Introduction
Cervical cancer is the third most common cancer
afflicting women, causing more than 500,000 new cancers and 274,000
deaths annually in the worldwide (1). With screening and treatment modalities
such as chemotherapy, surgery and radiotherapy, cervical cancer
incidence and mortality has decreased and survival has improved.
However, an ~80% increase in new cases has been reported annually
in developing countries and these cases are frequently advanced
cancers (2,3). Several studies have revealed that
sexually active women are susceptible to the human papillomavirus
(HPV) and that the majority of them are cured within 1–2 years.
However, HPV can persist in the cervical epithelium and progress to
cervical cancer (4). According to
the available data women infected with HPV-16 and −18 subtypes tend
to develop cervical cancer more often (5,6).
miRNAs are small non-coding endogenous RNAs 19–25
nucleotides in length that bind to the 3′-untranslated region
(3′-UTR) of their target mRNAs as gene regulators (7). miRNAs regulate gene expression by
translational suppression or mRNA degradation and influence
multiple cellular activities including viability, apoptosis and
signal transduction (5,8). Epidemiologic studies indicated that
genetic variations in the host may also contribute to the
pathogenesis of cervical cancer (9). For example, SNPs in miRNA precursors
may alter the expression of mature miRNA and change target
selection (10). miR-146a precursor
SNP (rs2910164) involves a G>C nucleotide substitution that
changes a G:U pair to a C:U (11).
Higher expression of miR-146a was observed in many solid types of
cancer, such as melanoma, breast and lung cancer (12–14). A
particular miR-146a was found to promote cellular viability
(15–18). However, the functions of miR-146a in
the development of cervical cancer remain unclear.
Multiple targets of miR-146a including interleukin
(IL)-1β, signal transducer, activator transcription 1 (Stat1),
IRAK1 and TRAF6 were observed in several cancer cells and these
targets are involved in cell viability, apoptosis and acute
inflammatory responses (19–22).
miR-146a was reported to limit IRAK1- and TRAF6-mediated signaling
acts as a negative feedback regulator in inflammatory settings
(23). IRAK1 and TRAF6 function as
signal transducers in the nuclear factor-κB (NF-κB) pathway that
activates iκB kinase (IKK) in response to pro-inflammatory
cytokines (24). NF-κB is known to
be a significant signaling factor involved in the progression of
cancers (25,26). Considerable progress towards
unraveling the molecular mechanisms underlying cervical cancer has
been recently achieved, however the identification of potential
novel therapeutic targets is necessary for improving the early
detection and treatment of cervical cancer.
In the present study, to investigate latent
functions of polymorphisms, we investigated the effect of SNP on
the expression of miR-146a in cervical cancer cells, and assessed
the association between miR-146a and both IRAK1 and TRAF6. We
observed that the overexpression of miR-146a promoted the viability
of cervical cancer cells via decreasing IRAK1 and TRAF6, providing
new insights into understanding cervical cancer.
Materials and methods
Cell lines and sample collection
Immortalized non-tumorigenic endocervical End1/E6E7
(CRL-2615) cells were purchased from the American Type Culture
Collection (ATCC; Manassas, VA, USA) and cultured in
keratinocyte-serum free medium (Gibco-BRL 17005-042; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 0.1 ng/ml human
recombinant EGF, 0.05 mg/ml bovine pituitary extract and additional
calcium chloride 44.1 mg/l (final concentration 0.4 mM). HeLa and
C-33A cervical cancer cells were obtained from the Shanghai
Institute of Biochemistry and Cell Biology, Chinese Academy of
Sciences (Shanghai, China). HeLa cells have been reported to
contain human papilloma virus 18 (HPV-18) sequences, while C-33A
cells and CRL-2615 were negative. Both HeLa and C-33A cells were
cultured in DMEM (HyClone, Logan, UT, USA) supplemented with 10%
fetal bovine serum (FBS; Sijiqing Biotechnology Co., Ltd.,
Hangzhou, China), 100 U/ml penicillin and 100 mg/ml streptomycin,
in a humidified atmosphere containing 5% CO2, at
37°C.
Cervical cancer samples were obtained between
January 2013 and January 2016 at the First Affiliated Hospital of
Nanjing Medical University. Twenty cervical cancer tissues and
fifteen specimens of normal cervical tissue were excised surgically
from patients who had previously provided informed written consent.
The study was approved by the Research Ethics Committee of Nanjing
Medical University, and informed consent was obtained from all
patients. Tumor samples and normal tissues were immediately frozen
and stored at −80°C until used. The tumor stage and grade was in
consistence with the International Federation of Gynecologists and
Obstetricians (FIGO).
Construction of the miRNA expression
vector and cell transfection
To investigate the effect of SNP on the expression
of miR-146a, pre-miR-146a consisting of a 370-bp DNA fragment was
amplified from a genomic DNA sample with a miR-146a rs2910164 G
allele or rs2910164 C allele, and cloned into pcDNA3.1(+)
expression plasmid vector (Invitrogen Life Technologies, Carlsbad,
CA, USA). Amplified DNA fragments were then sequenced to confirm
that there were no mutations. For transfections, CRL-2615, HeLa and
C-33A cells were transfected with expression plasmid vector
containing either the G or C allele of miR-146a rs2910164
(pre-miR-146a-G or pre-miR-146a-C), using Lipofectamine 2000
(Invitrogen Life Technologies) according to the manufacturer's
instructions. The pcDNA3.1(+) vector without an insert was used as
a negative control. Transfection efficiency was verified by
real-time PCR.
Quantitative real-time PCR
Total RNA was extracted from the cultured cells
using TRIzol reagent (Takara, Otsu, Japan), according to the
manufacturer's instructions. Bulge-loop miRNA quantitative
real-time (qRT)-PCR primer sets specific for miR-146a were designed
by Guangzhou RiboBio (Guangzhou, China) and expression levels were
normalized against U6. The following primers were used: TRAF6
forward, 5′-TTTGCTCTTATGGATTGTCCCC-3′ and reverse,
5′-CATTGATGCAGCACAGTTGTC-3′; IRAK1 forward,
5′-TGAGGAACACGGTGTATGCTG-3′ and reverse,
5′-GTTTGGGTGACGAAACCTGGA-3′; GAPDH forward, 5′- GAAGGTCG
GAGTCAACGGATTT-3′ and reverse, 5′-CTGGAAGATGGTGATGGGATTTC-3′. cDNA
was synthesized from 1 µg total RNA using random primers and
Superscript II Reverse Transcriptase (Takara) within 20 µl reaction
volumes. Quantitative real-time PCR was performed in an Applied
Biosystems StepOne Real-time PCR system (Thermo Fischer
Scientific), using SYBR-Green PCR kit (Takara) by normalizing to
GAPDH. The 2−∆∆Ct method was used to analyze the
relative miRNA or mRNA expression. All reactions were prepared in
three independent experiments.
Western blotting
Cells were harvested in RIPA lysis buffer containing
protease and phosphatase inhibitors, and the protein concentrations
were determined by BCA protein assay (Beyotime Institute of
Biotechnology, Shanghai, China). Protein samples were separated by
10% SDS-PAGE, and then transferred onto PVDF membranes (Millipore,
Billerica, MA, USA), blocked with TBST containing 5% skimmed milk
for 1 h at 37°C. Cells reacted with the following primary
antibodies: rabbit anti-TRAF6 antibody (1:500; cat. no. BS3684;
Bioworld Technology, Inc., St. Louis Park, MN, USA), rabbit
anti-IRAK1 antibody (1:1,000; cat. no. BS1541; Bioworld
Technology), mouse anti-cleaved caspase-3 antibody (1:1,000; cat.
no. 9661; Cell Signaling Technology, Danvers, MA, USA), cyclin D1
(1:1,000; cat. no. 2922; Cell Signaling Technology) and mouse
anti-β-actin antibody (1:5,000; cat. no. ab8226; Abcam, Cambridge,
MA, USA). After being washed in TBST at room temperature, the
membranes were incubated for 1 h at 37°C with a secondary
HRP-conjugated antibody (1:5,000; Beijing ZhongShan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) and were visualized using
an ECL detection kit (Amercontrol Biosciences, London, UK). Each
experiment was performed in triplicate.
Identification of potential miR-146a
targets and luciferase reporter assay
Potential targets of miR-146a were predicted and
analyzed using TargetScan (http://www.targetscan.org), miRanda (http://www.microrna.org) and PicTar softwares
(https://pictar.mdc-berlin.de).
TargetScan Release 7.0 was the primary source for target
identification and it offered 275 conserved targets, from which we
selected 10 top targets with the highest aggregate P-value. miRanda
and PicTar softwares were used to predict potential targets based
on mirSVR scores <1.50 and PicTar scores >5.0. A wild-type
3′-UTR of the IRAK1 or TRAF6 gene was cloned into the pMIR-Report
luciferase vector (Promega, Madison, WI, USA). Constructs carrying
the mutated fragment of the 3′-UTR of IRAK1 and TRAF6 mRNA without
the putative miR-146a binding sequences were used as the mutated
controls. Subsequently, 293 cells were transfected with the
reporter constructs together with a miR-146a-5p mimic or negative
control (NC), using Lipofectamine 2000 (Invitrogen Life
Technologies). Cells were harvested after 48 h and the activity was
assessed using a Modulus™ luminometer (Turner Biosystems; Thermo
Fisher Scientific). Renilla luciferase activity was used as
a control for transfection efficiency. All assays were conducted in
triplicate and the experiments were prepared in three independent
experiments. IRAK1 and TRAF6 genes were also verified in other
malignancies as previously reported (20).
Cell viability assay and cell-cycle
analysis
HeLa and C-33A cells were cultured at a density of
10,000 cells/well in 96-well plates. Cell culture continued for 24,
48 and 72 h after transfection, and cell viability were performed
using a Cell Counting Kit-8 (CCK-8; Dojindo Laboratories, Kumamoto,
Japan) according to the manufacturer's instructions. The absorbance
value of each well was assessed at 450 nm in a microplate reader.
The data were obtained from five independent cultures and
experiments were repeated in triplicate.
Forty-eight hours after transfection, cells were
harvested and washed twice with cold PBS, and the supernatant was
removed after centrifugation. Subsequently, the cells were
collected and fixed in 75% ice-cold ethanol and incubated at −20 °C
overnight. After being washed, they were stained with 50 mg/ml of
propidium iodide (PI) for DNA content analysis by flow cytometry on
a FACSCalibur system (BD Biosciences, Franklin Lakes, NJ, USA).
Results were expressed as a percentage of cells in each cell-cycle
phase.
Apoptosis analysis
Cells were lifted off the plates, washed twice with
PBS and determined with an Annexin V-FITC/propidium iodide (PI) kit
(BD Biosciences) at room temperature for 15 min in the dark, and
then analyzed by BD Biosciences FACS Calibur Flow Cytometry (BD
Biosciences) according to the manufacturer's protocol. All
experiments were performed independently, in triplicate.
Statistical analysis
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. The results are represented as
the means ± SD from three separate experiments or more. Statistical
analysis was performed by ANOVA for experiments involving more than
two groups. The two-tailed Student's t-test was performed for
comparisons with two groups and P<0.05 was considered to
indicate a statistically significant difference (*P<0.05;
**P<0.01).
Results
The expression of miR-146a in cervical
cancer and impact of SNP on the expression of miR-146a
Two types of human cervical cancer cells were first
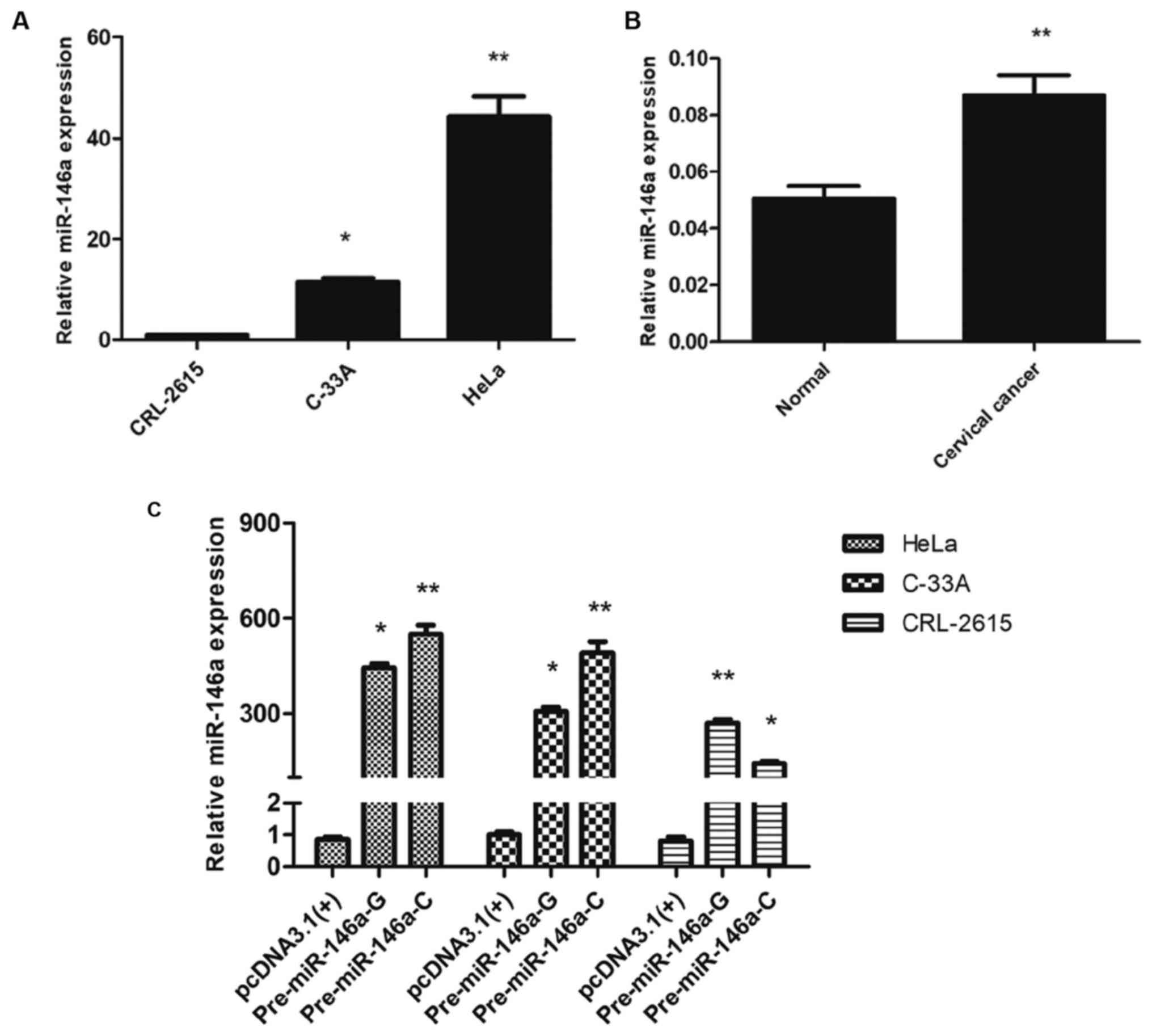
analyzed to quantify the expression levels of miR-146a. Our results
revealed that the expression of miR-146a was increased in these two
cell lines compared with the immortalized non-tumorigenic CRL-2615
cell line (Fig. 1A). Subsequently,
we assessed the expression levels of miR-146a in 20 cervical cancer
tissues and 15 normal cervical tissue samples. The results
demonstrated that the expression of miR-146a was significantly
upregulated in cancer tissues than that in normal tissues
(P<0.01, Fig. 1B). Collectively,
these results indicated that the high expression of miR-146a may be
involved as an oncogene in cervical cancer.
Transfection of recombinant expression plasmids into
HeLa and C-33A cervical cancer cell lines and the immortalized
non-tumorigenic CRL-2615 cell line revealed that the expression of
miR-146a was higher with the C allele compared to the G allele in
HeLa and C-33A cells, while the expression of miR-146a was lower
with the C allele compared to the G allele in CRL-2615 cells
(Fig. 1C). Thus the expression of
miR-146a was upregulated in cervical cancer, and the G>C
substitution in pre-miR-146a increased mature miR-146a expression
in cervical cancer cells, but exerted the opposite effect in
CRL-2615 cells.
Ectopic miR-146a effects on cell
viability and cervical cancer cell cycle
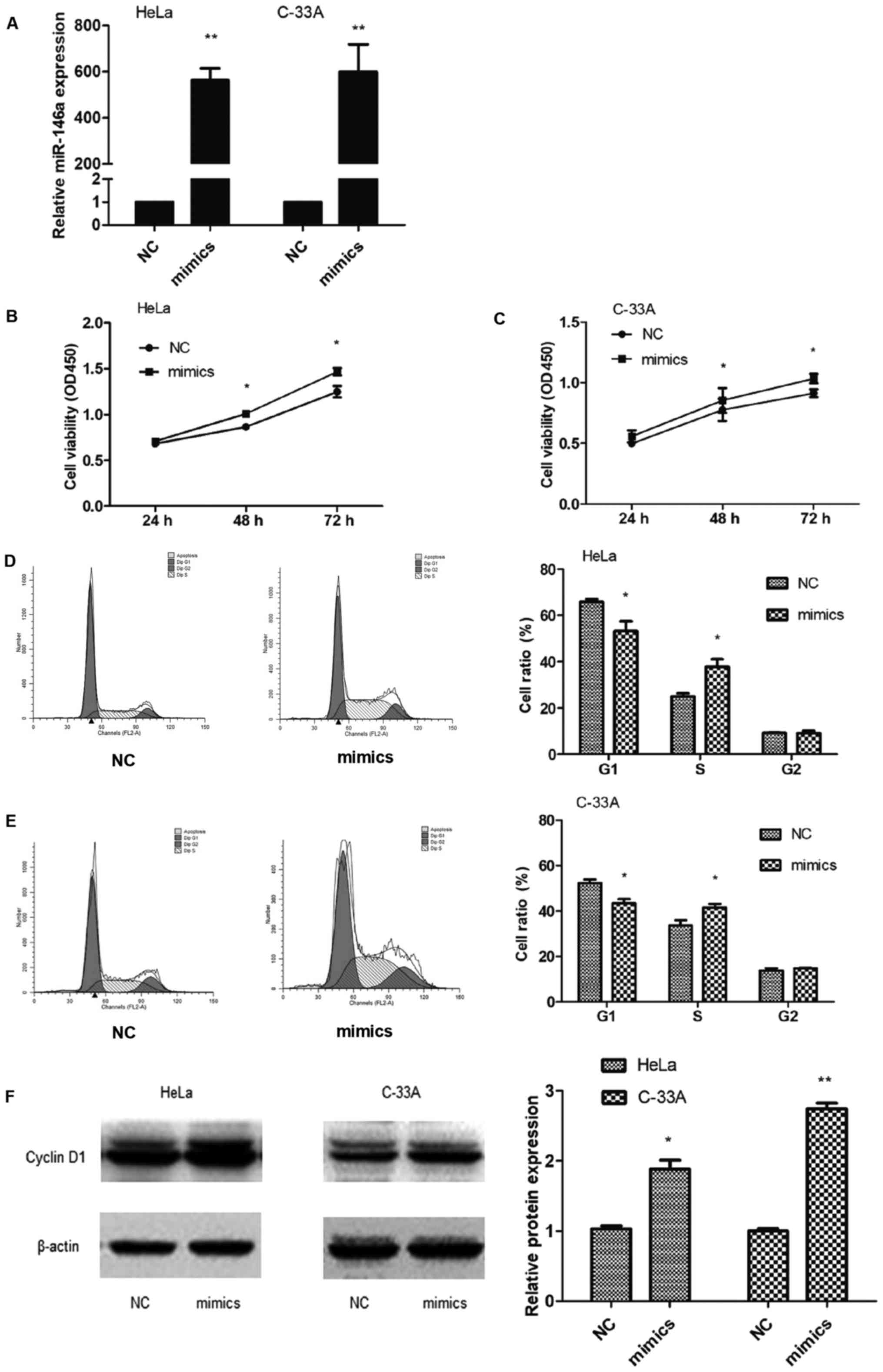
To explore the effect of miR-146a in cervical cancer
carcinogenesis, HeLa and C-33A cells were transiently transfected
with miR-146a mimic or negative control, and assessed for the
expression of miR-146a (Fig. 2A).
miR-146a significantly promoted cell growth in HeLa and C-33A cells
(Fig. 2B and C), indicating that
miR-146a exerted a growth-promoting function in cervical cancer
cells.
Given that miR-146a promotes the viability of
cervical cancer cells, we examined whether miR-146a influenced the
cell cycle progression of cervical cancer cells. The number of
cells in the G1 phase was significantly decreased after
transfection with miR-146a mimic in HeLa (P=0.048) and C-33A
(P=0.022) cells, while cells in the S phase were increased in HeLa
cells (P=0.018) and in C-33A cells (P=0.046) compared to the
controls (Fig. 2D and E).
Therefore, we hypothesized that miR-146a enhanced growth by
promoting cell cycle progression at the G1-to-S-phase transition.
The expression of the oncogene cyclin D1 in HeLa and C-33A cells
was also upregulated following the miRNA-146a mimic transfection
(Fig. 2F), indicating that
miRNA-146a may be an oncogenic regulator in cervical cancer
cells.
Effects of ectopic miR-146a on
apoptosis of cervical cancer cells
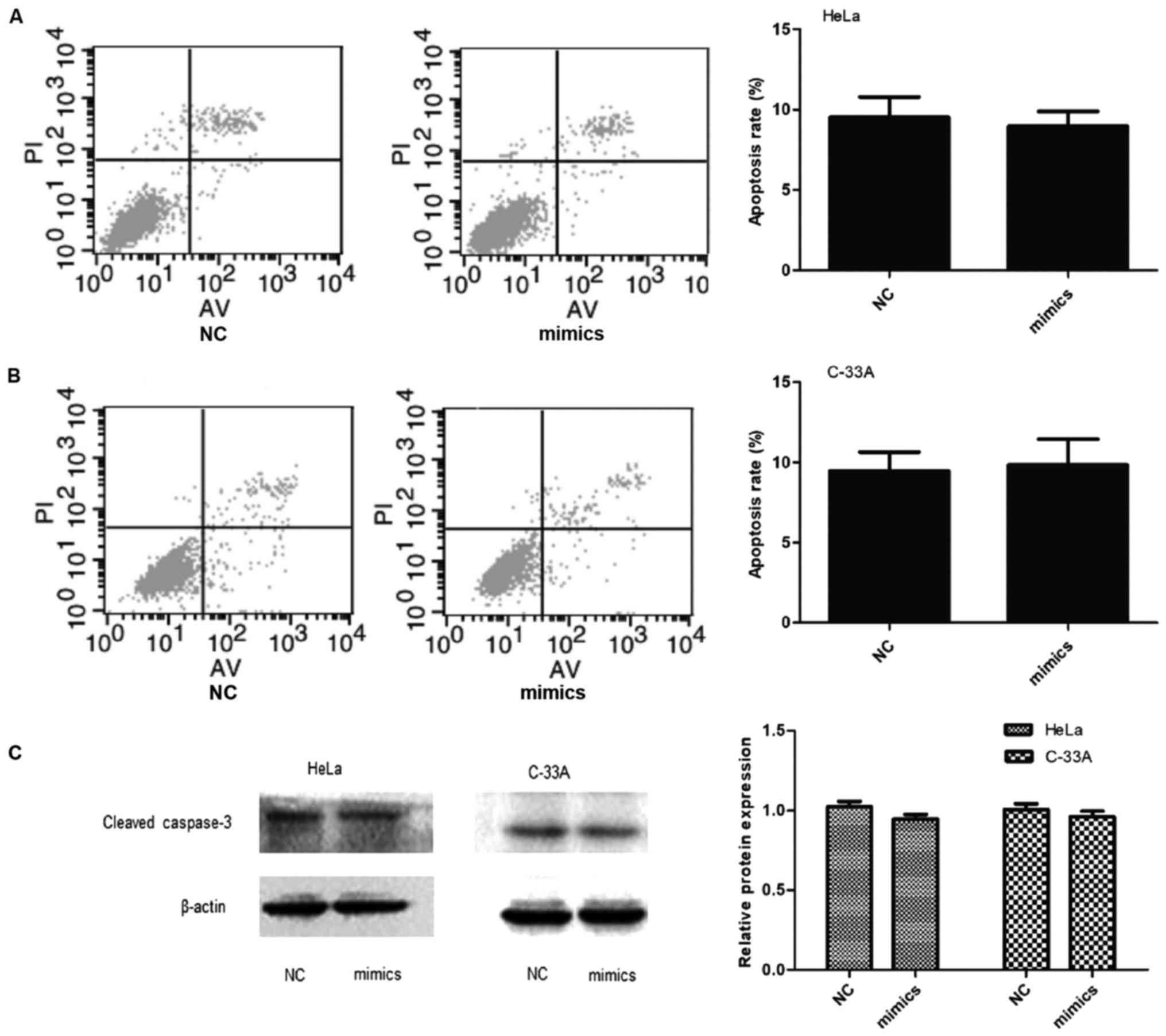
The number of early apoptotic cells was assessed by
flow cytometry and was not significantly decreased in miR-146a
mimic transfected cervical cancer cells (Fig. 3A and B, P>0.05). Western
immunoblotting analysis confirmed that cleaved caspase-3 protein
was not decreased in either cell line after miR-146a overexpression
(Fig. 3C, P>0.05). Thus,
miR-146a in cervical cancer cells does not modify apoptosis.
IRAK1 and TRAF6 are direct targets of
miR-146a in cervical cancer cells
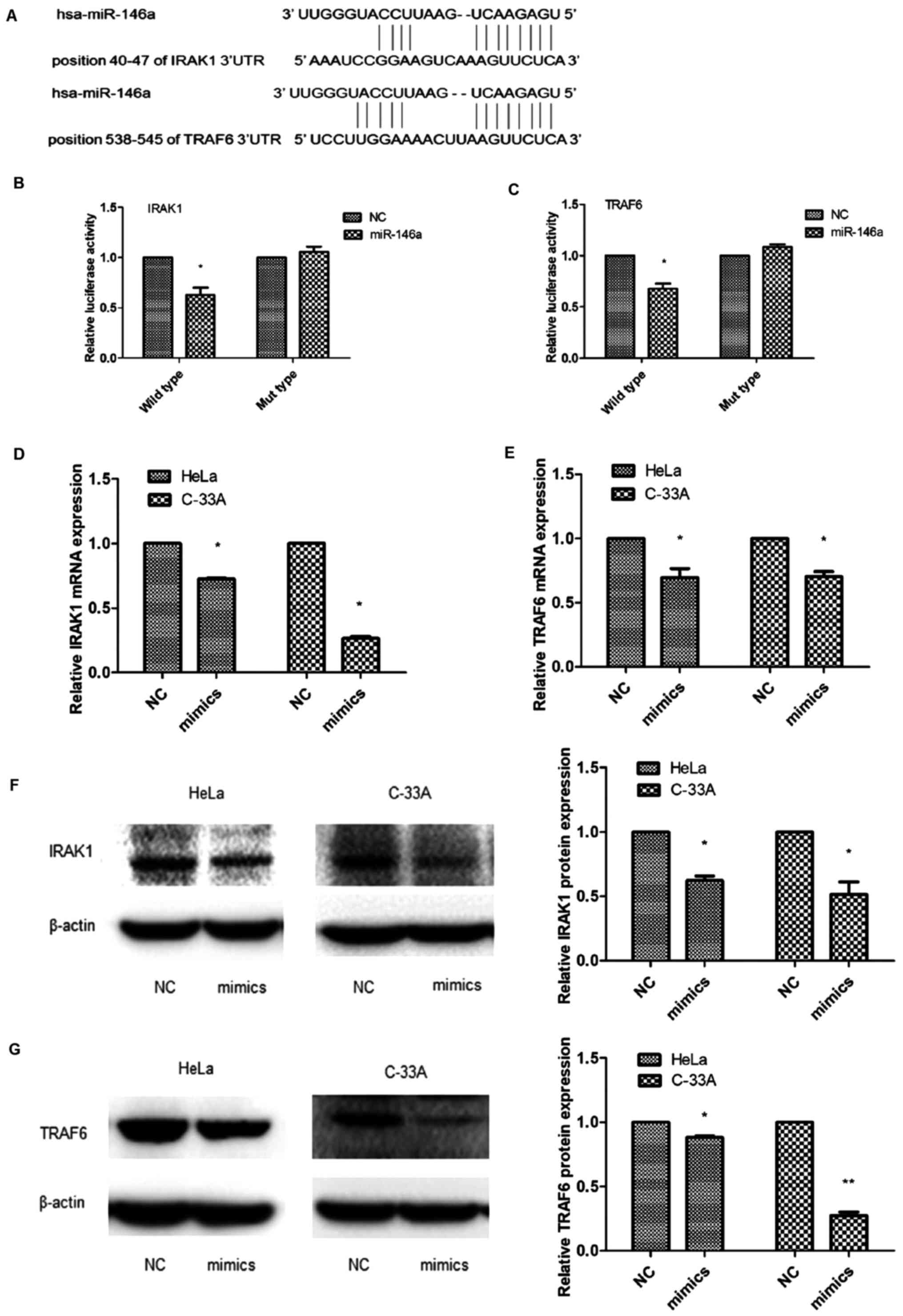
The use of target prediction tools such as
TargetScan, miRanda and PicTar predicted that miR-146a was a
putative regulator of IRAK1 and TRAF6 (Fig. 4A). To confirm that IRAK1 and TRAF6
were targets of miR-146a, we generated a pMIR-Report luciferase
vector containing wild-type 3′-UTR of the IRAK1 and TRAF6 genes. We
co-transfected these constructs into 293 cells together with a
miR-146a mimic or negative control, and then conducted luciferase
activity assays. As displayed in Fig.
4B and C, compared with the control, the overexpression of
miR-146a led to a significant reduction in the luciferase activity
of the wild-type IRAK1 and TRAF6 3′UTR reporter gene in 293 cells
(IRAK1 wild-type, TRAF6 wild-type, P<0.05). To explore the
mechanism of viability promotion induced by miR-146a, we
investigated whether miR-146a regulated the expression of IRAK1 and
TRAF6 in cervical cancer cells. Following miR-146a mimic
transfection, the expression of IRAK1 and TRAF6 mRNA (P<0.05;
Fig. 4D and E) and the protein
expression (P<0.05; Fig. 4F and
G) were decreased with ectopic expression of miR-146a.
miR-146a promotes cell viability by
targeting IRAK1 and TRAF6
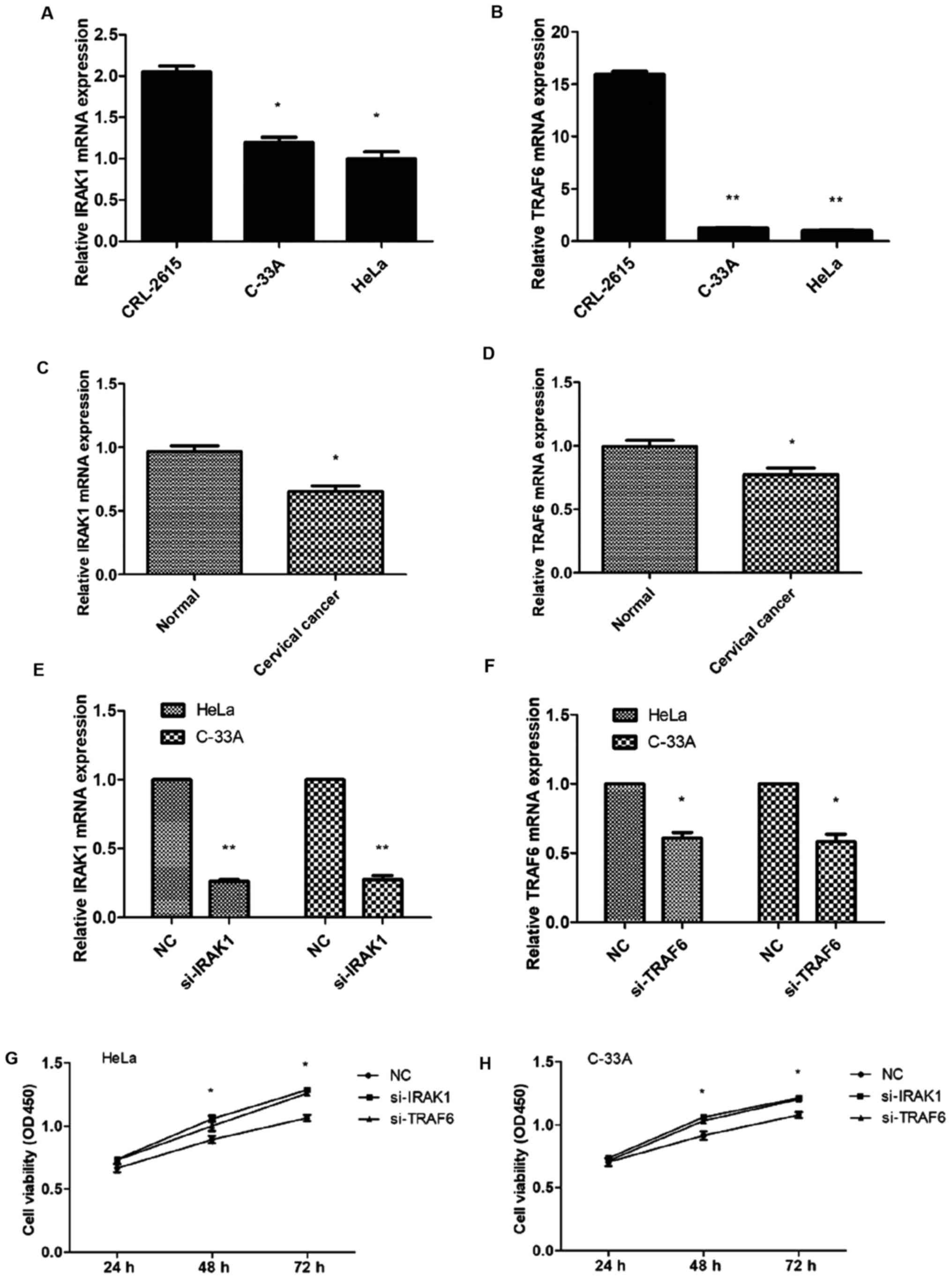
In HeLa and C-33A cells, the expression of IRAK1 and
TRAF6 mRNA was assessed and found decreased compared with CRL-2615
cells (Fig. 5A and B). There was no
difference between the HeLa and C-33A cells. RT-PCR was used to
investigate the mRNA expression levels of IRAK1 and TRAF6 in
cervical cancer tissues (n=20) and normal cervical tissues (n=15).
Both IRAK1 and TRAF6 mRNA expression levels were significantly
downregulated in cervical cancer tissues (Fig. 5C and D). To investigate whether
IRAK1 and TRAF6 were involved in miR-146a-mediated viability of
cervical cancer cells, we infected cervical cancer cells with IRAK1
and TRAF6 siRNAs to downregulate IRAK1 and TRAF6 (Fig. 5E and F). The CCK-8 assay
demonstrated that si-IRAK1 and si-TRAF6 significantly promoted cell
viability in HeLa and C-33A cells (P<0.05; Fig. 5G and H). These findings supported
our contention that miR-146a in cervical cancer cells promoted
tumor cell viability by inhibiting IRAK1 and TRAF6.
Discussion
Understanding tumor cell viability and its
underlying mechanisms can assist us in developing effective
therapeutic strategies that improve survival for patients with
cancer. In this context, miRNAs have been reported to be associated
with cancer cell viability, apoptosis, differentiation, adhesion
and migration (5,8,27).
Wang et al (28) used miRNA
array analyses to compare age-matched normal and cervical cancer
tissues and demonstrated that miR-146a was upregulated in cervical
cancer tissues, and that overexpression of miR-146a promoted cell
viability, while Sathyanarayanan et al (29) reported that miR-146a inhibited
viability, migration and invasion of human cervical cancer cells.
This could be due to the different cervical cancer cells and
diverse trials in their study. In the present study, we reported
that the expression of miR-146a increased in cervical cancer cells
compared with immortalized non-tumorigenic endocervical cells, and
that the expression of miR-146a was greater in HPV-18
sequence-positive HeLa cells than in HPV-18 sequence-negative C-33A
cells. Furthermore, miR-146a gain-of-function tests confirmed that
miR-146a acted as a tumor promoter in our study, and were in
accordance with the study of Wang et al (28).
Sequence variations in miRNA genes, including
pri-miRNAs and pre-miRNAs, could potentially influence the
processing of miRNAs (30). The
miRNA-146a precursor SNP (rs2910164) affects the expression of
mature miR-146a and contributes to a genetic predisposition toward
papillary thyroid carcinoma (11).
Preliminary evidence indicated that these effects were mediated
through target genes that included papillary thyroid carcinoma 1
gene (PTC1), IRAK1 and TRAF6, and that the C allele of mature
miR-146a was less effective in inhibiting these target genes
(11,23). We have previously reported that in
cervical cancer tissue, the miR-146a G>C polymorphism led to
increased production of miR-146a, and that the GG genotype was
associated with a higher risk of cervical cancer (31). In the present study, we discovered
that after transfection with recombinant plasmids, the expression
of miR-146a was higher in the C allele compared to the G allele in
cervical cancer cells, while miR-146a expression was reduced 2-fold
with respect to the C allele, compared to the G allele in CRL-2615
cells. The effect of SNP was not consistent in cervical cancer
cells and immortalized non-tumorigenic endocervical cells.
NF-κB is a significant signaling factor involved in
the development and progression of human cancers and it is the key
protein that has been documented as the critical target for therapy
in human cancers (25,32,33).
NF-κB has a key role in the innate and adaptive immune response of
the host and acts as a tumor suppressor in the initial stages of
HPV infection in cervical cancer cells, but appears to be
downregulated during cancer initiation (33). During the classical NF-κB activation
pathway, IRAK1 and TRAF6 were key regulators that induced the
upregulation of the transcription factor NF-κB activity.
Downregulation of IRAK1 and TRAF6 inhibited TNF-α-induced NF-κB
activation (34). Bhaumik et
al (24) reported that miR-146a
acted as a negative regulator of NF-κB activity by reducing the
metastatic potential of breast cancer cells. miR-146a prevented the
activation of NF-κB by targeting IRAK1 and TRAF6 in cardiomyocytes
and inflammatory monocytic cells (35). In the present study we reported that
the overexpression of miR-146a contributed to cervical cancer
carcinogenic processes via targeting IRAK1 and TRAF6. In addition,
overexpression of miR-146a decreased the expression of cyclin D1
which is an index of cell-cycle progression. The downregulation of
IRAK1 and TRAF6 inhibited the NF-κB activity which inhibited cell
growth, probably allowing HPV persistence and eventually, the
development of cervical cancer.
Liu et al (13) observed that miR-146a can prevent
tumor cell viability and enhance apoptosis as a tumor suppressor by
negatively regulating constitutive NF-κB activity in breast cancer
cells. However, aberrant expression of miR-146a did not affect
apoptosis in our study. Thus, the function of miR-146a may be
dependent on the cell type.
In conclusion, polymorphisms in miR-146a precursor
may be linked to the expression of miR-146a and may be a potential
target for cervical cancer therapy. As the etiology of cervical
cancer remains elusive, additional studies are required to confirm
our present findings.
Acknowledgements
Not applicable.
Funding
The present study was supported in part by a grant
from the Natural Science Foundation of Jiangsu Province (no.
BK2012878).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
SH and BD conceived and designed the study. QH, JS
and JL performed the experiments. QH wrote the manuscript. YC and
SH reviewed and edited the manuscript. QH and JS contributed
equally. All authors read and approved the manuscript and agree to
be accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The study was approved by the Research Ethics
Committee of Nanjing Medical University. Informed consent was
obtained from all patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
de Freitas AC, Gomes Leitão MC and Coimbra
EC: Prospects of molecularly-targeted therapies for cervical cancer
treatment. Curr Drug Targets. 16:77–91. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiffman M, Castle PE, Jeronimo J,
Rodriguez AC and Wacholder S: Human papillomavirus and cervical
cancer. Lancet. 370:890–907. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scheurer ME, Tortolero-Luna G and
Adler-Storthz K: Human papillomavirus infection: Biology,
epidemiology, and prevention. Int J Gynecol Cancer. 15:727–746.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Castellsagué X, Naud P, Chow SN, Wheeler
CM, Germar MJ, Lehtinen M, Paavonen J, Jaisamrarn U, Garland SM,
Salmerón J, et al: Risk of newly detected infections and cervical
abnormalities in women seropositive for naturally acquired human
papillomavirus type 16/18 antibodies: Analysis of the control arm
of PATRICIA. J Infect Dis. 210:517–534. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murchison EP, Stein P, Xuan Z, Pan H,
Zhang MQ, Schultz RM and Hannon GJ: Critical roles for Dicer in the
female germline. Genes Dev. 21:682–693. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Magnusson PK, Lichtenstein P and
Gyllensten UB: Heritability of cervical tumours. Int J Cancer.
88:698–701. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu M, Jolicoeur N, Li Z, Zhang L, Fortin
Y, L'Abbe D, Yu Z and Shen SH: Genetic variations of microRNAs in
human cancer and their effects on the expression of miRNAs.
Carcinogenesis. 29:1710–1716. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jazdzewski K, Murray EL, Franssila K,
Jarzab B, Schoenberg DR and de la Chapelle A: Common SNP in
pre-miR-146a decreases mature miR expression and predisposes to
papillary thyroid carcinoma. Proc Natl Acad Sci USA. 105:7269–7274.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zavala V, Pérez-Moreno E, Tapia T, Camus M
and Carvallo P: miR-146a and miR-638 in BRCA1-deficient triple
negative breast cancer tumors, as potential biomarkers for improved
overall survival. Cancer Biomark. 16:99–107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu R, Liu C, Chen D, Yang WH, Liu X, Liu
CG, Dugas CM, Tang F, Zheng P, Liu Y, et al: FOXP3 Controls an
miR-146/NF-κB Negative Feedback Loop That Inhibits Apoptosis in
Breast Cancer Cells. Cancer Res. 75:1703–1713. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang S, Liu F, Mao X, Huang J, Yang J,
Yin X, Wu L, Zheng L and Wang Q: Elevation of miR-27b by HPV16 E7
inhibits PPARγ expression and promotes proliferation and invasion
in cervical carcinoma cells. Int J Oncol. 47:1759–1766. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu F, Zhang S, Zhao Z, Mao X, Huang J, Wu
Z, Zheng L and Wang Q: MicroRNA-27b up-regulated by human
papillomavirus 16 E7 promotes proliferation and suppresses
apoptosis by targeting polo-like kinase2 in cervical cancer.
Oncotarget. 7:19666–19679. 2016.PubMed/NCBI
|
|
16
|
Peralta-Zaragoza O, Deas J, Meneses-Acosta
A, De la O-Gómez F, Fernández-Tilapa G, Gómez-Cerón C,
Benítez-Boijseauneau O, Burguete-García A, Torres-Poveda K,
Bermúdez-Morales VH, et al: Relevance of miR-21 in regulation of
tumor suppressor gene PTEN in human cervical cancer cells. BMC
Cancer. 16:2152016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leung CO, Deng W, Ye TM, Ngan HY, Tsao SW,
Cheung AN, Pang RT and Yeung WS: miR-135a leads to cervical cancer
cell transformation through regulation of β-catenin via a
SIAH1-dependent ubiquitin proteosomal pathway. Carcinogenesis.
35:1931–1940. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou X, Yue Y, Wang R, Gong B and Duan Z:
MicroRNA-145 inhibits tumorigenesis and invasion of cervical cancer
stem cells. Int J Oncol. 50:853–862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hwang WL, Jiang JK, Yang SH, Huang TS, Lan
HY, Teng HW, Yang CY, Tsai YP, Lin CH, Wang HW, et al:
MicroRNA-146a directs the symmetric division of Snail-dominant
colorectal cancer stem cells. Nat Cell Biol. 16:268–280. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Park H, Huang X, Lu C, Cairo MS and Zhou
X: MicroRNA-146a and microRNA-146b regulate human dendritic cell
apoptosis and cytokine production by targeting TRAF6 and IRAK1
proteins. J Biol Chem. 290:2831–2841. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Perry MM, Moschos SA, Williams AE,
Shepherd NJ, Larner-Svensson HM and Lindsay MA: Rapid changes in
microRNA-146a expression negatively regulate the IL-1beta-induced
inflammatory response in human lung alveolar epithelial cells. J
Immunol. 180:5689–5698. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Contreras JR, Palanichamy JK, Tran TM,
Fernando TR, Rodriguez-Malave NI, Goswami N, Arboleda VA, Casero D
and Rao DS: MicroRNA-146a modulates B-cell oncogenesis by
regulating Egr1. Oncotarget. 6:11023–11037. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hou J, Wang P, Lin L, Liu X, Ma F, An H,
Wang Z and Cao X: MicroRNA-146a feedback inhibits RIG-I-dependent
Type I IFN production in macrophages by targeting TRAF6, IRAK1, and
IRAK2. J Immunol. 183:2150–2158. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ito-Kureha T, Koshikawa N, Yamamoto M,
Semba K, Yamaguchi N, Yamamoto T, Seiki M and Inoue J: Tropomodulin
1 expression driven by NF-κB enhances breast cancer growth. Cancer
Res. 75:62–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kastrati I, Canestrari E and Frasor J:
PHLDA1 expression is controlled by an estrogen
receptor-NFκB-miR-181 regulatory loop and is essential for
formation of ER+ mammospheres. Oncogene. 34:2309–2316.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McManus MT: MicroRNAs and cancer. Semin
Cancer Biol. 13:253–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang X, Tang S, Le SY, Lu R, Rader JS,
Meyers C and Zheng ZM: Aberrant expression of oncogenic and
tumor-suppressive microRNAs in cervical cancer is required for
cancer cell growth. PLoS One. 3:e25572008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sathyanarayanan A, Chandrasekaran KS and
Karunagaran D: microRNA-146a inhibits proliferation, migration and
invasion of human cervical and colorectal cancer cells. Biochem
Biophys Res Commun. 480:528–533. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duan R, Pak C and Jin P: Single nucleotide
polymorphism associated with mature miR-125a alters the processing
of pri-miRNA. Hum Mol Genet. 16:1124–1131. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yue C, Wang M, Ding B, Wang W, Fu S, Zhou
D, Zhang Z and Han S: Polymorphism of the pre-miR-146a is
associated with risk of cervical cancer in a Chinese population.
Gynecol Oncol. 122:33–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sarkar FH, Li Y, Wang Z and Kong D:
Cellular signaling perturbation by natural products. Cell Signal.
21:1541–1547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lin Y, Bai L, Chen W and Xu S: The
NF-kappaB activation pathways, emerging molecular targets for
cancer prevention and therapy. Expert Opin Ther Targets. 14:45–55.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JE, Kim SY, Lim SY, Kieff E and Song
YJ: Role of Ca2+/calmodulin-dependent kinase II-IRAK1
interaction in LMP1-induced NF-κB activation. Mol Cell Biol.
34:325–334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gao M, Wang X, Zhang X, Ha T, Ma H, Liu L,
Kalbfleisch JH, Gao X, Kao RL, Williams DL, et al: Attenuation of
cardiac dysfunction in polymicrobial sepsis by microRNA-146a is
mediated via targeting of IRAK1 and TRAF6 expression. J Immunol.
195:672–682. 2015. View Article : Google Scholar : PubMed/NCBI
|