Introduction
Gastric cancer has a high incidence and mortality in
China (1). Because the symptoms of
early gastric cancer are not obvious, most cases of tumor invasion
and metastasis are diagnosed only when patients are hospitalized
(2). Low chemotherapy sensitivity
results in poor survival rates among gastric cancer patients
(2,3). Therefore, finding effective targets to
inhibit the growth and metastasis of gastric cancer is particularly
important. ERBB4 is a large transmembrane glycoprotein, and has
tyrosine kinase activity (4). Once
combined with epidermal growth factor (EGF), ERBB4 can activate the
related genes in the nucleus, thus promoting cell division and
proliferation (5,6). The expression of ERBB4 was revealed to
be increased in gastric, breast, and bladder cancer as well as head
and neck squamous cell carcinoma (7–10). In
addition, ERBB4 has been revealed to contribute to and play an
important role in tumor cell survival, proliferation, and motility
(11,12). ERBB4 has been demonstrated to be
overexpressed or with aberrant expression in a variety of solid
tumors especially in gastric cancer (13–16).
Therefore, ERBB4 may become a very important drug target for the
therapy of gastric cancer. Unfortunately, more research attention
has been paid to ERBB2 overlooking ERBB4. In the present study, we
investigated the effect of ERBB4 in the proliferation of gastric
cancer cells. We found that high ERBB4 levels were closely related
to the poor prognosis of gastric cancer patients. ERBB4 was highly
expressed in gastric cancer cell lines when compared to the normal
stomach cell line, GES. Clinical samples provided the same results.
We also found that ERBB4 regulated cell proliferation mainly
through the PI3K/Akt signaling pathway. Thus, ERBB4 may become a
potential therapeutic target for the treatment of gastric
cancer.
Materials and methods
Patients and tissue specimens
Gastric cancer tumor tissues with matched adjacent
non-tumor tissues were obtained from 27 patients that underwent
surgical treatment at Zhejiang Provincial People's Hospital
(Hangzhou, China). All patients provided a signed informed consent.
None of them had a history of chemotherapy or radiotherapy before
sampling, and the diagnosis of gastric cancer was pathologically
confirmed. This study was approved by the Institutional Ethics
Committee of Zhejiang Provincial People's Hospital.
Reagents and antibodies
RPMI-1640 was acquired from Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Fetal bovine serum (FBS) was
purchased from Gibco (Rockville, MD, USA). Inhibitors AST-1306 and
LY294002 were obtained from Selleck Chemicals (Houston, TX, USA). A
BCA Protein Assay kit was purchased from Beyotime Institute of
Biotechnology (Shanghai, China). Antibodies against β-actin (cat.
no. 4970S), ERBB4 (cat. no. 4795S), PI3K (cat. no. 4249S), Akt
(cat. no. 9272S), and p70S6K (cat. no. 2708S) and secondary
antibodies (cat. no. 7054S) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). All antibody dilution ratio
was 1:1,000. MTT was purchased from Beyotime Institute of
Biotechnology. Other chemicals were obtained from commercial
sources. An IHC staining kit was purchased from Thermo Fisher
Scientific, Inc.
Cell culture and transfection
Gastric cancer cell lines MNK-45 and SGC-7901 were
acquired from the Cell Bank of the Chinese Academy of Sciences
(Shanghai Institute of Cell Biology, Shanghai, China). MNK-45 and
SGC-7901 cells were cultured in RPMI-1640 containing 10% FBS and
maintained at 37°C in an atmosphere of 5% CO2 in a
humidified incubator. MNK-45 and SGC-7901 cells
(1.5×105) were seeded in 6-well plates, and incubated
for 12 h, then transfected with a lentiviral vector encoding small
interfering RNA targeting ERBB4 [lentiviral vector siRNA-ERBB4
(Lv-siRNA-ERBB4)] and negative control lentiviral vector (Lv-NC).
Lv-siRNA-ERBB4 and Lv-NC were synthesized by Shanghai GeneChem,
Co., Ltd. (Shanghai, China). ViralPlus Transduction Enhancer and
Polybrene were used for lentiviral vector transfection.
Western blot analysis
Cells or tissues were lysed using RIPA buffer
containing 1 mM PMSF protease inhibitor mixture. The total protein
concentration was assessed using the BCA assay and was equalized
with the extraction reagent. Protein (10 µg/lane) samples were
separated by 10% SDS-PAGE, and electrotransferred onto PVDF
nitrocellulose membranes The membranes were blocked with 5% skim
milk for 2 h at 37°C. Then, the membranes were incubated with
primary antibodies against β-actin (cat. no. 4970S), ERBB4 (cat.
no. 4795S), PI3K (cat. no. 4249S) Akt (cat. no. 9272S) and
secondary antibody anti-rabbit IgG (cat. no. 7054S; all from Cell
Signaling Technology, Danvers, MA, USA) and diluted 1:1,000.
Subsequently, a Gel imaging analysis system and Pierce Western Blot
Signal Enhancer (cat. no. 21050; Thermo Fisher Scientific, Inc.)
were used to detect the protein bands.
Cell proliferation assay
The cell proliferation of MNK-45 and SGC-7901 cells
was analyzed using flow cytometry. Briefly, the cells were plated
at a density of 1,000-10,000 cells/well in a 96-well plate, and
incubated at 37°C for 12, 24, 36, 48 and 60 h. At 12, 24, 48 or 60
h, the cells were incubated with RPMI-1640 medium containing 0.5
mg/ml MTT at 37°C for 4 h. Formazan crystals were dissolved with
150 µl DMSO. The absorbance of each well, including blanks, was
measured at 490 nm using an automatic microplate reader subsequent
to 10 min of oscillation. The formula of the cell growth inhibition
rate was as follows: Inhibition rate = (1 - absorbance of the COE
group/absorbance of the blank control group) ×100%.
In vivo imaging technology
In order to further examine the effect of ERBB4 on
the growth of gastric cancer cells in vivo, the animal
imaging technique was used to perform the assay in vivo.
Four-week-old nude mice were purchased from the Laboratory Animal
Center of Zhejiang University. All animal experiments were
performed in accordance with the guidelines of the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals. Human gastric cancer cell lines MNK-45 were transfected
with Lv-siRNA-ERBB4-GFP and Lv-NC-GFP and then transplanted
subcutaneously in nude mice. Tumor cells labeled with green
fluorescence were used for in vivo fluorescence imaging
after 7 days. The initial inoculum concentration of MNK-45 was
increased to 1×107 cells/ml. Tumors were formed ~7 days
after the injection. Tumors sizes were measured every 7 days using
an in vivo imaging system (PerkinElmer, Inc., Waltham, MA,
USA). The fluorescence signal reflecting the tumor sizes of the
mice were collected and analyzed using the in vivo imaging
system. This assay was approved by the Institutional Ethics
Committee of Zhejiang Provincial People's Hospital.
Immunohistochemistry
All specimens were fixed in neutral buffered
formalin and embedded in paraffin. The specimens were cut into 5-µm
sections, deparaffinized in xylene, and rehydrated in graded
ethanol. After non-specific binding sites were blocked by exposing
them to 10% normal goat serum in PBS for 20 min, the sections were
incubated overnight at 4°C using a series of antibodies (ERBB4,
1:200; PI3K, 1:200; Akt, 1:200). Following this incubation, the
slides were rinsed with PBS and incubated with biotinylated IgG for
20 min at 37°C. Finally, the sections were slightly counterstained
with hematoxylin for 30 sec before coverslip mounting.
Statistical analysis
The data processing was carried out using SPSS 19.0
statistical software. One-way analysis of variance (ANOVA) with
Dunnett's test was used to determine statistically significant
differences. All data were expressed as the mean ± standard
deviation (x±s). P<0.05 and P<0.01 indicated differences that
were statistically significant. All experiments were repeated at
least three times.
Results
ERBB4 is downregulated in gastric
cancer
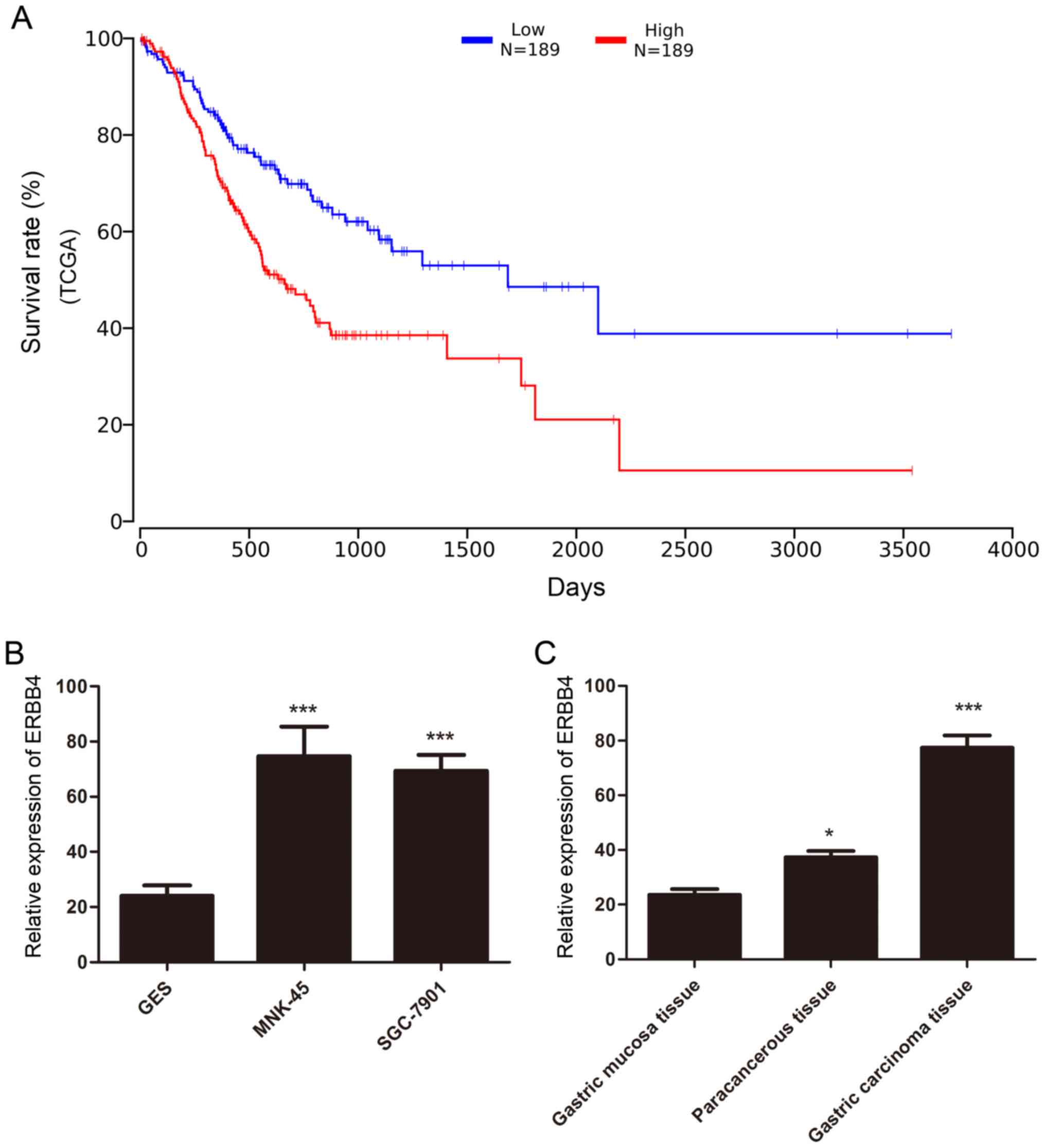
We analyzed 378 gastric cancer samples obtained from
The Cancer Genome Atlas (TCGA) datasets to identify critical ERBB4
involved in gastric cancer. The analysis demonstrated that high
ERBB4 levels in TCGA gastric cancer data were closely related to
the poor survival rate of gastric cancer patients suggesting that
ERBB4 was a prognostic indicator of gastric cancer (Fig. 1A). Quantification of the western
blot analysis was performed. In addition, western blot analysis
revealed that ERBB4 was significantly overexpressed in MNK-45 and
SGC-7901 gastric cancer cell lines compared to immortalized stomach
GES cells (Fig. 1B). Furthermore,
western blot analysis also revealed that ERBB4 was markedly
upregulated in tumor tissues compared to normal stomach tissues and
paracancerous tissue (Fig. 1C).
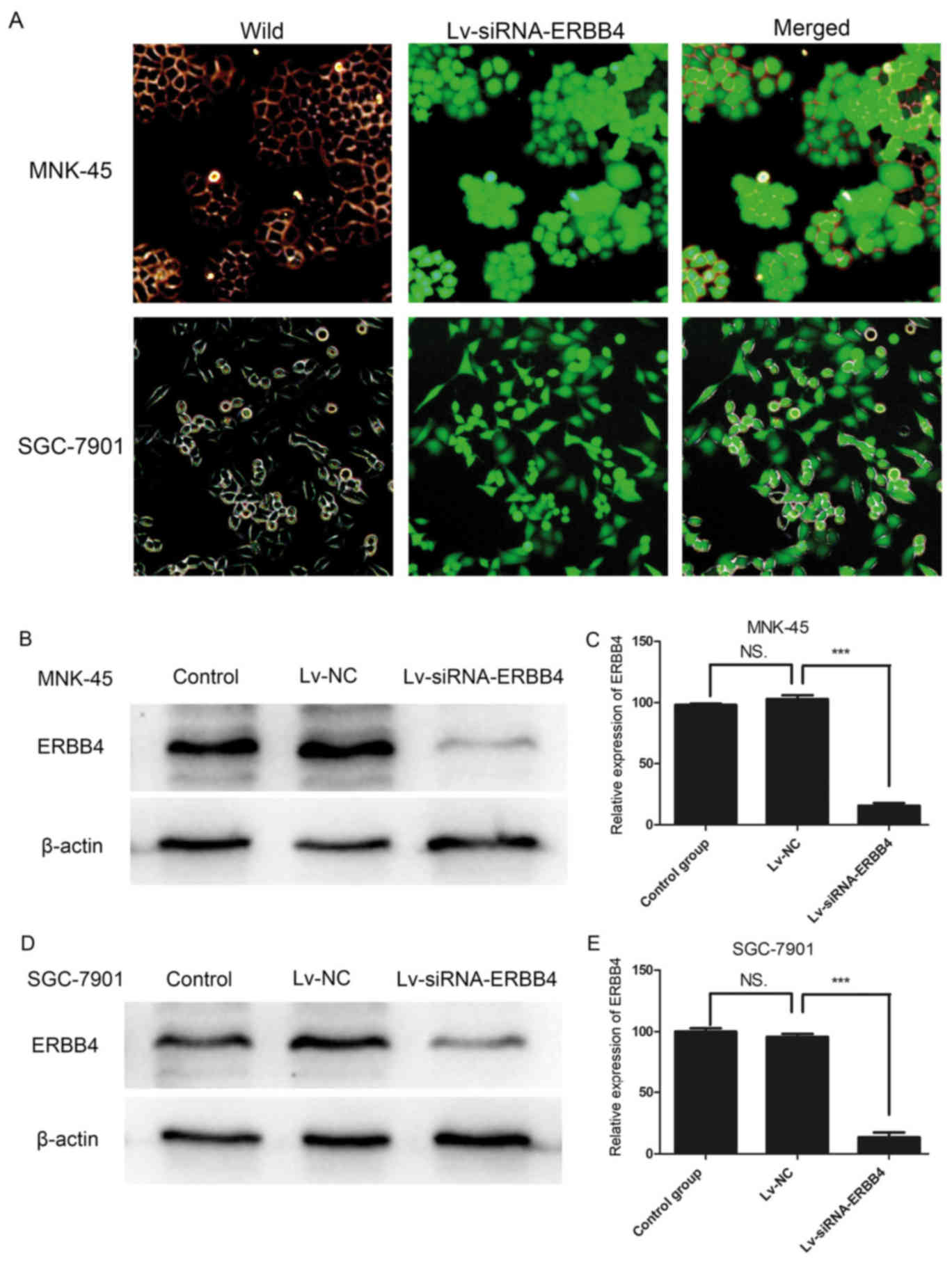
A lentivirus interferes with the
expression of ERBB4
To further study the function of ERBB4 in gastric
cancer, lentiviral vectors encoding siRNAs targeting ERBB4 (GenBank
accession number NC_000002.12) were constructed by Shanghai
GeneChem, Co., Ltd. Three different siRNAs targeting ERBB4
(Lv-siRNA-ERBB4) were designed to ensure the interference effect.
The three Lv-siRNA-ERBB4 vectors were transfected into MNK-45 and
SGC-7901 cells with ViralPlus Transduction Enhancer and Polybrene
so that their specificity for ERBB4 disruption could be determined.
The validated Lv-siRNA-ERBB4 was selected for the construction of
the lentiviral vector (Fig. 2A-C).
A non-silencing sequence was used as a negative control (Lv-NC).
Western blot analysis revealed that ERBB4 was markedly silenced by
Lv-siRNA-ERBB4 (Fig. 2D and E).
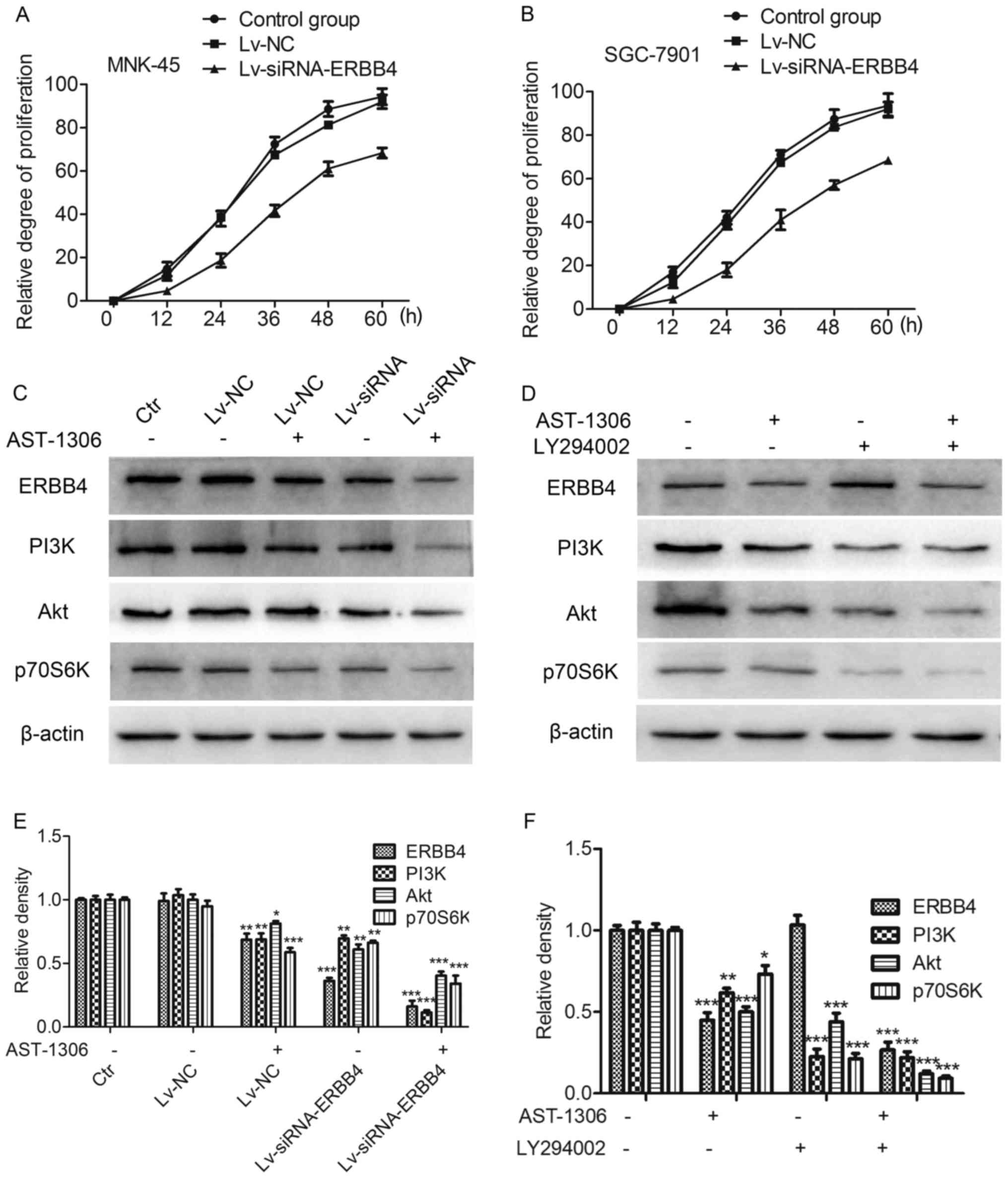
Effects of ERBB4 silencing on gastric
cancer cell proliferation and related signaling pathways
The MTT assay revealed that after Lv-siRNA silenced
ERBB4 expression, MNK-45 and SGC-7901 cell viability and cell
proliferation was decreased. Compared with the control group, the
cells after Lv-siRNA silenced ERBB4 expression exhibited a marked
growth inhibition (Fig. 3A and B).
Western blot analysis revealed that ERBB4 silencing, inhibited the
downstream PI3K/Akt signaling. Furthermore, the same results were
obtained with the ERBB4 inhibitor, AST-1306. In addition, the
PI3K/Akt signaling inhibitor LY294002 also provided further
evidence, constistent with the aforementioned results. (Fig. 3C-F).
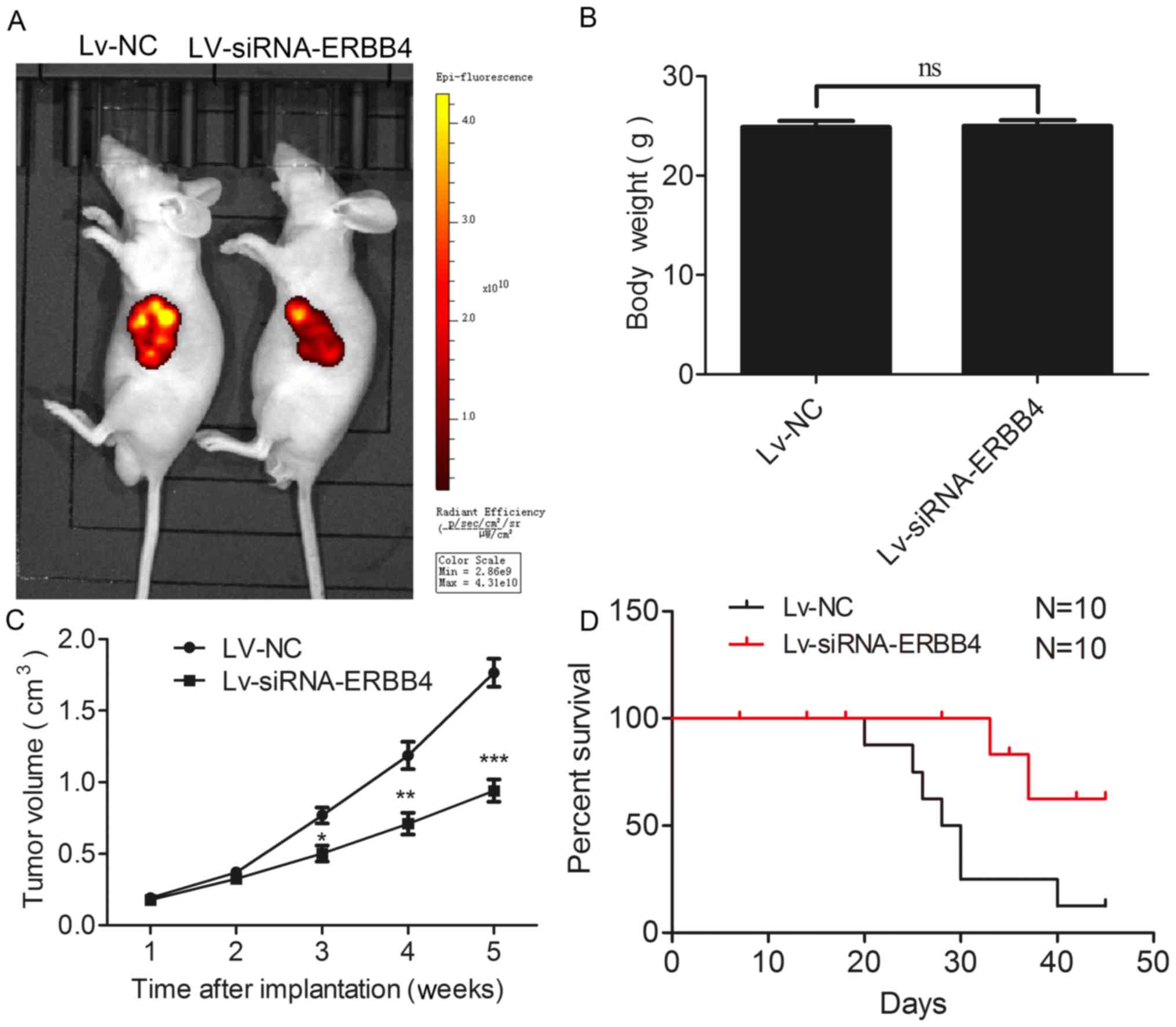
Silencing of ERBB4 expression
suppresses tumor growth in vivo and prolongs the survival time
To confirm the effects of ERBB4 in vivo, we
used a xenograft mouse model. The analysis demonstrated that Lv-NC
cells formed significantly larger tumors than the Lv-siRNA-ERBB4
group of cells in the nude mice (Fig.
4A). In addition, the body weight of nude mice exhibited no
difference (Fig. 4B). Furthermore,
the tumor volume was significantly diminished in the Lv-siRNA-ERBB4
group compared to the Lv-NC group (Fig.
4C). Notably, silencing of ERBB4 expression resulted in a
significantly enhanced survival time of the tumor-bearing nude mice
(Fig. 4D).
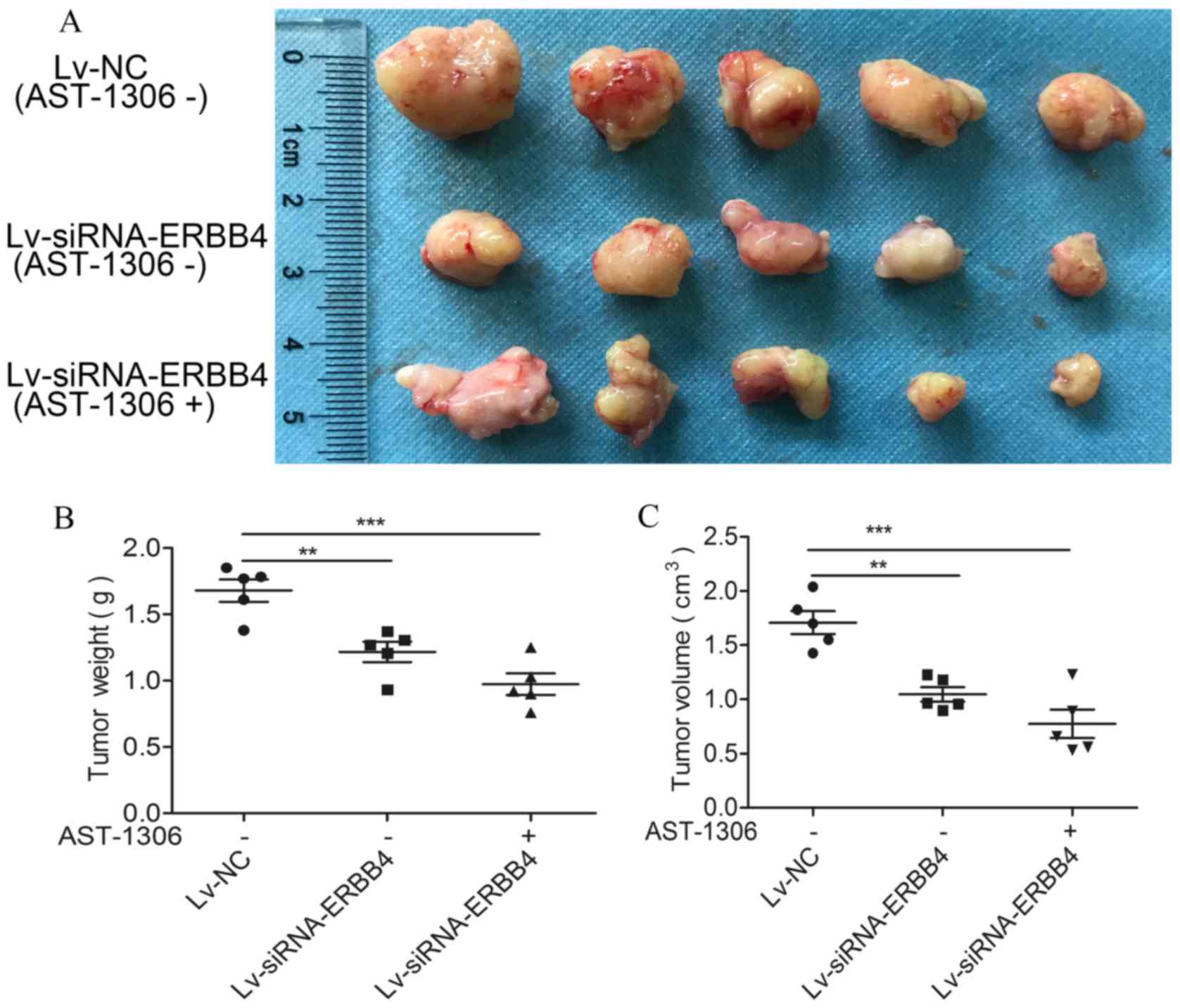
Lv-siRNA and AST-1306 markedly inhibit
the growth of transplanted tumors in nude mice
Lv-siRNA-ERBB4 significantly inhibited tumor growth
in nude mice. The size of the transplanted tumors in nude mice
treated with AST-1306 or transfected with the lentivirus was
significantly lower than that in the Lv-NC group (Fig. 5).
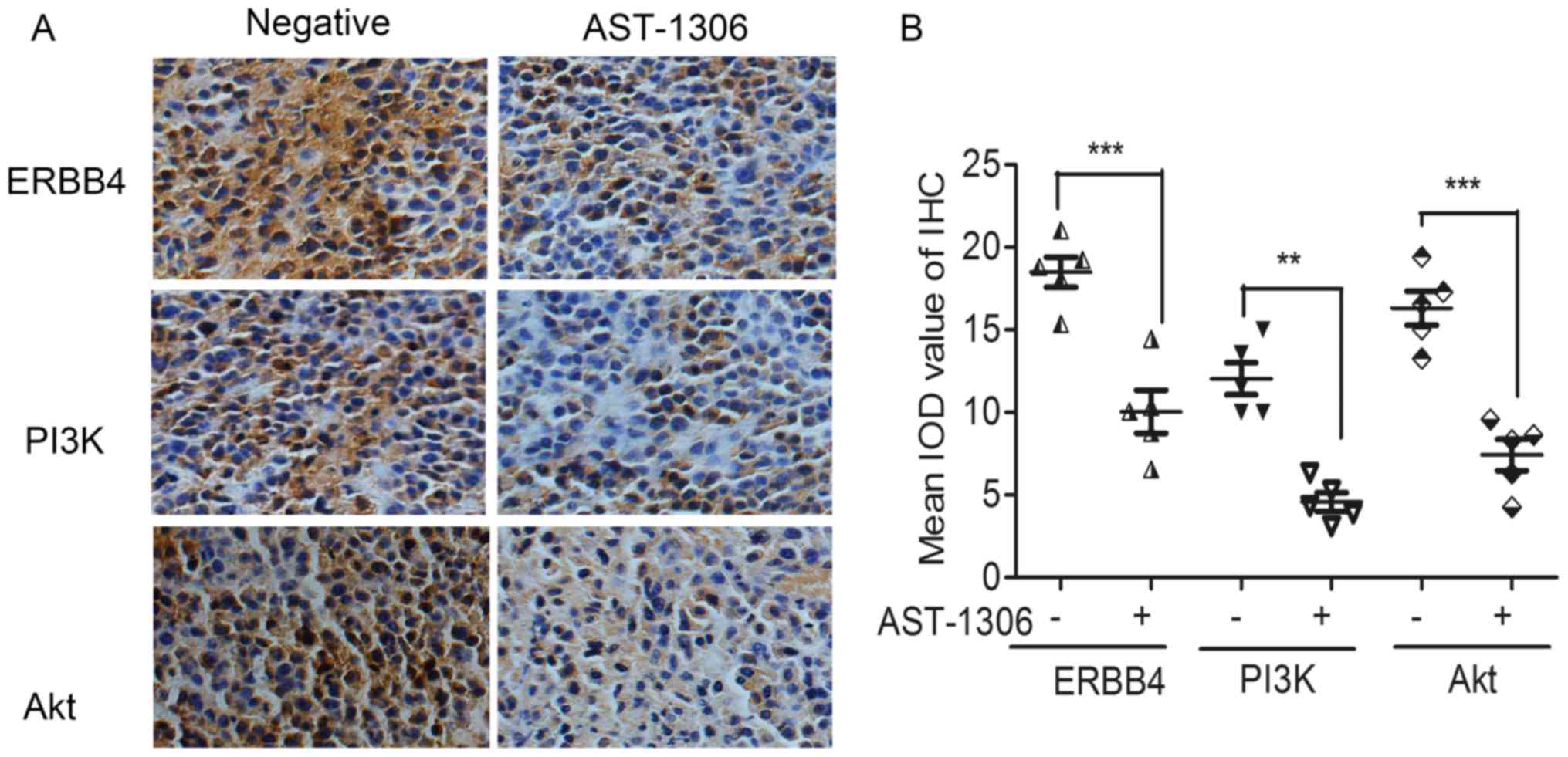
AST-1306 regulates ERBB4-PI3K/Akt
signaling pathways in vivo
To further ascertain the signal transduction pathway
mediating the anti-proliferation effects in vivo, xenografts
of nude mice were prepared as paraffin-embedded sections. The IHC
analysis revealed that the inhibitor of ERBB4 (AST-1306)
significantly downregulated the expression level of the
proliferation-related proteins PI3K and Akt (Fig. 6).
Discussion
Gastric cancer is one of the most common cancers
with the highest morbidity and mortality in China (1). Early screening of gastric cancer is an
important method for the treatment and prevention of gastric cancer
(17–20). However, there is no reliable and
specific molecular marker of gastric cancer. Therefore, it is
important to find a specific marker of gastric cancer. ERBB4
belongs to the family of tyrosine kinase receptors and is
overexpressed in many tumors especially in gastric cancer. In
recent years, the targeted therapy of epidermal growth factor
receptor (EGFR) family has achieved fruitful results in the
treatment of gastric cancer (21–24).
The EGFR family was determined to be highly expressed in gastric
cancer, and the positive rate of EGFR (ERBB1/HER1) was 40–60%
(25), human epidermal growth
factor receptor-2 (ERBB2/HER-2) ranged from 4 to 53% (26), HER-3/ERBB3 and HER-4/ERBB4 were 59
and 86%, respectively (27). The
positive expression rate of ERBB4 in gastric cancer was found to be
the highest, but few studies have been performed on ERBB4 in
gastric cancer. In the present study, we found that the high
expression of ERBB4 was closely related to the poor prognosis of
the patients. Inhibiting the expression of ERBB4 contributed to the
prolongation of the survival time of gastric cancer patients.
Through experiments, we found that ERBB4 was stably expressed in
gastric cancer tissues and cells relative to normal gastric mucosa
tissues and gastric mucosa cells. Therefore, we hypothesized that
ERBB4 may play an important role in the malignant behavior of
gastric cancer. It may become a target for predicting the prognosis
and treatment for gastric cancer patients. To further investigate
the role and mechanism of ERBB4 in gastric cancer, we used
molecular cloning techniques and a lentivirus to inhibit the
expression of ERBB4. The results revealed that the proliferation of
gastric cancer cells was markedly inhibited by silencing the
expression of ERBB4. Thus, ERBB4 can promote the proliferation of
gastric cancer cells. We further detected the key proteins of the
downstream signaling pathway of ERBB4 and found that inhibition of
ERBB4 expression would directly result in inactivation of the
downstream PI3K/Akt signaling pathway. In addition, the PI3K
signaling pathway can directly regulate cell proliferation through
its downstream proteins m-TOR and p70S6K (28–30).
These results revealed that ERBB4 can promote the proliferation of
gastric cancer cells through the PI3K/Akt signaling pathway. In
addition, the ERBB4 inhibitor (AST-1306) and the PI3K inhibitor
(LY-294002) experiments confirmed the aforementioned conclusion.
Collectively, these results indicated that ERBB4 mainly promotes
the proliferation of gastric cancer cells through the PI3K/Akt
signaling pathway. In order to investigate the role and mechanism
of ERBB4 on gastric cancer proliferation in vivo, xenograft
models of gastric cancer and an in vivo imaging system
(PerkinElmer, Inc.) were used for in vivo tests. The
xenograft models of the gastric cancer experiments suggested that
silencing of ERBB4 can markedly suppress the proliferation of
gastric cancer cells in vivo and prolong the survival time
of tumor-bearing nude mice.
In summary, ERBB4 promoted the proliferation of
gastric cancer cells via the PI3K/Akt/mTOR/p70S6K signaling
pathway. ERBB4 was significantly correlated with poor survival
time. Thus, our data revealed that ERBB4 is a potential prognostic
factor and a therapeutic target for gastric cancer.
Acknowledgements
Not applicable.
Funding
The research was funded by the Medicine and Health
Research Foundation of Zhejiang Province (project no.
2017KY018).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
JX and LG conceived and designed the study. JX, ZQ,
LG, GS and JL performed the experiments. JX wrote the paper. GS and
JL reviewed and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by
Institutional Ethics Committee of Zhejiang Provincial People's
Hospital (Hangzhou, China). All patients provided a signed informed
consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ni CY, Murphy MP, Golde TE and Carpenter
G: gamma-Secretase cleavage and nuclear localization of ErbB-4
receptor tyrosine kinase. Science. 294:2179–2181. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sordella R, Bell DW, Haber DA and
Settleman J: Gefitinib-sensitizing EGFR mutations in lung cancer
activate anti-apoptotic pathways. Science. 305:1163–1167. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan WL, Jain A, Takano A, Newell EW, Iyer
NG, Lim WT, Tan EH, Zhai W, Hillmer AM, Tam WL, et al: Novel
therapeutic targets on the horizon for lung cancer. Lancet Oncol.
17:e347–e362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li M, Zhang Z, Li X, Ye J, Wu X, Tan Z,
Liu C, Shen B, Wang XA, Wu W, et al: Whole-exome and targeted gene
sequencing of gallbladder carcinoma identifies recurrent mutations
in the ErbB pathway. Nat Genet. 46:872–876. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arteaga CL and Engelman JA: ERBB
receptors: From oncogene discovery to basic science to
mechanism-based cancer therapeutics. Cancer Cell. 25:282–303. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vlacich G and Coffey RJ: Resistance to
EGFR-targeted therapy: A family affair. Cancer Cell. 20:423–425.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hegde GV, de la Cruz CC, Chiu C, Alag N,
Schaefer G, Crocker L, Ross S, Goldenberg D, Merchant M, Tien J, et
al: Blocking NRG1 and other ligand-mediated Her4 signaling enhances
the magnitude and duration of the chemotherapeutic response of
non-small cell lung cancer. Sci Transl Med. 5:171ra182013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Settleman J: A therapeutic opportunity in
melanoma: ErbB4 makes a mark on skin. Cancer Cell. 16:278–279.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma X, Li L, Tian T, Liu H, Li Q and Gao Q:
Study of lung cancer regulatory network that involves erbB4 and
tumor marker gene. Saudi J Biol Sci. 24:649–657. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Broughton MN, Westgaard A, Paus E,
Øijordsbakken M, Henanger KJ, Naume B and Bjøro T: Specific
antibodies and sensitive immunoassays for the human epidermal
growth factor receptors (HER2, HER3, and HER4). Tumour Biol.
39:10104283177074362017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song G, Zhang H, Chen C, Gong L, Chen B,
Zhao S, Shi J, Xu J and Ye Z: miR-551b regulates
epithelial-mesenchymal transition and metastasis of gastric cancer
by inhibiting ERBB4 expression. Oncotarget. 8:45725–45735.
2017.PubMed/NCBI
|
|
15
|
Zhang M, Zhang L, Cui M, Ye W, Zhang P,
Zhou S and Wang J: miR-302b inhibits cancer-related inflammation by
targeting ERBB4, IRF2 and CXCR4 in esophageal cancer. Oncotarget.
8:49053–49063. 2017.PubMed/NCBI
|
|
16
|
Ohashi Y, Kumagai K, Miyata Y, Matsubara
R, Kitaura K, Suzuki S, Hamada Y and Suzuki R: Overexpression of
ErbB4 is an independent marker for lymph node metastasis in
Japanese patients with oral squamous cell carcinoma. Oral Surg Oral
Med Oral Pathol Oral Radiol. 122:313–321. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ueda Y, Fujishima H, Hirashita T,
Shiroshita H, Etoh T, Inomata M and Shiraishi N: Clinical impact of
small advanced gastric cancer (≤40 mm) in elderly patients: A
retrospective cohort study. Int J Surg. 45:131–137. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pereira MA, Ramos MFKP, Dias AR, Faraj SF,
Yagi OK, Safatle-Ribeiro AV, Maluf-Filho F, Zilberstein B,
Cecconello I, de Mello ES, et al: Risk factors for lymph node
metastasis in western early gastric cancer after optimal surgical
treatment. J Gastrointest Surg. 22:23–31. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chang JY, Shim KN, Tae CH, Lee KE, Lee J,
Lee KH, Moon CM, Kim SE, Jung HK, Jung SA, et al: Comparison of
clinical outcomes after endoscopic submucosal dissection and
surgery in the treatment of early gastric cancer: A
single-institute study. Medicine (Baltimore). 96:e72102017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bang CS, Park JM, Baik GH, Park JJ, Joo
MK, Jang JY, Jeon SW, Choi SC, Sung JK and Cho KB: Therapeutic
outcomes of endoscopic resection of early gastric cancer with
undifferentiated-type histology: A Korean ESD Registry Database
analysis. Clin Endosc. 50:569–577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pinto C, Di Fabio F, Barone C, Siena S,
Falcone A, Cascinu S, Llimpe Rojas FL, Stella G, Schinzari G,
Artale S, et al: Phase II study of cetuximab in combination with
cisplatin and docetaxel in patients with untreated advanced gastric
or gastro-oesophageal junction adenocarcinoma (DOCETUX study). Br J
Cancer. 101:1261–1268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Waddell T, Chau I, Cunningham D, Gonzalez
D, Okines AF, Okines C, Wotherspoon A, Saffery C, Middleton G,
Wadsley J, et al: Epirubicin, oxaliplatin, and capecitabine with or
without panitumumab for patients with previously untreated advanced
oesophagogastric cancer (REAL3): A randomised, open-label phase 3
trial. Lancet Oncol. 14:481–489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun GP, Sun Y, Xu RH, Xu JM, Li J, Wang
JW, Qin S, Feng JF, Ba Y, Shen L, et al: The Chinese subgroup from
a randomized phase III study of lapatinib in combination with
weekly paclitaxel versus weekly paclitaxel alone as second-line
treatment of HER2-amplified advanced gastric cancer (AGC) in Asian
countries. J Clin Oncol. 31 Suppl:a41092013.
|
|
24
|
Satoh T, Xu RH, Chung HC, Sun GP, Doi T,
Xu JM, Tsuji A, Omuro Y, Li J, Wang JW, et al: Lapatinib plus
paclitaxel versus paclitaxel alone in the second-line treatment of
HER2-amplified advanced gastric cancer in Asian populations: TyTAN
- a randomized, phase III study. J Clin Oncol. 32:2039–2049. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lieto E, Ferraraccio F, Orditura M,
Castellano P, Mura AL, Pinto M, Zamboli A, De Vita F and Galizia G:
Expression of vascular endothelial growth factor (VEGF) and
epidermal growth factor receptor (EGFR) is an independent
prognostic indicator of worse outcome in gastric cancer patients.
Ann Surg Oncol. 15:69–79. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chua TC and Merrett ND: Clinicopathologic
factors associated with HER2-positive gastric cancer and its impact
on survival outcomes - a systematic review. Int J Cancer.
130:2845–2856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayashi M, Inokuchi M, Takagi Y, Yamada H,
Kojima K, Kumagai J, Kawano T and Sugihara K: High expression of
HER3 is associated with a decreased survival in gastric cancer.
Clin Cancer Res. 14:7843–7849. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu X, Zhou X, Xu H, He Z, Shi X and Wu S:
SLC34A2 regulates the proliferation, migration, and invasion of
human osteosarcoma cells through PTEN/PI3K/AKT signaling. DNA Cell
Biol. 36:775–780, Epub ahead of print. 2017.PubMed/NCBI
|
|
29
|
Liang L, Gao C, Li Y, Sun M, Xu J, Li H,
Jia L and Zhao Y: miR-125a-3p/FUT5-FUT6 axis mediates colorectal
cancer cell proliferation, migration, invasion and pathological
angiogenesis via PI3K-Akt pathway. Cell Death Dis. 8:e29682017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fruman DA, Snapper SB, Yballe CM, Davidson
L, Yu JY, Alt FW and Cantley LC: Impaired B cell development and
proliferation in absence of phosphoinositide 3-kinase p85alpha.
Science. 283:393–397. 1999. View Article : Google Scholar : PubMed/NCBI
|