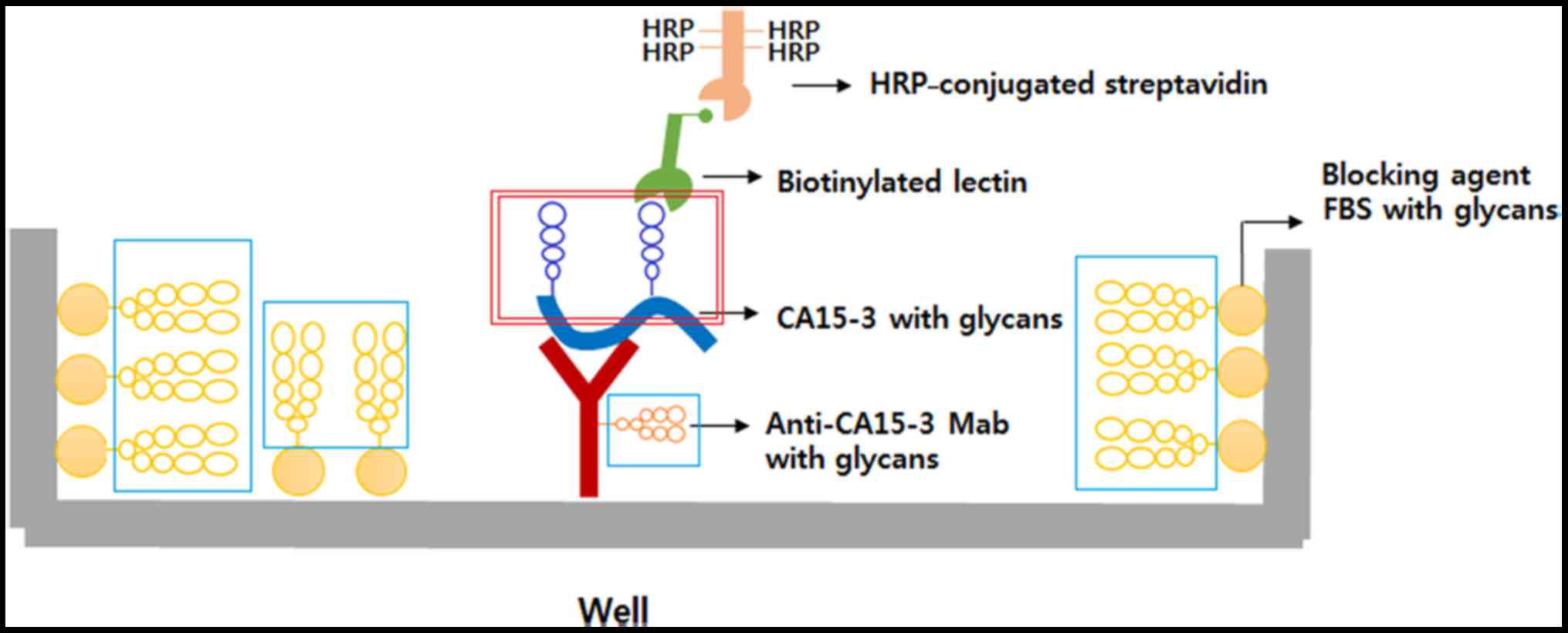
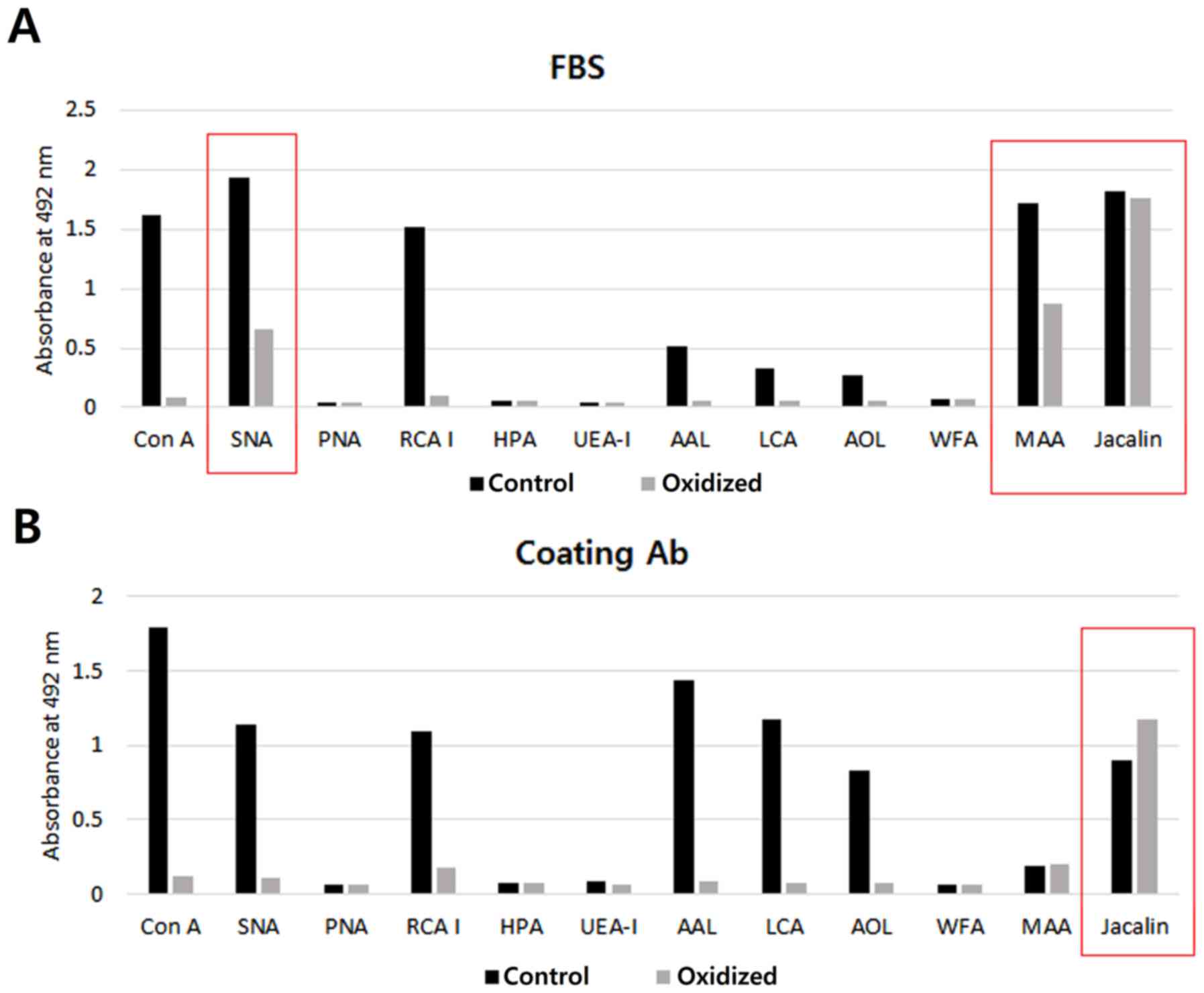
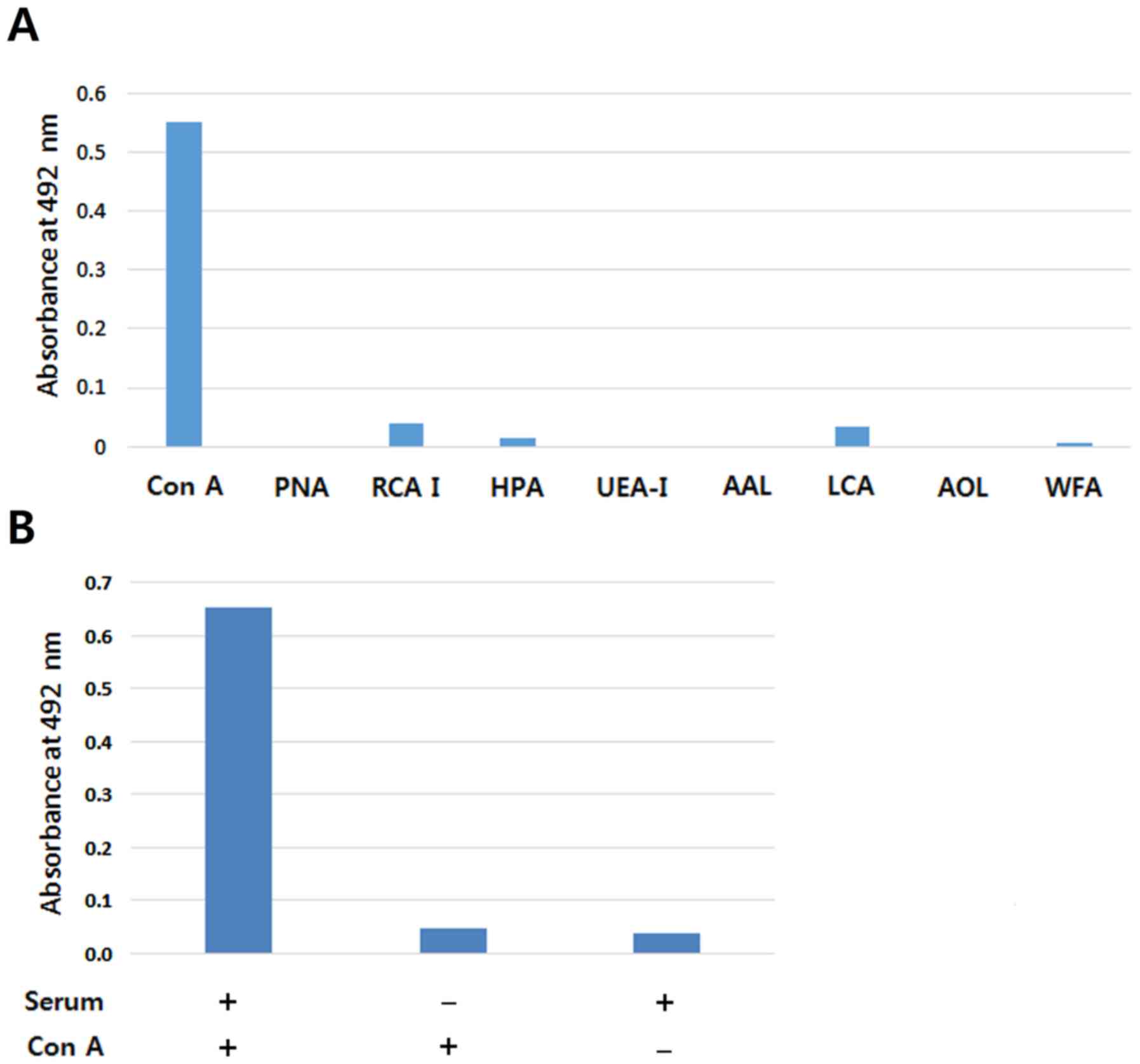
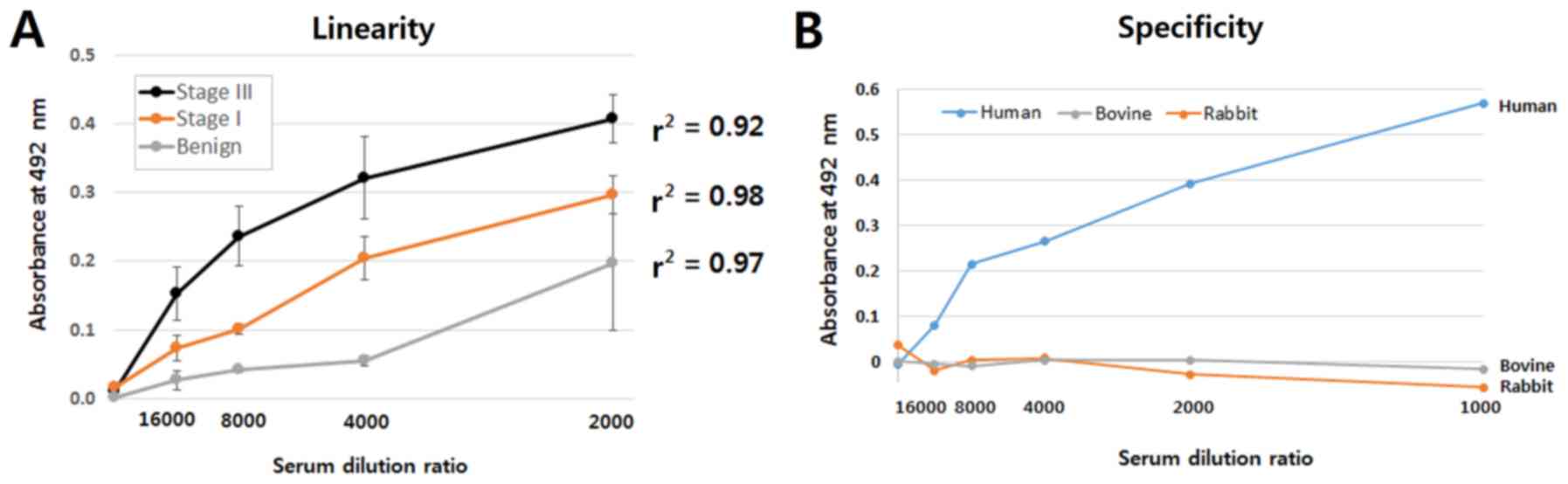
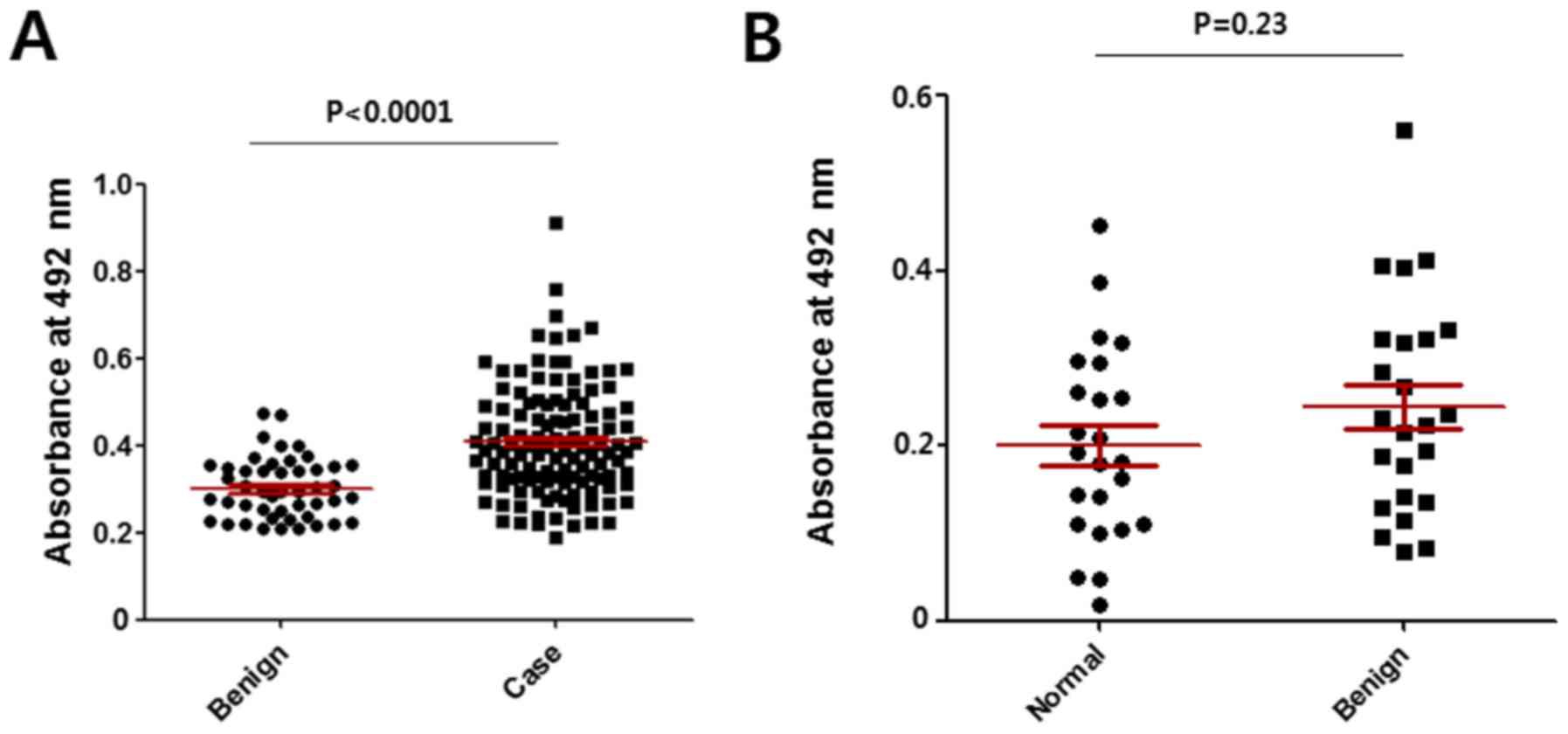
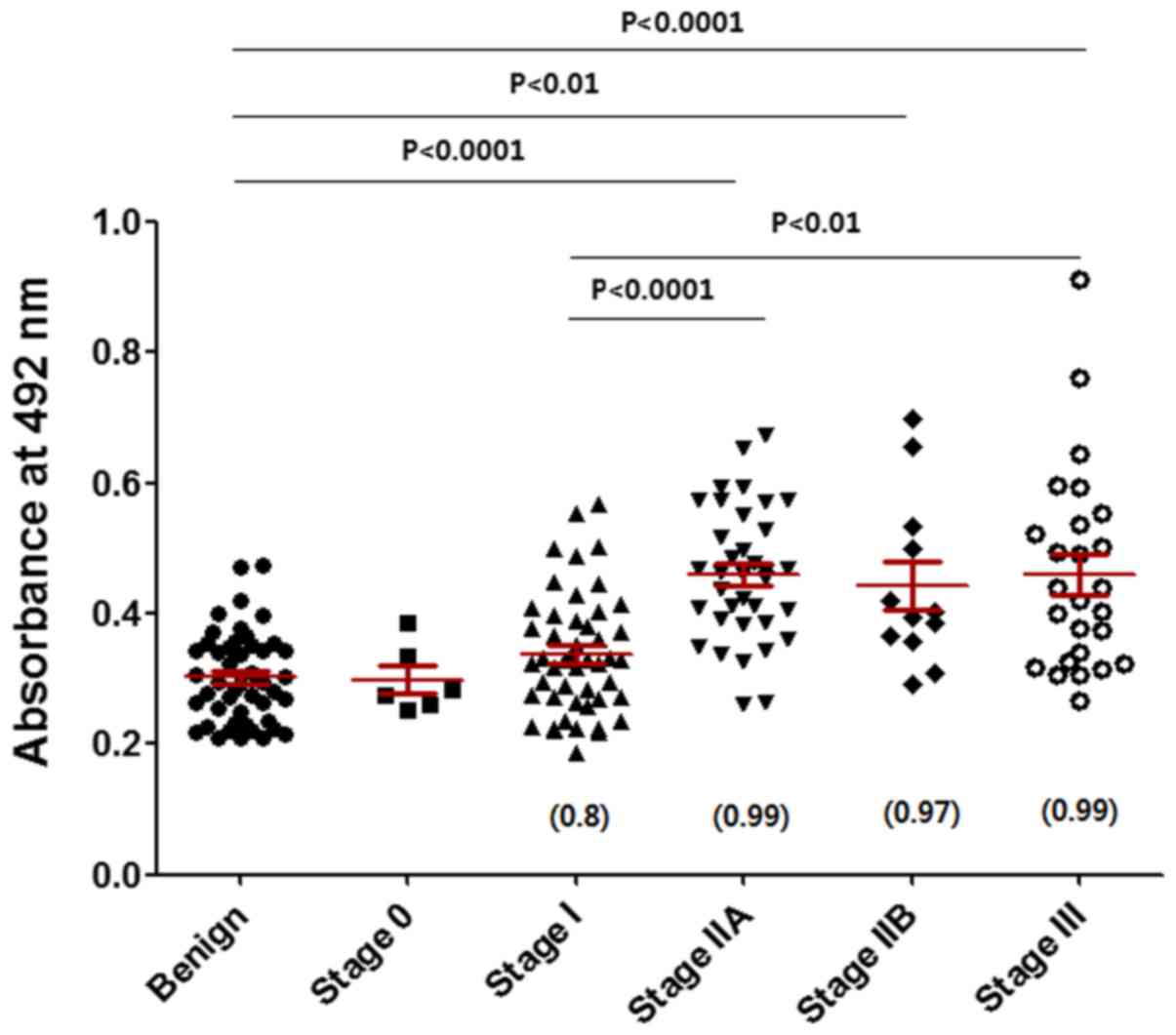
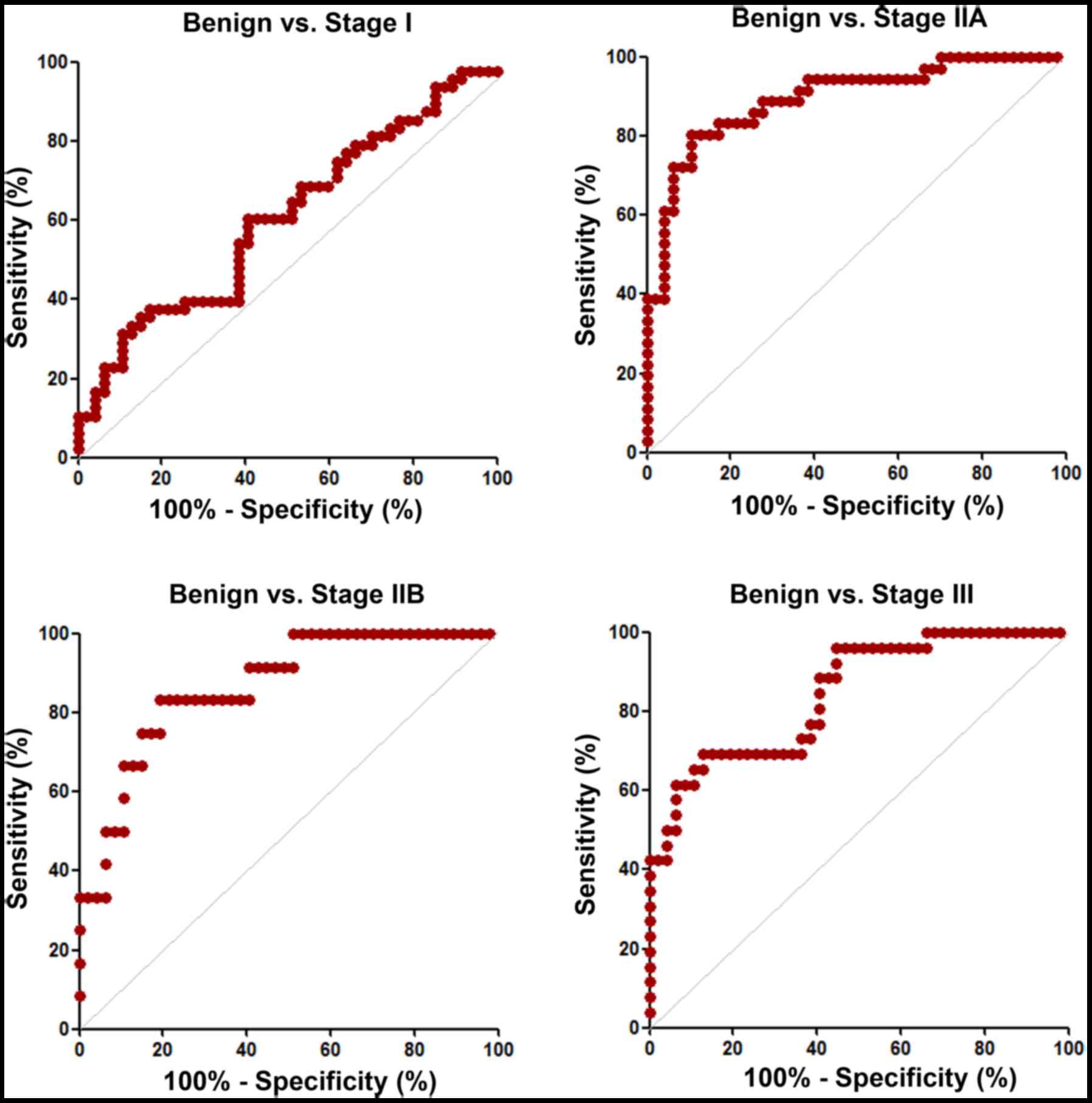
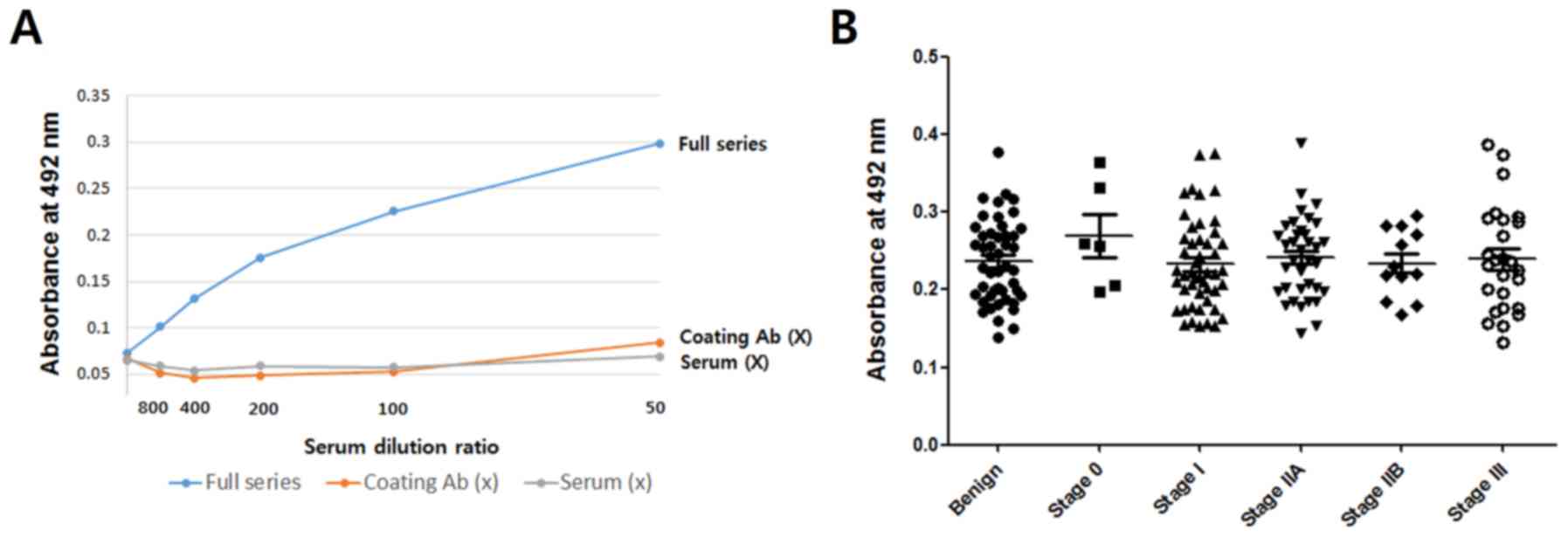
|
1
|
International WCR: Breast cancer
statistics. 16–January. 2015http://www.wcrf.org/int/cancer-facts-figures/data-specific-cancers/breast-cancer-statistics2015.
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global Cancer Statistics, 2012. Ca
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dumalaon-Canaria JA, Hutchinson AD,
Prichard I and Wilson C: What causes breast cancer? A systematic
review of causal attributions among breast cancer survivors and how
these compare to expert-endorsed risk factors. Cancer Causes
Control. 25:771–785. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Misek DE and Kim EH: Protein biomarkers
for the early detection of breast cancer. Int J Proteomics.
2011:3435822011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chou CP, Peng NJ, Chang TH, Yang TL, Hu C,
Lin HS, Huang JS and Pan HB: Clinical roles of breast 3T MRI, FDG
PET/CT, and breast ultrasound for asymptomatic women with an
abnormal screening mammogram. J Chin Med Assoc. 78:719–725. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nath S and Mukherjee P: MUC1: A
multifaceted oncoprotein with a key role in cancer progression.
Trends Mol Med. 20:332–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duffy MJ, Evoy D and McDermott EW: CA
15-3: Uses and limitation as a biomarker for breast cancer. Clin
Chim Acta. 411:1869–1874. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kufe D, Inghirami G, Abe M, Hayes D,
Justi-Wheeler H and Schlom J: Differential reactivity of a novel
monoclonal antibody (DF3) with human malignant versus benign breast
tumors. Hybridoma. 3:223–232. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Duffy MJ, Shering S, Sherry F, McDermott E
and O'Higgins N: CA 15-3: A prognostic marker in breast cancer. Int
J Biol Markers. 15:330–333. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harris L, Fritsche H, Mennel R, Norton L,
Ravdin P, Taube S, Somerfield MR, Hayes DF and Bast RC Jr: American
Society of Clinical Oncology: American society of clinical oncology
2007 update of recommendations for the use of tumor markers in
breast cancer. J Clin Oncol. 25:5287–5312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Munkley J and Elliott DJ: Hallmarks of
glycosylation in cancer. Oncotarget. 7:35478–35489. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Storr SJ, Royle L, Chapman CJ, Hamid UMA,
Robertson JF, Murray A, Dwek RA and Rudd PM: The O-linked
glycosylation of secretory/shed MUC1 from an advanced breast cancer
patient's serum. Glycobiology. 18:456–462. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lavrsen K, Madsen CB, Rasch MG, Woetmann
A, Odum N, Mandel U, Clausen H, Pedersen AE and Wandall HH:
Aberrantly glycosylated MUC1 is expressed on the surface of breast
cancer cells and a target for antibody-dependent cell-mediated
cytotoxicity. Glycoconj J. 30:227–236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kuzmanov U, Kosanam H and Diamandis EP:
The sweet and sour of serological glycoprotein tumor biomarker
quantification. BMC Med. 11:312013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hashim OH, Jayapalan JJ and Lee CS:
Lectins: An effective tool for screening of potential cancer
biomarkers. Peer J. 5:e37842017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Madiyalakan R, Kuzma M, Noujaim AA and
Suresh MR: An antibody-lectin sandwich assay for the determination
of CA125 antigen in ovarian cancer patients. Glycoconj J.
13:513–517. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bamrungphon W, Prempracha N, Bunchu N,
Rangdaeng S, Sandhu T, Srisukho S, Boonla C and Wongkham S: A new
mucin antibody/enzyme-linked lectin-sandwich assay of serum MUC5AC
mucin for the diagnosis of cholangiocarcinoma. Cancer Lett.
247:301–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HJ and Lee SJ: Antibody-based
enzyme-linked lectin assay (ABELLA) for the sialylated recombinant
human erythropoietin present in culture supernatant. J Pharm Biomed
Anal. 48:716–721. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cummings RD and Etzler ME: Antibodies and
lectins in glycan analysis. 2009.
|
|
20
|
Zhang L, Luo S and Zhang BL: The use of
lectin microarray for assessing glycosylation of therapeutic
proteins. MAbs. 8:524–535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bansil R and Turner BS: Mucin structure,
aggregation, physiological functions and biomedical applications.
Curr Opin Colloid In. 11:164–170. 2006. View Article : Google Scholar
|
|
22
|
Taylor C, Allen A, Dettmar PW and Pearson
JP: The gel matrix of gastric mucus is maintained by a complex
interplay of transient and nontransient associations.
Biomacromolecules. 4:922–927. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mahanta S, Fessler SP, Park J and Bamdad
C: A minimal fragment of MUC1 mediates growth of cancer cells. PLoS
One. 3:e20542008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee CS, Taib NAM, Ashrafzadeh A, Fadzli F,
Harun F, Rahmat K, Hoong SM, Abdul-Rahman PS and Hashim OH:
Unmasking heavily o-glycosylated serum proteins using perchloric
acid: Identification of serum proteoglycan 4 and protease c1
inhibitor as molecular indicators for screening of breast cancer.
PLoS One. 11:e1495512016.
|
|
25
|
Bhavanandan VP, Zhu Q, Yamakami K, Dilulio
NA, Nair S, Capon C, Lemoine J and Fournet B: Purification and
characterization of the MUC1 mucin-type glycoprotein, epitectin,
from human urine: Structures of the major oligosaccharide alditols.
Glycoconj J. 15:37–49. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hanisch FG, Stadie TRE, Deutzmann F and
PeterKatalinic J: MUC1 glycoforms in breast cancer - Cell line T47D
as a model for carcinoma-associated alterations of O-glycosylation.
Eur J Biochem. 236:318–327. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brockhausen I, Yang JM, Burchell J,
Whitehouse C and Taylor-papadimitriou J: Mechanisms underlying
aberrant glycosylation of muc1 mucin in breast-cancer cells. Eur J
Biochem. 233:607–617. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanson RL and Hollingsworth MA: Functional
consequences of differential o-glycosylation of MUC1, MUC4, and
MUC16 (downstream effects on signaling). Biomolecules. 6:E342016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stanley P: Golgi glycosylation. Cold
Spring Harb Perspect Biol. 3:a0051992011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Leoz ML, Young LJ, An HJ, Kronewitter
SR, Kim J, Miyamoto S, Borowsky AD, Chew HK and Lebrilla CB:
High-mannose glycans are elevated during breast cancer progression.
Mol Cell Proteomics. 10:M1102011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Biocompare: Bench tips: tips for reducing
elisa background. http://www.biocompare.com/Bench-Tips/122704-Tips-for-Reducing-ELISA-Background/2012
|
|
32
|
Kirwan A, Utratna M, O'Dwyer ME, Joshi L
and Kilcoyne M: Glycosylation-based serum biomarkers for cancer
diagnostics and prognostics. Biomed Res Int. 2015:4905312015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takeda Y, Shinzaki S, Okudo K, Moriwaki K,
Murata K and Miyoshi E: Fucosylated haptoglobin is a novel type of
cancer biomarker linked to the prognosis after an operation in
colorectal cancer. Cancer. 118:3036–3043. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thompson R, Creavin A, O'Connell M,
O'Connor B and Clarke P: Optimization of the enzyme-linked lectin
assay for enhanced glycoprotein and glycoconjugate analysis. Anal
Biochem. 413:114–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sharon N and Lis H: History of lectins:
From hemagglutinins to biological recognition molecules.
Glycobiology. 14:53R–62R. 2004. View Article : Google Scholar : PubMed/NCBI
|