Introduction
Chronic lymphocytic leukemia (CLL) remains an
incurable disease with an extremely variable course (1,2). It is
featured by a dynamic imbalance between the proliferation and
apoptosis of leukemia cells and by the accumulation of neoplastic B
lymphocytes co-expressing CD5 and CD19 antigens (3–6).
However, the underlying mechanism involved in the development of
this disease still remains unrevealed. In recent years, the role of
interleukin (IL)-9 in tumor immunity has obtained increasing
attention.
The cytokine IL-9 has largely been regarded as a Th2
cytokine that produces multifocal contributions to allergic disease
(7). Recent data revealed that IL-9
is involved in tumor immunity mediated by Treg cells and mast cells
(8). Its role in cell growth and
the anti-apoptotic process on multiple transformed cells also
suggests its effect in tumor progression. IL-9 has also been
revealed to participate in IL-2, −4, −7, −15 and −21 signaling
pathways. These pathways are mediated by heterodimeric receptors
that form a specific receptor chain with the common γ-chain members
of the STATs family (9). STAT3 is a
latent cytoplasmic transcription factor, originally discovered as a
transducer of signals from cell surface receptors to the nucleus.
Compelling evidence suggests that STAT3 is constitutively activated
in many cancers and plays a pivotal role in tumor growth and
metastasis. In addition, STAT3 regulates cellular proliferation,
invasion, migration, and angiogenesis, which are critical for
cancer metastasis (10).
MicroRNAs are considerably valuable indicators for
predicting the clinical behavior of CLL. Structurally, miRNAs are
short (19- to 25-nucleotide) RNAs, processed from hairpin loop
structures (pre-miRNAs; 60–110 nucleotides in length) that regulate
the expression of protein-coding genes as a result of imperfect
complementarity with targeting messenger RNAs (11). Several chromosomal abnormalities,
such as 11q-, 13q-, 17p- and trisomy 12, and molecular aberrations,
including loss or downregulation of miRNA-15a and −16-1and
overexpression of anti-apoptotic genes, have been identified in CLL
in recent years (12). In patients
with various IgVH and ZAP-70 kinase statuses a unique miRNA
signature was found to be differentially expressed, and the miRNA
signature was composed of the most frequently dysregulated miRNAs
in different hematological malignancies (such as miR-15/16, the
miR-29 family and miR-155). Recent studies have revealed that
miR-21 expression stratifiesthe survival of CLL patients with 17p-
as well as patients with various chromosomal aberrations,
suggesting that miR-21 expression could predict patient
survival.
Since IL-9, STAT3 and microRNAs are implicated in
tumor growth, the cross-talk pathways among these important factors
were examined. In the present study, we demonstrated that
expression of the pSTAT3 protein, miR-155 and miR-21 were
upregulated. Our results revealed that there was a novel
‘extracellular IL-9/STAT3/miR-155/miR-21/intracellular IL-9’
positive feedback system in CLL cells.
Materials and methods
Patients and samples
Peripheral blood mononuclear cells (PBMCs) were
isolated from heparinized blood obtained from 20 CLL patients who
were diagnosed with CLL for the first time at the Department of
Hematology, Shandong Provincial Hospital, from January 2010 to
December 2011. Samples from 10 healthy volunteers served as normal
controls. All patients had not received any treatment, and their
lymphocytes exceeded 90%. Density gradient centrifugation was used
to isolate the PBMCs from 10 healthy blood donors, and the PBMCs
were subjected to a preliminary phenotypic characterization.
Negative selection with antibody-coated magnetic
beads was used to obtain a >97% pure CD19+ B-cell
population, when residual non-B cells exceeded 10%.
The protocol was approved by the Shandong Provincial
Hospital Ethics Committee, and written informed consent was
obtained from all participants involved in this study.
Cell culture
The human CLL cell line MEC-1 was purchased from the
American Type Culture Collection (ATCC; Manassas, VA, USA) and
cultured at 37°C in 5% carbon dioxide. Fetal bovine serum (FBS;
10%) was added to Iscove's modified Dulbecco's medium (IMDM; both
from HyClone; GE Healthcare Life Sciences, Logan, UT, USA) to
culture the cells.
Transfection of cells
MEC-1 cells (2×105 cells/well) were
seeded in 24-well plates and incubated overnightand thenwere
transfected with the microRNA human miR-155, miR-21 and their
negative control miRNAs using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc., Shanghai, China) for 48 h according
to the manufacturer's instructions. The sequences were as follows:
miR-155 mimic sense, UUAAUGCUAAUCGUGAUAGGGGU and antisense,
CCCUAUCACGAUUAGCAUUAAUU; miR-21 mimic sense, UAGCUUAUCAGACUGAUGUUGA
and antisense, AACAUCAGUCUGAUAAGCUAUU; and mimic negative control
sense, 5′-UUCUCCGAACGUGUCACGUTT-3 and antisense,
5′-ACGUGACACGUUCGGAGAATT-3.
Knockdown of human STAT3 by RNA
interference (RNAi)
The RNAi target sequence to the human STAT3 gene
was: forward,
5′-AAGCAGCAGCTGAACAACATGTTCAAGAGACATGTTGTTCAGCTGCTGCTT-3′ and
reverse, 5′-AAGCAGCAGCTGAACAACATGTCTCTTGAACATGTTGTTCAGCTGCTGCTT-3′.
The sequences of small interfering RNA (siRNA) targeting the human
STAT3 gene and negative control siRNA were cloned into the
pGCsi.U6/neoRFP plasmid and generated to generate pGC-siSTAT3 by
Sangon Biotech Co., Ltd. (Shanghai, China). As a negative control,
a control-siRNA oligonucleotide duplex targeting no known human
genes was used: forward,
5′-CCGGTTCTCCGAACGTGTCACGTTTCAAGAGAACGTGACACGTTCGGAGAATTTTTG-3′ and
reverse,
5′-AATTCAAAAATTCTCCGAACGTGTCACGTTCTCTTGAAACGTGACACGTTCGGAGAA-3′.
pGC-siSTAT3 (0.8 µg) and pGC-siCtrl plasmids (0.8 µg) were
separately transfected into MEC-1 cells (2×105
cells/well, 24-well plates) using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc., Shanghai, China) (2.0 µl) following
the manufacturer's instructions for 4 h, after which time the
transfection medium was replaced with regular growth medium. Cells
were treated as indicated at 24 h after transfection.
Co-treatment or co-culture
experiments
To explore the effects of extracellular IL-9 on the
expression of pSTAT3 and intracellular IL-9 in MEC-1cells,
recombinant human IL-9 (rIL-9) was added into the medium. The final
concentration of rIL-9 was 20 ng/ml. For STAT3 inhibition, MEC-1
cells were pretreated with WP1066 (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) for 48 h and then were incubated with rIL-9. The
expression level of pSTAT3 and intracellular IL-9 was assessed by
western blotting after cells were co-cultured with rIL-9 for 0, 5,
15, 30, 60 and 120 min.
To elucidate the effects of miR-155 and miR-21 on
intracellular IL-9 production, MEC-1 cells and
miR-155/miR-21-transfected MEC-1 cells were stimulated with or
without WP1066 for 48 h. Cells were then collected, and the
expression of the IL-9 protein and mRNA were assessed.
Real-time quantitative polymerase
chain reaction (RT-qPCR)
Total RNA was extracted from PBMCs of CLL patients
and MEC-1 cell lines using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. The reverse transcription reaction and RT-qPCR
analysis were performed as previously described (15). Specific primers were bought from
Biosune (Shanghai, China), and the primer sequences are shown in
Table I.
 | Table I.The primer sequences. |
Table I.
The primer sequences.
| Gene Name | Sequence |
|---|
| IL-9 |
5′-CTCTGTTTGGGCATTCCCTCT-3′ |
|
|
5′-GGGTATCTTGTTTGCATGGTGG-3′ |
| miR-155 |
5′-TTAATGCTAATCGTGA-3′ |
|
|
5′-TTTGGCACTAGCACATT-3′ |
| miR-21 |
5′-TAGCTTATCAGACTGATG-3′ |
|
|
5′-TTTGGCACTAGCACATT-3′ |
| β-actin |
5′-CATTAAGGAGAAGCTGTGCT-3′ |
|
|
5′-GTTGAAGGTAGTTTCGTGGA-3′ |
| U6 |
5′-CTCGCTTCGGCAGCACA-3′ |
|
|
5′-AACGCTTCACGAATTTGCGT-3′ |
Western blot analysis
Total protein was extracted from PBMCs of CLL
patients and MEC-1 cells and B cells of healthy samples. The
determination of protein concentrations and detailed procedures of
the immunoblot analysis have been previously described (16). The antibody of GAPDH (1:1,000; cat.
no. sc-47724) was purchased from Santa Cruz Biotechnology, while
IL-9 (1:10,000; cat. no. B02925) was obtained from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China). All other antibodies,
STAT3 antibody (1:10,000; cat. no. ab76315) and pSTAT3 antibody
(1:1,000; cat. no. ab32500) were purchased from Abcam (Cambridge,
MA, USA).
Assessment of cell proliferation and
apoptosis
Both transfected MEC-1 cells and untransfected MEC-1
cells were seeded into 96-well plates at a concentration of
5×103 cells/well. The cells were treated with or without
WP1066 (10 nM) for 48 h previously to seeding.
Cells were then incubated with rIL-9 (20 ng/ml) for
0, 15, 30, 60, 90 and 120 min. At the indicated time-points, cell
proliferation was evaluated using Cell Counting Kit-8 (Beyotime
Institute of Biotechnology, Haimen, China) according to the
manufacturer's instructions. After cells were incubated with rIL-9
(20 ng/ml) for 120 min, fluorescein isothiocyanate (FITC)-Annexin V
and propidium iodide (PI) were used to analyze apoptotic and
necrotic cells (Neobioscience Co., Ltd., Shenzhen, China). Briefly,
Annexin V-FITC and PI were used to incubate cells (106)
for 10 min in the dark at room temperature. Cells were then
immediately analyzed with FACS can flow cytometer (Beckman Coulter,
Chicago, IL, USA). Viable cells are not stained. The necrotic cells
were Annexin V-FITC and PI-positive, whereas apoptotic cells were
Annexin V-FITC-positive and PI-negative.
Statistical analysis
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) for
Windows was used to perform statistical analyses. The numerical
data were statistically analyzed using the two-tailed Student's
t-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expression of pSTAT3, miR-155 and
miR-21 in CLL patients
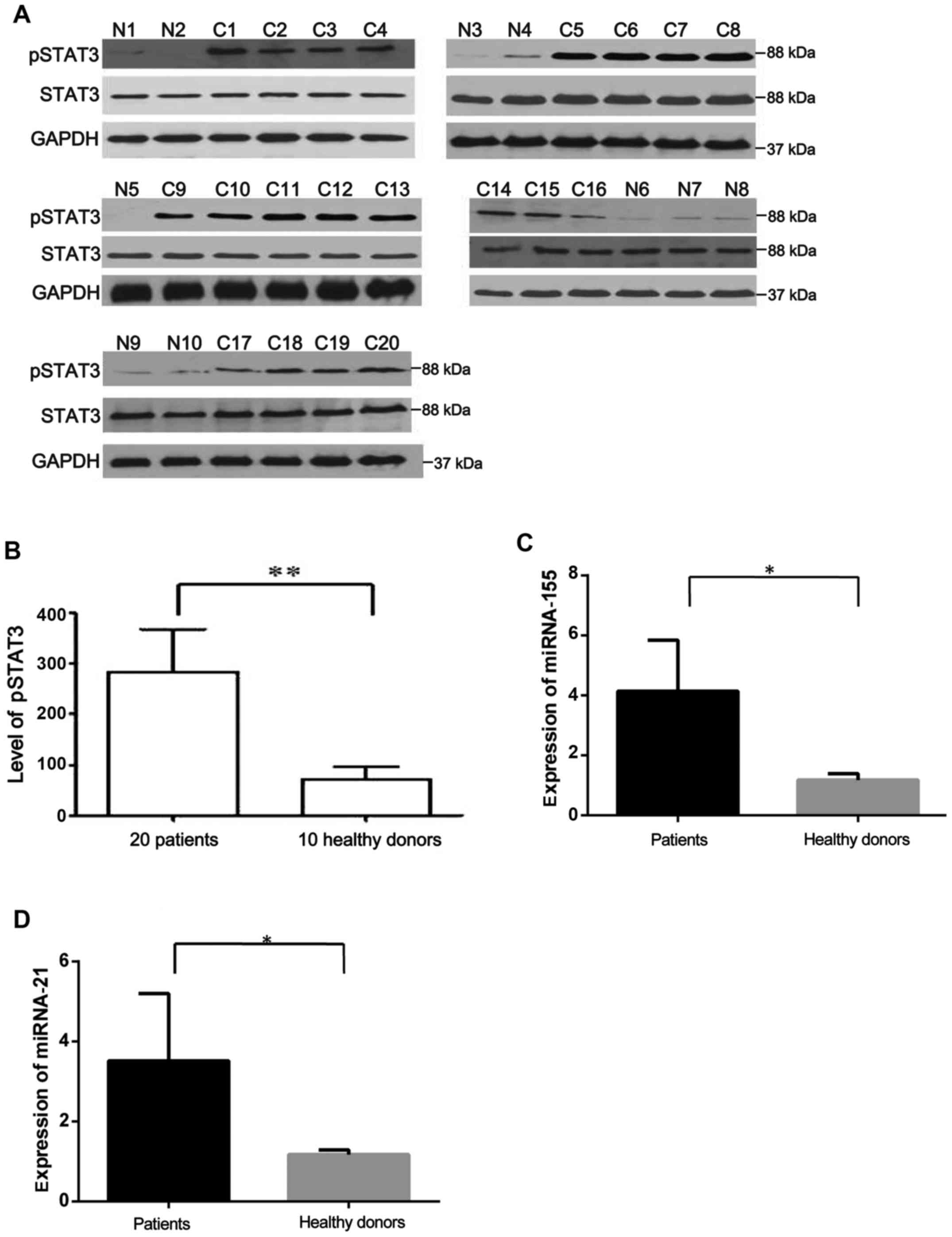
An elevated level of IL-9 was detected in sera from
20 out of 47CLL patients in our previous study (17,18).
Expression of STAT3 and pSTAT3 in PBMCs from the 20 CLL patients
with upregulated IL-9 and 10 healthy controls was detected using
western blotting. As shown in Fig. 1A
and B, the expression of pSTAT3 was higher in CLL patients than
that in healthy controls.
Expression of miR-155 and miR-21 in the 20 CLL
patients and 10 healthy controls was assessed using RT-qPCR. We
found that miR-155 and miR-21 were elevated in CLL samples compared
with the controls (Fig. 1C and
D).
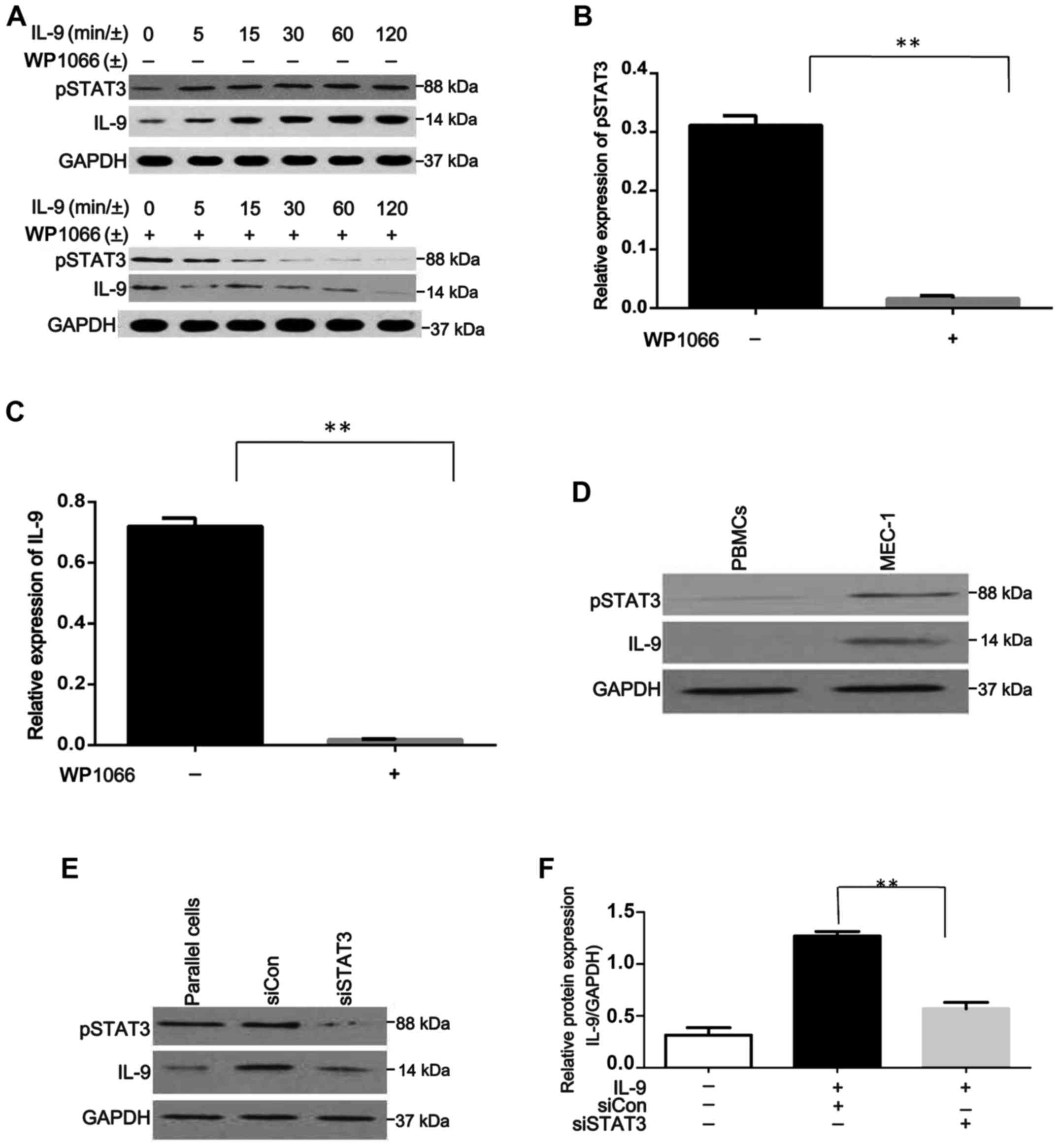
rIL-9 increases IL-9 expression through
phosphorylation of STAT3 in MEC-1 cells. The expression of IL-9 in
the medium of MEC-1 cells was assessed and no IL-9 was detected.
Then, rIL-9 was added to stimulate MEC-1 cells. The expression of
pSTAT3 and IL-9 are shown in Fig.
2A and the results revealed that the expression of pSTAT3 and
IL-9 increased in a time-dependent manner.
Furthermore, this increase could be suppressed by
Wp1066, which is a STAT3 inhibitor (Fig. 2B and C). Expression of pSTAT3 and
IL-9 were detected by western blotting, with the single band size
of 88 and 14 kDa, respectively, in peripheral blood mononuclear
cells (PBMCs) from healthy samples and human CLL cell line MEC-1,
after co-culture with rIL-9 for 120 min. pSTAT3 and IL-9 protein
levels were almost undetectable in PBMCs from healthy samples
(Fig. 2D).
To further explore the potential association between
IL-9 and pSTAT3 in CLL, the IL-9 protein was evaluated in
STAT3-depleted cells after co-culture with rIL-9 for 120 min
(Fig. 2E). As shown in Fig. 2F, STAT3 knockdown reduced IL-9
expression (P<0.0001). These results indicated that rIL-9
(extracellular IL-9) could stimulate MEC-1 cells to produce IL-9
through the phosphorylation of STAT3.
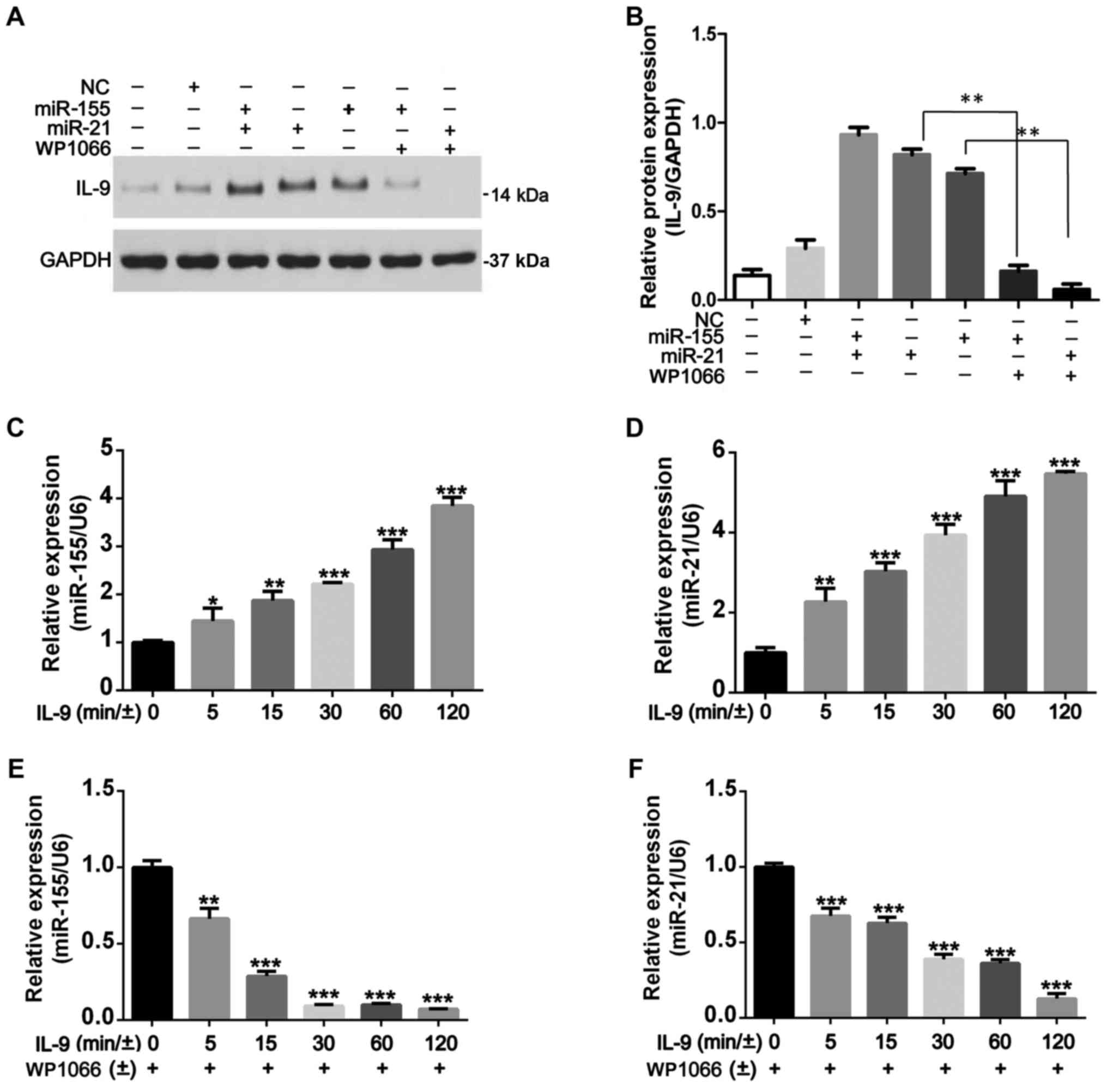
STAT3 phosphorylation regulates IL-9
production and is associated with miR-155 and miR-21
Since the phenomenon that activated STAT3 has a
marked effect on the upregulation of miR-155 and miR-21 expression
in CLL cells (19), we further
explored whether IL-9 levels would be affected by miR-155 and
miR-21 in CLL cells. After transfection with miR-155 and miR-21 for
48 h, rIL-9 (20 ng/ml) was used to stimulate MEC-1 cells for 120
min and then harvested to determine the expression of IL-9 using
western blotting. We determined that IL-9 expression was increased
in transfected cells when compared with untransfected ones and
negative controls. When miR-transfected MEC-1 cells were pretreated
with WP1066 for 48 h, WP1066 could suppress IL-9 production in
cells treated with rIL-9. The protein level of IL-9 was higher in
miR-155 and miR-21 transfected cells compared to miR-155 or miR-21
transfected cells, but there was no statistical significance
(Fig. 3A and B). In addition, we
demonstrated that overexpression of miR-155 and miR-21 could
promote the production of IL-9 in CLL cells. As shown in Fig. 3C and D, rIL-9 treatment promoted
time-dependent increase in the expression of miR-155 and miR-21,
which was significantly blocked by WP1066 (Fig. 3E and F). Given the upregulation of
pSTAT3 to miR-155 and miR-21 expression and the upregulation of
miR-155 and miR-21 to IL-9 production in CLL cells, it appeared
that the upregulation of IL-9 regulated by STAT3 phosphorylation
was mediated by miR-155 and miR-21.
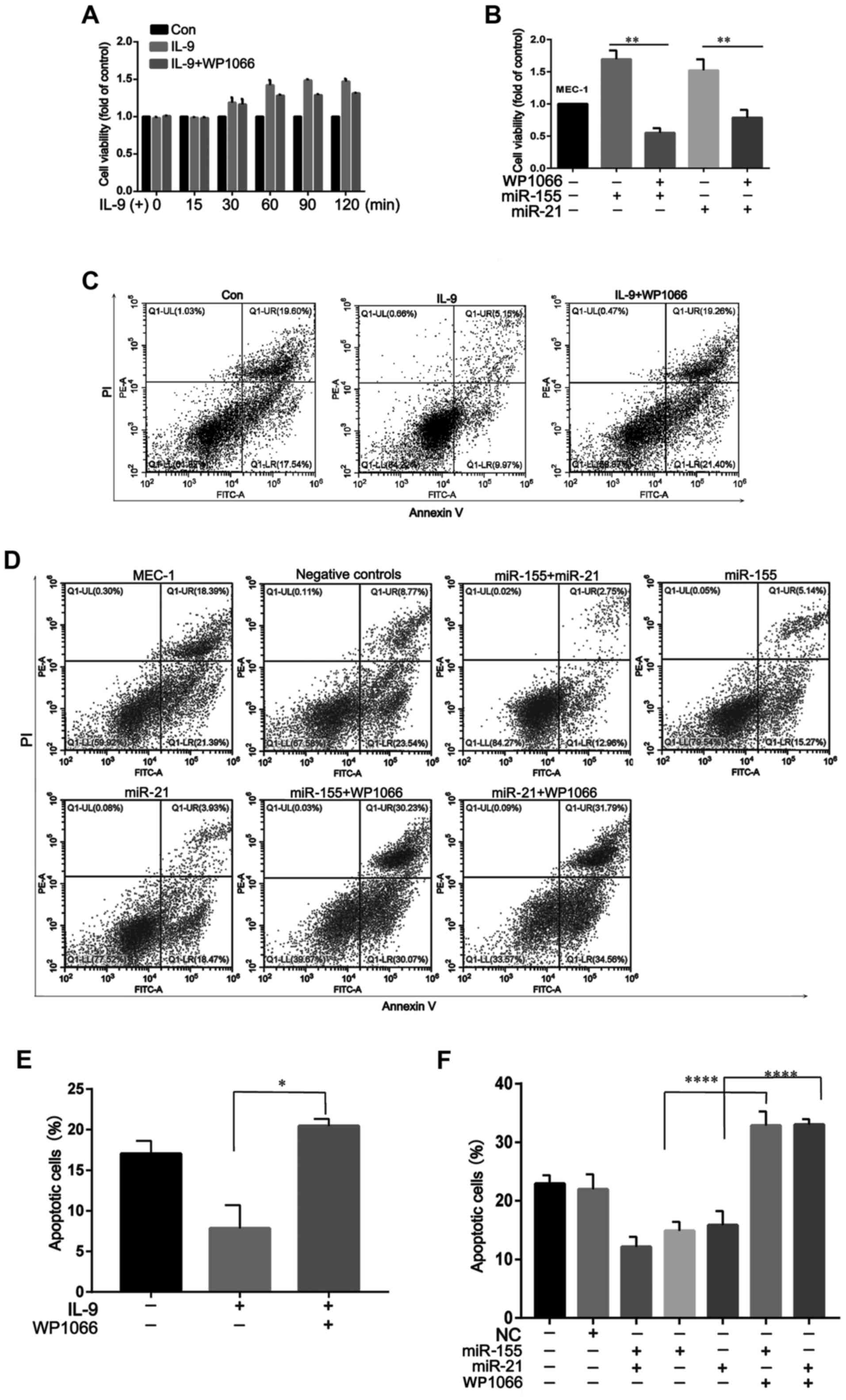
IL-9 promotes MEC-1 cell proliferation
and inhibits apoptosis
To determine the effect of extracellular IL-9 on the
cell growth of MEC-1cells, we first assayed its effect on the
proliferation of these cells. As shown in Fig. 4A, rIL-9 could promote MEC-1 cell
proliferation after co-culture for 30 min. However, pretreatment
with WP1066 could significantly suppress the proliferative effects
of rIL-9 on MEC-1 cells. Moreover, the proliferative effects of
rIL-9 on miR-155- or miR-21-transfected MEC-1 cells were more
apparent than MEC-1 cells. Pretreatment with WP1066 could also
suppress the proliferative effects of rIL-9 on transfected MEC-1
cells (Fig. 4B).
In addition to the promotion of cell proliferation,
other mechanisms could also participate in the regulation of
IL-9-mediated MEC-1 cell growth. In our following experiments, we
examined the effects of rIL-9 on apoptosis and necrosis of CLL. As
shown in Fig. 4C and E, rIL-9 could
reduce cell apoptosis to ~60% of the baseline level. Pretreatment
with WP1066 could significantly abolish the anti-apoptotic effects
of rIL-9 on MEC-1 cells. When cultured without rIL-9, the apoptosis
rate of miR-155/miR-21-transfected MEC-1 cells was lower than that
in MEC-1 cells and negative controls, which demonstrated that IL-9
produced by CLL cells itself could also inhibit CLL cell apoptosis.
Pretreatment with WP1066 could abolish the anti-apoptotic effects
of rIL-9 on transfected MEC-1 cells (Fig. 4D and F).
Discussion
In hematological malignancies, although aberrant
expression of IL-9 has been observed, the role of IL-9 in the
pathological significance of CLL has not been reported. In our
previous study, the results revealed an elevated level of IL-9 in
CLL patients. Upregulation of serum IL-9 is significantly
associated with the clinical stage, ZAP-70, B2M and IgVH in CLL
patients (17,18). In the present study, high expression
of pSTAT3, miR-155 and miR-21 were observed in PBMCs from CLL
patients. Furthermore, there was a complex link among IL-9, STAT3,
miR-155 and miR-21. Collectively, our results indicated novel
mechanisms involved in CLL pathology: i) extracellular IL-9 could
promote the generation of IL-9 in CLL cells; and ii) there is a
novel ‘extracellular IL-9/pSTAT3/miR-155/miR-21/intracellular IL-9’
positive feedback system in CLL cells.
IL-9 is a member of the common γ-chain family of
cytokines, and the IL-9receptor consists of the combination of the
cytokine-specific receptor IL-9 receptor-α (IL-9Rα) and the γ-chain
(9). In addition to its role in
immune responses, its growth and anti-apoptotic activities on
multiple transformed cells suggest potential effects in
hematological malignancies. The dysregulated expression of IL-9 has
been detected in biopsies or serum of patients with some
hematological malignancies, such as adult T-cell leukemia (ATL),
Hodgkin's disease (HD), an aplastic large cell lymphoma (ALCL), and
natural killer T (NKT)-cell lymphoma. All these studies provided
evidence that IL-9 plays an important role in pathogenesis of
hematological malignancies (20–22).
Our previous study indicated that IL-9 could contribute to CLL
progression (17,18). However, the pathogenic role of IL-9
in CLL remains largely unknown.
Research findings from cell culture and animal
models have revealed that the Jak/STAT signaling pathway, which can
be activated by many cytokines, including IL-9, plays a critical
role in hematological malignancies (23,24).
STAT3 mutations are consistent with the pathogenesis of chronic
lympho-proliferative disorders of NK cells and T-cell large
granular lymphocyte leukemia (25).
Mutations were found in exons 20 and 21, encoding the Src homology
2 domain. In acute myeloid leukemia cells, murine plasmacytomas,
and hybridomas, the autocrine secretion of interleukin-6 caused
phosphorylation of STAT3, Tyr705 and Ser727 (9,26),
including STAT3-mediated constitutive expression of SOCS-3 in
cutaneous T-cell lymphoma (27). In
addition, STAT3 also plays a pivotal role in the progression of
hematological malignancies, including CLL (28–30).
In the present study, our results revealed that pSTAT3 expression
was elevated in PBMCs from CLL patients. Moreover, extracellular
rIL-9 could induce STAT3 phosphorylation in the MEC-1 cell line.
The rIL-9-mediated pSTAT3 expression may be related to the
pathogenic role of CLL. Many studies have shown that the IL-9Rα
chain promoted the phosphorylation of JAK1 mutant and activation of
STAT, including STAT1, STAT3 and STAT5 (9,31,32)
and-IL-9 could not be expressed by Th2 and Th9 cells without
expression of STAT6 (33). To
assess the association between pSTAT3 and IL-9, MEC-1 cells were
chosen for our study. We found that extracellular IL-9 could not
only promote STAT3 phosphorylation but also increase IL-9
production by MEC-1 cells.
Since miR levels are dysregulated in CLL and STATs
are activated in CLL, we attempted to determine whether STATs
affect the transcription of miRNA genes in CLL cells. Recently,
studies revealed that STAT3 stimulation induced miR-155 and miR-21
upregulation in CLL cells (15,16).
In our study, we also demonstrated that miR-155 and miR-21
expression was upregulated in PBMCs from CLL patients. The changes
in miR-155 and miR-21 expression were accompanied by a substantial
increase of IL-9 expression. Furthermore, blocking STAT3 in
miR-155- and miR-21-transfected cells was associated with a
reduction of IL-9. These data revealed that IL-9 production induced
by pSTAT3 was mediated by miR-155 and miR-21. Since extracellular
IL-9 could not only promote STAT3 phosphorylation but also increase
IL-9 production in MEC-1 cells, we concluded that there was a novel
‘extracellular IL-9/pSTAT3/miR-155/miR-21-intracellular IL-9’
positive feedback system in CLL cells.
To explore the potential function of IL-9 in CLL
pathogenesis, we investigated the effects of rIL-9 on the
proliferation and apoptosis of CLL cells. After treatment with
rIL-9, the proliferation of MEC-1 cells increased, whereas their
apoptosis decreased. Also, STAT3 inhibitor could significantly
counteract the effects of rIL-9. Moreover, the proliferative
effects of rIL-9 on miR-155/miR-21-transfected MEC-1 cells were
more apparent than in MEC-1 cells. Although it was difficult to
determine whether the rIL-9 or the IL-9 produced by MEC-1 cells
played the dominant role in this process, the upregulation of IL-9
may play a role in the pathogenesis of CLL.
Collectively, we found that extracellular IL-9 could
not only activate the transcription of the STAT3 gene but also
upregulate the expression of miR-155 and miR-21. Consequently, the
STAT3-mediated expression of miR-155 and miR-21 promoted IL-9
secretion. Our findings provide a new explanation of the possible
molecular mechanism in the regulation of IL-9 in CLL. It may
provide valuable insights in understanding the cross-talk pathways
among IL-9, STAT3, miR-155 and miR-21, which may play a key role in
the pathogenesis of CLL.
The data we present here explored the cross-talk
pathways among IL-9, STAT3, miR-155 and miR-21in CLL. Indeed,
recent research has shown that IL-9 could contribute to CLL
progression. Our study provides evidence that the expression of
pSTAT3, miR-155 and miR-21 was increased in CLL with IL-9
upregulation. The STAT3 phosphorylation in MEC-1 cells stimulated
by rIL-9 was associated with a time-dependent increase in IL-9
secretion. Pretreatment with WP1066 eliminated the IL-9 secretion
despite the presence of rIL-9. IL-9 protein levels were increased
in miR-155-transfected MEC-1 cells and miR-21-transfected MEC-1
cells treated with rIL-9 at 120 min compared with untransfected
cells, which could be blocked by WP1066. In transfected MEC-1 cells
apoptosis inhibition and the proliferation enhanced by rIL-9 could
be blocked by WP1066. This perspective is particularly intriguing
in that extracellular IL-9 appears to not only activate the
transcription of the STAT3 gene but also to upregulate miR-155 and
miR-21 expression. Consequently, the STAT3-mediated translational
upregulation of miR-155 and miR-21 promoted IL-9 secretion.
Acknowledgements
Not applicable.
Funding
This study was partly supported by the National
Natural Science Foundation (nos. 81270598 and 81500124), the
Natural Science Foundations of Shandong Province (nos. Y2007C053,
2009ZRB14176 and ZR2012HZ003), the Technology Development Projects
of Shandong Province (nos. 2007GG10 and 2010GSF10250), the Major
Research Projects of Shandong Province (no. 2017GSF218007), the
Program of Shandong Medical Leading Talent, and the Taishan Scholar
Foundation of Shandong Province.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author upon reasonable
request.
Authors' contributions
CN and WX conceived and designed the study. CN, FLL
and LK performed the experiments. CN and LX wrote the paper. CN,
QHT, LPP and WX reviewed and edited the manuscript. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
The protocol was approved by the Shandong Provincial
Hospital Ethics Committee, and written informed consent was
obtained from all participants involved in this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CLL
|
chronic lymphocytic leukemia
|
|
pSTAT
|
phosphorylation of signal transducer
and activator of transcription
|
|
miRs
|
microRNAs
|
|
PBMCs
|
peripheral blood mononuclear cells
|
|
rIL-9
|
recombinant human IL-9
|
|
FITC
|
fluorescein isothiocyanate
|
|
PI
|
Annexin V and propidium iodide
|
|
ATL
|
adult T-cell leukemia
|
|
HD
|
Hodgkin's disease
|
|
ALCL
|
anaplastic large cell lymphoma
|
|
NKT
|
natural killer T cells
|
References
|
1
|
Negro R, Gobessi S, Longo PG, He Y, Zhang
ZY, Laurenti L and Efremov DG: Overexpression of the
autoimmunity-associated phosphatase PTPN22 promotes survival of
antigen-stimulated CLL cells by selectively activating AKT. Blood.
119:6278–6287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oi T, Asanuma K, Matsumine A, Matsubara T,
Nakamura T, Iino T, Asanuma Y, Goto M, Okuno K, Kakimoto T, et al:
STAT3 inhibitor, cucurbitacin I, is a novel therapeutic agent for
osteosarcoma. Int J Oncol. 49:2275–2284. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fecteau JF, Messmer D, Zhang S, Cui B,
Chen L and Kipps TJ: Impact of oxygen concentration on growth of
mesenchymal stromal cells from the marrow of patients with chronic
lymphocytic leukemia. Blood. 121:971–974. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rawstron AC, Böttcher S, Letestu R,
Villamor N, Fazi C, Kartsios H, de Tute RM, Shingles J, Ritgen M,
Moreno C, et al: European Research Initiative in CLL: Improving
efficiency and sensitivity: European Research Initiative in CLL
(ERIC) update on the international harmonised approach for flow
cytometric residual disease monitoring in CLL. Leukemia.
27:142–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Samuel S, Beljanski V, Van Grevenynghe J,
Richards S, Ben Yebdri F, He Z, Nichols C, Belgnaoui SM, Steel C,
Goulet ML, et al: BCL-2 inhibitors sensitize therapy-resistant
chronic lymphocytic leukemia cells to VSV oncolysis. Mol Ther.
21:1413–1423. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fang C, Zhuang Y, Wang L, Fan L, Wu YJ,
Zhang R, Zou ZJ, Zhang LN, Yang S, Xu W, et al: High levels of CD20
expression predict good prognosis in chronic lymphocytic leukemia.
Cancer Sci. 104:996–1001. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Soroosh P and Doherty TA: Th9 and allergic
disease. Immunology. 127:450–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tete S, Saggini A, Maccauro G, Rosati M,
Conti F, Cianchetti E, Tripodi D, Toniato E, Fulcheri M, Salini V,
et al: Interleukin-9 and mast cells. J Biol Regul Homeost Agents.
26:319–326. 2012.PubMed/NCBI
|
|
9
|
Hornakova T, Staerk J, Royer Y, Flex E,
Tartaglia M, Constantinescu SN, Knoops L and Renauld JC: Acute
lymphoblastic leukemia-associated JAK1 mutants activate the Janus
kinase/STAT pathway via interleukin-9 receptor alpha homodimers. J
Biol Chem. 284:6773–6781. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fionda C, Malgarini G, Soriani A, Zingoni
A, Cecere F, Iannitto ML, Ricciardi MR, Federico V, Petrucci MT,
Santoni A, et al: Inhibition of glycogen synthase kinase-3
increases NKG2D ligand MICA expression and sensitivity to NK
cell-mediated cytotoxicity in multiple myeloma cells: Role of
STAT3. J Immunol. 190:6662–6672. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen DL, Wang DS, Wu WJ, Zeng ZL, Luo HY,
Qiu MZ, Ren C, Zhang DS, Wang ZQ, Wang FH, et al: Overexpression of
paxillin induced by miR-137 suppression promotes tumor progression
and metastasis in colorectal cancer. Carcinogenesis. 34:803–811.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk
ML and Struhl K: STAT3 activation of miR-21 and miR-181b-1 via PTEN
and CYLD are part of the epigenetic switch linking inflammation to
cancer. Mol Cell. 39:493–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nakamura H, Taguchi A, Kawana K, Kawata A,
Yoshida M, Fujimoto A, Ogishima J, Sato M, Inoue T, Nishida H, et
al: STAT3 activity regulates sensitivity to tumor necrosis
factor-related apoptosis-inducing ligand-induced apoptosis in
cervical cancer cells. Int J Oncol. 49:2155–2162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hartman ML and Kilianska ZM: Lipoprotein
lipase: A new prognostic factor in chronic lymphocytic leukaemia.
Contemp Oncol (Pozn). 16:474–479. 2012.PubMed/NCBI
|
|
16
|
ENCODE Project Consortium, . Myers RM,
Stamatoyannopoulos J, Snyder M, Dunham I, Hardison RC, Bernstein
BE, Gingeras TR, Kent WJ, Birney E, Wold B, et al: A user's guide
to the encyclopedia of DNA elements (ENCODE). PLoS Biol.
9:e10010462011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen N, Lv X, Li P, Lu K and Wang X: Role
of high expression of IL-9 in prognosis of CLL. Int J Clin Exp
Pathol. 7:716–721. 2014.PubMed/NCBI
|
|
18
|
Chen N, Lu K, Li P, Lv X and Wang X:
Overexpression of IL-9 induced by STAT6 activation promotes the
pathogenesis of chronic lymphocytic leukemia. Int J Clin Exp
Pathol. 7:2319–2323. 2014.PubMed/NCBI
|
|
19
|
Barski A, Cuddapah S, Cui K, Roh TY,
Schones DE, Wang Z, Wei G, Chepelev I and Zhao K: High-resolution
profiling of histone methylations in the human genome. Cell.
129:823–837. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen J, Petrus M, Bryant BR, Nguyen Phuc
V, Stamer M, Goldman CK, Bamford R, Morris JC, Janik JE and
Waldmann TA: Induction of the IL-9 gene by HTLV-I Tax stimulates
the spontaneous proliferation of primary adult T-cell leukemia
cells by a paracrine mechanism. Blood. 111:5163–5172. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Matsushita K, Arima N, Ohtsubo H, Fujiwara
H, Hidaka S, Fukumori J and Tanaka H: Frequent expression of
interleukin-9 mRNA and infrequent involvement of interleukin-9 in
proliferation of primary adult T-cell leukemia cells and HTLV-I
infected T-cell lines. Leuk Res. 21:211–216. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ju W, Zhang M, Jiang JK, Thomas CJ, Oh U,
Bryant BR, Chen J, Sato N, Tagaya Y, Morris JC, et al: CP-690,550,
a therapeutic agent, inhibits cytokine-mediated Jak3 activation and
proliferation of T cells from patients with ATL and HAM/TSP. Blood.
117:1938–1946. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schuringa JJ, Wierenga AT, Kruijer W and
Vellenga E: Constitutive Stat3, Tyr705, and Ser727 phosphorylation
in acute myeloid leukemia cells caused by the autocrine secretion
of interleukin-6. Blood. 95:3765–3770. 2000.PubMed/NCBI
|
|
24
|
Brender C, Nielsen M, Kaltoft K, Mikkelsen
G, Zhang Q, Wasik M, Billestrup N and Odum N: STAT3-mediated
constitutive expression of SOCS-3 in cutaneous T-cell lymphoma.
Blood. 97:1056–1062. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Steele AJ, Prentice AG, Cwynarski K,
Hoffbrand AV, Hart SM, Lowdell MW, Samuel ER and Wickremasinghe RG:
The JAK3-selective inhibitor PF-956980 reverses the resistance to
cytotoxic agents induced by interleukin-4 treatment of chronic
lymphocytic leukemia cells: Potential for reversal of
cytoprotection by the microenvironment. Blood. 116:4569–4577. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goswami R, Jabeen R, Yagi R, Pham D, Zhu
J, Goenka S and Kaplan MH: STAT6-dependent regulation of Th9
development. J Immunol. 188:968–975. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rozovski U, Calin GA, Setoyama T, D'Abundo
L, Harris DM, Li P, Liu Z, Grgurevic S, Ferrajoli A, Faderl S, et
al: Signal transducer and activator of transcription (STAT)-3
regulates microRNA gene expression in chronic lymphocytic leukemia
cells. Mol Cancer. 12:502013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li P, Grgurevic S, Liu Z, Harris D,
Rozovski U, Calin GA, Keating MJ and Estrov Z: Signal transducer
and activator of transcription-3 induces microRNA-155 expression in
chronic lymphocytic leukemia. PLoS One. 8:e646782013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shaim H, Estrov Z, Harris D, Sanabria
Hernandez M, Liu Z, Ruvolo P, Thompson PA, Ferrajoli A, Daher M,
Burger J, et al: The CXCR4-STAT3-IL-10 pathway controls the
immunoregulatory function of chronic lymphocytic leukemia and is
modulated by lenalidomide. Front Immunol. 8:17732018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
He S, Liao G, Liu Y, Huang L, Kang M and
Chen L: Overexpression of STAT3/pSTAT3 was associated with poor
prognosis in gastric cancer: A meta-analysis. Int J Clin Exp Med.
8:20014–20023. 2015.PubMed/NCBI
|
|
31
|
Carretero R, Wang E, Rodriguez AI,
Reinboth J, Ascierto ML, Engle AM, Liu H, Camacho FM, Marincola FM,
Garrido F, et al: Regression of melanoma metastases after
immunotherapy is associated with activation of antigen presentation
and interferon-mediated rejection genes. Int J Cancer. 131:387–395.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Randhawa J, Ostojic A, Vrhovac R, Atallah
E and Verstovsek S: Splenomegaly in myelofibrosis - new options for
therapy and the therapeutic potential of Janus kinase 2 inhibitors.
J Hematol Oncol. 5:432012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mathé E, Nguyen GH, Funamizu N, He P,
Moake M, Croce CM and Hussain SP: Inflammation regulates microRNA
expression in cooperation with p53 and nitric oxide. Int J Cancer.
131:760–765. 2012. View Article : Google Scholar : PubMed/NCBI
|