Introduction
Although the incidence of gastrointestinal cancer is
lower than that of other cancers, it is the second leading cause of
cancer-related deaths worldwide (1). Gastric cancer begins in the inner
lining of cells, causing abnormal hyperplasia that leads to
ulceration, inflammation and ultimately tumour formation (2). More than 50% of distal gastric cancer
patients can be cured, however, early diagnosis accounts for only
10–20% of all cases (3). The
mortality rate of gastric cancer has declined significantly with
the latest advances in diagnosis and treatment. However, most
patients are diagnosed at an advanced stage, which is difficult to
cure (4). For patients with
node-positive (T1 N1) and muscle-invasive (T2 N0) disease,
radiotherapy and chemotherapy maybe the most effective treatment
measures. However, patients receiving radiotherapy and chemotherapy
may have problems with chemoresistance and toxic side-effects.
Thus, further studies are needed in order to develop new treatment
strategies.
Increasingly, researchers are focusing on naturally
occurring compounds from dietary sources (5). Cucurbitacins, which are found in
plants from the Cucurbitaceae family, are tetracyclic triterpenes.
Cucurbitacins exhibit moderate to high toxicity, but they contain
structural properties that may aid in future chemotherapy
modalities. These properties make them potential antitumour agents
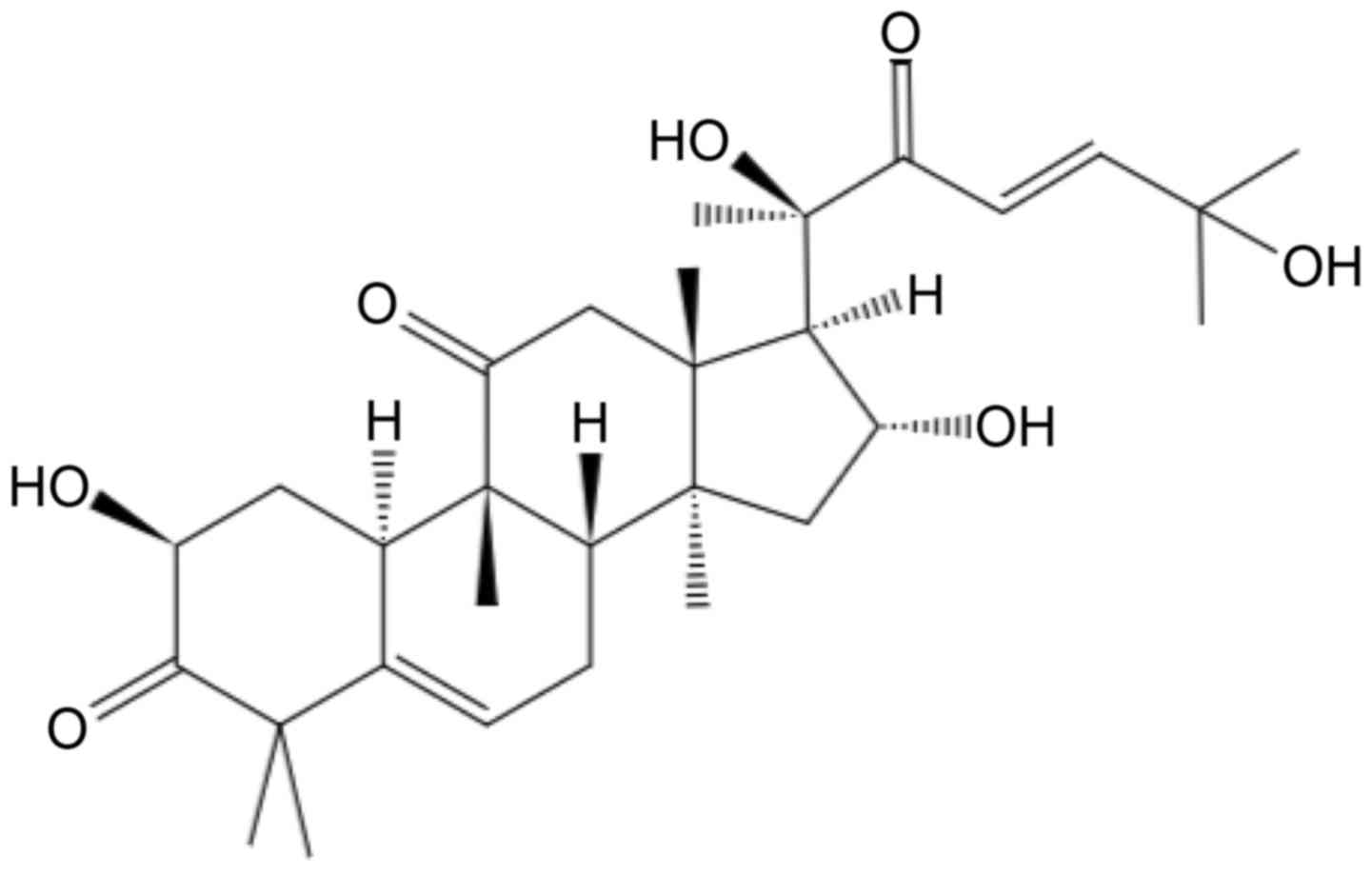
(5,6). Cucurbitacin D (CuD; Fig. 1) is a member of the Cucurbitaceae
family. Studies have reported the anticancer activity of CuD in
various cancer models (5,7,8). CuD
has been reported to inhibit the growth of cervical cancer cells
(5) and doxorubicin-resistant human
breast carcinoma cells (8). In
addition, CuD has been found to suppress the heat shock protein 90
(HSP90) chaperone machinery (9).
These data indicated that CuD potentially affects gastric cancer.
The aim of the present study was to investigate the in vitro
and in vivo effects of CuD on gastric cancer growth and
apoptosis.
The progression of gastric cancer occurs through
various oncogenic pathways such as the nuclear factor-κB, phospho
inositide 3-kinase/protein kinase B (PI3K/Akt) and Wnt/β-catenin
pathways (10). Among these, the
PI3K/Akt pathway is an important signalling pathway that regulates
cell survival, growth, metabolism and chemotherapy resistance
(11). Gastric cancer is
characterized by a high rate of somatic cell turnover via the
PI3K/Akt pathway, which indicates that PI3K/Akt may be an effective
therapeutic target (12). In the
present study, we observed that CuD effectively triggered gastric
cancer cell apoptosis by inhibiting Akt and activating the
inducible nitric oxide synthase (iNOS) pathway.
Materials and methods
Cells and drugs
AGS, SNU1 and Hs746T cell lines were purchased from
Corbioer Company (Nanjing, China). Shanghai Winherb Medical S&T
Development Co. Ltd. (Shanghai, China) provided purified CuD. All
the primary antibodies including those for iNOS (diluted at
1:1,000; cat. no. 13120), Bax (diluted at 1:1,000; cat. no. 2722),
B-cell lymphoma 2 (Bcl-2; cat. no. 2870), C-caspase-9 (diluted at
1:1,000; cat. no. 9509P), cytochrome c (diluted at 1:1,000;
cat. no. 4272), phosphorylated and total Akt (diluted at 1:1,000;
cat. no. 4060/4691), mechanistic target of rapamycin (mTOR, cat.
no. 2971/2983), sr6 (diluted at 1:1,000; cat. no. 9204/2708) and
glyceraldehyde 3-phosphate dehydrogenase (GAPDH; diluted at
1:1,000; cat. no. 5174) were obtained from Cell Signaling
Technology, Inc. (Danvers, MA, USA). The Akt activator SC79 was
obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany.) The
iNOS inhibitor L-canavanine was purchased from MedChemExpress
(Monmouth Junction, NJ, USA).
Cell proliferation assay
Cell proliferation and viability were assessed by
Cell Counting Kit-8 (CCK-8) assay (Beyotime Institute of
Biotechnology, Haimen, China). Cells were seeded into a 96-well
plate. After the cells were treated with CuD, CCK-8 solution (10
µl) was added to each well. After 4 h of incubation, an
enzyme-linked immunosorbent assay (ELISA) (Synergy HT; BioTek
Instruments, Inc., Winooski, VT, USA) was used to the determine
absorbance at 450 nm.
TUNEL staining
Apoptosis was detected by a terminal
deoxynucleotidyl transferase 2′-deoxyuridine-5′-triphosphate
nick-end labelling (TUNEL) assay (ApopTag Plus Fluorescein In
Situ Apoptosis Detection kit; Millipore, Darmstadt, Germany).
After washing with phosphate-buffered saline (PBS; Gibco, Grand
Island, NY, USA), the cells were fixed with 1% paraformaldehyde in
PBS. TUNEL reagents (EMD Millipore, Billerica, MA, USA) were used
to stain the apoptotic cells and 4′,6-diamidino-2-phenylindole
(DAPI; Invitrogen; Life Technologies Carlsbad, CA, USA) was used to
stain the DNA. A microscope (Olympus BX51TRF; Olympus Corp., Tokyo,
Japan) was used to analyse and count positive cells.
ROS generation
Reactive oxygen species (ROS) were detected with a
ROS Assay kit (Cell Biolabs, Inc., San Diego, CA, USA). A
cell-permeable fluorogenic probe 2′,7′-dichlorodihydrofluorescin
diacetate (DCFH-DA) was used to label ROS. A fluorometric plate
reader (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used
to quantify fluorescence at 480/530 nm.
ATP level
The levels of ATP were detected by an ATP assay kit
(S0026; Beyotime Institute of Biotechnology). After the cells were
treated with CuD, cells lysates were collected and incubated with
ATP detection solution (100 µl). A fluorometric plate reader was
used to determine the levels of ATP. Standard curve method was used
to calculate the ATP concentration in each group.
Intracellular Ca2+
level
The level of intracellular Ca2+ was
assessed using Fluo-3/AM (S1056; Beyotime Institute of
Biotechnology). After treatment, D-Hanks balanced salt solution
(D-HBBS; Jinuo Co., Ltd., Shanghai, China) (without
Ca2+, Mg2+ and phenol red) was used to wash
cells. Then, cells were incubated with Fluo-3/AM (5 µM) for 60 min
at 37°C. After being washed in D-HBBS for 30 min, a microplate
reader was used to determine the fluorescent absorbance value, with
representative intracellular Ca2+ levels.
Nitric oxide (NO) production
NO production was detected using Griess method kit
(S0021; Beyotime Institute of Biotechnology) according to the
instructions of the NO assay kit.
Western blot analysis
Cell lysates were collected using RIPA lysis buffer.
Total protein (50 µg) was used for SDS-PAGE. After being
transferred into immobilon-FL transfer membranes (IPFL00010;
Millipore, Billerica, MA, USA), proteins were incubated with the
primary antibody overnight at 4°C, followed by incubation with
secondary antibodies for 1 h. A two-coloured infrared imaging
system (Odyssey; LI-COR Biosciences, Lincoln, NE, USA) was used to
scan. GAPDH protein was used as the reference protein.
Xenograft tumour model
All animal experiments were performed according to
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (NIH Publications no. 8023, revised 1978). All
animal experiments were approved by the Committee on the Use of
Live Animals of The First Affiliated Hospital of Zhengzhou
University. Male BALB/c-nu/nu nude mice (6–8 weeks) were purchased
from the Beijing HFK Bioscience and housed in specific
pathogen-free conditions under approved institutional animal care
and use protocols. Mice received injection of 1×106 AGS
cell suspension. After reaching ~200 mm3 tumour volume,
the mice were treated with CuD (intraperitoneal injection, 1 mg/kg,
once daily), SC79 (intraperitoneal injection, 20 mg/kg, once daily)
or combination of CuD with SC79 for 20 days. The tumor size was
measured by vernier caliper measurement of the tumor size and the
short diameter. Tumour volumes were calculated using the following
formula: Length × width2 × 0.5236.
Statistical analysis
We used the SPSS 17.0 (SPSS, Inc., Chicago, IL, USA)
for statistical analysis. All data were expressed as the mean ±
standard deviation. The difference among groups was assessed by
analysis of variance (ANOVA). The difference between groups was
assessed by Student's t-test. We considered P<0.05 to indicate a
statistically significant difference.
Results
Effects of CuD on gastric cancer cell
proliferation
Under normal conditions, human gastric cancer cell
lines (AGS, SNU1 and Hs746T) have a fast growth rate. In the
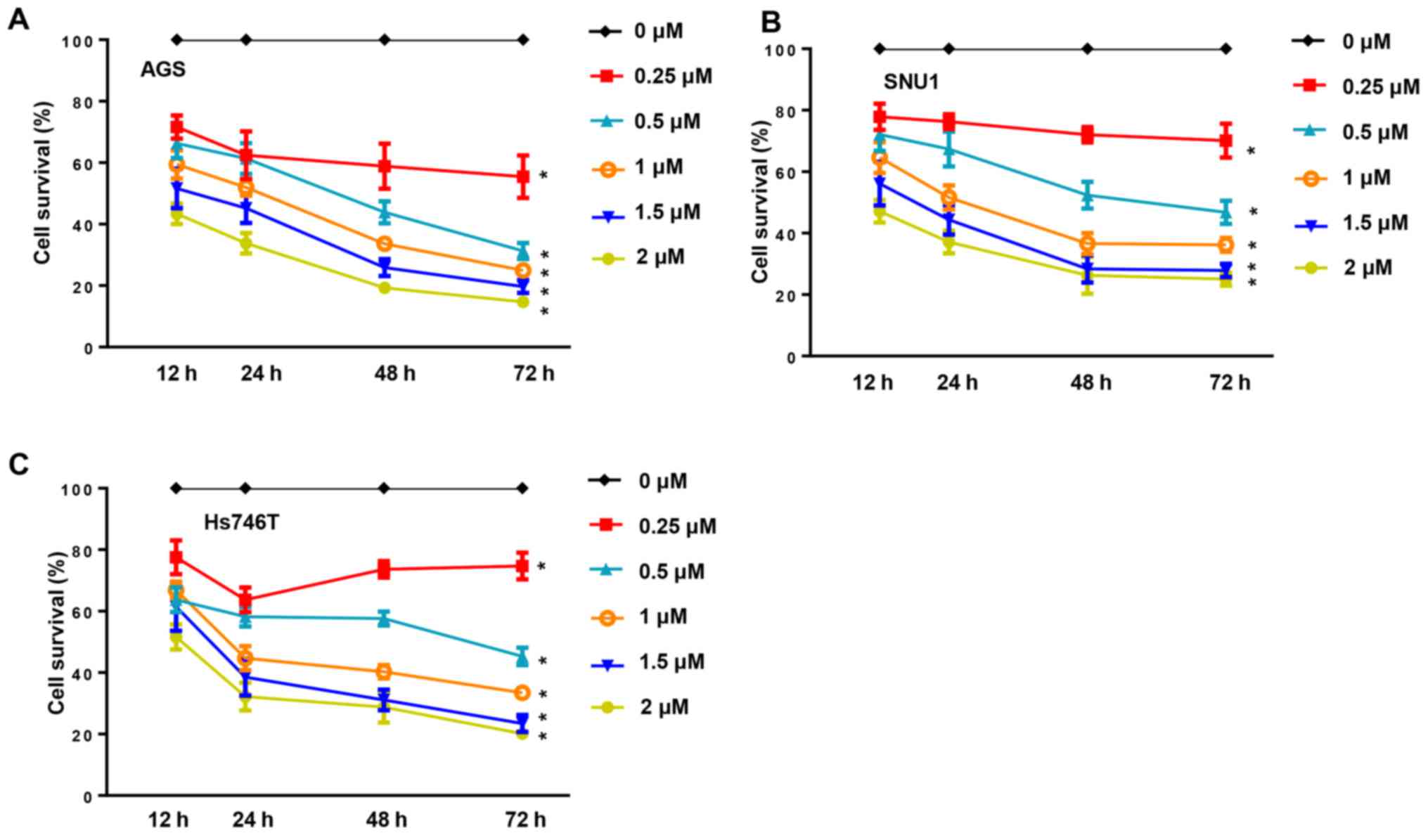
present study, cancer cell proliferation was inhibited in a dose-
and time-dependent manner when the cells were incubated with CuD
(0.25, 0.5, 1, 1.5 and 2 µM) for 12, 24, 48 and 72 h (Fig. 2). The anti-growth effect of CuD was
more significant in the AGS cell line, indicating that the AGS cell
line was more sensitive to CuD.
CuD increases ROS generation in
gastric cancer cells
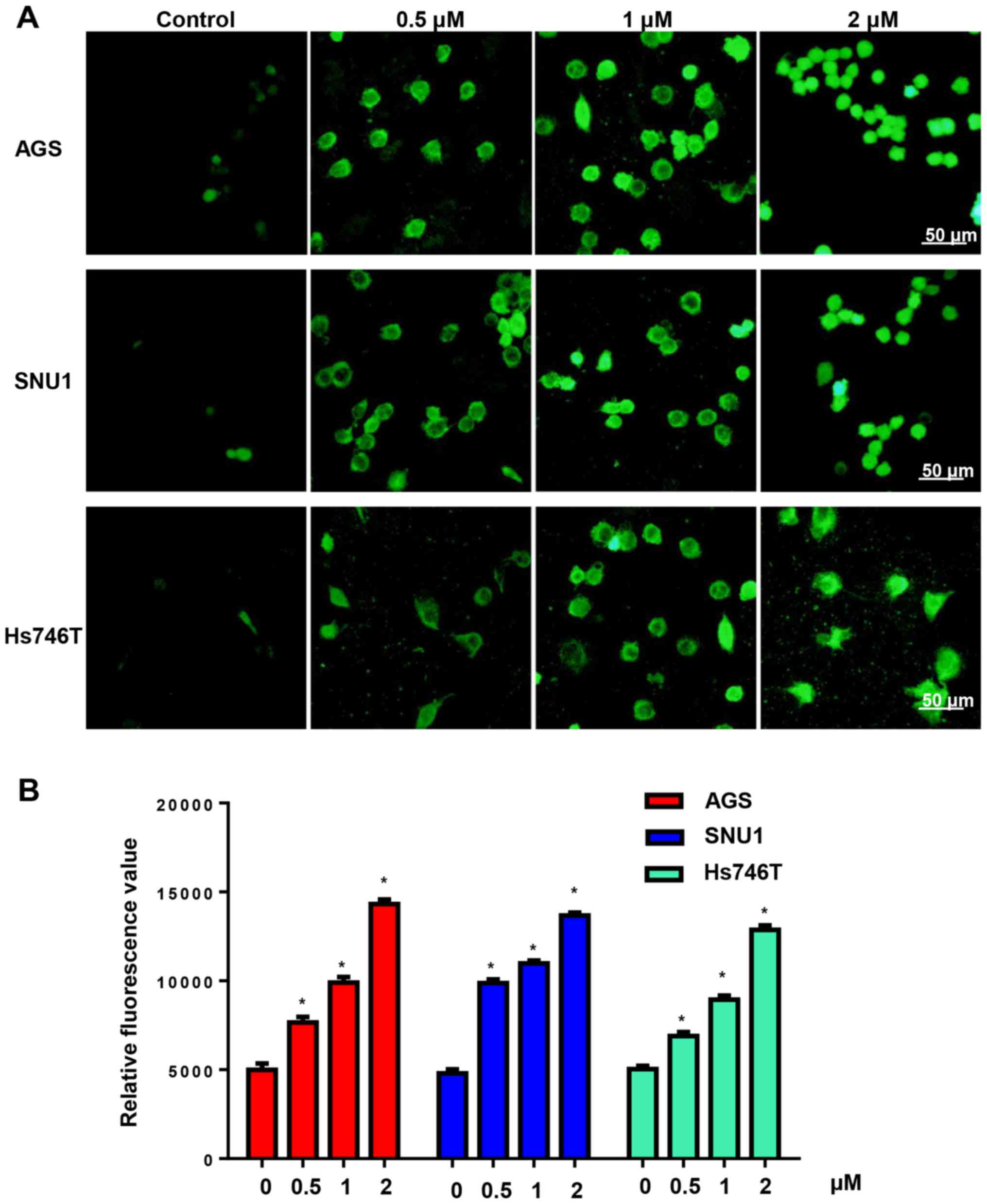
Increasing the generation of ROS is an effective
antitumour property of the cucurbitacin family. We detected ROS
generation after cells were treated with CuD (0.5, 1 and 2 µM) for
24 h. We found that CuD increased the ROS levels of gastric cancer
cell lines in a dose-dependent manner (Fig. 3).
Effects of CuD on gastric cancer cell
apoptosis
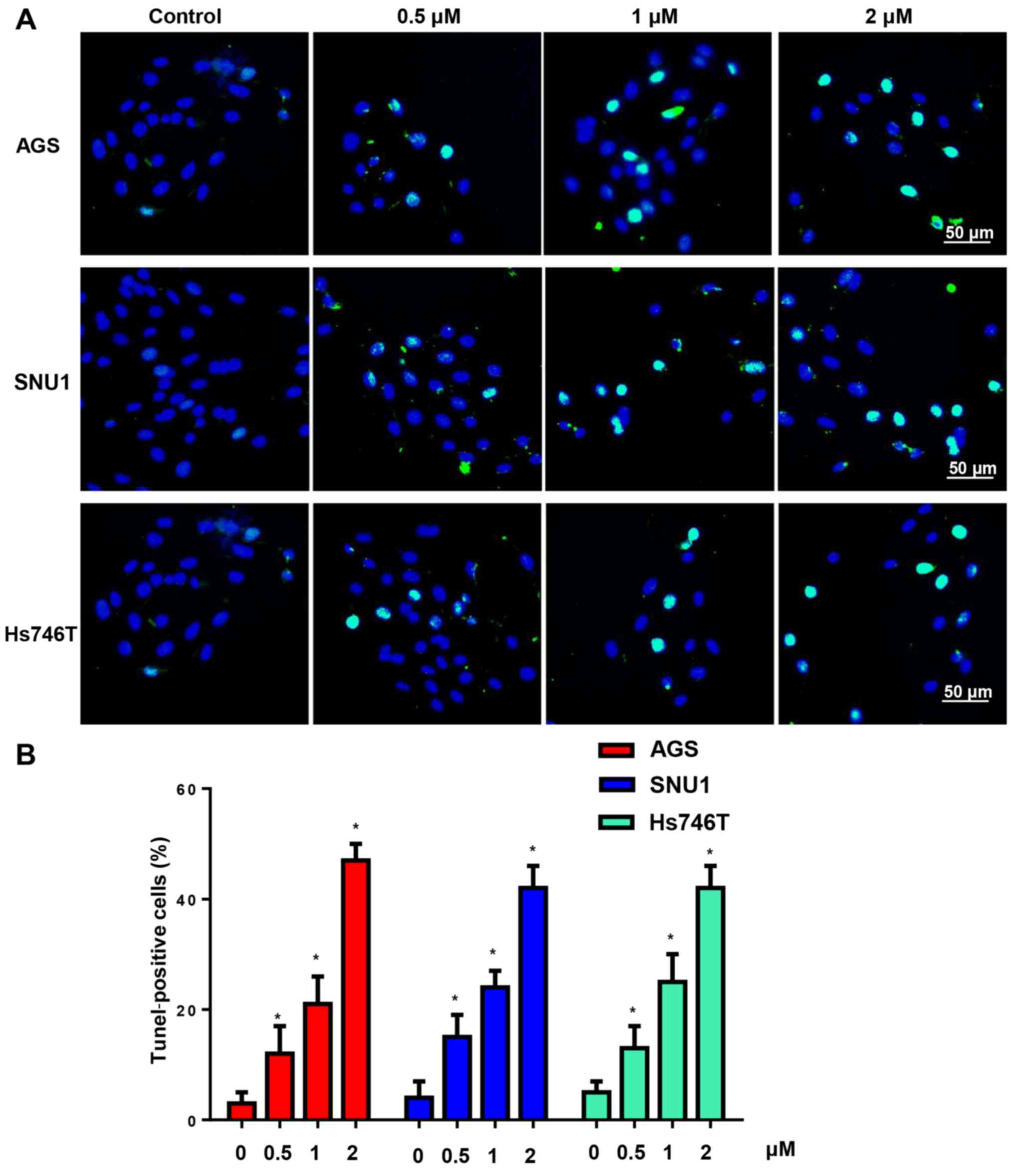
Subsequently, TUNEL staining was used to determine
the effect of CuD on gastric cancer cell apoptosis. After
incubation with CuD (0.5, 1 and 2 µM) for 24 h, the gastric cancer
cells had a high ratio of apoptosis-positive to apoptosis-negative
cells, which was dose-dependent (Fig.
4).
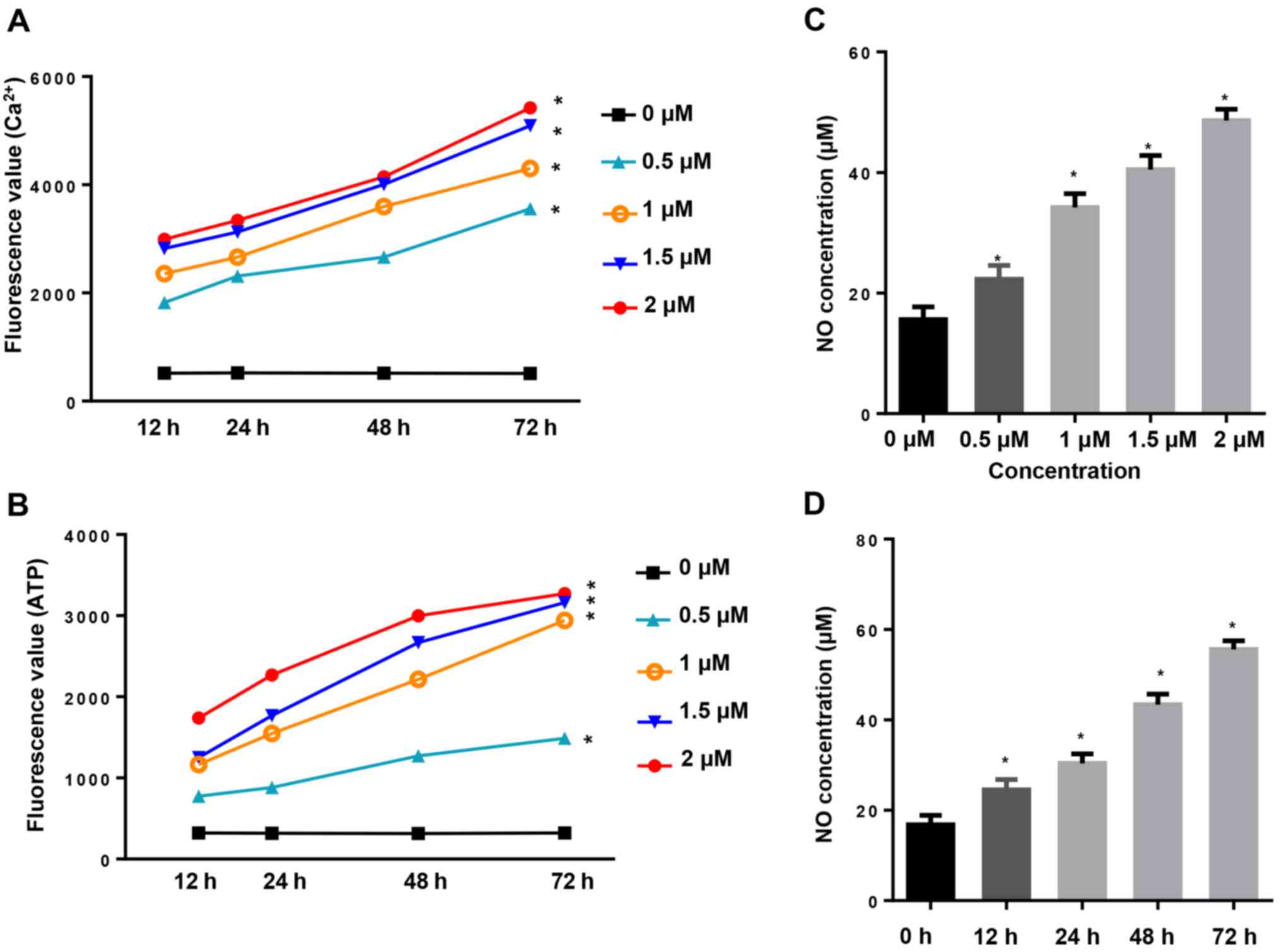
CuD increases intracellular
Ca2+, ATP and NO concentration
Increased Ca2+ fluxion and generation of
ATP are closely related to cell apoptosis. In the present study,
intracellular Ca2+ and ATP generation were assessed by
their fluorescence absorbance values. Our results revealed that
incubation of AGS cells with CuD (0.5, 1, 1.5 and 2 µM) for 12, 24,
48 and 72 h increased the levels of intracellular Ca2+
(Fig. 5A) and ATP (Fig. 5B) in a dose- and time-dependent
manner. Intracellular nitric oxide (NO) production in the culture
media was assessed as nitrite concentration. We observed that
incubation of AGS cells with CuD (0.5, 1, 1.5 and 2 µM) for 12, 24,
48 and 72 h markedly increased NO production both in a dose- and
time-dependent manner (Fig. 5C and
D).
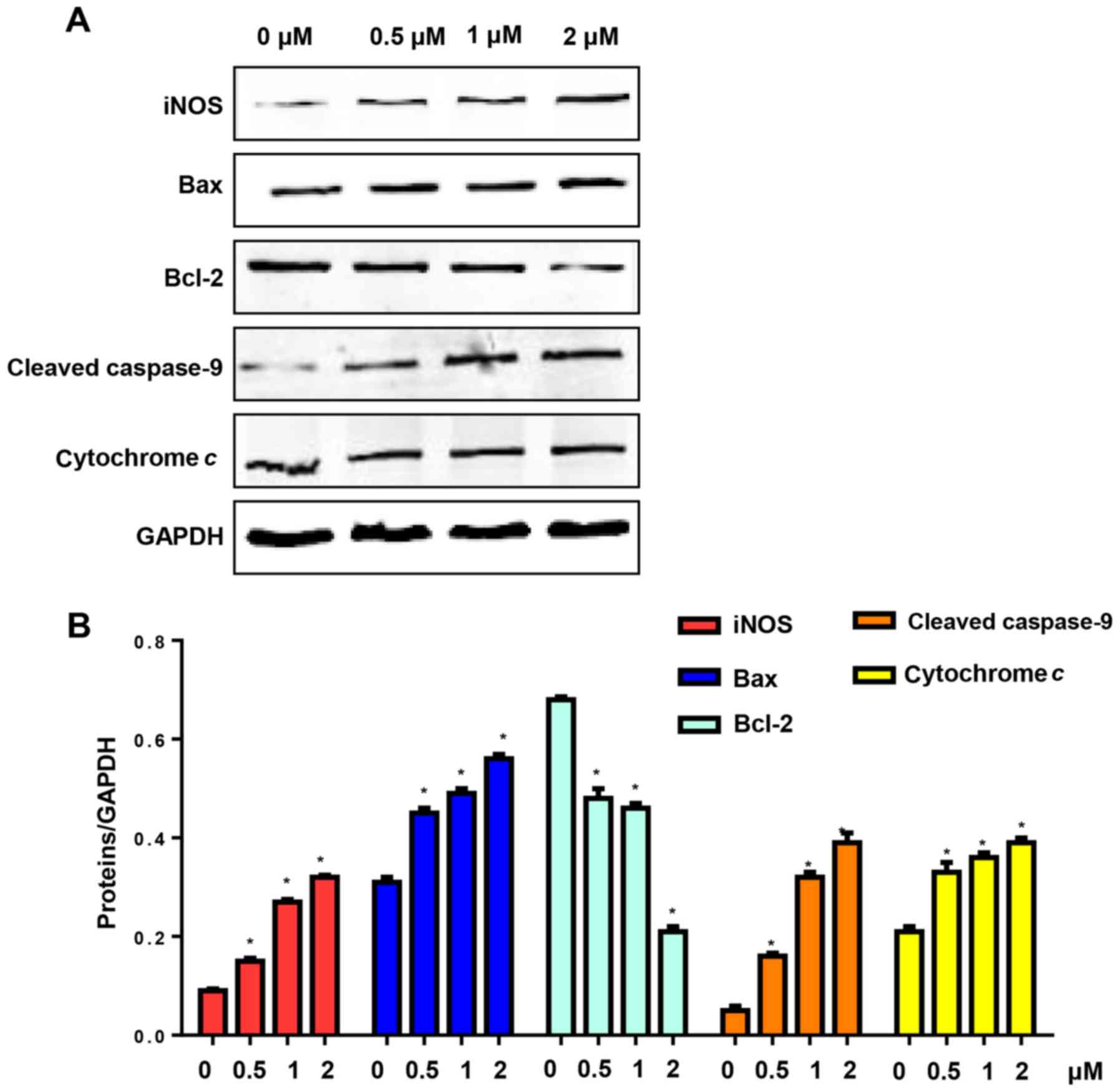
CuD triggers the activation of the
mitochondrial pathway in gastric cancer cell lines
NO production is triggered by NO synthase (NOS).
Apoptosis is triggered by intrinsic and extrinsic apoptotic
pathways. Therefore, we evaluated the expression of iNOS and the
mitochondrial pathway in AGS cells after treatment with CuD (0.5, 1
and 2 µM) for 24 h. Our results revealed that CuD increased the
expression level of iNOS, which triggered the generation of NO. CuD
also increased Bax levels and reduced the expression of Bcl-2,
triggering the activation of caspase-9, as well as the release of
cytochrome c in AGS cells (Fig.
6).
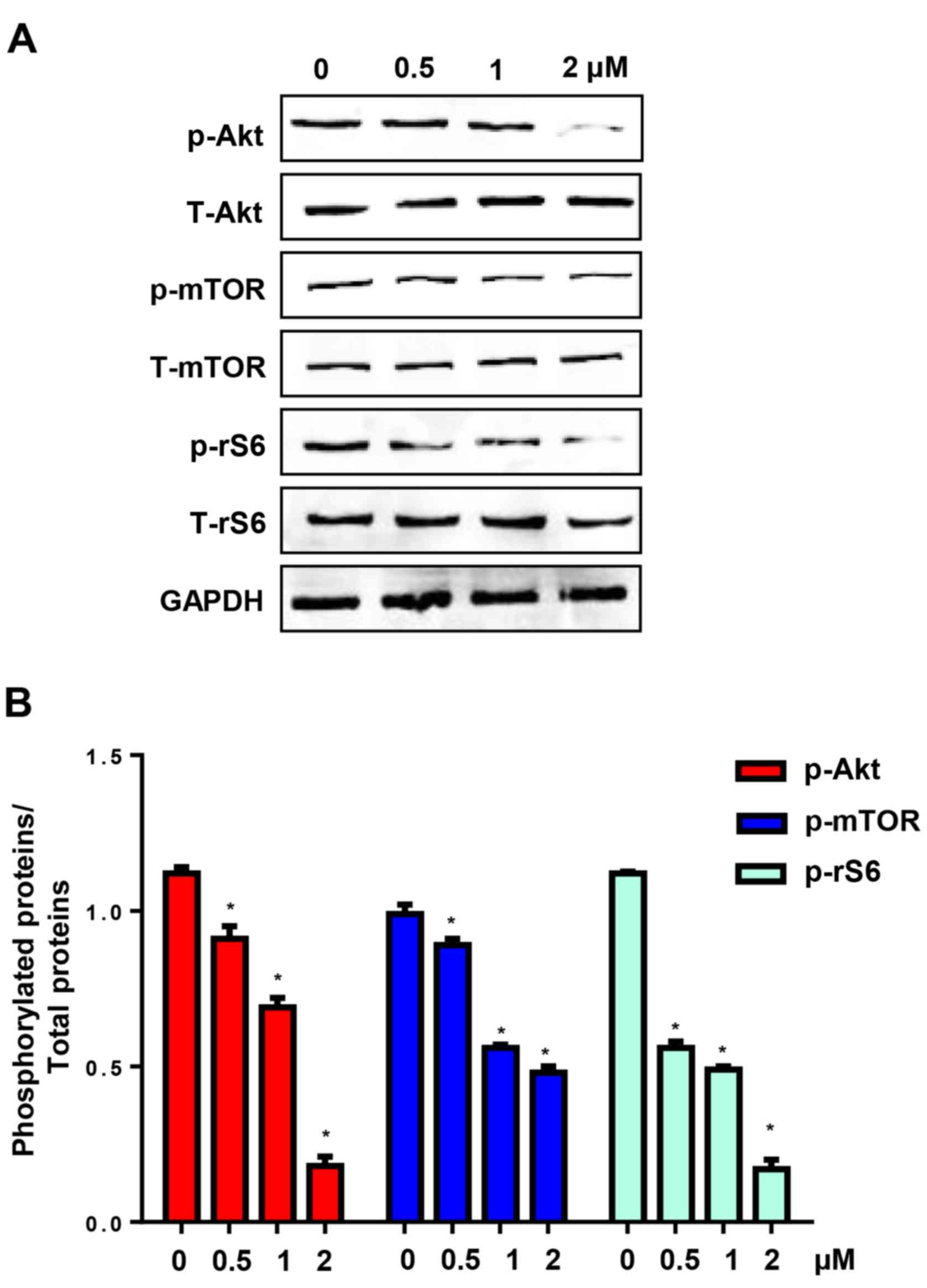
CuD inhibits Akt-mediated signalling
pathway
The Akt pathway is involved in many processes
associated with the poor prognosis of cancer, such as pro-survival,
anti-apoptosis, metastases and chemotherapy resistance (11). CuD has been reported to inhibit Akt
in schwannoma and meningioma cells (13). We found that incubation of AGS cells
with CuD (0.5, 1 and 2 µM) for 24 h significantly reduced the
levels of phosphorylated Akt, mTOR (a downstream protein of the Akt
pathway) and ribosomal S6 protein (rS6) (Fig. 7). These data indicated that CuD
targeted the Akt signalling pathway in gastric cancer cells.
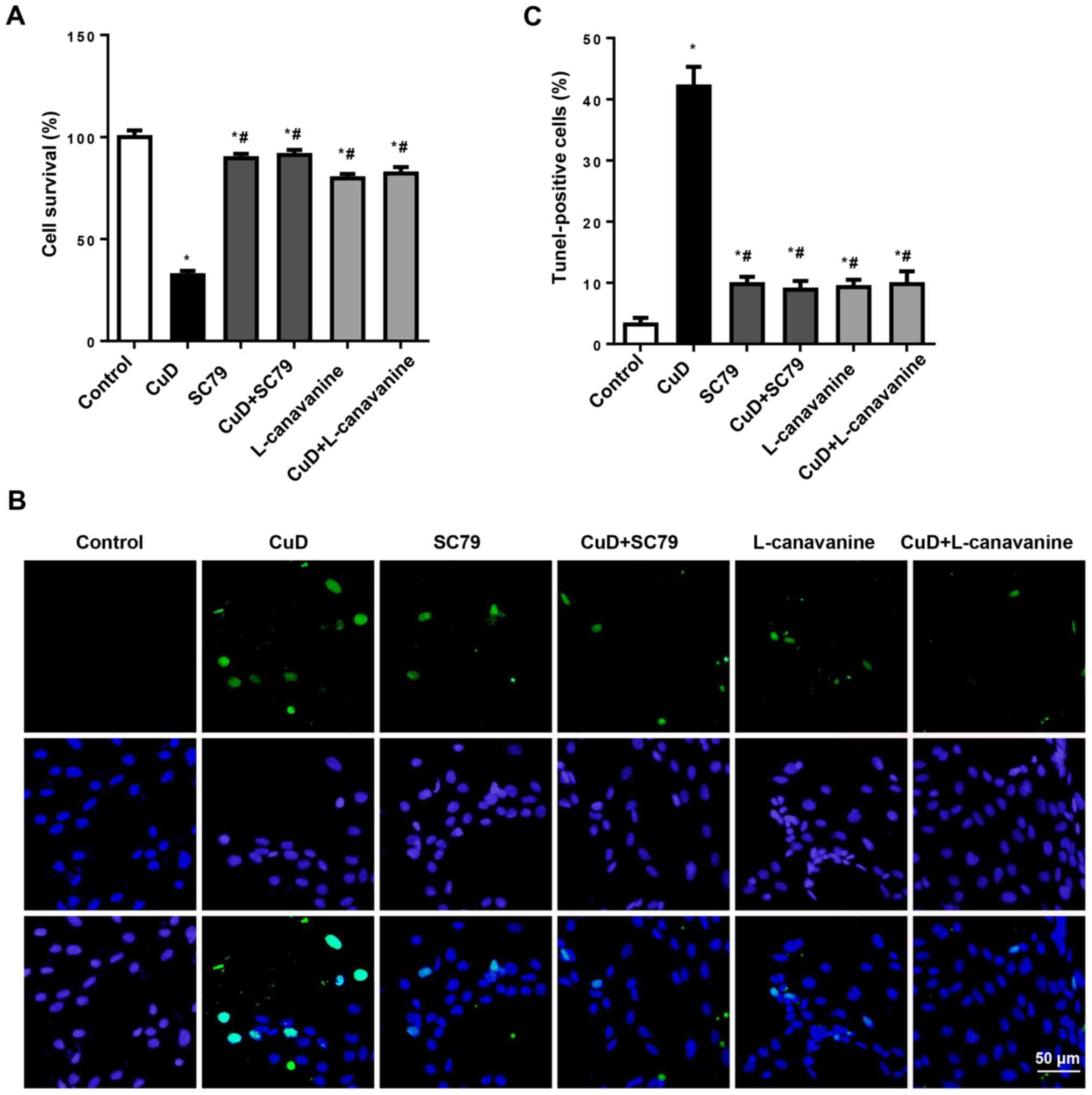
CuD targets Akt and iNOS
To confirm that the effects of CuD were dependent on
Akt and iNOS, AGS cells were treated with SC79 (4 µg/ml) or
L-canavanine (1 mM) and CuD (2 µM) for 24 h. We observed that both
SC79 and L-canavanine reversed the antiproliferative and
pro-apoptotic effects of CuD in AGS cells (Fig. 8). This indicated that CuD targeted
Akt and iNOS.
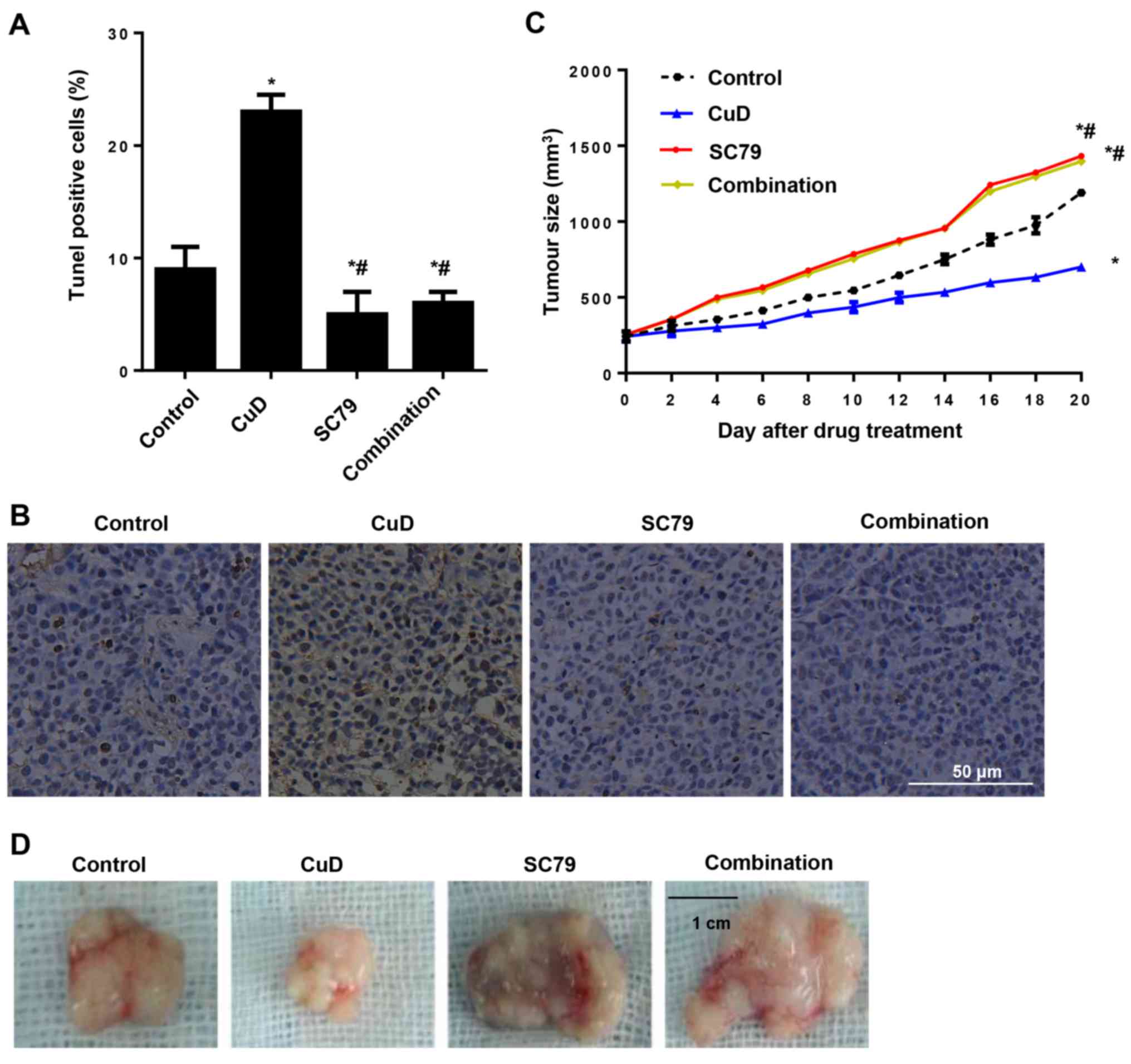
Effects of CuD on gastric cancer in a
xenograft mouse model
The effects of CuD on gastric cancer were confirmed
by an in vivo xenograft-mouse model. After tumours became
palpable, the mice were treated with either CuD and/or SC79 for 20
days. We found that CuD induced tumour cell apoptosis and arrested
tumour growth in vivo. In contrast, SC79 inhibited tumour
cell apoptosis and augmented tumour growth. In addition, SC79
reversed the anti-growth effect of CuD (Fig. 9).
Discussion
Gastric cancer, one of most common malignant tumours
and the second leading cause of cancer-related deaths worldwide, is
the fourth most common cancer worldwide (14). Although the incidence of gastric
cancer varies greatly among countries, in developing countries the
incidence of gastric cancer is >70% (3). The efficacy of chemotherapy in the
treatment of local and metastatic gastric cancer is limited by
chemoresistance, which results in treatment failure. Complementary
and alternative medicine can be used to overcome these limitations.
Plant derived natural products have attracted widespread attention
because they are less toxic (15).
In the present study, we found that cucurbitacin D (CuD) induced
apoptosis and subsequently inhibited the growth of gastric cancer
cells, making it a potential therapeutic agent for the treatment of
gastric cancer.
Oxidative stress, characterized by the imbalance of
ROS and anti-oxidants, has been implicated in the pathogenesis of
several diseases (16). At lower
concentrations, ROS are important signalling molecules involved in
cellular proliferation, migration and apoptosis. At higher
concentrations, most ROS are harmful to cells due to the
accumulation of irreversible damage to proteins, lipids and most
importantly, to DNA, leading to mutations and cell death (17). Our functional experiment revealed
that CuD significantly promoted ROS generation in gastric cancer
cells.
Increased proliferation, diminished differentiation
and reduced apoptosis are the features of tumour cells. Therefore,
the most effective target in cancer therapy is promotion of
apoptosis (18). The pro-apoptotic
property of cucurbitacins makes them a potential antitumour agent
(8,15,19).
In addition, CuD reportedly induced cell apoptosis in human T cell
leukaemia (7), breast carcinoma
(8), human endometrial and ovarian
cancer cells (20) and cervical
cancer (5). Our results indicated
that CuD induced apoptosis in AGS, SNU1 and Hs746T gastric cancer
cells. Mitochondrial membrane permeabilization (MMP) is closely
associated with cancer cell death. Mitochondrial Ca2+
overload drives ROS generation, triggering MMP and release of
pro-apoptotic factors, leading to cell apoptosis and death
(21). Furthermore, high
concentrations of NO derived from iNOS inhibit the expression of
Bcl-2, activate caspase and subsequently promote the release of
cytochrome c, leading to cell death (22). In the present study, CuD raised MMP
levels and increased iNOS expression and NO levels, which triggered
modulation of Bcl-2 proteins and cleavage of caspase-9 and promoted
cytochrome c release from the mitochondria. Various
oncogenic pathways participate in the progression of gastric
cancers such as, nuclear factor-κB, PI3K/Akt and Wnt/β-catenin
(18). Among these, Akt regulates
cell survival, growth, metabolism and chemotherapy resistance
(11). Furthermore, Akt regulates
cell survival by targeting multiple downstream proteins. One
downstream protein is mTOR, which can be activated through mTOR
complex 1 (mTORC1). After activation, mTOR induces activity of
ribosomal protein S6 kinase β-1 (S6K1), which promotes cell
proliferation (11,23). Alteration of the Akt/mTOR pathway is
the second most common cause of cancer in humans.
Immunohistochemistry staining has shown that 74% of gastric cancers
have high Akt expression, but genomic sequencing has demonstrated
that 1–3% of gastric cancers have high Akt expression (11,24).
In the present study, CuD effectively inhibited Akt signalling in
gastric cancer cells. Our in vivo study consistently
demonstrated that CuD exerted anti-growth effects in the in
vivo xenograft tumour model, however, those anti-growth effects
could be reversed by an Akt activator.
In the present study, the caspase-9 activity was
assessed by the ratio of cleaved caspase-9 to GAPDH, not the ratio
of cleaved caspase-9 to total caspase-9. This may cause some
inaccuracy. Secondly, whether CuD affected equally Akt and iNOS
signalling was not clear. In addition, whether Akt and iNOS
signalling are the only target of CuD anti-gastric cancer effects
remain unknown. Future studies including a rescue assay to
demonstrate that CuD induces apoptosis via inhibition of Akt and
activation of the iNOS pathway are needed.
In conclusion, CuD inhibited gastric cancer in
vitro and in vivo by inducing iNOS/NO signalling and
suppressing the Akt pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZYZ and ZLF conceived and designed the study. ZYZ,
ZLF and WCF performed the experiments. ZLF and ZYZ wrote the
manuscript. WCF reviewed and edited the manuscript. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
All animal experiments were performed according to
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (NIH Publications no. 8023, revised 1978). All
animal experiments were approved by the Committee on the Use of
Live Animals of The First Affiliated Hospital of Zhengzhou
University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Takahashi T, Saikawa Y and Kitagawa Y:
Gastric cancer: Current status of diagnosis and treatment. Cancers.
5:48–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patel TN, Roy S and Ravi R: Gastric cancer
and related epigenetic alterations. Ecancermedicalscience.
11:7142017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gastric Cancer Treatment
(PDQ®): Health Professional VersionPDQ Cancer
Information Summaries. Bethesda, MD: 2002
|
|
4
|
Hu B, El Hajj N, Sittler S, Lammert N,
Barnes R and Meloni-Ehrig A: Gastric cancer: Classification,
histology and application of molecular pathology. J Gastrointest
Oncol. 3:251–261. 2012.PubMed/NCBI
|
|
5
|
Sikander M, Hafeez BB, Malik S, Alsayari
A, Halaweish FT, Yallapu MM, Chauhan SC and Jaggi M: Cucurbitacin D
exhibits potent anti-cancer activity in cervical cancer. Sci Rep.
6:365942016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Sun Y, Wu Y and Zhang J:
Cucurbitacin E inhibits osteosarcoma cells proliferation and
invasion through attenuation of PI3K/AKT/mTOR signaling. Biosci
Rep. Sep 21–2016.(Epub ahead of print). View Article : Google Scholar
|
|
7
|
Nakanishi T, Song Y, He C, Wang D, Morita
K, Tsukada J, Kanazawa T and Yoshida Y: Autophagy is associated
with cucurbitacin D-induced apoptosis in human T cell leukemia
cells. Med Oncol. 33:302016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ku JM, Kim SR, Hong SH, Choi HS, Seo HS,
Shin YC and Ko SG: Cucurbitacin D induces cell cycle arrest and
apoptosis by inhibiting STAT3 and NF-κB signaling in
doxorubicin-resistant human breast carcinoma (MCF7/ADR) cells. Mol
Cell Biochem. 409:33–43. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hall JA, Seedarala S, Rice N, Kopel L,
Halaweish F and Blagg BS: Cucurbitacin D is a disruptor of the
HSP90 chaperone machinery. J Nat Prod. 78:873–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hudler P: Challenges of deciphering
gastric cancer heterogeneity. World J Gastroenterol.
21:10510–10527. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tran P, Nguyen C and Klempner SJ:
Targeting the Phosphatidylinositol-3-kinase pathway in gastric
cancer: Can omics improve outcomes? Int Neurourol J. 20 Suppl
2:S131–S140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fang WL, Huang KH, Lan YT, Lin CH, Chang
SC, Chen MH, Chao Y, Lin WC, Lo SS, Li AF, et al: Mutations in
PI3K/AKT pathway genes and amplifications of PIK3CA are associated
with patterns of recurrence in gastric cancers. Oncotarget.
7:6201–6220. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Spear SA, Burns SS, Oblinger JL, Ren Y,
Pan L, Kinghorn AD, Welling DB and Chang LS: Natural compounds as
potential treatments of NF2-deficient schwannoma and meningioma:
Cucurbitacin D and goyazensolide. Otol Neurotol. 34:1519–1527.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cheng XJ, Lin JC and Tu SP: Etiology and
prevention of gastric cancer. Gastrointest Tumors. 3:25–36. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lui VW, Yau DM, Wong EY, Ng YK, Lau CP, Ho
Y, Chan JP, Hong B, Ho K, Cheung CS, et al: Cucurbitacin I elicits
anoikis sensitization, inhibits cellular invasion and in vivo tumor
formation ability of nasopharyngeal carcinoma cells.
Carcinogenesis. 30:2085–2094. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marengo B, Nitti M, Furfaro AL, Colla R,
Ciucis CD, Marinari UM, Pronzato MA, Traverso N and Domenicotti C:
Redox homeostasis and cellular antioxidant systems: Crucial players
in cancer growth and therapy. Oxid Med Cell Longev.
2016:62356412016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morry J, Ngamcherdtrakul W and Yantasee W:
Oxidative stress in cancer and fibrosis: Opportunity for
therapeutic intervention with antioxidant compounds, enzymes, and
nanoparticles. Redox Biol. 11:240–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xu W, Yang Z and Lu N: Molecular targeted
therapy for the treatment of gastric cancer. J Exp Clin Cancer Res.
35:12016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma G, Luo W, Lu J, Ma DL, Leung CH, Wang Y
and Chen X: Cucurbitacin E induces caspase-dependent apoptosis and
protective autophagy mediated by ROS in lung cancer cells. Chem
Biol Interact. 253:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ishii T, Kira N, Yoshida T and Narahara H:
Cucurbitacin D induces growth inhibition, cell cycle arrest, and
apoptosis in human endometrial and ovarian cancer cells. Tumour
Biol. 34:285–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen LD, Liu ZH, Zhang LF, Yao JN and Wang
CF: Sanggenon C induces apoptosis of colon cancer cells via
inhibition of No production, iNOS expression and ROS activation of
the mitochondrial pathway. Oncol Rep. 38:2123–2131. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Vannini F, Kashfi K and Nath N: The dual
role of iNOS in cancer. Redox Biol. 6:334–343. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sasaki T and Kuniyasu H: Significance of
AKT in gastric cancer (Review). Int J Oncol. 45:2187–2192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Almhanna K, Strosberg J and Malafa M:
Targeting AKT protein kinase in gastric cancer. Anticancer Res.
31:4387–4392. 2011.PubMed/NCBI
|