Introduction
Renal cell carcinoma (RCC) is commonly diagnosed in
urological malignant tumors. In 2016, the American Cancer Society
estimated that the number of new kidney cancer cases would reach
almost 62,000 in the United States, accounting for 1 in 20 new
diagnoses in men and 3% for women (1). An estimated 14,000 Americans will
succumb to kidney cancer this year. In China, RCC is also one of
the common diseases in urology. Projected age-standardized
incidence rate (per 100,000) of kidney cancer reaches 2.4 in males
and 1.0 in females (2). Although
scientists have not identified exact causes of RCC occurrence,
accumulating evidence suggests that obesity, hypertension, smoking,
alcohol, occupational exposure to trichloroethylene, and genetic
factors are risk factors for this disease (3).
ALDOA, also named ALDA, GSD12 and
HEL-S-87p, encodes the protein aldolase A (or
fructose-bisphosphate aldolase), which is a glycolytic enzyme that
catalyzes reversible conversion of fructose-1,6-bisphosphate to
glyceraldehyde 3-phosphate and dihydroxyacetone phosphate. Three
different genes encode three aldolase isozymes (A, B and C).
Aldolase A is the major aldolase in early embryos and adult muscles
and is expressed lowly in adult liver, kidneys, and intestines
(4). Aldolase A deficiency is
associated with hemolytic anemia and severe rhabdomyolysis
(5–7). High expression of ALDOA is related to
lung squamous cell carcinoma (8),
highly metastatic pancreatic cancer (9) and colorectal cancer (10). Researchers discovered that RCC
patients feature elevated serum aldolase A compared with those with
other urological tumors and benign urological diseases (11). A recent study identified ALDOA as
candidate marker of late-stage clear cell RCC (12). However, further studies are still
warranted to determine the mechanism of tumorgenesis and
progression with aberrant ALDOA expression in RCC.
ALDOA has been reported to promote
epithelial-mesenchymal transition (EMT) and migration in lung
squamous cell carcinoma (8). EMT
has been widely demonstrated to contribute to cancer dissemination
and progression. EMT causes downregulation of epithelial markers,
most notably E-cadherin, and upregulation of mesenchymal markers,
such as N-cadherin and vimentin (13). Kidney organogenesis involves
mesenchymal-epithelial transition from original mesenchymal cells
and formation of renal vesicles and tubules and maturation of
nephrons (14). In RCC, this
transition reverses, leading to EMT and dedifferentiation. The
Wnt/β-catenin signaling pathway is one of the major signaling
pathways involved in RCC (15).
β-catenin, a transcriptional coactivator, emerges as a key molecule
in canonical Wnt signaling. Caspi et al reported that ALDOA
regulates the Wnt signaling pathway (16).
In the present study, we aimed to determine the
expression and function of ALDOA in RCC tissues and cells and its
possible mechanism utilizing small interfering RNA (siRNA) and
overexpression plasmids. Our results revealed that downregulated
ALDOA expression could affect aberrant expression of E-cadherin,
N-cadherin, and vimentin in RCC cells and inactivate the
Wnt/β-catenin signaling pathway. These observations revealed that
ALDOA may be a novel biomarker and provide a potential therapeutic
strategy for treatment of RCC.
Materials and methods
Patients and tissue microarray
(TMA)
A total of 139 RCC tissues were obtained from
patients who were treated by radical nephrectomy or partial
nephrectomy between February 2008 and May 2011 at the First
Affiliated Hospital of Nanjing Medical University (Nanjing, China).
Pathologists confirmed identification of tumor tissues. None of the
patients had been treated by either radiotherapy or chemotherapy.
This study was approved by the Medical Ethics Committee of the
hospital. Table I summarizes
clinical and pathological features of 139 patients.
 | Table I.Association between ALDOA expression
and clinicopathological factors in 139 RCC patients. |
Table I.
Association between ALDOA expression
and clinicopathological factors in 139 RCC patients.
|
| ALDOA
expression |
|
|
|---|
|
|
|
|
|
|---|
| Variables | Negative (−) | Positive (+) | Strong positive
(++) | P-value | n | Proportion (%) |
|---|
| Sex |
|
|
| 0.004 |
|
|
|
Male | 6 | 41 | 37 |
| 84 | 60.4 |
|
Female | 11 | 13 | 31 |
| 55 | 39.6 |
| Age, median
(range), years | 55 (37–75) | 55 (20–86) | 57 (27–81) | 0.763 | 56 |
|
| Side |
|
|
| 0.621 |
|
|
|
Left | 9 | 24 | 36 |
| 69 | 49.6 |
|
Right | 8 | 30 | 32 |
| 70 | 50.4 |
| Surgical
procedure |
|
|
| 0.175 |
|
|
| Partial
nephrectomy | 2 | 11 | 21 |
| 34 | 24.5 |
| Radical
nephrectomy | 15 | 43 | 47 |
| 105 | 75.5 |
| Pathological
type |
|
|
| 0.026 |
|
|
| Clear
cell renal cell carcinoma | 17 | 53 | 56 |
| 126 | 90.6 |
|
Papillary carcinoma | 0 | 0 | 5 |
| 5 |
3.6 |
|
Chromophobe renal cell
carcinoma | 0 | 1 | 6 |
| 7 |
5.0 |
|
Other | 0 | 0 | 1 |
| 1 |
0.7 |
| Maximum diameter of
the tumor, cm |
|
|
| 0.452 |
|
|
| ≤4 | 10 | 25 | 35 |
| 70 | 50.4 |
|
>4–7 | 5 | 22 | 23 |
| 50 | 36.0 |
|
>7–10 | 1 | 7 | 7 |
| 15 | 10.8 |
|
>10 | 1 | 0 | 3 |
| 4 |
2.9 |
| Metastasis |
|
|
| 0.020 |
|
|
|
Yes | 2 | 1 | 12 |
| 15 | 10.8 |
| No | 15 | 53 | 56 |
| 124 | 89.2 |
| Histological
grade |
|
|
| 0.033 |
|
|
| Highly
differentiated | 15 | 42 | 45 |
| 102 | 73.4 |
|
Moderately differentiated | 1 | 12 | 18 |
| 31 | 22.3 |
| Poorly
differentiated | 1 | 0 | 5 |
| 6 |
4.3 |
TMAs were constructed using the aforementioned 139
RCC tissues. All tissues were pathologically confirmed as RCC. An
experienced pathologist reviewed hematoxylin and eosin slides
again. Representative areas of specimens were identified and
marked. Tissue cores measuring 2 mm from marked areas were selected
from donor blocks and transferred to recipient paraffin blocks of
TMA. Paraffin blocks were sectioned to produce serial 4-µm
sections. Then, immunohistochemical studies were performed on
positively-charged slides mounted with 4 µm of TMA paraffin
blocks.
Immunohistochemistry
Sections from TMA paraffin blocks were removed from
the incubator and successively dewaxed in xylene I and xylene II
for 10 min. Subsequently, the slides were rehydrated with
sequential ethanol washes, starting at 100%, followed by 95%, 85%,
and 75%, and then incubated for 10 min in 3%
H2O2 to block endogenous peroxidase. Antigen
retrieval was performed for 10 min by a steam pressure cooker
containing citrate buffer. Then, the samples were blocked with
5–10% animal serum for 10 min and incubated with the appropriate
antibody against ALDOA monoclonal antibody (1:100; cat. no.
H00000226-M02; Abnova, Tapei, Taiwan) at 37°C for 2 h and 4°C
overnight. After 5 min of washing with phosphate-buffered saline
(PBS) thrice, slides were cultured in peroxidase-conjugated goat
anti-mouse IgG (H+L) secondary antibody (1:1,000; cat. no. ZB-2305;
ZSGB-BIO, Inc., Beijing, China) for 20 min. After two 5-min washes
in PBS, reactions were visualized with a fresh substrate solution
containing 3,3′-diaminobenzidine. Sections were counterstained with
hematoxylin, dehydrated and dewaxed. Slides were sealed and
analyzed by optical microscopy.
Evaluation of staining
Evaluation of protein staining was performed
independently by two experienced pathologists who had no knowledge
of obtained clinical and pathological data. Results of
immunostaining for ALDOA were determined according to a previously
described scoring system (17,18).
Percentages of positive tumor cells were estimated in at least five
areas at an ×400 magnification and assigned with one of the
following quantitative scores: 0, 0–5%; 1, 6–25%; 2, 26–50%; 3,
51–75%; and 4, 76–100%. Intensity of positive staining was scored
as follows: 1, low; 2, moderate; and 3, strong. Finally, a total
score (0–12) for each sample was calculated by multiplying the
quantitative score by the intensity score. Scores 0 to 4, 5 to 8,
and 9 to 12 indicated negative (−), positive (+), and strong
positive (++) expression of ALDOA, respectively.
Cell culture and tissue samples
Human RCC cell lines (769-P, 786-0, ACHN and Caki-1)
and normal renal proximal tubular cells (HK-2) were purchased from
the Cell Bank Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China). Cells of 769-P and 786-0 lines were
maintained in Roswell Park Memorial Institute (RPMI)-1640 (Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), ACHN and HK-2
cells were maintained in Dulbecco's modified Eagle's medium, and
Caki-1 was cultured in McCoy's 5A (both from Gibco; Thermo Fisher
Scientific, Inc.). All media were supplemented with 10% fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) within a
humidified atmosphere containing 5% CO2 at 37°C.
Following the Local Ethics Committee of the First
Affiliated Hospital of Nanjing Medical University (Nanjing, China),
21 paired tumor specimens and normal tissue samples for the
detection of ALDOA expression were obtained with informed consent
from RCC patients who had undergone radical nephrectomy or partial
nephrectomy at the Department of Urology of the First Affiliated
Hospital of Nanjing Medical University (Nanjing, China). Fresh
samples were obtained during surgery, immediately frozen in liquid
nitrogen, and stored at −80°C until further analysis.
Identification of tumor tissues and adjacent normal tissues was
confirmed by histopathological examination.
Cell transfection
Cells from 786-0, Caki-1 and 769-P lines were seeded
at a density of 1×106 cells/well in 6-well plates at 70%
confluence on the day before transfection. 786-0 and Caki-1 cell
transfection was performed with Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. Cells grown in 6-well plates were transfected with
100 pM of synthetic ALDOA siRNA or negative control (NC). 769-P
cells were transfected with ALDOA overexpression plasmids or vector
controls using DNAfectin™ Plus (Applied Biological Materials (ABM),
Richmond, BC, Canada), according to the manufacturer's
instructions. Cells overexpressing ALDOA were defined as the OV
group, while cells transfected with the vector alone were defined
as the NC group. Six hours post-transfection with siRNA or NC and
16 h post-transfection with overexpression plasmids or vector
controls, culture medium was replaced with RPMI-1640 or McCoy's 5A
containing FBS. Table II contains
the sequences of ALDOA siRNA and NC. All used siRNA and NC were
designed and synthesized by Shanghai GenePharma Co., Ltd,.,
(Shanghai, China). ALDOA overexpression plasmids were constructed
and purchased from Obio Technology Ltd. (Shanghai, China). Total
RNA was collected 24 h after transfection and used for reverse
transcription-polymerase chain reaction (RT-PCR) analysis to
evaluate ALDOA expression. Total protein was prepared 60 h after
transfection and used for western blot analysis. Other parts of the
cells were used for cell proliferation, cell cycle, cell colony
formation, and cell migration and invasion assays.
 | Table II.The sequences of siRNA and NC. |
Table II.
The sequences of siRNA and NC.
| Name |
| Sequences |
|---|
| ALDOA-siRNA-1 | Sense |
5′-GCCUUGCCUGUCAAGGAAATT-3′ |
|
| Anti-sense |
5′-UUUCCUUGACAGGCAAGGCTT-3′ |
| ALDOA-siRNA-2 | Sense |
5′-GCGUUGUGUGCUGAAGAUUTT-3′ |
|
| Anti-sense |
5′-AAUCUUCAGCACACAACGCTT-3′ |
| ALDOA-siRNA-3 | Sense |
5′-GCCAGUAUGUGACCGAGAATT-3′ |
|
| Anti-sense |
5′-UUCUCGGUCACAUACUGGCTT-3′ |
| Negative control
(NC) | Sense |
5′-UUCUCCGAACGUGUCACGUTT-3′ |
|
| Anti-sense |
5′-ACGUGACACGUUCGGAGAATT-3′ |
RNA isolation and RT-PCR
Total RNA was isolated from tissue samples or
cultured cell lines by using TRIzol (Invitrogen; Thermo Scientific,
Inc.) according to the manufacturer's instructions. Total RNA was
reverse-transcribed into cDNAs using PrimeScript™ RT Master Mix
(Perfect Real-Time) (Takara Biotechnology, Co., Ltd., Dalian,
China) in accordance with the manufacturer's instructions.
Conditions of reverse transcription were as follows: 37°C for 15
min, 85°C for 5 sec, and 4°C until the end of the procedure. RNA
and cDNA concentrations were assessed by NanoDrop (Thermo Fisher
Scientific, Inc.). ALDOA expression was analyzed by RT-PCR using
SYBR Green assay in accordance with the manufacturer's instructions
(Applied Biosystems; Thermo Fisher Scientific, Inc.). Relative
expression of ALDOA was calculated using the 2−∆∆Ct
method. The following primer sequences were used: ALDOA: forward
5′-CGGGAAGAAGGAGAACCTG-3′ and reverse 5′-GACCGCTCGGAGTGTACTTT-3′;
and β-actin: forward 5′-ACTGGAACGGTGAAGGTGAC-3′ and reverse
5′-AGAGAAGTGGGGTGGCTTTT-3′ (synthesized by Invitrogen, Shanghai,
China). RT-PCR was performed under the following conditions: 50°C
for 2 min, 95°C for 2 min; 40 cycles at 95°C for 15 sec and 60°C
for 1 min; and 95°C for 15 sec, 60°C for 1 min and 95°C for 15 sec.
Reactions were performed and analyzed by Applied Biosystems StepOne
Plus Real-Time PCR System (Applied Biosystems; Thermo Fisher
Scientific, Inc.). All reactions were run in triplicate.
Cell proliferation assay
Cells of 786-0, Caki-1 and 769-P lines were
transfected to investigate the influence of ALDOA on the
proliferative capacity of RCC cells. After 48 h of transfection,
the cells were seeded onto 96-well plates at a density of
2×103 cells/well and cultured for 24, 48, 72 and 96 h.
Cell proliferation was determined using Cell Counting Kit-8 (CCK-8;
Dojindo Molecular Technologies, Kumamoto, Japan) following the
manufacturer's protocol. Absorbance was detected at an optical
density (OD) of 450 nm by a spectrophotometer. In each group, three
wells were assessed at each time-point.
Cell cycle assay
For the cell cycle analysis, 1×105 cells
were harvested 48 h after transfection, washed twice with ice-cold
PBS, and fixed with 70% ethanol at −20°C overnight. Cells were
incubated with 50 mg/ml of propidium iodide and 1 mg/ml RNase for
30 min in the dark at room temperature. Using flow cytometry,
treated cells were analyzed to determine distribution of cell cycle
stages after transfection. At least 100,000 cells were necessary
for each sample. Experiments were performed in triplicate.
Colony formation assay
To determine the long-term effects of ALDOA on cell
colony formation, 48 h after transfection, the cells were seeded in
culture dishes at a density of 1×103 cells/dish and
cultured at 37°C with medium exchange. Cells were grown for 10 days
to form colonies contained more than 50 cells, which were stained
with crystal violet, photographed, and counted under a
fluorescence-inverted microscope (×100 magnification).
Cell migration and invasion
assays
To investigate the possible effects of ALDOA on
metastasis of RCC cells, migration and invasion assays were
performed after transfection for 48 h. For the migration assays,
2×104 cells in 200 µl of serum-free medium were placed
in the upper chamber of the Transwell (pore size, 8 µm; BD
Biosciences, San Jose, CA, USA). For the invasion assays,
5×104 cells in 200 µl of serum-free medium were placed
in the upper chamber coated with Matrigel (BD Biosciences) in
accordance with the manufacturer's protocol. The lower chamber was
filled with 500 µl of media containing 20% FBS. After incubating
the cells for 24 h at 37°C, the cells remaining in the upper
membrane were removed by cotton swab, and those on the lower
membrane surface were fixed in methanol and stained with crystal
violet. Five random fields were photographed and counted under an
optical microscope (×200 magnification). All experiments were
performed in triplicate.
Protein isolation and western blot
analysis
To further study the effects of ALDOA on protein
changes in signaling pathways related to cell proliferation and
metastasis in RCC cells, we isolated proteins from transfected
cells and performed a western blot assay. Cells were washed thrice
in PBS and lysed using radioimmunoprecipitation assay buffer
(KeyGen Biotech Co., Ltd., Nanjing, Jiangsu, China) supplemented
with protease inhibitors at 4°C for 30 min. The protein
concentration was measured using a BCA kit (Beyotime Institute of
Biotechnology, Nantong, Jiangsu, China), according to the
manufacturer's instructions. The same amounts of proteins were
electrophoresed in 10% sodium dodecyl sulfate polyacrylamide gel,
transferred to a polyvinylidene fluoride membrane (EMD Millipore,
Billerica, MA, USA), blocked for 2 h with 5% non-fat milk at room
temperature, and incubated with primary antibodies at 4°C
overnight. Then, the membrane was incubated with horseradish
peroxidase-conjugated secondary antibody for 2 h after washing
thrice with Tris-buffered saline and 0.1% Tween-20. Antibodies
against ALDOA (1:400; cat. no. H00000226-M02; Abnova, Tapei,
Taiwan), actin (1:3,000; cat. no. ab179467; Abcam, Cambridge, UK),
E-cadherin (1:1,000; cat. no. 3195), N-cadherin (1:1,000; cat. no.
13116), vimentin (1:1,000; cat. no. 5741), β-catenin (1:1,000; cat.
no. 8480), phospho-β-catenin (1:1,000; cat. no. 9567), cyclin D1
(1:1,000; cat. no. 2978), Met (1:1,000; cat. no. 8198), matrix
metalloproteinase-7 (MMP-7) (1:1,000; cat. no. 3801) and c-Myc
(1:1,000; cat. no. 5605) (Cell Signaling Technology, Danvers, MA,
USA) were used in western blot analysis in accordance with the
manufacturer's instructions. Blots were detected using enhanced
chemiluminescence (Thermo Fisher Scientific, Inc.). The protein
levels were determined by normalization to actin.
Statistical analyses
SPSS Statistics 20 software package (IBM Corp.
Armonk, NY, USA) and GraphPad Prism 5 (GraphPad Software, Inc., La
Jolla, CA, USA) were used for statistical analysis. We used
Chi-square test to analyze the relationship between ALDOA
expression and clinicopathological factors. Univariate survival was
assessed using the Kaplan-Meier curve and log-rank test. Results
are presented as the mean ± standard deviation (SD). Differences
between two groups were analyzed using Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
High expression of ALDOA in RCC tumor
tissues
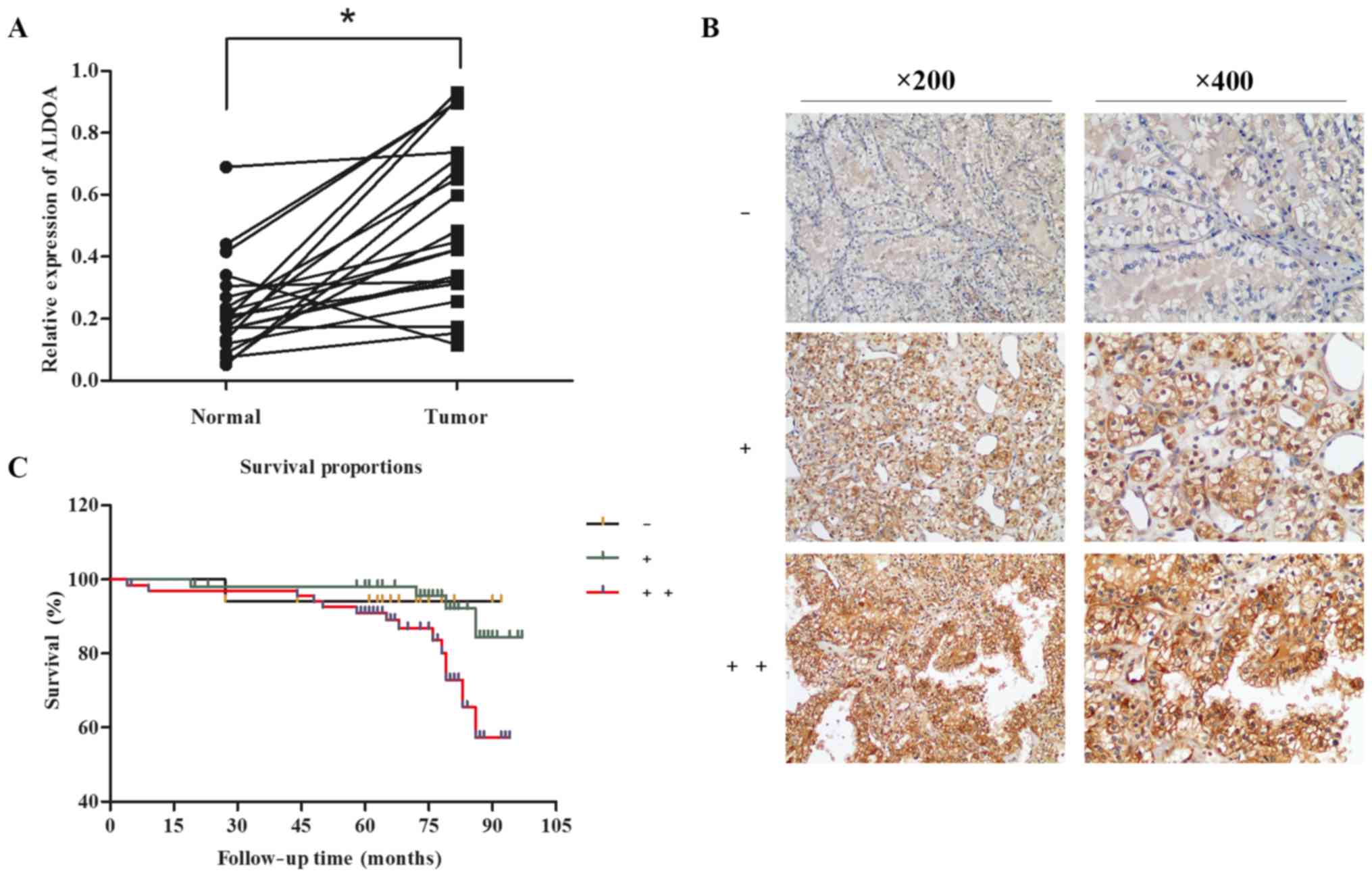
To detect ALDOA expression pattern in RCC, we first
examined mRNA levels of ALDOA in 21 patients diagnosed with the
disease. RT-PCR was performed to investigate ALDOA expression in 21
paired RCC tissues and adjacent non-tumor tissues. Compared with
paired adjacent non-tumor tissues, RCC samples presented
significantly upregulated ALDOA levels (20/21) (P<0.0001;
Fig. 1A). To investigate the
presence of similar changes at the translational level, we
determined ALDOA protein expression in 139 cases of RCC. The TMA of
139 RCC tissues and immunohistochemistry staining revealed that
percentages of positive and strong positive ALDOA expression
reached 38.8% (54/139) and 48.9% (68/139), respectively (ALDOA
expression rate = 87.8%, Fig.
1B).
Next, we used the Chi-square test to evaluate the
relationship of ALDOA expression and clinicopathological factors of
RCC. The results revealed the absence of a significant difference
between the ALDOA expression and constituent ratio in the different
groups of the maximum diameter of the tumor (P=0.452) (Table I). However, ALDOA expression was
significantly associated with metastasis (P=0.020) and histological
grade (P=0.033) (Table I).
Kaplan-Meier analysis of patient data revealed
notably shorter overall survival time of higher ALDOA-expressing
individuals than those with lower ALDOA expression (P=0.0341)
(Fig. 1C). The overall survival
rates of the negative, positive and strong positive ALDOA
expression groups were 94.1, 92.6 and 79.4%, respectively. Thus,
high expression of ALDOA in RCC patients contributed to advanced
RCC and poorer survival. Then, we carried out research on the
biological roles of ALDOA in RCC cell lines to further study the
aforementioned findings.
ALDOA is upregulated in RCC cell
lines
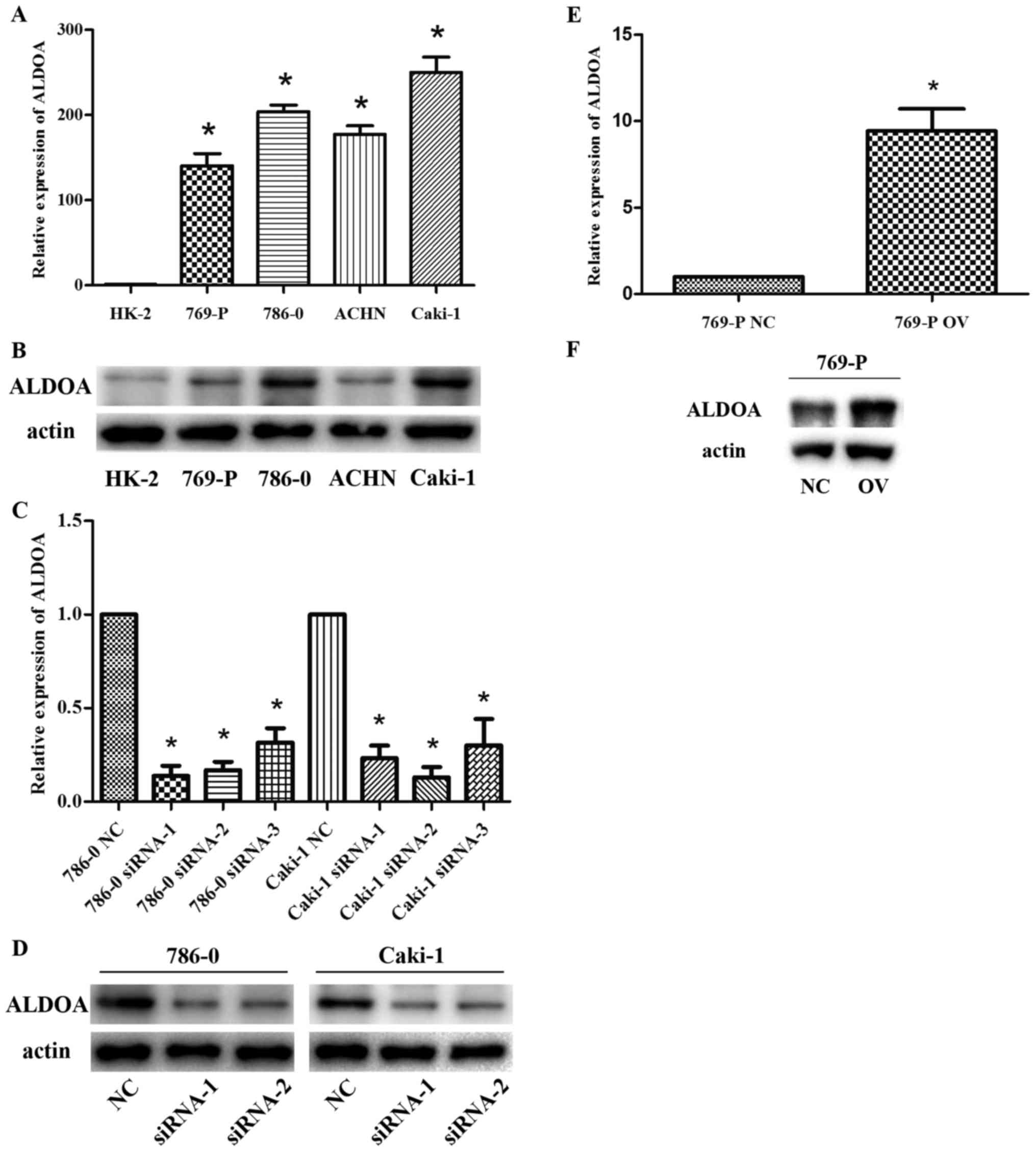
The expression level of ALDOA was determined in four
RCC cell lines (769-P, 786-0, ACHN and Caki-1) and in normal renal
proximal tubular cell line (HK-2) by RT-PCR and western blot
analysis. Expression of ALDOA was significantly higher in RCC cell
lines than in HK-2 cells (P<0.01; Fig. 2A and B). Among the four RCC cell
lines, 786-0 and Caki-1 exhibited the highest ALDOA level and 769-P
exhibited the lowest ALDOA level. Thus, 786-0, Caki-1 and 769-P
were selected for subsequent cell functional experiments.
To investigate the effects of ALDOA in RCC cells, we
knocked down ALDOA expression by transfecting siRNA and
overexpressed ALDOA by transfecting overexpression plasmids. ALDOA
expression in RCC samples was significantly lower with ALDOA-siRNA
transfection than that in NC (P<0.05; Fig. 2C and D) and markedly upregulated
after ALDOA overexpression plasmid transfection (P=0.008; Fig. 2E and F). Due to more efficient
transfection we used the first and second ALDOA-siRNA in the
following experiments.
ALDOA promotes cell proliferation of
RCC cells
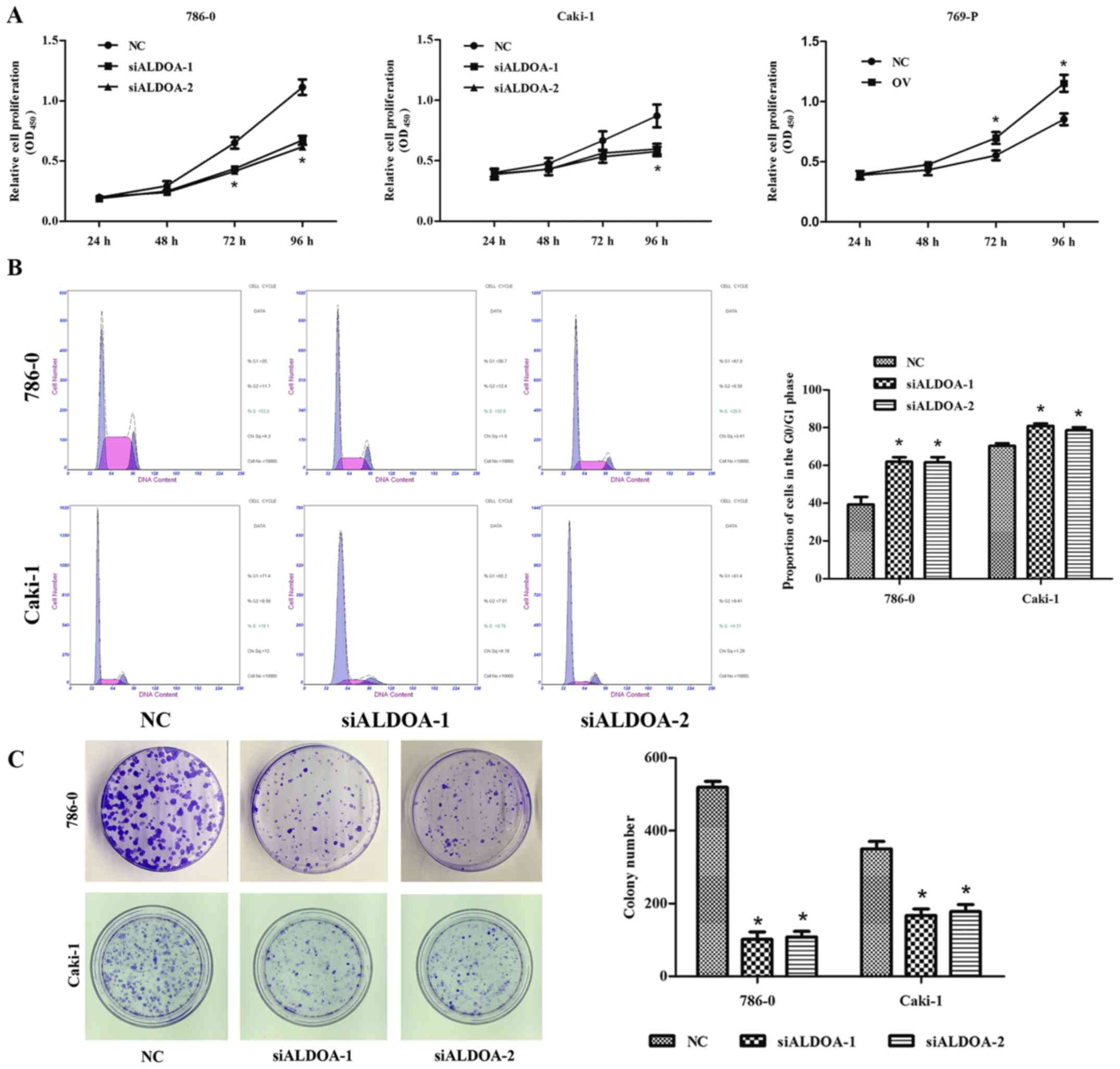
CCK-8 assay was performed to assess cell
proliferation in 786-0, Caki-1 and 769-P cells after transfection.
Compared with the NC group, a significant difference was detected
at 72 and 96 h in 786-0 and 769-P cells and at 96 h in Caki-1 cells
(P<0.05; Fig. 3A). Flow
cytometric analysis was performed to explore the effects of
inhibition on cell proliferation. Analysis revealed remarkably
higher percentages of 786-0 and Caki-1 cells transfected with
ALDOA-siRNA in the G0/G1 phase than those transfected with NC
siRNA, indicating that ALDOA can induce G0/G1 cell cycle arrest in
RCC cells (P<0.05; Fig. 3B).
ALDOA promotes colony formation of RCC
cells
Colony formation was observed after transfection
with ALDOA-siRNA and NC siRNA. Compared with the NC group,
downregulation of ALDOA significantly inhibited colony formation in
786-0 and Caki-1 cell lines (P<0.001; Fig. 3C).
ALDOA promotes migration and invasion
in RCC cells
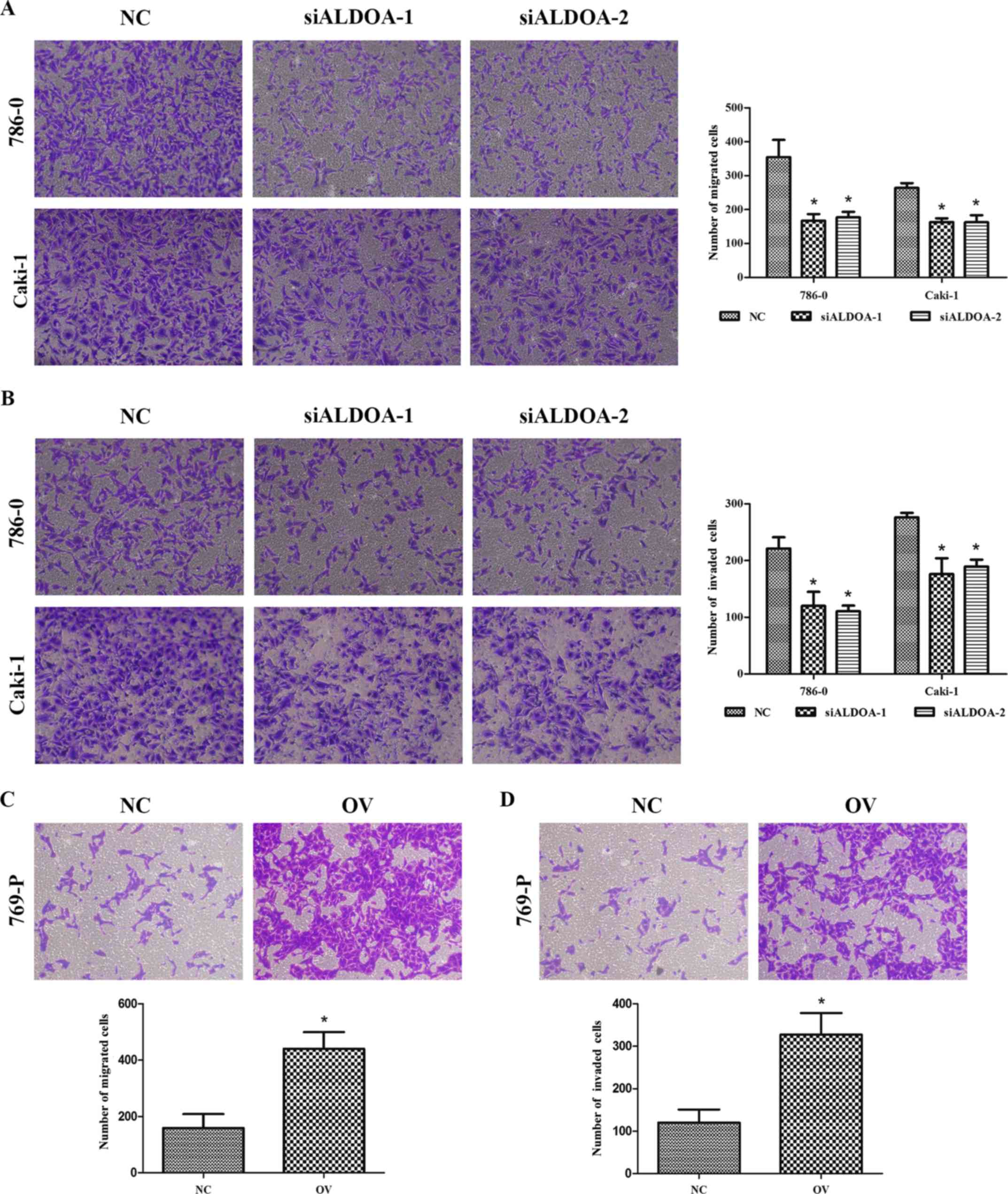
We used Transwell assays to detect changes in
migration and invasion capabilities after knocking down and
upregulating ALDOA expression. The migration assay indicated that
downregulation of ALDOA significantly suppressed migration
capability in 786-0 and Caki-1 cells compared with NC (P<0.05;
Fig. 4A). Similarly, the invasion
assay indicated that downregulation of ALDOA inhibited invasion
capability in 786-0 and Caki-1 cells compared with NC (P<0.01;
Fig. 4B). Conversely,
overexpression of ALDOA enhanced the migration and invasion of
769-P cells (P<0.001; Fig. 4C and
D). These results revealed that ALDOA may play an important
role in RCC progression.
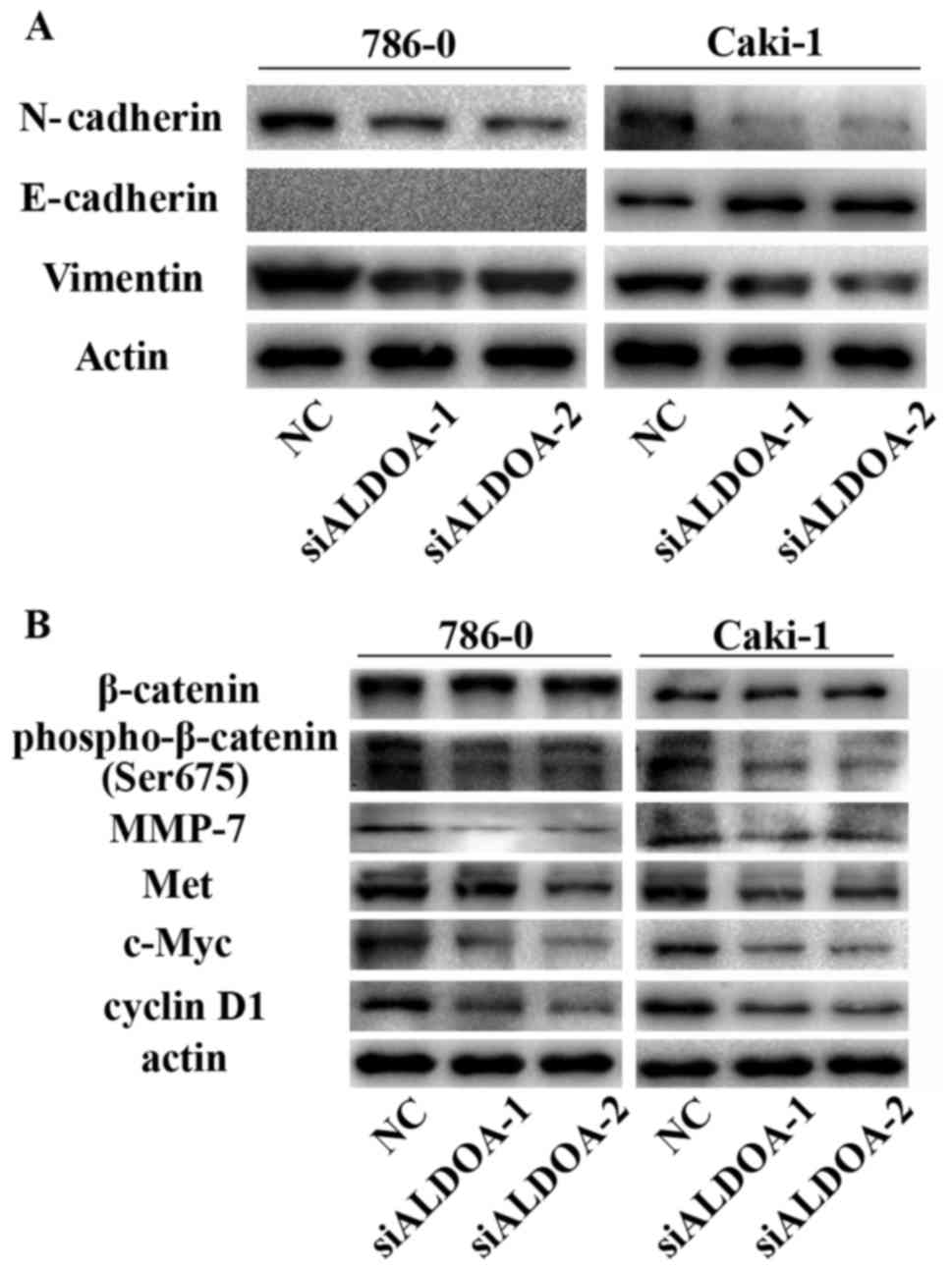
ALDOA promotes EMT in RCC cells
As aforementioned, EMT is a key process in RCC
progression and metastasis. Considering the effect of ALDOA on RCC
cell migration and invasion, we further investigated whether ALDOA
could affect EMT markers in RCC cells using western blot analysis.
The results revealed a gain in E-cadherin expression in Caki-1
cells and loss of N-cadherin and vimentin in both 786-0 and Caki-1
after transfection with ALDOA-siRNA (Fig. 5A), suggesting that ALDOA induced
EMT.
ALDOA may activate the Wnt/β-catenin
signaling pathway
Our aforementioned results revealed that ALDOA
promoted RCC cell proliferation, colony formation, migration and
invasion, and EMT. Thus, to assess the mechanism by which ALDOA
contributed to RCC progression, we examined the effect of transient
ALDOA downregulation on the Wnt/β-catenin signaling pathway, which
is involved in tumor development. Although no difference was
detected in total β-catenin expression, phospho-β-catenin (Ser675)
was effectively reduced in ALDOA-siRNA-transfected RCC cells
(Fig. 5B). Downstream target
proteins, such as MMP-7, Met, c-Myc, and cyclin D1, were
significantly decreased in ALDOA-downregulated RCC cells (Fig. 5B). All these findings revealed that
ALDOA may induce cell proliferation and metastasis through the
Wnt/β-catenin signaling pathway.
Discussion
Given the improvement of people's health awareness
and inspection technology, incidence of RCC, which is one of the
common malignant tumors of the urinary system, has increased
recently. However, in patients with localized RCC, probability of
metastasis remains as high as 40%; and tumor metastasis of these
patients results in a median survival time of approximately 6–12
months, with a 5-year survival rate of 9% (19). As radiotherapy and chemotherapy are
insensitive to RCC, surgical resection remains the first choice for
treatment (19). Therefore, in
absence of other effective therapies, further studies on the
mechanism of genesis and development of RCC and finding new
biomarkers are necessary for promoting early diagnosis and
treatment.
ALDOA participates not only in glycolysis but also
in tumor development and affects prognosis with aberrant expression
in some tumors (8–10,20).
In the present study, we first used RT-PCR to confirm high
expression of ALDOA in RCC tissues and cells in comparison with
normal ones, suggesting that increased expression of ALDOA may be
related to the occurrence of RCC. Then, TMA and
immunohistochemistry were performed to analyze 139 RCC tissue
samples. Strong positive expression of ALDOA accounted for 48.9%
(68/139), positive expression reached 38.8% (54/139), and negative
expression totaled 12.2% (17/139), further supporting high ALDOA
expression in RCC, and this condition may be related to
oncogenesis. Our clinical data indicated that patients with strong
positive expression of ALDOA featured higher incidence of
metastasis and worse histological differentiation. However, tumor
size did not exhibit any association with ALDOA expression. During
follow-up of the 139 RCC patients, Kaplan-Meier analysis revealed
that negative ALDOA expression resulted in the longest survival
among patients, whereas those with strong positive ALDOA expression
exhibited the shortest survival period. All these results strongly
suggest that ALDOA possesses clinical value as a prognostic factor
for RCC.
Considering the confirmed high ALDOA expression in
RCC cell lines by RT-PCR and western blot analysis, siRNA and
overexpression plasmid transfection approaches were employed to
investigate the potential biological effects of ALDOA on RCC cell
function. Two independent siRNAs significantly decreased the
expression of ALDOA in 786-0 and Caki-1 cells, and the
overexpressed plasmid effectively upregulated ALDOA expression in
769-P cells. We observed that ALDOA knockdown significantly
inhibited growth of 786-0 and Caki-1 cells, resulting from the
arrest in the G0/G1 phase of the cell cycle. The G1 phase starts
from mitosis to the period before DNA replication, in which RNA and
ribosomes are synthesized. When arrested in the G1 phase, the cells
remain in the G0 phase, where cell division stops, thus inhibiting
cell proliferation. Western blot analysis revealed decreased
expression of protein cyclin D1 in RCC cells transfected with
ALDOA-siRNA compared with NC. Cyclin D1 is a critical target of
proliferative signals in the G1 phase and is rapidly synthesized
and accumulated in the nucleus in the G1 phase and disappears as
cells proceed to the S phase (21).
Overexpression of cyclin D1 is considered to be associated with
early-stage cancer and progression (22). Thus, decreased cyclin D1 expression
may induce arrest in the G1 phase after downregulating ALDOA
expression. Combined with colony formation, we speculated that
ALDOA may promote RCC cell proliferation. Previous studies reported
that EMT, which plays an important role in cancer invasion and
metastasis, induces physical translocation of cancer cells to
distant organs and their development into metastatic lesions
(23,24). Our data indicated that ALDOA
overexpression increased the migration and invasion abilities of
RCC cells, while ALDOA knockdown significantly reduced these
abilities by blocking EMT, downregulating the expression of
N-cadherin and vimentin in 786-0 and Caki-1 cells, and upregulating
the expression of E-cadherin in Caki-1 cells. Coinciding with
results reported in literature (25,26),
E-cadherin was not detected in 786-0 cells. These findings revealed
that ALDOA may function as a tumor promoter in RCC.
Recently, a research study reported that aldolase
proteins can regulate novel Wnt signaling; for example, ALDOA
activates Wnt signaling by disrupting glycogen synthase kinase 3
(GSK-3) and β-axin interaction and targeting axin to the
dishevelled-induced signalosomes (16). The Wnt signaling pathway is involved
in many cellular processes, including proliferation, migration,
differentiation, movement, and survival (27). In unstimulated cells, the β-catenin
destruction complex, which contains scaffolding protein axin,
adenomatous polyposis coli protein, GSK-3 α/β, and casein
kinase-1α, maintains extremely low cytosolic and nuclear levels of
β-catenin by promoting phosphorylation and ubiquitination of
β-catenin (28,29). In the present study, we revealed
that ALDOA knockdown reduced the expression of phospho-β-catenin
(Ser675). Previous studies have demonstrated that β-catenin can be
phosphorylated by protein kinase A (PKA) at site Ser675, and
phosphorylation by PKA promotes transcriptional activity and
binding of β-catenin to its transcriptional coactivator (30,31).
Thus, knockdown of ALDOA expression decreased transcriptional
activity of β-catenin at the nuclear level. Expression of
Wnt/β-catenin-activated target proteins MMP-7, Met, c-Myc and
cyclin D1 decreased after ALDOA knockdown. MMP-7, Met and c-Myc are
important factors of tumor invasion, angiogenesis, carcinogenesis,
and apoptosis (32–34), whereas cyclin D1 is associated with
the cell cycle (22). Thus, we
speculated that ALDOA may influence RCC progression through the
Wnt/β-catenin signaling pathway.
In summary, our results revealed that ALDOA was
significantly upregulated in RCC. Positive ALDOA expression was
associated with metastasis, histological differentiation, and
prognosis of RCC patients. Silencing ALDOA expression in RCC cells
by specific siRNA significantly decreased their proliferative,
migratory, and invasive abilities, while ALDOA overexpression
increased these abilities. ALDOA may serve as a potential tumor
promoter in RCC by EMT and the Wnt/β-catenin signaling pathway.
Consequently, ALDOA may be a competent candidate target for the
diagnosis and therapy of RCC. Further studies are still required to
research the mechanism and assess the role of ALDOA in vivo
in the future.
Acknowledgements
We would like to thank all the colleagues who helped
us on designing and performing the experiments.
Funding
This study was supported by the National Natural
Science Foundation of China (nos. 81372757 and 81570676) and the
Science and Education Health Project of Jiangsu Province for
important talent (no. RC2011055).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
MG, ZW and XJ conceived and designed the study. ZH,
YH, YT and MB performed the experiments. Analysis and
interpretation of data was conducted by ZH, YL and SW. ZH and YT
wrote the paper. ZH, CQ, JQ and QC reviewed and edited the
manuscript. All authors read and approved the manuscript and agree
to be accountable for all aspects of the research in ensuring that
the accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
This study was approved by the Medical Ethics
Committee of the First Affiliated Hospital of Nanjing Medical
University and informed consent was obtained from the patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang L, Parkin DM, Ferlay J, Li L and Chen
Y: Estimates of cancer incidence in China for 2000 and projections
for 2005. Cancer Epidemiol Biomarkers Prev. 14:243–250.
2005.PubMed/NCBI
|
|
3
|
Chow WH, Dong LM and Devesa SS:
Epidemiology and risk factors for kidney cancer. Nat Rev Urol.
7:245–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kajita E, Moriwaki J, Yatsuki H, Hori K,
Miura K, Hirai M and Shiokawa K: Quantitative expression studies of
aldolase A, B and C genes in developing embryos and adult tissues
of Xenopus laevis. Mech Dev. 102:283–287. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yao DC, Tolan DR, Murray MF, Harris DJ,
Darras BT, Geva A and Neufeld EJ: Hemolytic anemia and severe
rhabdomyolysis caused by compound heterozygous mutations of the
gene for erythrocyte/muscle isozyme of aldolase,
ALDOA(Arg303X/Cys338Tyr). Blood. 103:2401–2403. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kishi H, Mukai T, Hirono A, Fujii H, Miwa
S and Hori K: Human aldolase A deficiency associated with a
hemolytic anemia: Thermolabile aldolase due to a single base
mutation. Proc Natl Acad Sci USA. 84:8623–8627. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miwa S, Fujii H, Tani K, Takahashi K,
Takegawa S, Fujinami N, Sakurai M, Kubo M, Tanimoto Y, Kato T, et
al: Two cases of red cell aldolase deficiency associated with
hereditary hemolytic anemia in a Japanese family. Am J Hematol.
11:425–437. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du S, Guan Z, Hao L, Song Y, Wang L, Gong
L, Liu L, Qi X, Hou Z and Shao S: Fructose-bisphosphate aldolase a
is a potential metastasis-associated marker of lung squamous cell
carcinoma and promotes lung cell tumorigenesis and migration. PLoS
One. 9:e858042014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ji S, Zhang B, Liu J, Qin Y, Liang C, Shi
S, Jin K, Liang D, Xu W, Xu H, et al: ALDOA functions as an
oncogene in the highly metastatic pancreatic cancer. Cancer Lett.
374:127–135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng Y, Li X, Wu M, Yang J, Liu M, Zhang
W, Xiang B, Wang X, Li X, Li G, et al: New prognosis biomarkers
identified by dynamic proteomic analysis of colorectal cancer. Mol
Biosyst. 8:3077–3088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Takashi M, Zhu Y, Nakano Y, Miyake K and
Kato K: Elevated levels of serum aldolase A in patients with renal
cell carcinoma. Urol Res. 20:307–311. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Neely BA, Wilkins CE, Marlow LA,
Malyarenko D, Kim Y, Ignatchenko A, Sasinowska H, Sasinowski M,
Nyalwidhe JO, Kislinger T, et al: Proteotranscriptomic analysis
reveals stage specific changes in the molecular landscape of
clear-cell renal cell carcinoma. PLoS One. 11:e01540742016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He H and Magi-Galluzzi C:
Epithelial-to-mesenchymal transition in renal neoplasms. Adv Anat
Pathol. 21:174–180. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rivera MN and Haber DA: Wilms' tumour:
Connecting tumorigenesis and organ development in the kidney. Nat
Rev Cancer. 5:699–712. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Banumathy G and Cairns P: Signaling
pathways in renal cell carcinoma. Cancer Biol Ther. 10:658–664.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Caspi M, Perry G, Skalka N, Meisel S,
Firsow A, Amit M and Rosin-Arbesfeld R: Aldolase positively
regulates of the canonical Wnt signaling pathway. Mol Cancer.
13:1642014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tong X, Li K, Luo Z, Lu B, Liu X, Wang T,
Pang M, Liang B, Tan M, Wu M, et al: Decreased TIP30 expression
promotes tumor metastasis in lung cancer. Am J Pathol.
174:1931–1939. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cheng G, Wang S, Li X, Li S, Zheng Y,
Zhang L, Bao M, Liang C, Huang Z, Liu Y, et al: Positive expression
of NR6A1/CT150 as a predictor of biochemical recurrence-free
survival in prostate cancer patients. Oncotarget. 8:64427–64439.
2016.PubMed/NCBI
|
|
19
|
van Spronsen DJ, de Weijer KJ, Mulders PF
and De Mulder PH: Novel treatment strategies in clear-cell
metastatic renal cell carcinoma. Anticancer Drugs. 16:709–717.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Long F, Cai X, Luo W, Chen L and Li K:
Role of aldolase A in osteosarcoma progression and metastasis: In
vitro and in vivo evidence. Oncol Rep. 32:2031–2037. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baldin V, Lukas J, Marcote MJ, Pagano M
and Draetta G: Cyclin D1 is a nuclear protein required for cell
cycle progression in G1. Genes Dev. 7:812–821. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diehl JA: Cycling to cancer with cyclin
D1. Cancer Biol Ther. 1:226–231. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chaffer CL and Weinberg RA: A perspective
on cancer cell metastasis. Science. 331:1559–1564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hill B, De Melo J, Yan J, Kapoor A, He L,
Cutz JC, Feng X, Bakhtyar N and Tang D: Common reduction of the Raf
kinase inhibitory protein in clear cell renal cell carcinoma.
Oncotarget. 5:7406–7419. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Langner C, Ratschek M, Rehak P, Schips L
and Zigeuner R: Expression of MUC1 (EMA) and E-cadherin in renal
cell carcinoma: a systematic immunohistochemical analysis of 188
cases. Mod Pathol. 17:180–188. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Clevers H: Wnt/beta-catenin signaling in
development and disease. Cell. 127:469–480. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rubinfeld B, Albert I, Porfiri E, Fiol C,
Munemitsu S and Polakis P: Binding of GSK3beta to the
APC-beta-catenin complex and regulation of complex assembly.
Science. 272:1023–1026. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kimelman D and Xu W: beta-catenin
destruction complex: Insights and questions from a structural
perspective. Oncogene. 25:7482–7491. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Taurin S, Sandbo N, Qin Y, Browning D and
Dulin NO: Phosphorylation of beta-catenin by cyclic AMP-dependent
protein kinase. J Biol Chem. 281:9971–9976. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hino S, Tanji C, Nakayama KI and Kikuchi
A: Phosphorylation of beta-catenin by cyclic AMP-dependent protein
kinase stabilizes beta-catenin through inhibition of its
ubiquitination. Mol Cell Biol. 25:9063–9072. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Coussens LM, Fingleton B and Matrisian LM:
Matrix metalloproteinase inhibitors and cancer: Trials and
tribulations. Science. 295:2387–2392. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sattler M and Salgia R: The MET axis as a
therapeutic target. Update Cancer Ther. 3:109–118. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grandori C, Cowley SM, James LP and
Eisenman RN: The Myc/Max/Mad network and the transcriptional
control of cell behavior. Annu Rev Cell Dev Biol. 16:653–699. 2000.
View Article : Google Scholar : PubMed/NCBI
|