Introduction
As one of the most prevalent malignancies worldwide,
lung cancer is the leading cause of cancer-associated mortality
with a 5-year survival rate of only 15% (1,2). Among
all lung cancer cases, non-small cell lung cancer (NSCLC) accounts
for the most common diagnosed type. Although great progress has
been made in NSCLC treatment, cisplatin (DDP), a first-line
chemotherapy drug, remains the mainstay of clinical therapy against
NSCLC. Unfortunately, during sequential treatment with DDP, its
efficacy is often limited due to the development of resistance,
which is a major contributor to the relapse and prognosis of NSCLC
(3–5). Despite the great efforts in the
elucidation of the causes for tumor resistance to DDP, at present,
the underlying mechanisms are not clearly understood.
Over the last few decades, due to its importance in
a variety of biological functions ranging from physiological and
pathophysiological processes, the gasotransmitter H2S
has attracted much interest (6–8).
Compared with its key role of acting as a vasodilator,
neuromodulator and inflammatory signaling mediator, the field of
H2S and cancer is a new and expanding research area.
Although emerging findings have demonstrated that H2S
dysregulation has a significant effect on cell proliferation/cell
death, cellular bioenergetic production and cellular redox
homeostasis, the role of H2S in cancer development and
progression is controversial and paradoxical (9). While many reports show that inhibition
of H2S biosynthesis exerts anticancer effects in
vitro and in vivo, other studies show that
H2S donors of various types exert anticancer actions
(10). These inconsistent findings
in the field of H2S research indicate that, in different
types of human cancers, the underlying mechanisms regulating the
delicate balance between the pro-cancer and anticancer effects
induced by H2S require further investigation.
In mammalian cells, endogenous H2S is
derived primarily from the metabolism of L-cysteine and
homocysteine by the catalysis of cystathionine γ-lyase (CSE) and
cystathionine β-synthase (CBS). CSE is found predominantly in the
cardiovascular system and muscle tissues. In comparison, CBS
activity is higher than CSE in the brain, nervous system and liver
(11,12). In close relation to H2S
generation, it is not surprising that the expression/activity
changes in endogenous H2S-producing enzymes have been
found in many cancers, including colon, liver, ovarian, breast,
gastric and prostate cancers (13,14).
Moreover, recent data have demonstrated that manipulation of
H2S-producing enzymes or administration of
H2S donors can sensitize some types of cancer cells to
concomitant chemotherapy (15).
These findings imply that there might be a conceivable connection
between H2S and tumor chemoresistance. Given that some
laboratory and clinical studies have shown that both CBS and CSE
have been simultaneously identified in human lungs (16,17),
it is important to assess the effect of H2S on DDP
resistance in NSCLC.
The present study aimed to investigate the role of
H2S in the chemosensitivity of NSCLC cells to cisplatin
in vitro. In cisplatin-resistant A549/DDP cells, decreased
H2S production and downregulation of CBS were observed
compared with these parameters in A549 cells. The treatment
A549/DDP cells with H2S donor (NaHS) did not only reduce
cisplatin resistance through regulation of cell proliferation and
apoptosis, but also significantly inhibited the cell migration and
invasion capacities. Furthermore, various signaling proteins
associated with these key molecular events, including total p53
(t-p53), phosphorylated p53 (p-p53), p21, Bax, Bcl-xL, matrix
metalloproteinase-2 (MMP-2) and MMP-9, were detected to explore the
potential molecular mechanisms.
Materials and methods
Chemicals and reagents
Sodium hydrogen sulfide (NaHS), a donor of
H2S, was obtained from Sigma Chemical Co. (Merck KGaA,
Darmstadt, Germany), stored at 4°C and protected from sunlight.
Cisplatin was purchased from Qilu Pharmacy Ltd. Co. (Jinan,
Shandong, China). Cy-NO2 fluorescence probe for
H2S detection was kindly donated by Professor Fabiao Yu
of the Key Laboratory of Coastal Zone Environmental Processes,
Yantai Institute of Coastal Zone Research, Chinese Academy of
Sciences. The rabbit anti-human antibodies for CBS (cat. no.
sc-133208), CSE (cat. no. sc-119499), t-p53 (cat. no. FL-393),
p-p53 (cat. no. sc-135772), p27 (cat. no. sc-1641), caspase-3 (cat.
no. sc-56053), Bax (cat. no. PA5-11378), Bcl-xL (cat. no.
sc-136132) and β-actin (cat. no. sc-47778) were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). MMP-2 (cat. no.
MAB3308) and MMP-9 (cat. no. AB19016) antibodies were obtained from
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Horseradish
peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody
(cat. no. KC-RB-035) was obtained from KangChen Bio-tech, Inc.
(Shanghai, China).
Cell culture and treatment
The two NSCLC cell lines, A549 and
cisplatin-resistant A549/DDP cells, were purchased from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). The cell
lines were cultured in RPMI-1640 medium (Gibco; Thermo Fisher
Scientific, Inc.) containing 10% fetal bovine serum (FBS;
Invitrogen Life Technologies; Thermo Fisher Scientific, Inc.), 100
U/ml penicillin and 100 µg/ml streptomycin. A humidified incubator
was maintained at 37°C with 5% CO2. NaHS and cisplatin
were dissolved in RPMI-1640 medium at the indicated concentrations
and were changed every 12 h throughout the experiments.
Flow cytometric analysis of
intracellular H2S with a fluorescence probe
The intracellular H2S evaluated using a
Cy-NO2 fluorescence probe was performed as previously
described (18). Briefly, to
compare the intracellular H2S concentrations in the A549
and A549/DDPcells, the cells were cultured in RPMI-1640 medium
containing 10% FBS for 24 h at 37°C. The medium was then replaced
with RPMI-1640 medium containing 10% FBS and the Cy-NO2
fluorescence probe (10 µmol/l), in which the cells were incubated
for 1 h at 37°C. The cells were then harvested and washed twice
with ice-cold phosphate-buffered saline (PBS). The fluorescence
signal intensity of intracellular H2S was determined
using the FACS FC500 flow cytometer with excitation and emission
wavelengths of 755 and 789 nm, respectively. To compare the
intracellular H2S concentrations under different NaHS
treatments in the A549/DDP cells, the cells treated with the
indicated doses of NaHS (0, 200, 400, 600, 800 and 1,000 µmol/l)
were cultured in RPMI-1640 medium containing 10% FBS for 24 h at
37°C followed by the aforementioned detection procedures.
Detection of cell viability by MTT
assay
To measure the effect of NaHS on the cell viability
of A549/DDP cells, the cells were treated with different
concentrations of NaHS according to previous publications (19,20).
Briefly, the A549/DDP cells were seeded into 96-well flat plates
(1.5×104 cells/well) and then treated with or without
various concentrations of NaHS (0, 200, 400, 800 or 1,000 µmol/l)
for 24 h at 37°C. The group without NaHS treatment was used as a
control. Following incubation, 5 mg/ml MTT (10 µl/well) was added
to the media and the cells were further incubated in an atmosphere
of 5% CO2 at 37°C for 4 h. After removal of the
supernatant, 100 µl dimethyl sulfoxide was added to determine the
OD value at 570 nm using a microtiter plate reader (ELX800; BioTek
Instruments, Inc., Winooski, VT, USA). To calculate the percentage
of cell viability, the means of the optical density (OD) in the
indicated groups in triplicate were used. Cell viability (%) = (OD
treatment group/OD control group) × 100%.
Measurement of the chemosensitivity to
cisplatin of the A549/DPP cells following NaHS treatment
The half maximal inhibitory concentration
(IC50) of the A549/DDP cells was determined by the MTT
assay. Briefly, A549/DDP cells with or without NaHS treatment were
incubated with the indicated doses of cisplatin (0, 2, 4, 6, 8 and
10 µg/ml) for 24 h followed by the aforementioned detection
procedures of the MTT assay. The IC50 value was
calculated by nonlinear regression analysis with GraphPad Prism 5.0
(GraphPad Software Inc., San Diego, CA, USA), using the
dose-response with variable slope function. Every step was
conducted at least three times.
Analysis of the cell cycle
distribution and apoptosis using flow cytometry
For cell cycle analysis, A549/DDP cells were
harvested and washed with ice-cold PBS, and then fixed with 70%
ethanol (v/v) overnight at −20°C. Fixed cells were washed with
ice-cold PBS twice and then resuspended in PBS containing propidium
iodide (PI) (50 µg/ml)/RNase A (50 µg/ml) for 10 min. For cell
apoptosis analysis, A549/DDP cells were double-stained with Annexin
V-FITC (5 µg/ml) and PI (5 µg/ml). Finally, both cell cycle and
apoptosis were analyzed using a flow cytometer (FACS FC500; Beckman
Coulter, Inc., Brea, CA, USA).
Transwell migration and invasion
assays
Cell motility was assessed using Transwell chambers
(8.0-µm pore size; 6.5-mm diameter insert; Corning Inc., Corning,
NY, USA) uncoated (migration assay) or coated (invasion assay) with
Matrigel following the manufacturer's instructions. Approximately
2×104 A549/DDP cells were seeded into the upper
Transwell chambers in 500 µl serum-free medium with or without NaHS
(800 µmol/l). The bottom chamber was filled with 10% FBS RPMI-1640
medium which was used as a chemoattractant. After being cultured
for 24 or 48 h, cells on the upper side of the inserts were removed
and then fixed in 4% paraformaldehyde and finally stained with 0.1%
crystal violet solution. For quantification, the migratory and
invasive cells were counted in 10randomly selected fields under a
light microscope with ×200 magnification. Triplicate experiments
were performed with each group, and the means and standard
deviations were calculated.
Western blot analyses
A549/DDP cells from a 25 cm2 flask
following the various treatments were washed twice in ice-cold PBS
and lysed in 100 µl RIPA lysis buffer on ice. Cell lysates were
then centrifuged at 14,000 × g for 20 min at 4°C. The supernatant
was recovered and the protein concentration was detected by
Coomassie Blue Fast staining solution (Beyotime, Haimen, China).
Proteins (20–40 µg) were subjected to sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then
transferred to polyvinylidene difluoride (PVDF) membranes. After
incubation in a blocking buffer (containing 10% non-fat milk and
0.1% Tween-20) for 2 h, the membranes were immunoblotted with
specific antibodies overnight with gentle agitation at 4°C. The
dilutions for the primary antibodies used are as follows: CBS
(1:500), CSE (1:500), t-p53 (1:1,000), p-p53 (1:1,000), p21
(1:1,000), caspase-3 (1:500), Bax (1:500), Bcl-xL (1:500), MMP-2
(1:1,000) and MMP-9 (1:1,000). β-actin (1:500) was used as a
control. After being washed for 5 min, the membranes were incubated
with secondary antibodies conjugated to horseradish peroxidase
(1:3,000) for detection. Finally, images were captured using a
FluorChem FC2 gel imaging system (Alpha Innotech, San Leandro, CA,
USA).
Statistical analysis
SPSS 19.0 software (IBM SPSS, Inc., Armonk, NY, USA)
was used for statistical analysis. Data are expressed as the mean ±
SD. The statistical significance of the results between each group
was evaluated using one-way ANOVA or t-test. Differences
were considered significant at P<0.05. All experiments were
repeated at least three times.
Results
Endogenous H2S production
and expression levels of CBS and CSE in A549 and A549/DDP
cells
To determine the role of H2S in NSCLC,
using a Cy-NO2 fluorescence probe, we first test whether
there are differences in H2S production between A549 and
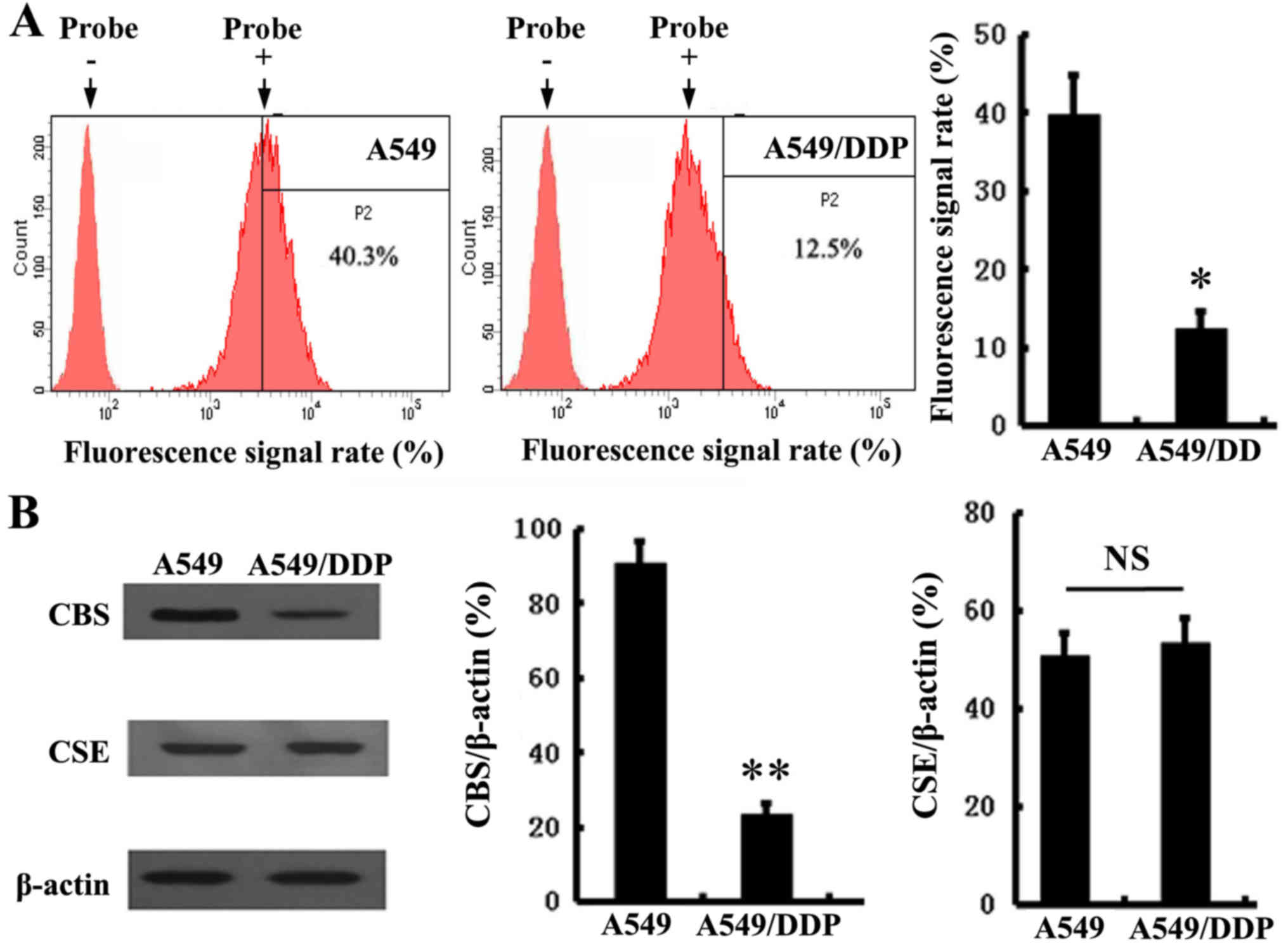
cisplatin-resistant A549/DDP cells. As shown in Fig. 1A, we found that the production of
hydrogen sulfide in the A549/DDP cells was distinctly decreased,
compared with the A549 cells. Then, as the main endogenous
H2S-producing enzymes, the expression levels of CSE and
CBS were detected in both cell lines. The results showed that the
expression of CBS (Fig. 1B) was
significantly downregulated in the A549/DDP cells. In contrast,
there was no difference in CSE expression between the A549 and
A549/DDP cells.
NaHS inhibits cell viability of the
A549/DDP cells
To test the effect of exogenous H2S on
the cell viability of A549/DDP cells, we performed MTT assays using
various doses (0, 200, 400, 800 and 1,000 µmol/l) of NaHS (a donor
of H2S) for 24 h. The MTT assay showed that NaHS
significantly inhibited the viability of the A549/DDP cells in a
dose-dependent manner (Fig. 2A).
Since the inhibitory effect of H2S on the viability of
the A549/DDP cells was most significant following treatment with
800 µmol/l NaHS, this dose of NaHS was used in all subsequent
experiments with different treatment. Moreover, the decrease in
cell viability was accomplished by increased intracellular
H2S after NaHS treatment of A549/DDP cells, which was
indicated by enhanced fluorescence signals at 789 nm emission
(Fig. 2B).
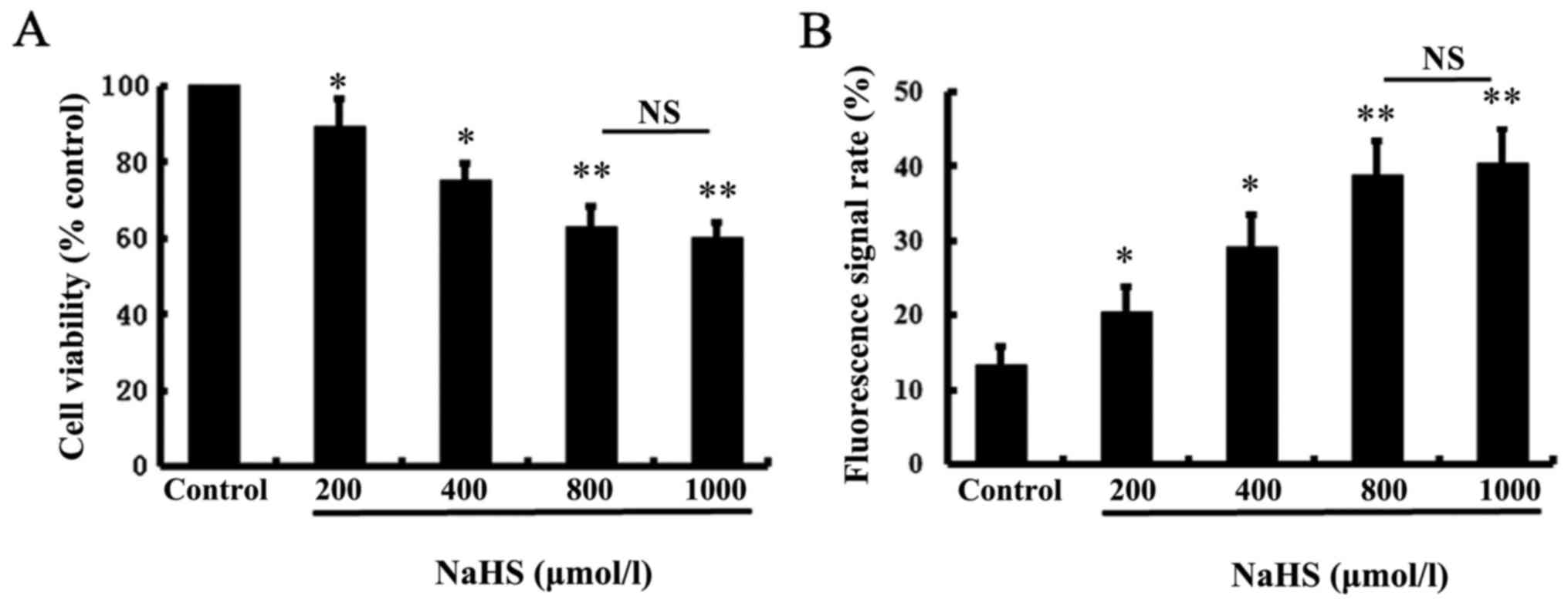 | Figure 2.Effect of NaHS on A549/DDP cell
viability. Cell viability was tested using the MTT assay. (A) The
cell viability was significantly reduced following treatment of
NaHS at the indicated concentrations (200, 400, 800, or 1,000
µmol/l) for 24 h. (B) Intracellular H2S was
significantly increased following treatment of NaHS at the
indicated concentrations (200, 400, 800, or 1,000 µmol/l) for 24 h.
Intracellular H2S was measured by flow cytometry, with
excitation/emission wavelengths of 755/789 nm. The group without
NaHS treatment was used as a control. Data are expressed as the
mean ± SD. NS, not significant; *P<0.05, **P<0.01 vs. the
control. |
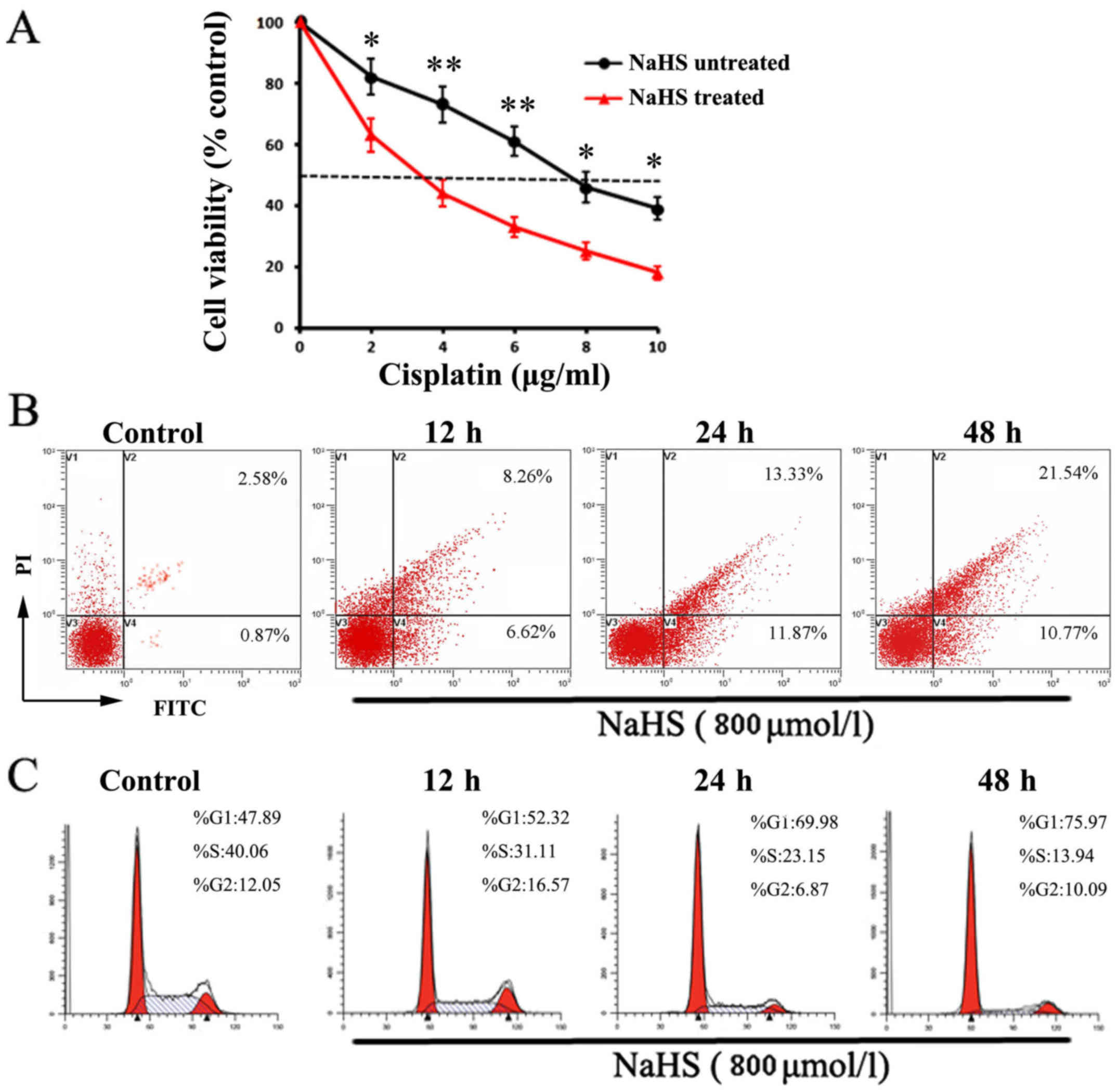
Effect of NaHS treatment on cisplatin
sensitization of A549/DDP cells
In order to determine whether NaHS treatment can
influence the cisplatin sensitization of A549/DDP cells, the cells
were incubated with various concentrations of cisplatin with or
without NaHS (800 µmol/l) for 24 h. It was demonstrated that,
without NaHS treatment, the IC50 of A549/DDP cells for
cisplatin was 7.82±0.58 µg/ml. In contrast, when A549/DDP cells
treated with NaHS (800 µmol/l) and cisplatin in combination, the
IC50 decreased to 3.63±0.27 µg/ml (Fig. 3A). These observations indicate that
NaHS administration sensitized the A549/DDP cells to cisplatin.
Pro-apoptotic and antiproliferative
effect of NaHS onA549/DDP cells
To further explore the reasons for the effect of
NaHS on the viability of A549/DDP cells, flow cytometric analyses
were performed to investigate the differences in cell apoptosis and
proliferation with or without NaHS treatment (800 µmol/l) at 12, 24
and 48 h, respectively. As shown in Fig. 3B, compared with the control group,
the levels of cell apoptosis in the NaHS-treated groups were
gradually increased. After 24 and 48 h of incubation, the apoptosis
rates in the NaHS-treated cells were >25.2 and 32.31% with
significant changes, respectively. Similarly, in comparison with
the control group, the percentage of NaHS-treated cells in the G1
phase was increased (69.98 and 75.97%, respectively) and the
percentage in the S stage was decreased (23.15 and 13.94%,
respectively) following 24 and 48 h of intervention, and the
differences were significant (Fig.
3C).
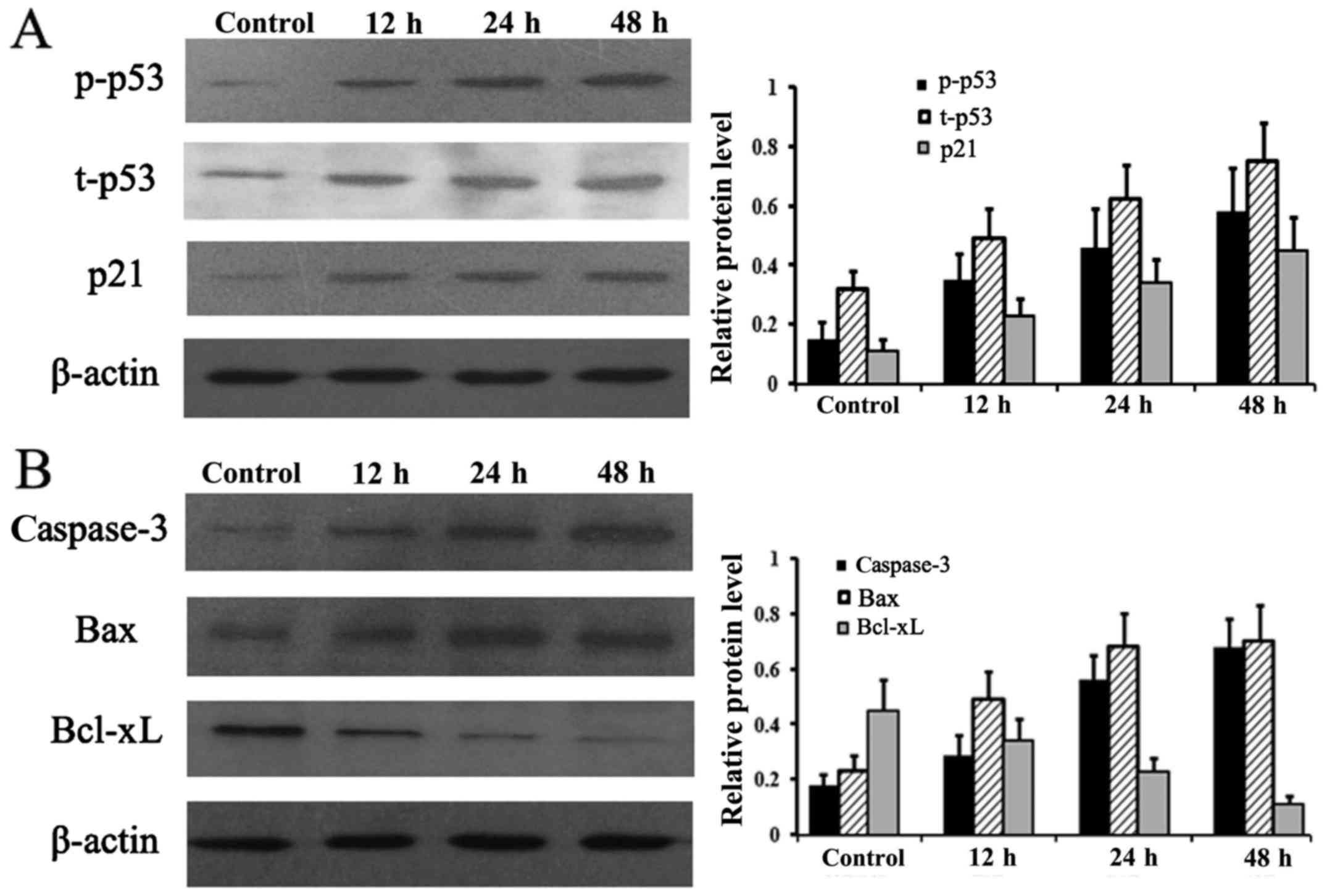
Expression levels of apoptosis- and
proliferation-associated proteins
To analyze the molecular changes during the
NaHS-induced decrease incell viability, protein levels of t-p53,
p-p53, p21, caspase-3, Bax and Bcl-xL were examined at different
times. As shown in Fig. 4A and B,
compared with the untreated control group, the levels of t-p53,
p-p53, p21, caspase-3 and Bax were considerably increased after
NaHS treatment in a time-dependent manner. However, Bcl-xL was
markedly downregulated after NaHS incubation (Fig. 4B).
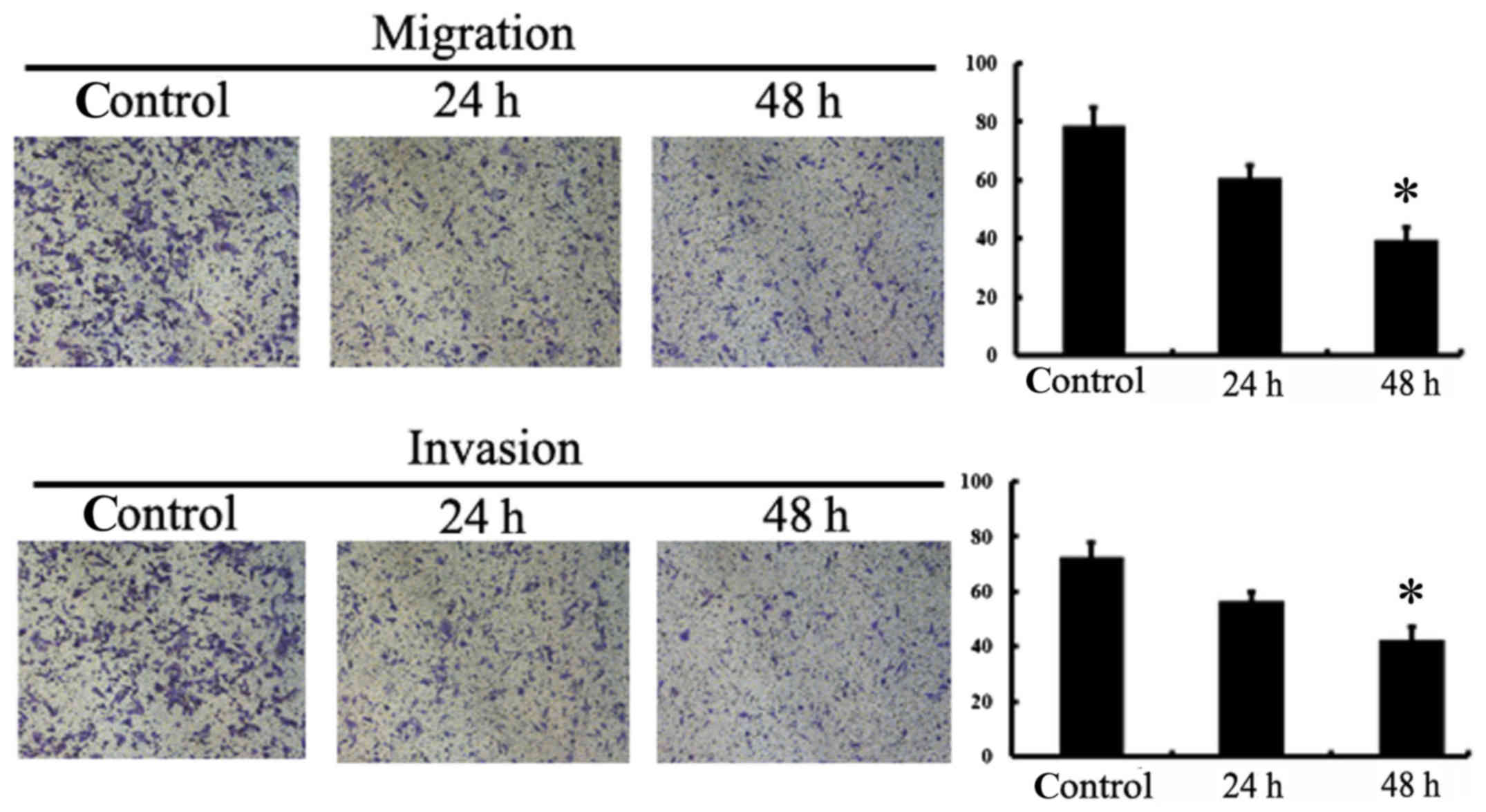
NaHS inhibits the migration and
invasion of A549/DDP cells
To evaluate the contribution of NaHS on cell
migration and invasion, we added NaHS to the upper inserts of
Transwell chambers. The results showed that, at various time points
(24 and 48 h), the numbers of cells that infiltrated the membrane
was significantly reduced after NaHS treatment, indicating the
inhibitory effects of NaHS on the migration and invasion of
A549/DDP cells (Fig. 5). Moreover,
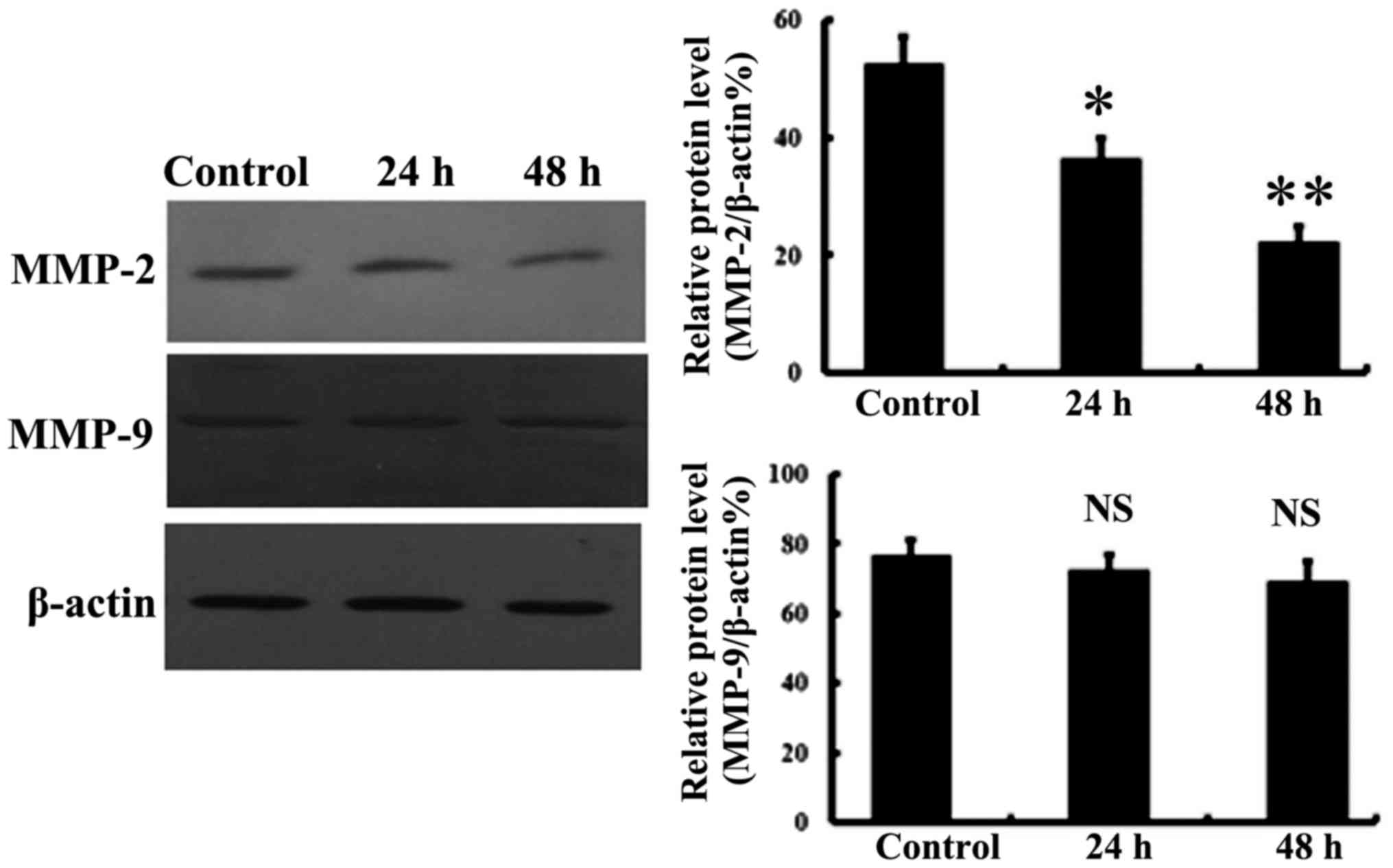
MMP-2 and MMP-9 expression was assessed to further determine the
mechanisms involved in the reduced cell migration and invasion
following NaHS treatment. As shown in Fig. 6, MMP-2 expression was significantly
attenuated following 800 µmol/l NaHS treatment, but there was no
significant effect of NaHS observed at the MMP-9 level.
Discussion
As a great challenge in the treatment of lung
cancer, drug resistance has become increasingly severe. Thus, it is
urgent to elucidate the underlying mechanisms so as to improve the
efficacy of chemotherapy drugs, such as cisplatin (DDP) (21). Research over the past few decades
has identified that the cause of DDP resistance involves multiple
factors, such as drug transport and metabolism, DNA repair, cell
survival and apoptosis (22).
Although numerous studies have focused on investigations to
elucidate the roles of H2S in different types of human
cancers in vitro and in vivo, literature concerning
the effect of H2S on cisplatin resistance in cancer is
limited. Based on the fact that H2S exerts an important
role in most biological processes, it can be anticipated that
H2S may also contribute to the induction of DDP
resistance.
To test our hypothesis, we first detected the
concentration of endogenous H2S with a fluorescent probe
in A549 and A549/DDP cell lines. It was found that the production
of H2S was definitely elevated in A549 cells when
compared with the production in A549/DDP cells. The immunoblotting
assays implied that cystathionine β-synthase (CBS), rather than
cystathionine γ-lyase (CSE), appeared to play a major role in
H2S production due to its significantly increased
expression in A549 cells. Based on these findings, we speculated
that the difference in the H2S pathway between A549 and
A549/DDP cells may contribute to, at least in part, their
phenotypic variance, such as resistance to DDP. To this end, we
first observed the effect of NaHS, an exogenous H2S
donor, at different concentrations on A549/DDP cell viability. MTT
assay revealed that the doses of NaHS from 200 to 1,000 µmol/l
markedly inhibited cell proliferation, leading to a decrease in
cell viability which reached a peak at 800 µmol/l. These
observations were confirmed by the observation of apoptosis
induction and cell cycle arrest. Taking into account that normal
cellular homeostasis is maintained through a balance between the
processes of cell proliferation and cell death (e.g. apoptosis), it
is reasonable to believe that changes in cell proliferation and/or
apoptosis after the treatment of H2S may influence the
proportion of surviving cells in the A549/DDP cell population,
namely, cell viability. Moreover, our results demonstrated that the
significant inhibitory effect was likely related to the increased
cisplatin efficacy of A549/DDP cells due to the finding that 800
µmol/l NaHS treatment shifted the IC50 of cisplatin from
7.82 to 3.63 µg/ml. In addition, the strong influence of NaHS on
the inhibition of migration and invasion abilities of A549/DDP
cells was also observed in the present study.
Consistent with our data, previous studies have
shown the anticancer activity of H2S in a range of
cancers through the role of this gas in triggering cell death
and/or inhibiting cell proliferation (23–25).
In addition, several investigations have provided evidence that
H2S is involved in the efficacy of cancer radiotherapy
and chemotherapy. In cultured MDA-MB-231 cells, De Preter et
al found that tumor cells after NaHS administration were more
sensitive to irradiation compared with those that received
irradiation alone, implying that NaHS may act as a potential
radiosensitizer in some solid cancers (26). In contrast, Bhattacharyya and
colleagues found high expression of CBS in ovarian tumor samples
and inhibition of CBS sensitized several ovarian cancer cell lines
to cisplatin (15). Similarly, in
lung adenocarcinoma, a recent study reported that a decrease in
H2S biosynthesis through inhibition of
H2S-producing enzymes can sensitize certain lung cancer
cell lines to chemotherapeutic agents in vitro and in
vivo (16). These apparent
discrepancies might be attributed to several factors which should
be a concern in further investigations. Above all, cellular
H2S can be metabolized through two pathways, known as
enzymatic (endogenous) and nonenzymatic (exogenous) processes.
Since an increasing amount of evidence suggests that exogenously
administered and/or endogenously produced H2S could
exhibit two obviously opposite functions on the growth of cancer
cells, there may be a delicate balance between the pro-cancer and
anticancer effects induced by H2S (20,27).
Therefore, it can be anticipated that the net effects of
H2S on cell characteristics are the result of the
balance of both pathways. The bell-shaped pharmacology of
H2S, whereby lower (endogenous) H2S
production tends to promote, while higher (generated from
exogenously added H2S donors) tends to inhibit cancer
cell proliferation, should also be kept in mind (28). These observations imply that the
paradoxical actions of H2S in cancer can also result
from the different production of this gasotransmitter. Accordingly,
the source and dosage of H2S should be determined for
future research to make the data more consistent and explicit.
It has been well recognized that the anticancer
activity of cisplatin is largely depended on its ability to
increase DNA damage and cell apoptosis, both of which can be
controlled by p53 (29–31). Therefore, to provide further insight
into the potential mechanisms involved in the effect of
H2S on the biological behaviors of A549/DDP cells, we
then focused our investigation on p53 pathway members associated
with these key molecular events, such as cell cycle, apoptosis and
migration. Research demonstrated that p53 is not only a key player
in carcinogenesis, but is also associated with resistance to
established cytotoxic anticancer drugs, including cisplatin
(32,33). In the present study, the enhancement
of total p53 and p-p53 in A549/DDP cells after NaHS treatment was
consistent with previous reports that restoring p53 apoptotic
function in advanced cancer through modulation its expression and
activation is often essential for sensitivity toward
chemotherapeutic drugs (34,35).
Analyses of several downstream effectors of p53 offer more detailed
clues to the antitumor potential of NaHS. On the one hand,
upregulation of p21 after NaHS exposure indicated that fewer
A549/DDP cells can proceed through the G1 checkpoint to S phase
because of cell cycle arrest controlled by p21. On the other hand,
cell death was promoted by NaHS treatment through changes in
apoptosis-related proteins, such as anincreaseincaspase-3 and an
increase in the Bax/Bcl-xL ratio. Metastasis is another fatal
characteristic of malignant tumors, especially for advanced or late
stage cancer. The association of MMPs with cancer metastasis has
raised considerable interest due to their ability to cleave the
extracellular matrix (ECM) allowing cancer cells to invade adjacent
tissues or spread to distant organs. Among the known MMPs, MMP-2
and MMP-9 have been thought to be key enzymes due to their capacity
to degrade gelatin as well as type IV collagen, the central
component of the basement membrane. Recent in vitro studies
using diverse cancer cell lines showed that the inhibitory effects
of NaHS or H2S on cell invasion maybe through the
downregulation of MMP-2 (36). In
line with these previous findings, A549/DDP cells treated with 800
µmol/l NaHS exhibited diminished MMP-2 expression, implying the
involvement of MMPs in the participation of invasion and migration
of A549/DDP cells.
It should be noted that the present study has some
limitations which should be considered for future assessments.
Firstly, it is well-known that the development of drug resistance
in cancer is complicated and heterogeneous. The present study only
indicated that there may be a relation between H2S
production and the cisplatin-resistant phenotype in A549/DDP cells.
To determine whether there is a causal association between
H2S and the development of cisplatin resistance in
A549/DDP cells, further intensive studies should be performed
(37). Secondly, although the study
found that p53 and its downstream signaling members are involved in
the effect of NaHS on A549/DDP cells, the detailed mechanism(s) of
p53 regulation were not demonstrated. The crosstalk network that
exists between p38 MAPK and p53 in NaHS-induced apoptosis of glioma
cells may provide a valuable clue to elucidate the reasons for p53
activation under NaHS challenge (19). Finally, further investigations are
warranted in order to extend these findings to animal in
vivo studies and clinical sample detections.
In summary, this study demonstrated that NaHS
exposure can increase the efficacy of cisplatin in A549/DDP cells.
The anticancer effect of NaHS treatment was evidenced by inhibition
of proliferation, induction of apoptosis and suppression of
invasion. These phenotypic changes in cell functions may be
mediated by the activation of p53, which in turn may alter the
expression of downstream targets, such as p21, caspase-3, Bax,
Bcl-xL and MMP-2. These findings imply that NaHS administration may
be a potential therapeutic strategy for the treatment of NSCLC with
cisplatin resistance.
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural
Science Foundation of China (no. 81502536), the Natural Science
Foundation of Shandong Province (nos. ZR2014HL056 and ZR2015HL060)
and the Program of Shandong Medical and Health Science and
Technology Development (no. 2014WS0186).
Availability of data and materials
Not applicable.
Authors' contributions
YM and FJ conceived and designed the study. YM, ZHY,
XMD, JQG, JXH and YY performed the experiments. ZHY and FJ wrote
the paper. YM, ZHY, YY and FJ reviewed and edited the manuscript.
All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma LY, Xie XW, Ma L, Pang JL, Xiong XM,
Zheng HD, Shen XL, Wen ZG and Wang HY: Downregulated long
non-coding RNA TRPM2-AS inhibits cisplatin resistance of non-small
cell lung cancer cells via activation of p53-p66shc pathway. Eur
Rev Med Pharmacol Sci. 21:2626–2634. 2017.PubMed/NCBI
|
|
2
|
Gao J, Meng Q, Zhao Y, Chen X and Cai L:
EHD1 confers resistance to cisplatin in non-small cell lung cancer
by regulating intracellular cisplatin concentrations. BMC Cancer.
16:4702016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fennell DA, Summers Y, Cadranel J, Benepal
T, Christoph DC, Lal R, Das M, Maxwell F, Visseren-Grul C and Ferry
D: Cisplatin in the modern era: The backbone of first-line
chemotherapy for non-small cell lung cancer. Cancer Treat Rev.
44:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sarin N, Engel F, Kalayda GV, Mannewitz M,
Cinatl J Jr, Rothweiler F, Michaelis M, Saafan H, Ritter CA, Jaehde
U and Frötschl R: Cisplatin resistance in non-small cell lung
cancer cells is associated with an abrogation of cisplatin-induced
G2/M cell cycle arrest. PLoS One. 12:e01810812017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rose MC, Kostyanovskaya E and Huang RS:
Pharmacogenomics of cisplatin sensitivity in non-small cell lung
cancer. Genomics Proteomics Bioinformatics. 12:198–209. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hellmich MR, Coletta C, Chao C and Szabo
C: The therapeutic potential of cystathionine β-synthetase/hydrogen
sulfide inhibition in cancer. Antioxid Redox Signal. 22:424–448.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu D, Si W, Wang M, Lv S, Ji A and Li Y:
Hydrogen sulfide in cancer: Friend or foe? Nitric Oxide. 50:38–45.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wallace JL and Wang R: Hydrogen
sulfide-based therapeutics: Exploiting a unique but ubiquitous
gasotransmitter. Nat Rev Drug Discov. 14:329–345. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rose P, Moore PK and Zhu YZ: H2S
biosynthesis and catabolism: New insights from molecular studies.
Cell Mol Life Sci. 74:1391–1412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Szabo C: Gasotransmitters in cancer: From
pathophysiology to experimental therapy. Nat Rev Drug Discov.
15:185–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao Y, Biggs TD and Xian M: Hydrogen
sulfide (H2S) releasing agents: Chemistry and biological
applications. Chem Commun. 50:11788–11805. 2014. View Article : Google Scholar
|
|
12
|
Guo FF, Yu TC, Hong J and Fang JY:
Emerging roles of hydrogen sulfide in inflammatory and neoplastic
colonic diseases. Front Physiol. 7:1562016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee ZW, Teo XY, Tay EY, Tan CH, Hagen T,
Moore PK and Deng LW: Utilizing hydrogen sulfide as a novel
anti-cancer agent by targeting cancer glycolysis and pH imbalance.
Br J Pharmacol. 171:4322–4336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Szabo C, Coletta C, Chao C, Módis K,
Szczesny B, Papapetropoulos A and Hellmich MR: Tumor-derived
hydrogen sulfide, produced by cystathionine-β-synthase, stimulates
bioenergetics, cell proliferation, and angiogenesis in colon
cancer. Proc Natl Acad Sci USA. 110:12474–12479. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bhattacharyya S, Saha S, Giri K, Lanza IR,
Nair KS, Jennings NB, Rodriguez-Aguayo C, Lopez-Berestein G, Basal
E, Weaver AL, et al: Cystathionine beta-synthase (CBS) contributes
to advanced ovarian cancer progression and drug resistance. PLoS
One. 8:e791672013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Szczesny B, Marcatti M, Zatarain JR,
Druzhyna N, Wiktorowicz JE, Nagy P, Hellmich MR and Szabo C:
Inhibition of hydrogen sulfide biosynthesis sensitizes lung
adenocarcinoma to chemotherapeutic drugs by inhibiting
mitochondrial DNA repair and suppressing cellular bioenergetics.
Sci Rep. 6:361252016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Russo A, Saide A, Cagliani R, Cantile M,
Botti G and Russo G: RpL3 promotes the apoptosis of p53 mutated
lung cancer cells by down-regulating CBS and NF-κB upon 5-FU
treatment. Sci Rep. 6:383692016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang R, Yu FB, Chen LX, Chen H, Wang LJ
and Zhang WW: A highly selective turn-on near-infrared fluorescent
probe for hydrogen sulfide detection and imaging in living cells.
Chem Commun. 48:11757–11759. 2012. View Article : Google Scholar
|
|
19
|
Zhao L, Wang Y, Yan Q, Lv W, Zhang Y and
He S: Exogenous hydrogen sulfide exhibits anti-cancer effects
though p38 MAPK signaling pathway in C6 glioma cells. Biol Chem.
396:1247–1253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH,
Li L, Moore PK and Deng LW: The slow-releasing hydrogen sulfide
donor, GYY4137, exhibits novel anti-cancer effects in vitro and in
vivo. PLoS One. 6:e210772011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen Y, Gao Y, Zhang K, Li C, Pan Y, Chen
J, Wang R and Chen L: MicroRNAs as regulators of cisplatin
resistance in lung cancer. Cell Physiol Biochem. 37:1869–1880.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galluzzi L, Vitale I, Michels J, Brenner
C, Szabadkai G, Harel-Bellan A, Castedo M and Kroemer G: Systems
biology of cisplatin resistance: Past, present and future. Cell
Death Dis. 5:e12572014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chattopadhyay M, Kodela R, Nath N,
Dastagirzada YM, Velázquez-Martínez CA, Boring D and Kashfi K:
Hydrogen sulfide-releasing NSAIDs inhibit the growth of human
cancer cells: A general property and evidence of a tissue
type-independent effect. Biochem Pharmacol. 83:715–722. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu YC, Wang XJ, Yu L, Chan FK, Cheng AS,
Yu J, Sung JJ, Wu WK and Cho CH: Hydrogen sulfide lowers
proliferation and induces protective autophagy in colon epithelial
cells. PLoS One. 7:e375722012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lv M, Li Y, Ji MH, Zhuang M and Tang JH:
Inhibition of invasion and epithelial-mesenchymal transition of
human breast cancer cells by hydrogen sulfide through decreased
phospho-p38 expression. Mol Med Rep. 10:341–346. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Preter G, Deriemaeker C, Danhier P,
Brisson L, Pham Cao TT, Grégoire V, Jordan BF, Sonveaux P and
Gallez B: A fast hydrogen sulfide-releasing donor increases the
tumor response to radiotherapy. Mol Cancer Ther. 15:154–161. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu M, Wu L, Montaut S and Yang G:
Hydrogen sulfide signaling axis as a target for prostate cancer
therapeutics. Prostate Cancer. 2016:81085492016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu D, Li M, Tian W, Wang S, Cui L, Li H,
Wang H, Ji A and Li Y: Hydrogen sulfide acts as a double-edged
sword in human hepatocellular carcinoma cells through
EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Sci Rep.
7:51342017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hientz K, Mohr A, Bhakta-Guha D and
Efferth T: The role of p53 in cancer drug resistance and targeted
chemotherapy. Oncotarget. 8:8921–8946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pflaum J, Schlosser S and Müller M: p53
family and cellular stress responses in cancer. Front Oncol.
4:2852014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lane D and Levine A: p53 research: The
past thirty years and the next thirty years. Cold Spring Harb
Perspect Bio. 2:a0008932010.
|
|
32
|
Davaadelger B, Duan L, Perez RE, Gitelis S
and Maki CG: Crosstalk between the IGF-1R/AKT/mTORC1 pathway and
the tumor suppressors p53 and p27 determines cisplatin sensitivity
and limits the effectiveness of an IGF-1R pathway inhibitor.
Oncotarget. 7:27511–27526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Leung EL, Fraser M, Fiscus RR and Tsang
BK: Cisplatin alters nitric oxide synthase levels in human ovarian
cancer cells: Involvement in p53 regulation and cisplatin
resistance. Br J Cancer. 98:1803–1809. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Häcker S, Karl S, Mader I, Cristofanon S,
Schweitzer T, Krauss J, Rutkowski S, Debatin KM and Fulda S:
Histone deacetylase inhibitors prime medulloblastoma cells for
chemotherapy-induced apoptosis by enhancing p53-dependent Bax
activation. Oncogene. 30:2275–2281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gregorc V, Ludovini V, Pistola L, Darwish
S, Floriani I, Bellezza G, Sidoni A, Cavaliere A, Scheibel M, De
Angelis V, et al: Relevance of p53, bcl-2 and Rb expression on
resistance to cisplatin-based chemotherapy in advanced non-small
cell lung cancer. Lung Cancer. 39:41–48. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang L, Qi Q, Yang J, Sun D, Li C, Xue Y,
Jiang Q, Tian Y, Xu C and Wang R: An anticancer role of hydrogen
sulfide in human gastric cancer cells. Oxid Med Cell Longev.
2015:6364102015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo W, Cheng ZY and Zhu YZ: Hydrogen
sulfide and translational medicine. Acta Pharmacol Sin.
34:1284–1291. 2013. View Article : Google Scholar : PubMed/NCBI
|