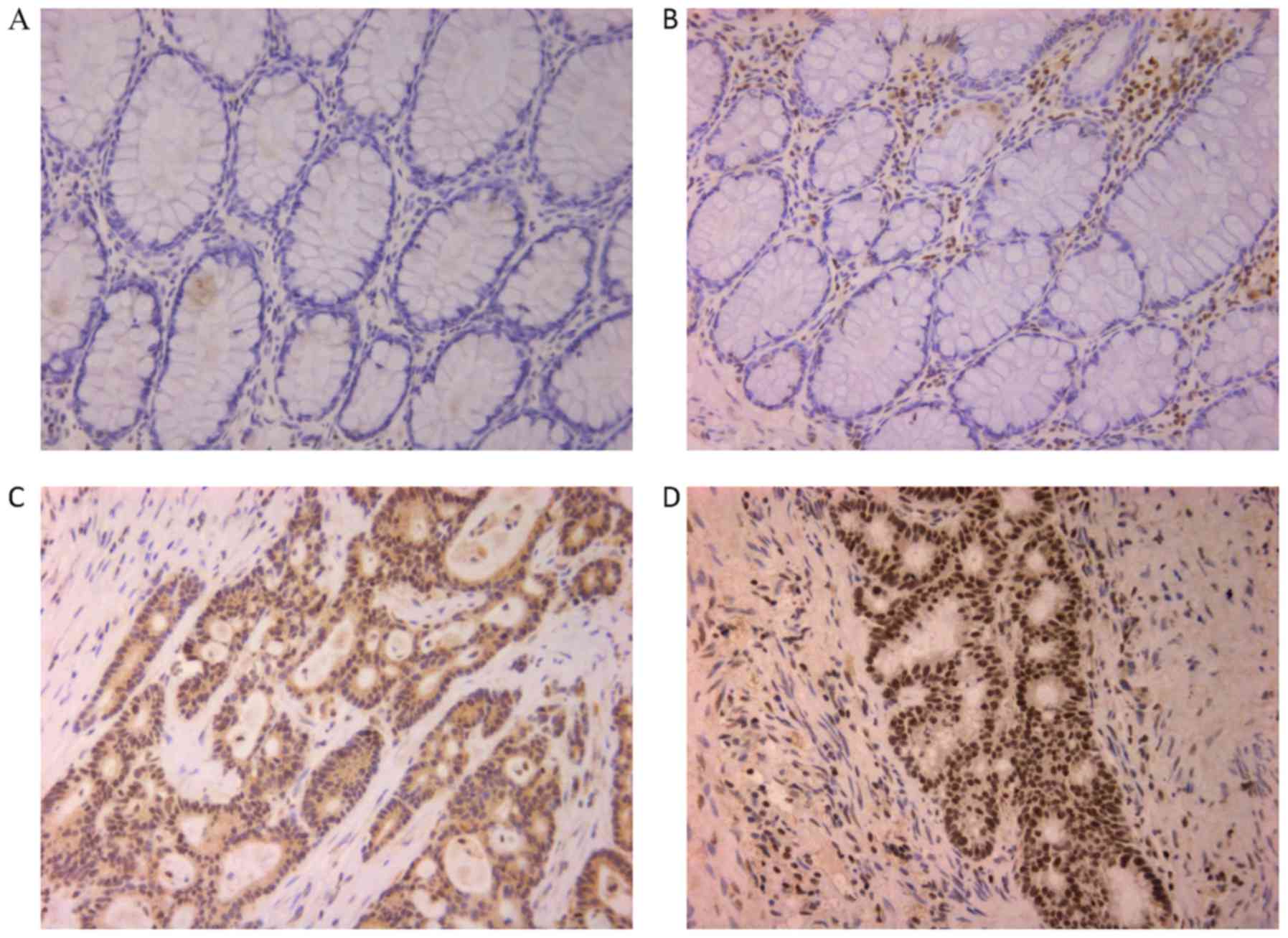
Immunohistochemistry was used to detect the
expression of β-arrestin2 in CRC and normal colon tissues.
Forty-one CRC and 18 healthy tissues were isolated. Significantly
higher expression of β-arrestin2 protein was observed in CRC
tissues compared to healthy tissues (P<0.05; Fig. 1; Table
I). The positive rate of β-arrestin2 expression in CRC was
60.98% (25/41), while it was 27.78% (5/18) in the healthy tissues.
Furthermore, β-arrestin2 was mainly expressed in the cytoplasm and
nucleus (Fig. 1C and D). In
summary, these data indicated that β-arrestin2 has an important
role in the initiation and development of CRC. However, no
correlation between β-arrestin2 expression in CRC and
clinicopathological characteristics, including TNM stage, tumor
volume and CEA was found (Table
II).
 | Table I.The expression of β-arrestin2 in
colon epithelial cells of normal colon mucosal tissue and
colorectal cancer tissue. |
Table I.
The expression of β-arrestin2 in
colon epithelial cells of normal colon mucosal tissue and
colorectal cancer tissue.
| Group | Positive | Negative | P-value |
|---|
| Normal colon
mucosal tissue | 5 | 13 | 0.043 |
| Colorectal cancer
tissue | 25 | 16 |
|
 | Table II.The correlation between the
expression of β-arrestin2 and patient clinicopathological
characteristics. |
Table II.
The correlation between the
expression of β-arrestin2 and patient clinicopathological
characteristics.
|
|
| β-arrestin2 |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | Cases | Positive | Positive ratio | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 21 | 14 | 0.67 | >0.05 |
|
Female | 20 | 11 | 0.55 |
|
| Age (years) |
|
|
|
|
|
≥60 | 31 | 20 | 0.65 | >0.05 |
|
<60 | 10 | 5 | 0.5 |
|
| Clinical stage |
|
|
|
|
|
I+II | 20 | 12 | 0.6 | >0.05 |
|
III+IV | 21 | 15 | 0.71 |
|
| Histopathological
type |
|
|
|
|
| Mucoid
adenocarcinoma | 6 | 5 | 0.83 | >0.05 |
|
Non-mucoid adenocarcinoma | 35 | 22 | 0.63 |
|
| pT |
|
|
|
|
|
pT1-3 | 20 | 15 | 0.75 | >0.05 |
|
pT4 | 21 | 12 | 0.57 |
|
| pN |
|
|
|
|
|
pN0 | 20 | 12 | 0.6 | >0.05 |
|
pN1-2 | 21 | 15 | 0.71 |
|
| pM |
|
|
|
|
|
pM0 | 33 | 22 | 0.67 | >0.05 |
|
pM1-2 | 8 | 5 | 0.63 |
|
| Lymph node
metastasis |
|
|
|
|
|
Negative | 22 | 12 | 0.55 | >0.05 |
|
Positive | 19 | 13 | 0.68 |
|
| Liver
metastasis |
|
|
|
|
|
Negative | 36 | 24 | 0.67 | >0.05 |
|
Positive | 5 | 3 | 0.6 |
|
| Peritoneal
dissemination |
|
|
|
|
|
Negative | 37 | 25 | 0.68 | >0.05 |
|
Positive | 4 | 2 | 0.5 |
|
| Grade |
|
|
|
|
|
Well-moderate | 28 | 15 | 0.54 | >0.05 |
|
Poor | 13 | 10 | 0.77 |
|
| Tumor volume
(mm) |
|
|
|
|
|
<50 | 20 | 12 | 0.6 | >0.05 |
|
≥50 | 21 | 13 | 0.62 |
|
| General type |
|
|
|
|
|
Non-ulcerative type | 15 | 10 | 0.67 | >0.05 |
|
Ulcerative type | 26 | 17 | 0.65 |
|
| CEA |
|
|
|
|
|
≥10 | 13 | 8 | 0.62 | >0.05 |
|
<10 | 28 | 17 | 0.61 |
|
β-arrestin2 downregulation has no
effect on LoVo cell proliferation, migration and invasion
To explore the role of β-arrestin2 in colon cancer
biological behavior (proliferation, invasion and migration) in
vitro, β-arrestin2 was downregulated in LoVo cells
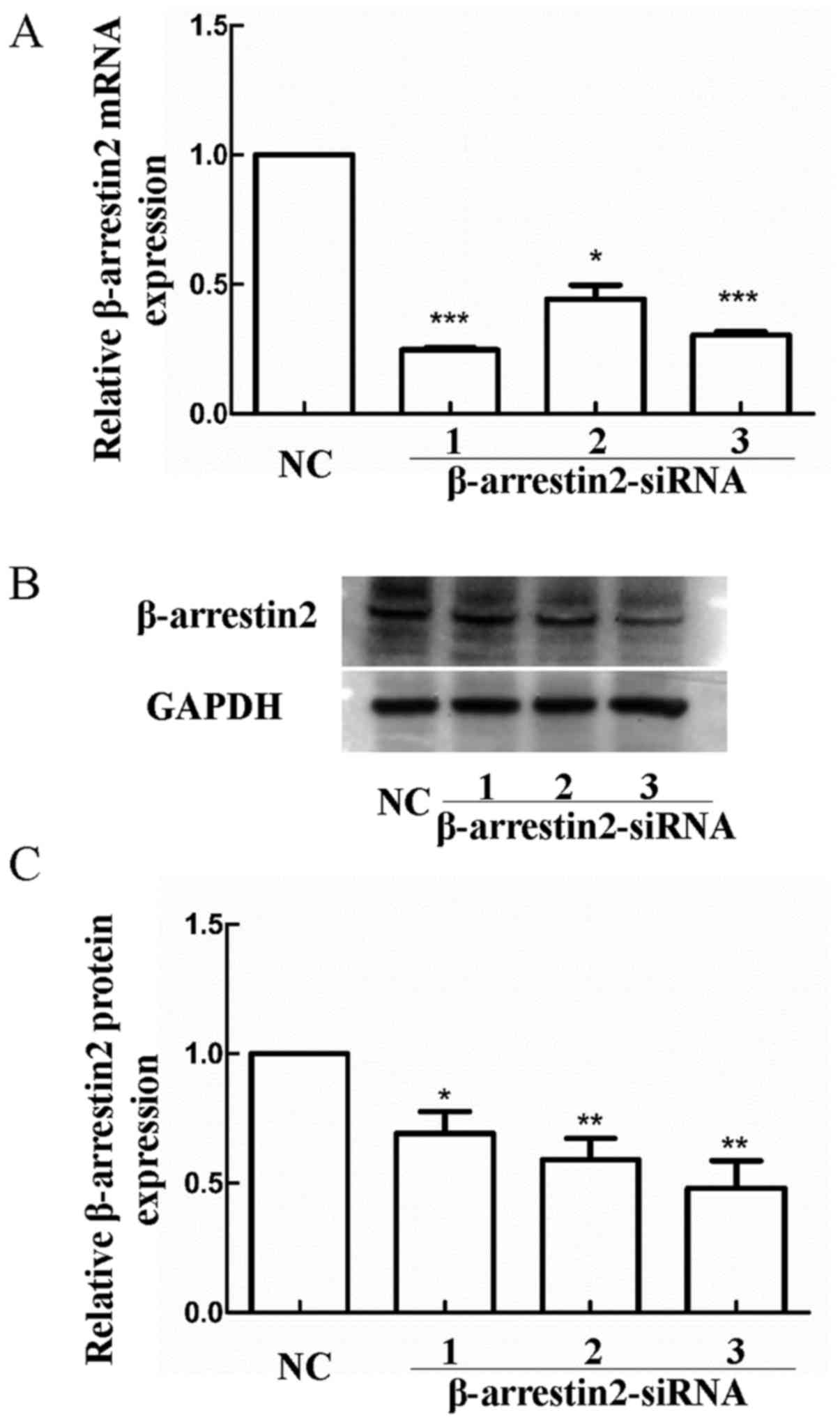
(β-arrestin2-siRNA group). Reduced β-arrestin2 expression was
confirmed by RT-PCR and western blot analysis (Fig. 2). Notably, no significant difference
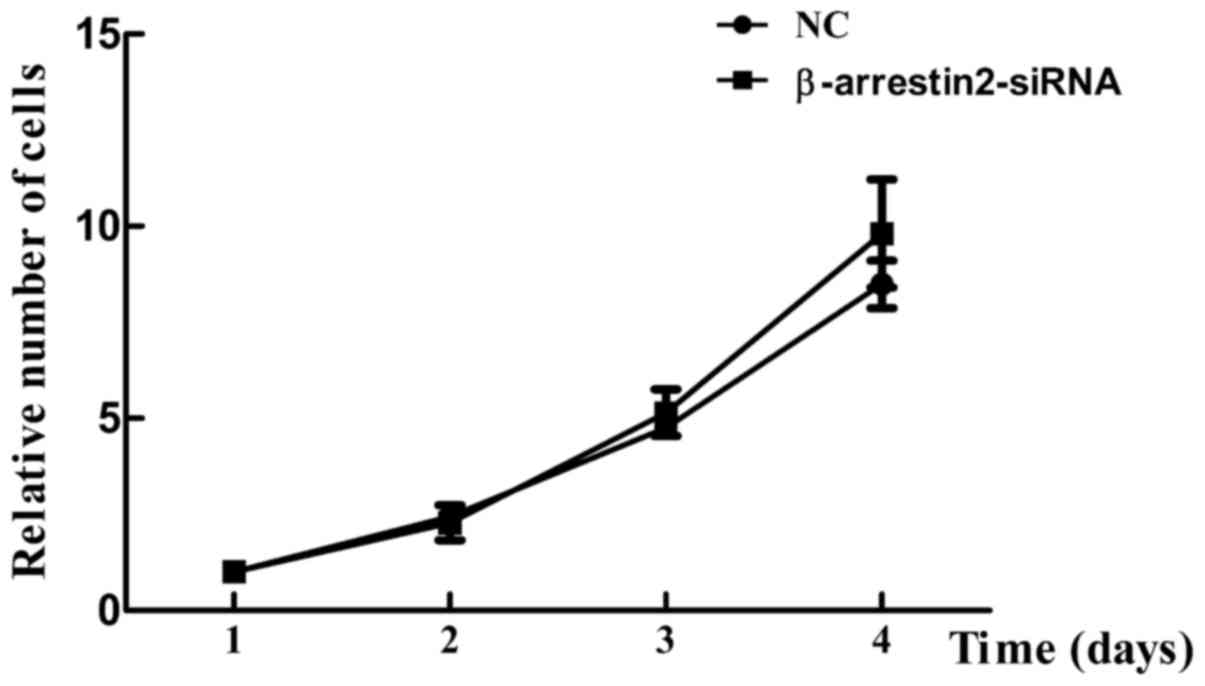
in cell viability was observed between the NC group and
β-arrestin2-siRNA group (Fig. 3),
indicating that β-arrestin2 downregulation was not associated with
cell proliferation.
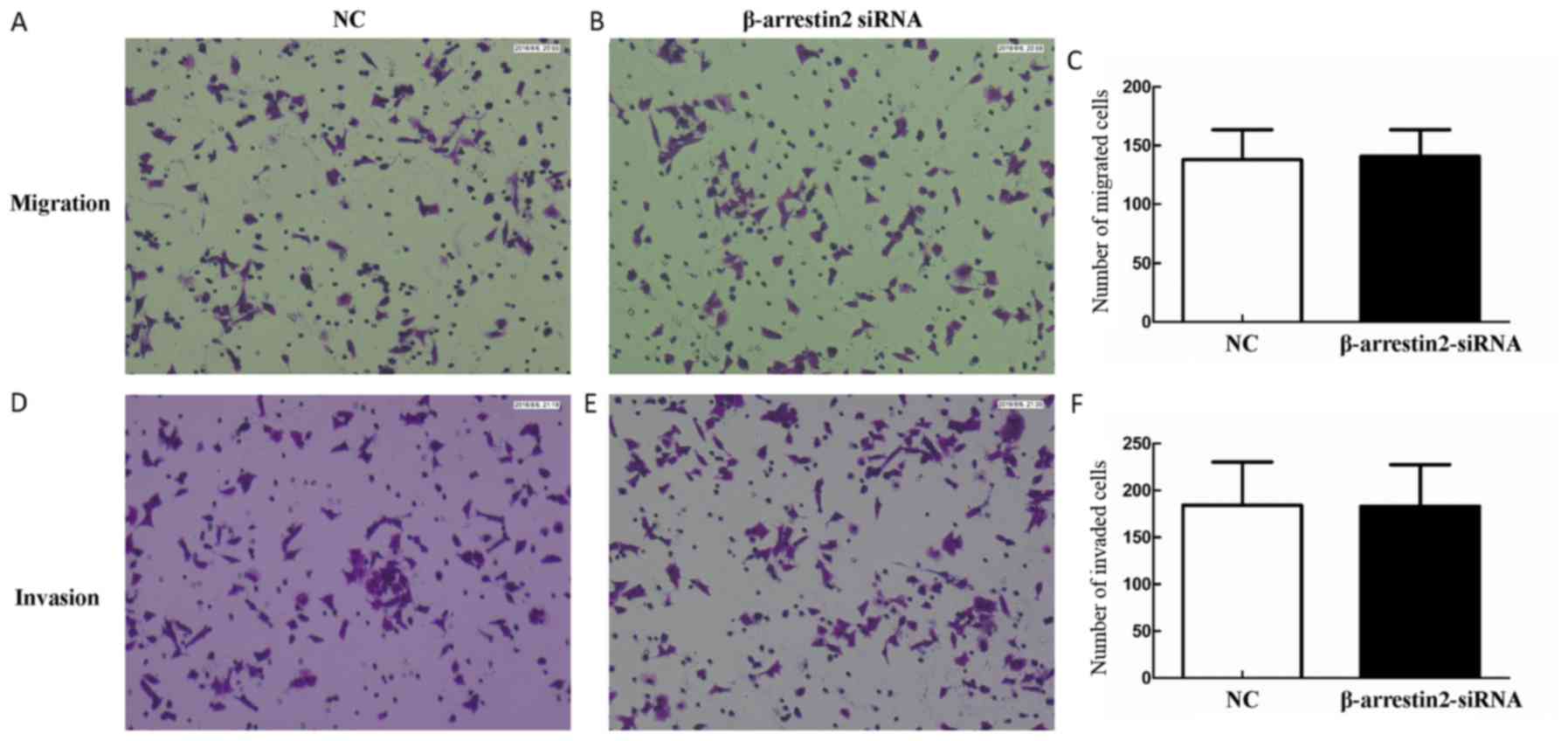
To determine the effect of β-arrestin2 on cell
migration and invasion, Transwell assay was performed. No
significant difference in cell migration and invasion between the
NC group and β-arrestin2-siRNA group was observed (migration,
137.86±44.29 vs. 140.78±39.06, P>0.05; invasion, 184.34±79.26
vs. 183.25±76.61, P>0.05) (Fig.
4). These results demonstrated that β-arrestin2 downregulation
was not associated with cell invasion and migration.
β-arrestin2 downregulation inhibits
the 5-FU-induced CRC cell apoptosis
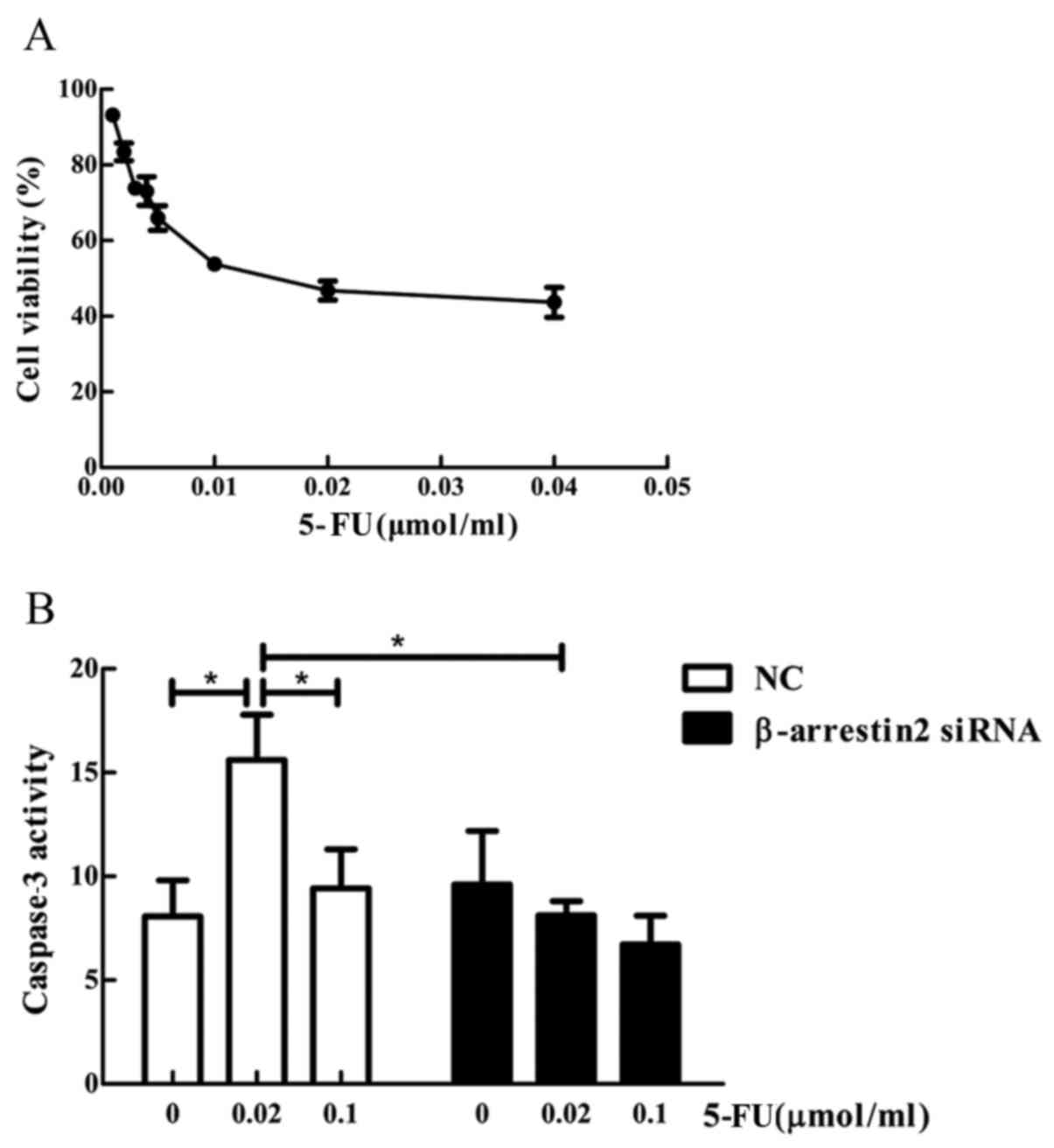
5-FU was selected to induce apoptosis of LoVo colon
cancer cells in vitro. CCK-8 assay was used to determine the
cell viability pre- and post- treatment. In the NC group, no
increase in caspase-3 activity was observed by increasing 5-FU
concentration, while, after treatment with 0.02 µmol/ml 5-FU,
decreased caspase-3 expression was observed in the
β-arrestin2-siRNA group compared with the NC group (15.614±3.781
vs. 8.133±1.173, P<0.05) (Fig.
5).
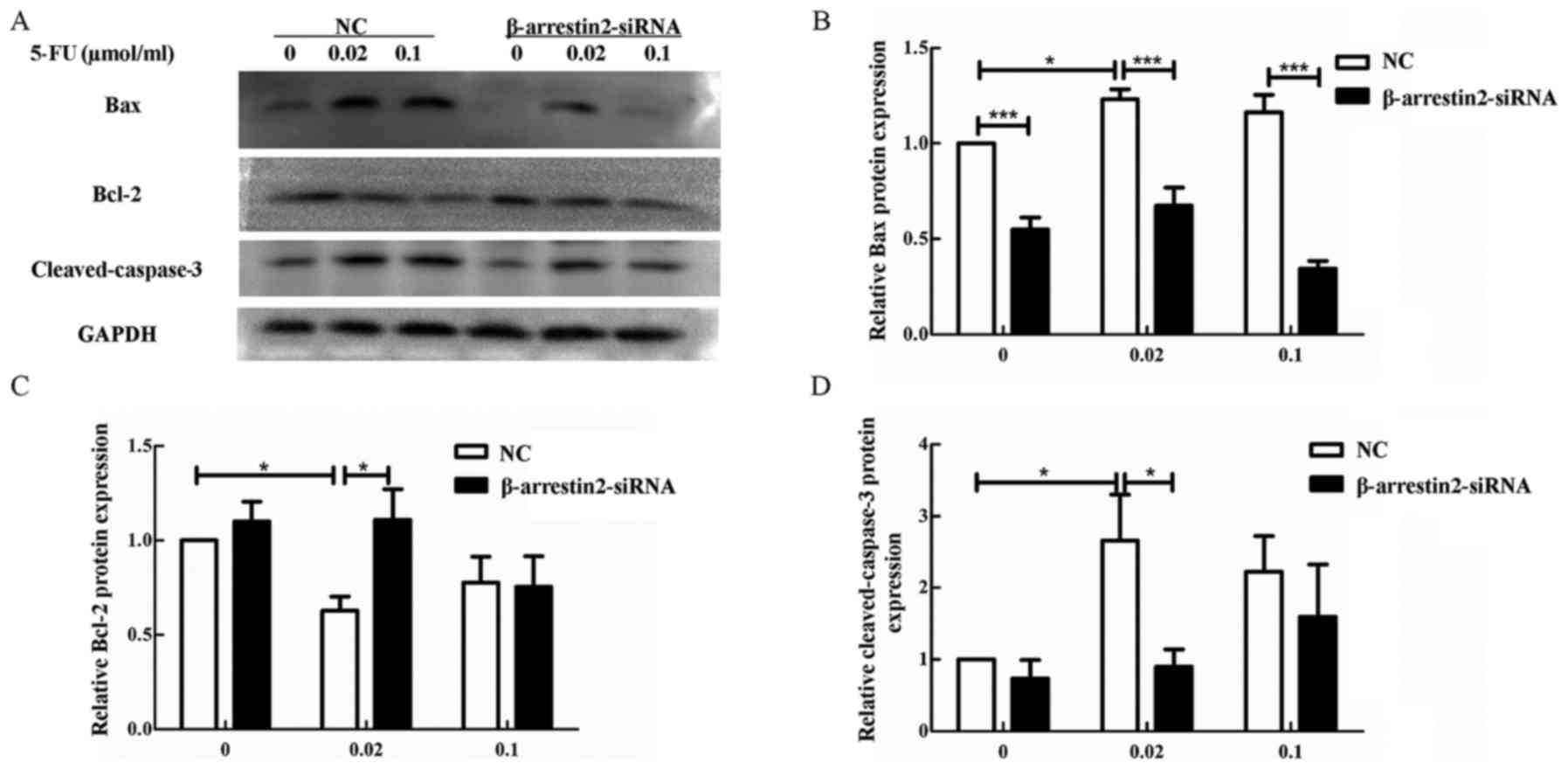
To further confirm our results, we detected the
apoptotic protein by western blot analysis. In the NC group, the
pro-apoptotic protein Bax and cleaved-caspase-3 were increased,
while the anti-apoptotic protein Bcl-2 was decreased after
stimulation with 0.02 µmol/ml 5-FU compared with the untreated
group (0 µmol/ml 5-FU) (Fig. 6,
P<0.05). Furthermore, the opposite effect was observed in the
β-arrestin2-siRNA group after stimulation with 0.02 µmol/ml 5-FU.
Cleaved-caspase-3 and Bax were significantly decreased, while Bcl-2
was increased in the β-arrestin2-siRNA group compared to NC group
(Fig. 6, P<0.05).
Altogether, the above mentioned data indicated that
β-arrestin2 downregulation inhibited the 5-FU-induced CRC cell
apoptosis, by reducing the expression of the pro-apoptotic proteins
cleaved-caspase-3 and Bax.
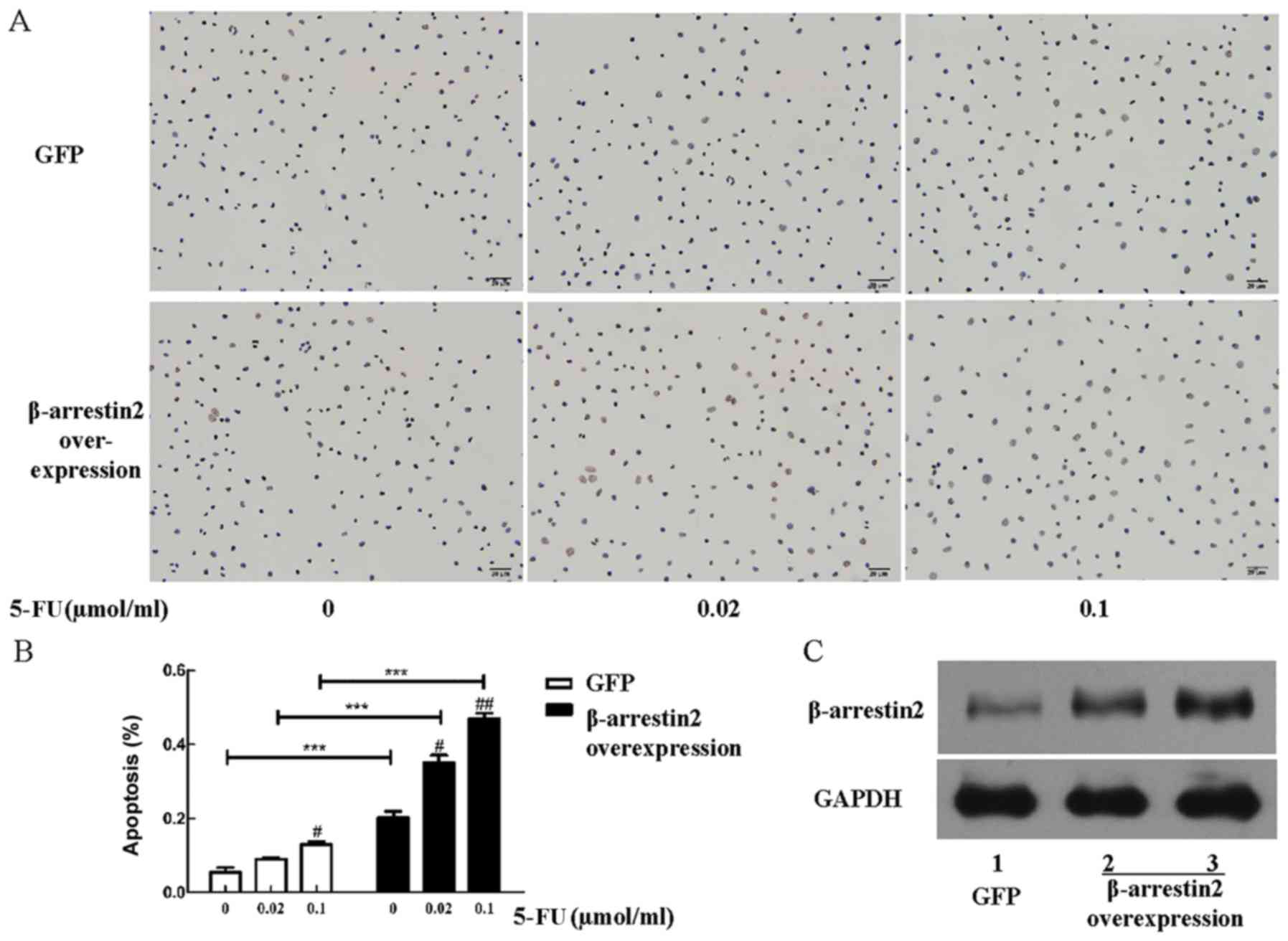
β-arrestin2 overexpression enhances
apoptosis after 5-FU treatment
In the present study we explored the effect of
β-arrestin2 overexpression on apoptosis after 5-FU treatment. The
human colon cancer cell line HCT116 was selected for the
β-arrestin2 overexpression experiment. Briefly, apoptosis of HCT116
cells increased with the concentration of 5-FU and enhanced
significantly after β-arrestin2 overexpression (Fig. 7A and B), indicating that the
overexpression of β-arrestin2 plays an important part in cell
apoptosis.
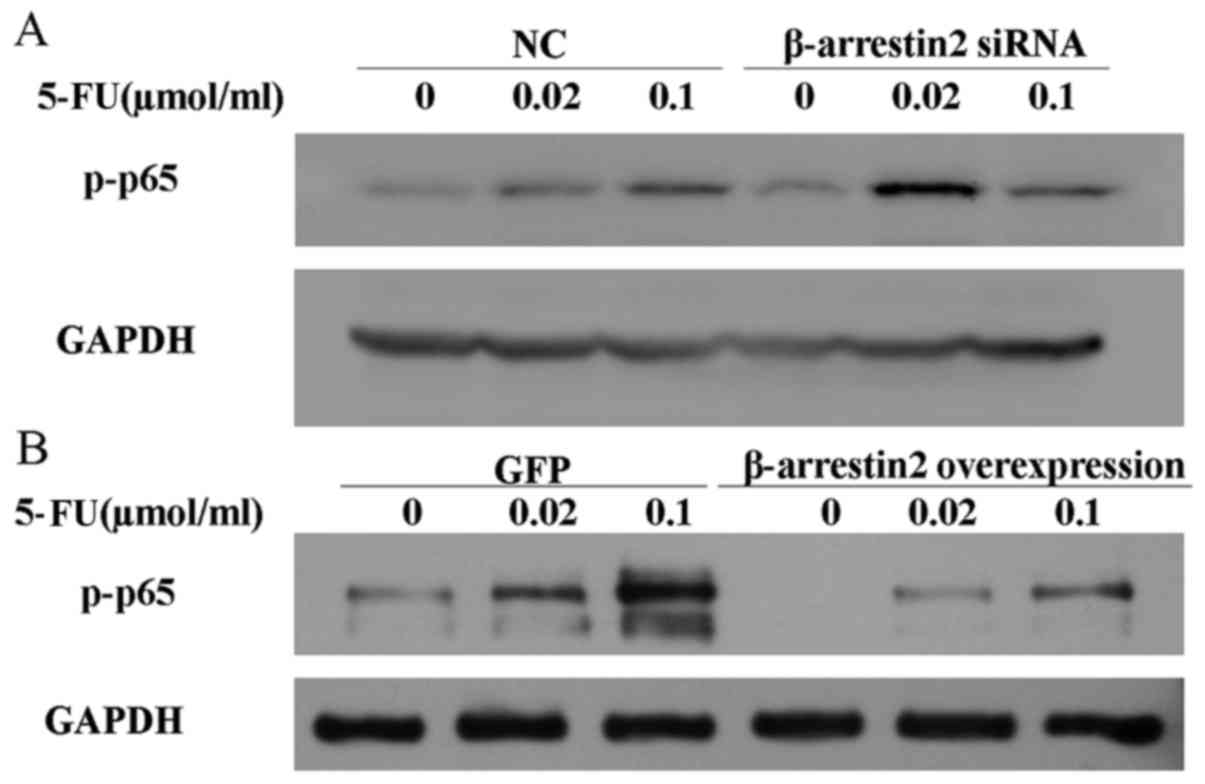
β-arrestin2 inhibits the expression of
p-p65
It has been proved that the NF-κB activation is
associated with the development of chemoresistance to 5-FU in colon
and breast cancer cells. In addition, β-arrestin2 is a negative
element for the NF-κB activity. Therefore, we questioned whether
the effect of β-arrestin2 on apoptosis depends on NF-κB activity.
The expression of p-p65 was determined by western blot analysis
after the downregulation and the overexpression of β-arrestin2. As
displayed in Fig. 8, in the cells
were β-arrestin2 was downregulated, the level of p-p65 increased
after stimulation with 0.02 µmol/ml 5-FU, while it decreased in the
cells were β-arrestin2 was overexpressed. Collectively, these data
demonstrated that β-arrestin2 was involved in 5-FU-induced cell
apoptosis in an NF-κB-dependent manner.
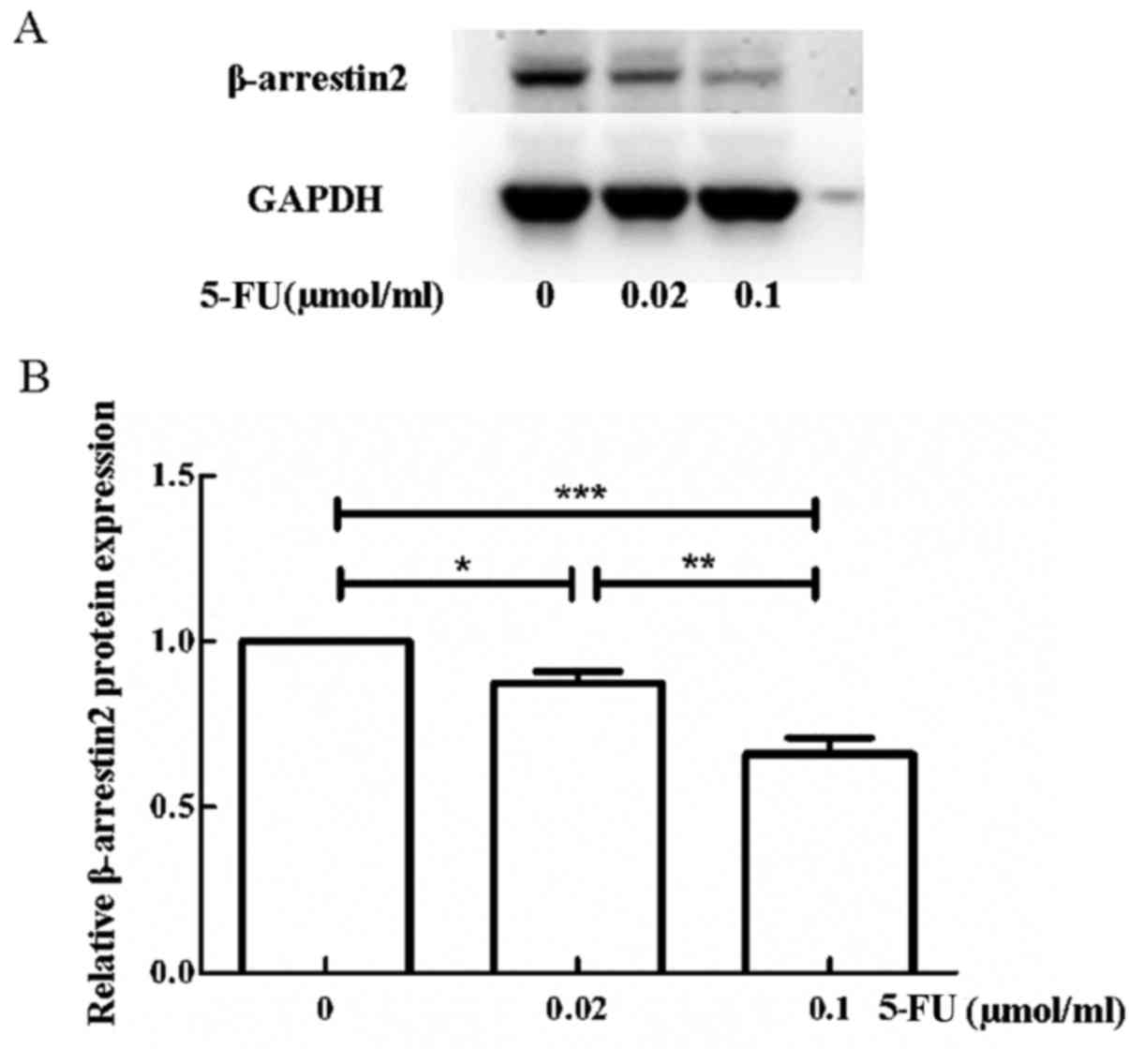
β-arrestin2 expression decreases with
5-FU stimulation
Since β-arrestin2 has a pro-apoptotic effect, we
explored the β-arrestin2 expression of the LoVo cells after
stimulation with 5-FU. As displayed in Fig. 9, β-arrestin2 expression of LoVo
cells decreased with 5-FU stimulation.
Discussion
CRC is a common cancer with a high mortality rate
especially in metastatic CRC (1,3).
Although many different methods have been used for the treatment of
CRC, 5-FU is considered the first line treatment of CRC. However,
5-FU has been shown to be effective only in 31% of cases when used
as a sole drug for CRC. Understanding the mechanisms that lead to
the unsatisfactory effect of CRC could be extremely beneficial in
identifying effective therapies for CRC. The present study revealed
that β-arrestin2 was essential in 5-FU-stimulated apoptotic
responses, and it delineated the downstream biochemical pathways
responsible for 5-FU-stimulated, β-arrestin2-mediated pro-apoptotic
effect. Thereby, it revealed a novel potential mechanism for 5-FU
effect mediated by β-arrestin2.
β-arrestin2, also known as arrestin3 and ARRB2, has
been initially known for its ability to mediate the desensitization
of GPCR signaling (19).
Accordingly, it can interact with GPCR acting as a scaffold protein
for the recruitment of many cytoplasmic signaling proteins, such as
c-Src (5). β-arrestin2 can mediate
agonist-induced signaling leading to the signaling pathway
activation of ERK, MAPK and PI3K, and the inhibition of NF-κB
(7,8). Thus, β-arrestin2 is involved in many
cellular processes associated with cell proliferation, migration,
invasion and apoptosis. Growing evidence indicates that by
affecting cellular responses, β-arrestin2 has many additional
effects that are related to cancer initiation and progression
(9,10).
Many studies have confirmed that β-arrestin2 is
abnormally expressed in cancer. Compared with normal tissues,
β-arrestin2 is overexpressed in many cancers, such as breast cancer
(20). However, the expression of
β-arrestin2 decreases in hepatocellular cancer and non-small cell
lung cancer (15,21). To confirm the role of β-arrestin2 in
CRC, we first examined the expression of β-arrestin2 in CRC tissues
and normal colon tissues. The obtained results demonstrated that
β-arrestin2 expression in CRC was significantly increased compared
with normal colon tissues, which means that β-arrestin2 may exert a
different regulatory function in CRC. It has been established that
low β-arrestin2 expression in hepatocellular cancer was correlated
with aggressive pathological features, including advanced tumor
stage, metastasis, poor cell differentiation and large tumor size
(15). Nonetheless, its role in CRC
is still not well understood. In contrast, β-arrestin2
overexpression is not associated with CRC clinicopathological
features, such as tumor volume, TNM stage and CEA level.
Furthermore, β-arrestin2 expression may serve as a prognosis
indication. Lower β-arrestin2 expression indicates poor prognosis
in NSCLC and hepatocellular cancer (15,21).
We will follow the survival time of CRC patients in our next study.
It will clarify the relation between the survival rate of CRC
patients and β-arrestin2 expression. The role of β-arrestin2 in
cancer is very distinct which could mean it is tissue-specific.
Although clinical data have not revealed a clear
role of β-arrestin2 in CRC, we obtained colon cancer cells for
in vitro experiments. β-arrestin2 has progressive and
restrictive double functions in cell proliferation (11,13,14).
β-arrestin2 promotes the proliferation of chronic myelogenous
leukemia and castration-resistant prostate cancer, but inhibits
lung cancer growth (13,14). However, in the present study the
proliferation rate did not change following β-arrestin2
downregulation in CRC, which was in accordance with results
previously reported by Bonnans et al (12), where β-arrestin2 depletion caused
only 33% of the tumors in ApcΔ14/+ mice while the tumor
size revealed no alterations compared to the WT ApcΔ14/+
mice (12). As for the effect of
β-arrestin2 on metastasis, it suppresses the migration and invasion
of hepatocellular cancer and lung cancer, but promotes breast
cancer (14–16,20,22).
In the present study, there were no differences in cell migration
and invasion capacity after the β-arrestin2 downregulation,
indicating that β-arrestin2 did not affect the proliferation,
migration and invasion capacity of colon cancer cells. Each cancer
has its specific mechanisms of initiation and development.
β-arrestin2 is a multifunctional protein that is involved in many
cellular responses and signal pathways (7,8).
Accordingly, we postulated that the role of β-arrestin2 in cancer
depends on the organizational specificity.
Accumulating evidence has uncovered the role of
β-arrestin2 in apoptosis (23,24)
and the dual effect of β-arrestin2 in apoptosis (24–27).
Under different conditions, β-arrestin2 can be pro-apoptotic or
anti-apoptotic (28–30). In the present study, 5-FU, as a core
drug for CRC, was selected as a drug-interfering factor. Caspase-3
activation is a hallmark for apoptosis. We examined the activity of
caspase-3 using caspase-3 activity assay kit and determined the
cleaved-caspase-3, Bax and Bcl-2 expression by western blot
analysis. Our results revealed that the activity of caspase-3 was
suppressed, the expression of pro-apoptotic protein Bax and
cleaved-caspase-3 was decreased and the anti-apoptotic protein
Bcl-2 was increased after β-arrestin2 downregulation. Thus, these
results indicated that β-arrestin2 downregulation prevented colon
cancer cells from 5-FU-induced apoptosis. To obtain a better
understanding of β-arrestin2 function on apoptosis, we
overexpressed the expression of β-arrestin2 in human colon cancer
cell line HCT116 and found that the cell apoptosis induced by 5-FU
increased after β-arrestin2 overexpression. This indicated that
β-arrestin2 promoted 5-FU-induced apoptosis in colon cancer cells.
In a previous study by Zeng et al (29) it has been proved that β-arrestin2
promotes inflammation-induced epithelial apoptosis through ER
stress/PUMA in colitis. Liu et al (17) demonstrated that β-arrestin2
deficiency was associated with radiation-induced intestinal crypt
progenitor cell apoptosis through protracted NF-κB activation and
suppression of PUMA. Consequently, we surmised that β-arrestin2 can
be pro-apoptotic in the intestinal crypt cells. However, additional
studies need to be performed in order to confirm this
hypothesis.
We have proved that β-arrestin2 promoted
5-FU-induced apoptosis in colon cancer cells but the underlying
mechanisms remain to be investigated. The activation of the NF-κB
pathway has shown to be enhanced in a variety of cancers, including
renal cancer, CRC and prostate cancer (31–33).
It has been proved that NF-κB activation is associated with the
development of chemoresistance to 5-FU in CRC and gastric cancer
(34,35). Many chemotherapy drugs have been
demonstrated to be more effective when combining the inhibition of
the NF-κB pathway (36–39). β-arrestin2 has been proved to
inhibit the NF-κB activation through its direct interaction with
IкBα or by inhibiting TRAF6 self-ubiquitination (40,41).
Accordingly, we postulated that β-arrestin2 promoted apoptosis by
inhibiting the NF-κB activation. The increase of p-p65 following
5-FU stimulation in the LoVo and HCT116 cells is a firm proof of
chemoresistance. As we expected, the level of p-p65 increased by
0.02 µmol/ml 5-FU stimulation after the β-arrestin2 downregulation
in LoVo cells, and decreased after β-arrestin2 overexpression in
HCT116 cells. Accordingly, we concluded that β-arrestin2 promoted
apoptosis by inhibiting the NF-κB activation.
Furthermore, we noticed that the caspase-3 activity
was not enhanced upon stimulation with 0.1 µmol/ml 5-FU compared
with the 0 µmol/ml 5-FU stimulation. We also demonstrated that
β-arrestin2 decreased with 5-fu stimulation. Based on these
findings, we postulated that the expression of β-arrestin2
decreased to a low level following stimulation by 5-FU.
Furthermore, β-arrestin2 in CRC can be indicative of sensitivity to
chemotherapy and can even serve as a marker of prognosis, which
could be proved by further studies.
In conclusion, our results demonstrated the role of
β-arrestin2 in CRC. β-arrestin2 promoted 5-FU-induced apoptosis via
the NF-κB pathway and could serve as a favorable prognostic
biomarker for CRC.
Acknowledgements
We would like to thank Dr Tiezheng Sun for his
technical support.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 812246) and the
Peking University People's Hospital Research and Development Funds
(no. 2118000542).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YZ and YL conceived and designed the study. WR, TW,
QZ, FL and FG performed the experiments. XH and JZ offered the
technical support. WR wrote the manuscript. YZ reviewed and edited
the manuscript. All authors read and approved the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Human Ethics Review Board of the Peking University People's
Hospital (Beijing, China).
Consent for publication
Written informed consents for publication of their
clinical details were obtained from the patient or their
parents.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
siRNA
|
small interfering RNA
|
|
NC
|
negative control
|
|
GPCR
|
G protein-coupled receptor
|
|
PCR
|
polymerase chain reaction
|
|
NSCLC
|
non-small cell lung cancer
|
References
|
1
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Douaiher J, Ravipati A, Grams B, Chowdhury
S, Alatise O and Are C: Colorectal cancer-global burden, trends,
and geographical variations. J Surg Oncol. 115:619–630. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lefkowitz RJ and Shenoy SK: Transduction
of receptor signals by beta-arrestins. Science. 308:512–517. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Luttrell LM and Lefkowitz RJ: The role of
beta-arrestins in the termination and transduction of
G-protein-coupled receptor signals. J Cell Sci. 115:455–465.
2002.PubMed/NCBI
|
|
7
|
Lefkowitz RJ, Rajagopal K and Whalen EJ:
New roles for beta-arrestins in cell signaling: Not just for
seven-transmembrane receptors. Mol Cell. 24:643–652. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Barki-Harrington L and Rockman HA:
Beta-arrestins: Multifunctional cellular mediators. Physiology.
23:17–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sobolesky PM and Moussa O: The role of
β-arrestins in cancer. Prog Mol Biol Transl Sci. 118:395–411. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu S, Wang D, Wu J, Jin J, Wei W and Sun
W: Involvement of β-arrestins in cancer progression. Mol Biol Rep.
40:1065–1071. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fereshteh M, Ito T, Kovacs JJ, Zhao C,
Kwon HY, Tornini V, Konuma T, Chen M, Lefkowitz RJ and Reya T:
β-Arrestin2 mediates the initiation and progression of myeloid
leukemia. Proc Natl Acad Sci USA. 109:12532–12537. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bonnans C, Flacelière M, Grillet F, Dantec
C, Desvignes JP, Pannequin J, Severac D, Dubois E, Bibeau F,
Escriou V, et al: Essential requirement for β-arrestin2 in mouse
intestinal tumors with elevated Wnt signaling. Proc Natl Acad Sci
USA. 109:3047–3052. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Duan X, Kong Z, Liu Y, Zeng Z, Li S, Wu W,
Ji W, Yang B, Zhao Z and Zeng G: β-Arrestin2 contributes to cell
viability and proliferation via the down-regulation of FOXO1 in
castration-resistant prostate cancer. J Cell Physiol.
230:2371–2381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raghuwanshi SK, Nasser MW, Chen X,
Strieter RM and Richardson RM: Depletion of beta-arrestin-2
promotes tumor growth and angiogenesis in a murine model of lung
cancer. J Immunol. 180:5699–5706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun WY, Hu SS, Wu JJ, Huang Q, Ma Y, Wang
QT, Chen JY and Wei W: Down-regulation of β-arrestin2 promotes
tumour invasion and indicates poor prognosis of hepatocellular
carcinoma. Sci Rep. 6:356092016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goertzen CG, Dragan M, Turley E, Babwah AV
and Bhattacharya M: KISS1R signaling promotes invadopodia formation
in human breast cancer cell via β-arrestin2/ERK. Cell Signal.
28:165–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu Z, Tian H, Jiang J, Yang Y, Tan S, Lin
X, Liu H and Wu B: β-Arrestin-2 modulates radiation-induced
intestinal crypt progenitor/stem cell injury. Cell Death Differ.
23:1529–1541. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jing X, Zhang H, Hu J, Su P, Zhang W, Jia
M, Cheng H, Li W and Zhou G: β-arrestin 2 is associated with
multidrug resistance in breast cancer cells through regulating MDR1
gene expression. Int J Clin Exp Pathol. 8:1354–1363.
2015.PubMed/NCBI
|
|
19
|
DeWire SM, Ahn S, Lefkowitz RJ and Shenoy
SK: Beta-arrestins and cell signaling. Annu Rev Physiol.
69:483–510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li TT, Alemayehu M, Aziziyeh AI, Pape C,
Pampillo M, Postovit LM, Mills GB, Babwah AV and Bhattacharya M:
Beta-arrestin/Ral signaling regulates lysophosphatidic
acid-mediated migration and invasion of human breast tumor cells.
Mol Cancer Res. 7:1064–1077. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Z, Tong W, Tan Z, Wang S and Lin P: The
clinical significance of β-arrestin 2 expression in the serum of
non-small cell lung cancer patients. Zhongguo Fei Ai Za Zhi.
14:497–501. 2011.(In Chinese). PubMed/NCBI
|
|
22
|
Alemayehu M, Dragan M, Pape C, Siddiqui I,
Sacks DB, Di Guglielmo GM, Babwah AV and Bhattacharya M:
β-Arrestin2 regulates lysophosphatidic acid-induced human breast
tumor cell migration and invasion via Rap1 and IQGAP1. PLoS One.
8:e561742013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharma D and Parameswaran N: Multifaceted
role of β-arrestins in inflammation and disease. Genes Immun.
16:499–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Revankar CM, Vines CM, Cimino DF and
Prossnitz ER: Arrestins block G protein-coupled receptor-mediated
apoptosis. J Biol Chem. 279:24578–24584. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ahn S, Kim J, Hara MR, Ren XR and
Lefkowitz RJ: {beta}-Arrestin-2 mediates anti-apoptotic signaling
through regulation of BAD phosphorylation. J Biol Chem.
284:8855–8865. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang P, Gao H, Ni Y, Wang B, Wu Y, Ji L,
Qin L, Ma L and Pei G: Beta-arrestin 2 functions as a
G-protein-coupled receptor-activated regulator of oncoprotein Mdm2.
J Biol Chem. 278:6363–6370. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Luan B, Zhang Z, Wu Y, Kang J and Pei G:
Beta-arrestin2 functions as a phosphorylation-regulated suppressor
of UV-induced NF-kappaB activation. EMBO J. 24:4237–4246. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun X, Zhang Y, Wang J, Wei L, Li H,
Hanley G, Zhao M, Li Y and Yin D: Beta-arrestin 2 modulates
resveratrol-induced apoptosis and regulation of Akt/GSK3β pathways.
Biochim Biophys Acta. 1800:912–918. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zeng LX, Tao J, Liu HL, Tan SW, Yang YD,
Peng XJ, Liu ZH, Jiang J and Wu B: β-Arrestin2 encourages
inflammation-induced epithelial apoptosis through ER stress/PUMA in
colitis. Mucosal Immunol. 8:683–695. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang F, Liu J, Fu H, Wang J, Li F, Yue H,
Li W, Zhao J and Yin D: GSK-3β promotes PA-induced apoptosis
through changing β-arrestin 2 nucleus location in H9c2
cardiomyocytes. Apoptosis. 21:1045–1055. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oya M, Takayanagi A, Horiguchi A, Mizuno
R, Ohtsubo M, Marumo K, Shimizu N and Murai M: Increased nuclear
factor-kappa B activation is related to the tumor development of
renal cell carcinoma. Carcinogenesis. 24:377–384. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lind DS, Hochwald SN, Malaty J, Rekkas S,
Hebig P, Mishra G, Moldawer LL, Copeland EM III and Mackay S:
Nuclear factor-kappa B is upregulated in colorectal cancer.
Surgery. 130:363–369. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lessard L, Mes-Masson AM, Lamarre L, Wall
L, Lattouf JB and Saad F: NF-kappa B nuclear localization and its
prognostic significance in prostate cancer. BJU Int. 91:417–420.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Camp ER, Li J, Minnich DJ, Brank A,
Moldawer LL, MacKay SL and Hochwald SN: Inducible nuclear
factor-kappaB activation contributes to chemotherapy resistance in
gastric cancer. J Am Coll Surg. 199:249–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Körber MI, Klingenbrunner S, Bartsch R,
Steger GG and Mader RM: NF-κB addiction and resistance to
5-fluorouracil in a multi-stage colon carcinoma model. Int J Clin
Pharmacol Ther. 51:35–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Endo F, Nishizuka SS, Kume K, Ishida K,
Katagiri H, Ishida K, Sato K, Iwaya T, Koeda K and Wakabayashi G: A
compensatory role of NF-κB to p53 in response to 5-FU-based
chemotherapy for gastric cancer cell lines. PLoS One. 9:e901552014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kwon OH, Kim JH, Kim SY and Kim YS:
TWEAK/Fn14 signaling mediates gastric cancer cell resistance to
5-fluorouracil via NF-κB activation. Int J Oncol. 44:583–590. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Shakibaei M, Mobasheri A, Lueders C, Busch
F, Shayan P and Goel A: Curcumin enhances the effect of
chemotherapy against colorectal cancer cells by inhibition of NF-κB
and Src protein kinase signaling pathways. PLoS One. 8:e572182013.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu B, Guo X, Mathew S, Armesilla AL,
Cassidy J, Darling JL and Wang W: Triptolide simultaneously induces
reactive oxygen species, inhibits NF-kappaB activity and sensitizes
5-fluorouracil in colorectal cancer cell lines. Cancer Lett.
291:200–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao H, Sun Y, Wu Y, Luan B, Wang Y, Qu B
and Pei G: Identification of beta-arrestin2 as a G protein-coupled
receptor-stimulated regulator of NF-kappaB pathways. Mol Cell.
14:303–317. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Tang Y, Teng L, Wu Y, Zhao X and
Pei G: Association of beta-arrestin and TRAF6 negatively regulates
Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol.
7:139–147. 2006. View
Article : Google Scholar : PubMed/NCBI
|