Introduction
Melanoma is one of the most aggressive types of skin
cancer, and is the most deadly cutaneous neoplasm (1). In Western populations, although it
accounts for less than 5% of all skin cancers, melanoma is one of
the most common cancers among young adults and is one of the most
common causes of death (2,3). Although melanoma is relatively rare,
the increasing incidence over the past two decades has raised a
major health concern, and once metastasis to distant organ occurs,
the prognosis becomes extremely poor. Metastatic melanoma is
resistant to many types of chemotherapeutic agents (4). To date, dacarbazine is considered to
be the reference single agent for the management of advanced
melanoma, and dacarbazine treatment exhibits effective responses in
~15–25% of patients (4). However,
in metastatic melanoma patients, the 6-year median survival rate is
extremely low (5).
The embryonic protein Nodal, belongs to the
transforming growth factor (TGF)-β family, and plays critical roles
in maintaining the self-renewal capacity and pluripotency of human
embryonic stem cells (hESCs) (6,7).
Emerging evidence has revealed its involvement in promoting the
growth and progression of various types of cancer, including
melanoma, glioma, breast, prostate and endometrial cancer (8–12). In
both melanoma and its CSC subpopulation, accounting for 0.5% of
melanoma cancer cells, activated Nodal/Activin signaling promotes
tumorigenicity and maintains self-renewal capacity (13). In most cases, Nodal induces signal
transduction after being cleaved by the proprotein convertases
Furin or Pace4 to remove an N-terminal prodomain from precursor
Nodal (proNodal) (14). A recent
report also revealed that proNodal, but not mature Nodal, induces
Nodal signaling transduction (15).
However, it is still unknown which forms of Nodal, proNodal or
mature Nodal regulates CSC properties.
Hypoxia, as one of the factors critical in the
biological processes of CSCs, plays crucial roles in the activation
of pathways involved in the maintenance of CSC functions (16,17),
and is implicated in the development of chemoresistance. It was
recently uncovered that hypoxia exposure converted non-cancer stem
cells to CSCs in the melanoma cancer cell line A375 (18). Furthermore, Quail et al found
that, in melanoma and breast cancer cells, hypoxia induced Nodal
expression and caused promotion of cell invasion and angiogenic
phenotypes, suggesting that Nodal may also be regulated by hypoxia
in pancreatic CSCs (19).
In the present study, we investigated the effect of
hypoxia exposure on Nodal expression and CSC properties in melanoma
cancer cells. Our study revealed that hypoxia-induced Nodal
enhanced CSC properties, including self-renewal capacity, sphere
forming ability and chemoresistance. Moreover, both proNodal and
mature Nodal induced by hypoxia exposure contributed to a higher
tumorigenic potential.
Materials and methods
Cell culture, sphere formation and
treatments
Human melanoma cancer cell line, A375, was purchased
from the American Type Culture Collection (ATCC; Manassas, VA, USA)
and cultured in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS), 100 units/ml
penicillin and 100 µg/ml streptomycin (Life Technologies; Thermo
Fisher Scientific, Inc., Waltham, MA, USA).
For sphere formation, 2×105 cells were
seeded in each 6-well plate and cultured with DMEM-F12 (1:1) medium
containing human basic fibroblast growth factor (bFGF; 10 ng/ml;
PeproTech, Rocky Hill, NJ, USA), human epidermal growth factor
(EGF; 20 ng/ml; PeproTech) and 2% B27 (Life Technologies; Thermo
Fisher Scientific, Inc.). Every three days, the medium was
half-refreshed.
Hypoxic cultures were carried out in a humidified
hypoxia workstation Invivo2 400 model (Baker Ruskinn
Technology Ltd., Bridgend, UK). Briefly, medium was pre-exposed to
20% O2, 5% O2, 2% O2, 1%
O2, or 0.5% O2 balanced with nitrogen and 5%
CO2. Then, the cells were cultured in pre-treated medium
and maintained in corresponding conditions for 24 or 48 h.
Western blotting
Cells were lysed with Laemmli sample buffer (50 mM
Tris pH 6.8, 1.25% SDS, 10% glycerol) and heated at 100°C for 15
min for denaturalization. SDS-PAGE electrophoresis (6–12% gradient)
was performed to fractionate total protein. Blots were then
incubated with the following primary antibodies purchased from
Abcam: CD44 (cat. no. ab157107), CD133 (cat. no. ab19898), Nanog
(cat. no. ab21624), Sox2 (cat. no. ab97959), β-actin (cat. no.
ab8226), cleaved Nodal (cat. no. ab81287), proNodal (cat. no.
ab109317), ALK4 (cat. no. ab109300), ALK7 (cat. no. ab111121),
Smad2/3 (cat. no. ab202445), p-Smad2 (cat. no. ab53100), p-Smad3
(cat. no. ab52903), HK-II (cat. no. ab104832), Glut-I (cat. no.
ab115730), PDK-1 (cat. no. 110025) and HIF-1α (cat. no. ab113642).
Primary antibodies were diluted at 1:1,000 in PBS and incubated
with the blotted membrane at room temperature for 2 h. After three
washes with PBS supplemented with 0.1% Tween-20 at room
temperature, goat anti-mouse secondary antibody (cat. no. ab97040)
(for β-actin, PDK-1 and HIF-1α) was used; for other antibodies,
goat anti-rabbit secondary antibody (cat. no. ab7090) was used.
β-actin was used as a loading control. Proteins were detected using
ECL (Life Technologies; Thermo Fisher Scientific, Inc.) and
visualized on X-ray film (Kodak, Japan).
Lentivirus production and cell
infection
We obtained lentivirus-based shRNA constructs
targeting human Nodal (shNodal;
5′-CCGGGCGGTTTCAGATGGACCTATTCTCGAGAATAGGTCCATCTGAAACCGCTTTTTG-3′)
(LV-shNodal) as well as a control shRNA-expressing plasmid
(shScrambled;
5′-CCGGCTATGGACGCTCTTATGTACTGGCGCCAGTACATAAGAGCGTCCATAGTTTTTG-3′)
(LV-shScrambled). The oligonucleotides were cloned into the
shRNA-pGCL-GFP lentiviral vector (Shanghai Genchem, Shanghai,
China), respectively. Infectious viruses were produced by
contransfecting the lentiviral vectors and packaging constructs
(pHelper1.0 and pHelper2.0) into 293T cells using Lipofectamine™
reagent (Life Technologies; Thermo Fisher Scientific, Inc.).
The lentiviruses containing the Nodal coding
sequences (LV-Nodal) for overexpressing Nodal were produced by
Genomeditech Company (Shanghai, China), which were labeled with
RFP. Lentiviruses containing the empty vector (LV-vector) were used
as a negative control. Cells were transduced with the packaged
lentiviruses at a multiplicity of infection (MOI) of 1:10
(cell:virus) (20) and 72-h later,
the transduced cells were subsequently collected for further
analysis.
Glucose uptake assay
For measuring glucose uptake, 1×106 cells
were plated in 6-well plates overnight for attaching. The cells
were incubated in glucose-free medium for 30 min at 37°C in a
CO2 incubator and then 2-[3H]deoxyglucose at
1 µCi was added into the medium for a 2-h incubation. The cells
were washed with ice-cold PBS three times, and transferred to
scintillation vials for counting as described previously (21,22).
Lactate measurements
Cells (1×106) were plated in 6-well
plates overnight and the medium was replaced with serum-free medium
for 12 h. The supernatant was collected and diluted with PBS. For
measuring lactate, the EnzyChrom™ L-Lactate assay kit (BioAssay
Systems, Hayward, CA, USA) was employed following the
manufacturer's instruction and was measured at 565 nm using a
microplate reader (Synergy 2 Multi-Mode Microplate Reader; BioTek,
Winooski, VT, USA).
ATP production
Cells (1×106) were plated in 6-well
plates and allowed to adhere overnight. ATP Lite assay kit
(PerkinElmer, Inc., Waltham, MA, USA) was employed to measure ATP
production following the manufacturer's instruction.
Reactive oxygen species (ROS)
detection
Total intracellular ROS was assessed by flow
cytometry using the dichlorofluorescein (DCF) oxidation assay. The
intracellular ROS oxidizes cleaved DCFH-DA which enters into cells.
Target cells (5×105) were incubated with DCFH-DA (10 µM)
for 1 h at 37°C, followed by 3 washes with ice-cold PBS, and ROS
fluorescence was analyzed using 3 laser Navios flow cytometers
(Beckman Coulter, Brea, CA, USA) with green fluorescence channel
(FL1).
Cell death analysis
Cells (5×104) were collected, washed in
PBS and stained with 1 µg/ml PI for 30 min in darkness. The stained
cells were washed with ice-cold PBS for three times and analyzed by
flow cytometry using 3 laser Navios flow cytometers. PI-positive
cells were regarded as dead cells, and the percentage of dead cells
was determined.
Cell viability assay
Cells were grown in 96-well plates, and viable cell
numbers were determined with the CellTiter-Glo Luminescent Cell
Viability Assay kit (Promega, Madison, WI, USA) following the
manufacturer's guide.
Transwell cell invasion assays
Matrigel (Millipore Corp., Darmstadt, Germany) was
diluted 1:2 in DMEM/F-12 and 60 µl of diluted Matrigel was added to
the upper chamber of Transwell plates. Then, the chambers were
incubated at 37°C in a 5% CO2 incubator for 2 h.
Single-cells were resuspended in DMEM/F-12 and seeded into the
upper chamber at a density of 2×104 cells/well.
Subsequently, 600 µl of DMEM/F-12 supplemented with 20 ng/ml EGF,
10 ng/ml bFGF and 2% B27 was added to the lower chamber. After a
24-h incubation, the inserts were collected, and the cells on the
lower surface were fixed in 4% paraformaldehyde and stained with
crystal violet for 15 min. The cells in five random views were
counted under a X71 (U-RFL-T) fluorescence microscope (Olympus,
Melville, NY, USA).
Cloning of cleavage-resistant Nodal
precursor (mut-proNodal)
The expression vector for mut-proNodal has been
described (23) and the lentiviral
vector containing mut-proNodal coding sequence (LV-mut-proNodal)
was packaged as previously described (23).
Serial replating assay
Cells were replated at a clonal density (2,000
cells/well) and cultured in serum-free medium supplemented with 2%
B-27, 10 ng/ml EGF and 20 ng/ml bFGF. The medium was half-replaced
every 3 days. After 10 days, PBS-washed cells were fixed with 4%
paraformaldehyde and stained with 0.1% crystal violet for 10 min
and washed again with PBS, and the colonies were counted. For
replating, the same amount of cells was plated in serum-free
medium. After 10 days, the same procedure was performed three
times.
Statistical analysis
All data were normalized to control values for each
assay and are presented as mean ± standard deviation (SD).
Multigroup comparisons of the means were carried out by one-way
analysis of variance (ANOVA) test with post hoc contrasts by
Student-Newman-Keuls test. Significance was chosen as
P<0.05.
Results
Hypoxia upregulates the expression of
Nodal protein in A375 CSCs
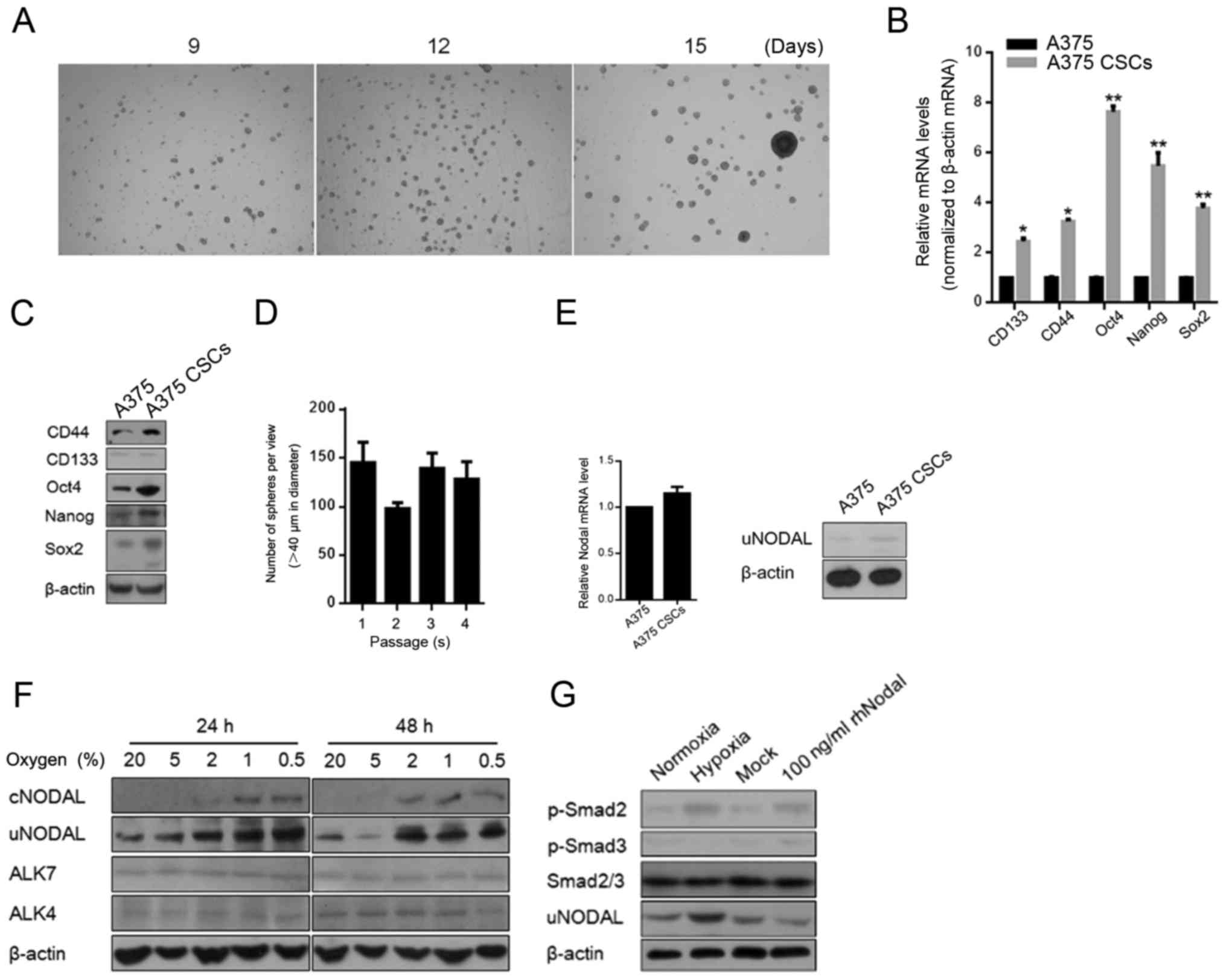
Serum-free medium culturing was used for enriching
the stem-like cells from A375 cells in this study. After 14 days of
culturing, spheres were completely formed and showed morphological
similarity with embryonic tissues (Fig.
1A). In order to characterize the stem-like characteristics of
the A375 subpopulation enriched using SFM culturing, we examined
the expression levels of several stem cell markers (CD133, CD44,
OCT4, Nanog and SOX2) and found that all were upregulated in the
A375 CSCs compared to levels in the A375 cells at both the mRNA and
protein levels (Fig. 1B and C). We
further used in vitro serial replating assay to examine the
self-renewal capacity of the A375 CSCs and the results confirmed
the stem-like property (Fig. 1D).
Nodal is reported to be restricted to embryonic tissues and hESCs,
and re-emerges during tumorigenesis in several types of cancers
(24). This promoted us to assess
the differential expression of Nodal in A375 and A375 CSCs.
Unexpectedly, compared to A375, no detectable difference in Nodal
was found in the A375 CSCs (Fig.
1E).
For detecting the regulatory factor of tumor
progression, hypoxia, which is an important environmental change in
many cancers, was employed to determine its effect on the
regulation of Nodal protein expression. We exposed A375 CSCs to
varying levels of O2 for 24 and 48 h. Uncleaved Nodal
protein (uncleaved NODAL, uNODAL, 39 kDa) and cleaved and secreted
Nodal protein (cleaved NODAL, cNODAL, 15 kDa) were detected in
whole cell lysate and in concentrated medium by ultrafiltration
individually. The data showed that exposure of A375 CSCs to varying
O2 concentrations (0.5–20% O2) for 24 and 48
h upregulated the Nodal protein level at concentrations ≤2%
O2 (Fig. 1F). We also
detected the expression pattern of Nodal receptor, ALK4/7, and no
detectable changes were observed (Fig.
1F). Surprisingly, hypoxic exposure undetectably disturbed
Nodal mRNA levels, indicating its post-transcriptional regulation
on Nodal (data not shown). We then investigated whether Nodal/Nodal
receptors are coupled to Smad signaling pathway by detecting
phosphorylated Smad2/3 in A375 CSCs. Western blot analysis showed
that Smad2, but not Smad3, was phosphorylated in A375 CSCs exposed
to hypoxia at oxygen concentration of 0.5% and co-incubated with
100 ng/ml rhNodal (as a positive control) (Fig. 1G).
Hypoxic-induced Nodal enhances glucose
uptake and promotes glycolysis
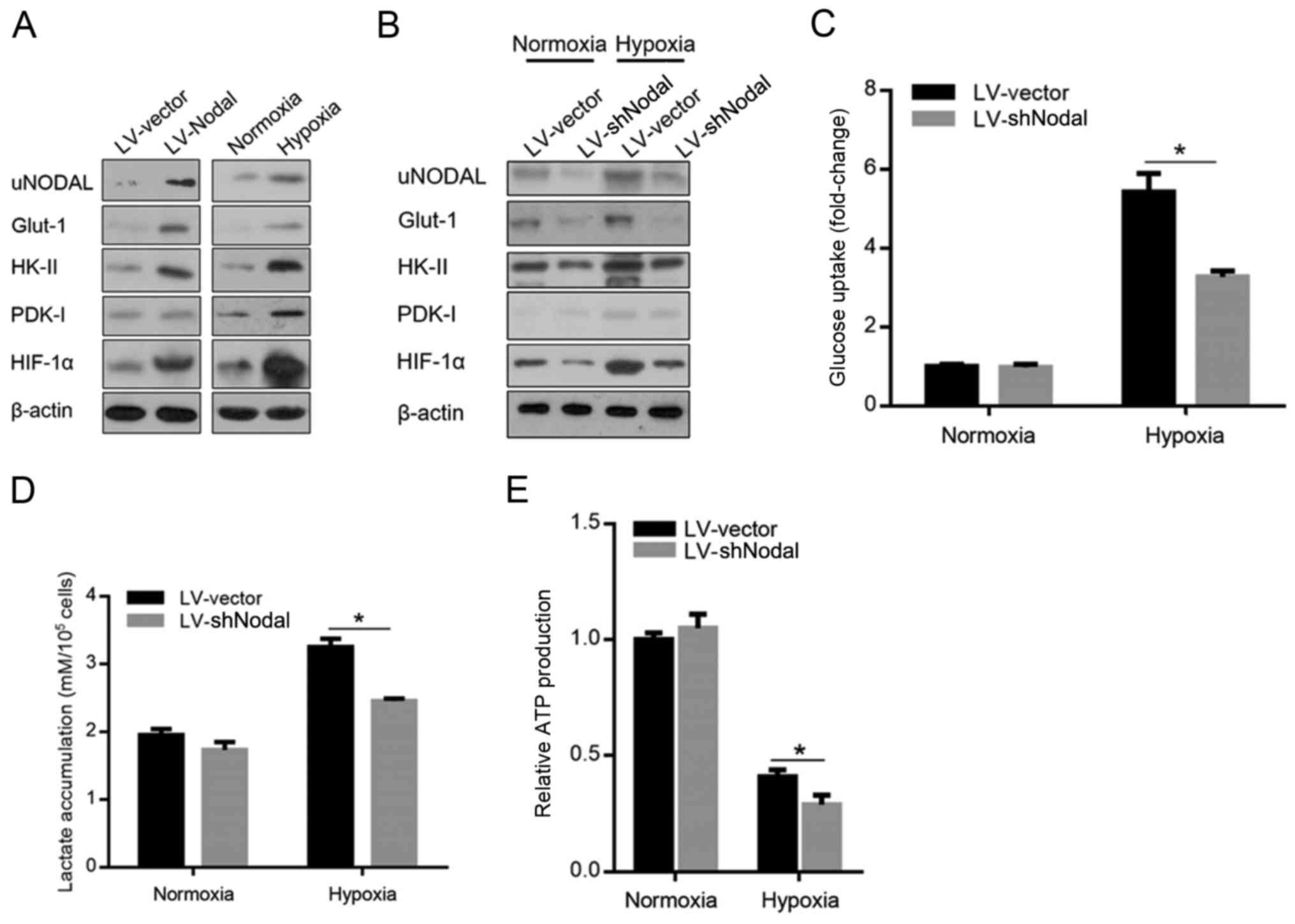
Nodal protein expression was reported to be
positively correlated with hexokinase (HK)-II, glucose transporter
(Glut)-1 and pyruvate dehydrogenase kinase (PDK)-1 by activating
hypoxia-inducible factor (HIF)-1α, which is induced by hypoxic
exposure and directly regulates energy metabolism (25–27).
Firstly, by introducing Nodal, we confirmed the positive
correlation of Nodal with HK-II, Glut-1, PDK-1 and HIF-1α in A375
CSCs (Fig. 2A), and hypoxia
exposure exerted similar effects. For ascertaining whether
hypoxia-induced Nodal contributes to the upregulation of HIF-1α,
HK-II, Glut-1 and PDK-1, in hypoxia-induced A375 CSCs transfected
with Nodal shRNA, semi-quantitative western blotting was performed.
As shown in Fig. 2B, hypoxia
exposure markedly upregulated all these proteins, and Nodal
knockdown detectably attenuated their protein levels. In
hypoxic-exposed A375 CSCs, knockdown of Nodal significantly
decreased 2-[3H]deoxyglucose uptake (Fig. 2C) and lactate accumulation (Fig. 2D). We next assessed whether Nodal
contributes to the regulation of ATP production. As expected, Nodal
knockdown negatively affected ATP production (Fig. 2E).
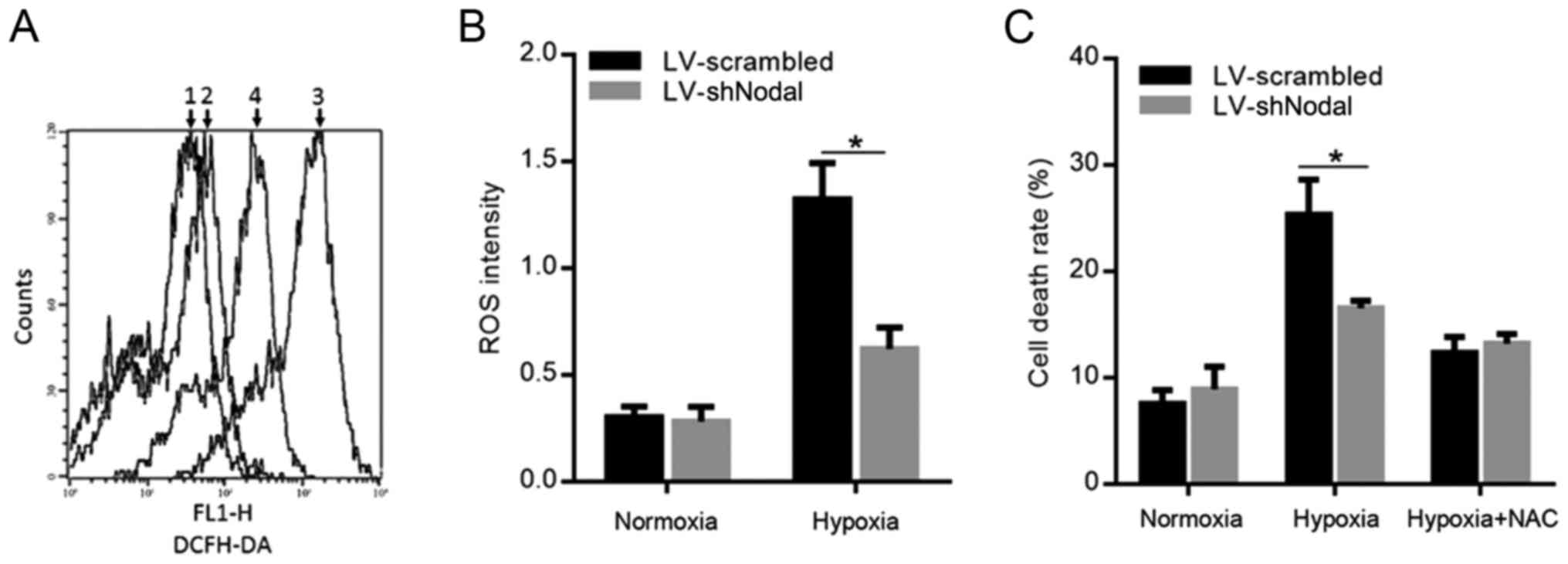
Nodal expression prevents excess ROS
production and apoptosis in A375 CSCs
It has been reported that promotion of glycolysis
prevents excess ROS production (28). Thus, we examined whether Nodal
expression regulates ROS production. As shown in Fig. 3A, hypoxia exposure promoted ROS
production and knockdown of Nodal markedly decreased accumulated
ROS, which was confirmed quantitatively (Fig. 3B). As ROS is important for
hypoxia-induced cell death, we analyzed whether Nodal modulates
ROS-induced cell death in A375 CSCs. Knockdown of Nodal expression
sensitized A375 CSCs against hypoxia-induced cell death, whereas
the addition of ROS scavenger, N-acetyl-cysteine (NAC) slightly
abolished the effect of Nodal, suggesting that Nodal enhances ROS
detoxification mechanisms (Fig.
3C).
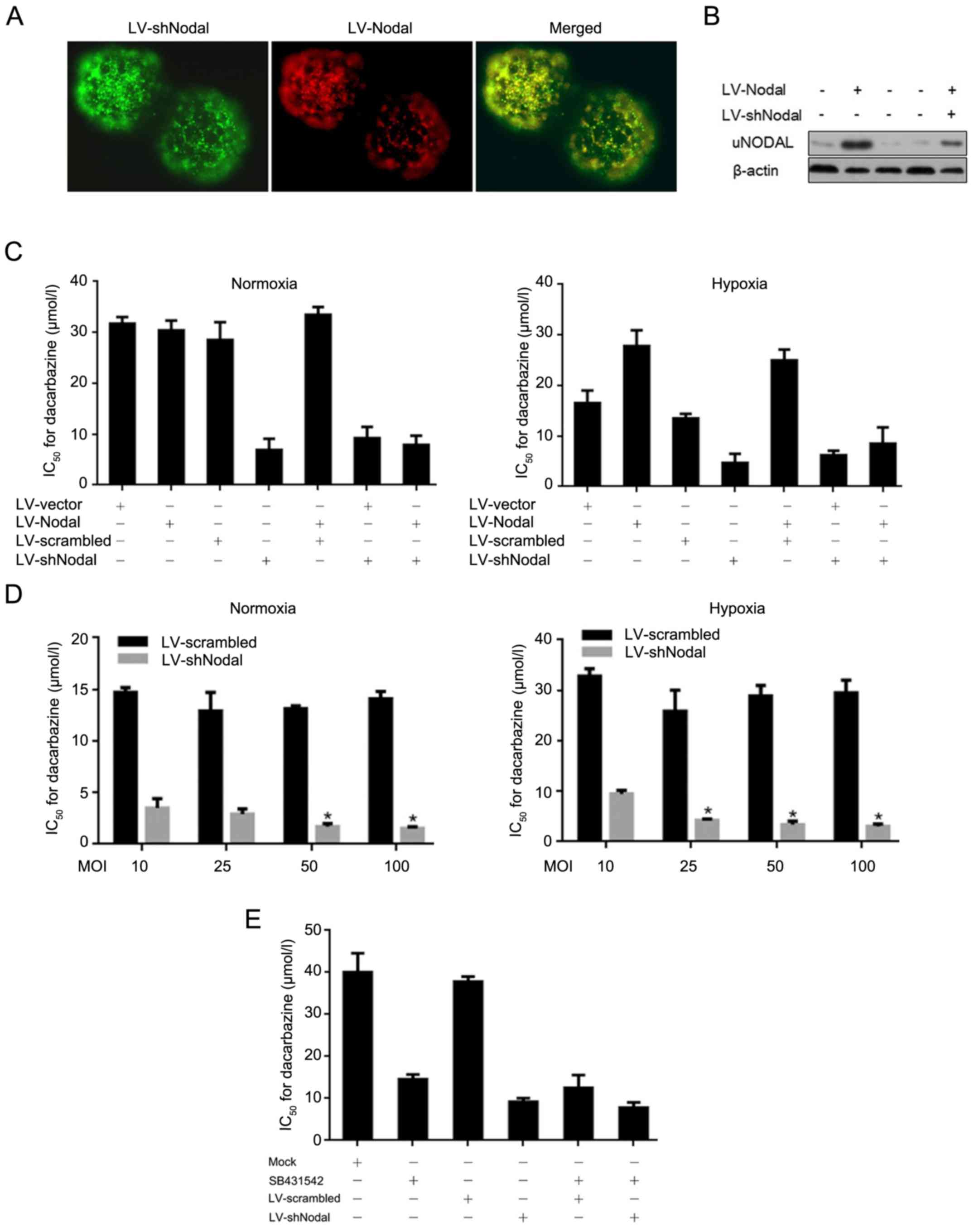
Hypoxic-induced Nodal partially
contributes to decarbazine resistance in A375 CSCs
For investigating the roles of Nodal in A375 CSCs,
we produced lentiviral particles coding for shRNA targeting the
Nodal mRNA (LV-shNodal and LV-scrambled) and for overexpressing
Nodal mRNA (LV-Nodal and LV-vector). The infectious efficacy and
Nodal protein levels were detected (Fig. 4A and B). A375 CSCs were infected
with LV-Nodal, LV-shNodal, or LV-Nodal/LV-shNodal to determine
their IC50 value for dacarbazine, respectively. As
expected, Nodal induced by hypoxic exposure and overexpressed by
lentiviral infection desensitized A375 CSCs to dacarbazine, and
knockdown of Nodal by LV-shNodal sensitized A375 CSCs to
dacarbazine, indicating the critical role of Nodal protein in
dacarbazine response (Fig. 4C). For
further confirm the necessity of Nodal on dacarbazine
desensitization, A375 CSCs were infected by LV-Nodal or LV-shNodal
at MOI 10, 25, 50 and 100 to determine their IC50. With
the decrease in Nodal protein, A375 CSCs presented more sensitivity
to dacarbazine (Fig. 4D). However,
in hypoxic-exposed A375 CSCs, upregulation of Nodal protein caused
no detectable changes in the sensitization to dacarbazine, possibly
because of its high endogenous level (data not shown). We then
blocked the Nodal signal by SB431542 treatment in A375 CSCs to
ascertain whether uncleaved precursor (proNodal) is involved in
dacarbazine response. Surprisingly, after SB431542 blockage,
knockdown of Nodal still sensitized hypoxic-exposed A375 CSCs to
dacarbazine, indicating that proNodal also contributes to
dacarbazine resistance in an undetermined manner (Fig. 4E).
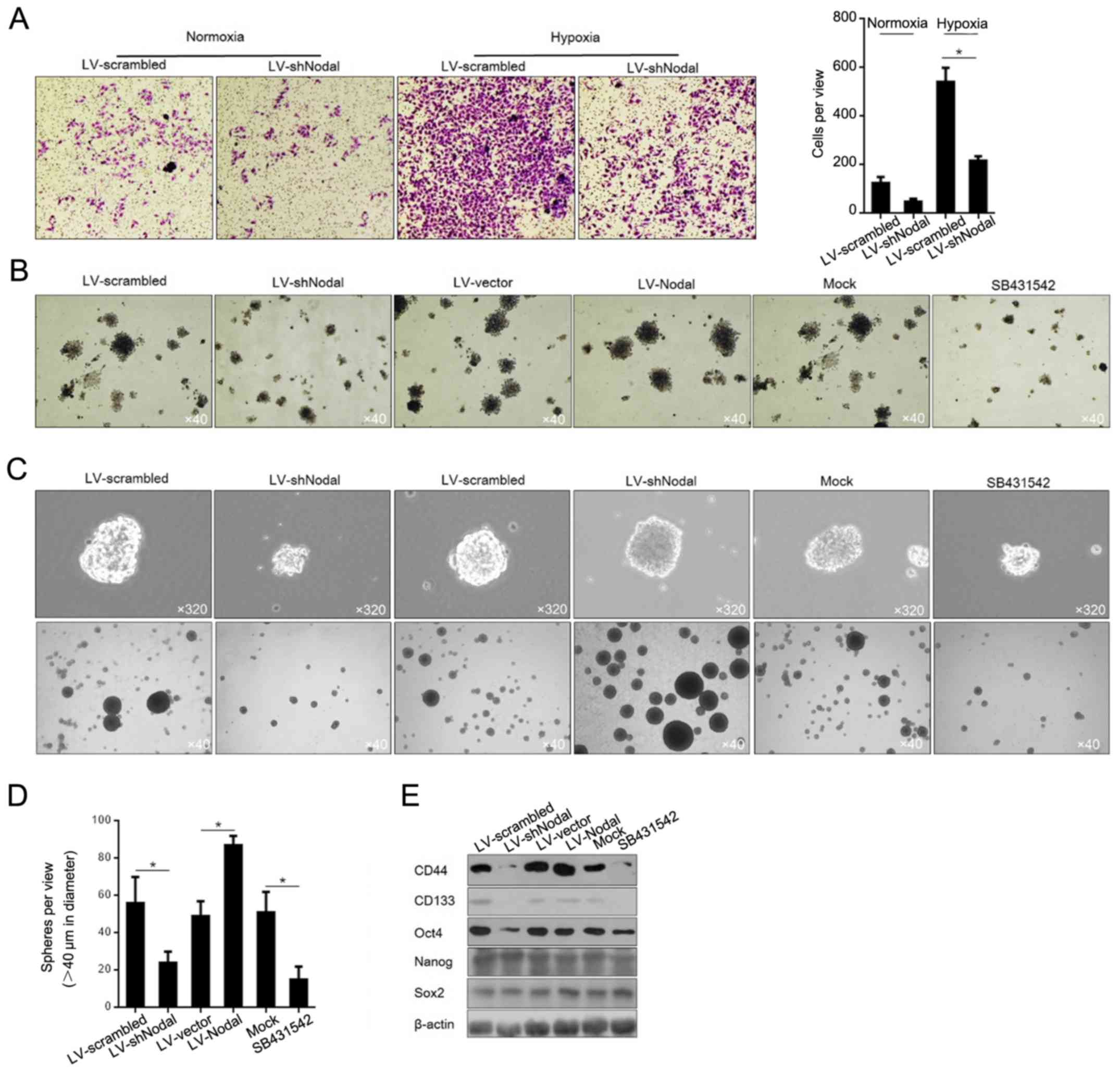
Hypoxic-induced Nodal promotes cell
invasion and maintains cell stemness, but not proliferation in A375
CSCs
Because of its tight association with malignancies
in cancer and CSCs (29,30), we assessed the effects of
hypoxic-induced Nodal on proliferation, invasion and preservation
of stemness. Hypoxia exposure slightly affected the distribution of
cell cycle phases and cell viability (data not shown), possibly due
to high endogenous Nodal protein level. Then, the invasiveness
affected by hypoxia exposure was detected, and the results showed
that hypoxia exposure promoted invasion via upregulation of Nodal
in A375 CSCs (Fig. 5A). For
evaluating the capacity of self-renewal properties in A375 CSCs,
colony formation and sphere formation assays were employed. The
colony formation results demonstrated that both knockdown of Nodal
expression and blockage by SB431542 in A375 CSCs apparently reduced
colony formation (Fig. 5B). To
further characterize the effects of hypoxia-induced Nodal on A375
CSCs, we overexpressed Nodal by lentiviral infection to simulate
upregulation of hypoxic-induced Nodal, and to determine the sphere
formation ability. Similarly, upregulated Nodal expression promoted
the formation of spheres in the A375 CSCs in SFM (Fig. 5C). Simultaneously, both knockdown of
Nodal expression and blockage by SB431542 in A375 CSCs caused
dissociation of spheres (Fig. 4C).
We counting the spheres ≥40 µm in diameter, and found that Nodal
expression promoted the sphere forming rate, and Nodal knockdown
and SB431542 exposure diminished the sphere forming rate (Fig. 5D). By considering the regulatory
roles of Nodal on stemness maintenance, we next evaluated its
effects on related genes, including CD44, CD133, Oct4, Nanog, and
Sox2. Without disturbing Nanog and Sox2 levels, expression of Nodal
was positively correlated with CD44, CD133 and Oct4 (Fig. 5E), which was consistent with its
effect on spheroid morphology.
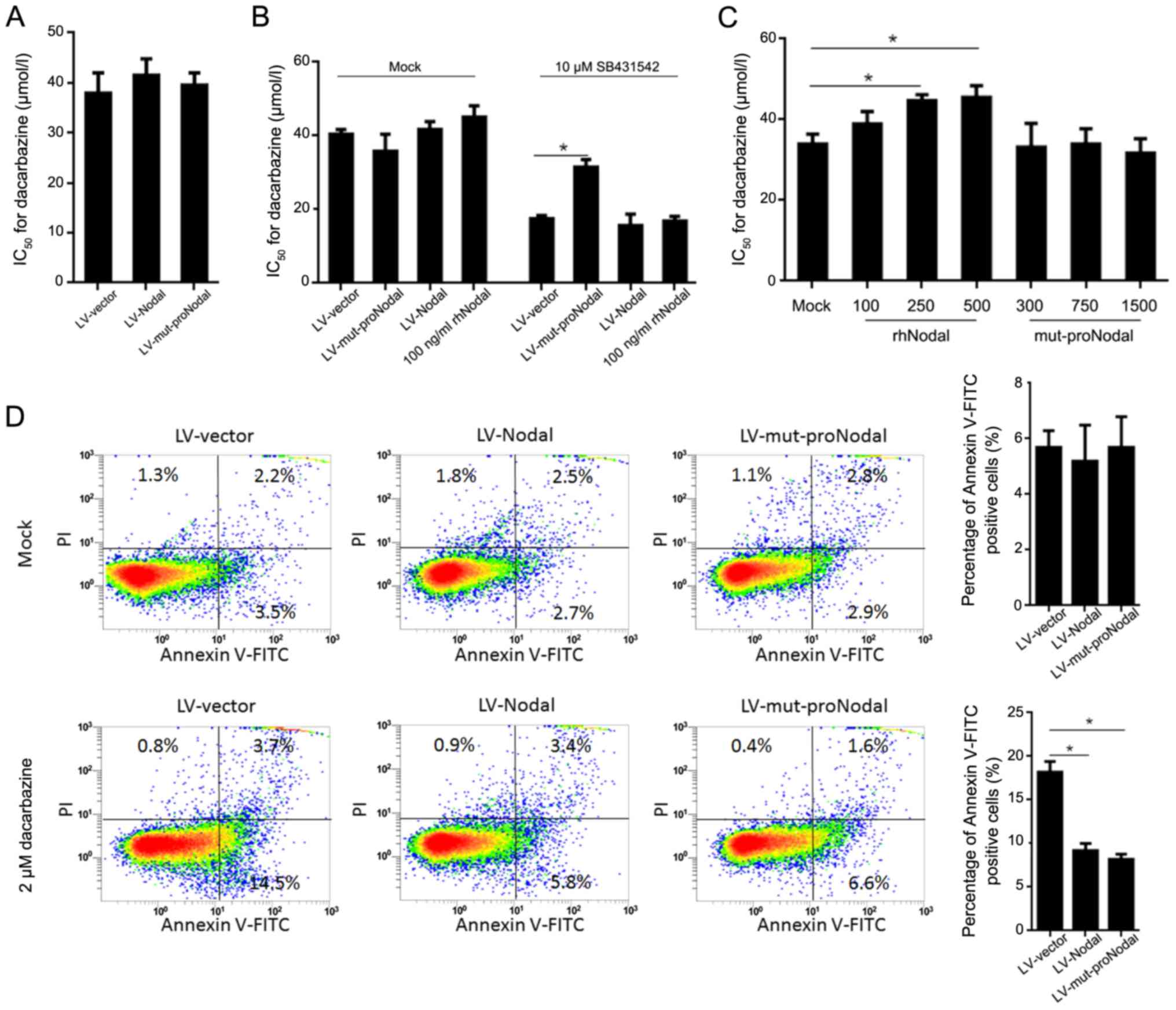
Both cleaved Nodal and proNodal
contribute to dacarbazine resistance in A375 CSCs
Nodal is secreted as a precursor protein by
endoproteolytic cleavage. It has been revealed that proNodal
displays a subset of activities at physiological concentrations
(31–33). By considering the potential
involvement of proNodal in physiological processes of A375 CSCs, we
investigated whether the precursor and/or cleaved Nodal contribute
to dacarbazine resistance. We first examined whether a recombinant
Nodal cleavage mutant (mut-proNodal) which is resistant to specific
cleavage can regulate dacarbazine sensitization in A375 CSCs. In
A375 CSCs infected with LV-mut-proNodal, dacarbazine resistance was
not disturbed, and SB431542-pretreated A375 CSCs were desensitized
to dacarbazine after LV-mut-proNodal infection (Fig. 6A and B). For ascertaining whether
mut-proNodal functions via recognizing the Nodal receptor, we
exposed A375 CSCs to purified mut-proNodal and found that, rhNodal
but not mut-proNodal exposure desensitized A375 CSCs to dacarbazine
and was reversed by SB431542 co-exposure (Fig. 6C). For further confirming the
apoptosis induced by dacarbazine treatment, Annexin V-PI staining
was analyzed by flow cytometry. The results showed that, after 2 µM
dacarbazine treatment for 24 h, introduction of mut-proNodal
significantly reduced the apoptotic rate, with a robustness similar
to that observed for LV-Nodal (Fig.
6D). Unexpectedly, introduction of mutant-proNodal failed to
regulate self-renewal capacity and sphere formation (data not
shown), indicating that proNodal might function via an independent
undetermined manner.
Discussion
In the present study, we report that hypoxia
exposure of A375 CSCs enhanced the CSC phenotype, tumor formation,
invasion and chemoresistance. Hypoxia exposure upregulated Nodal
protein and subsequently activated downstream signaling pathways.
Blockage of Nodal pathway using ALK-4/5/7 inhibitor (SB431542; 10
µM) inhibited the CSC properties of hypoxia-exposed A375 CSCs.
Moreover, it was revealed that hypoxic-induced Nodal enhanced
glucose uptake and promoted glycolysis, and subsequently exerted
protective effects on A375 CSCs via preventing ROS
accumulation.
Nodal plays essential roles in the processes of
embryonic development and maintains the pluripotent properties of
stem cells during embryogenesis (34,35).
Generally, Nodal expression is specifically expressed in embryonic
tissues and hESCs and is undetectable in most highly differentiated
tissues. However, it is found to be upregulated and contributes to
increasing cancer cell aggressiveness, tumorigenicity and
metastasis in several types of cancer, including melanoma, breast
cancer and melanoma (36–38). These findings indicate that the
abnormal expression of Nodal promotes cancer cell proliferation,
invasion and migration and inhibits apoptosis. It was reported that
hypoxia exposure induces Nodal expression predominantly mediated
via an HIF-1α-dependent pathway in melanoma cells. However, the
effect of hypoxia exposure on melanoma CSCs is still unknown. In
the present study, to further explore the regulatory effect of
hypoxia exposure on Nodal expression and CSC properties in A375
CSCs, we exposed A375 CSCs to hypoxia and found that Nodal was
induced after hypoxia exposure. Overexpression of Nodal increased
the number and size of spheroids and promoted colony formation. In
addition, semi-quantitative western blot analysis revealed that
induced Nodal protein resulted in increased expression of CD44,
CD133 and Oct4. For confirming whether induced Nodal expression by
hypoxia exposure was responsible for these changes in expression,
Nodal was knocked down using shNodal which subsequently resulted in
a reversal of CSC properties in A375 CSCs in regards to the
expression of CSC markers. ALK4/5/7 blockage using SB431542
presented similar effects on CSC properties of A375 CSCs with Nodal
knockdown, revealing that Nodal signaling was necessary for
regulating A375 CSC features.
Nodal is a member of the TGF-β superfamily of
secreted proteins that signals through the serine/threonine kinase
receptor family triggering the phosphorylation of Smad2 and 3
(6,7). It is worth testing the regulation of
Nodal induced by hypoxia on TGF-β in further studies. It was
reported that Nodal protein expression is positively correlated
with HK-II, Glut-I and PDK-1 via activation of HIF-1α (24,25).
Consistently, in the present study, it is found that, Nodal
upregulated by hypoxia exposure promoted HK-II, Glut-1, PDK-1 and
HIF-1α in A375 CSCs. Knockdown of Nodal remarkably attenuated
hypoxic-induced upregulation of glycolysis-associated genes,
indicating the critical role of Nodal in controlling glycolysis. By
promoting glycolysis, hypoxic-induced Nodal prevented ROS
accumulation and thus exerted protective effects on A375 CSCs.
Instead of imaging ROS staining in spheres, we quantified ROS
accumulation due to technical difficulty. However, it is still
unknown how Nodal regulates this process.
Nodal is widely known as an inducer for
mesendogermal genes and gastrulation movements within the epiblast
after being activated and secreted after cleavage (32,39).
It was also found that uncleaved Nodal (proNodal) governs the
expression of downstream target genes, including sonic hedgehog
(shh) and FGFR3 transcriptionally or post-transcriptionally,
indicating that proNodal is potentially involved in physiological
regulation (15). Processed Nodal
activates Nodal/Activin signaling to maintain quiescence and
chemoresistance in melanoma cancer stem cells (40), however, whether proNodal takes part
in these processes remains unknown. In the present study,
introduction of mut-proNodal desensitized A375 CSCs pretreated with
10 µM SB431542, indicating that it functions independent of the
ALK4/7 receptor. This was further confirmed by exposure of A375
CSCs to purified rhNodal or mut-proNodal, in which mut-proNodal
failed to influence the chemosensitivity of the A375 CSCs to
dacarbazine. By performing apoptotic analysis, introduction of
mut-proNodal significantly induced desensitization of A375 CSCs to
dacarbazine, and revealed it functions intracellularly independent
with cell surface receptor ALK4/7.
In conclusion, this study provides novel information
concerning the effects of hypoxia exposure on Nodal expression and
functions in A375 CSCs. Our results revealed that hypoxia exposure
upregulated Nodal expression and activated Nodal signal on Smad2/3,
contributed to maintain stemness and promotes malignanct potential,
including invasion, sphere formation and colony formation.
Importantly, uncleaved Nodal precursor, proNodal, desensitized A375
CSCs to dacarbazine, without disturbing self-renewal capacity and
sphere formation, independent of mature Nodal receptor ALK4/7.
Further research is required to reveal the novel mechanism. Taken
together, we revealed that hypoxia-induced Nodal promoted malignant
behavior and chemoresistance in melanoma cancer cell A375.
Acknowledgements
The authors thank Ms H.M. Shi for English
editing.
Funding
No funding was received.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
LH and CY conceived and designed the study. LH, CJJ,
WX, HE and ZZY performed the experiments. LH wrote the manuscript.
CY reviewed and edited the manuscript. All authors read and
approved the manuscript and agree to be accountable for all aspects
of the research in ensuring that the accuracy or integrity of any
part of the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Cell lines were used in the study. No human tissues
were used nor animal studies were carried out and thus no ethical
approval was required.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no conflict of
interests.
References
|
1
|
Miller AJ and Mihm MC: Melanoma. N Engl J
Med. 355:51–65. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Godar DE: Worldwide increasing incidences
of cutaneous malignant melanoma. J Skin Cancer. 2011:8584252011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reed KB, Brewer JD, Lohse CM, Bringe KE,
Pruitt CN and Gibson LE: Increasing incidence of melanoma among
young adults: An epidemiological study in Olmsted County,
Minnesota. Mayo Clin Proc. 87:328–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bedia C, Casas J, Andrieu-Abadie N,
Fabriàs G and Levade T: Acid ceramidase expression modulates the
sensitivity of A375 melanoma cells to decarbazine. J Biol Chem.
286:28200–28209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mouawad R, Sebert M, Michels J, Bloch J,
Spano JP and Khayat D: Treatment for metastatic malignant melanoma:
Old drugs and new strategies. Crit Rev Oncol Hematol. 74:27–39.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brennan J, Norris DP and Robertson EJ:
Nodal activity in the node governs left-right asymmetry. Genes Dev.
16:2339–2344. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takenaga M, Fukumoto M and Hori Y:
Regulated nodal signaling promotes differentiation of the
definitive endoderm and mesoderm from ES cells. J Cell Sci.
120:2078–2090. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Topczewska JM, Postovit LM, Margaryan NV,
Sam A, Hess AR, Wheaton WW, Nickoloff BJ, Topczewski J and Hendrix
MJ: Embryonic and tumorigenic pathways converge via Nodal
signaling: Role in melanoma aggressiveness. Nat Med. 12:925–932.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee CC, Jan HJ, Lai JH, Ma HI, Hueng DY,
Lee YC, Cheng YY, Liu LW, Wei HW and Lee HM: Nodal promotes growth
and invasion in human gliomas. Oncogene. 29:3110–3123. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lawrence MG, Margaryan NV, Loessner D,
Collins A, Kerr KM, Turner M, Seftor EA, Stephens CR, Lai J; APC
BioResource, ; et al: Reactivation of embryonic nodal signaling is
associated with tumor progression and promotes the growth of
prostate cancer cells. Prostate. 71:1198–1209. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Quail DF, Zhang G, Walsh LA, Siegers GM,
Dieters-Castator DZ, Findlay SD, Broughton H, Putman DM, Hess DA
and Postovit LM: Embryonic morphogen nodal promotes breast cancer
growth and progression. PLoS One. 7:e482372012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Papageorgiou I, Nicholls PK, Wang F,
Lackmann M, Makanji Y, Salamonsen LA, Robertson DM and Harrison CA:
Expression of nodal signalling components in cycling human
endometrium and in endometrial cancer. Reprod Biol Endocrinol.
7:1222009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lonardo E, Hermann PC, Mueller MT, Huber
S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S,
Rodriguez-Arabaolaza I, Ramirez JC, et al: Nodal/Activin signaling
drives self-renewal and tumorigenicity of pancreatic cancer stem
cells and provides a target for combined drug therapy. Cell Stem
Cell. 9:433–446. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fuerer C, Nostro MC and Constam DB:
Nodal·Gdf1 heterodimers with bound prodomains enable
serum-independent nodal signaling and endoderm differentiation. J
Biol Chem. 289:17854–17871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ellis PS, Burbridge S, Soubes S, Ohyama K,
Ben-Haim N, Chen C, Dale K, Shen MM, Constam D and Placzek M:
ProNodal acts via FGFR3 to govern duration of Shh expression in the
prechordal mesoderm. Development. 142:3821–3832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cabarcas SM, Mathews LA and Farrar WL: The
cancer stem cell niche-there goes the neighborhood? Int J Cancer.
129:2315–2327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu H, Wang D, Liu Y, Su Z, Zhang L, Chen
F, Zhou Y, Wu Y, Yu M, Zhang Z and Shao G: Role of the
Hypoxia-inducible factor-1 alpha induced autophagy in the
conversion of non-stem pancreatic cancer cells into
CD133+ pancreatic cancer stem-like cells. Cancer Cell
Int. 13:1192013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quail DF, Taylor MJ, Walsh LA,
Dieters-Castator D, Das P, Jewer M, Zhang G and Postovit LM: Low
oxygen levels induce the expression of the embryonic morphogen
Nodal. Mol Biol Cell. 22:4809–4821. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou S, Kurt-Jones EA, Cerny AM, Chan M,
Bronson RT and Finberg RW: MyD88 intrinsically regulates CD4 T-cell
responses. J Virol. 83:1625–1634. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
dos Santos SC, Tenreiro S, Palma M, Becker
J and Sá-Correia I: Transcriptomic profiling of the
Saccharomyces cerevisiae response to quinine reveals a
glucose limitation response attributable to drug-induced inhibition
of glucose uptake. Antimicrob Agents Chemother. 53:5213–5223. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Walsh MC, Smits HP, Scholte M and van Dam
K: Affinity of glucose transport in Saccharomyces cerevisiae is
modulated during growth on glucose. J Bacteriol. 176:953–958. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Constam DB and Robertson EJ: Regulation of
bone morphogenetic protein activities by prodomains and proprotein
convertases. J Cell Biol. 144:139–149. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Vo BT and Khan SA: Expression of nodal and
nodal receptors in prostate stem cells and prostate cancer cells:
Autocrine effects on cell proliferation and migration. Prostate.
71:1084–1096. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lai JH, Jan HJ, Liu LW, Lee CC, Wang SG,
Hueng DY, Cheng YY, Lee HM and Ma HI: Nodal regulates energy
metabolism in glioma cells by inducing expression of
hypoxia-inducible factor 1α. Neuro Oncol. 15:1330–1341. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Papandreou I, Cairns RA, Fontana L, Lim AL
and Denko NC: HIF-1 mediates adaptation to hypoxia by actively
downregulating mitochondrial oxygen consumption. Cell Metab.
3:187–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang F, Zhang GY, Xing T, Lu ZY, Li JH,
Peng C, Liu GH and Wang NS: Renalase contributes to the renal
protection of delayed ischaemic preconditioning via the regulation
of hypoxia-inducible factor-1α. J Cell Mol Med. 19:1400–1409. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jian SL, Chen WW, Su YC, Su YW, Chuang TH,
Hsu SC and Huang LR: Glycolysis regulates the expansion of
myeloid-derived suppressor cells in tumor-bearing hosts through
prevention of ROS-mediated apoptosis. Cell Death Dis. 8:e27792017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Elliott RL and Blobe GC: Role of
transforming growth factor beta in human cancer. J Clin Oncol.
23:2078–2093. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Glasgow E and Mishra L: Transforming
growth factor-beta signaling and ubiquitinators in cancer. Endocr
Relat Cancer. 15:59–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beck S, Le Good JA, Guzman M, Ben Haim N,
Roy K, Beermann F and Constam DB: Extraembryonic proteases regulate
nodal signaling during gastrulation. Nat Cell Biol. 4:981–985.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ben-Haim N, Lu C, Guzman-Ayala M,
Pescatore L, Mesnard D, Bischofberger M, Naef F, Robertson EJ and
Constam DB: The nodal precursor acting via activing receptors
induces mesoderm by maintaining a source of its convertases and
BMP4. Dev Cell. 11:313–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eimon PM and Harland RM: Effects of
heterodimerization and proteolytic processing on derriere and nodal
activity: Implications for mesoderm induction in xenopus.
Development. 129:3089–3103. 2002.PubMed/NCBI
|
|
34
|
Strizzi L, Postovit LM, Margaryan NV,
Seftor EA, Abbott DE, Seftor RE, Salomon DS and Hendrix MJ:
Emerging roles of nodal and Cripto-1: From embryogenesis to breast
cancer progression. Breast Dis. 29:91–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen MM: Nodal signaling: Developmental
roles and regulation. Development. 134:1023–1034. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Duan W, Li R, Ma J, Lei J, Xu Q, Jiang Z,
Nan L, Li X, Wang Z, Huo X, et al: Overexpression of nodal induces
a metastatic phenotype in pancreatic cancer cells via the Smad2/3
pathway. Oncotarget. 6:1490–1506. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Strizzi L, Postovit LM, Margaryan NV,
Lipavsky A, Gadiot J, Blank C, Seftor RE, Seftor EA and Hendrix MJ:
Nodal as a biomarker for melanoma progression and a new therapeutic
target for clinical intervention. Expert Rev Dermatol. 4:67–78.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Postovit LM, Margaryan NV, Seftor EA,
Kirschmann DA, Lipavsky A, Wheaton WW, Abbott DE, Seftor RE and
Hendrix MJ: Human embryonic stem cell microenvironment suppresses
the tumorigenic phenotype of aggressive cancer cells. Proc Natl
Acad Sci USA. 105:4329–4334. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Brennan J, Lu CC, Norris DP, Rodriguez TA,
Beddington RS and Robertson EJ: Nodal signaling in the epiblast
patterns the early mouse embryo. Nature. 411:965–969. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cioffi M, Trabulo SM, Sanchez-Ripoll Y,
Miranda-Lorenzo I, Lonardo E, Dorado J, Vieira Reis C, Ramirez JC,
Hidalgo M, Aicher A, et al: The miR-17-92 cluster counteracts
quiescence and chemoresistance in a distinct subpopulation of
pancreatic cancer stem cells. Gut. 64:1936–1948. 2015. View Article : Google Scholar : PubMed/NCBI
|