Introduction
Gastrointestinal stromal tumors (GISTs) are the most
common mesenchymal tumors of the gastrointestinal (GI) tract, and
arise from interstitial cells of Cajal (ICCs) or their precursor
cells (1–5). GISTs tend to form well-circumscribed,
protruding nodules without diffuse infiltration, so that local
resection is often initially an adequate therapy (3,6–8). A 1–2
cm surgical safety margin is thought to be required for grossly and
microscopically complete resection of GISTs (8–10),
referred to as an R0 resection in the Union for International
Cancer Control (UICC) system (11).
Novitsky et al (9)
demonstrated that all 50 surgically removed GISTs with a grossly
1–2 cm margin beyond the tumors exhibited microscopic negative
margins ranging from 0.2 to 4.5 cm. Based on this, Everett et
al (10) deduced a possible
0.5-cm length microscopic extension of GISTs. To the best of our
knowledge, however, detailed histopathological examination of such
laterally extending or spreading lesions of GISTs has not been
previously conducted. Therefore, we examined the incidence and the
histopathological features of these laterally spreading lesions of
GISTs in the present study.
Materials and methods
Patients and GISTs
We examined a total of 52 GISTs grossly completely
removed from 50 patients, which were retrieved from the surgical
pathology files (1994–2016, October) of the Department of
Pathology, Japan Self-Defense Forces Central Hospital, and the
surgical pathology files (1996–2017, April) of the Division of
Pathology, Mishuku Hospital, Tokyo, Japan. All GISTs were confirmed
to be immunohistochemically positive for KIT (1:100; polyclonal;
cat. no. A4502; Dako; Agilent Technologies, Inc., Santa Clara, CA,
USA) and CD34 (1:100; NU-4A1; cat. no. 413361; Nichirei
Biosciences, Inc., Tokyo, Japan). Clinical findings were obtained
from medical charts and request forms for surgical pathology
examination. Patients consisted of 39 men and 11 women, and ranged
in age from 33 to 88 years (mean, 63.9 years). GISTs were located
on the esophagus (2), stomach (38),
small intestine (11 in total: Duodenum, 2; jejunum, 2; ileum, 1;
and not otherwise specified, 6) and cecum (1). Sixteen minute or small GISTs were
incidentally detected in other diseases. The present study was a
retrospective study, which was performed according to the
Declaration of Helsinki, and was approved by the Medical Research
Ethics Committees of the Japan Self-Defense Forces Central Hospital
(approval no. 28-014) and Mishuku Hospital (approval no.
2016-04).
GIST examination
In the present study, we defined ‘exophytic’ GISTs,
as those with serosal protruding extramural components constituting
>50% of the tumor volume. ‘Dumbbell’ -shaped GISTs were also
found (2), but were re-classified
in this study as either exophytic or non-exophytic, according to
the aforementioned definition. Exophytic GISTs attached to the GI
wall with a relatively narrow pedicle were called ‘pedunculated’
GISTs. When a laterally spreading lesion was present, we calculated
its length from the outline of the main GIST or from the pedicle of
the pedunculated GIST. Histology of GISTs was divided into 2 types,
that is, spindle- or epithelioid-cell predominant. The risk
category of each GIST was evaluated using the Joensuu criteria
(12). All 10–20% buffered
formalin-fixed and paraffin-embedded representative specimens were
available. Select serial 4 µm-thick sections were re-cut and
immunostained for discovered on GIST 1 (DOG1) (1:100; clone no. K9;
cat. no. NCL-L-DOG-1; Leica Biosystems, Newcastle, UK), α-smooth
muscle action (SMA) (clone no. 1A4; cat. no. 412021; Nichirei
Biosciences, Inc., Tokyo, Japan; prediluted) and S-100 protein
(S-100) (polyclonal; cat. no. 422091; Nichirei Biosciences, Inc.;
prediluted).
Statistical analysis
Associations between the laterally spreading
features and other clinicopathological findings were analyzed using
the Chi-square test with the Yates' correlation and the unpaired
t-test. Significance was set at P<0.05.
Results
Table I summarizes
the clinicopathological findings of the 52 GISTs. Of the 52 GISTs,
29 (56%) were exophytic, 8 (15%) were pedunculated, 8 (15%) were
dumb-bell shaped, 13 (25%) exhibited ulceration, 43 (83%)/9 (17%)
were spindle-cell/epithelioid-cell predominant, 13 (25%) had a high
mitotic rate (>5/50 high-power fields), 14 (27%) exhibited
mucosal invasion, and 8 (15%) contained skeinoid fibers. Focal SMA
and S-100 positivity was found in 14 (27%) and 4 (8%) GISTs,
respectively. In 38 GISTs immunostained for DOG1, 37 (97%)
exhibited positivity. Eleven (21%) and 10 GISTs (19%) were assessed
as intermediate and high risk, respectively. Laterally spreading
features were identified in 7 GISTs (13%). In 2 GISTs, the
laterally spreading tumor cells involved the surgical margin, and
were referred to as an R1 resection according to the UICC system
(11). No other R1 cases were
found.
 | Table I.Clinicopathological findings of 52
GISTs removed from 50 patients. |
Table I.
Clinicopathological findings of 52
GISTs removed from 50 patients.
| Patients [n=50
patients] |
|
| Age
(years) | 33–88 (mean,
63.9) |
| Sex,
male/female, n | 39/11 |
| GIST location |
|
|
Esophagus/stomach/small
intestine/large intestine, n (%) | 2 (4%)/38 (73%)/11
(21%)/1 (2%) |
| Tumor
size (cm) | 0.09–20 (mean,
4.56) |
| Macroscopic type |
|
|
Exophytica/pedunculatedb/dumbbell-shaped, n (%) | 29 (56%)/8 (15%)/8
(15%) |
| Microscopic
findings |
|
|
Ulceration, n (%) | 13 (25%) |
|
Spindle-cell/epithelioid-cell
predominant, n (%) | 43 (83%)/9 (17%) |
| High
mitotic rate (>5 per 50 HPFs), n (%) | 13 (25%) |
| Mucosal
invasion, n (%) | 14 (27%) |
| Skeinoid
fibers, n (%) | 8 (15%) |
| Laterally
spreading features, n (%) | 7 (13%) |
| R1
resectionc, n (%) | 2 (4%) |
| Immunohistochemical
positivity |
|
|
KIT/CD34/DOG1/focal SMA/focal
S-100 protein, n (%) | 52 (100%)/52
(100%)/37 (97%)d/14
(27%)/4 (8%) |
| Risk category of
GISTe |
|
| Very
low/low/intermediate/high, n (%) | 15 (29%)/16 (31%)/11
(21%)/10 (19%) |
A summary of the clinicopathological findings of
laterally spreading GISTs is provided in Table II. Of these, 1 arose in the
jejunum, while the other 6 were present on the gastric walls,
including the posterior wall of fundus (2 cases), corpus (3 cases)
and antral anterior wall (1 case). Five (71%) were exophytic, while
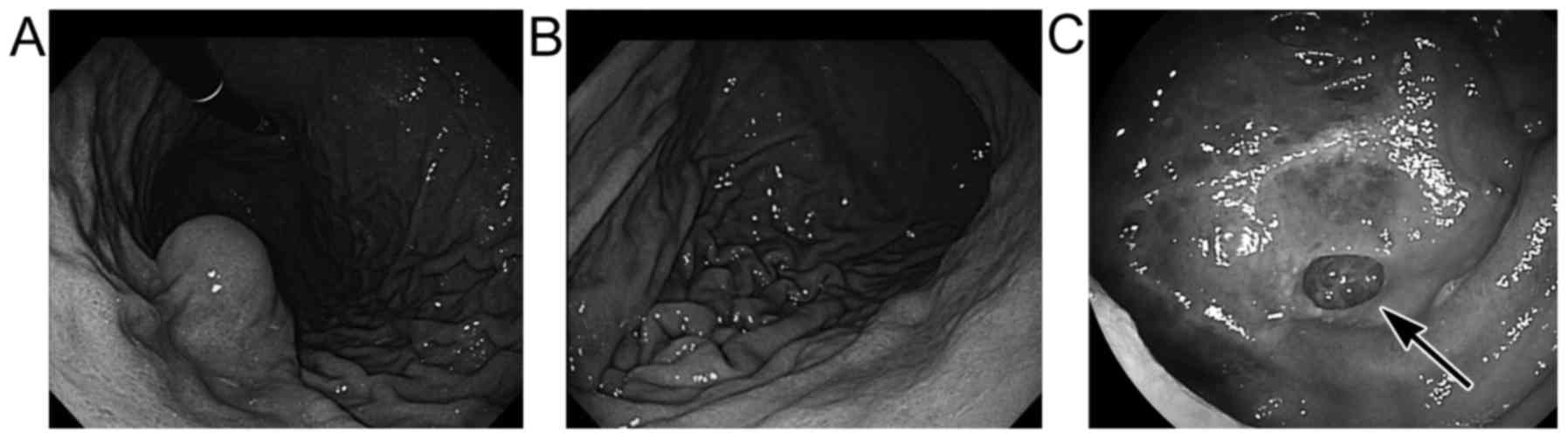
4 exhibited pedunculated features. Endoscopically, submucosal
tumor-like protrusions or mildly elevated features were observed in
4 GISTs (Fig. 1A; cases 1, 3, 5 and
6) and concomitant ulceration was found in one (case 6). However,
these endoscopic features did not appear to be different from those
of other GISTs without laterally spreading lesions. The other 3
laterally spreading GISTs (cases 2, 4 and 7) exhibited no elevated
tumorous features endoscopically (Fig.
1B and C), although one of them (case 7) had well-demarcated,
depressed lesions (Fig. 1C).
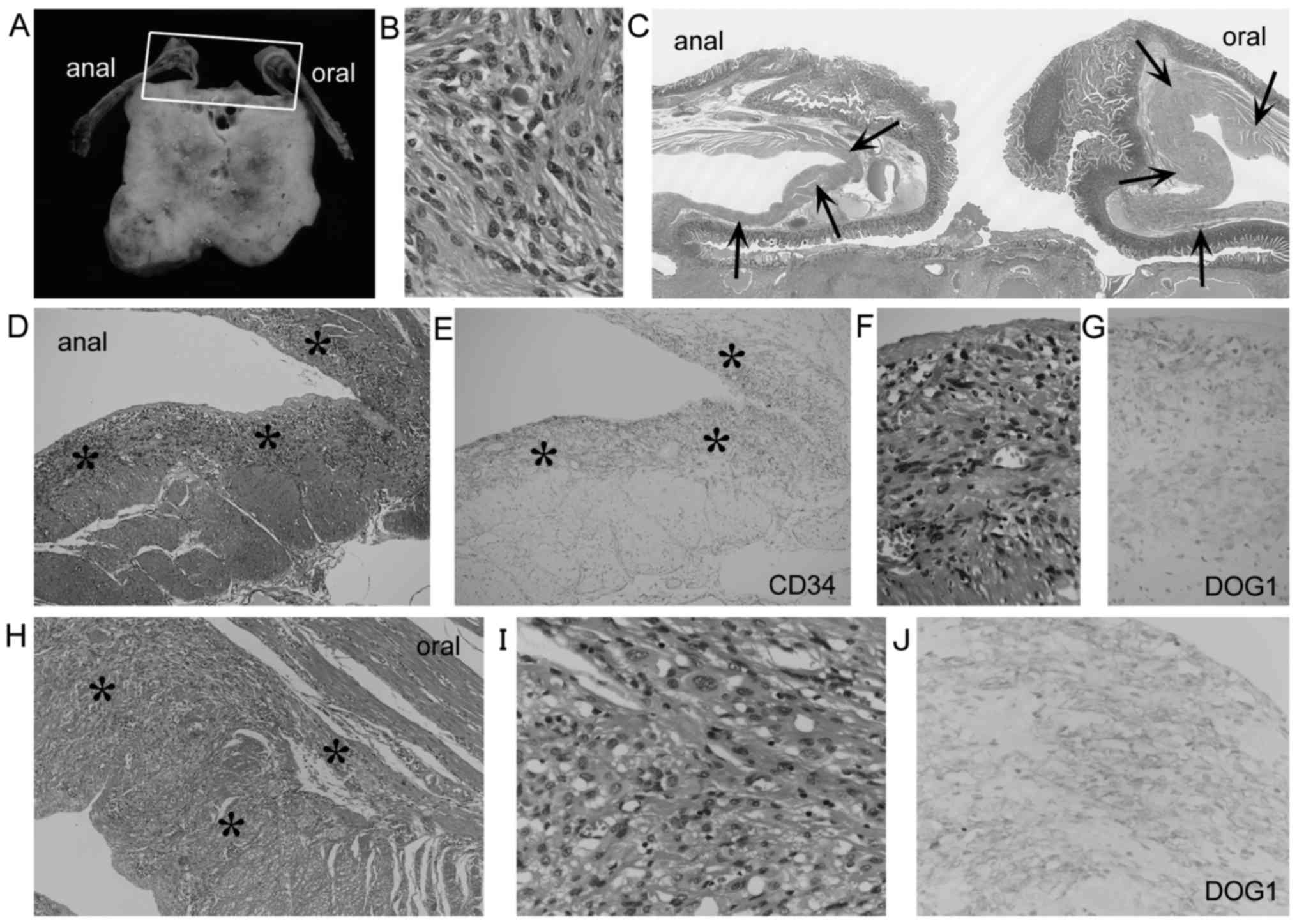
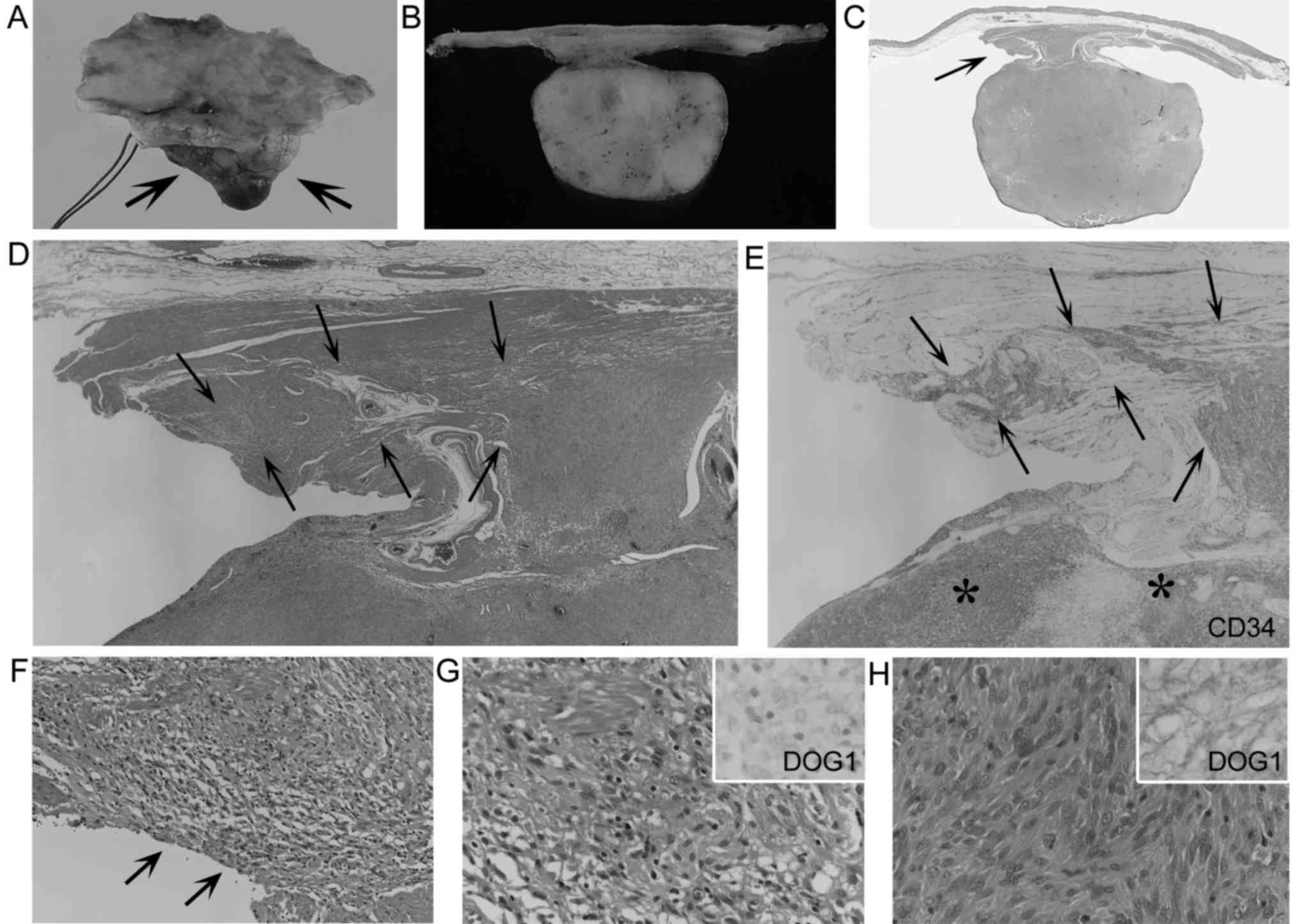
Histologically, the laterally spreading features were not found in
the mucosa or submucosa, and were localized within the muscularis
propria without significant thickening adjacent to the main GISTs
(Figs. 2–4). The lengths of these spreading lesions
ranged from 0.12 to 0.7 cm (mean, 0.3 cm). In the 2 R1 resection
cases, the removed muscular layers adjacent to the main GISTs were
relatively limited, and their surgical margins were involved by the
spreading lesions (Fig. 2C and F).
These spreading cells histologically resembled those of tumor cells
of the main GISTs in 4 cases (Figs. 2G
and H, and 3B, F and I). In
another 3 cases, the spreading cells consisted of more slender
spindle cells with smaller nuclei, compared with tumor cells of the
main GISTs (Fig. 4D and F).
Compared with immunohistochemical features of the main GISTs,
KIT+ and DOG1 staining of the spreading lesions were
similar in 4 cases (Fig. 4E and G),
but were weaker or diminished in the other 3 cases (Fig. 2G, inset and H, inset). There were no
differences of CD34+ staining between the main GISTs and
the spreading lesions (Figs. 2E and
4C). No SMA or S-100 positivity was
found in the spreading spindle cells. One patient (case 6)
succumbed to the disease 2.5 years after the surgery. In this case,
the autopsy revealed a 5.5-cm recurrent growth of the GIST at the
gastric excision site and multinodular peritoneal seeding in the
upper abdominal cavity. The other 6 patients with laterally
spreading GISTs were alive 0.4–19.2 years after the surgery, with
no evidence of disease.
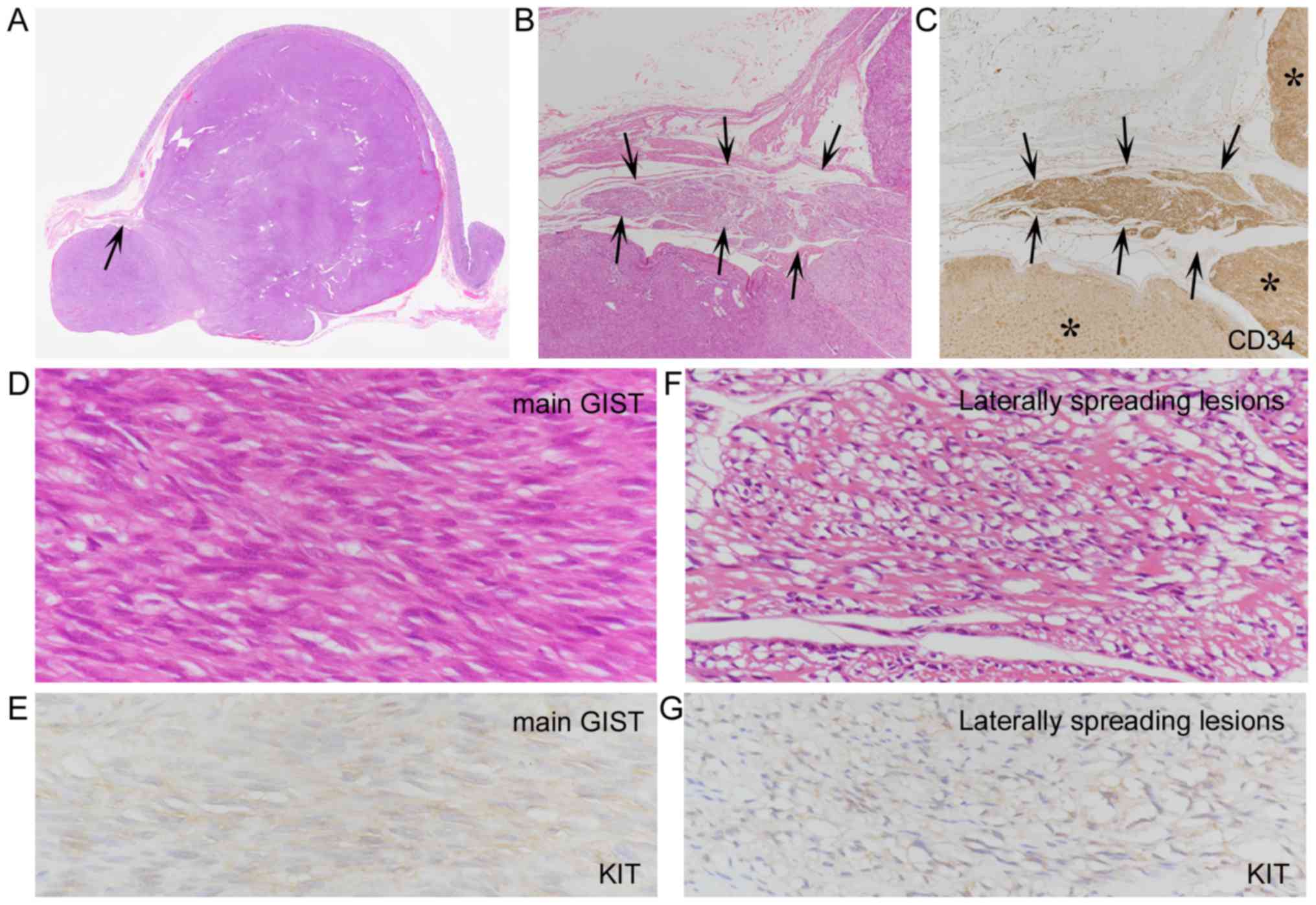
 | Figure 2.Laterally spreading gastrointestinal
stromal tumor (GIST) (case 2). (A and B) An exophytic GIST (A,
arrows) and a cut section (B) revealing a pedunculated shape. (C)
H&E-stained loupe view showing widely removed mucosal and
submucosal margins with limited non-thickened muscularis propria
(arrow). (D and E) Low-power view of H&E-stained intramuscular
spreading lesions (D, arrows), and their CD34+ features
(E, arrows), resembling those of the main GIST (E, asterisks)
(magnification, ×20 for both). (F) H&E-stained moderate-power
view of intramuscular spreading lesions, which involve the surgical
margin (arrows) (magnification, ×200). (G and H) H&E-stained
high-power view of laterally spreading tumor cells (G) and tumor
cells of the main GIST (H), showing some resemblance between both
types of cells. Discovered on GIST 1 (DOG1)+ features of
laterally spreading tumor cells (G, inset) were weaker than those
of the main GIST (H, inset) (magnification, ×400 for all). |
 | Table II.Clinicopathological findings of 7
GISTs showing laterally spreading features. |
Table II.
Clinicopathological findings of 7
GISTs showing laterally spreading features.
|
|
|
|
|
|
|
|
| Findings of laterally
spreading lesions |
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|---|
| Case | Age/sex | Location | Size (cm) | Macroscopic type | Micro-scopic
type | Mucosal invasion | Risk
categorya | Length (cm) | Surgical margin | Histology | KIT/DOG1 | Follow-up
(years) |
|---|
| 1 | 49/M | G-C-GC | 3.8 | Exob, Non-pedc | Sp | None | Low | 0.15 | Negative | More
slendere |
Similarg | ANED, 7.2 |
| 2 | 53/M | G-C-PW | 2.5 | Exob, Pedc | Ep | None | Low | 0.3 | Positive |
Similarf | Weakh | ANED, 2.3 |
| 3 | 78/M | G-A-AW | 4.8 | Exob, Pedc | Ep | None | Low | 0.7 | Positive | More
slendere |
Similarg | ANED, 1 |
| 4 | 68/F | G-C-LC | 16.8 | Exob, Pedc | Sp | None | High | 0.12 | Negative |
Similarf |
Similarg | ANED, 19.2 |
| 5 | 67/M | G-F-PW | 2.8 |
Non-exob,d | Sp | None | Low | 0.12 | Negative |
Similarf |
Similarg | ANED, 13.8 |
| 6 | 88/M | G-F-PW | 4 |
Non-exob | Sp | Present | Int | 0.18 | Negative | More
slendere | Weakh | DOD, 2.5 |
| 7 | 76/M | Jejunum | 6 | Exob, Pedc | Sp | Present | Int | 0.51 | Negative |
Similarf | Weakh | ANED, 0.4 |
The laterally spreading features were significantly
associated with pedunculated GISTs (P=0.006), but not with older
age (P=0.312), sex (P=0.969), tumor location (gastric or
non-gastric) (P=0.725), tumor size (P=0.430), exophytic type
(P=0.626), dumb-bell shape (P=0.634), ulceration (P=0.977),
microscopic type (spindle- or epithelioid-cell predominant)
(P=0.757), high mitotic rate (P=0.815), mucosal invasion (P=0.666),
skeinoid fibers (P=0.634), or higher risk category (high and
intermediate risk category) (P=0.872) (Table III).
 | Table III.Relationship between the laterally
spreading GISTs and other clinicopathological variables. |
Table III.
Relationship between the laterally
spreading GISTs and other clinicopathological variables.
|
| Presence of
laterally spreading lesions of GISTs |
|
|---|
|
|
|
|
|---|
| Variables | Yes (n=7) | No (n=45) | P-value |
|---|
| Age, range (mean),
years | 49–88 (68.4) (n=7
patients) | 33–81 (63.2) (n=43
patients) | 0.312 |
| Sex (Male/female),
n | 6/1 (n=7
patients) | 33/10 (n=43
patients) | 0.969 |
|
Gastric/non-gastric, n | 6/1 | 32/13 | 0.725 |
| Tumor size (mean),
cm | 2.5–16.8
(5.81) | 0.09–20 (4.37) | 0.430 |
| Exophytic
typea, n | 5 | 24 | 0.626 |
| Pedunculated
typeb, n | 4 | 4 | 0.006d |
| Dumbbell-shaped
features, n | 1 | 7 | 0.634 |
| Ulceration, n | 2 | 11 | 0.977 |
|
Spindle-cell/epithelioid-cell predominant,
n | 5/2 | 38/7 | 0.757 |
| High mitotic rate
(>5 per 50 HPFs), n | 1 | 12 | 0.815 |
| Mucosal invasion,
n | 2 | 13 | 0.666 |
| Skeinoid fibers,
n | 1 | 7 | 0.634 |
| Risk
categoryc,
intermediate risk + high risk, n | 3 | 17 | 0.872 |
Discussion
The present study revealed unique laterally
spreading features in 13% of a total of 52 GISTs. These spreading
lesions were harbored within the otherwise normal-looking adjacent
muscularis propria, so that recognition would be challenging either
radiologically, endoscopically, or surgically. In fact, the present
study did not reveal endoscopic features or signs specific to the
laterally spreading lesions. These spreading lesions were not
extensive (range, 0.12–0.7 cm), but infrequently (29% of the 7
cases) involved the muscular surgical margins. These findings not
only support the generally accepted concept that R0 resection
requires a 1 to 2-cm safety margin (8–10), but
also indicate that such safety margins should be applied to the
muscularis propria, not the mucosa or submucosa. In addition, the
present study demonstrated a close relationship between laterally
spreading lesions and the pedunculated shape of GISTs. Therefore,
surgeons should pay attention to the ≥1-cm muscular safety margins
for R0 resection, particularly for pedunculated GISTs, even if
these muscular walls are not thickened or tumorous.
In some cases, laterally spreading tumor cells were
somewhat different from those of the main tumors. Compared with the
pathological features of the main GISTs, laterally spreading
lesions consisted of more slender tumor cells with smaller nuclei
(in 3 cases), and showed weaker or diminished KIT/DOG1+
staining (in 3 cases). However, the former 3 cases were not
identical to the latter 3 cases, and the slender morphological
features of laterally spreading tumor cells did not appear to be
associated with the weaker or diminished KIT/DOG1+
staining. On the other hand, the more slender morphological
features of laterally spreading tumor cells somewhat resembled
those of ICC hyperplasia (4,6,13).
It is known that ICCs can be positive for DOG1 (14,15).
These findings raise the possibility that some laterally spreading
lesions may represent a hyperplastic reaction of ICCs caused,
secondarily, by torque from the stalk of the pedunculated GISTs.
However, the other 4 spreading lesions identified were composed of
tumor cells resembling those of the main GISTs, suggesting true
intramuscular extension of the tumor cells.
The most important risk factors associated with
GISTs are anatomic location, tumor size, and mitotic activity, all
of which are included in the widely accepted risk-stratification
criteria (1–6,12).
Tumor rupture is another independent risk factor of GISTs, despite
its rarity (3–7,12).
Mucosal invasion, high cellularity, and increased microvessel
density may be additional indicators of poor prognosis (1,3,4,6).
Some authors noted high recurrence or poor prognosis in incomplete
resection cases of GISTs compared with complete resection cases
(8,16). However, DeMatteo et al
(17) concluded that
microscopically positive surgical margins did not influence patient
outcomes. Zhi et al (18)
demonstrated that a microscopically positive surgical margin can
impact the disease-free survival of patients with GISTs, but had no
influence on overall survival. Therefore, the prognostic
significance of incomplete GIST resection remains controversial
(6,9,10,19).
In the present study, both patients that had laterally
spreading-related R1 resection were alive without recurrence 1–2.3
years after surgery, demonstrating that they were not associated
with unfavorable outcomes. However, the recurrence or metastases of
GISTs may occur many years later (6), so that further follow-up is required.
In addition, one patient with a laterally spreading GIST succumbed
to the disease 2.5 years after surgery, although the surgical
margin in this case was free of tumor cells and the spreading
lesions exhibited ICC hyperplasia-like slender morphology.
Therefore, regarding the clinicopathological significance of the
laterally spreading lesions, further investigations of a larger
series of GISTs are needed.
To the best of our knowledge, the present study is
the first to histologically describe the laterally spreading
features of GISTs, which occurred in 13% of the GISTs evaluated.
These spreading lesions may contribute to R1 resection, albeit
uncommonly. For R0 resection, the ≥1-cm muscular safety margin
should be required, particularly in cases of pedunculated
GISTs.
Acknowledgements
The authors thank Mr. Kenji Okada and Mr. Shin-ichi
Katori for their excellent technical assistance.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SM conceived and designed the study. MM provided
examined materials. SM, KM, and HT performed the histopathological
examination. SM and YU collected appropriate references. SM wrote
the paper. SM, MM, YU, KS, KM and HT reviewed and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Medical Research Ethics Committees of the Japan Self-Defense Forces
Central Hospital (Tokyo, Japan) and Mishuku Hospital (Tokyo,
Japan).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DOG1
|
discovered on GIST 1
|
|
GI
|
gastrointestinal
|
|
GIST
|
gastrointestinal stromal tumor
|
|
ICCs
|
interstitial cells of Cajal
|
|
S-100
|
S-100 protein
|
|
α-SMA
|
α-smooth muscle actin
|
|
UICC
|
Union for International Cancer
Control
|
References
|
1
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: Pathology and prognosis at different sites. Semin
Diag Pathol. 23:70–83. 2006. View Article : Google Scholar
|
|
2
|
Miettinen M, Fletcher CD, Kindblom LG and
Tsui WM: Mesenchymal tumours of the oesophagus; mesenchymal tumours
of the stomach; mesenchymal tumours of the small intestine;
mesenchymal tumours of the colon and rectumWHO Classification of
Tumours of The Digestive System. Bosman FT, Carneiro F, Hruban RH
and Theise ND: 4th edition. International Agency for Research on
Cancer; Lyon: pp. 35–36, pp74-79, pp115-116, pp181-182. 2010
|
|
3
|
Rosai J: Rosai and Ackerman's surgical
pathology. 10th edition. Mosby/Elsevier; Philadelphia, PA: 2011,
View Article : Google Scholar
|
|
4
|
Rubin BP: GIST and EGISTEnzinger and
Weiss's soft tissue tumors. Goldblum JR, Folpe AL and Weiss SW: 6th
edition. Elsevier/Saunders; Philadelphia, PA: pp. 569–590. 2014
|
|
5
|
Miettinen MM, Corless CL, Debiec-Rychter
M, Fletcher JA, Lasota J, Rubin BP and Sciot R: Gastrointestinal
stromal tumorWHO Classification of Tumours of Soft Tissue and Bone.
Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: 4th edition.
International Agency for Research on Cancer; Lyon: pp. 164–167.
2013
|
|
6
|
Grant NG and Noffsinger AE: Mesenchymal
tumorsFenoglio-Preiser's Gastrointestinal Pathology. Noffsinger AE:
4th edition. Wolters Kluwer; Philadelphia, PA: pp. 1141–1222.
2017
|
|
7
|
Dematteo RP, Heinrich MC, El-Rifai WM and
Demetri G: Clinical management of gastrointestinal stromal tumors:
Before and after STI-571. Hum Pathol. 33:466–477. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gouveia AM, Pimenta AP, Capelinha AF, de
la Cruz D, Silva P and Lopes JM: Surgical margin status and
prognosis of gastrointestinal stromal tumor. World J Surg.
32:2375–2382. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Novitsky YW, Kercher KW, Sing RF and
Heniford BT: Long-term outcomes of laparoscopic resection of
gastric gastrointestinal stromal tumors. Ann Surg. 243:738–747.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Everett M and Gutman H: Surgical
management of gastrointestinal stromal tumors: Analysis of outcome
with respect to surgical margins and technique. J Surg Oncol.
98:588–593. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brierley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumours. 8th edition.
Wiley-Blackwell; Hoboken: 2017
|
|
12
|
Joensuu H: Risk stratification of patients
diagnosed with gastrointestinal stromal tumor. Hum Pathol.
39:1411–1419. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Agaimy A and Wünsch PH: Sporadic Cajal
cell hyperplasia is common in resection specimens for distal
oesophageal carcinoma. A retrospective review of 77 consecutive
surgical resection specimens. Virchows Arch. 448:288–294. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Espinosa I, Lee CH, Kim MK, Rouse BT,
Subramanian S, Montgomery K, Varma S, Corless CL, Heinrich MC,
Smith KS, et al: A novel monoclonal antibody against DOG1 is a
sensitive and specific marker for gastrointestinal stromal tumors.
Am J Surg Pathol. 32:210–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miettinen M, Wang ZF and Lasota J: DOG1
antibody in the differential diagnosis of gastrointestinal stromal
tumors: A study of 1840 cases. Am J Surg Pathol. 33:1401–1408.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Langer C, Gunawan B, Schüler P, Huber W,
Füzesi L and Becker H: Prognostic factors influencing surgical
management and outcome of gastrointestinal stromal tumours. Br J
Surg. 90:332–339. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
DeMatteo RP, Lewis JJ, Leung D, Mudan SS,
Woodruff JM and Brennan MF: Two hundred gastrointestinal stromal
tumors. Recurrence patterns and prognostic factors for survival.
Ann Surg. 231:51–58. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhi X, Jiang B, Yu J, Røe OD, Qin J, Ni Q,
Sun L, Xu M, Zhu J and Ma L: Prognostic role of microscopically
positive margins for primary gastrointestinal stromal tumors: A
systematic review and meta-analysis. Sci Rep. 6:215412016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McCarter MD, Antonescu CR, Ballman KV,
Maki RG, Pisters PWT, Demetri GD, Blanke CD, von Mehren M, Brennan
MF, McCall L, et al: Microscopically positive margins for primary
gastrointestinal stromal tumors: Analysis of risk factors and tumor
recurrence. J Am Coll Surg. 215:53–60. 2012. View Article : Google Scholar : PubMed/NCBI
|