Introduction
Gastric cancer (GC) is one of the most common
digestive system tumors and patients suffering from GC are usually
diagnosed with late-stage disease. Systemic chemotherapy is the
most effective treatment to prolong patient survival and improve
the quality of life of patients with advanced GC. Yet, the
occurrence of resistance to chemotherapy drugs greatly reduces the
clinical efficacy of chemotherapy in GC (1). Thus, identification of potential
biomarkers of adverse survival outcomes as well as an in-depth
study of the mechanisms underlying drug resistance are crucial for
the treatment of GC.
NIK- and IKKβ-binding protein (NIBP), also known as
trafficking protein particle complex 9 (TRAPPC9), is a type of
NF-κB signaling pathway regulating factor that has been detected in
human nerve cells (2–4). Moreover, the NF-κB signaling pathway
is a pivotal mediator for both physiological and pathological
events in cell proliferation, apoptosis as well as in oncogenesis
(5,6). Hence, NIBP may regulate activation of
the NF-κB signaling pathway in tumorigenesis as well as in
progression. Previous studies have demonstrated that NIBP is
aberrantly and highly expressed in colorectal cancer, while
overexpression of NIBP was found to be significantly correlated
with tumor differentiation, clinical stage and metastasis (7,8).
Additionally, it was found that lentiviral-mediated silencing of
NIBP expression inhibited colon cancer cell growth, invasion and
metastasis, and this may be related to regulation of the activation
of the NF-κB signaling pathway (9).
Nevertheless, little is known concerning the functional relevance
of NIBP expression, in particular, the effect of NIBP on the
chemoresistance in GC.
Epithelial-mesenchymal transition (EMT) is the
process whereby epithelial cells undergo the dissolution of
cell-cell adhesion and loss of apico-basolateral polarity and
transform into mesenchymal cells. Downregulation of epithelial
markers and upregulation of mesenchymal markers are the most
conspicuous molecular characteristics of EMT. It has been confirmed
that EMT is responsible for the progression of various types of
malignancies in pathological conditions (10). Emerging evidence indicates that EMT
not only promotes migratory and invasive capacity of cancer cells,
but it is also the key to acquisition of chemoresistance (11). Furthermore, suppression of the
process of EMT was found to improve sensitivity to
chemotherapeutics in genetically engineered mouse models with
deletion of Snail or Twist (12).
Research has shown that EMT is induced by activation of the NF-κB
signaling pathway, resulting in cisplatin-treatment resistance of
bladder cancer (13). Our previous
research indicated that the chemotherapy sensitivity of GC cells
was enhanced through suppression of the NF-κB signaling pathway
following treatment with Ginkgo biloba extract 761 (EGb 761)
(14).
Cancer stem cells (CSCs) are a subgroup of cells
which possess self-renewal capacity, drive tumorigenesis,
maintenance, relapse and therapy resistance (15). CD133 is a surface glycoprotein which
has been recognized as a universal CSC marker in many types
carcinomas. Accumulating evidence indicates that CD133 is also a
functional factor involved in tumorigenesis and tumor progression
(16). It has been reported that
CD133 overexpression facilitated tumor invasiveness and metastasis
by induction of EMT, which was found to be related to activation of
the NF-κB signaling pathway in pancreatic cancer (17). In addition, following the deletion
of CD133 by ultrasound-targeted microbubble destruction technique
(UTMD), EMT was significantly inhibited with the accompanying
suppression of the NF-κB signaling pathway in vitro and
in vivo (18). The above
studies demonstrate that CD133 serves as a critical activation
factor to participate in constitution of the EMT regulatory network
of the NF-κB signaling pathway. In the light of these results, it
was hypothesized that NIBP may activate the NF-κB signaling pathway
and contribute to the chemoresistance of GC by regulating
CD133-induced EMT. Meanwhile, EGb 761 may reverse drug resistance
through the suppression of EMT regulated by NIBP-mediated NF-κB
signaling pathway.
Materials and methods
Drug preparation and cell culture
EGb 761® was purchased from Dr Willmar
Schwabe Pharmaceuticals (Karlsruhe, Germany). Human gastric cancer
cell line SGC-7901 and CDDP-resistant gastric cancer cell line
SGC-7901/CDDP were provided by the Chinese Academy of Sciences
(Beijing, China). The cells were grown in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal calf serum (FCS; Gibco; Thermo Fisher Scientific), 100
U/ml penicillin and 100 µg/ml streptomycin, at 37°C in a 5%
CO2 humidified atmosphere. SGC-7901/CDDP cells were
conventionally maintained in the above RPMI-1640 medium containing
5 µg/ml cis-diamminedichloroplatinum (II) (CDDP) (Hospira Australia
Pty Ltd., Mulgrave, VIC, Australia).
Clinical data
Forty patients with GC who underwent curative
resection were enrolled in the present study from January 2016 to
January 2017 at The First Affiliated Hospital of Guangxi Medical
University (Nanning, Guangxi, China). There were 30 males and 10
females among the 40 patients and the age of the patients ranged
from 35 to 74 years. Written informed consent was provided by each
patient. Samples of tumor and normal gastric tissues were obtained
at the time of surgery; for normal tissue collection, samples were
taken at least 10 cm from the gastric cancer edge. The specimens
were fixed using 4% paraformaldehyde. After dehydration,
transparent and paraffin-embedding, the specimens were constructed
into 4-µm-thick serial section. For all specimens, a definite
diagnosis of GC was made by pathology. The research was authorized
by the Medical Ethics Committee of The First Affiliated Hospital of
Guangxi Medical University, Guangxi, China.
Immumohistochemical staining
Tissue sections were fixed with 0.01 mol/l citric
acid for 5 min and blocked with normal goat serum. The sections
were then incubated with rabbit polyclonal anti-NIBP (1:200; cat.
no. 16014-1-AP; Proteintech Group, Wuhan, China), rabbit monoclonal
anti-NF-κB p65 (1:200; cat. no. 8242; Cell Signaling Technology,
Beverly, MA, USA) and rabbit polyclonal anti-NF-κB p-p65 antibodies
(1:200; cat. no. 3031; Cell Signaling Technology) for 1 h at room
temperature, and more steps were strictly complied with according
to the SP immunohistochemical kit (ZSGB Biotech, Beijing, China)
instructions. Positive control was provided by the manufacturers,
and using phosphate-buffered saline (PBS) instead of primary
antibody as a negative control. The positive staining was evaluated
by combining staining intensity with percentage of positive cells
to conduct an analysis of half quantitative. The intensity of
staining was scored as: no staining, 0; light yellow, 1; yellow, 2;
and brown, 3. The percentage of positive cells was scored as:
positive cells <5%, 0; positive-staining cells 5–25%, 1;
positive cells 26–50%, 2; and positive cells >50%, 3. A weighted
score was produced by adding the scores of the staining intensity
and the percentage of positive cells. The weighted scores ≤2 were
defined as negative; >2 were defined as positive.
Cell viability analysis
Effects of EGb 761 and CDDP on cell viability were
assessed by using MTT assay. MTT was purchased from Sigma-Aldrich
(Merck KGaA, Darmstadt, Germany). Briefly, cells were cultured in
96-well plate and exposed to various concentrations of EGb 761
and/or CDDP for 24 h, the culture medium was discarded and 20 ml of
MTT (5 mg/ml) was added to each well for 4 h. The formazan crystals
were then dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich;
Merck KGaA) and the absorbance was assessed at 570 nm with a
microplate reader (BioTek Instruments, Inc., Winooski, VT,
USA).
Real-time fluorescent quantitative
PCR
SGC-7901 cells were treated with 2 µg/ml CDDP with
or without EGb 761 (360 µg/ml) for 24 h, and SGC-7901/CDDP cells
were treated in 4 µg/ml CDDP with or without EGb 761 (360 µg/ml)
for 24 h. RNA was extracted from cells by using TRIzol reagent
(Takara Biotechnology, Co., Ltd., Dalian, China). Then, 1 µg DNAse
I-treated RNA was used to synthesis cDNA for the subsequent
real-time fluorescence PCR amplification according to the Reverse
Transcription kit (Takara Biotechnology, Co., Ltd.) instructions.
The specific primer sequences for NIBP were
5′-GAACTGCCTTAGCCCTGAAGACATA-3′ (forward) and
5′-AGCCTTGATGCACGCTTCC-3′ (reverse), generating a fragment of 109
bp. The specific primer sequences for NF-κB p65 were
5′-ACCTCGACGCATTGCTGTG-3′ (forward) and 5′-CTGGCTGATCTGCCCAGAAG-3′
(reverse), generating a fragment of 145 bp. The primers for β-actin
were 5′-GCACCGTCAAGGCTGAGAAC-3′ (forward) and
5′-TGGTGAAGACGCCAGTGGA-3′ (reverse), generating a fragment of 138
bp. The SYBR Green PCR Master Mix kit (Takara Biotechnology, Co.,
Ltd.) was used and quantitative PCR analysis was carried out on the
LightCycler 480 (Roche Diagnostics, Indianapolis, IN, USA). The
extent of NIBP and NF-κB p65 mRNA expression were quantitated by
using Image-Pro Plus software (Media Cybernetics, Rockville, MD,
USA).
Western blot analysis
SGC-7901/CDDP and SGC-7901 cells were pretreated
with the appropriate concentration of EGb 761 or CDDP (refer to
real-time fluorescent quantitative PCR section). Related proteins
from the SGC-7901 and SGC-7901/CDDP cells were prepared using a
Beyotime Biotechnology cytoplasmic kit (Beyotime Institute of
Biotechnology, Shanghai, China) according to the kit instructions.
All proteins were separated on SDS-PAGE gels for electrophoresis,
and transferred onto polyvinylidene fluoride (PVDF) membranes.
Then, the membranes were blocked with PBST (PBS containing 0.05%
Tween-20) containing 5% non-fat dry milk for 1 h, and each membrane
was incubated with rabbit polyclonal anti-NIBP (1:1,000; cat. no.
16014-1-AP; Proteintech Group), rabbit monoclonal anti-NF-κB p65
(1:1,000; cat. no. 8242; Cell Signaling Technology), rabbit
polyclonal anti-NF-κB p-p65 (1:1,000; cat. no. 3031; Cell Signaling
Technology), rabbit monoclonal anti-vimentin antibody (1:1,000;
cat. no. 5741; Cell Signaling Technology), rabbit polyclonal
anti-ZO-1 (1:500; cat. no. 21773-1-AP; Proteintech Group), rabbit
polyclonal anti-CD133 (1:500; cat. no. 18470-1-AP; Proteintech
Group) and rabbit polyclonal anti-GAPDH antibody (1:5,000; cat. no.
10494-1-AP; Proteintech Group) overnight at 4°C. Finally, the
membranes were probed with the appropriate secondary antibodies
(1:500; cat. no. E032720; EarthOx, San Francisco, CA, USA) for 1 h
at room temperature. The generating protein bands were visualized
and analyzed by Odyssey CLx Infrared Imaging system (LI-COR
Biosciences, Lincoln, NE, USA).
Flow cytometric analysis
Cells were seeded in 6-well plates at
5×105 cells/well, starved overnight in RPMI-1640 medium
with 0.5% serum and incubated with the appropriate concentration of
EGb761 or CDDP (refer to real-time fluorescent quantitative PCR
section). Then, the cells were collected and washed with cold PBS
twice. The cells were then gently resuspended in 400 µl binding
buffer, and 5 µl Annexin V-FITC was added to the above cell
solution. The cells were gently vortexed, incubated for 10 min at
4°C avoiding light; 10 µl propidium iodide (PI) was added and the
cells were cultured for another 5 min. Samples were analyzed using
FACSCalibur flow cytometry (BD Biosciences, San Jose, CA, USA) and
CellQuest software (BD Biosciences) was used to analyze the
results.
Statistical analysis
All results are presented as the mean ± SD. All the
experiments were repeated at least three times. SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA) was used for statistical analysis.
The data were analyzed using the Student's t-test or ANOVA test.
The frequencies in the different groups were evaluated using the
Fisher's exact test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemistry analysis of NIBP,
NF-κB p65 and p-p65 in GC tissues
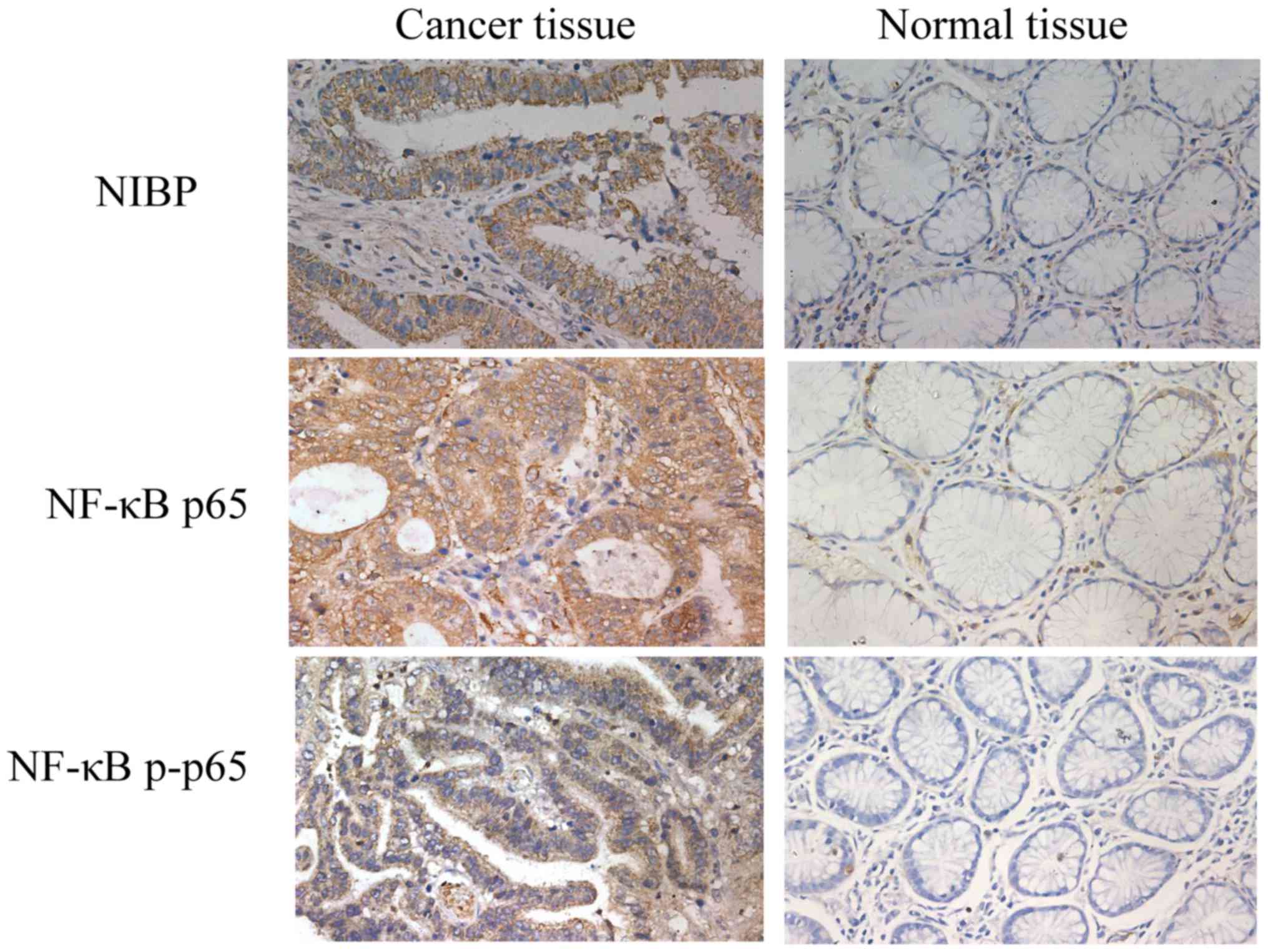
Immunohistochemical analysis of tissue sections
showed that positive NIBP staining was mainly observed in the
cytoplasm, and positive NF-κB p65 and p-p65 staining was detected
in the cell nucleus and cytoplasm (Fig.
1). The percentages of positive expression of NIBP, NF-κB p65
and p-p65 in the GC tissues were significantly higher than those
determined in the normal tissues (Table
I).
 | Table I.Expression of NIBP, NF-κB p65 and
p-p65 in the gastric cancer and normal gastric tissues. |
Table I.
Expression of NIBP, NF-κB p65 and
p-p65 in the gastric cancer and normal gastric tissues.
|
|
| NIBP+ | NF-κB
p65+ | NF-κB
p-p65+ |
|---|
|
|
|
|
|
|
|---|
| Samples | N | n (%) | P-value | n (%) | P-value | n (%) | P-value |
|---|
| Normal tissues | 40 | 14 (35) | 0.002 | 17 (42.5) | 0.006 | 15
(37.5) | 0.007 |
| Cancer tissues | 40 | 29 (72.5) |
| 30 (75) |
| 28
(70) |
|
Clinical significance of NIBP, NF-κB
p65 and p-p65 in GC tissues
As shown in Table
II, the expression levels of NIBP, NF-κB p65 and p-p65 were
closely related to tumor invasion depth, differentiation degree,
pathological stage and lymphatic metastasis. Furthermore, there was
a closely correlation between the expression of NIBP and NF-κB p65
(p-p65) (Table III) in GC
tissues.
 | Table II.Correlation of the
clinicopathological characteristics of the gastric cancer tissues
and NIBP, NF-κB p65 and p-p65 expression. |
Table II.
Correlation of the
clinicopathological characteristics of the gastric cancer tissues
and NIBP, NF-κB p65 and p-p65 expression.
|
|
| NIBP |
| NF-κB p65 |
| NF-κB p-p65 |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristics | N | − | + | P-value | − | + | P-value | − | + | P-value |
|---|
| Age (years) |
|
|
| 0.455 |
|
| 0.103 |
|
| >0.999 |
|
<50 | 11 | 4 | 7 |
| 5 | 6 |
| 3 | 8 |
|
|
≥50 | 29 | 7 | 22 |
| 5 | 24 |
| 9 | 20 |
|
| Sex |
|
|
| 0.418 |
|
| 0.232 |
|
| >0.999 |
|
Male | 30 | 7 | 23 |
| 6 | 24 |
| 9 | 21 |
|
|
Female | 10 | 4 | 6 |
| 4 | 6 |
| 3 | 7 |
|
| Serosal
invasion |
|
|
| 0.029 |
|
| 0.018 |
|
| 0.011 |
|
Negative | 14 | 7 | 7 |
| 7 | 7 |
| 8 | 6 |
|
|
Positive | 26 | 4 | 22 |
| 3 | 23 |
| 4 | 22 |
|
| Histological
grade |
|
|
| 0.006 |
|
| 0.025 |
|
| 0.018 |
| Well
and moderately differentiated | 18 | 9 | 9 |
| 8 | 10 |
| 9 | 9 |
|
| Poorly
differentiated | 22 | 2 | 20 |
| 2 | 20 |
| 3 | 19 |
|
| TNM stage |
|
|
| 0.012 |
|
| 0.028 |
|
| 0.005 |
|
I+II | 19 | 9 | 10 |
| 8 | 11 |
| 10 | 9 |
|
|
III+IV | 21 | 2 | 19 |
| 2 | 19 |
| 2 | 19 |
|
| Lymph node
metastasis |
|
|
| 0.014 |
|
| 0.007 |
|
| 0.041 |
|
Negative | 10 | 6 | 4 |
| 6 | 4 |
| 6 | 4 |
|
|
Positive | 30 | 5 | 25 |
| 4 | 26 |
| 6 | 24 |
|
 | Table III.Correlation between the expression of
NF-κB p65 and p-p65 and NIBP in the gastric cancer tissues. |
Table III.
Correlation between the expression of
NF-κB p65 and p-p65 and NIBP in the gastric cancer tissues.
|
| NF-κB p65 |
|
| NF-κB p-p65 |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
|
| − | + | Correlation | P-value | − | + | Correlation | P-value |
|---|
| NIBP |
|
|
| <0.001 |
|
|
| <0.001 |
| − | 7 | 4 | 0.550 |
| 8 | 3 | 0.574 |
|
| + | 3 | 26 |
|
| 4 | 25 |
|
|
Expression levels of NIBP, NF-κB p65,
p-p65, vimentin, ZO-1 and CD133 in GC cells
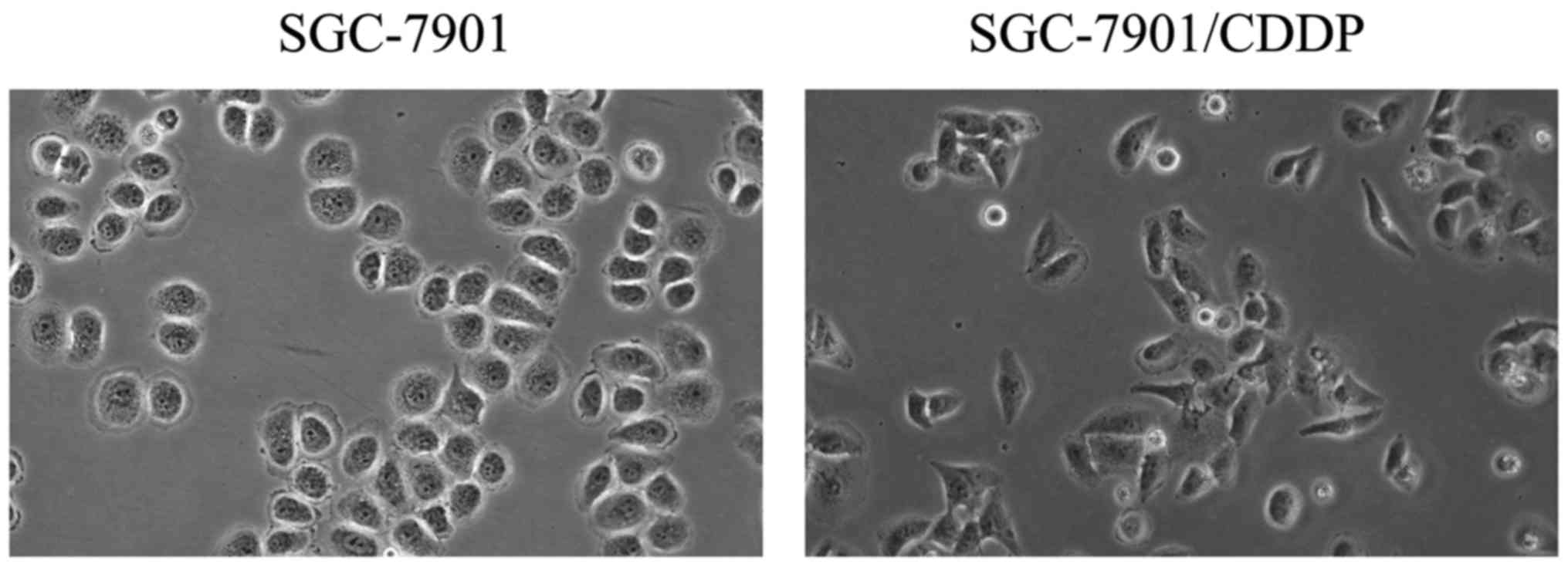
Morphological observation displayed huge
morphological differences between SGC-7901/CDDP cells which
exhibited spindle-like mesenchymal appearance and SGC-7901 cells
which displayed cobblestone-like epithelial shape (Fig. 2).
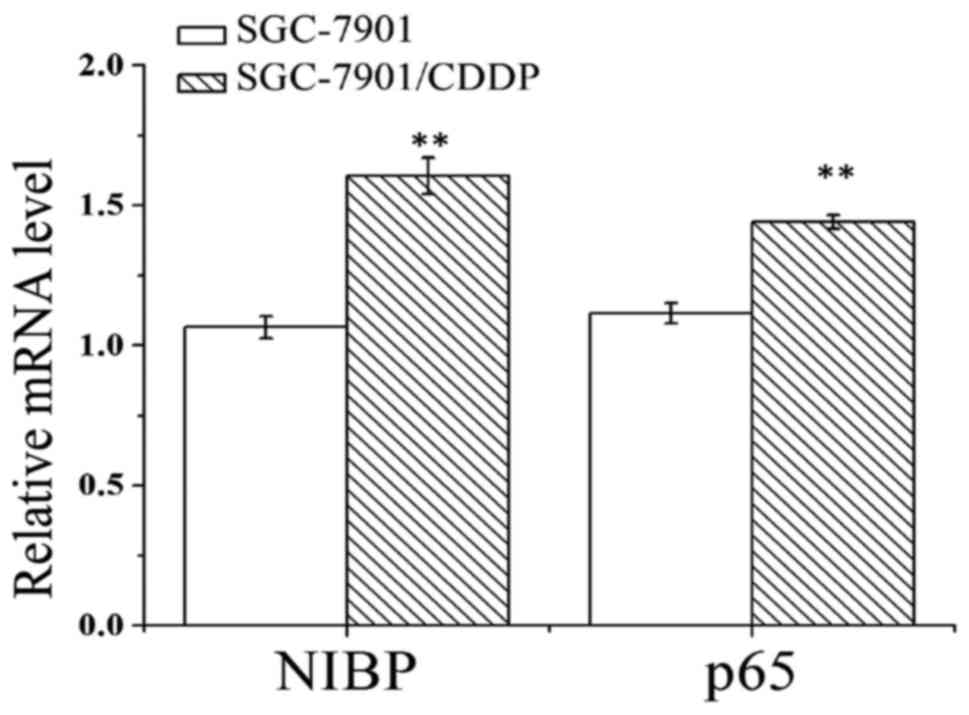
The results indicated that the mRNA expression
levels of NIBP and NF-κB p65 in the SGC-7901/CDDP cells were
enhanced as compared to those of SGC-7901 cells (Fig. 3). In line with the results of
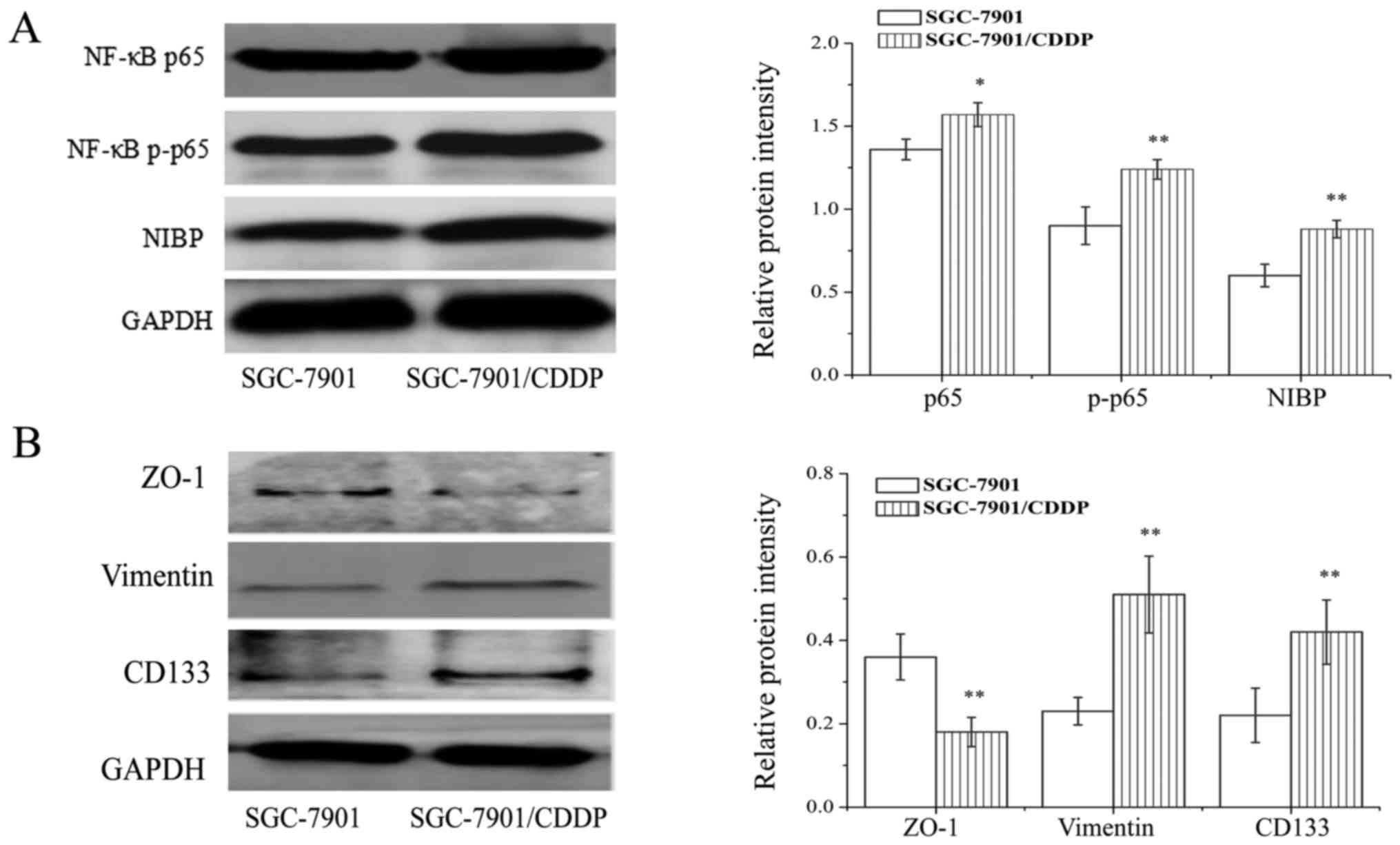
real-time PCR, the protein expression of NIBP, NF-κB p65 and p-p65
in SGC-7901/CDDP cells was higher than levels noted in the SGC-7901
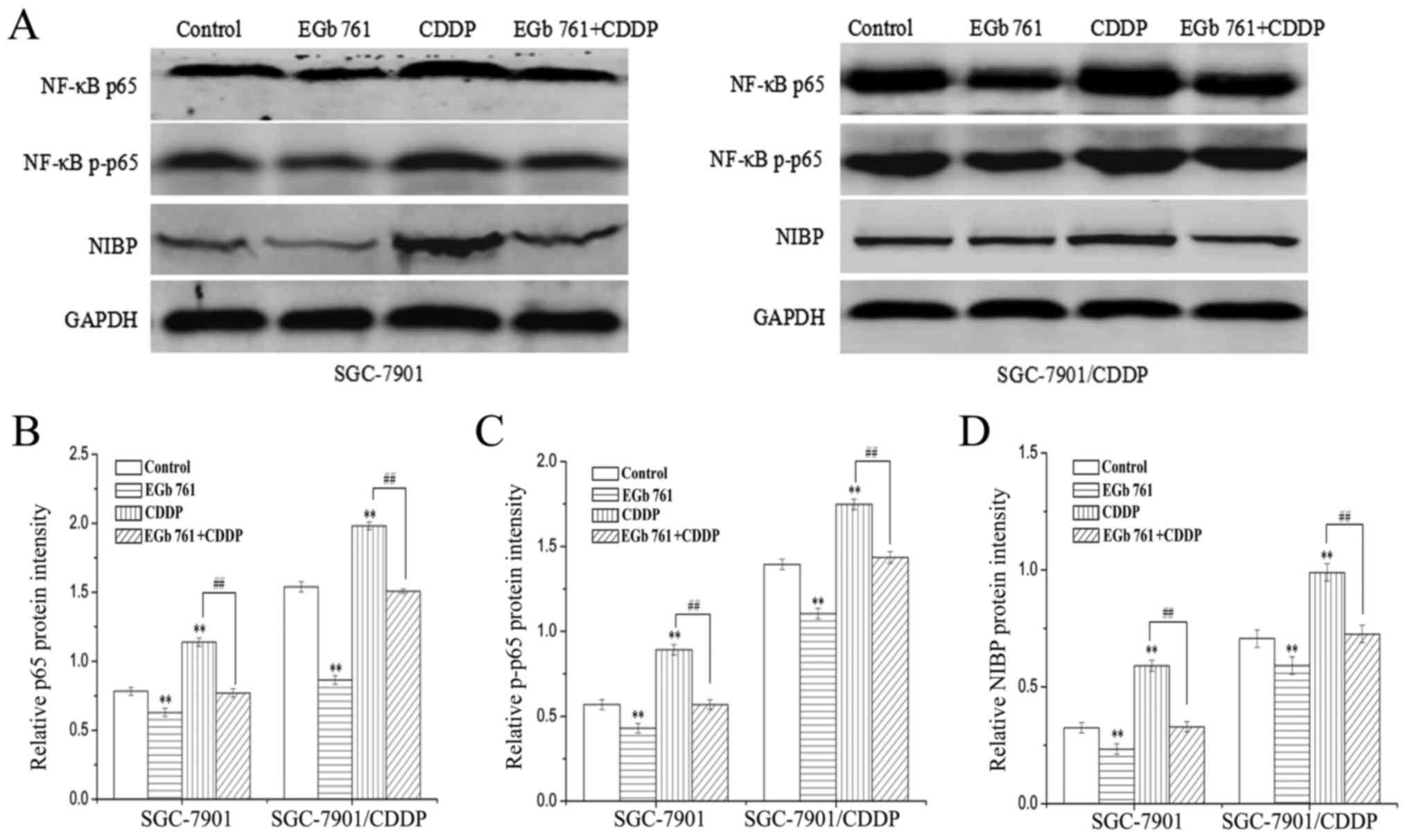
cells (Fig. 4A) (P<0.01).
Compared with the SGC-7901 cells, the expression levels of vimentin
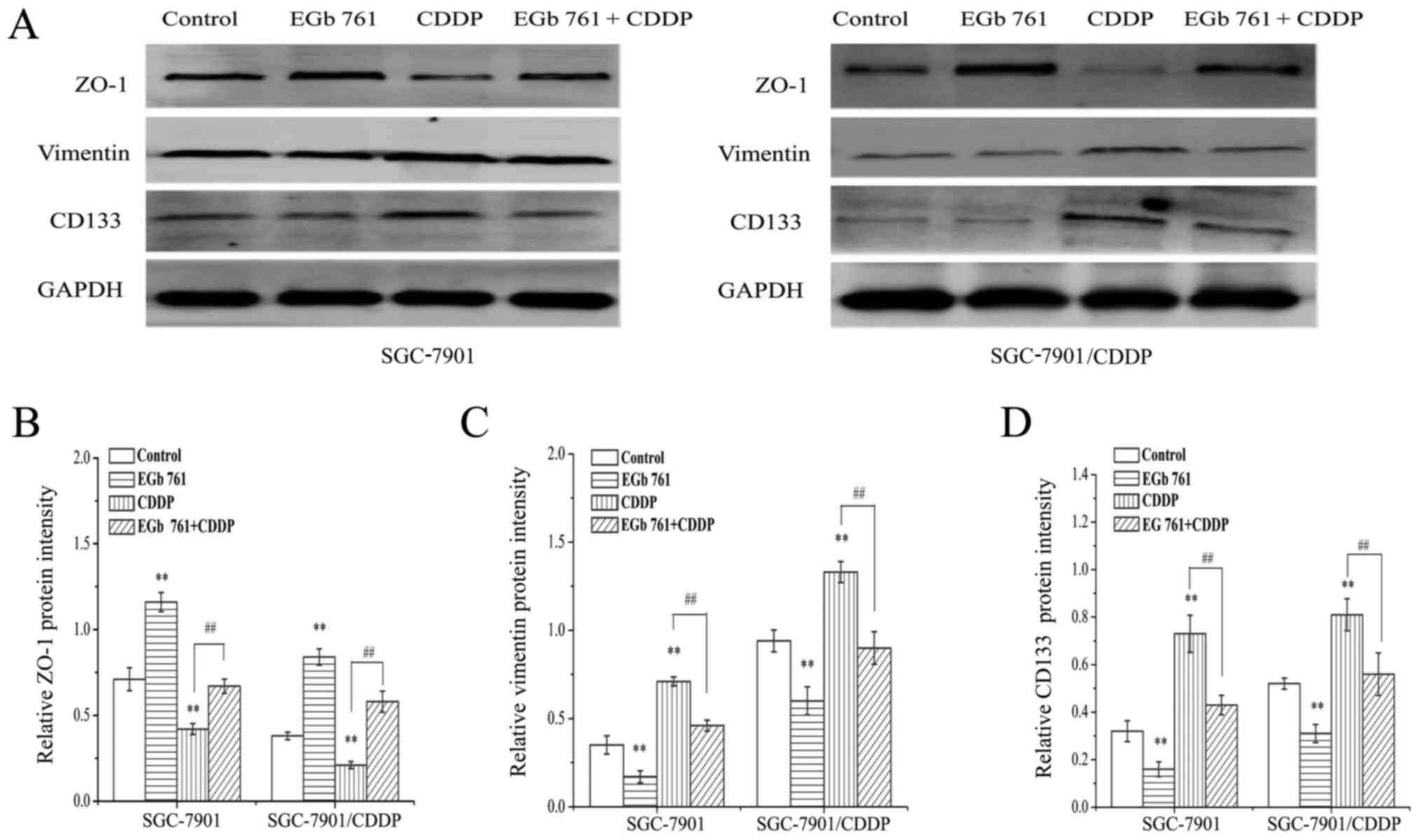
and CD133 were increased in the SGC-7901/CDDP cells, while the
expression of ZO-1 was decreased (Fig.
4B).
Effects of EGb 761 on the expression
of NIBP, NF-κB p65, p-p65, CD133 and EMT induced by CDDP
Compared with the control group, the mRNA expression
levels of NIBP and NF-κB p65 were significantly increased in the
CDDP group, and following combination treatment of EGb 761 and
CDDP, the mRNA expression of NIBP and NF-κB p65 induced by CDDP
were significantly attenuated in both cell lines (Fig. 5). Consistently, the protein
expression levels of NIBP, NF-κB p65 and p-p65 were increased after
treatment with CDDP and decreased following the combined treatment
with EGb 761 and CDDP (Fig. 6).
The protein expression of vimentin and CD133 were
increased, while the expression of ZO-1 was decreased in the CDDP
group, as compared to that in the control group. Following the
combination treatment with EGb761 and CDDP, the expression levels
of vimentin and CD133 induced by CDDP were significantly decreased,
while expression of ZO-1 suppressed by CDDP was significantly
increased (Fig. 7).
Effect of CDDP and EGb761 on the
proliferation and apoptosis of GC cells
The proliferation of SGC-7901 and SGC-7901/CDDP
cells were markedly suppressed following treatment with CDDP or
EGb761 in a dose-dependent manner, and the proliferation inhibition
of CDDP in SGC-7901 cells was significant greater than that of
SGC-7901/CDDP cells (Fig. 8A).
However, there was no significant difference between the
proliferation inhibition of EGb761 in the SGC-7901 and
SGC-7901/CDDP cells (Fig. 8B),
suggesting that SGC-7901/CDDP cells were not resistant to EGb761.
The proliferation inhibition of CDDP was significantly enhanced by
the combined treatment with EGb761 in a dose-dependent manner in
both cell lines (Fig. 8C and D).
The flow cytometric analysis revealed that the cell apoptosis
induced by CDDP was elevated following the combined treatment with
EGb761 and CDDP in both cell lines (Fig. 9).
Discussion
NIK- and IKKβ-binding protein (NIBP) (also known as
TRAPPC9, trafficking protein particle complex 9), as a significant
transcription protein interacting with NIK and IKKβ, plays a
crucial role in a number of fundamental pathophysiological
processes. Increasing evidence indicates that NIBP overexpression
is seemingly involved in the genesis and development of breast
cancer and colon cancer by activation of the NF-κB signaling
pathway (7,9). In the present study, NIBP was found
extensively expressed in gastric cancer (GC) tissues and there was
a closely correlation between the overexpression of NIBP and tumor
invasion depth, differentiation degree, clinical stage and
lymphatic metastasis. Thus, NIBP may contribute to the oncogenesis
and progression of gastric carcinoma. Our previous studies
confirmed that NIBP expression is linked with tumor
differentiation, clinical stage and metastasis in colorectal cancer
(7,8). Further research confirmed that the
expression of NF-κB p65 and NF-κB p-p65 is closely related to the
overexpression of NIBP in GC tissues. Hence, NIBP overexpression
may exert negative influence on the prognosis of GC by regulating
the NF-κB signaling pathway.
In cancer cells, the NF-κB signaling pathway is
involved in cell proliferation, metastasis and chemotherapy
resistance (19,20). Previous evidence indicates that an
increase in the expression of IKKβ and NF-κB p-p65 was promoted
with the expression of NIBP, which indicates that NIBP serves as an
activator in the NF-κB canonical pathways (2). In the present study, the NF-κB
signaling pathway was markedly activated by NIBP in the
drug-resistant GC cells, which indicated that NIBP is involved in
chemotherapy resistance in GC cells. Similar findings were reported
in mouse intestinal nerve cells. The activation of NF-κB p65 was
enhanced with the overexpression of NIBP, and the activation of
NF-κB p65 was inhibited with the suppression of NIBP expression by
using shRNA (4).
Epithelial-mesenchymal transition (EMT) is a process
whereby epithelial cells depolarize and lose intercellular adhesion
components and tight junction, with a corresponding phenotypic
conversion from an epithelial phenotype into a fibroblast-like
mesenchymal morphology (10). In
the present study, SGC-7901 cells exhibited a cuboidal or
cobblestone shape, however, SGC-7901/CDDP cells displayed a
distinct elongated spindle or shuttle appearance. Furthermore,
prominent cisplatin resistance was exhibited in the SGC-7901/CDDP
cells, along with decreased expression of epithelial cell adhesion
molecule ZO-1 and increased expression of mesenchymal cell marker
vimentin. Research has indicated that EMT confers chemotherapy
refractory properties to various types of malignancies and
increases resistance against chemotherapeutics (11,21).
Blocking EMT by knockdown of Snail effectively sensitizes cancer
cells to chemotherapy and radiotherapy (22). Our previous study showed that EGb
761 enhanced the chemotherapy sensitivity of GC cells via
suppression of the NF-κB signaling pathway (14). In the present study, it was
confirmed that the chemo-refractory phenotype was reversed by EGb
761 by suppressing NIBP, the NF-κB signaling pathway and EMT.
Research suggests that the activation of NF-κB weakens the effects
of chemotherapy drugs on tumor cells and that it is associated with
enhanced multi-drug resistance (23,24).
Furthermore, cisplatin results in acquisition of chemoresistance
via triggering EMT through the NF-κB signaling pathway (12,25).
Therefore, the NIBP/NF-κB/EMT axis may be involved in the
chemoresistance of GC.
CD133, a robust CSC marker, impacts patient
prognosis and chemosensitivity of gastric carcinoma and pancreatic
cancer (16,26). It was found that the expression of
CD133 was markedly increased in SGC-7901/CDDP cells when compared
with that in the SGC-7901 cells in the present study. Compelling
evidence indicates that CD133 acts as a upstream promoter of EMT
progression, rather than just a stem cell marker (27). CD133 modulates EMT to exert its
molecular function in pancreatic cancer and non-small cell lung
cancer (28,29). These findings demonstrated that
CD133-modulated EMT may be critical for developing chemoresistance
in GC. In the present study, CDDP-induced expression of CD133 and
EMT was suppressed following inhibition of NIBP-mediated NF-κB
signaling pathway by combination treatment of EGb 761 and CDDP.
Growing evidence suggests that CD133 expression induces EMT through
activation of the NF-κB signaling pathway in tumors (17,18).
Taken together, overexpression of NIBP may endow GC cells the
capability of chemo-refractory properties via the NF-κB/CD133/EMT
cascade.
Summarily, the NIBP-mediated NF-κB signaling pathway
may contribute to oncogenesis, progression and chemotherapeutic
drug resistance by mediating EMT in GC. Inhibition of NIBP may
reverse drug resistance via the NF-κB/CD133/EMT cascade in gastric
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (no. 81460380), the Natural
Science Foundation of Guangxi, China (no. 2011GXNSFA018182), the
Project Foundation from the Health Department of Guangxi, China
(no. GZKZ 10-107) and the Innovation of Project of Guangxi Graduate
Education (no. YCBZ2017035).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SQL and JAH conceived and designed the study. ZHF,
LYZ, WHW and NQ performed the experiments. ZHF and SQL wrote the
paper. ZHF, SQL, CYX, MBQ and LYZ reviewed and edited the
manuscript. All authors read and approved the manuscript and agreed
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The research was authorized by the Medical Ethics
Committee of The First Affiliated Hospital of Guangxi Medical
University, Guangxi, China. Written informed consent was provided
by each patient.
Consent for publication
This manuscript is approved by all participants for
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang D and Fan D: Multidrug resistance in
gastric cancer: recent research advances and ongoing therapeutic
challenges. Expert Rev Anticancer Ther. 7:1369–1378. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hu WH, Pendergast JS, Mo XM, Brambilla R,
Bracchi-Ricard V, Li F, Walters WM, Blits B, He L, Schaal SM, et
al: NIBP, a novel NIK and IKK(beta)-binding protein that enhances
NF(kappa)B activation. J Biol Chem. 280:29233–29241. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zong M, Satoh A, Yu MK, Siu KY, Ng WY,
Chan HC, Tanner JA and Yu S: TRAPPC9 mediates the interaction
between p150Glued and COPII vesicles at the target
membrane. PLoS One. 7:e299952012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang Y, Bitner D, Filho Pontes AA, Li F,
Liu S, Wang H, Yang F, Adhikari S, Gordon J, Srinivasan S and Hu W:
Expression and function of NIK- and IKK2-binding protein (NIBP) in
mouse enteric nervous system. Neurogastroenterol Motil. 26:77–97.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoesel B and Schmid JA: The complexity of
NF-κB signaling in inflammation and cancer. Mol Cancer. 12:862013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Vaiopoulos AG, Athanasoula KC and
Papavassiliou AG: NF-kappaB in colorectal cancer. J Mol Med.
91:1029–1037. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu C, Qin M, Tan L, Liu S and Huang J:
NIBP impacts on the expression of E-cadherin, CD44 and vimentin in
colon cancer via the NF-κB pathway. Mol Med Rep. 13:5379–5385.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tan L, Liu SQ, Qin MB, et al: Relationship
between expression of NIBP and noncanonical NF-κB signaling:
Clinical significance in colon carcinoma. World Chin J Diges.
23:1238–1246. 2015. View Article : Google Scholar
|
|
9
|
Zhang Y, Liu S, Wang H, Yang W, Li F, Yang
F, Yu D, Ramsey FV, Tuszyski GP and Hu W: Elevated NIBP/TRAPPC9
mediates tumorigenesis of cancer cells through NF-κB signaling.
Oncotarget. 6:6160–6178. 2015.PubMed/NCBI
|
|
10
|
Thiery JP, Acloque H, Huang RYJ and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shang Y, Cai X and Fan D: Roles of
epithelial-mesenchymal transition in cancer drug resistance. Curr
Cancer Drug Targets. 13:915–929. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng X, Carstens JL, Kim J, Scheible M,
Kaye J, Sugimoto H, Wu CC, LeBleu VS and Kalluri R:
Epithelial-to-mesenchymal transition is dispensable for metastasis
but induces chemoresistance in pancreatic cancer. Nature.
527:525–530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun Y, Guan Z, Liang L, Cheng Y, Zhou J,
Li J and Xu Y: NF-kappaB signaling plays irreplaceable roles in
cisplatin-induced bladder cancer chemoresistance and tumor
progression. Int J Oncol. 48:225–234. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mao YB, Liu SQ, Tan L, Zhou Q and Huang
JA: EGb761 enhances cisplatin and etoposide-induced apoptosis of
human gastric cancer SGC-7901 cells. World Chin J Diges.
21:3330–3337. 2013. View Article : Google Scholar
|
|
15
|
Soltanian S and Matin MM: Cancer stem
cells and cancer therapy. Tumour Biol. 32:425–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weng CC, Kuo KK, Su HT, Hsiao PJ, Chen YW,
Wu DC, Hung WC and Cheng KH: Pancreatic tumor progression
associated with CD133 overexpression: Involvement of increased TERT
expression and epidermal growth factor receptor-dependent Akt
activation. Pancreas. 45:443–457. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nomura A, Banerjee S, Chugh R, Dudeja V,
Yamamoto M, Vickers SM and Saluja AK: CD133 initiates tumors,
induces epithelial-mesenchymal transition and increases metastasis
in pancreatic cancer. Oncotarget. 6:8313–8322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu YM, Li XF, Liu H and Wu XL:
Ultrasound-targeted microbubble destruction-mediated downregulation
of CD133 inhibits epithelial-mesenchymal transition, stemness and
migratory ability of liver cancer stem cells. Oncol Rep.
34:2977–2986. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sakuma Y, Yamazaki Y, Nakamura Y,
Yoshihara M, Matsukuma S, Koizume S and Miyagi Y: NF-κB signaling
is activated and confers resistance to apoptosis in
three-dimensionally cultured EGFR-mutant lung adenocarcinoma cells.
Biochem Biophys Res Commun. 423:667–671. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zou W, Ma X, Hua W, Chen B and Cai G:
Caveolin-1 mediates chemoresistance in cisplatin-resistant ovarian
cancer cells by targeting apoptosis through the Notch-1/Akt/NF-κB
pathway. Oncol Rep. 34:3256–3263. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang D, Duan H, Huang H, Tong X, Han Y,
Ru G, Qu L, Shou C and Zhao Z: Cisplatin resistance in gastric
cancer cells is associated with HER2 upregulation-induced
epithelial-mesenchymal transition. Sci Rep. 6:205022016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang K, Jiao X, Liu X, Zhang B, Wang J,
Wang Q, Tao Y and Zhang D: Knockdown of snail sensitizes pancreatic
cancer cells to chemotherapeutic agents and irradiation. Int J Mol
Sci. 11:4891–4902. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu W, Yang JL, Wang YL, Wang H, Yao M,
Wang L, Gu JJ, Cai Y, Shi Y and Yao DF: Reversal of multidrug
resistance of hepatocellular carcinoma cells by metformin through
inhibiting NF-κB gene transcription. World J Hepatol. 8:985–993.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou W, Fu XQ, Zhang LL, Zhang J, Huang X,
Lu XH, Shen L, Liu BN, Liu J, Luo HS, et al: The
AKT1/NF-kappaB/Notch1/PTEN axis has an important role in
chemoresistance of gastric cancer cells. Cell Death Dis.
4:e8472013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miow QH, Tan TZ, Ye J, Lau JA, Yokomizo T,
Thiery JP and Mori S: Epithelial-mesenchymal status renders
differential responses to cisplatin in ovarian cancer. Oncogene.
34:1899–1907. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jia ZF, Wu YH, Cao DH, Cao XY, Jiang J and
Zhou BS: Polymorphisms of cancer stem cell marker gene CD133
are associated with susceptibility and prognosis of gastric cancer.
Future Oncol. 13:979–989. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moon YH, Kim D, Sohn HM and Lim W: Effect
of CD133 overexpression on the epithelial-to-mesenchymal transition
in oral cancer cell lines. Clin Exp Metastasis. 33:487–496. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding Q, Yoshimitsu M, Kuwahata T, Maeda K,
Hayashi T, Obara T, Miyazaki Y, Matsubara S, Natsugoe S and Takao
S: Establishment of a highly migratory subclone reveals that CD133
contributes to migration and invasion through
epithelial-mesenchymal transition in pancreatic cancer. Hum Cell.
25:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tu Z, Xie S, Xiong M, Liu Y, Yang X, Tembo
KM, Huang J, Hu W, Huang X, Pan S, et al: CXCR4 is involved in
CD133-induced EMT in non-small cell lung cancer. Int J Oncol.
50:505–514. 2017. View Article : Google Scholar : PubMed/NCBI
|