Introduction
Autophagy is an important mechanism for regulating
the process of cell growth and death and is a highly conserved
process which can maintain the metabolic balance and preserve
stable environmental energy to maintain cell metabolic needs
(1). Autophagy can inhibit the
chromosomal instability by inhibiting protein aggregation, as well
as organelle and chromosome damage. Tumor cells with apoptotic
dysfunction can maintain long-term survival by autophagy which
reduces cell necrosis, inflammation and genetic damage. Autophagic
abnormality is closely related to the occurrence and development of
hepatocellular carcinoma (HCC). Ding et al (2) found that the lower autophagic activity
of liver cells at a precancerous stage was closely related to the
malignant behavior and prognosis of HCC, and there was loss of the
expression of the autophagy-related gene Beclin-1 during the
carcinogenesis of HCC induced by chemical carcinogens. Autophagy
can inhibit the occurrence of HCC, however autophagy occurred in
liver cancer cells when they were under hypoxia, drug or toxic
chemical damage. Cytoplasmic vesicles made from double membrane
coated material to be degraded form the autophagosomes, and then
the autophagosomes and lysosomes combine to form autolysosome. The
degraded substances in vesicles are digested, hydrolyzed and
released into the cytoplasm to be used again and aid liver cancer
cells to tolerate hypoxic conditions and resist chemotherapy.
Therefore, autophagy plays a role of ‘double-edged sword’ in the
occurrence and development of tumors. Under different conditions,
the promoting or inhibiting effect of autophagy on the
proliferation of tumor cells mainly depends on the cell state and
survival environment as well as the stimulating factors (3–5).
Bufalin, which is derived from the traditional Chinese medicine
named ‘Chan Su’, has strong toxicity, can destroy the
rough-surfaced endoplasmic reticulum of mitochondria in tumor cells
by inhibiting the synthesis of DNA and RNA in tumor cells, induce
differentiation and apoptosis of tumor cells, and inhibit tumor
angiogenesis, and then inhibit the proliferation of tumor cells
(6–8). Our previous study indicated that
bufalin could significantly inhibit proliferation, invasion and
metastasis of liver cancer cells by blocking cell cycle at the S
and G2 phase in BEL-7402 hepatoma cells, and the inhibitive
efficacy was time- and dose-dependent (9). Bufalin can inhibit the downstream
target molecules of MMP-2 and MMP-9 in liver cancer cells by
influencing the expression of Gli1 protein in the Hh-signaling
pathway. Bufalin can upregulate the expression of E-cadherin and
downregulate the expression of β-catenin and VEGF in liver cancer
cells by influencing the Gli3 protein expression of the
Hh-signaling pathway. Bufalin combined with the inhibitors of
Hh-signaling pathway can significantly reduce the malignant
biological behavior of liver cancer cells via the Hh-signaling
pathway (10). Our previous studies
revealed that bufalin inhibited the EMT of HCC cells by increasing
the inter-membrane E-cadherin/β-catenin complex. This is the key
mechanism of bufalin against cell proliferation, invasion and
metastasis of HCC cells (11,12).
However, the exact mechanism of inhibiting HCC growth and inducing
apoptosis of liver cancer cells has not yet been fully elucidated.
In the present study we examined cultured human liver cancer cells
and observed the effects of bufalin on liver cancer cell
proliferation by influencing the cell autophagic state. Therefore,
the present study provided new theoretical basis for developing new
related molecular targeted drugs to treat HCC by studying the
effects of bufalin on liver cancer cell autophagy.
Materials and methods
Reagents
Bufalin, purchased from Sigma Chemical Co. (St.
Louis, MO, USA), was dissolved in anhydrous alcohol at a
concentration of 10−1 mol/l and stored at 4°C. High
glucose Dulbecco's modified Eagle's medium (DMEM) and fetal bovine
serum (FBS) were purchased from Gibco Laboratories (Gaithersburg,
MD, USA). In addition, 5-fluorouracil (5-FU) was purchased from
Shanghai Xudong Haipu Pharmaceutical Co., Ltd. (Shanghai, China)
and 3-methyladenine autophagy inhibitor (3-MA) was obtained from
Gibco Laboratories and was diluted to 5 mmol/l DMEM containing 10%
FBS. Chloroquine autophagy inhibitor (CQ; Sigma Corporation of
America, Ronkonkoma, NY, USA), was diluted to 5 µg/ml DMEM
containing 10% FBS. Acridine orange and Cell Counting Kit-8 (CCK-8)
were obtained from Sigma Corporation of America.
Cell lines
HCC-LM3 cells were obtained from the Liver Cancer
Institute of Zhongshan Hospital Αffiliated to Fudan University. The
cells were cultured in high-glucose DMEM supplemented with 10% FBS,
100 U/ml of penicillin and 100 µg/ml of streptomycin in a
humidified atmosphere with 5% CO2 in air at 37°C.
Subsequently, the cells in the logarithmic growth phase were
collected for the following experiments.
Grouping
HCC-LM3 cells were cultured in vitro and were
divided into the following groups: Control, 5-FU, bufalin, bufalin
+ 3-MA, bufalin + CQ and bufalin + 3-MA + CQ.
Cell proliferation assay
The HCC-LM3 cells were cultured in high-glucose DMEM
supplemented with 10% FBS in a humidified atmosphere with 5%
CO2 at 37°C. Then, the cells in the logarithmic growth
phase in each group, were pretreated with autophagy inhibitors 3-MA
(the effective dose concentration of 5 mmol/l) and/or CQ (the
effective dose concentration of 5 µg/ml) for 12 h in 96-well
plates. Subsequently, 5 ml DMEM plus 10% FBS was injected into
culture plates in the control group, 5 ml 5-FU (186 µg/ml,
IC50 of 5-FU for HCC-LM3 cells at 24 h) was injected
into culture plates in the 5-FU group, 5 ml bufalin (0.12 µg/ml,
IC50 of bufalin for HCC-LM3 cells at 24 h) was injected
into culture plates in the bufalin, the bufalin + 3-MA, the bufalin
+ CQ and the bufalin + 3-MA + CQ groups. The cells in each group
were cultured in a humidified atmosphere with 5% CO2 at
37°C for 24 h. The culture medium in each group was discarded, and
then 100 µl CCK-8 was injected into the culture plates of each
group in the dark. Following incubation in a humidified atmosphere
with 5% CO2 at 37°C for 1.5 h, the absorbance at 450 nm
was detected by an automatic microplate spectrophotometer
(SpectraMax 190; Molecular Devices, Sunnyvale, CA, USA). Each
experiment was performed in triplicate. The cell inhibition ratio
was calculated using the following formula: Growth inhibition ratio
(%) = [1 - (average absorbance of the treated group-average
absorbance of the blank group)/(average absorbance of the control
group-average absorbance of the blank group)] × 100%.
Analysis of the autophagosome and
autophagolysosome formation of liver cancer cells
The HCC-LM3 cells were cultured in high-glucose DMEM
supplemented with 10% FBS in a humidified atmosphere with 5%
CO2 at 37°C. Subsequently, the cells in the logarithmic
growth phase were plated at a density of 2.5×106
cells/ml and then at 100 µl/well in 6-well plates. Twelve hours
later, the cells in each group were pretreated with autophagy
inhibitors 3-MA (5 mmol/l) and/or CQ (5 µg/ml) for 12 h. In
addition, 5 ml DMEM plus 10% FBS was injected into culture plates
in the control group, 5 ml 5-FU (186 µg/ml) was injected into
culture plates in the 5-FU group, 5 ml bufalin (0.12 µg/ml) was
injected into culture plates in the bufalin, the bufalin + 3-MA,
the bufalin + CQ and the bufalin + 3-MA + CQ groups. The cells in
each group were cultured in a humidified atmosphere with 5%
CO2 at 37°C for 24 h. The cells in each group were
collected by centrifugation (2,504 × g at 4°C for 10 min), fixed
with 2.5% glutaraldehyde solution for 12 h, and then fixed with 1%
Osmium tetroxide solution for 2 h. Finally, the gradient ethanol
solution was used to dehydrate the cells and the cells were
embedded with epoxy resin. The embedded cells in each group were
cut into 70 nm ultrathin sections, and stained with saturated
uranyl acetate and lead acetate solution, then observed and imaged
under transmission electron microscope (Hitachi 120 kV transmission
electron microscope HT7800; Hitachi, Tokyo, Japan).
Analysis of cell acidic vesicle
formation
The HCC-LM3 cells were cultured in high-glucose DMEM
supplemented with 10% FBS in a humidified atmosphere with 5%
CO2 at 37°C. Subsequently, the cells in the logarithmic
growth phase were plated at a density of 2.5×106
cells/ml and then at 100 µl/well in 24-well plates. Twelve hours
later, the cells in each group were pretreated with autophagy
inhibitors 3-MA (5 mmol/l) and/or CQ (5 µg/ml) for 12 h. In
addition, 5 ml DMEM plus 10% FBS was injected into culture plates
in the control group, 5 ml 5-FU (186 µg/ml) was injected into
culture plates in the 5-FU group, 5 ml bufalin (0.12 µg/ml) was
injected into culture plates in the bufalin, the bufalin + 3-MA,
the bufalin + CQ and the bufalin + 3-MA + CQ groups. The cells in
each group were cultured in a humidified atmosphere with 5%
CO2 at 37°C for 24 h. The cells in each group were
collected by centrifugation (2,504 × g at 4°C for 10 min) and
stained with 500 l acridine orange solution (1acrml) for 5 min; a
portion of the HCC-LM3 cells in each group were rinsed with 0.01 M
PBS three times. Then the HCC-LM3 cell in each group were observed
under the fluorescence microscope (Olympus bioluminescence
microscope BX53; Olympus Corp., Tokyo, Japan), and imaged randomly
at 5 visual fields. The red fluorescence intensity was assesse by
the ImageJ software (V1.48u; National Institutes of Health,
Bethesda, MD, USA).
In addition, a part of the HCC-LM3 cells in each
group was rinsed with 0.01 M PBS three times. The cells in each
group were digested with trypsin for 20 sec, the digestion was
terminated with 1 ml 0.01 M PBS containing 5% serum. The cells in
each group were collected by centrifugation (402 × g at 4°C for 5
min) and resuspended in the 0.5 ml 0.01 M PBS containing 5% serum.
The fluorescence intensity at 488 nm was evaluated by flow
cytometry; the fluorescence intensity is expressed by FL3/FL1.
Protein expression assay with western
blot analysis
HCC-LM3 cells were cultured in high-glucose DMEM
supplemented with 10% FBS in a humidified atmosphere with 5%
CO2 at 37°C. Subsequently, the cells in the logarithmic
growth phase were plated at a density of 1.5×105
cells/ml, and then 100 µl/well in 6-well plates. Twelve hours
later, the cells in each group were pretreated with autophagy
inhibitors 3-MA (5 mmol/l) and or CQ (5 µg/ml) for 12 h. In
addition, 5 ml 5-FU (186 µg/ml) was injected into culture plates in
the 5-FU group, 5 ml bufalin (0.12 µg/ml) was injected into culture
plates in the bufalin, the bufalin + 3-MA, the bufalin + CQ and the
bufalin + 3-MA + CQ groups. The cells in each group were cultured
in a humidified atmosphere with 5% CO2 at 37°C for 12,
24 and 48 h. The cells in each group were collected by
centrifugation (2,504 × g at 4°C for 10 min) and the proteins of
cells in each group were obtained with cell lysis solution. Using
western blotting, the protein expression of LC, LC3-II, P62 and
Beclin-1 was detected in the HCC-LM3 cells of each group.
Statistical analysis
Data were analyzed by analysis of variance using
SPSS 18.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5 (SAS
Institute, Inc., Cary, NC, USA). All data are presented as the mean
± SD. The inhibition rate and percentage were calculated using
χ2 test. One-way ANOVA test or Student's t-test were
used to analyze the other data. P-values <0.05 were considered
to indicate a statistically significant difference.
Results
The effect of bufalin combined with
autophagy inhibitors on the proliferation of liver cancer
cells
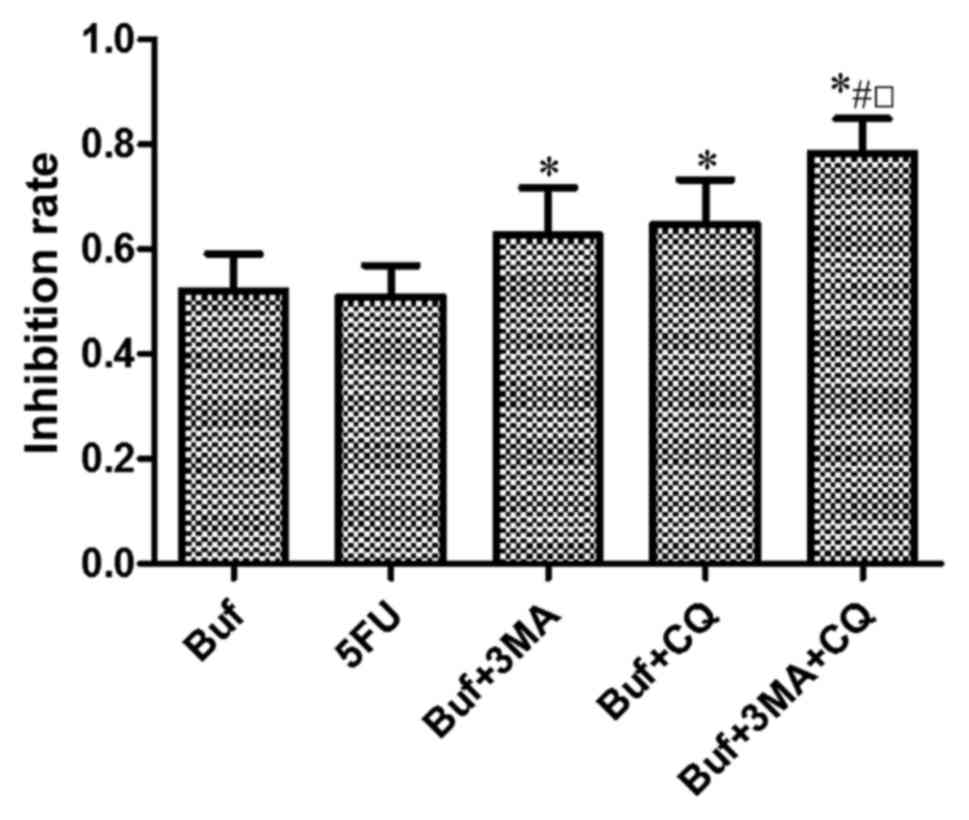
Compared with the control group, bufalin
significantly inhibited the growth of HCC-LM3 cells. Compared with
the bufalin group, autophagy inhibitors 3-MA or CQ significantly
enhanced the inhibitory effect of bufalin on the growth of HCC-LM3
cells. The inhibitory effect of bufalin combined with 3-MA and CQ
on the growth of HCC-LM3 cells was strongest (F=6.58, P<0.05).
There was no significant difference of the inhibitory effect on the
growth of HCC-LM3 cells between the bufalin + 3-MA group and the
bufalin + CQ group (F=6.58, P>0.05) (Fig. 1).
The effect of bufalin combined with
autophagy inhibitors on the formation of autophagosomes in liver
cancer cells
Compared with the control group, bufalin induced the
increase of autophagosomes in HCC-LM3 cells. After the HCC-LM3
cells were pretreated with the autophagy inhibitors 3-MA or CQ for
12 h, the autophagosomes induced by bufalin for 24 h in HCC-LM3
cells decreased significantly (F=13.27, P<0.05). This finding
indicated that the autophagy inhibitors effectively inhibited the
autophagosome formation in HCC-LM3 cells induced by bufalin
(Fig. 2 and Table I).
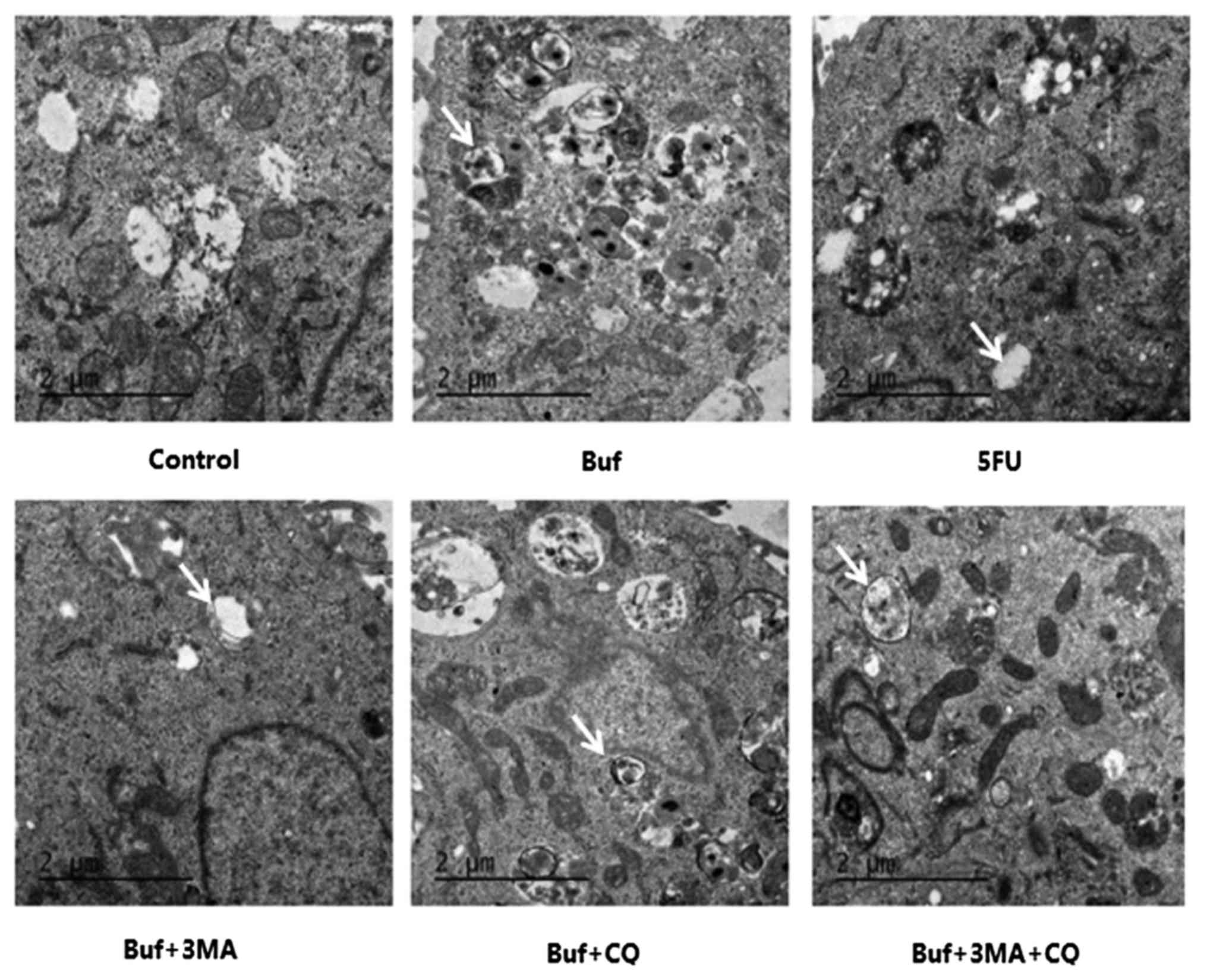 | Figure 2.The autophagosome formation in HCC-LM3
cells when bufalin alone or in combination with autophagy
inhibitors is used for 24 h [transmission electron microscope
(TEM), magnification ×11.500]. The autophagosome in HCC-LM3 cells
is indicated with white arrows. The drug concentration of 3-MA, CQ,
5-FU and bufalin was 5 mmol/l, 5, 186 and 0.12 µg/ml, respectively.
Bufalin induced the increase of autophagosomes in HCC-LM3 cells
(Buf, bufalin group). Following the pretreatment of HCC-LM3 cells
with autophagy inhibitors, the autophagosomes induced by bufalin in
HCC-LM3 cells decreased significantly (in buf + 3-MA, buf + CQ, buf
+ 3-MA + CQ groups). |
 | Table I.Influence of bufalin alone or combined
with autophagy inhibitors on the formation of autophagosome in
HCC-LM3 cells for 24 h (mean ± SD, n=3). |
Table I.
Influence of bufalin alone or combined
with autophagy inhibitors on the formation of autophagosome in
HCC-LM3 cells for 24 h (mean ± SD, n=3).
|
| Control group | Bufalin group | 5-FU group | Buf + 3-MA group | Buf + CQ group | Buf + 3-MA + CQ
group |
|---|
| No. of autophagosomes
in HCC-LM3 cells |
0.00±0.00a | 13.60±4.12 | 11.67±3.10 |
2.53±3.86a |
4.28±3.22a |
3.12±2.71a |
The effect of bufalin combined with
autophagy inhibitors on the formation of acidic vesicles in liver
cancer cells
Compared with the control group, bufalin induced the
increase of acidic vesicles in HCC-LM3 cells. After the HCC-LM3
cells were pretreated with autophagy inhibitors 3-MA or CQ for 12
h, the acidic vesicles induced by bufalin for 24 h in HCC-LM3 cells
decreased significantly (F=24.58, P<0.05). There was no
significant difference among the bufalin + 3-MA, the bufalin + CQ
and the bufalin + 3-MA + CQ groups (F=24.58, P>0.05). This
finding indicated that the autophagy inhibitors effectively
inhibited the acidic vesicle formation in HCC-LM3 cells induced by
bufalin, however the inhibitory effect of autophagy inhibitors was
irrelevant to the type of autophagy inhibitors (Figs. 3 and 4; Table
II).
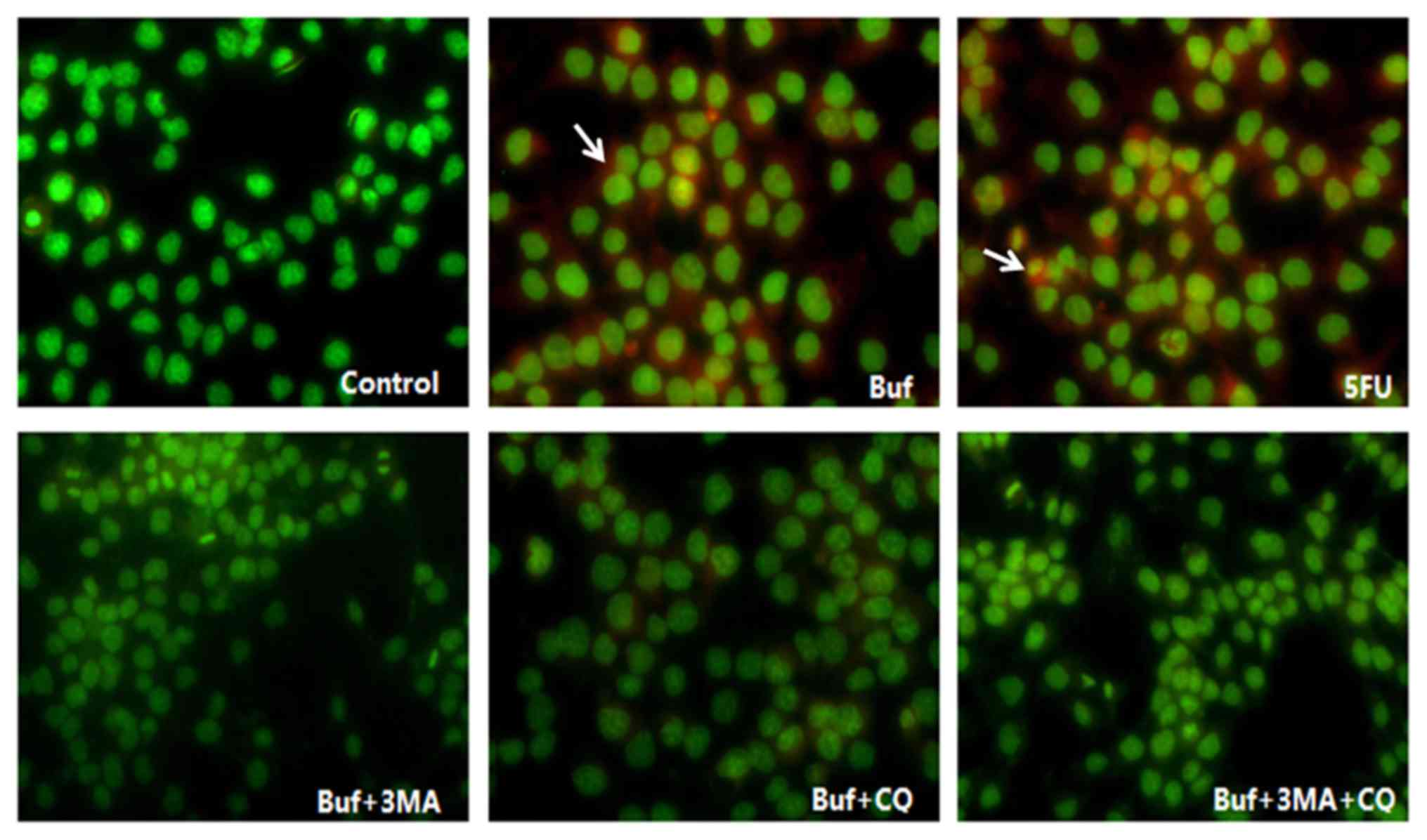 | Figure 3.Acidic vesicle formation in HCC-LM3
cells when bufalin alone or in combination with autophagy
inhibitors is used for 24 h (red fluorescence, magnification ×200).
The drug concentration of 3-MA, CQ, 5-FU and bufalin was 5 mmol/l,
5, 186 and 0.12 µg/ml, respectively. The acidic vesicles in HCC-LM3
cells are indicated with white arrows. Bufalin induced the increase
of acidic vesicles in HCC-LM3 cells in the bufalin group (red
fluorescence intensity in the bufalin vs. the control group,
F=13.10, P<0.05). After the HCC-LM3 cells were pretreated with
autophagy inhibitors, the acidic vesicles in HCC-LM3 cells
decreased significantly in the bufalin + 3-MA, the bufalin + CQ and
the bufalin + 3-MA + CQ group (vs. the bufalin group; F=13.10,
P<0.05), however, there was no significant difference of the
acidic vesicles in HCC-LM3 cells between the bufalin + 3-MA, the
bufalin + CQ and the bufalin + 3-MA + CQ groups (vs. the bufalin +
3-MA + CQ group; F=13.10, P<0.05). |
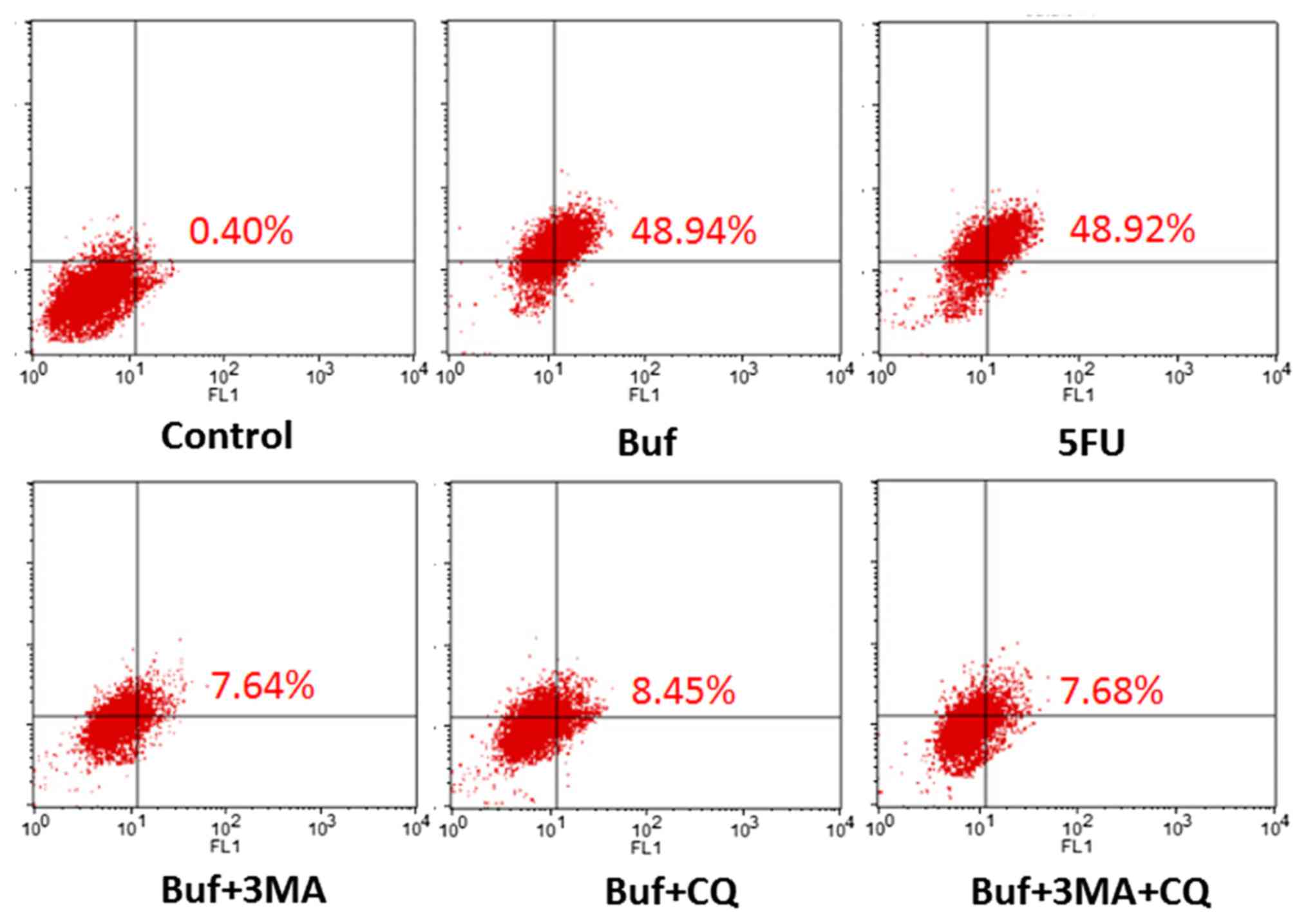 | Figure 4.The percentage of HCC-LM3 cells
containing acidic vesicles detected by flow cytometry following
treatment with bufalin alone or in combination with autophagy
inhibitors for 24 h. The drug concentration of 3-MA, CQ, 5-FU and
bufalin was 5 mmol/l, 5, 186 and 0.12 µg/ml, respectively. Compared
with the control group, the percentage of HCC-LM3 cells containing
acidic vesicles significantly increased when bufalin was used for
24 h (48.9 vs. 0.40%, F=24.58, P<0.05. Compared with the bufalin
group, the percentage of liver cancer cells containing acidic
vesicles in the bufalin + 3-MA, the bufalin + CQ and the bufalin +
3-MA + CQ group significantly decreased when bufalin was used for
24 h (48.9 vs. 7.64, 8.45 and 7.68%; F=24.58, P<0.05, Table III). |
 | Table II.Influence of bufalin alone or combined
with autophagy inhibitors on the fluorescence intensity of acidic
vesicles in HCC-LM3 cells for 24 h (mean ± SD, n=3). |
Table II.
Influence of bufalin alone or combined
with autophagy inhibitors on the fluorescence intensity of acidic
vesicles in HCC-LM3 cells for 24 h (mean ± SD, n=3).
|
| Control group | Bufalin group | 5-FU group | Buf + 3-MA group | Buf + CQ group | Buf + 3-MA + CQ
group |
|---|
| Fluorescence
intensity of acidic vesicles in HCC-LM3 cells |
9614.25±1361.30a |
44910.15±1361.30a |
45121.69±1361.30a |
4768.559±1361.30a |
5031.209±1361.30a |
4211.579±1361.30a |
The effect of bufalin combined with
autophagy inhibitors on the expression of autophagy-related
proteins in liver cancer cells for 12 h
Compared with the bufalin + 3-MA group, the
expression of LC3-I in HCC-LM3 cells significantly decreased after
bufalin alone or in combination with autophagy inhibitors was used
to treat the HCC-LM3 cells for 12 h (F=2.37, P<0.05). There was
no significant difference of the expression of LC3-I in the HCC-LM3
cells between the other groups (F=2.37, P>0.05). Compared with
the bufalin group, the expression of LC3-II in HCC-LM3 cells
significantly decreased in the bufalin + 3-MA group. Compared with
the bufalin and bufalin + 3-MA group, the expression of LC3-II in
HCC-LM3 cells significantly increased in the bufalin + CQ group.
Compared with the bufalin + CQ group, the expression of LC3-II in
HCC-LM3 cells significantly decreased in the bufalin + 3-MA + CQ
group (F=2.63, P<0.05). Compared with the control group, the P62
expression of HCC-LM3 cells significantly decreased in the bufalin
group. Compared with the bufalin, the bufalin + 3-MA and the
bufalin+CQ groups, the expression of P62 in HCC-LM3 cells
significantly increased in the bufalin + 3-MA + CQ group (F=3.63,
P<0.05). Compared with the control group, the expression of
Beclin-1 in HCC-LM3 cells significantly increased in the bufalin
group. Compared with the bufalin group, the expression of Beclin-1
in HCC-LM3 cells significantly decreased in the bufalin + 3-MA and
the bufalin + 3-MA + CQ groups. Compared with the bufalin + 3-MA
group, the expression of Beclin-1 in HCC-LM3 cells significantly
increased in the bufalin + CQ group. Compared with the bufalin + CQ
group, the expression of Beclin-1 in HCC-LM3 cells significantly
decreased in the bufalin + 3-MA + CQ group (F=4.33, P<0.05)
(Fig. 5).
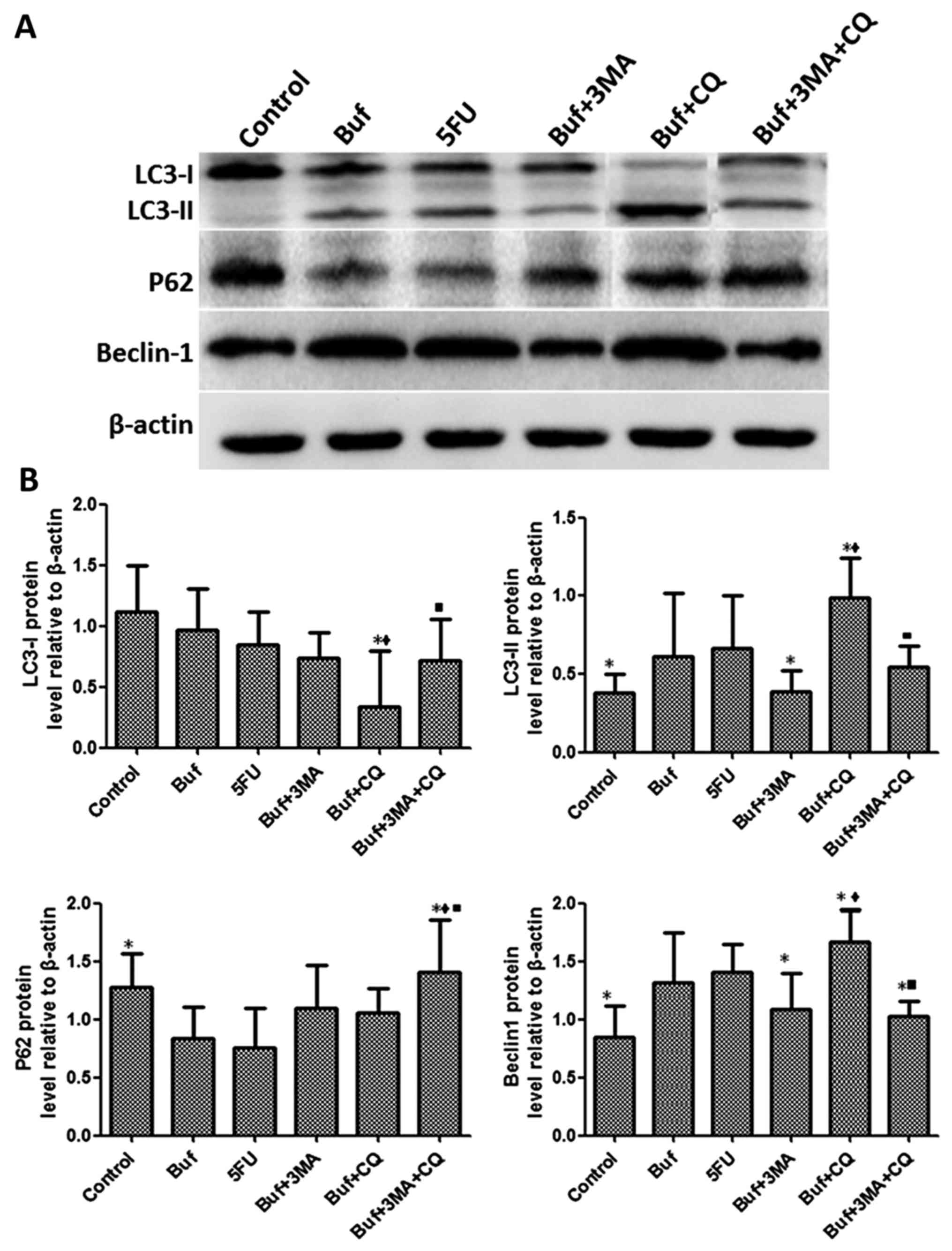 | Figure 5.Bufalin affects cell autophagy by
influencing the expression of autophagy related proteins in liver
cancer cells at 12 h. (A) The expression of LC3-I, LC3-II, P62 and
Beclin-1 in HCC-LM3 cells was detected by western blot analysis
after bufalin alone or in combination with autophagy inhibitors was
used to treat the HCC-LM3 cells for 12 h. (B) The different
expression of LC3-I, LC3-II, P62 and Beclin-1 in HCC-LM3 cells was
analyzed after bufalin alone or in combination with autophagy
inhibitors was used to treat the HCC-LM3 cells for 12 h. The drug
concentration of 3-MA, CQ, 5-FU and bufalin was 5 mmol/l, 5, 186
and 0.12 µg/ml, respectively. *P<0.05 vs. the bufalin group;
♦P<0.05, the bufalin + 3-MA vs. the bufalin + CQ, the
bufalin + 3-MA + CQ group; ■P<0.05, the bufalin + CQ
vs. the bufalin + 3-MA + CQ group. |
The effect of bufalin combined with
autophagy inhibitors on the expression of autophagy-related
proteins in liver cancer cells for 24 h
Compared with the control group, the expression of
LC3-I in HCC-LM3 cells significantly decreased in the bufalin group
after bufalin alone or in combination with autophagy inhibitors was
used to treat the HCC-LM3 cells for 24 h. Compared with the bufalin
group, the expression of LC3-I in HCC-LM3 cells significantly
increased in the bufalin + CQ and the bufalin + 3-MA + CQ groups
(F=5.60, P<0.05). Compared with the control group, the
expression of LC3-II in HCC-LM3 cells significantly increased in
the bufalin group. Compared with the bufalin group, the expression
of LC3-II in HCC-LM3 cells significantly decreased in the bufalin +
3-MA group and the bufalin + 3-MA + CQ group. Compared with the
bufalin + 3-MA group, the expression of LC3-II in HCC-LM3 cells
significantly increased in the bufalin + CQ group (F=4.97,
P<0.05). Compared with the control group, the expression of P62
in HCC-LM3 cells significantly decreased in the bufalin group.
Compared with the bufalin group, the expression of P62 in HCC-LM3
cells significantly increased in the bufalin + 3-MA, the bufalin +
CQ and the bufalin + 3-MA + CQ groups (F=5.92, P<0.05). Compared
with the control group, the expression of Beclin-1 in HCC-LM3 cells
significantly increased in the bufalin group. Compared with the
bufalin group, the expression of Beclin-1 in HCC-LM3 cells
significantly decreased in the bufalin + 3-MA group. Compared with
the bufalin + 3-MA group, the expression of Beclin-1 in HCC-LM3
cells significantly increased in the bufalin + CQ and the bufalin +
3-MA + CQ groups (F=5.33, P<0.05) (Fig. 6).
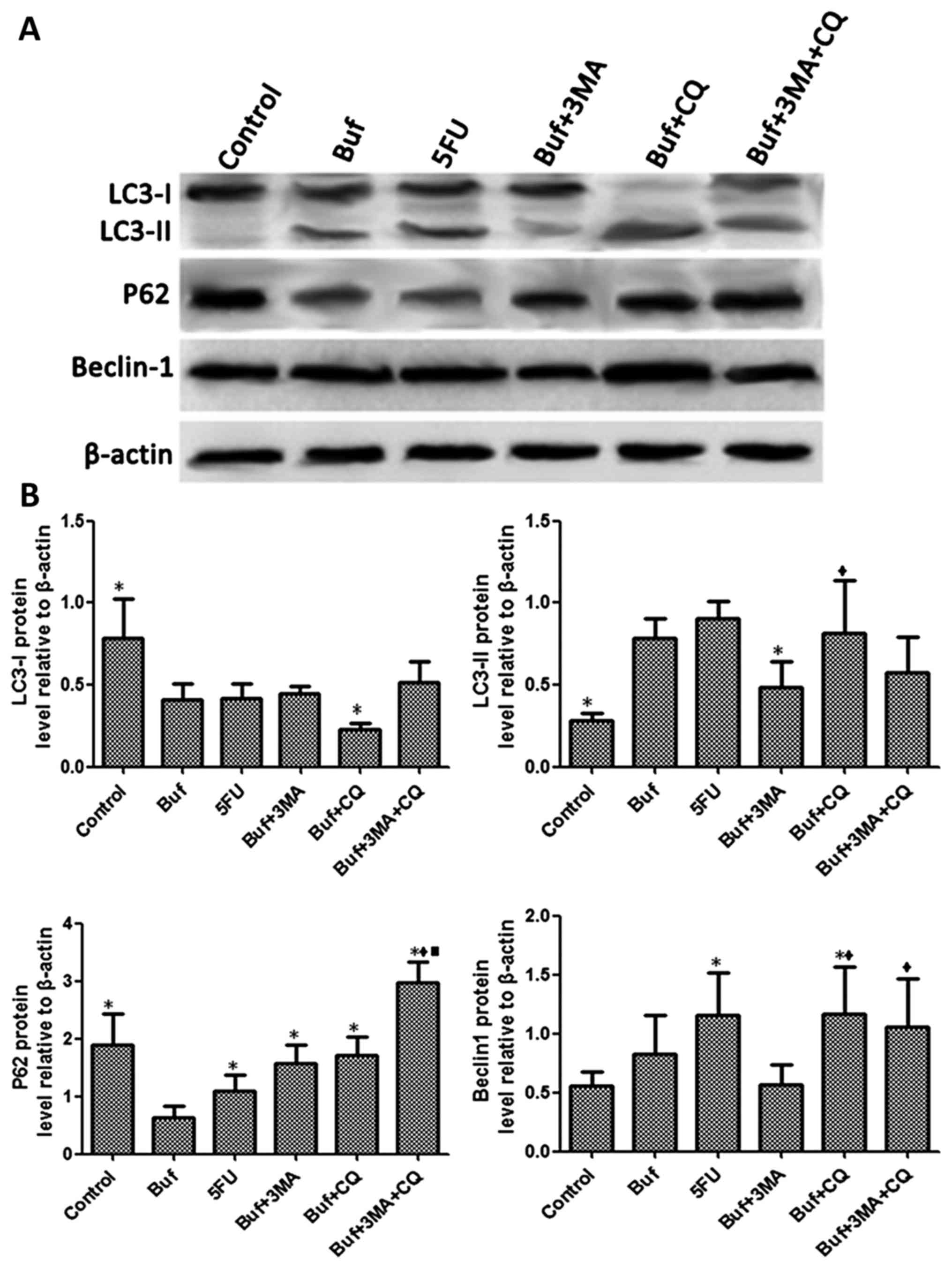 | Figure 6.Bufalin affects cell autophagy by
influencing the expression of autophagy related proteins in liver
cancer cells at 24 h. (A) The expression of LC3-I, LC3-II, P62 and
Beclin-1 in HCC-LM3 cells was detected by western blot analysis
after bufalin alone or in combination with autophagy inhibitors was
used to treat the HCC-LM3 cells for 24 h. (B) The different
expression of LC3-I, LC3-II, P62 and Beclin-1 in HCC-LM3 cells was
analyzed after bufalin alone or and in combination with autophagy
inhibitors was used to treat the HCC-LM3 cells for 24 h. The drug
concentrations of 3-MA, CQ, 5-FU and bufalin were 5 mmol/l, 5, 186
and 0.12 µg/ml, respectively. *P<0.05 vs. the bufalin group;
♦P<0.05, the bufalin + 3-MA vs. the bufalin + CQ, the
bufalin + 3-MA + CQ groups; ■P<0.05, the bufalin + CQ
vs. the bufalin + 3-MA + CQ group. |
The effect of bufalin combined with
autophagy inhibitors on the expression of autophagy-related
proteins in liver cancer cells for 48 h
Compared with the bufalin group, the LC3-I
expression of HCC-LM3 cells significantly increased in the bufalin
+ 3-MA group and the bufalin + CQ group after bufalin alone or/and
in combination with autophagy inhibitors was used to treat the
HCC-LM3 cells for 48 h. Compared with the bufalin + CQ group, the
expression LC3-I in HCC-LM3 cells significantly decreased in the
bufalin + 3-MA + CQ group (F=1.32, P<0.05). Compared with the
control group, the expression of LC3-II in HCC-LM3 cells
significantly increased in the bufalin group. Compared with the
bufalin group, the expression of LC3-II in HCC-LM3 cells
significantly decreased in the bufalin + 3-MA group (F=1.96,
P<0.05). Compared with the control group, the expression of P62
in HCC-LM3 cells significantly decreased in the bufalin group.
Compared with the bufalin group, the expression of P62 in HCC-LM3
cells significantly increased in the bufalin + 3-MA + CQ group
(F=5.37, P<0.05). Compared with the bufalin group, the
expression of Beclin-1 in HCC-LM3 cells significantly decreased in
the bufalin + 3-MA and the bufalin + 3-MA + CQ groups and the
expression Beclin-1 in HCC-LM3 cells significantly increased in the
bufalin + CQ group. Compared with the bufalin + 3-MA group, the
expression of Beclin-1 in HCC-LM3 cells significantly increased in
the bufalin + CQ group. Compared with the bufalin + CQ group, the
expression of Beclin-1 in HCC-LM3 cells significantly decreased in
the bufalin + 3-MA + CQ group (F=15.84, P<0.05) (Fig. 7).
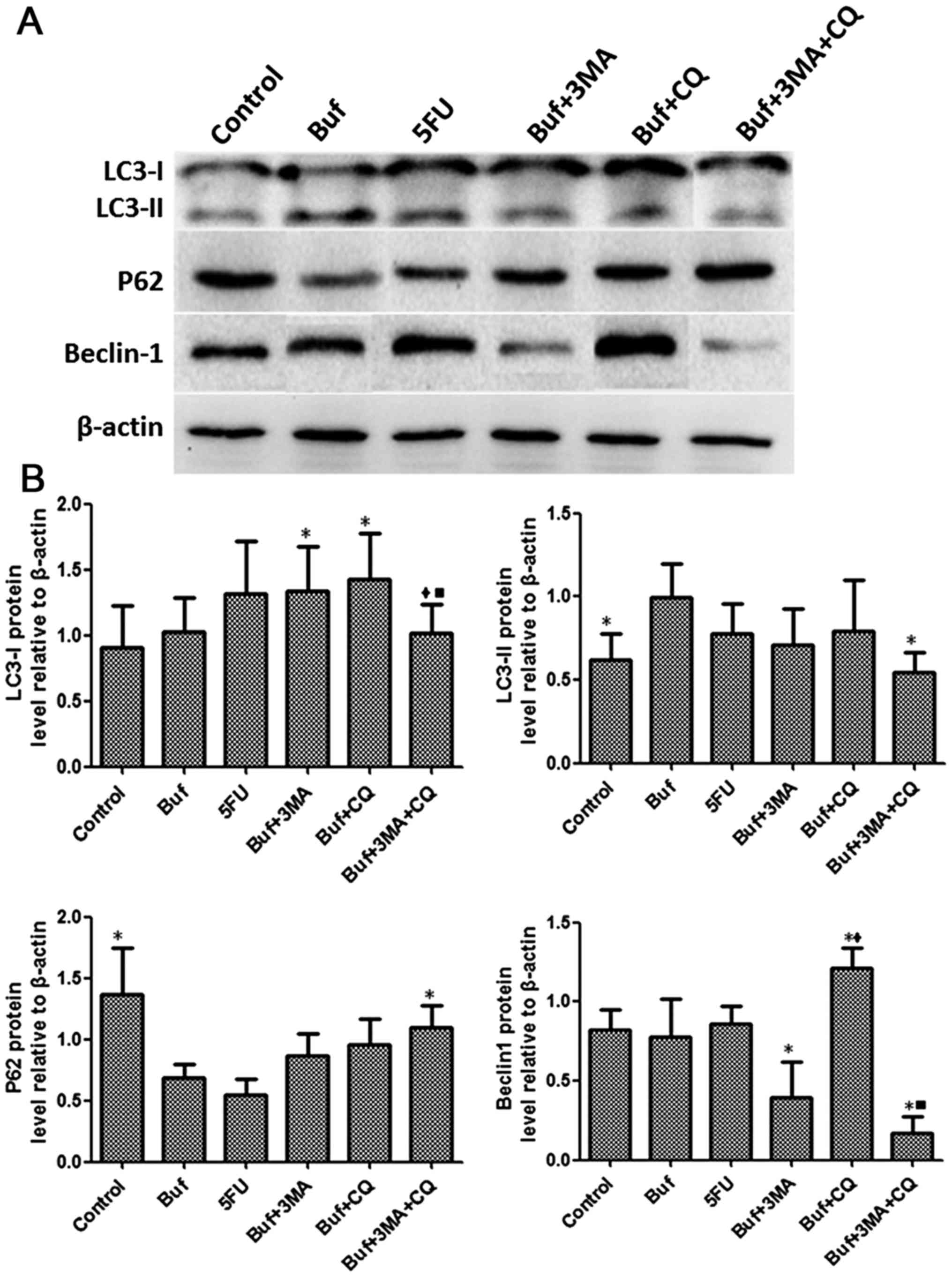 | Figure 7.Bufalin affects cell autophagy by
influencing the expression of autophagy related proteins in liver
cancer cells at 48 h. (A) The expression of LC3-I, LC3-II, P62 and
Beclin-1 in HCC-LM3 cells was detected by western blot analysis
after bufalin alone or/and in combination with autophagy inhibitors
was used to treat HCC-LM3 cells for 48 h. (B) The different
expression of LC3-I, LC3-II, P62 and Beclin-1 in HCC-LM3 cells was
analyzed after bufalin alone or/and in combination with autophagy
inhibitors was used to treat HCC-LM3 cells for 48 h. The drug
concentration of 3-MA, CQ, 5-FU and bufalin was 5 mmol/l, 5, 186
and 0.12 µg/ml, respectively. *P<0.05 vs. the bufalin group;
♦P<0.05, the bufalin + 3-MA vs. the bufalin + CQ, the
bufalin + 3-MA + CQ group; ■P<0.05, the bufalin + CQ
vs. the bufalin + 3-MA + CQ group. |
Discussion
Autophagy can lead to cancer cell death and inhibit
tumor growth. However, autophagy can also destroy the organelles
and proteins, enhance the ability of tumor cell to resist to severe
environmental changes and protect tumor cells, thereby promoting
tumor growth. Autophagy plays a dual role in the protection and
inhibition of the proliferation of liver cancer cells and its
mechanism involves the interaction between multiple genes and
factors. LC3, P62 and Beclin-1 are closely related to autophagy
proteins. When autophagy occurs in cells, LC3-I combines with
phosphatidylethanolamine on the surface of autophagic vacuoles to
form LC3-II. LC3-II binds specifically to the surface of the
autophagic vesicle membrane and is involved in the regulation of
autophagic vesicle formation (13).
At the late stage of autophagy, the autophagosome fuses with
lysosome to degrade LC3-II by hydrolases (14,15).
P62 is the substrate and regulatory protein of autophagy (16). At the initial stage of autophagy,
misfolded proteins are not able to be normally transported and
aggregate in the cytoplasm to form the aggresomes. The aggresomes
undergo ubiquitination and recruit greater polymers through the P62
protein. Abnormal aggregation in tumor tissue can be recognized by
regulatory proteins or receptors that bind to the Atg8 protein
(13,17–19).
Autophagy can inhibit tumor growth by scavenging the P62 protein in
mice with autophagy defects (13).
Beclin-1 is an essential molecule in the formation of
autophagosomes, which regulates the formation and maturation of
autophagosomes in mammals. Ding et al (2) observed that the expression of
autophagy-related protein Beclin-1 in HCC tissues was significantly
lower than that in peripheral normal tissues and the expression
level of Beclin-1 was correlated with the extent of malignancy of
HCC. Duan et al (20)
observed that Beclin-1 upregulated the expression of PI3KC3 and
induced autophagy in ovarian cancer cells. Li et al
(21) observed that the
autophagosomes and LC3-II protein increased significantly in liver
cancer cells (HepG2, BEL-7402 cells) and P62 protein decreased
significantly after the liver cancer cells were deprived of
nutrients in vitro. In addition they observed that the
invasive ability of the liver cancer cells was significantly
enhanced due to nutritional deprivation.
Furthermore, 3-MA and CQ are autophagy inhibitors;
3-MA can inhibit the autophagosome formation by inhibiting the
intracellular phosphatidylinositol three phosphate kinase. CQ can
inhibit the autophagosome formation by inhibiting the proteolytic
enzymes of intracellular lysosomes. The present study indicated
that bufalin significantly inhibited the growth of liver cancer
cells. The inhibitory effect of bufalin was significantly enhanced
when combined with autophagy inhibitors 3-MA or CQ. Furthermore,
the inhibitory effect of bufalin on the growth of liver cancer
cells was strongest when combined with 3-MA and CQ. Bufalin could
induce the increase of autophagosomes in HCC-LM3 cells, whereas
when the HCC-LM3 cells were pretreated with autophagy inhibitors
3-MA or CQ, the autophagosomes induced by bufalin in HCC-LM3 cells
decreased markedly. Bufalin induced the decrease of autolysosomes
in HCC-LM3 cells in combination with autophagy inhibitors 3-MA or
CQ. This finding indicated that the autophagy inhibitors could
effectively inhibit the autophagosome formation induced by bufalin
in HCC-LM3 cells. Autophagy is involved in the proliferation of
liver cancer cells. The inhibitory effect of bufalin on liver
cancer cells could be affected by interfering with autophagy. The
autophagy inhibitors could synergistically enhance the inhibitory
effect of bufalin on the proliferation of liver cancer cells by
interfering with autophagy.
Under normal circumstances, P62 protein locates in a
specific site of autophagosomes in early autophagy and is broken
down by hydrolases of the lysosomes when autophagolysosomes form.
When P62 protein cannot be degraded and begins to accumulate, it
indicates that the autophagy process may be inhibited (22). The LC3-I protein can be decomposed
to transform into the LC3-I protein when autophagy occurs in cells.
The LC3-II protein can be degraded by proteolytic enzymes of the
lysosomes when autophagolysosomes form. The increase of the LC3-II
protein and the decrease of the LC3-I protein in cells, is an
indication that autophagy occurs in cells (23). Beclin-1 binds to different molecules
to form a complex that regulates the formation and maturation of
autophagosomes. When autophagolysosomes form, Beclin-1 is degraded
by proteolytic enzymes of the lysosomes (24). The present study indicated that
bufalin upregulated the protein expression of LC3-II and Beclin-1,
as well as downregulated the expression of p62 in HCC-LM3 cells.
These protein expression changes induced autophagy in HCC-LM3
cells. Combined with autophagy inhibitor 3-MA, bufalin
downregulated the protein expression of LC3-II and Beclin-1, and
upregulated the expression of p62 in HCC-LM3 cells, thereby
inhibited autophagy in HCC-LM3 cells. Combined with autophagy
inhibitor CQ, bufalin upregulated the protein expression of LC3-II,
Beclin-1 and p62 in HCC-LM3 cells. This observation may be related
to the inhibition of proteolytic enzyme activity by CQ, thereby the
degradation of proteolytic enzymes on P62, LC3- II and Beclin-1
proteins in HCC-LM3 cells can be inhibited (25). Concerning the inhibitory effect of
bufalin on P62, LC3-II and Beclin-1 proteins in HCC-LM3 cells there
was no significant difference between the bufalin + 3-MA and the
bufalin + 3-MA + CQ group. These results indicated that the
inhibitory effect of 3-MA on autophagy-related proteins in liver
cancer cells could not be enhanced in combination with CQ. The main
reason may be that CQ inhibits the autophagolysosome degradation by
inhibiting the role of proteolytic enzyme of lysosome, thereby
inhibits the final stage of autophagy. Conversely, 3-MA inhibits
the phosphatidylinositol three phosphate kinase to block the
formation of autophagosomes, thereby inhibits the early stage of
autophagy. When the two inhibitors are combined, 3-MA can prevent
most of the autophagy from entering the terminal stage, thus
preventing CQ from functioning normally (21,25).
In conclusion, the present study indicated that
bufalin induced autophagy in liver cancer cells by upregulating the
protein expression of LC3-II and Beclin-1 and by downregulating the
protein expression of P62. Autophagy inhibitors significantly
enhanced the inhibitory effect of bufalin on the growth of liver
cancer cells by interfering with the protein expression of LC3-II,
Beclin-1 and P62 to inhibit the autophagy of liver cancer cells.
These findings indicate that the therapeutic efficacy of bufalin on
HCC can be improved through targeting autophagy-related proteins.
It provide important theoretical basis on developing molecular
targeted drugs for autophagy-related proteins of HCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
Research Project of Medical Key Specialty of Putuo Sistrict,
Shanghai (no. B-162).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
ZPF and QJM conceived and designed the study. ZPF
and SX performed the experiments. ZPF and QJM wrote the paper. QJM
and LQ reviewed and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All experimental protocols were approved by the
Institutional Review Board of the Department of Laboratory Animal
Science of the Second Military Medical University (Shanghai,
China).
Consent for publication
Not applicable.
Competing interests
The authors state that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FBS
|
fetal bovine serum
|
|
5-FU
|
5-fluorouracil
|
|
3-MA
|
3-methyladenine
|
|
CQ
|
chloroquine
|
References
|
1
|
Hale AN, Ledbetter DJ, Gawriluk TR and
Rucker EB III: Autophagy: Regulation and role in development.
Autophagy. 9:951–972. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai
Z, Shi GM, Wang XY, Ke AW, Wu B, et al: Association of autophagy
defect with a malignant phenotype and poor prognosis of
hepatocellular carcinoma. Cancer Res. 68:9167–9175. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Du H, Yang W, Chen L, Shi M, Seewoo V,
Wang J, Lin A, Liu Z and Qiu W: Role of autophagy in resistance to
oxaliplatin in hepatocellular carcinoma cells. Oncol Rep.
27:143–150. 2012.PubMed/NCBI
|
|
4
|
Guo XL, Li D, Hu F, Song JR, Zhang SS,
Deng WJ, Sun K, Zhao QD, Xie XQ, Song YJ, et al: Targeting
autophagy potentiates chemotherapy-induced apoptosis and
proliferation inhibition in hepatocarcinoma cells. Cancer Letter.
320:171–179. 2012. View Article : Google Scholar
|
|
5
|
Choi KS: Autophagy and cancer. Exp Mol
Med. 44:109–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang CL and Zhu YQ: Advances in research
on antitumor activity of toads. Nat Product Res Dev. 12:67–72.
2000.
|
|
7
|
Han JT, Chen XY and Xu RC: Advances in
pharmacological activities of bufalin. Chin Remed Clin. 2:120–122.
2002.
|
|
8
|
Chen XY, Hu WL, Xu RC, Chen L and Qian J:
Effect of bufalin on cytotoxicity and growth related gene
expression of human hepatoma cell line SMMC 7721. Chin J Pharmacol
Toxicol. 15:293–296. 2001.
|
|
9
|
Gai JQ, Qin JM and Fan YZ: Experimental
study on bufalin inhibiting hepatocellular carcinoma proliferation
and invasion. World Chin J Digestol. 22:1921–1927. 2014. View Article : Google Scholar
|
|
10
|
Sheng X, Sun X, Sun K, Sui H, Qin J and Li
Q: Inhibitory effect of bufalin combined with Hedgehog signaling
pathway inhibitors on proliferation and invasion and metastasis of
liver cancer cells. Int J Oncol. 49:1513–1524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Change J, Sun K, Sheng X and Qin JM:
Experimental study of bufalin on inhibiting cell proliferation and
apoptosis in liver can cer cells with high metastatic potential.
Chin J Exp Surg. 32:2388–2391. 2015.
|
|
12
|
Gai JQ, Sheng X, Qin JM, Sun K, Zhao W and
Ni L: The effect and mechanism of bufalin on regulating
hepatocellular carcinoma cell invasion and metastasis via
Wnt/β-catenin signaling pathway. Int J Oncol. 48:338–348. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pankiv S, Clausen TH, Lamark T, Brech A,
Bruun JA, Outzen H, Øvervatn A, Bjørkøy G and Johansen T:
p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of
ubiquitinated protein aggregates by autophagy. J Biol Chem.
282:24131–24145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Levine B and Yuan J: Autophagy in cell
death: An innocent convict? J Clin Invest. 115:2679–2688. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tanida I, Nishitani T, Nemoto T, Ueno T
and Kominami E: Mammalian Apg12p, but not the Apg12p. Apg5p
conjugate, facilitates LC3 processing. Biochem Biophys Res Commun.
296:1164–1170. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tanida I, Ueno T and Kominami E: In vitro
assays of lipidation of Mammalian Atg8 homologs. Curr Protoc Cell
Biol. 64:11.20.1–13. 2014. View Article : Google Scholar
|
|
17
|
Zhou ZW, Li YX, He ZX, Pan ST, Yang Y,
Zhang X, Chow K, Yang T, Qiu JX, Zhou Q, et al: Induction of
apoptosis and autophagy via sirtuin1- and PI3K/Akt/mTOR-mediated
pathways by plumbagin in human prostate cancer cells. Drug Des
Devel Ther. 9:1511–1554. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Komatsu M, Waguri S, Koike M, Sou YS, Ueno
T, Hara T, Mizushima N, Iwata J, Ezaki J, Murata S, et al:
Homeostatic levels of p62 control cytoplasmic inclusion body
formation in autophagy-deficient mice. Cell. 131:1149–1163. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Inoue D, Suzuki T, Mitsuishi Y, Miki Y,
Suzuki S, Sugawara S, Watanabe M, Sakurada A, Endo C, Uruno A, et
al: Accumulation of p62/SQSTM1 is associated with poor prognosis in
patients with lung adenocarcinoma. Cancer Sci. 103:760–766. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duan ZL, Peng ZL and Wang ZH: Expression
and involved signal transduction pathway of autophagy gene Beclin 1
in epithelialovarian cancer. Sichuan Da Xue Xue Bao Yi Xue Ban.
38:239–242. 2007.(In Chinese). PubMed/NCBI
|
|
21
|
Li Z, Yang B, Guo Y, Zheng QC, Peng Y, Ke
WB, Zhang L and Xiong J: Starvation-induced autophage promotes
invasion of hepatocellular carcinoma cells. Acta Med Univ Sci
Technol Huangzhong. 41:513–517. 2012.
|
|
22
|
Ferrari V and Cutler DJ: Uptake of
chloroquine by human erythrocytes. Biochem Pharmacol. 39:753–762.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hao G, Sun TS and Li SG: Advances in
detection of autophagy in mammals. Chin J Clinicians. 6:1531–1533.
2012.
|
|
24
|
Kovács AL, Molnár K and Seglen PO:
Inhibition of autophagic sequestration and endogenous protein
degradation in isolated rat hepatocytes by methylated adenosine
derivatives. FEBS Lett. 134:194–196. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen S, Zhou L, Zhang Y, Leng Y, Pei XY,
Lin H, Jones R, Orlowski RZ, Dai Y and Grant S: Targeting
SQSTM1/p62 induces cargo loading failure and converts autophagy to
apoptosis via NBK/Bik. Mol Cell Biol. 34:3435–3449. 2014.
View Article : Google Scholar : PubMed/NCBI
|